Open Access Article
Open Access ArticleNear-infrared luminescence and high thermal stability of Rb2NaScF6:Cr3+ phosphor for spectroscopy applications†
Lingxiang
Chu
,
Yi
Qin
,
Tao
Yang
,
Jing
Wan
,
Qiang
Zhou
 *,
Huaijun
Tang
*,
Huaijun
Tang
 ,
Yanqing
Ye
and
Zhengliang
Wang
,
Yanqing
Ye
and
Zhengliang
Wang
 *
*
Key Laboratory of Green Chemistry Materials in University of Yunnan Province, Key Laboratory of Advanced Synthetic Chemistry (Yunnan Minzu University) of National Ethnic Affairs Commission, School of Chemistry and Environment, Yunnan Minzu University, Kunming 650500, P. R. China. E-mail: q-zhou@ymu.edu.cn; wangzhengliang@foxmail.com
First published on 13th September 2023
Abstract
The search for chromium-doped near-infrared phosphor for spectroscopy application has attracted widespread concern. Herein, we prepared a double-perovskite fluoride phosphor, Rb2NaScF6:Cr3+ (abbreviated as RNSF:Cr), with wide blue excitation and near-infrared emission bands using a co-precipitation method. The crystal and electronic structures, as well as the luminescent properties were systematically investigated. Evidences show that RNSF offers a weak coordination environment and a wide bandgap for Cr3+ doping. By virtue of the weak electron–phonon coupling effect and the large activation energy, a high photoluminescence thermal stability was observed at 150 °C, with a ratio of 92% of that at 25 °C. In view of the high thermal stability, RNSF:Cr phosphor was incorporated into phosphor-converted light-emitting diodes to evaluate its potential use for near-infrared spectroscopy applications.
Introduction
As an up-and-coming analytical technique, near-infrared (NIR) spectroscopy plays a crucial role in multifunctional application fields of night vision, food inspection and non-contact biomedical imaging owing to the invisibility, strong penetration and non-destruction benign natures of NIR light.1–3 Nowadays, phosphor-converted light-emitting diode (pc-LED) consisting of NIR phosphor with a blue LED chip that offers the benefits of high efficiency, compactness and long lifetime is considered to be the most popular technical solution to emit NIR light and realize portable and real-time detection of product quality and human health.4,5 Considering the vital role of colour conversion in pc-LED device, NIR phosphor with customized spectral features of blue excitation and NIR emission is critically important.6A series of luminescent activators, i.e., Eu2+, Fe3+, Mn4+ and Cr3+, have been utilized one after another to generate NIR emission.7–10 Considering the spectral adaption with commercial blue LED chip and Mn4+ luminescence always locates in red and deep red regions, Cr3+ is considered as a preferable choice for the generation of NIR emission.11 Compared with oxide host, i.e., Mg7Ga2GeO12 or Li2MgZrO4,12,13 the low phonon energy as well as the weak electron–phonon coupling (EPC) strength of fluoride enables its attraction to produce efficient Cr3+-doped NIR phosphor. To date, Cr3+-doped fluorides have attracted widespread attention. The unique [Ar]3d3 electron configuration and the low phonon energy of fluoride empower the generation of Cr3+d–d transition that transfers high-energy blue light to low-energy NIR light in weak crystal field. Until now, a plenty of Cr3+-doped fluoride NIR phosphors have been reported, for instance, Zhou and his colleagues recently prepared a Cr3+-doped K2LiScF6 fluoride phosphor through a combination of hydrothermal and cation exchange methods. However, it presents a medium thermal stability (75%@150 °C).14 Liang and his co-workers prepared a novel LiInF4:Cr3+ phosphors with an ultra-large full width at half-maximum (FWHM) of 210 nm for vein imaging through a hydrothermal method, but its luminescence ratio only preserves 27% at 100 °C.15 It should be noted that photoluminescence (PL) thermal stability is a decisive indicator for evaluating the phosphor's application potential since the high working temperature of LED devices, resulting in the exploration of NIR phosphors with excellent PL thermal stability is of great significance.16–18 Recently, a series of Cr3+-doped fluorides have been endowed high popularity due to their stable structures that enable the excellent thermal stability of Cr3+. For example, Zhou et al. presented a Cs2NaAl3F12:Cr3+ NIR phosphor with an outstanding luminescence maintenance ratio of 101% at 150 °C for vein imaging application.19 Our group also reported a highly thermo-stable LiMgAlF6:Cr3+ far-red-emitting phosphor (93.9%@150 °C) for plant growth lighting.20 However, despite these successful strides, it was found that some examples of Cr3+-activated fluoride NIR phosphors exhibit relatively low PL thermal stability.21,22 Therefore, to address this issue, preparing Cr3+-doped fluoride NIR phosphor with high PL thermal stability is still worthy of investigation.
