Open Access Article
Open Access ArticleRemarkably enhanced upconversion luminescence in Na+ codoped spinel nanoparticles for photothermal cancer therapy and SPECT imaging†
Annu
Balhara
ab,
Santosh K.
Gupta
 *ab,
Nidhi
Aggarwal
c,
Swapnil
Srivastava
c,
Jiban Jyoti
Panda
*ab,
Nidhi
Aggarwal
c,
Swapnil
Srivastava
c,
Jiban Jyoti
Panda
 *c,
Sourav
Patra
ad,
Avik
Chakraborty
ae,
Sutapa
Rakshit
ae and
Rubel
Chakravarty
ad
*c,
Sourav
Patra
ad,
Avik
Chakraborty
ae,
Sutapa
Rakshit
ae and
Rubel
Chakravarty
ad
aHomi Bhabha National Institute, Anushaktinagar, Mumbai 400094, India. E-mail: santoshg@barc.gov.in
bRadiochemistry Division, Bhabha Atomic Research Centre, Trombay, Mumbai 400085, India
cInstitute of Nano Science and Technology, Mohali, Punjab, India. E-mail: jyoti@inst.ac.in
dRadiopharmaceuticals Division, Bhabha Atomic Research Centre, Trombay, Mumbai 400085, India
eRadiation Medicine Centre, Bhabha Atomic Research Centre, Parel, Mumbai 400012, India
First published on 4th October 2023
Abstract
Glioblastoma multiforme (GBM) is the most fatal brain tumor and chemo/radiotherapeutic options and other palliative care have not fetched much success in its management due to the highly heterogeneous nature of GBM tissue and the presence of the blood–brain barrier. Impressively, Na+ co-doped ZnAl2O4:Ho3+, Yb3+ upconversion nanoparticles (UCNPs) with remarkably enhanced upconversion luminescence (UCL) have demonstrated good cellular uptake, bio/cyto compatibility and anticancer efficacy in C6 glioma cells. The % cell viability of C6 cells treated with UCNPs decreased to 46% under 980 nm near infrared (NIR) laser exposure exhibiting an excellent potential in photothermal therapy (PTT). Laser power dependence studies explain the UC mechanism and the role of Na+ ions in the UC luminescence enhancement is also investigated using density functional theory (DFT) calculations and positron annihilation lifetime spectroscopy (PALS). The Na+ ion codoping resulted in a significant lowering of zinc vacancies in ZnAl2O4:Ho3+, Yb3+, indicating its effective role in eliminating defect-induced non-radiative channels. Intrinsically (166-Holmium) radiolabeled ZnAl2O4:Yb3+, 166Ho3+, Na+ has also shown great potential towards in vivo single-photon emission computed tomography (SPECT) imaging. The results presented herein highlight the potential of this highly upconvertible molecule for dual modality SPECT/optical imaging for therapeutic and theranostic applications as well as for photothermal cancer therapy.
1. Introduction
Cancer is a life-threatening malady affecting a vast number of people worldwide. According to the global statistics, cancer accounted for approximately 10 million deaths and more than 19.3 million fresh cases in the year 2020.1 Theranostic agents are promising for modern biomedical applications as they play both diagnostic and therapeutic roles.2,3 Luminescent nanomaterials have attracted significant attention for image-guided cancer therapy and nanomedicine based applications. In the recent past, the use of multifunctional luminescent materials as imaging probes for multiplexed imaging and photo-triggered targeted drug delivery in the tumor tissues has proved to be an area of great interest for researchers.4 Bioimaging modalities for tumor diagnosis include optical imaging, positron emission tomography (PET), magnetic resonance imaging (MRI), computed tomography (CT), and single-photon emission computed tomography (SPECT). Moreover, combining photothermal therapy (PTT) triggering agents with multiple bioimaging modalities, such as optical imaging and SPECT/CT offers a new direction for in vivo theranostics.5 Multi-modal imaging is gaining attention as it offers better imaging contrast and sensitivity for the diagnosis of tumors.6 Anticancer agents performing bioimaging and therapeutics simultaneously are in demand for next-generation theranostics. Image-guided photothermal therapy offers potential non-invasive cancer therapeutics, but the major limitation associated with the traditional phototherapy involves the use of harmful ultraviolet (UV) light sources along with its demerit of lower tissue penetration.7 NIR-absorbing upconversion nanoparticles (UCNPs) are endowed with a remarkable tissue penetration power, and a unique capability of converting NIR to UV light in order to carry out the photoreaction necessary for its application as a theranostic agent.8,9 UCNPs are conventionally functionalized with anticancer molecules, photothermal agents, and photo-sensitizers, which could possibly absorb the released high energy photons and produce ROS to aid in cancer cell killing. Wang and co-workers have depicted heightened in vivo photodynamic effect by loading a photosensitizer, Chlorin e6 (Ce6), in PEGylated UCNPs for cancer therapy.10 In the recent past, lanthanide-doped UCNPs have emerged to be of great potential for biomedicine platforms owing to their exceptional physico-chemical and biological properties, viz., higher anti-Stokes shift, longer fluorescence lifetime, high photo and chemical stability, low cytotoxicity, and lower background scattering.11,12 Many lanthanide-doped-UCNPs are also known to convert the laser energy into thermal energy when delivered to the tumor tissues, thereby mediating the photothermal death of the tumorous tissue.13 Tsai and co-workers demonstrated NIR-triggered phototherapeutic effects mediated by angiopep-2 functionalized UCNPs in brain glioblastoma models.14 A plethora of UCNP-based nanosystems, such as core–shell UCNPs,15,16 polymer-coated UCNPs,17 SiO2 functionalized UCNPs,18 and metal-UCNPs,19,20 have been primarily explored as theranostic agents in cancer.Upconversion luminescence (UCL) in lanthanide (Ln3+) doped phosphors has been thoroughly investigated by researchers and many reviews have summarized the progress made in the development of UC phosphors,21,22 where Er3+, Ho3+, and Tm3+ doped phosphors have received more attention owing to strong UCL that could be ascribed to their ladder-like energy levels.23 The Ho3+ doped materials are known for the upconversion of two or more NIR photons resulting in the red, green, and NIR emissions but display weak UCL due to spin forbidden 4f–4f transitions and the poor absorption of NIR photons.21,23 Though the luminescence can be enhanced by increasing the doping concentrations, the rare earth ions as dopants have low-doping optimal concentrations limited by the concentration quenching observed at higher dopant concentrations.21 Therefore, Yb3+ having large absorption coefficient for the NIR region is essentially codoped in Ho3+ doped phosphors as a sensitizer for efficient NIR pumping and energy transfer to activator Ho3+ ions.23,24 Several studies have reported the boost in the UCL in different Ho3+/Yb3+ co-doped hosts.23–27 The rational choice of hosts also impacts the luminescence characteristics of the phosphors and a good host should satisfy particular criteria. Since halide and oxysulfide based hosts suffer from poor photostability, hygroscopicity, and toxicity, suitable oxide-based compounds are preferred and they possess superior UCL properties.23
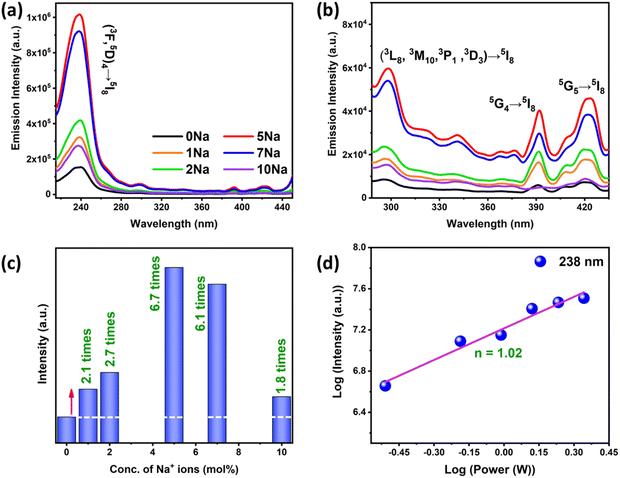
Another emerging application of Ln3+ doped UCNPs is virus and bacterial deactivation that is triggered by effective ultraviolet-C (UV-C) emission in the effective germicidal region (220–280 nm).28 Phosphors emitting UV-C light can be an alternative for current UV lamps (mercury lamps at 254 nm) with lower efficiency, mainly for the microbial disinfection of water. Though the UV-C upconversion is achievable by co-doping of Ho3+ ions, which can be attributed to their richness of intermediate energy levels, studies exploring the UV-C emission by Ho3+ doped phosphors are still rare. In 2010, Chen et al.29 reported UV-C emission in NaYF4:Yb3+/Ho3+ powders under 970 nm excitation, but could only achieve very weak emission at 247 nm even at a high pump power of 37 W cm−2, which is not suitable for practical applications.
