Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlling simonkolleite crystallisation via metallic Zn oxidation in a betaine hydrochloride solution
Shaoqing
Qu
a,
Eftychios
Hadjittofis
ab,
Francisco
Malaret
 cd,
Jason
Hallett
cd,
Jason
Hallett
 c,
Rachel
Smith
a and
Kyra Sedransk
Campbell
c,
Rachel
Smith
a and
Kyra Sedransk
Campbell
 *a
*a
aThe University of Sheffield, Department of Chemical and Biological Engineering, Sheffield, UK. E-mail: k.sedransk@sheffield.ac.uk
bUCB Pharma SA Belgium, Brussels, Belgium
cImperial College London, Department of Chemical Engineering, London, UK
dNanomox Ltd., London, UK
First published on 2nd March 2023
Abstract
Zinc oxide nanoparticles, with a hexagonal flake structure, are of significant interest across a range of applications including photocatalysis and biomedicine. Simonkolleite (Zn5(OH)8Cl2·H2O), a layered double hydroxide, is a precursor for ZnO. Most simonkolleite synthesis routes require precise pH adjustment of Zn-containing salts in alkaline solution, and still produce some undesired morphologies along with the hexagonal one. Additionally, liquid-phase synthesis routes, based on conventional solvents, are environmentally burdensome. Herein aqueous ionic liquid, betaine hydrochloride (betaine·HCl), solutions are used to directly oxidise metallic Zn, producing pure simonkolleite nano/microcrystals (X-ray diffraction analysis, thermogravimetric analysis). Imaging (scanning electron microscopy) showed regular and uniform hexagonal simonkolleite flakes. Morphological control, as a function of reaction conditions (betaine·HCl concentration, reaction time, and reaction temperature), was achieved. Different growth mechanisms were observed as a function of the concentration of betaine·HCl solution, both traditional classical growth of individual crystals and non-traditional growth patterns; the latter included examples of Ostwald ripening and oriented attachment. After calcination, simonkolleite's transformation into ZnO retains its hexagonal skeleton; this produces a nano/micro-ZnO with a relatively uniform shape and size through a convenient reaction route.
Introduction
The use of zinc oxide (ZnO) as an additive crosses a diverse range of applications, including skincare,1 photocatalysis,2 electronics,3 and biomedicine.4 Some applications require tightly controlled ZnO, whilst others do not currently have the same high specification requirements. The use of highly-specified ZnO is for applications where specific functionalities of ZnO5 are achieved, these function are linked to particular structural features (including at the nanoscale). Thus, the development of ZnO micro and nanomaterials has been of significant interest;6 more applications may benefit as the both the structure–property relationships are better understood and the ability to achieve these structures is improved. A wide breadth of ZnO morphologies have been reported, using various synthetic approaches, including rod,7 spherical,8 needle-like,9 nanodisk,10 and more complex assembled structures.11,12 Some of the synthetic routes look to intermediates to produce additional morphologies. Li13 reported nanosheets of ZnO formed using simonkolleite as an intermediate; this pathway could serve to synthesise (thin) hexagonal nano and micro disks, on a large scale.Simonkolleite (Zn5(OH)8Cl2·H2O) is a layered double hydroxide (LDH) which was first reported by Schmetzer et al.14 (Fig. 1). The crystal comprises a stack, along the c-axis, of flat layers; these layers are composed of octahedron oxyhydrogen complexes centered on zinc (light grey) and tetrahedrons of the ZnO3Cl complex (dark grey). The structure is completed with water molecules between layers15,16 and Cl atoms preferentially directed into the same interlayer space.
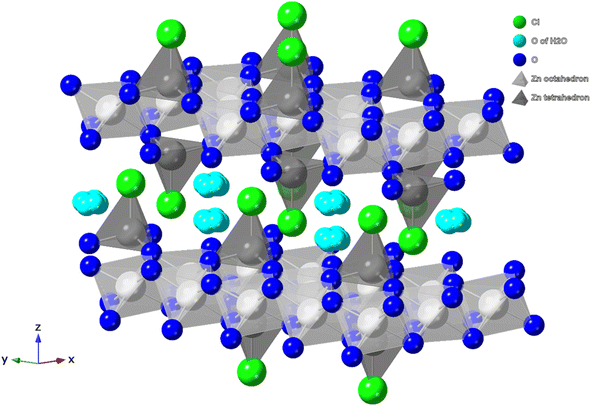 | ||
| Fig. 1 The layered double hydroxide crystal structure of simonkolleite.15,60 Zinc containing octahedron and tetrahedrons are shown (light and dark grey, respectively) with the interlayer spacing containing water. N. b. H atoms are not shown. | ||
Interest in simonkolleite itself stems from several desirable properties. The layered structure has excellent potential as a cation exchange material,17 is popular as a filler for nanocomposites,18 has been employed as a drug carrier in systems requiring slow-release,19–21 and is appealing as a catalyst or sieve.22
Synthesis of simonkolleite has been reported, including by Li,13via precipitation in an ammonia solution, using ZnCl2 as the zinc source. Subsequent calcination of the simonkolleite produced both sheet-like and spindle-like ZnO structures, with a thickness of about 40 nm. Nanosheet ZnO has been confirmed to have good activity as a photocatalyst. However, this process lacks key environmental and sustainability credentials, which severely detracts from its potential in future deployment; the concerns include: (1) the volatility and corrosiveness of ammonia, (2) the precise pH control reportedly required, and (3) the unsustainable and environmentally-damaging waste stream produced. To improve the sustainability of this method, Aida23 reports the use of hibiscus flower extract. However, this change is incremental and does not address the high concentration of ZnCl2 required as a reactant. Other conventional synthetic methods, such as using sodium hydroxide to carry out precipitation, neither provide good morphological control of the products nor a greener chemistry.24–26
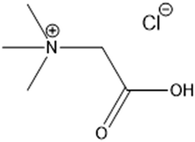
Betaine hydrochloride (betaine·HCl) is in the family of choline carboxylate ionic liquids (Fig. 2). It is composed of a hydrophilic carboxylic tail with a hydrophobic quaternary ammonium salt head. Its amphoteric structure leads to specific aggregation behaviours in the liquid phase, distinguishing it from conventional solvents or salts. It has been suggested that the hydrogen bonding of betaine·HCl cation and water molecules can serve as a mechanism to link two or more molecules; this then serves as a basis for the formation of aggregates.27,28 The self-assembly behaviour of betaine·HCl molecules may affect crystal growth; thus, it has the potential to be used to adjust, and possibly control, crystal structure.29 Furthermore, betaine·HCl can be synthesised from biomass in a sustainable and economical way.30 Compared with conventional solvents or other ionic liquids, such as imidazolium-based ionic liquids, betaine·HCl has lower toxicity and is easily degraded without significant environmental impact.31–33 It has been employed in the metal industry, e.g. rare-earth metal recovery34 and metal complex synthesis.35,36 Our previous work have demonstrated betaine·HCl can dissolve Zn, and has been reported to undergo complexation.37,38 The implications, therefore, include the possibility of inducing the precipitation of simonkolleite with a specific nano/micro structure.39
Currently, to the best of our knowledge, simonkolleite synthesis routes employing conventional solvents rely on precise pH adjustment to prevent competing reactions (in turn, resulting in the formation of other zinc complexes like Zn(OH)2).18 In previous work, an alternative method was developed to produce simonkolleite from zinc using aqueous solutions of 1-butyl-3-methyl-imidazolium chloride.40 Ionic liquid 1-butyl-3-methyl-imidazolium chloride is employed to catalytically oxidise metal Zn. However, the reaction pathway to simonkolleite is both slow and goes through multiple intermediate species. The consequence of this is both a variety of products (including Zn(OH)2, for example) and the yield of simonkolleite is relatively low. It exposed that conventional ionic solutions is difficult to control the morphology of the zinc product, by virtue of the solvent alone without adding additional reagents.