In this work, we prepared a NIR-emitting RNSF:Cr phosphor with high PL thermal stability through a co-precipitation method. The crystal and electronic structures, as well as the luminescent properties of RNSF:Cr were characterized and investigated. Evidences show that RNSF offers a weak crystal field with a wide bandgap (6.838 eV) for Cr3+ doping. Benefiting from the weak EPC strength (S = 1.15) and large activation energy (ΔE = 0.251 eV), RNSF:Cr exhibits an outstanding thermal stability at 150 °C, with a retention rate of 92% of that at room temperature (RT). Based on this observation, NIR pc-LED devices were prepared and used as radiation sources by coating RNSF:Cr powders on blue LED chips for non-destructive vein imaging and night vision applications. The results indicate that the as-synthesized RNSF:Cr phosphor has promising use in pc-LED device for NIR spectroscopy applications.
Experimental
Materials and preparation
Rubidium carbonate (Rb2CO3, AR), sodium fluoride (NaF, AR), scandium oxide (Sc2O3, AR), ammonium hydrogen fluoride (NH4HF2, AR), chromium nitrate nonahydrate (Cr(NO3)3·9H2O, AR), HF (40 wt% in H2O) and absolute ethanol were purchased from Shanghai Aladdin Biochemical Technology Co. Ltd., China to synthesize RNSF:Cr phosphor. All raw materials were used as supplied without any further purification. (NH4)3CrF6 was prepared using the reported literature method.23 RNSF:Cr phosphor was synthesized through a co-precipitation method at RT. To demonstrate this, we describe the synthesis procedure for RNSF:0.03Cr sample as an example. Typically, 2.5 mmol of Sc2O3 was weighed and dissolved in 5 mL of HF solution under magnetically stirring to form a homogeneous solution. Next, 5 mmol of Rb2CO3, 5 mmol of NaF and 0.15 mmol of (NH4)3CrF6 were successively added into the solution under stirring at RT for 8 h. Then, the light green precipitates were collected, washed and centrifuged with absolute ethanol for several times, and finally dried at 80 °C for 6 h to obtain the final RNSF:0.03Cr product for later characterization. Different molar ratios of (NH4)3CrF6 and Sc2O3 were employed to synthesize a series of RNSF:Cr products with various dopant contents.The chip-on-board (COB) blue chip (Guhoon Optoelectronics Co., Ltd., China) and the as-obtained NIR phosphor were used to fabricate NIR pc-LED device with mass ratio between the UV structural adhesive (Shenzhen Tegu New Materials Co., Ltd., China) and the NIR phosphor of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The LED device was dried at 120 °C for 60 min followed by photoelectric tests.
1. The LED device was dried at 120 °C for 60 min followed by photoelectric tests.
Characterization and computation
The crystal structure and phase purity of the as-prepared RNSF:Cr sample were characterized using a Bruker D8 Advance X-ray diffractometer (XRD) with Cu Kα radiation (λ = 0.15406 nm). The operating voltage, current, scanning rate, angular range, and step size were 40 kV, 40 mA, 10° min−1, 10°–70°, and 0.02°, respectively. The powder diffraction data for Rietveld analysis were carried out on the same diffractometer with a scanning rate of 1° min−1 and an angular range from 5 to 120°. Electron paramagnetic resonance (EPR) spectrum was collected from a Bruker EMXplus-6/1 spectrometer. The morphology and elemental composition were detected from an FEI Nova NanoSEM 450 scanning electron microscope (SEM) with an energy-dispersive X-ray spectrometer (EDS) attachment. The photoluminescence excitation (PLE) and PL spectra, internal quantum efficiency (IQE), and lifetime were recorded from an Edinburgh FLS1000 fluorescence spectrophotometer with an additional integrating sphere. The diffuse reflectance (DR) spectrum was recorded by a Hitachi UH4150 UV-Vis-NIR spectrophotometer using BaSO4 as a reference. The concentration- and temperature-dependent PL spectra were obtained using an AVANTES Avaspec Mini 2048CL-SHB3 fiber spectrophotometer with a temperature heating attachment for warming up and a 460 nm laser diode as the excitation source. Thermogravimetric (TG) data were acquired by a Netzsch TG 209 F1 under nitrogen atmosphere with a heating rate of 10 °C min−1 ranging from 30 to 1000 °C. The electroluminescence spectra of the pc-LEDs were examined by an OHSP-350M LED fast-scan spectrophotometer (Hangzhou Hopoo Light & Color Technology Co., Ltd.). The demonstration images were captured by a Canon XA10E industrial camera loaded with NIR and natural modes. Thermographs of LED device recorded at different driven currents were taken with NIR imaging mode.The Rietveld XRD refinement was performed on a General Structure Analysis System (GSAS) software. The crystal structure was constructed by using a Visualization for Electronic and Structural Analysis (VESTA Ver. 3.4.4) software. The energy bands and electronic structures were estimated by using a CASTEP module of the Materials Studio package based on density functional theory (DFT).