The desired UCL intensity enhancement, controllable emission, chromaticity, and high color purity required for emerging applications could not be achieved by merely codoping Yb3+ ions as higher concentrations of RE3+ are detrimental to UCL.30 Therefore, researchers have focussed on crystal field modulation, which is an effective way to improve upconversion emission intensity essential for practical applications.31 The same could be achieved by codoping of non-luminous centres that play the role of local field modifiers and surface enhancers, leading to the successful increment in photoluminescence intensity. In recent years, many publications showcased the role of alkali ion (Li+, Na+, and K+) and Mg2+ codoping in boosting the UCL and DC emission, in order to achieve tunable emission.30–35 In the last decade, some publications have revealed the role of alkali metals in improving both UC and DC emission intensity, with majority of them focussing on Li+ ion co-doping in different Ho3+ activated host matrices.33,36 The effect of Na+ codoping has been also explored in phosphors, thereby enhancing emission efficiency32,34,35,37,38 but there are very few reports in the literature that emphasize the effect of Na+ codoping in Ho3+/Yb3+ doped materials. Among many oxide hosts activated with RE3+ ions, ZnAl2O4 with a cubic-type spinel lattice is a potential host due to its excellent properties like low phonon energy, non-toxicity, high photochemical and thermal stability, low cost, radiation stability, hydrophobicity, and wide band gap (∼3.8 eV).39–41 Spinels consisting of Zn2+ ions as RE3+ activated hosts (AB2O4:RE3+) have been explored for their efficient emission properties and ability to incorporate RE3+ ions at tetrahedral (A2+) and octahedral (B3+) sites. Moreover, an enhancement in UCL was obtained in Ho3+/Yb3+ doped spinels, by codoping of non-luminous centers as charge compensators.42,43 To the best of our knowledge, Ho3+/Yb3+ codoping in the zinc aluminate host and the effect of Na+ co-doping on UCL have not been reported so far.
These favorable characteristics of the zinc aluminate spinel host and the biomedical potential of Ho3+ coupled with its suitable radiological half-life have pushed us to explore the potential of ZnAl2O4:Ho3+ sensitized with Yb3+ and co-doped with Na+ for SPECT imaging-guided targeted drug or radionuclide delivery.44–46 Radioactive labeling is considered as a fundamental phenomenon in biology-related applications. Lohar et al. have highlighted the potential of Ho-166 for intra-arterial radiation therapy owing to its excellent radiological properties [half-life = 26.8 h, high energy β− emission with Emax ∼ 1.85 MeV (50.0%) and 1.74 MeV (∼49%), and 80.6 keV γ-photon emission] and feasibility for its production on a large scale via simple 165Ho (n, γ) 166Ho reaction with suitable specific activity in moderate flux research nuclear reactors.45 The use of photoreactions to provide modulated delivery of a diagnostic or therapeutic agent to the diseased biological tissues as in cancer has been explored widely in the recent past.7 Therefore, intrinsically radiolabelled Ho3+ doped UCNPs present the potential for multi-mode SPECT/optical imaging, which utilizes integrated nuclear imaging and fluorescence imaging-guided glioma therapy.
Thus, this study reports a new upconverting material ZnAl2O4:Ho3+, Yb3+, Na+ and an effort was made to enhance and tune the UC emissions of Ho3+ ions on NIR excitation via codoping of Na+ ions. The ZnAl2O4 host has been selected for the Ho3+/Yb3+ codoping, due to its efficient photoluminescence properties. In this regard, a series of ZnAl2O4:1%Ho3+, 5%Yb3+, x%Na+ (x = 0 to 10 mol %) samples were synthesized using the solid-state route. The role of Na+ ions in boosting the visible, blue, and UV-C UC emissions was identified using the photoluminescence studies, lifetime decay measurements, PALS, and DFT studies. The mechanisms of UC processes were studied with the help of UC emission intensity dependencies on the 980 nm laser power. Additionally, herein, for the first time, we have tried to explore solely our upconverting nanomaterial (without any further down processing or with any carrier/coating) as a new-modality for glioma therapy. We investigated the in vitro behaviour and anticancer efficacy of our novel upconverting nanomaterial in C6 rat-glioma cells to establish it as a prospective candidate for future glioma therapy. We further explored this material for in vivo SPECT imaging using intrinsically radiolabeled ZnAl2O4:Yb3+, 166Ho3+, Na+. Its radiochemical purity was established using thin-layer chromatography. The in vitro stability of intrinsically radiolabeled ZnAl2O4:166Ho3+ (166Ho is a radioactive isotope) was determined under physiological conditions over a period of ∼6 half-lives of 166Ho. The in vivo stability of intrinsically radiolabeled ZnAl2O4:Yb3+, 166Ho3+, Na+ was demonstrated by SPECT/CT imaging in healthy Wistar rats.
The experimental section consists of synthesis, instrumentation details, computational methodology, production of 166Ho, synthesis and photothermal effect of ZnAl2O4:Yb3+, Ho3+, Na+, cellular uptake studies performed in C6 cells, in vitro biocompatibility study performed in fibroblast cells, in vitro anticancer efficacy of UCNPs determined in C6 glioma cells, scratch wound healing assay, synthesis of intrinsically radiolabeled ZnAl2O4:Yb3+, 166Ho3+, Na+, and preclinical studies with intrinsically radiolabeled ZnAl2O4:Yb3+, 166Ho3+, Na+, which are elaborated in the ESI† as S1–S11, respectively.