Betaine·HCl can oxidise metal zinc directly, offering an alternative to 1-butyl-3-methyl-imidazolium chloride, inducing a high concentration of Zn2+ ions. However, its differences from 1-butyl-3-methyl-imidazolium chloride present an opportunity to reduce, and potentially all-together avoid, the formation of most competing products or the presence of polymorphism. Meanwhile, it is also different from conventional acids like HCl to form hydrogen and stop at ZnCl2.41 Without manual pH adjusting, Zn in betaine hydrochloride solution, can stably produce high-purity, regular hexagonal sheet-like simonkolleite, which outperforms conventional acids. Thus, this economical green ionic liquid, betaine·HCl, presents excellent potential in synthesising simonkolleite nano/microparticles, which can be subsequently calcined into ZnO nanosheets. Meanwhile, the expected waste stream is deacidified betaine, which is easy to be reused by acidification.42,43
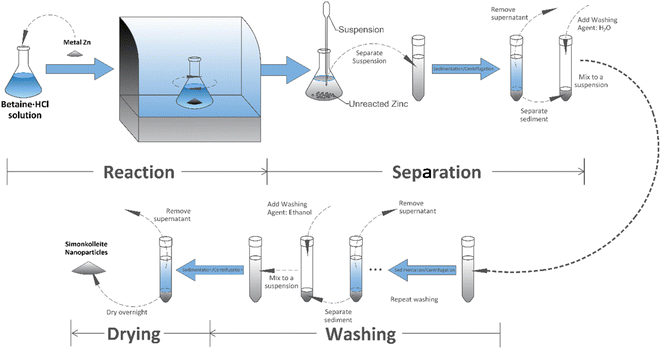
Herein, this work is based on the utilisation of recycled scrap metal Zn to synthesise shape-controllable simonkolleite crystals. The conventional acid and additives are replaced by betaine hydrochloride, as an environmentally-benign solvent to produce simonkolleite through the direct oxidation of a solid Zn metal substrate. Adopting mild and environmentally friendly betaine acid minimised the potential risk to operators and the environment. Simultaneously, this process also eliminates the need to adjust the pH of the solution. It developed a ‘one-pot’ method, which simplified the complexity of the operation as much as possible, to achieve precise regulation of the morphology of simonkolleite. Whilst there are sustainability benefits to removing this salt, in fact, its addition through the solid metal is grounded in more fundamental reasoning. The slow release of zinc ions into the liquid phase is achieved by the moderate reaction rate of betaine·HCl with zinc. The zinc concentration in the liquid phase is essentially being managed, with a view towards maintaining a favourable concentration range for the formation of well-controlled simonkolleite crystals. This ingenious solvent utilisation method has potential in the crystallisation industry. The products were characterised by X-ray diffraction (XRD), thermogravimetric analysis (TGA), and scanning electron microscopy (SEM) to verify the purity of the product simonkolleite and the feasibility of converting it into flaky ZnO. Using this pathway, relatively pure simonkolleite with flaky hexagonal nanocrystals can be obtained. This study mainly investigated the morphological effects of betaine·HCl concentration, reaction time, and reaction temperature on the simonkolleite formed. Additionally, it also obtained ZnO nanoparticles by calcination experiments to verify the feasibility of producing flaky hexagonal ZnO.
Experimental methods
This experiment used metal zinc (Zn grains, 20–35 mesh, 99.8%, Sigma-Aldrich) as the Zn source. Other reagents included betaine·HCl (betaine hydrochloride, PCR reagent, >99.9%, Sigma-Aldrich), methanol (anhydrous, 9, 9.8%, Sigma-Aldrich) and deionised water. All reagents were used as supplied (i.e. without any further purification or treatment).Synthesis of simonkolleite
| Serial number | Betaine·HCl concentration/mass% | Mass of solution/g | Mass of Zn grains/g | Reaction temperature/°C | Reaction time/days |
|---|---|---|---|---|---|
| 1 | 10 | 15 | 3 | 40 | 1 |
| 2 | 3 | ||||
| 3 | 5 | ||||
| 4 | 7 | ||||
| 5 | 15 | ||||
| 6 | 40 | 1 | |||
| 7 | 3 | ||||
| 8 | 5 | ||||
| 9 | 7 | ||||
| 10 | 15 | ||||
| 11 | 40 | 10 | 2 | 160 | 1 |
The products also were characterised by thermogravimetric analysis (TGA 4000, PerkinElmer, Inc.). For each sample, ca. 15 mg was transferred into a crucible. A N2 gas environment with a flow rate of 20 mL min−1 was used. The sample was heated from 30 °C to 800 °C at a rate of 1 °C min−1 and held for 30 minutes. Subsequently, the sample, now calcined, was cooled to room temperature at 5 °C min−1.
Results and discussion
Three variables involved in the synthesis process of simonkolleite were considered: betaine·HCl concentration, reaction time, and reaction temperature. Two betaine·HCl concentrations were probed (‘low’ and ‘high’ water content) as a function of time (up to 15 days) at 40 °C; a separate case investigated the effect of elevated temperature (160 °C).To illustrate key features of the product characterisation, a case study (10% betaine·HCl solution for ten days) is presented in detail below. Subsequently, a discussion of the morphological control of simonkolleite particles (size and thickness), as a function of varying reaction conditions (betaine·HCl concentration, reaction time, and reaction temperature) is presented.
Product characterisation
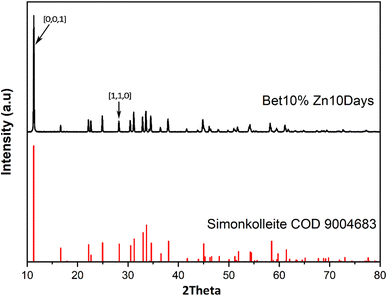
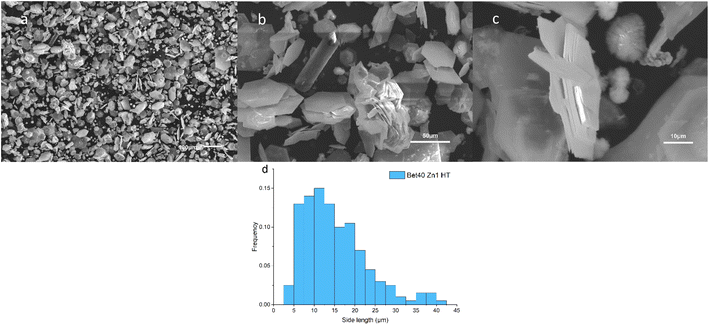
The product from 10% of betaine·HCl after a 10 day reaction period is used as the exemplar herein to illustrate the characterisation undertaken. As previously described, the solid product was recovered through washing steps; subsequent analysis was executed to obtain information on composition and morphology of the solid particles using XRD, SEM, and TGA.For the separated product, a white powder, has XRD peaks strongly consistent with simonkolleite (Zn5(OH)8Cl2·H2O) (Fig. 4), suggesting the synthesised product has relatively high purity. The product has a strong peak where 2θ is ca. 11°, corresponding to the [0,0,1] crystal plane of simonkolleite.13,44 The intensity of the peak attributed to the [0,0,1] plane implies that the end-face is, by far, the dominant face exposed by the crystal. Moreover, it indicates that the growth along the c-axis is inhibited. Taken together this describes the crystals presenting as flat, rather than elongated rods.