Results and discussion
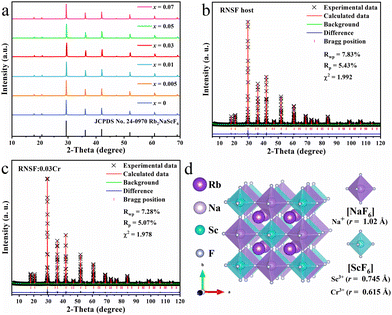
Fig. 1a shows the XRD patterns of RNSF:xCr (x = 0-0.07) samples with various dopant concentrations, in which all diffraction peaks are well indexed into the standard JCPDS card for the cubic Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (225) structure of RNSF (JCPDS No. 24-0970). The intense diffraction peaks and the impurity-free patterns suggest the high degree of crystallinities and the single phases of the as-obtained RNSF:Cr samples, verifying the incorporation of Cr3+ ion does not change the cubic structure of RNSF host, even with the highest doping content.24
m (225) structure of RNSF (JCPDS No. 24-0970). The intense diffraction peaks and the impurity-free patterns suggest the high degree of crystallinities and the single phases of the as-obtained RNSF:Cr samples, verifying the incorporation of Cr3+ ion does not change the cubic structure of RNSF host, even with the highest doping content.24
 | ||
| Fig. 1 (a) XRD patterns of RNSF:xCr (x = 0–0.07) samples. (b) and (c) Rietveld refinement results of RNSF host and RNSF:0.03Cr sample. (d) Crystal structure diagram of RNSF host. | ||
Fig. 1b and c exhibit the refined XRD patterns of RNSF host and a selected RNSF:0.03Cr sample, which were carried out on the GSAS program for the purpose of checking the structural difference after Cr3+ doping. The satisfactory RWP, RP, χ2 factors, that 7.83%, 5.43%, 1.992 for RNSF and 7.28%, 5.07%, 1.978 for RNSF:0.03Cr, suggest that the refinement results are dependable and the crystal structure of RNSF:0.03Cr accords well with RNSF host.25 The refined lattice parameters of RNSF (a = b = c = 8.6098 Å, V = 638.2544 Å3 and Z = 4) and RNSF:0.03Cr (a = b = c = 8.6085 Å, V = 637.9565 Å3 and Z = 4) are listed in Table S1 in ESI,† further verifying that the RNSF:0.03Cr sample is single phase. On the basis of the crystallographic information file established by the refinement results, the crystal structure of RNSF was constructed by using the VESTA program. The built RNSF crystal model is displayed in Fig. 1d, where the coordination environment surrounding Sc and Na can be clearly observed. Apparently, each Sc or Na is connected with six F to form two kinds of octahedral cages, i.e., [NaF6] and [ScF6], in this structural system, while each Rb occupies 12-coordinated cavity to form a regular [RbF12] polyhedron. The three kinds of polyhedral connect with each other into a double perovskite network with Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (225) space group of cubic phase. Considering the ionic radius and ionic charge between Na+ (1.02 Å), Sc3+ (0.745 Å) and Cr3+ (0.615 Å) with the same coordination number, Cr3+ is inclined to substitute the lattice site of Sc3+ since the same valence state and the acceptable ionic radius difference.26 This is also the reason why RNSF:0.03Cr has a shortened lattice parameter and a smaller unit cell volume than those of RNSF host.
m (225) space group of cubic phase. Considering the ionic radius and ionic charge between Na+ (1.02 Å), Sc3+ (0.745 Å) and Cr3+ (0.615 Å) with the same coordination number, Cr3+ is inclined to substitute the lattice site of Sc3+ since the same valence state and the acceptable ionic radius difference.26 This is also the reason why RNSF:0.03Cr has a shortened lattice parameter and a smaller unit cell volume than those of RNSF host.