2. Results and discussion
2.1. Structure and phase studies
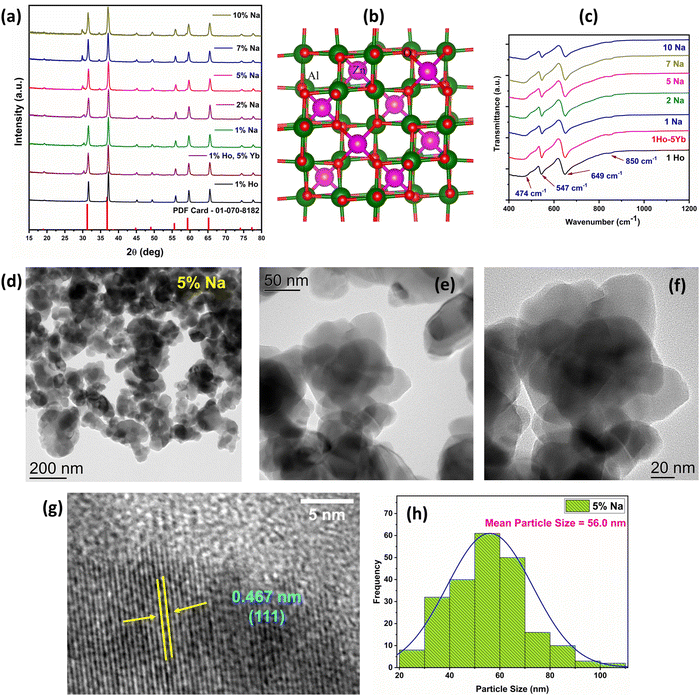
Fig. 1(a) demonstrates the XRD patterns of doped ZnAl2O4:1%Ho3+ and codoped ZnAl2O4:1%Ho3+, 5%Yb3+, x%Na+ (x = 0, 1, 2, 5, 7 and 10 mol%) samples. Scheme 1 demonstrates the synthesis of ZnAl2O4:Ho3+, Yb3+, Na+ UCNPs by the solid-state reaction. The diffraction peaks attained in the XRD patterns can be assigned to the formation of the cubic ZnAl2O4 spinel phase in all the doped and codoped ZnAl2O4 samples (PDF card – 01-070-8182). A small peak located at a 2θ value of ∼30° appears in all the Yb3+ codoped samples and can be attributed to Yb2O3 residue. Fig. 1(b) shows the crystal structure of ZnAl2O4 with Zn2+ ions occupying tetrahedral and Al3+ ions occupying octahedral sites in cubic normal spinel arrangement. The substitution of Ho3+ (0.901 Å) and Yb3+ (0.868 Å) ions having large ionic radii in Zn2+ (0.74 Å) sites is the likely substitution. There is a large size mismatch for Ho3+, Yb3+ ions and Al3+ (0.535 Å) and substitution in Al3+ sites is unlikely. Due to the large ionic radius of Na+ ions (1.02 Å), Na+ ions also occupy Zn2+ sites. We synthesized a Ho3+ and Na+ ion codoped Yb2O3 sample to investigate the contribution from Ho3+ ions present in the Yb2O3 impurity phase in the upconversion emissions. The XRD pattern for the same is provided in Fig. S1 (ESI†). | ||
| Scheme 1 Schematic illustration demonstrating the synthesis of ZnAl2O4:1%Ho3+, 5%Yb3+, x%Na+ (x = 0, 1, 2, 5, 7, and 10 mol%) UCNPs. | ||
Before we move ahead and exploit the positive aspects of the dopant, sensitizer, and sodium ions, we wanted to check the feasibility of zinc aluminate spinel to successfully accommodate tri-doping species, viz, Ho3+, Yb3+, and Na+. To explore the feasibility of formation of the tri-doped system, we have calculated defect formation energies. The details about the computational calculations are provided in Section S2.1 (ESI†). As can be seen from Table S1 (ESI†), the calculated defect formation energy for Ho3+ is negative (−1.20 eV), indicating that doping is thermodynamically feasible. Interestingly, the defect formation energy becomes more negative when codoped with Yb3+ and Na+ (−5.51 eV). This indicates that the tri-doping is highly favoured over single element doping.
FTIR spectroscopy was performed to get an insight about the phase and local structure of ZnAl2O4. Fig. 1(c) depicts the FTIR spectrum of doped and codoped ZnAl2O4 samples and the absorption bands at 649 cm−1, 547 cm−1, and 474 cm−1 can be assigned to the symmetric stretching, symmetric bending, and asymmetric stretching modes of the AlO6 groups, respectively.40 The FTIR spectroscopy reveals some inversion in the ZnAl2O4 spinel structure that could be inferred from the appearance of a less intense shoulder band at ∼850 cm−1 due to tetrahedral AlO4 groups.40
2.2. Morphostructural and elemental analysis
The information regarding the morphology and mean particle size could be inferred from the FE-SEM images presented in Fig. S2 (ESI†). The particles in the doped and codoped ZnAl2O4:Ho3+, Yb3+, Na+ samples have a spherical morphology and uneven particle size distribution. The EDS spectra of ZnAl2O4:1%Ho3+, ZnAl2O4:1%Ho3+, 5%Yb3+, and ZnAl2O4:1%Ho3+, 5%Yb3+, 5%Na+ samples are presented in Fig. S3 (ESI†), which clearly show the successful incorporation of Yb3+ and Ho3+ ions. The incorporation of Na+ ions in the lattice of ZnAl2O4 was studied using laser-induced breakdown spectroscopy (LIBS) and the LIBS emission spectra clearly show the presence of Na atomic emission doublet lines at 589.995 nm and 589.592 nm (Fig. S4a, ESI†), indicating successful incorporation in the parent crystal. Further description regarding the information obtained from LIBS is provided in Section S2.4 and Fig. S4b, c (ESI†). TEM images of the 5 mol% Na+ codoped ZnAl2O4 sample having maximum UCL were collected, which revealed an almost spherical morphology and average particle size of 56.0 nm (Fig. 1(d)–(f)). The HRTEM image shows (111) lattice planes of ZnAl2O4 with a d-spacing of 0.4667 nm, consistent with the spinel structure (Fig. 1(g)), which indicates the high crystallinity of the sample. The particle size distribution curve is given in Fig. 1(h) and particle sizes are found in the range of 20 to 130 nm.2.3. Time resolved photoluminescence
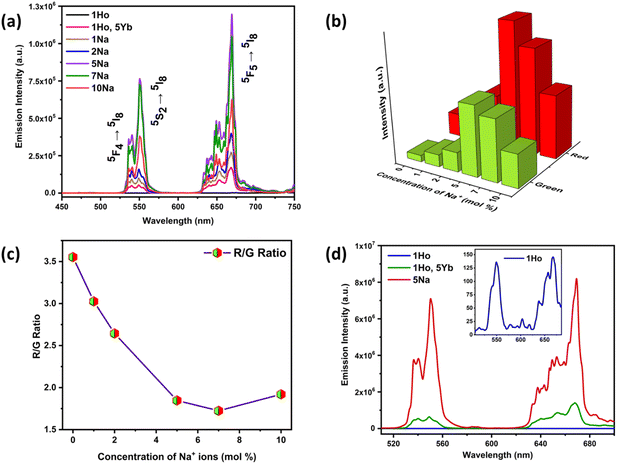
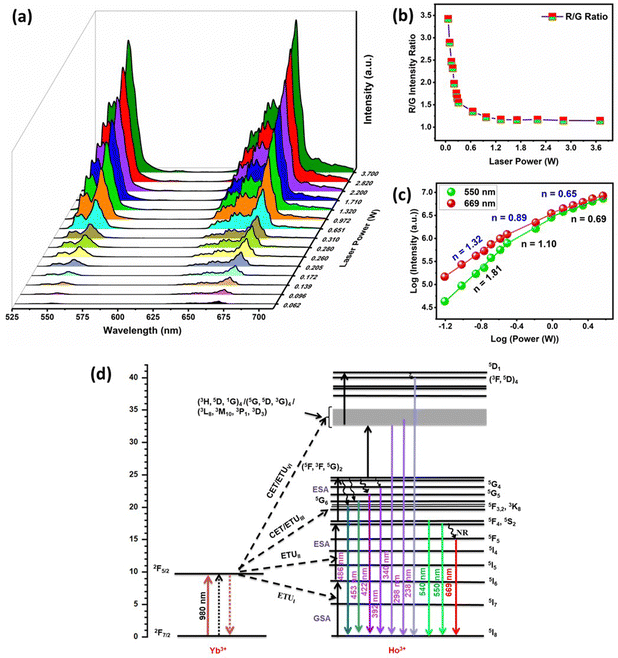
The peak positions were not altered by Yb3+ codoping and the boost in UCL intensity occurred as a result of energy transfer between Yb3+–Ho3+ pairs that can be ascribed to efficient absorption of 980 nm photons by Yb3+ ions. The 980 nm NIR light can efficiently excite Yb3+ ions in the ground state 2F7/2 to the 2F5/2 excited state due to the large absorption cross-section of Yb3+ for 980 nm photons.35,36,47 The energy released during the de-excitation of photons from the 2F5/2 energy level of Yb3+ ions was transferred to Ho3+ ions in their ground state (5I8). The schematic energy level diagram in Fig. 3(d) represents different mechanisms of energy transfer between Yb3+–Ho3+ pairs, which include energy transfer upconversion (ETU), ground state absorption (GSA), excited state absorption (ESA), and cooperative energy transfer (CET).36 The 5I6 excited state of Ho3+ ions was populated via the GSA mechanism due to energy transfer from Yb3+ ions (ETUI). The Ho3+ ions in the 5I6 energy level can populate the 5F4/5S2 excited states by mechanisms, (i) the absorption of 980 nm photons by Ho3+ ions through ESA, and (ii) energy transfer from Yb3+ ions through ETUII. Moreover, the ETUII mechanism is usually more probable in comparison to ESA that is more feasible at higher pump powers.35 The excited Ho3+ ions present in the 5F4/5S2 energy levels relax to the ground state (5I8) by emitting photons of 540 and 550 nm (green emission), and by non-radiative relaxation (NR) to the 5F5 state, followed by the emission of a 669 nm photon (red emission).