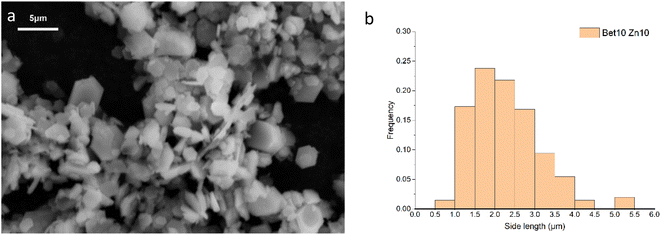
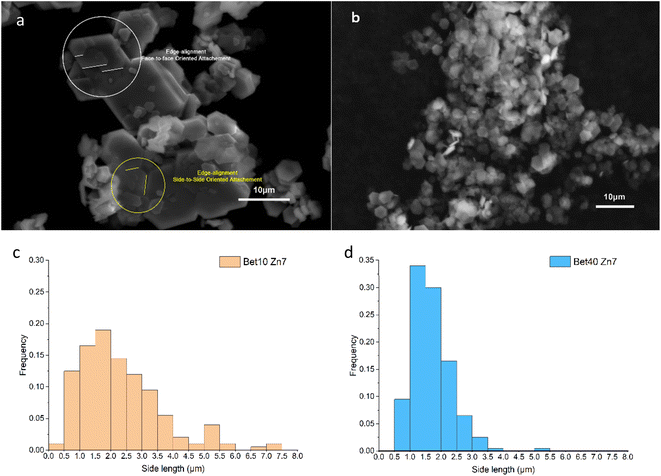
The synthesised simonkolleite was imaged (Fig. 5a) using SEM. These images are consistent and regular hexagonal flake morphology is observed, which is consistent with the typical crystal form of simonkolleite and in good agreement with the analysis of the XRD diffraction pattern obtained. The clean, sharp edges of the crystals are notable and provide strong evidence that product is fully crystalline; this is also consistent with XRD where peaks show good definition. Neither technique gives indication of amorphous material.
The length of one edge of an individual hexagonally shaped crystal was selected as a metric to quantify particle size. The regularity of hexagon side lengths was assessed and found that the edge length variance of three sides for one crystal was, on average for n = 200, <0.01 μm, the effective limit of measurement accuracy and confidence. As such, hexagons measured could be considered regular hexagons within the measurement limit. The distribution of side lengths for n = 200 was in the range between 0.5 μm and 5.5 μm. Due to the clustering of crystals, the thickness cannot be measured reliably; one can only assuredly state that the thickness of a single crystal is <0.5 μm. The presence of clustering and/or sticking will be discussed in later sections. The product characterisation by thermogravimetric analysis (TGA), consistent with previously reported studies for simonkolleite,45 is discussed in a later section.
Effects of reaction conditions on the morphology
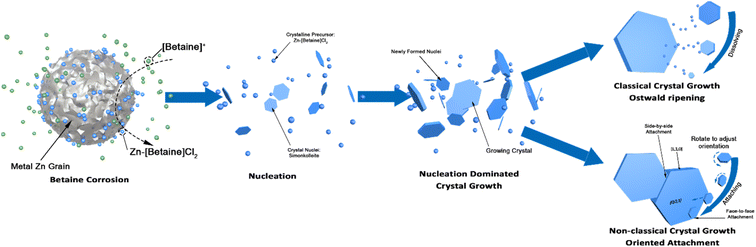
Crystal morphologies are often closely linked to functional properties, providing significant importance in understanding how specific morphologies are achieved. The crystal morphologies of simonkolleite are influenced by the synthesis conditions, including betaine·HCl concentration, reaction temperature, and reaction time.The wide distribution in the 10% betaine·HCl case can be attributed to two different non-classical crystal growth mechanisms: Ostwald ripening46 and oriented attachment (Fig. 7). Initially in the solution, the interaction between metal zinc and betaine·HCl results in the release of crystalline precursors, which are most likely Zn-[betaine]Cl2.47,48 In the early growth stages, the simonkolleite crystals then grow following the classical growth mechanism, from crystalline precursors, into tiny (and growing) crystals.49 However, not all crystal precursors will follow this pathway. When the level of supersaturation decreases, non-classical crystallisation mechanisms will overtake the classical mechanism. This shift will cause tiny crystals to support simonkolleite growth through a sacrificial mechanism, Ostwald ripening. The tiny crystals dissolve, and their dissolution then supports the further growth of other pre-existing (larger) crystals.50
In addition to growth through the Ostwald ripening process, there is also evidence of growth by oriented attachment. Through imaging two observations are made which support the existence of this second mechanism: (1) large-scale aggregation amongst individual crystals and (2) multiple tiny crystals oriented and attached to large crystal surfaces. In particular, a common approach to oriented attachment can be described as face-to-face attaching; the [0,0,1] planes are stacked on each other, resulting in the tiny single crystals merging into thicker large crystals after alignment. Another type of attachment, side-to-side, is also observed; large crystals undergo ordered fusion in the thin side plane. Through these side-to-side connections, multiple hexagons can be aggregated into complex flat clusters at higher scales.51
The oriented attachment is a non-classical mechanism impacting the crystal coalescence process.52 Multiple crystals are induced by the anisotropy of each plane or interaction, such as face-specific van der Waals forces and electrostatic interactions, adjusting the contact angle between crystals to minimise the surface energy.53 Whether it is the attachment of tiny crystals or the fusion of large crystals, the alignment is preferential over disorder. Therefore, it can be deduced that the crystal formation of simonkolleite in 10% of betaine·HCl is affected by the combined action of Ostwald ripening and oriented attachment.
By contrast to the simonkolleite formed at 10%, that which was precipitated from a 40% betaine·HCl solution exhibits a more uniform and smaller size distribution. The crystals are easily identifiable individually and have a single side particle length mean of ca. 1.7 μm and median of ca. 1.6 μm (Fig. 6b and d). The crystals made at these ‘low’ water content conditions have a significantly thinner appearance (so much so that they appear nearly translucent under the SEM). Evidence of growth patterns here are different, with indications pointing to nucleation-dominated crystallisation (not growth-dominant crystallisation). Notably, there is also no observed directional attachment or coalescence.