The EPR spectrum provided in Fig. S1 of ESI† verifies the valence state of trivalent chromium ion and once again confirms that Cr3+ activator has entered RNSF host lattice. The SEM and elemental mapping images shown in Fig. S2 (ESI†) suggest that RNSF:Cr product consists of micro-sized octahedral particulates, in which Cr3+ activators are homogeneously dispersed. Fig. 2a presents the calculated electronic structure of RNSF host on the basis of first principles of DFT. Both the valence band maximum (VBM) and conduction band minimum (CBM) are situated at the G point of Brillouin zone, suggesting the direct bandgap of the host material. The estimated bandgap (6.838 eV) between VBM and CBM is wide enough to accommodate impurity Cr3+ energy levels for the generation of Cr3+ NIR emission, which contributes to suppressing thermal ionization and thereby achieving high thermal stability.27,28 The upper part of the VB is dominated by F p orbitals whist the lower part of the CB is composed of Sc d orbitals, illustrating that the luminescent properties of Cr3+ in RNSF host highly depend on [ScF6] octahedral (Fig. 2b).29
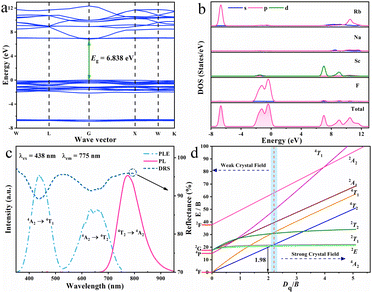 | ||
| Fig. 2 (a) and (b) Band structure, partial and total densities of states of RNSF host. (c) PLE, PL and DR spectra of RNSF:0.03Cr product. (d) Tanabe-Sugano diagram for Cr3+ in octahedral symmetry. | ||
Fig. 2c gives the PLE, PL, and DR spectra of the RNSF:0.03Cr sample monitored at RT. The PLE spectrum was recorded upon 775 nm light irradiation and consisted of two excitation bands locating at blue and red regions that perfectly fit with the two distinct absorption bands of the DR spectrum, which are originated from the spin-allowed transitions of Cr3+ from 4A2 ground state to 4T1 and 4T2 excited states, respectively.30 Notably, the blue excitation band is much stronger than the red one, suggesting that RNSF:Cr phosphor could be combined with a commercial InGaN chip to assemble a NIR pc-LED device.31 Additionally, two dips located at 633 and 660 nm are inspected from the 4A2 → 4T2 transition, which are associated with Fano antiresonance between weakly coupled 2E/2T1 and strongly coupled 4T2 levels, as a result, excitation peak reduces and additional peak generates.32,33 Upon 438 nm light excitation, the PL spectrum exhibits a NIR emission band extending from 650 to 950 nm with peak location at 775 nm and FWHM of 95 nm, which is attributed to Cr3+: 4T2 → 4A2 transition in weak coordination environment.34,35 The NIR emission can be verified by calculating the ratio of Dq/B for the estimation of crystal field strength surrounding Cr3+ ion being weak or strong with a dividing point of 2.3. The lower Dq/B ratio means the weaker crystal field strength. In this case, the Dq, B and Dq/B values are calculated to be 1552 cm−1, 782 cm−1 and 1.98, respectively, according to the PLE peak energies of 4T1 (22831 cm−1) and 4T2 (15528 cm−1) levels. This result illustrates that Cr3+ ions undergo a weak crystal field in RNSF host, resulting in a NIR emission band in this system.36 The estimation result is marked in the Tanabe-Sugano diagram of Cr3+ shown in Fig. 2d. The measured IQE value of RNSF:0.03Cr phosphor is 48.95%, indicating its potential for NIR pc-LED application.
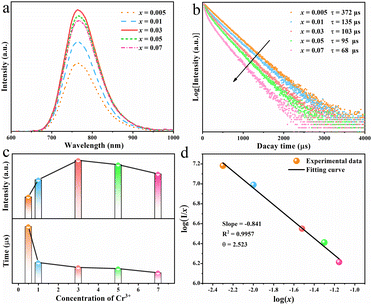
For a deep understanding of the effect of dopant concentration on the luminescent properties of the as-prepared phosphor, the concentration-dependent PL spectra of RNSF:xCr (x = 0.005–0.07) were measured at RT and the results are shown in Fig. 3a. Apparently, the rising Cr3+ content (x value) prompts the increase of PL intensity until it reaches x = 0.03, and then causes the declination of PL intensity due to the concentration quenching effect (the upper part of Fig. 3c). Therefore, the optimal x value for RNSF:xCr is determined to be x = 0.03, at which RNSF:0.03Cr has the highest PL intensity and is employed for the subsequent measurements. The corresponding PL decay curves of RNSF:xCr examined at RT are shown in Fig. 3b. All of them abide to a single exponential function, verifying Cr3+ ion substitutes the single lattice site of Sc3+ in RNSF matrix.37 Increasing the x value from 0.005 to 0.07, the fitted lifetime descends from 372 to 68 μs (the lower part of Fig. 3c), which is attributable to the intensified non-radiation transition among Cr3+ activators.38 Plotting the relationship between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (I/x) and log
(I/x) and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (x) on the basis of the Dexter theory, the critical distance between neighbouring Cr3+ ions was calculated, which can be used to explain the specific concentration quenching mechanism.39 In this circumstance, the critical distance Rc is calculated to be 21.66 Å and the θ value is fitted as 2.523, as shown in Fig. 3d, strongly demonstrating that the energy transfer among the nearest or next nearest Cr3+ ions is the primary reason for concentration quenching of Cr3+ in RNSF host.40,41