The integral intensity ratios for red and green UC emissions were calculated for all the codoped Ho3+/Yb3+/Na+ samples of ZnAl2O4, in the region of 620–695 nm for red and 520–580 nm for green UC emission. Interestingly, the red to green UC emission ratio (R/G ratio) showed a significant decrease up to a codoping concentration of 7 mol% of Na+ ions from 3.22 to 1.72 as the Na+ ion concentration increases and then, a slight rise in R/G ratio is observed for 10 mol% (Fig. 2(c)). This suggests that the green UC emission intensity got amplified by higher times in comparison with the red UC emission. This was very much visibly evident from Fig. S6a and S7 (ESI†) as well where the Commission Internationale de L’Eclairage (CIE) coordinate values hinge towards the yellowish-red region and become more and more greener at higher sodium ion concentration, resulting in the change of CIE coordinates from (0.438, 0.535) to (0.387, 0.604). Thus, the color tunability in the ZnAl2O4:Ho3+, Yb3+, Na+ phosphor material has been achieved and the color purity values increased from 92.4% to 98.0% by variation in the codoping concentrations of Na+ ions. Multi-color emissive UCNPs have potential for multiplexed bioimaging and multiple signals can be acquired simultaneously, which improves the bioimaging resolution. The description for color purity and correlated color temperature (CCT) calculations are provided in Section S2.5 (ESI†). Hence, the codoping of Na+ ions is a potential strategy to tune the UC emission along with enhancing the UC emission intensity.
The UC emission spectra of the doped ZnAl2O4:1%Ho3+ sample demonstrated peaks at 550 and 669 nm with a very low signal to noise ratio at a pump power excitation of 0.310 W. Therefore, the spectra of doped ZnAl2O4:1%Ho3+ were recorded at a higher pump power of 2.82 W for showcasing the effect of Yb3+ and Na+ codoping in the Ho3+ doped sample. The comparison of singly doped 1%Ho3+, codoped 1%Ho3+/5%Yb3+, and Ho3+/Yb3+/5%Na+ samples of ZnAl2O4 is shown in Fig. 2(d) and the inset demonstrates the zoomed in UC emission spectra of the ZnAl2O4:1%Ho3+ sample. In our case, the green and red emissions in the codoped ZnAl2O4:Ho3+–Yb3+ samples were enhanced by 4 × 103 times and 6.9 × 103 times, respectively. Moreover, we have achieved a remarkable increment by 3.5 × 104 times in the green and 3.2 × 104 times in the red UC emissions via Na+/Yb3+ codoping. The codoping of Na+ ions in the codoped 1%Ho3+/5%Yb3+ sample resulted in an enhancement of green and red UC emissions by 8.6 and 5.0 times, respectively. Table S2 (ESI†) tabulates some of the recent reports wherein non-fluorescent metal ions are used as crystal field modulators to enhance UC emission in Ho3+–Yb3+ codoped phosphors. It was quite evident from this table that the current spinel-based phosphor with sodium ion as a field modulator and charge compensator not only emerged victorious in terms of intensity enhancement but also was being able to produce UV-C and visible UC and short-wave IR by down conversion as well as Na+ concentration and laser power-dependent induced tunability. At the same time, our material showed the potential to serve as a theranostic and anticancer agent. The luminescence decay curves for 550 and 669 nm UC emissions are shown in Fig. S8 (ESI†) and the detailed analysis and consequences of Na+ codoping on the decay lifetimes are presented in Section S2.6 (ESI†).
| I ∝ Pn | (1) |
The occupation of Zn2+ sites by Ho3+ and Yb3+ ions creates Zn2+ deficiencies in the ZnAl2O4 matrix and charge imbalance. The codoping with Na+ ions maintains the charge balance:
| 4Zn2+ = Ho3+ + Yb3+ + 2Na+ | (2) |
Consequently, the Na+ doping lowers the structural defects, thereby reducing the probability of non-radiative processes. Hence, the UCL increases by increasing the doping concentration of Na+ ions until 5 mol%. The decrease in UCL at higher Na+ ion concentration was observed and at higher doping concentrations Na+ ions were compelled to occupy interstitial sites, resulting in the generation of new defect centers that reduces UCL.55 To prove it further and get a better insight, we carried out PALS measurement, which is considered one of the most sensitive techniques for understanding defect evolution in inorganic materials. The analysis of PALS data is provided in detail in Section S2.7 (ESI†). The first two lifetime components and their intensities in all the samples are given in Fig. S11 (ESI†) as a function of %Na+ codoping in ZnAl2O4 doped with 1%Ho and 5%Yb. PALS results suggest that initial codoping of Na+ partially removes defects, while at concentration higher than 5%, the charge compensation is not effective. The size of Na+ is higher than that of all the other ions and it seems to be causing distortions in the lattice that is reflected as an increase in average positron lifetime. The positron lifetimes decreased with codoping of Na+ initially and increased later. The average positron lifetime too increased more drastically at higher concentration of Na+. The positron lifetimes seem to be inversely correlated with PL that lower the positron lifetimes, higher the emission or the defects or cation vacancy removal is enhancing the PL intensities.