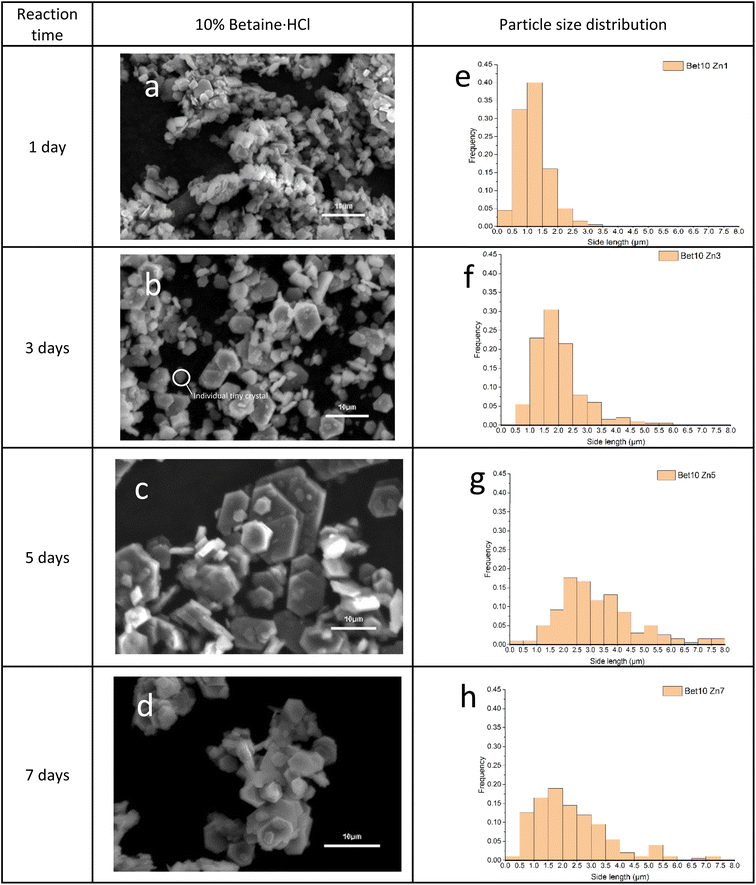
Simonkolleite single-crystals formed in 10% betaine·HCl solutions maintain a hexagonal shape, as a function of time, during the crystallisation growth process (i.e. no observation of any significant transition or polymorphism) (Fig. 8). With increasing time, the hexagonal crystals show an increase in size of both the face and the plate thickness. After one day in 10% betaine·HCl solution (Fig. 8a), the crystals appear as separate hexagonal sheets, suggesting a classical nucleation and growth sequence of mechanisms. The crystal flakes' mean side length was ca. 1.3 μm (Fig. 8e). The thickness of the flakes observed was still relatively thin. With increasing time, two significant changes occurred. Initially, the primary individual crystals increased in size in the [1,0,0] and [1,1,0] directions. A shift in the average side length of the hexagons is evidenced across the period from one to seven days. At seven days, the side length reached a mean of ca. 2.3 μm. However, the increasing average is only one metric; the particle distribution is gradually widening with time. This latter phenomenon is unsurprising as various merging and aggregation behaviours are clearly occurring (Fig. 8b–d), where the various pathways differentially alter the crystal growth, and therefore size (Fig. 8f–h).
During this process, imaging suggests that the number of tiny crystals gradually declined with increasing time. It can be observed that some individual tiny crystals were still present in the third-day sample, while they were rarely found in the seventh-day sample. This pathway illustrates the previously discussed process of nucleation and growth. In the early phase, tiny crystals may grow; however, with increasing time, the majority of the tiny crystal population either have grown themselves or have been incorporated into larger-diameter structures by Ostwald ripening or oriented attachment.
Therefore, single crystals rarely grow individually and independently to a diameter >5 μm. This is evidenced by the fact that crystals of this size no longer appear to be from a single nucleus, and notably the hexagonal shape is maintained regardless of multiple size crystals coordinating. Considered all together, this continues to provide substantial evidence of both Ostwald ripening54 and oriented attachment55,56 occurring in the crystallisation process, furthermore this then also indicates the self-assembly capability of simonkolleite.
Precipitation of crystal product in the 40% of betaine·HCl was not observed, nor could any product be precipitated, after one day. The first solid precipitation was separated successfully after two days (Fig. 9a). Although the product in 40% concentration remains hexagonal shape, the crystal growth process is different from the 10% case. The particle size increased with increasing reaction time; however, the crystal size distribution is not broadening with time as was seen in the 10% case. Individual crystals have a mean side length of ca. 1.5 μm; no crystals' side lengths were ever measured to be >4 μm (Fig. 9e–h). The crystals' thickness can only be assessed semi-quantitatively, due to the nature of the sample and imaging; however, there is virtually no evidence of increasingly thick crystals, as is seen in the 10% case. The crystals do not exhibit attachment mechanisms (of either the face or side), leaving most as individual crystals without agglomeration. The relatively narrow size distribution also suggests that Ostwald ripening is either uncommon or does not occur at all.
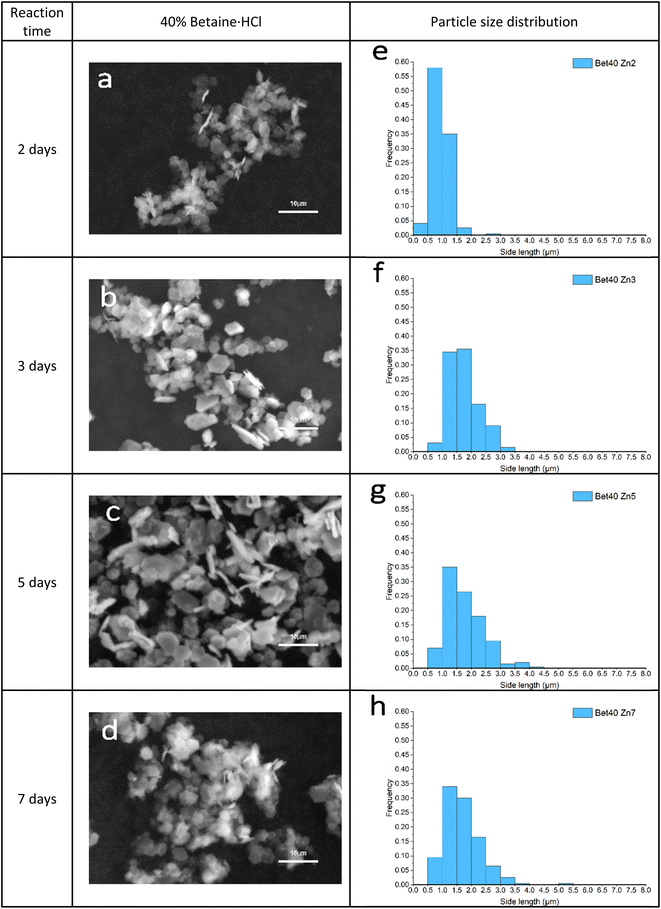 | ||
| Fig. 9 (a–d) SEM images of simonkolleite crystal growth synthesised in 40% of betaine·HCl over time; (e–h) corresponded PSD maps statistical the side lengths of 200 hexagonal crystals. | ||
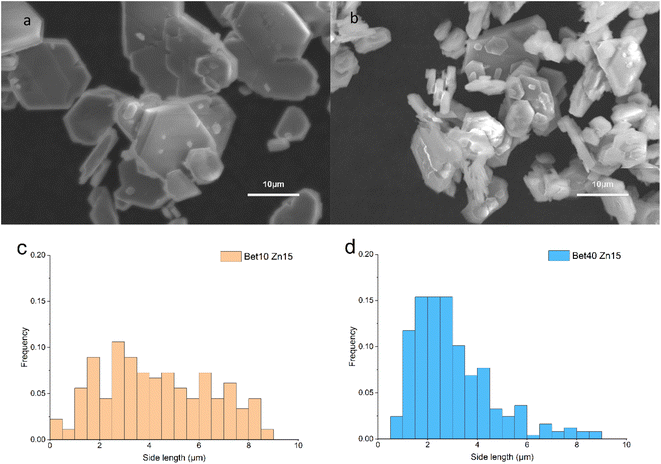
Whilst distinctions were obvious in the samples assessed at seven days, a qualitative look at a longer reaction time of 15 days (Fig. 10) shows fewer notable differences. In both cases, there are several key features observed: (1) some crystals are >10 μm in diameter and (2) there is evidence of attachment and/or merging. What does remain distinguishable, however, is that the particle size distributions are consistent with what was observed at earlier time steps. For 40% betaine·HCl the distribution is narrower, with a ca. 90% of the crystals between 0 and 5 μm for side length. By contrast, the 10% betaine·HCl is much broader in its distribution and with no real peak; to provide a comparison, the proportion of crystals between 0 and 5 μm is only 61.5%.