(x) on the basis of the Dexter theory, the critical distance between neighbouring Cr3+ ions was calculated, which can be used to explain the specific concentration quenching mechanism.39 In this circumstance, the critical distance Rc is calculated to be 21.66 Å and the θ value is fitted as 2.523, as shown in Fig. 3d, strongly demonstrating that the energy transfer among the nearest or next nearest Cr3+ ions is the primary reason for concentration quenching of Cr3+ in RNSF host.40,41
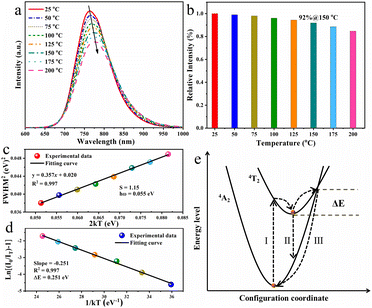
The working temperature of LED device may reach 150 °C or even higher because of the accumulated heat during the operation process, resulting in the investigation on the thermal quenching behaviour of the as-obtained RNSF:0.03Cr phosphor is of great importance.42Fig. 4a depicts the temperature-dependent PL spectra of RNSF:0.03Cr recorded from 25 to 150 °C, in which the PL intensity diminishes monotonically with the elevated ambient temperatures. When the ambient temperature is increased to 150 °C, the integral PL intensity still preserves 92% of the starting value at 25 °C (Fig. 4b). This ratio is conspicuously higher than a number of Cr3+-activated fluorides (Table S2 in ESI†), indicating the superior anti-thermal quenching performance of the RNSF:0.03Cr NIR phosphor and its potential in NIR pc-LED devices. Moreover, the spectral peak position has a red-shift trend and the corresponding FWHM gradually expands as the temperature increases from 25 to 150 °C. The former phenomenon is attributed to the declined crystal field strength and the intensified non-radiative transitions resulted from lattice expansion at high temperatures, and the latter is always ascribed to the enhanced EPC strength that employs the Huang–Rhys factor (S) as an indicator.43 The equation of FWHM2 = 5.57 × S × (hω)2 × (1 + 2kT/hω) can be used to describe the EPC strength by plotting the relationship between FWHM2 and 2kT, in which hω stands for the phonon energy, and k is the Boltzmann constant.44 The trustworthy fitting results are shown in Fig. 4c, from which a linear fitting curve with an estimated S value of 1.15 is obtained, demonstrating that the Cr3+ activators undergo a low EPC strength in RNSF host to achieve high PL thermal stability.
In order to elucidate the high PL thermal stability of RNSF:Cr phosphor, the activation energy (ΔE) was employed to evaluate the thermal quenching resistance of the titled material by using the modified Arrhenius formula of IT = I0/[1 + A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−ΔE/kT)], where I0 and IT represent the integral PL intensities at 25 °C and a given thermodynamic temperature, respectively, k is 8.629 × 10−5 eV K−1 and A stands for a constant.45 By plotting ln
exp(−ΔE/kT)], where I0 and IT represent the integral PL intensities at 25 °C and a given thermodynamic temperature, respectively, k is 8.629 × 10−5 eV K−1 and A stands for a constant.45 By plotting ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [(I0/IT)−1] versus 1 kT−1, a reliable linear fitting curve with a slope of −0.251 is attained, as shown in Fig. 4d, suggesting that the ΔE value is estimated to be 0.251 eV. This value is much larger than RT thermal disturbance energy (0.026 eV), indicating that the energy barrier for non-radiative relaxation is hard to overcome and logically leads to the high thermal stability.46 The configuration coordinate diagram shown in Fig. 4e can be used to elaborate the thermal quenching behaviour of RNSF:Cr phosphor. Under blue light radiation, electrons of Cr3+ can be excited from the low-energy 4A2 state to the high-energy 4T2 level through pathway I. At RT, most of the electrons return to the ground state via pathway II with NIR emission, and the rest of them relax to the ground state in the form of non-radiative transition. At elevated temperatures, the electrons at 4T2 state could be excited to the intersection point of the 4T2 and 4A2 energy levels, and then get back to the ground state via a non-radiative form of way III. The energy barrier between the lowest position and the intersection point is defined as the activation energy (ΔE). Obviously, large ΔE is beneficial for the reduction of non-radiative transition because it is hard to overcome the energy barrier, thus leading to the excellent PL thermal stability.47 Moreover, The TG curve shown in Fig. S3 (ESI†) further confirms the excellent thermochemical stability of RNSF:Cr phosphor, which starts to decompose at a high temperature of 943 °C.