The Kroger–Vink notation for either Ho3+ or Yb3+ substituted at Zn2+ should be represented with eqn (3) and (4) as mentioned below:
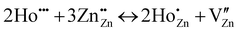 | (3) |
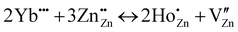 | (4) |
It is reported that these negatively charged zinc vacancies provide additional pathways for non-radiative transitions and successfully quench the UC and DC luminescence by absorbing the photon energy emitted by the active holmium ion centres.56 The inclusion of sodium ions increased the UCL emission intensity as they decreased the amount of negatively charged zinc vacancies by acting as a charge compensator created as a result of aliovalent substitution of Ho3+/Yb3+ at the Zn2+ site as well as by acting as a crystal field modulator. But it was the significant reduction in the density of cation vacancies that is considered the main reason for the enhancement in UCL/DCL intensity on sodium ion codoping. In addition, the effect of Ho3+, Yb3+, and Na+ ion codoping in the zinc aluminate lattice has been investigated using DFT calculations and the results are discussed in Section S2.8 (ESI†) and we have analysed the electronic structure (Fig. S12a–c, ESI†).
2.4. The photothermal effect of ZnAl2O4:1%Ho3+, 5%Yb3+, 5%Na+ UCNPs
As a consequence of the high NIR absorbing property, we anticipated that ZnAl2O4:1%Ho3+, 5%Yb3+, 5%Na+ UCNPs would demonstrate remarkable photothermal behaviour to act as a photothermal agent for destructing the cancer cells. To evaluate their photothermal effect, 1 mg mL−1 solution of UCNPs was exposed to a 980 nm laser for 10 mins and any increment in the surrounding media temperature was recorded using a thermometer. It was interesting to observe that the temperature of UCNP solution increased from 20 °C to 50 °C in a time span of 10 min (Fig. S14, ESI†), confirming the photothermal nature of the UCNPs, as compared to the control water sample, where no significant temperature enhancement was observed.58 The results were indicative of the conversion of light energy into thermal energy on laser irradiation of the UCNPs subsequently leading to the generation of heat that could aid in the photothermal triggered death of the cells in tumor tissues. Thus, this upconvertible nanophosphor has the potency to serve as a photothermal based therapeutic modality in cancer.592.5. Cellular uptake studies of the UCNPs performed in C6 cells
To determine the ability of our UCNPs to get internalized into the cancer cells, their cellular uptake behaviour was evaluated in C6 glioma cells. For the study, cells were treated with rhodamine tagged UCNPs at a concentration of 40 μg mL−1 and uptake was analysed via confocal microscopy. As indicative of our results (Fig. 5), a time-dependent uptake of UCNPs was observed in the cells. The red fluorescence due to rhodamine depicted a successful distribution of our UCNPs in the C6 cells. As compared to the control cells, a higher fluorescence signal was observed in the cells exposed to the UCNPs as the time lapsed from 2 h to 8 h.60,61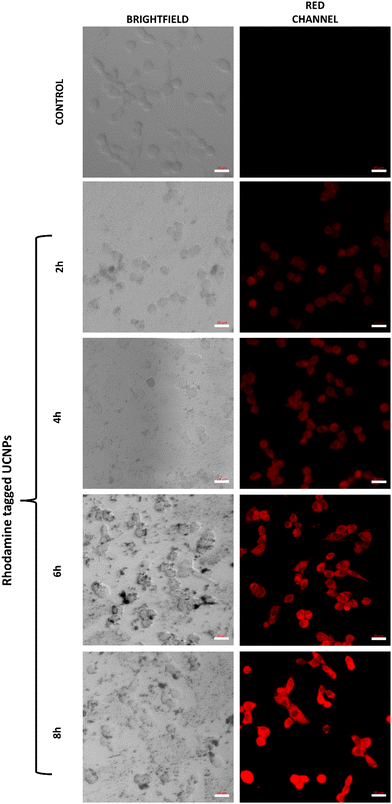 | ||
| Fig. 5 Cellular uptake of ZnAl2O4:1%Ho3+, 5%Yb3+, 5%Na+ UCNPs as determined in C6 glioma cells after different time periods using confocal microscopy. Scale: 20 μm. | ||
2.6. Biocompatibility study of the UCNPs performed in fibroblast cells
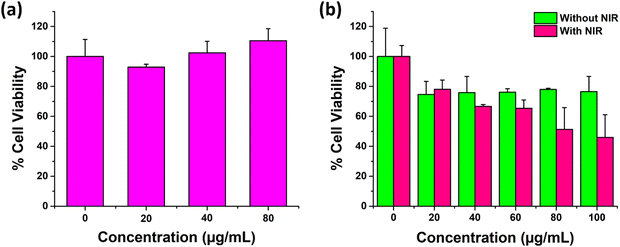
For cellular experiments, UCNPs were properly dispersed in aqueous media following sonication. We further carried out dynamic light scattering based studies (Fig. S15, ESI†) whose results depicted a fair dispersion of the particles in aqueous media with a PDI of 0.4. For a system to be translated as a therapeutic modality or as a drug delivery carrier in biological tissues, it is highly essential that the system depict promising compatibility with a healthy biological environment. Thus, the cytotoxicity of the UCNPs was determined in non-cancerous fibroblast L929 cells. As shown in Fig. 6(a), the percent cell viability of L929 fibroblast cells incubated for 24 h with the varying concentration of UCNPs was observed to be equal to or greater than 100%. Thereby, the results indicated an excellent bio and cytocompatibility of the as prepared UCNPs in L929 cells.622.7. Anti-cancer efficacy of the UCNPs determined in C6 glioma cells
After the confirmation of a superior cellular uptake and favourable in vitro biocompatibility, UCNPs were next evaluated for their anticancer efficacy in C6 glioma cells by MTT assay. The study was conducted at different concentrations of UCNPs in two groups, viz., without NIR and with NIR (980 nm NIR, at 1 W cm−2 for 5 mins) laser exposure in C6 glioma cells for 24 h. The results indicated that the percent cell viability of C6 cells exposed to 100 μg mL−1 UCNPs decreased to approximately 46% under NIR laser exposure as compared to the 76.5% observed in the absence of the NIR laser. Also, the cell viability decreased concomitantly with an increase in the particle concentration from 20 μg mL−1 to 100 μg mL−1 in a gradual fashion (Fig. 6(b)). From these results, it was observed that the ZnAl2O4:1%Ho3+, 5%Yb3+, 5%Na+ UCNPs depicted a concentration dependent cellular toxicity in the C6 cell line, which was further enhanced upon NIR irradiation due to the activation of their photothermal properties. As indicative of the photothermal property of our UCNPs, the heat generated through laser irradiation was the underlying cause for a higher observed cancer cell killing ability of the particles in the presence of the laser.60,632.8. Scratch wound healing assay
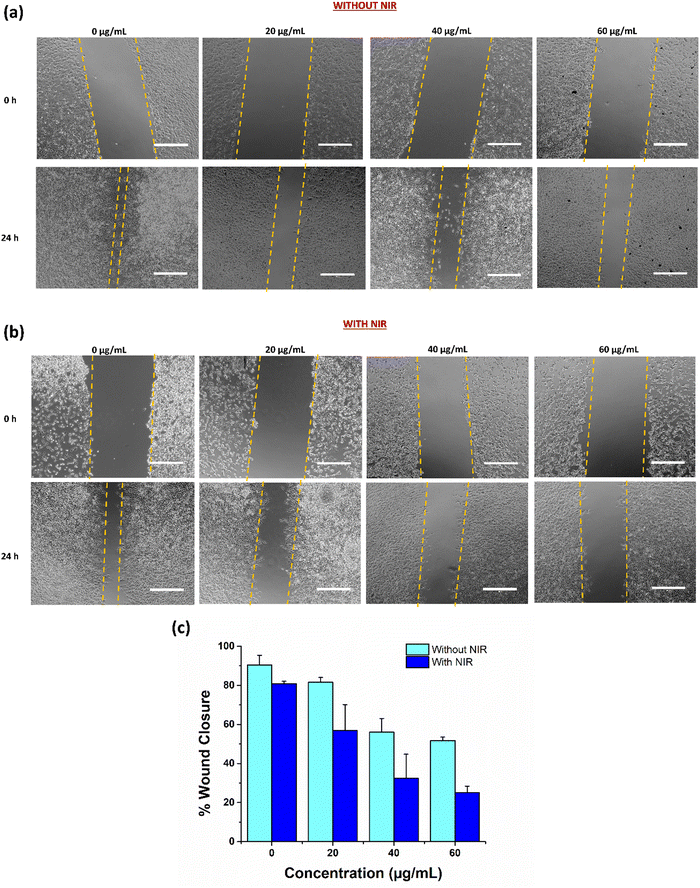
After the successful depiction of anticancer ability of UCNPs under NIR conditions, we next investigated their anti-migratory potential in C6 glioma cells by a scratch wound healing assay. Scratch assay has been reported to be a standard method to study cell migration. The ability of the cells to migrate across the wound in the presence of treatment at different concentrations with NIR and without NIR was studied to explore the anti-metastatic activity of our UCNPs.64 The results as demonstrated in Fig. 7(a) and (b), indicated a fortunate concentration dependent inhibition of cancer cell migration under NIR exposure in C6 cells. After 24 h of incubation, the wound distance was measured and analysed using the ImageJ software. Higher inhibition in C6 cell migration was observed with a higher concentration of treatment in the presence of a 980 nm laser. The wound healing capability of cells at 60 μg mL−1 after 24 h of incubation was uncertain and insignificant, resulting in a diminished migration potential of cells as compared to the control cells. Further, quantitatively, percentage wound healing was estimated using the ImageJ software, which depicted an insignificant wound closure with only 25.17% of closure in cells treated with 60 μg mL−1 UCNPs in the presence of a laser as compared to around 80% wound closure in control cells (0 μg mL−1) under similar NIR conditions (Fig. 7(c)). The cell migration inhibition potential improved with increasing concentration of the UCNPs, inferring a concentration dependent anti-migratory effect, which may further strengthen their anticancer effect in glioma.652.9. Preclinical studies with intrinsically radiolabeled ZnAl2O4:1%Ho3+, 5%Yb3+, 5%Na+
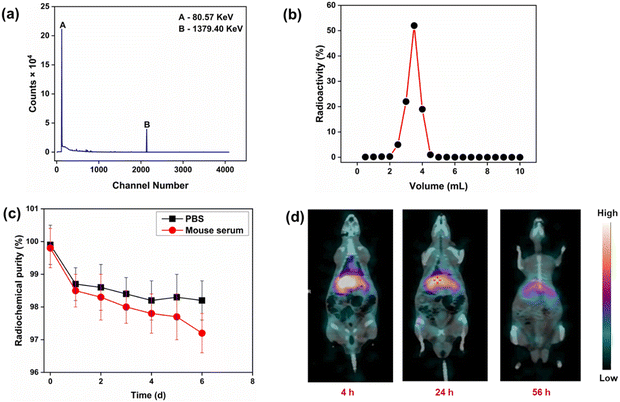
The radionuclidic purity of the synthesized intrinsically radiolabeled ZnAl2O4:5%Yb3+, 166Ho3+, 5%Na+ (166Ho is a radioactive isotope) was determined using γ-spectrometry. Only characteristic peaks corresponding to 166Ho could be determined in the γ-ray spectrum (Fig. 8(a)), demonstrating its high radionuclidic purity (>99.99%) for preclinical studies. The radiochemical purity of ZnAl2O4:5%Yb3+, 166Ho3+, 5%Na+ was determined using size exclusion chromatography (Fig. 8(b)). As expected, ZnAl2O4:5%Yb3+, 166Ho3+, 5%Na+ was eluted in 2.5–5 mL fractions, while free 166Ho3+ was eluted after 6 mL fractions. From this study, the radiochemical purity of ZnAl2O4:5%Yb3+, 166Ho3+, 5%Na+ was determined to be 98.5 ± 0.7%. This was further corroborated by radio-TLC study (Fig. S16, ESI†). In vitro stability of intrinsically radiolabeled ZnAl2O4:5%Yb3+, 166Ho3+, 5%Na+ was determined under physiological conditions over a period of ∼6 half-lives of 166Ho (Fig. 8(c)). During this period, the radiochemical stability decreased marginally in both the media from ∼99% to 97%. This study amply demonstrated that intrinsically radiolabeled ZnAl2O4:5%Yb3+, 166Ho3+, 5%Na+ would retain its radiochemical integrity when administered for in vivo use.As a proof of concept, intrinsically radiolabeled ZnAl2O4:5%Yb3+, 166Ho3+, 5%Na+ was administered intravenously in healthy Wistar rats. In vivo SPECT/CT imaging at different time points demonstrated the uptake of radioactivity in the liver (Fig. 8(d)). There was no uptake of radioactivity in the bladder, which indicated that the radiolabeled agent did not disintegrate in vivo.66 With the passage of time, the radiolabeled ZnAl2O4:5%Yb3+, 166Ho3+, 5%Na+ cleared from the biological system via the hepatobiliary route, which is expected for inorganic formulations.66 This is the expected clearance pattern of radiolabeled nanoparticles when administered in vivo.44,66 No accumulation of radioactivity in the skeleton or bladder was observed, which proved that the radiolabeled material maintained its integrity in vivo and the free 166Ho3+ ions did not leach out of the radiolabeled material.44,66 This study amply demonstrated the suitability of the radiolabeled material for use as a SPECT imaging probe. Owing to its luminescent properties, intrinsically radiolabeled ZnAl2O4:5%Yb3+, 166Ho3+, 5%Na+ could potentially be used for dual modality SPECT/optical imaging. Since 166Ho is an excellent therapeutic radioisotope,44,45 this class of intrinsically radiolabeled nanoformulations holds promise for use in theranostic applications.
3. Conclusion
The ZnAl2O4:Ho3+, Yb3+, Na+ samples, upon 980 nm excitation, show intense visible and short wavelength UV-C UC emissions along with long wave infrared radiation downconversion, which have been demonstrated rarely to date in Ho3+/Yb3+ doped UC phosphors. The Na+ ion codoping resulted in a large boost in UCL, which is essential for practical implementations. We experimentally observed the lowering of zinc vacancies in ZnAl2O4:Ho3+, Yb3+ on co-doping with Na+, indicating its effective role as a charge compensator and crystal field modulator. The excellent photophysical properties, high color purity and color tunability are attainable by adjusting the composition of Na+ ions and the incident laser power. We demonstrated the use of the intrinsically radiolabeled ZnAl2O4:166Ho3+, Yb3+, Na+ phosphor for dual-modality SPECT/optical imaging and cancer treatment.Additionally, promising in vitro results were obtained with fibroblast and C6 glioma cell lines, conclusively demonstrating the biocompatibility and effective laser-triggered anticancer potential of carrier/drug-free ZnAl2O4:Ho3+, Yb3+, Na+ UCNPs. The proof of concept ZnAl2O4:166Ho3+, Yb3+, Na+ SPECT imaging experiments revealed high radiochemical purity and integrity, good in vitro and in vivo stability, clearance via the hepatobiliary route, fast pharmacokinetics, and no detectable radioactivity accumulating in the bladder, skeleton or other organs, all of which are valuable for future work pairing 166Ho with therapeutic isotopes. Further, the impressive, intense UV-C emission could be crucial in safe disinfection and medical applications, especially in a threatening crisis like the COVID-19 pandemic.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
AB and SKG would like to acknowledge Dr J. Bahadur, SSPD for providing SEM images, Dr Kathi Sudarshan for PALS measurement, Dr B. Modak for DFT calculations, Dr Manoj Mohapatra in active holmium sample preparation and Dr Arnab Sarkar, FCD for LIBS measurements. The authors acknowledge the lead role of Dr Sudipta Chakraborty, Head, Radiochemicals Section, Radiopharmaceuticals Division, Bhabha Atomic Research Centre in production of radioisotopes in the Dhruva reactor. We would like to acknowledge Dr Praveen Kumar, School of Materials Sciences, Indian Association for the Cultivation of Science, Kolkata for help in TEM measurements. JJP would like to acknowledge Har-Gobind Khorana Young Innovative Biotechnologist Award from DBT (BT/13/IYBA/2020/08) and partial support from the DBT grant BT/PR36632/NNT/28/1694/2020 and SERB-Power grant (SPG/2021/002910-G) for funding. Bhabha Atomic Research Centre (BARC) is a government of India funded research insitute and this work was funded by BARC.References
- J. Ferlay, M. Ervik, F. Lam, M. Colombet, L. Mery, M. Piñeros, A. Znaor, I. Soerjomataram and F. Bray, Cancer, 2018, 3, 2019 Search PubMed.
- W. He, M. You, Z. Li, L. Cao, F. Xu, F. Li and A. Li, Sens. Actuators, B, 2021, 334, 129673 CrossRef CAS.
- Y. Wang, S. Song, S. Zhang and H. Zhang, Nano Today, 2019, 25, 38–67 CrossRef CAS.
- K. Zhang, Q. Zhao, S. Qin, Y. Fu, R. Liu, J. Zhi and C. Shan, J. Colloid Interface Sci., 2019, 537, 316–324 CrossRef CAS.
- C. Deng, M. Zheng, J. Xin and F. An, J. Colloid Interface Sci., 2023, 651, 384–393 CrossRef CAS.
- K. Heinzmann, L. M. Carter, J. S. Lewis and E. O. Aboagye, Nat. Biomed. Eng., 2017, 1, 697–713 CrossRef PubMed.
- G. Lee and Y. I. Park, Nanomaterials, 2018, 8, 511 CrossRef PubMed.
- D. M. Samhadaneh, G. A. Mandl, Z. Han, M. Mahjoob, S. C. Weber, M. Tuznik, D. A. Rudko, J. A. Capobianco and U. Stochaj, ACS Appl. Bio Mater., 2020, 3, 4358–4369 CrossRef CAS PubMed.
- K. Zhang, Z. Wan, H. Jiang, X. Xiao, Q. Feng, Y. Meng and Y. Yu, Mater. Des., 2021, 203, 109597 CrossRef CAS.
- C. Wang, H. Tao, L. Cheng and Z. Liu, Biomaterials, 2011, 32, 6145–6154 CrossRef CAS PubMed.
- K. Du, J. Feng, X. Gao and H. Zhang, Light: Sci. Appl., 2022, 11, 222 CrossRef CAS PubMed.
- K. Kaur, B. K. Sahu, K. Swami, M. Chandel, A. Gupta, L.-H. Zhu, J. P. Youngblood, S. Kanagarajan and V. Shanmugam, ACS Appl. Mater. Interfaces, 2022, 14, 27507–27514 CrossRef CAS PubMed.
- P. Li, Y. Yan, B. Chen, P. Zhang, S. Wang, J. Zhou, H. Fan, Y. Wang and X. Huang, Biomater. Sci., 2018, 6, 877–884 RSC.
- Y.-C. Tsai, P. Vijayaraghavan, W.-H. Chiang, H.-H. Chen, T.-I. Liu, M.-Y. Shen, A. Omoto, M. Kamimura, K. Soga and H.-C. Chiu, Theranostics, 2018, 8, 1435 CrossRef CAS PubMed.
- Z. Chu, T. Tian, Z. Tao, J. Yang, B. Chen, H. Chen, W. Wang, P. Yin, X. Xia and H. Wang, Bioact. Mater., 2022, 17, 71–80 CrossRef CAS.
- J. Hu, R. Wang, R. Fan, Z. Huang, Y. Liu, G. Guo and H. Fu, J. Lumin., 2020, 217, 116812 CrossRef CAS.
- L. Zhang, C. Cao, N. Kaushik, R. Y. Lai, J. Liao, G. Wang, N. Ariotti, D. Jin and M. H. Stenzel, Biomacromolecules, 2022, 23, 2572–2585 CrossRef CAS PubMed.
- N. Li, X. Wen, J. Liu, B. Wang, Q. Zhan and S. He, Opt. Mater. Express, 2016, 6, 1161–1171 CrossRef CAS.
- L. Cheng, K. Yang, Y. Li, X. Zeng, M. Shao, S.-T. Lee and Z. Liu, Biomaterials, 2012, 33, 2215–2222 CrossRef CAS PubMed.
- B. Dong, S. Xu, J. Sun, S. Bi, D. Li, X. Bai, Y. Wang, L. Wang and H. Song, J. Mater. Chem., 2011, 21, 6193–6200 RSC.
- S. Wen, J. Zhou, K. Zheng, A. Bednarkiewicz, X. Liu and D. Jin, Nat. Commun., 2018, 9, 1–12 CrossRef CAS.
- Y. Zhang, X. Zhu and Y. Zhang, ACS Nano, 2021, 15, 3709–3735 CrossRef CAS.
- K. Li and R. V. Deun, Inorg. Chem., 2019, 58, 6821–6831 CrossRef CAS.
- B. P. Kore, A. Kumar, L. Erasmus, R. E. Kroon, J. J. Terblans, S. J. Dhoble and H. C. Swart, Inorg. Chem., 2018, 57, 288–299 CrossRef CAS PubMed.
- M. K. Mahata, T. Koppe, K. Kumar, H. Hofsäss and U. Vetter, Sci. Rep., 2020, 10, 1–12 CrossRef PubMed.
- H. Suo, C. Guo, W. Wang, T. Li, C. Duan and M. Yin, Dalton Trans., 2016, 45, 2629–2636 RSC.
- P. Tadge, I. Martín, S. Rai, S. Sapra, T. Chen, V. Lavín, R. Yadav and S. Ray, J. Lumin., 2022, 252, 119261 CrossRef CAS.
- X. Wang, Y. Chen, F. Liu and Z. Pan, Nat. Commun., 2020, 11, 1–8 CrossRef CAS PubMed.
- G. Chen, C. Yang, B. Aghahadi, H. Liang, Y. Liu, L. Li and Z. Zhang, J. Opt. Soc. Am. B, 2010, 27, 1158–1164 CrossRef CAS.
- L. Xing, W. Yang, J. Lin, M. Huang and Y. Xue, Sci. Rep., 2017, 7, 1–7 CrossRef.
- X. Zhou, J. Qiao and Z. Xia, Chem. Mater., 2021, 33, 1083–1098 CrossRef CAS.
- X. Gao, W. Zhang, X. Wang, X. Huang and Z. Zhao, J. Alloys Compd., 2022, 893, 162265 CrossRef CAS.
- T. V. Gavrilović, D. J. Jovanović, L. V. Trandafilović and M. D. Dramićanin, Opt. Mater., 2015, 45, 76–81 CrossRef.
- S. K. Gupta, K. Sudarshan, A. K. Yadav, R. Gupta, D. Bhattacharyya, S. N. Jha and R. M. Kadam, Inorg. Chem., 2018, 57, 821–832 CrossRef CAS PubMed.
- S. Sinha, M. K. Mahata, H. Swart, A. Kumar and K. Kumar, New J. Chem., 2017, 41, 5362–5372 RSC.
- R. Yadav, R. Yadav, A. Bahadur and S. Rai, RSC Adv., 2016, 6, 51768–51776 RSC.
- S. Chang, J. Fu, X. Sun, G. Bai, G. Liu, K. Wang, L. Xu, Q. Wei, T. Meier and M. Tang, Inorg. Chem., 2022, 61, 3746–3753 CrossRef CAS PubMed.
- F. Chun, B. Zhang, H. Liu, W. Deng, W. Li, M. Xie, C. Luo and W. Yang, Dalton Trans., 2018, 47, 17515–17524 RSC.
- M. E. Foley, R. W. Meulenberg, J. R. McBride and G. F. Strouse, Chem. Mater., 2015, 27, 8362–8374 CrossRef CAS.
- R. E. Rojas-Hernandez, F. Rubio-Marcos, I. Romet, A. Del Campo, G. Gorni, I. Hussainova, J. F. Fernandez and V. Nagirnyi, Inorg. Chem., 2022, 61, 11886–11896 CrossRef CAS PubMed.
- G. N. Starostin, I. A. Zvonareva, D. A. Medvedev and S. V. Zvonarev, Ceram. Int., 2022, 48, 35606–35613 CrossRef CAS.
- Monika, R. S. Yadav, A. Bahadur and S. B. Rai, J. Phys. Chem. C, 2020, 124, 10117–10128 CrossRef CAS.
- R. S. Yadav, A. Rai and S. B. Rai, Sci. Rep., 2021, 11, 1–17 CrossRef PubMed.
- G. Gaikwad, N. Rohra, C. Kumar, S. Jadhav, H. D. Sarma, L. Borade, S. Chakraborty, S. Bhagwat, P. Dandekar and R. Jain, J. Drug Delivery Sci. Technol., 2021, 63, 102485 CrossRef CAS.
- S. Lohar, S. Jadhav, R. Chakravarty, S. Chakraborty, H. D. Sarma and A. Dash, Appl. Radiat. Isot., 2020, 161, 109161 CrossRef CAS PubMed.
- D. Ni, W. Bu, S. Zhang, X. Zheng, M. Li, H. Xing, Q. Xiao, Y. Liu, Y. Hua, L. Zhou, W. Peng, K. Zhao and J. Shi, Adv. Funct. Mater., 2014, 24, 6613–6620 CrossRef CAS.
- X. Jiang, Z. Zhang, T. Zhang, C. Li, Z. Leng, W. Yang, X. Wang, X. Shi, X. Zhang and H. Sha, J. Lumin., 2022, 245, 118759 CrossRef CAS.
- Q. Su, H.-L. Wei, Y. Liu, C. Chen, M. Guan, S. Wang, Y. Su, H. Wang, Z. Chen and D. Jin, Nat. Commun., 2021, 12, 4367 CrossRef CAS.
- S. Yan, Y. Liang, Y. Chen, J. Liu, D. Chen and Z. Pan, Dalton Trans., 2021, 50, 8457–8466 RSC.
- M. Malinowski, Z. Frukacz, M. Szuflińska, A. Wnuk and M. Kaczkan, J. Alloys Compd., 2000, 300, 389–394 CrossRef.
- M. Malinowski, M. Kaczkan, A. Wnuk and M. Szuflinska, J. Lumin., 2004, 106, 269–279 CrossRef CAS.
- W. Carnall, P. Fields and K. Rajnak, J. Chem. Phys., 1968, 49, 4450–4455 CrossRef CAS.
- J. Suyver, A. Aebischer, S. García-Revilla, P. Gerner and H. Güdel, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 125123 CrossRef.
- Y. Peng, L. Xu, J. Peng, T. Wang, Q. Wang, Y. Li, Z. Yin, J. Han, J. Qiu and Z. Yang, J. Lumin., 2022, 252, 119269 CrossRef CAS.
- A. S. Kumar, R. A. Kumar and R. Vijayakumar, J. Mater. Sci.: Mater. Electron., 2022, 33, 21297–21310 CrossRef CAS.
- S. K. Gupta, K. Sudarshan, P. S. Ghosh, A. P. Srivastava, S. Bevara, P. K. Pujari and R. M. Kadam, J. Mater. Chem. C, 2016, 4, 4988–5000 RSC.
- Z. Zhang, Y. Yang, M. Zhao, L. Lu, F. Zhang and Y. Fan, ACS Appl. Bio Mater., 2022, 5, 2935–2942 CrossRef CAS PubMed.
- K. Du, P. Lei, L. Dong, M. Zhang, X. Gao, S. Yao, J. Feng and H. Zhang, Appl. Mater. Today, 2020, 18, 100497 CrossRef.
- Y. Xing, L. Li, X. Ai and L. Fu, Int. J. Nanomed., 2016, 11, 4327 CrossRef CAS PubMed.
- M. Buchner, P. G. Calavia, V. Muhr, A. Kröninger, A. J. Baeumner, T. Hirsch, D. A. Russell and M. J. Marín, Photochem. Photobiol. Sci., 2019, 18, 98–109 CrossRef CAS PubMed.
- B. Chen, Y. Wang, Y. Guo, P. Shi and F. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 2327–2335 CrossRef CAS PubMed.
- R. Lv, D. Wang, L. Xiao, G. Chen, J. Xia and P. N. Prasad, Sci. Rep., 2017, 7, 15753 CrossRef PubMed.
- M. Nahorniak, O. Pop-Georgievski, N. Velychkivska, M. Filipová, E. Rydvalová, K. Gunár, P. Matouš, U. Kostiv and D. Horák, Life, 2022, 12, 1383 CrossRef CAS PubMed.
- K. Ovejero Paredes, D. Díaz-García, V. García-Almodóvar, L. Lozano Chamizo, M. Marciello, M. Díaz-Sánchez, S. Prashar, S. Gómez-Ruiz and M. Filice, Cancers, 2020, 12, 187 CrossRef PubMed.
- S. Chibh, K. Kaur, U. K. Gautam and J. J. Panda, Nanoscale, 2022, 14, 715–735 RSC.
- R. Chakravarty, A. Guleria, S. Jadhav, C. Kumar, A. K. Debnath, H. D. Sarma and S. Chakraborty, Ind. Eng. Chem. Res., 2020, 59, 22492–22500 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma00376k |
| This journal is © The Royal Society of Chemistry 2023 |