The first point to address is the appearance of Ostwald ripening, oriented attachment, and assembly behaviours in 40% betaine·HCl concentration at this extended time, whereas these phenomena were not apparent in the assessment at seven days. It can be speculated that the crystallisation precursor concentration remains higher (likely supersaturated) and lasts for longer in the case with 40% betaine·HCl, as compared to that with 10%. This higher, and longer lingering, concentration can be explained by the higher concentration of betaine·HCl resulting in more Zn-containing ions,13,44 which, in turn, provide a key reactant to forming a longer-lasting supply of the simonkolleite precursor, [Zn(betaine)2Cl2] (Fig. 11).
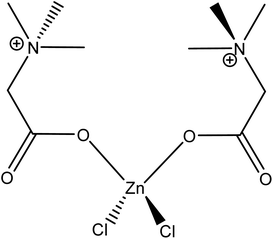 | ||
| Fig. 11 Presumed impurity Zn–betaine complex chemical formula [Zn(betaine)2Cl2].48 | ||
Thus, in the high concentration of betaine·HCl case (40%), the high supersaturation of precursors is the driving force for crystallisation during the initial period, from 2 to 7 days, leading to the critical crystal size remaining at a low level. As such, non-classical behaviours (namely Ostwald ripening) are not preferential during this period. From a thermodynamic standpoint, the surface energy of smaller crystal nuclei is always greater than that of a large crystal; therefore, the growth velocity of crystals is inversely proportional to size (i.e. tiny crystals have a higher rate of growth than large crystals). Newly produced tiny crystals can always catch up in size to larger crystals growing more slowly; overall, this results in narrowing of the crystal size distribution with time (‘size-distribution focusing’).57 However, this classical growth process is limited when the chain is broken, i.e. inadequate precursor concentration (e.g. zinc ions are not available). In this scenario, the supersaturation in the liquid phase then decreases (including dropping below supersaturation levels), resulting in Ostwald ripening overtaking as the dominant crystal growth mechanism. Of course, in cases with 10% betaine·HCl solutions the first period, with classical nucleation exists only briefly, if at all.
A second, competitive, behaviour of betaine·HCl molecules may also be in play, which must be considered, as it has the potential to act as a type of surfactant and, therefore, impact the crystal growth. The tail carboxyl group of betaine·HCl molecules releases hydrogen and leaves the carboxylate anion after the oxidation of metal zinc. The result is the formation of the zwitterion, [betaine]-, which can complex with zinc ions present on the polar face of the crystal. This would make it, effectively, an end-capping agent and inhibit growth along the c-axis direction (the [0,0,1] direction). As a result of this inhibition, this would promote the formation of sheet-like structures58 and encourage thinner structures. Herein, the particles formed at 40% betaine·HCl do appear thinner than those made in 10%. Additionally, there is significant evidence of other competing behaviours still occurring. Namely, signs of Ostwald ripening are apparent where tiny crystals in the solution can orientate and attach to large flakes to form a new layer. Monomers can stick to the raised edges of a new layer to further develop the layer. It allows simonkolleite to grow in the [0,0,1] direction and eventually form a thick hexagonal multilayer structure.
Simonkolleite calcination
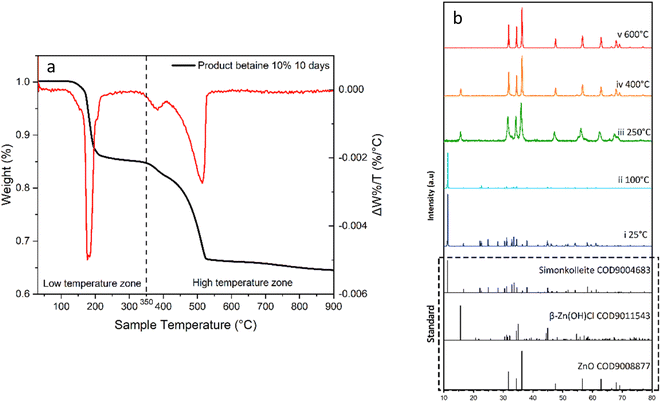
The simonkolleite product was calcined (oxygen-free); SEM imaging was used to demonstrate whether the structural characteristics (i.e. hexagonal shape) were maintained (Fig. 13). The calcination was monitored by thermogravimetric analysis to 900 °C (Fig. 14a). This sample was subsequently characterised by XRD to confirm the conversion to ZnO and. To further establish the decomposition pathway, five individual calcination experiments were conducted to different temperatures (25, 100, 250, 400, and 600 °C) and characterised by XRD (Fig. 14b) on simonkolleite made with 10% of betaine·HCl for ten days.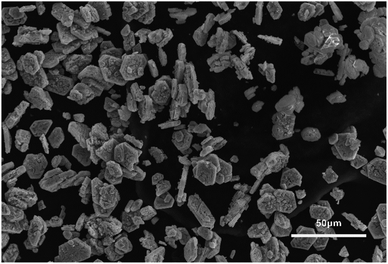 | ||
| Fig. 13 An SEM image of ZnO (confirmed by XRD) taken after the calcination (oxygen-free to 900 °C) of simonkolleite, which itself was formed in 10% of betaine for 15 days. | ||
Three key thermal decomposition reactions of simonkolleite have been identified at <350 °C. The XRD characterisation of the product calcined at 250 °C reveals the Zn(OH)Cl and ZnO (Fig. 14b, iii. green); there is no evidence of simonkolleite. The simonkolleite first decomposes into zinc oxide and 2β-Zn(OH)Cl at 170 °C (water is also released); if this reaction it goes to completion, a mass loss of 13.05% is expected (eqn (1)61).
| Zn5(OH)8Cl2·H2O → 3ZnO + 2β-Zn(OH)Cl + 4H2O (g) | (1) |
Subsequently, 2β-Zn(OH)Cl is further decomposed into zinc oxide (ZnO), hydrated zinc chloride (ZnCl2·0.25H2O), and water at 220 °C (eqn (2)). The water-derived mass loss is 2.44% theoretically, with reaction completion.
| 2β-Zn(OH)Cl → ZnO + ZnCl2·0.25H2O + 0.75H2O(g) | (2) |
| ZnCl2·0.25H2O → 0.25ZnO + 0.5HCl(g) + 0.75ZnCl2 | (3) |
Consider the following cases, where all low-temperature zone reactions occur eqn (1)–(3) to completion. This results in a theoretical cumulative overall mass loss of 18.8%. Experimentally, the mass loss at 250 °C is 16.1%, presenting quite good agreement with the calculated expected loss and simultaneously indicating that all three reactions do not go to completion. The latter statement is consistent with the XRD results where no simonkolleite remains (i.e. eqn (1) goes to completion), but evidence of 2β-Zn(OH)Cl is apparent (i.e. eqn (2) does not go to completion).
Complex decomposition reactions can occur in the high-temperature zone (i.e. above 350 °C). Herein, a significant mass drop is observed in this high-temperature zone reaching an eventual residual mass ca. 67% (of the original mass). The mass loss predominantly occurs between 350 °C to 525 °C, with a small continuing decline after 525 °C. At a calcination temperature of 400 °C, the composition of the calcined product remains 2β-Zn(OH)Cl and ZnO. Notably, a characteristic peak of 2β-Zn(OH)Cl (2θ = 15.603°, [0,0,−2]) is decreasing (Fig. 14b, iv orange) relative to the spectrum at 250 °C. Simultaneously, the intensity of ZnO peaks (2θ = 31.773°, [−1,0,0]; 34.420°, [0,0,−2]; 36.256°, [−1,0,−1]) is increasing relatively speaking. When the temperature is increased to 600 °C, there is no longer evidence of 2β-Zn(OH)Cl and only peaks attributable to ZnO remain (Fig. 14b, v. red).