[(I0/IT)−1] versus 1 kT−1, a reliable linear fitting curve with a slope of −0.251 is attained, as shown in Fig. 4d, suggesting that the ΔE value is estimated to be 0.251 eV. This value is much larger than RT thermal disturbance energy (0.026 eV), indicating that the energy barrier for non-radiative relaxation is hard to overcome and logically leads to the high thermal stability.46 The configuration coordinate diagram shown in Fig. 4e can be used to elaborate the thermal quenching behaviour of RNSF:Cr phosphor. Under blue light radiation, electrons of Cr3+ can be excited from the low-energy 4A2 state to the high-energy 4T2 level through pathway I. At RT, most of the electrons return to the ground state via pathway II with NIR emission, and the rest of them relax to the ground state in the form of non-radiative transition. At elevated temperatures, the electrons at 4T2 state could be excited to the intersection point of the 4T2 and 4A2 energy levels, and then get back to the ground state via a non-radiative form of way III. The energy barrier between the lowest position and the intersection point is defined as the activation energy (ΔE). Obviously, large ΔE is beneficial for the reduction of non-radiative transition because it is hard to overcome the energy barrier, thus leading to the excellent PL thermal stability.47 Moreover, The TG curve shown in Fig. S3 (ESI†) further confirms the excellent thermochemical stability of RNSF:Cr phosphor, which starts to decompose at a high temperature of 943 °C.
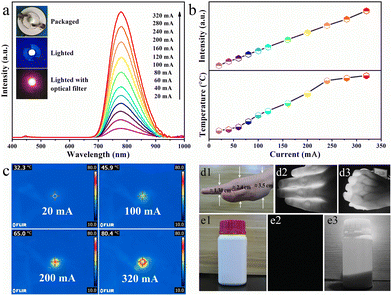
Benefiting from the excellent thermal stability (92%@150 °C) and NIR emission of RNSF:0.03Cr phosphor, a NIR pc-LED device was assembled by coating the as-prepared NIR phosphor on a blue InGaN chip. The device photographs and the corresponding PL spectra operated at various driven currents in the range of 20-320 mA are provided in Fig. 5a. It is obvious that the emission spectra are composed of two parts. One is the strong NIR emission assigned to Cr3+:4T2 → 4A2 transition, and the other is the negligible blue emission generated by the blue InGaN chip. Obviously, with the increase of driven current, the integral NIR emission intensity shows a continuous increasing trend without light saturation, as shown in the upper part of Fig. 5b, further verifying the excellent PL thermal stability of RNSF:Cr phosphor. Temperature and thermographs of the working LED device under various driven currents captured by a NIR imaging camera are shown in the lower part of Fig. 5b and c, in which the working temperature of the fabricated NIR pc-LED increased from 32.3 °C to 80.4 °C induced by the accumulated heat at higher driven currents. Thus, one can conclude that RNSF:Cr phosphor could be stably used in LED device since its high PL thermal stability. Moreover, as the rising driven current, the NIR output power of the LED device continuously increases and the photoelectric conversion efficiency decreases monotonously (Fig. S4 in ESI†), which are mainly attributable to the decreasing IQE of the blue LED chip at higher temperatures.48
In view of the remarkable characteristics of NIR light with non-destruction and penetrability, the manufactured pc-LED device is employed as lighting source for spectroscopy applications of vein imaging and night vision. Considering the absorption of NIR radiation by hemoglobin in human blood, vein imaging analysis was carried out, as shown in Fig. 5d1–3. It turns out that NIR light can easily penetrate ∼1.3 cm thick fingers and ∼2.4 cm thick palm, in which the vein distribution can be clearly observed without any damage to human hand. Moreover, Fig. 5e1–3 exhibit the photographs of opaque reagent bottle that are captured by a camera with natural and NIR modes using the NIR pc-LED as radiation source. Apparently, the photograph of the plastic bottle with white body and red cap is clearly witnessed under natural light illumination, whist nothing is captured without exposure to natural light. However, when the assembled NIR pc-LED device is lighted on, the opaque plastic bottle, as well as the chemical powders inside, are clearly visualized by a NIR camera. These clear demonstration images strongly confirm the potential use of the thermally stable RNSF:Cr phosphor for vein imaging and night vision applications.
Conclusion
In this work, a double perovskite RNSF:Cr fluoride phosphor with blue excitation and NIR emission band was synthesized through a co-precipitation method. The crystal and electronic structures, as well as the luminescent properties were investigated systematically. RNSF:Cr presents a NIR emission band owing to Cr3+:4T2 → 4A2 spin-allowed transition under blue light excitation, and possesses excellent thermal stability at 150 °C with a retention rate of 92% of that at RT, which is originated from the weak EPC strength (S = 1.15) surrounding Cr3+ activators and the large activation energy (ΔE = 0.251 eV) of RNSF:Cr phosphor. By coating RNSF:Cr NIR phosphor on blue InGaN chips, its potential uses in pc-LED devices were explored in different spectroscopy applications.Author contributions
L. Chu, Y. Qin and T. Yang – investigation, writing and editing; J. Wan – calculation; Q. Zhou – methodology, conceptualization, validation, review, supervision and funding acquisition; H. Tang – investigation and review; Y. Ye – review and supervision; Z. Wang – methodology, conceptualization, review and supervision.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22065039 and 22365034) and the Yunnan Fundamental Research Projects (202101AT070072).References
- V. Rajendran, M. H. Fang, W. T. Huang, N. Majewska, T. Lesniewski, S. Mahlik, G. Leniec, S. M. Kaczmarek, W. K. Pang, V. K. Peterson, K. M. Lu, H. Chang and R. S. Liu, J. Am. Chem. Soc., 2021, 143, 19058–19066 CrossRef CAS PubMed.