In the higher temperature region the removal of ZnCl2, via either eqn (4) (direct vaporisation from melt state) or eqn (6), occurs. The remaining 2β-Zn(OH)Cl, which was not converted in the low temperature range, can degrade in the high temperature region via two alternative pathways (eqn (5) or (7)) (Fig. 15).
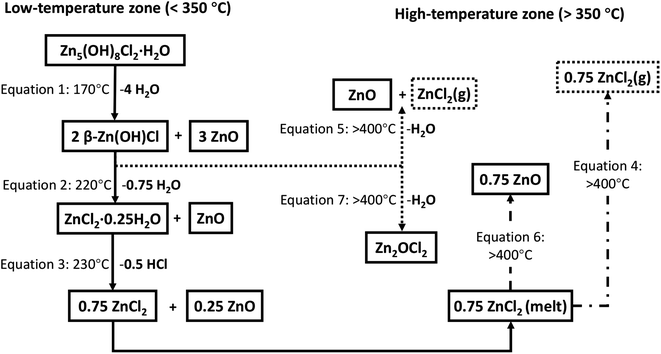 | ||
| Fig. 15 Schematic of a series of reaction equations for the decomposition process of simonkolleite; the residual substances are represented in the solid box, and volatile substances are represented by the dashed box. The arrows represent the reaction direction of the thermal decomposition process, and the solid arrows represent the most likely reactions.45,62,63 | ||
Several potential contributing mechanisms can be considered in this high-temperature conversion. Whilst various mechanisms are possible, it is critical to consider that ZnCl2 is highly hygroscopic and does not tend to melt and evaporate in the presence of water. Anhydrous ZnCl2 is thermodynamically unstable and readily absorbs water from the environment to form hydrates. Therefore, the moisture of calcining environment impacts the decomposition process.64 Jones' research63 suggests ZnCl2 can melt at 320 °C and may vaporise at >400 °C (eqn (4)).
| 0.75ZnCl2(melt) ⇄ 0.75ZnCl2(g) | (4) |
A kinetically dominated reaction releasing water and ZnCl2 (eqn (5)) can take 2β-Zn(OH)Cl reactant and form ZnO. The theoretical volatilisation temperature of ZnCl2 is 400 °C, potentially contributing to the significant mass loss observed between 400 and 535 °C.
| 2β-Zn(OH)Cl → ZnO + ZnCl2(g) + H2O(g) | (5) |
Considering the hygroscopic nature of ZnCl2, an alternative decomposition route of the hydrate, ZnCl2·0.25H2O, likely contributes to the mass losses observed. The result of this pathway are losses from gaseous HCl and H2O along with solid ZnO (eqn (6)). A second alternative pathway for the decomposition of 2β-Zn(OH)Cl has been suggested (eqn (7)).62,65
| 0.75ZnCl2 + 1.5H2O(g) → 0.75 [ZnCl2·2H2O] → 0.75 ZnO + 1.5HCl(g) + 0.75H2O(g) | (6) |
| 2β-Zn(OH)Cl → Zn2OCl2 + H2O(g) | (7) |
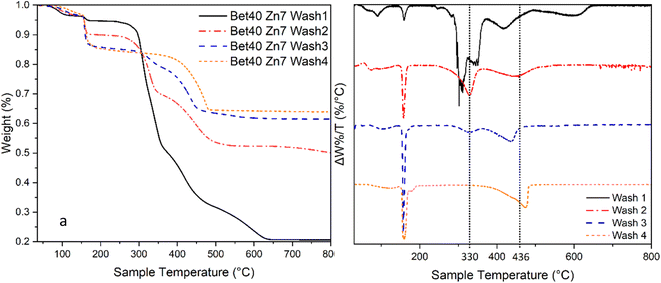
The onset of mass loss at 350 °C suggests that there may be either (1) the humidity in the calciner reducing the volatilisation temperature of ZnCl2 or (2) trapped volatile impurities released in this temperature range (Fig. 16). There is significant evidence that the latter explanation is the cause of this apparent early onset of mass loss. When varying degrees of washing were implemented, there was a significant impact on the losses measured in the range of 330–400 °C. Samples washed fewer times, i.e. higher retention of Zn–betaine complexes in particular, were observed. It is suggested that it is the Zn–betaine complex48,66 (Fig. 11), rather than pure betaine·HCl, because its decomposition temperature (330 °C) is significantly higher than betaine·HCl itself (ca. 250 °C).67,68 As can be seen, the peak at ca. 330 °C is notable because it is virtually eliminated with adequate washing. Therefore, this loss contributes ca. 3% of the total mass loss, leaving 11.2% attributable to the high decomposition reactions of 2β-Zn(OH)Cl and ZnCl2.
Conclusion
In this work, hexagonal flakes of simonkolleite (Zn5(OH)8Cl2·H2O) have been successfully synthesised in a betaine·HCl solution resulting in the direct oxidation of ZnO (oxidative ionothermal synthesis). The product was characterised by XRD and TGA, where high purities of simonkolleite were reached. The size of the simonkolleite crystals, including thickness and diameter, can be adjusted by betaine·HCl concentration, reaction time, and reaction temperature.The concentration of betaine·HCl is a dominant parameter in crystal size; overall, higher betaine·HCl concentration tested (40%) resulted in smaller and thinner simonkolleite flakes than the lower betaine·HCl (10%). Simonkolleite formed at 40% results in crystals preferentially forming individual flakes and do not show a significant tendency towards aggregation or other coalescence behaviours. By contrast, simonkolleite formed with 10% betaine solution showed distinctly different behaviours with the appearance of Ostwald ripening and attachment growth. The result, in these simonkolleite crystals, is that they appear as aggregates rather than as individual crystals. At a longer time for reaction, 15 days, the difference distribution in crystal size was quite notable for the different reaction conditions. The elevated reaction temperature tested showed a broader range of crystals morphologies, by contrast to the tests conducted at 40 °C, which consistently only show hexagonal flake structures. In addition, the calcination of simonkolleite to ZnO retains the hexagonal structure on the micron scale. This investigative work highlights how the production of hexagonally-shaped simonkolleite, or ZnO, can be obtained using an environmentally benign solution. Under suitable conditions, the crystal size can be controlled to be relatively uniform, and the distribution is within 0.5 microns. Moreover, control of the simonkolleite morphology and understanding of the chemistry of formation provide underpinning knowledge to exploit this method for production.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
KSC would like to acknowledge the Royal Society and EPSRC for her Dorothy Hodgkin Research Fellowship (DHF150014).References
- H. Yin and P. S. Casey, ZnO nanorod composite with quenched photoactivity for UV protection application, Mater. Lett., 2014, 121, 8–11 CrossRef CAS.
- C. B. Ong, L. Y. Ng and A. W. Mohammad, A review of ZnO nanoparticles as solar photocatalysts: synthesis, mechanisms and applications, Renewable Sustainable Energy Rev., 2018, 81, 536–551 CrossRef CAS.
- Z. M. Al-Asady, A. H. Al-Hamdani and M. A. Hussein, Study the optical and morphology properties of zinc oxide nanoparticles, AIP Conf. Proc., 2020, 2213, 020061 CrossRef CAS.
- A. O. Dikovska, et al., Fabrication of ZnO nanostructures and their application in biomedicine, Nanophotonics, 2012, 8424, 84242Q Search PubMed.
- Z. X. Lin, Y. A. Zhang, Y. Ye, X. T. Zhou and T. L. Guo, Synthesis and photoelectric properties of ZnO nanostructure with different morphologies via hydrothermal method, Mater. Technol., 2012, 27, 350–354 CrossRef CAS.
- A. Kolodziejczak-Radzimska and T. Jesionowski, Zinc oxide-from synthesis to application: a review, Materials, 2014, 7, 2833–2881 CrossRef CAS PubMed.
- Q. Yao, et al., One-step solvothermal deposition of ZnO nanorod arrays on a wood surface for robust superamphiphobic performance and superior ultraviolet resistance, Sci. Rep., 2016, 6, 1–11 CrossRef PubMed.
- G. Otis, M. Ejgenberg and Y. Mastai, Solvent-free mechanochemical synthesis of zno nanoparticles by high-energy ball milling of ε-zn(Oh)2 crystals, Nanomaterials, 2021, 11, 1–12 CrossRef PubMed.
- K. P. Raj and K. Sadayandi, Effect of temperature on structural, optical and photoluminescence studies on ZnO nanoparticles synthesised by the standard co-precipitation method, Phys. B, 2016, 487, 1–7 CrossRef CAS.
- S. H. Jung, et al., Sonochemical preparation of shape-selective ZnO nanostructures, Cryst. Growth Des., 2008, 8, 265–269 CrossRef CAS.
- I. Yavari, A. R. Mahjoub, E. Kowsari and M. Movahedi, Synthesis of ZnO nanostructures with controlled morphology and size in ionic liquids, J. Nanopart. Res., 2009, 11, 861–868 CrossRef CAS.
- X. Liang, D. Mei, M. Cao, D. Qu and B. Deng, Effects of structural patterns and degree of crystallinity on the performance of nanostructured ZnO as anode material for lithium-ion batteries, J. Alloys Compd., 2015, 455–462, DOI:10.1016/j.jallcom.2014.11.195.
- Y. Li, Y. Zou and Y. Hou, Synthesis and characterisation of simonkolleite nanodisks and their conversion into ZnO nanostructures, Cryst. Res. Technol., 2011, 46, 305–308 CrossRef CAS.
- K. Schmetzer, G. Scnorrer-Koehler and O. Medenbach, Wülfingite, e-Zn(OH)2, and simonkolleite, Zn5(OH)8Cl2·H2O, two new minerals from Richelsdorf, Hesse, FRG, Neues Jahrb. Mineral., Monatsh., 1985, 151, 145–154 Search PubMed.
- F. C. Hawthorne and E. Sokolova, Simonkolleite, Zn5(OH)8Cl2(H2O), a decorated interrupted-sheet structure of the form [MΦ2]4, Can. Mineral., 2002, 40, 939–946 CrossRef CAS.
- K. Schmetzer, G. Scnorrer-Koehler and O. Medenbach, Wülfingite, e-Zn(OH)2, and simonkolleite, Zn5(OH)8Cl2·H2O, two new minerals from Richelsdorf, Hesse, FRG, Neues Jahrb. Mineral., Monatsh., 1985, 151, 145–154 Search PubMed.
- T. Stanimirova, Exchange reactions of zinc hydroxide-sulfate minerals in halide solutions, Appl. Clay Sci., 2019, 168, 396–408 CrossRef CAS.
- S. Nakagaki, et al., Natural and synthetic layered hydroxide salts (LHS): recent advances and application perspectives emphasizing catalysis, Prog. Solid State Chem., 2021, 64, 100335 CrossRef CAS.
- H. Nabipour and M. H. Sadr, Layered zinc hydroxide-ibuprofen nanohybrids: Synthesis and characterisation, Bull. Mater. Sci., 2015, 38, 1561–1567 CrossRef CAS.
- R. Adnan, C. Wong, J. Stanslas, M. Hussein and A. Latip, Release Behavior and Toxicity Profiles towards A549 Cell Lines of Ciprofloxacin from its Layered Zinc Hydroxide Intercalation Compound, 2013 Search PubMed.
- J. Liu, J. Wang and X. Zhang, Intercalation, Characterisation and release behavior of imidacloprid into layered hydroxide salts by coupling of (3-glycidyloxypropyl)trimethoxysilane, Colloids Surf., A, 2018, 553, 42–49 CrossRef CAS.
- T. Hara, J. Kurihara, N. Ichikuni and S. Shimazu, Size control of catalytic reaction space by intercalation of alkylcarboxylate anions into Ni-Zn mixed basic salt interlayer: application for knoevenagel reaction in water, Chem. Lett., 2010, 39, 304–305 CrossRef CAS.
- M. S. Aida, et al., {ZnO} and Simonkolleite Nanocomposite Synthesis via Green Chemistry Using Hibiscus Flower Extract, ECS J. Solid State Sci. Technol., 2021, 10, 123016 CrossRef.
- J. An, M. J. Trujillo-Rodríguez, V. Pino and J. L. Anderson, Non-conventional solvents in liquid phase microextraction and aqueous biphasic systems, J. Chromatogr. A, 2017, 1500, 1–23 CrossRef CAS PubMed.
- J. P. Hallett and T. Welton, Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2, Chem. Rev., 2011, 111, 3508 CrossRef CAS PubMed.
- K. Binnemans, P. T. Jones, Á. Manjón Fernández and V. Masaguer Torres, Hydrometallurgical Processes for the Recovery of Metals from Steel Industry By-Products: A Critical Review, J. Sustain. Metall., 2020, 6, 505 CrossRef.
- A. Amorim da Costa and J. E. S. Leite, Molecular association of betaine and betaine hydrochloride in aqueous solutions – a study by Raman spectroscopy, Biochim. Biophys. Acta, Gen. Subj., 2001, 1525, 161–166 CrossRef CAS PubMed.
- M. Di Gioacchino, F. Bruni and M. A. Ricci, Aqueous solution of betaine: Hydration and aggregation, J. Mol. Liq., 2020, 318, 114253 CrossRef CAS.
- H. Cölfen and S. Mann, Higher-order organisation by mesoscale self-assembly and transformation of hybrid nanostructures, Angew. Chem., Int. Ed., 2003, 42, 2350–2365 CrossRef PubMed.
- N. S. Kaisheva, A. S. Kaishev and M. v. Larsky, Post-Alcohol Corn Dregs as a Pharmaceutical Source for Obtaining Nitrogen-Containing Betaines, Pharm. Chem. J., 2022, 56, 262–269 CrossRef CAS.
- M. Petkovic, et al., Novel biocompatible cholinium-based ionic liquids—toxicity and biodegradability, Green Chemistry, 2010, 12, 643–649 RSC.
- N. Muhammad, et al., Synthesis and physical properties of choline carboxylate ionic liquids, J. Chem. Eng. Data, 2012, 57, 2191–2196 CrossRef CAS.
- P. H. Yancey, M. E. Clark, S. C. Hand, R. D. Bowlus and G. N. Somero, Living with water stress: Evolution of osmolyte systems, Science, 1982, 217, 1214–1222 CrossRef CAS PubMed.
- L. Stoy, V. Diaz and C. H. Huang, Preferential Recovery of Rare-Earth Elements from Coal Fly Ash Using a Recyclable Ionic Liquid, Environ. Sci. Technol., 2021, 55, 9209–9220 CrossRef CAS PubMed.
- L. Wiehl, et al., Structural and magnetic properties of betaine adducts with transition metals: I. ((CH3)3NCH2COO) 3MnMCl4 with M ≤ Mn2+, Co2+, Zn2+, J. Phys.: Condens. Matter, 2006, 18, 11067–11079 CrossRef CAS.