- Z. Jia, C. Yuan, Y. Liu, X. J. Wang, P. Sun, L. Wang, H. Jiang and J. Jiang, Light Sci. Appl., 2020, 9, 86–95 CrossRef CAS PubMed.
- M. H. Fang, Z. Bao, W. T. Huang and R. S. Liu, Chem. Rev., 2022, 122, 11474–11513 CrossRef CAS PubMed.
- J. Zhong, Y. Zhou, F. Du, H. Zhang, W. Zhao and J. Brgoch, ACS Appl. Mater. Interfaces, 2021, 13, 31835–31842 CrossRef CAS PubMed.
- C. Yuan, R. Li, Y. Liu, L. Zhang, J. Zhang, G. Leniec, P. Sun, Z. Liu, Z. Luo, R. Dong and J. Jiang, Laser Photonics Rev., 2021, 15, 2100227 CrossRef CAS.
- D. Wen, H. Liu, Y. Guo, Q. Zeng, M. Wu and R. S. Liu, Angew. Chem., Int. Ed., 2022, 134, e202204411 CrossRef.
- J. Qiao, S. Zhang, X. Zhou, W. Chen, R. Gautier and Z. Xia, Adv. Mater., 2022, 34, e2201887 CrossRef PubMed.
- G. Liu, S. Zhang and Z. Xia, Opt. Lett., 2023, 48, 1296–1299 CrossRef CAS PubMed.
- S. Li, Q. Zhu, X. Li, X. Sun and J. G. Li, J. Alloys Compd., 2020, 827, 154365 CrossRef CAS.
- J. Wang, X. Han, Y. Zhou, Z. Wu, D. Liu, C. Zeng, S. Cao and B. Zou, J. Phys. Chem. Lett., 2023, 14, 1371–1378 CrossRef CAS PubMed.
- N. Ma, W. Li, B. Devakumar, Z. Zhang and X. Huang, Mater. Today Chem., 2021, 21, 100512 CrossRef CAS.
- J. Xiang, X. Zhou, X. Zhao, Z. Wu, C. Chen, X. Zhou and C. Guo, Laser Photonics Rev., 2023, 17, 2200965 CrossRef CAS.
- X. Zhou, J. Xiang, J. Zheng, X. Zhao, H. Suo and C. Guo, Mater. Chem. Front., 2021, 5, 4334–4342 RSC.
- Y. Wang, J. Feng, P. Chen, J. Huo, Q. Zhang, S. Liu, M. Tang, J. Li and J. Zhou, Dalton Trans., 2023, 52, 10071–10078 RSC.
- L. Song, S. Liang, W. Nie, X. He, J. Hu, F. Lin and H. Zhu, Inorg. Chem., 2023, 62, 11112–11120 CrossRef CAS PubMed.
- J. Xiang, J. Zheng, X. Zhao, X. Zhou, C. Chen, M. Jin and C. Guo, Mater. Chem. Front., 2022, 6, 440–449 RSC.
- Q. Fan, J. Li, J. Yang, Y. Zhou, Q. Zhou and Z. Wang, Mater. Res. Bull., 2023, 158, 112065 CrossRef CAS.
- D. Huang, H. Zhu, Z. Deng, H. Yang, J. Hu, S. Liang, D. Chen, E. Ma and W. Guo, J. Mater. Chem. C, 2021, 9, 164–172 RSC.
- K. Chen, S. Jia, X. Zhang, Z. Shao, Y. Zhou, T. Fan, T. Yu and T. Deng, Inorg. Chem., 2023, 62, 7964–7975 CrossRef CAS PubMed.
- X. Hu, X. Li, Q. Yang, Y. Ye, Z. Wang, Q. Zhou, H. Tang and Q. Wang, J. Lumin., 2023, 263, 120095 CrossRef CAS.
- X. Zhang, K. Chen, T. Deng, J. Yuan, R. Zhou, T. Yu, Y. Zhou and E. Song, Mater. Today Chem., 2022, 26, 101194 CrossRef CAS.
- W. Nie, Y. Li, J. Zuo, Y. Kong, W. Zou, G. Chen, J. Peng, F. Du, L. Han and X. Ye, J. Mater. Chem. C, 2021, 9, 15230–15241 RSC.
- F. He, E. Song, Y. Zhou, H. Ming, Z. Chen, J. Wu, P. Shao, X. Yang, Z. Xia and Q. Zhang, Adv. Funct. Mater., 2021, 31, 2103743 CrossRef CAS.
- X. Zhang, S. Qing, Y. Qin, Q. Zhou, J. Wan, H. Tang and Z. Wang, J. Lumin., 2023, 254, 119531 CrossRef CAS.
- W. Nie, L. Yao, G. Chen, S. Wu, Z. Liao, L. Han and X. Ye, Dalton Trans., 2021, 50, 8446–8456 RSC.
- S. Qing, X. Zhang, T. Yang, L. Chu, Y. Zhou, J. Wan, Z. Wang, H. Tang and Q. Zhou, Dalton Trans., 2022, 51, 14214–14220 RSC.
- J. Qiao, J. Zhao, Q. Liu and Z. Xia, J. Rare Earths, 2019, 37, 565–572 CrossRef CAS.
- H. Zhang, J. Zhong, F. Du, L. Chen, X. Zhang, Z. Mu and W. Zhao, ACS Appl. Mater. Interfaces, 2022, 14, 11663–11671 CrossRef CAS PubMed.
- Y. Yan, M. Shang, S. Huang, Y. Wang, Y. Sun, P. Dang and J. Lin, ACS Appl. Mater. Interfaces, 2022, 14, 8179–8190 CrossRef CAS PubMed.
- J. Lai, W. Shen, J. Qiu, D. Zhou, Z. Long, Y. Yang, K. Zhang, I. Khan and Q. Wang, J. Am. Ceram. Soc., 2020, 103, 5067–5075 CrossRef CAS.
- S. Ding, P. Feng, J. Cao, X. Ma and Y. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 44622–44631 CrossRef CAS PubMed.
- A. Lempicki, L. Andrews, S. J. Nettel, B. C. McCollum and E. I. Solomon, Phys. Rev. Lett., 1980, 44, 1234–1237 CrossRef CAS.
- O. Taktak, H. Souissi and S. Kammoun, J. Lumin., 2015, 161, 368–373 CrossRef CAS.
- S. Liu, H. Cai, S. Zhang, Z. Song, Z. Xia and Q. Liu, Mater. Chem. Front., 2021, 5, 3841–3849 RSC.
- Y. Zhou, C. Li and Y. Wang, Adv. Opt. Mater., 2022, 10, 2102246 CrossRef CAS.
- S. Adachi, J. Lumin., 2021, 232, 117844 CrossRef CAS.
- S. Miao, Y. Liang, D. Chen, R. Shi, X. Shan, Y. Zhang, F. Xie and X. J. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 53101–53110 CrossRef CAS PubMed.
- T. Tan, S. Wang, J. Su, W. Yuan, H. Wu, R. Pang, J. Wang, C. Li and H. Zhang, ACS Sustainable Chem. Eng., 2022, 10, 3839–3850 CrossRef CAS.
- Y. Chen, F. Liu, Z. Zhang, J. Hong, M. S. Molokeev, I. A. Bobrikov, J. Shi, J. Zhou and M. Wu, J. Mater. Chem. C, 2022, 10, 7049–7057 RSC.
- J. Chen, C. Guo, Z. Yang, T. Li and J. Zhao, J. Am. Ceram. Soc., 2016, 99, 218–225 CrossRef CAS.
- L. You, R. Tian, T. Zhou and R. J. Xie, Chem. Eng. J., 2021, 417, 129224 CrossRef CAS.
- Q. Song, Z. Liu, H. Jiang, Z. Luo, P. Sun, G. Liu, Y. Liu, H. Jiang and J. Jiang, J. Am. Ceram. Soc., 2021, 104, 5235–5243 CrossRef CAS.
- F. Zhao, H. Cai, Z. Song and Q. Liu, Chem. Mater., 2021, 33, 3621–3630 CrossRef CAS.
- C. Li and J. Zhong, Chem. Mater., 2022, 34, 8418–8426 CrossRef CAS.
- Q. Zhou, L. Dolgov, A. M. Srivastava, L. Zhou, Z. Wang, J. Shi, M. D. Dramićanin, M. G. Brik and M. Wu, J. Mater. Chem. C, 2018, 6, 2652–2671 RSC.
- Y. Wang, Z. Wang, G. Wei, Y. Yang, S. He, J. Li, Y. Shi, R. Li, J. Zhang and P. Li, Adv. Opt. Mater., 2022, 10, 2200415 CrossRef CAS.
- Z. Liao, C. Li, J. Zhong, Y. Li and W. Zhao, Dalton Trans., 2023, 52, 2853–2862 RSC.
- Y. Zhang, Y. Liang, S. Miao, D. Chen, S. Yan and J. Liu, Inorg. Chem. Front., 2021, 8, 5186–5194 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Details to the SEM and elemental mapping images, EPR spectrum, TG curve, and crystallographic parameters after Cr3+ doping. NIR output power and photoelectric conversion efficiency of LED device, and some typical Cr3+-doped fluoride phosphors. See DOI: https://doi.org/10.1039/d3ma00522d |
| This journal is © The Royal Society of Chemistry 2023 |