- P. Nockemann, B. Thijs, K. Van Hecke, L. Van Meervelt and K. Binnemans, Polynuclear metal complexes obtained from the task-specific ionic liquid betainium bistriflimide, Cryst. Growth Des., 2008, 8, 1353–1363 CrossRef CAS.
- P. Nockemann, et al., Task-specific ionic liquid for solubilising metal oxides, J. Phys. Chem. B, 2006, 110, 20978–20992 CrossRef CAS PubMed.
- C. Xiao-Ming and T. C. W. Mak, Metal-betaine interactions VII. Crystal and molecular structures of aquadichloro(pyridine betaine)zinc(II), dichlorobis(pyridine betaine)zinc(II) and dichlorobis(betaine)zinc(II) monohydrate, Inorg. Chim. Acta, 1991, 182, 139–144 CrossRef.
- C. Yao, A. Xie, Y. Shen, W. Zhu and J. Zhu, Graphene oxide used as a surfactant to induce the flower-like ZnO microstructures: Growth mechanism and enhanced photocatalytic properties, Cryst. Res. Technol., 2014, 49, 982–989 CrossRef CAS.
- F. Malaret, J. Hallett and K. Sedransk Campbell, Oxidative Ionothermal Synthesis for Micro and Macro Zn-based Materials, Mater. Adv., 2020, 1, 3597 RSC.
- S. Abd El Wanees, et al., Controlling of H2 gas production during Zn dissolution in HCl solutions, J. Mol. Liq., 2017, 248, 943–952 CrossRef CAS.
- N. Araji, et al., Synthesis of maleic and fumaric acids from furfural in the presence of betaine hydrochloride and hydrogen peroxide, Green Chem., 2017, 19, 98–101 RSC.
- A. K. Jangir, N. Bhawna, G. Verma, S. Pandey and K. Kuperkar, Design and thermophysical characterisation of betaine hydrochloride-based deep eutectic solvents as a new platform for CO2 capture, New J. Chem., 2022, 46, 5332–5345 RSC.
- S. Cousy, N. Gorodylova, L. Svoboda and J. Zelenka, Influence of synthesis conditions over simonkolleite/ZnO precipitation, Chem. Pap., 2017, 71, 2325–2334 CrossRef CAS.
- A. Moezzi, M. Cortie and A. McDonagh, Transformation of zinc hydroxide chloride monohydrate to crystalline zinc oxide, Dalton Trans., 2016, 45, 7385–7390 RSC.
- R. D. Vengrenovich, B. V. Ivanski and A. V. Moskalyuk, Generalized Lifshits-Slezov-Wagner distribution, J. Exp. Theor. Phys., 2007, 104, 906–912 CrossRef CAS.
- T. Viswanathan, Water purification compositions and the method of producing the same, J. Franklin Inst., 2020, 139–144 Search PubMed.
- C. Xiao-Ming and T. C. W. Mak, Metal-betaine interactions VII. Crystal and molecular structures of aquadichloro(pyridine betaine)zinc(II), dichlorobis(pyridine betaine)zinc(II) and dichlorobis(betaine)zinc(II) monohydrate, Inorg. Chim. Acta, 1991, 182, 139–144 CrossRef.
- V. I. Kalikmanov Classical nucleation theory, in Nucleation theory, Springer, 201317–41 Search PubMed.
- D. V. Alexandrov, On the theory of Ostwald ripening: Formation of the universal distribution, J. Phys. A: Math. Theor., 2015, 48, 035103 CrossRef.
- J. J. De Yoreo, et al., Crystallisation by particle attachment in synthetic, biogenic, and geologic environments, Science, 2015, 349, 6247 CrossRef PubMed.
- M. Niederberger and H. Cölfen, Oriented attachment and mesocrystals: Non-classical crystallisation mechanisms based on nanoparticle assembly, Phys. Chem. Chem. Phys., 2006, 8, 3271–3287 RSC.
- M. L. Sushko, Understanding the driving forces for crystal growth by oriented attachment through theory and simulations, J. Mater. Res., 2019, 34, 2914–2927 CrossRef CAS.
- D. V. Alexandrov, On the theory of Ostwald ripening: Formation of the universal distribution, J. Phys. A: Math. Theor., 2015, 48, 035103 CrossRef.
- B. B. V. Salzmann, M. M. Van Der Sluijs, G. Soligno and D. Vanmaekelbergh, Oriented Attachment: From Natural Crystal Growth to a Materials Engineering Tool, Acc. Chem. Res., 2021, 54, 787–797 CrossRef CAS PubMed.
- M. Niederberger and H. Cölfen, Oriented attachment and mesocrystals: Non-classical crystallisation mechanisms based on nanoparticle assembly, Phys. Chem. Chem. Phys., 2006, 8, 3271–3287 RSC.
- Y. Yin and A. P. Alivisatos, Colloidal nanocrystal synthesis and the organic–inorganic interface, Nature, 2005, 437, 664–670 CrossRef CAS PubMed.
- M. H. Jung and M. J. Chu, Synthesis of hexagonal ZnO nanodrums, nanosheets and nanowires by the ionic effect during the growth of hexagonal ZnO crystals, J. Mater. Chem. C, 2014, 2, 6675–6682 RSC.
- F. C. Hawthorne and E. Simonkolleite Sokolova, Zn5(OH)8Cl2(H2O), a decorated interrupted-sheet structure of the form [MΦ2]4, Can. Mineral., 2002, 40, 939–946 CrossRef CAS.
- T. Shinagawa, M. Watanabe, J. I. Tani and M. Chigane, (0001)-Oriented Single-Crystal-Like Porous ZnO on ITO Substrates via Quasi-Topotactic Transformation from (001)-Oriented Zinc Hydroxychloride Crystals, Cryst. Growth Des., 2017, 17, 3826–3833 CrossRef CAS.
- S. Cousy, L. Svoboda and Z. Jiří, Basic Precipitation of Simonkolleite Nanoplatelets, Nanocon, 2013, 16–19 Search PubMed.
- S. H. Son and F. Tsukihashi, Vapor pressure measurement of zinc oxychloride, J. Phys. Chem. Solids, 2005, 66, 392–395 CrossRef CAS.
- F. Jones, H. Tran, D. Lindberg, L. Zhao and M. Hupa, Thermal stability of zinc compounds, Energy Fuels, 2013, 27, 5663–5669 CrossRef CAS.
- F. Jones, H. Tran, D. Lindberg, L. Zhao and M. Hupa, Thermal stability of zinc compounds, Energy Fuels, 2013, 27, 5663–5669 CrossRef CAS.
- A. Moezzi, M. Cortie and A. McDonagh, Transformation of zinc hydroxide chloride monohydrate to crystalline zinc oxide, Dalton Trans., 2016, 45, 7385–7390 RSC.
- T. Viswanathan, Water purification compositions and the method of producing the same, J. Franklin Inst., 2020, 139–144 Search PubMed.
- J. Suuronen, I. Pitkänen, H. Halttunen and R. Moilanen, Formation of the main gas compounds during thermal analysis and pyrolysis. Betaine and betaine monohydrate, J. Therm. Anal. Calorim., 2002, 69, 359–369 CrossRef CAS.
- C. Nunes, J. Han, D. C. Monkhouse and R. Suryanarayanan, Preformulation Studies to Meet the Challenges in the Manufacture of Betaine Solid Dosage Form, J. Pharm. Sci., 2004, 93, 38–47 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |