Open Access Article
Open Access ArticleConstructing amorphous/amorphous heterointerfaces in nickel borate/boride composites for efficient electrocatalytic methanol oxidation†
Ping
Chen
,
Sai
Zhang
,
Yu
Fan
,
Wenlong
Yang
 * and
Xiliang
Luo
* and
Xiliang
Luo
 *
*
Key Laboratory of Optic-electric Sensing and Analytical Chemistry for Life Science, MOE, Key Laboratory of Analytical Chemistry for Life Science in Universities of Shandong, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, P. R. China. E-mail: wlyang@qust.edu.cn; xiliangluo@qust.edu.cn
First published on 1st February 2023
Abstract
Owing to the inherently torpid kinetics involving an intricate multi-electron redox process, the methanol oxidation reaction (MOR) severely restrains the efficiency of direct methanol fuel cells (DMFCs). Herein, we put forward a room-temperature liquid-phase reaction strategy to construct an amorphous/amorphous heterojunction structure in a nickel borate/boride composite. It is found that the two sides of the heterointerface are composed of amorphous nickel boride (a-NixB) nanoparticles and amorphous nickel borate (a-Ni-Bi) ultrathin nanosheets. Experimental measurements reveal that the amorphous/amorphous heterostructure endows the catalyst with enhanced charge transport capacity and stepped-up formation of electroactive NiOOH species, both of which contribute considerably to optimizing the catalytic kinetics for the MOR. As a result of the highly exposed active area and better intrinsic reactivity of catalytic sites, the amorphous Ni-Bi/NixB (a-Ni-Bi/NixB) hybrid catalyst exhibits outstanding electrocatalytic MOR activity with a low onset potential of 0.38 V vs. Ag/AgCl and a large current density of 213 mA cm−2 at a potential of 0.6 V vs. Ag/AgCl, which can be comparable to the best MOR performance of highly active Ni-based catalysts to date. This work opens new opportunities to design and synthesize efficient MOR electrocatalysts.
Introduction
Direct methanol fuel cells (DMFCs) have emerged as a clean and renewable energy conversion technology that can directly accomplish electrical output by converting chemical energy, and they hold great promise to address the growing energy demand and environmental problem.1–3 Nevertheless, a DMFC's overall conversion efficiency is frustratingly impeded by the kinetically slow methanol oxidation reaction (MOR) correlative to its complicated multi-electron transfer pathway, together with disadvantageous routes for the evolution of detrimental intermediates.4,5 To date, despite the fact that a series of noble metals such as Pt- and Pd-based materials have been broadly recognized as the most effective MOR electrocatalysts to promote the sluggish methanol oxidation process, their extreme scarcity and daunting expensiveness as well as easy poisoning unfortunately render the realization of their large-scale utilization impractical for future commercialization.6,7 In such regard, numerous research attempts have been unhesitatingly made to achieve affordable transition-metal-based catalysts on account of their desirable advantages of abundant reserves, low cost, ease of availability and reliable electrochemical properties.8–11 Even so, limited by their slow charge transfer, deficient active sites and poor intrinsic catalytic activity, the electrocatalytic MOR performance of these materials is still far behind what we expect and needs significant promotion so as to catch up with the requirement of the commercial application of DMFCs.12–14As reported recently, heterointerface engineering, especially for constructing heterostructures based on ultrathin two-dimensional (2D) nanomaterials, has attracted considerable attention in the electrochemical field owing to the impressive synergetic effects between ultrathin 2D structures and atomic-level coupled interactions of different components on electrocatalysis, which can not only allow for more exposed heterojunctions as catalytically active centres on the surface of catalysts, but also appreciably modulate the surface charge distribution for optimizing the reaction kinetics, as a consequence, dramatically improving the catalytic performance of electrocatalysts.15–20 To date, relevant research studies have mainly concentrated on crystalline/crystalline heterostructures by virtue of their well-defined heterointerfaces along with available synthetic strategies and structural characterization methods. Nowadays, increasing efforts have been intensely devoted to pursuing amorphous/crystalline heterostructures since amorphous electrocatalysts usually exhibit remarkable superiority to their crystalline counterparts in facilitating the electrocatalytic process.21–24 Therefore, it can be deliberately anticipated that the amorphous/amorphous heterojunction confined in ultrathin 2D structures will increase drastically both the number and intrinsic reactivity of active sites, enabling the realization of highly active electrocatalysts for the MOR. However, to the best of our knowledge, the construction of amorphous/amorphous heterostructures in ultrathin 2D nanosheets has rarely been reported to date, which is mainly due to the following aspects: (1) the isotropic nature of amorphous materials certainly makes the formation of nanostructures with ultrathin 2D configuration challenging (the growth mechanism of which relies principally on the inherent directionality of lattices). (2) The connatural metastability of the amorphous state in solution renders it very prone to transform into a crystalline state, inevitably leading to the formation of amorphous/crystalline heterostructures. (3) The understanding of the underlying relationships between amorphous/amorphous heterostructures and the MOR performance of electrocatalysts still remains scarce, primarily ascribed to the substantial difficulties in constructing such heterostructures and concerned theoretical simulations.25,26 Thus, it is particularly desirable yet tricky to design and synthesize advanced electrocatalysts with abundant amorphous/amorphous heterostructures for optimizing the MOR kinetics, aiming to accomplish high-efficiency methanol electro-oxidation.
As a kind of positive electrode material for wide application in energy conversion and storage, transition-metal boride and borate have been demonstrated as attractive electrocatalysts with compelling catalytic activity and stability for small-molecule (H2O, urea, methanol, and so forth) oxidation in a strongly alkaline medium.27–29 Notably, both ultrathin nickel-based boride and borate nanosheets in an amorphous state have been achieved using a mature wet chemical strategy.30–32 More preferably, amorphous metal boride is very susceptible to common oxidants and can be oxidized spontaneously into amorphous metal oxides and/or hydroxides once exposed to air or oxygen,33 giving a giant opportunity to constitute an eligible amorphous/amorphous heterostructure for strengthening the MOR performance of non-noble metal electrocatalysts.
Inspired by the abovementioned discussions, herein, we construct an appealing amorphous/amorphous heterostructure consisting of amorphous nickel boride (denoted as a-NixB) nanoparticles on amorphous nickel borate (denoted as a-Ni-Bi) ultrathin nanosheets through a rapid and simple room-temperature aqueous reaction under ambient conditions. Intriguingly, thanks to the synergistic effect between the ultrathin 2D configuration and amorphous/amorphous hetero-interfaces, the as-prepared amorphous Ni-Bi/NixB (denoted as a-Ni-Bi/NixB) hybrid catalyst is found to deliver promising electrocatalytic performance towards methanol oxidation, which affords a large anodic current density reaching up to 213 mA cm−2 at 0.6 V vs. Ag/AgCl, roughly 36.5 and 10.0 times higher than those of pure a-NixB (5.84 mA cm−2) and a-Ni-Bi (21.4 mA cm−2), respectively. Meanwhile, taking the a-Ni-Bi/NixB hybrid structures as a proof-of-concept example, we shed light on the substantial efficacy of amorphous/amorphous heterostructures in boosting the electrocatalytic MOR process. This work may present a new perspective for designing and synthesizing highly efficient electrocatalysts for future energy conversion applications.
Results and discussion
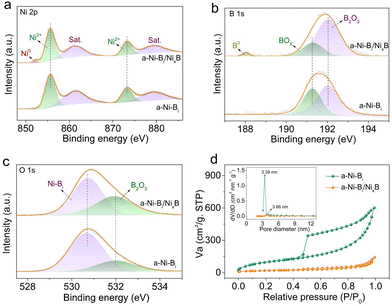
In this study, the fabrication of a-Ni-Bi/NixB hybrid structures was easily accomplished by implementing a facile liquid-phase reaction at room temperature. As illustrated in Fig. 1(a), black precipitation appears instantly after adding BH4− to nickel ammonia (Ni[NH3]62+) aqueous solution. As demonstrated before, the strong reducibility of BH4− brings about the fast formation of the a-NixB phase along with the reduction of Ni(II) to Ni(0). Simultaneously, the hydrolysis of BH4− gives rise to numerous borate anions that will immediately combine with nickel cations to generate an a-Ni-Bi phase, resulting in the establishment of a-Ni-Bi/NixB heterostructures. It should be noted that the presence of a-NixB can in turn catalyze the hydrolysis reaction of BH4− to stimulate in situ creation of more a-Ni-Bi surrounding the as-formed a-NixB, endowing robust contact between the two components at the heterointerface.34 In this composite, the a-NixB component will gradually disappear with the assistance of bubbling oxygen during the aqueous reaction, eventually causing the formation of a bare a-Ni-Bi sample. First, powder X-ray diffraction (XRD) analysis was performed to characterize the phase composition of the resulting products. As observed from the XRD patterns in Fig. 1(b), a broad diffraction peak at around 45° in the case of a-Ni-Bi/NixB discloses the amorphous nature of the a-NixB component, while two evident diffraction peaks at 33.4° and 59.8° are readily assigned to the amorphous Ni3(BO3)2 phase (JCPDS no. 26-1284), matching well with previously reported results.35 This explicitly demonstrates the coexistence of a-Ni-Bi and a-NixB phases in such hybrid structures. Of particular interest, the discernible disappearance of the as-mentioned broad diffraction peak in the XRD pattern of a-Ni-Bi reflects the consequent acquirement of the bare a-Ni-Bi sample upon oxygen treatment. Subsequently, transmission electron microscopy (TEM) was carried out to investigate the structural information of the samples. The TEM image in Fig. 1(c) indicates that the a-Ni-Bi component in the as-synthesized hybrid typically exhibits sheet-like morphology and its almost transparent feature signifies the ultrathin thickness of nanosheets. From the TEM image in Fig. S1 (ESI†), the thickness of the curled edge of nanosheets can be identified to be about 5.0 nm. As can be seen, the a-NixB component, in the form of nanoparticles with an average size of ∼50 nm (highlighted by yellow dashed circles), seems to be anchored evenly on the surface of a-Ni-Bi nanosheets, demonstrating their highly compatible structure with each other. Notably, the high-resolution TEM (HRTEM) image in Fig. 1(d) shows negligible crystal fringes in both a-Ni-Bi and a-NixB sides, verifying the dominantly amorphous characteristics of the two components. Meanwhile, a close look visibly demonstrates the interface between the a-NixB nanoparticle and the a-Ni-Bi nanosheet (marked by the orange dashed line), convincingly validating the successful construction of the a-Ni-Bi/NixB heterostructures. As expected, no obvious particle-like morphology can be identified from the TEM image in Fig. 1(e), which strictly confirms the decomposition of the a-NixB nanoparticles upon continuous oxygen bubbling, thereby leaving the pure a-Ni-Bi nanosheets.X-ray photoelectron spectroscopy (XPS) was conducted to survey the surface composition and chemical state of the a-Ni-Bi nanosheets and the a-Ni-Bi/NixB hybrid. As displayed in Fig. 2(a), the high-resolution Ni 2p XPS spectra in the case of a-Ni-Bi nanosheets exhibit two dominant Ni 2p3/2 and Ni 2p1/2 peaks at 855.7 and 873.2 eV, accompanied by two satellite peaks at 861.4 and 879.4 eV, typically indicative of the presence of bivalent nickel. While in the case of the a-Ni-Bi/NixB hybrid, an additional peak at 852.3 eV can be well detected, which unambiguously authenticates the existence of metallic Ni0 belonging to the NixB nanoparticles.36 As shown in Fig. 2(b), the B 1s spectra of a-Ni-Bi nanosheets can be clearly deconvoluted into two distinct peaks at 191.3 and 192.0 eV, both of which are assignable to the binding energy of B–O in boron oxide species. In addition to that, the peak located at 188.0 eV corresponds well to the signal of elemental boron in the case of the a-Ni-Bi/NixB hybrid, further corroborating the existence of NixB.33 In the O1s spectrum (Fig. 2(c)), a pair of fitted peaks centered at 530.8 and 532.0 eV can be respectively attributed to the B–O–Ni bond in the a-Ni-Bi phase and the B–O bond in B2O3.37,38 Concomitantly, the relative amount of the B–O–Ni species was briefly quantified in terms of the integral area of the corresponding peak, revealing that the relative content of the a-Ni-Bi phase in the a-Ni-Bi/NixB hybrid increases distinctly with the operation of bubbling oxygen, which is in good accordance with the aforementioned results and discussion. In addition, N2 adsorption–desorption tests were conducted to gain more surface structural information and insights into the pore structure of the obtained samples. According to the adsorption–desorption isotherm curves in Fig. 2(d), an obvious type-IV hysteresis loop can be recognized for both samples, representing the presence of plentiful mesopores correlative to appreciable slit-shaped pores, which probably originated from the sheet-like morphology of a-Ni-Bi. Evidently, Barrett–Joyner–Halenda (BJH) pore size distribution profiles (inset of Fig. 2(d)) display one prominent sharp peak corresponding to the pore diameters of about 3.39 and 3.66 nm respectively for a-Ni-Bi and a-Ni-Bi/NixB samples (enlarged image in Fig. S2, ESI†), further proving the existence of abundant mesopores with a narrow range of size distribution. Also, this can be vividly confirmed by the TEM image in Fig. S3 (ESI†). Impressively, the a-Ni-Bi nanosheets show a much larger BJH pore volume of 0.936 cm3 g−1 with respect to the a-Ni-Bi/NixB hybrid sample (0.222 cm3 g−1). Moreover, the Brunauer–Emmett–Teller (BET) surface area of the a-Ni-Bi sample is found to be 369.0 m2 g−1, roughly 5.7 times higher than that of the a-Ni-Bi/NixB hybrid sample (70.3 cm2 g−1). These results suggest that the a-Ni-Bi component makes a significant contribution to the specific surface area of the a-Ni-Bi/NixB hybrid sample due to its ultrathin 2D structure, which is favorable for the sufficient exposure of active sites for expediting the surface catalytic reaction.
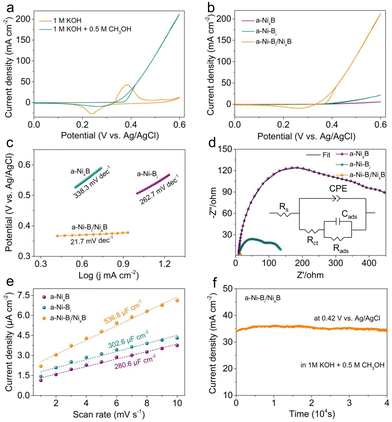
In order to investigate the influence of amorphous/amorphous heterostructures on the electrochemical behaviors, a sequence of measurements was preliminarily carried out in a classic three-electrode system. In this study, a-NixB nanoparticles were also synthesized as an essential reference using a similar liquid-phase reaction and the corresponding TEM image and XRD pattern are presented in Fig. S4 (ESI†). Prior to collecting data, all working electrodes need to be activated by consecutive cyclic voltammetry (CV) tests until the current signal reaches stabilization. Fig. 3(a) shows the CV curves of the a-Ni-Bi/NixB hybrid sample recorded at different scan rates from 10 to 150 mV s−1 in 1 M KOH solution. As can be observed, the cathodic and anodic peaks located at around 0.35 V vs. Ag/AgCl are associated with the reversible Ni2+/Ni3+ redox couple.39 It is apparent that the a-Ni-Bi/NixB hybrid shows a smaller potential gap (∼100 mV) between the oxidation and reduction peak in the light of the CV curve at a scan rate of 10 mV s−1, as compared to those of a-NixB (∼170 mV) and a-Ni-Bi (∼180 mV) (Fig. S5, ESI†), which portends the more advantageous electrical conductivity and reversibility of the a-Ni-Bi/NixB hybrid electrode.40 Also, the redox peak intensity increases progressively with the increase of scan rate, exhibiting a good linear relationship between them (Fig. 3(b)). Furthermore, on the basis of the average of anodic and cathodic results, the surface coverage of the Ni2+/Ni3+ redox species (Γ*) for each electrode can be investigated. As summarized in Fig. 3(d), the Γ* values for the a-Ni-Bi/NixB hybrid, a-NixB and a-Ni-Bi electrodes are respectively estimated to be 3.77 × 10−7, 3.96 × 10−8 and 4.86 × 10−8 mol cm−2, because of which the a-Ni-Bi/NixB hybrid electrode shows a significantly greater oxidation peak current in comparison with the other two electrodes. This manifests the higher surface coverage of the Ni2+/Ni3+ redox species on the a-Ni-Bi/NixB hybrid catalyst during the electro-oxidation process. Moreover, both the anodic and cathodic peak current densities are linearly proportional to the square root of scan rates (Fig. 3(c)), revealing that the vigorous transformation between Ni(OH)2 and NiOOH species in alkaline media is a diffusion-controlled process related to the solid-phase body of proton diffusion as reported before.41 Consequently, the proton diffusion coefficient (D) is viewed as an imperative parameter for describing the electro-oxidation property of catalysts, which is evaluated by means of the Randles–Sevcik equation42 together with the corresponding linear slopes in Fig. 3(c). As seen in Fig. 3(d), the a-Ni-Bi/NixB hybrid electrode delivers the highest D value of 2.35 × 10−7 cm2 s−1 among the three electrodes, nearly two orders of magnitude larger than those for the a-NixB electrode (2.58 × 10−9 cm2 s−1) and the a-Ni-Bi electrode (3.82× 10−9 cm2 s−1), which demonstrates the sharply promoted Ni(OH)2/NiOOH redox process and thus the preferential generation of catalytically active NiOOH species at the amorphous/amorphous interface region, suggesting that the a-Ni-Bi/NixB hybrid electrode can drive the MOR at a more negative onset potential.
With a view to shedding light on the efficacy of the amorphous/amorphous heterojunction structure on the electrocatalytic MOR performance, CV measurements were carried out in 1 M KOH electrolyte with and without 0.5 M methanol at a sweep rate of 50 mV s−1. As shown in Fig. 4(a), the a-Ni-Bi/NixB hybrid electrode exhibits a drastic boost in anodic current density after the addition of methanol, providing direct proof of its impactful electrochemical response towards methanol oxidation. By contrast, upon the addition of methanol, the slightly enhanced anodic current densities in the cases of the other two electrodes signify their mightily inferior electrocatalytic MOR performance (Fig. S6, ESI†). In terms of the CV curves obtained in 1 M KOH containing 0.5 M methanol (Fig. 4(b)), the hybrid catalyst of a-Ni-Bi/NixB presents a lower onset potential (0.38 V vs. Ag/AgCl) and a greater anodic current density with respect to the a-NixB and a-Ni-Bi catalysts, cogently demonstrating its superior electrocatalytic activity for the MOR. In particular, the a-Ni-Bi/NixB hybrid electrode requires merely a low potential of 387 mV vs. Ag/AgCl to drive a current density of 10 mA cm−2, but that for the a-Ni-Bi electrode is found to be 506 mV vs. Ag/AgCl. Significantly, the a-Ni-Bi/NixB electrode affords quite a large current density reaching up to 213 mA cm−2 at a potential of 0.6 V vs. Ag/AgCl, about 36.5 and 10.0 times higher than those of the a-NixB and a-Ni-Bi electrodes, respectively. This value can be comparable to those of the most active MOR electrocatalysts reported recently under similar conditions (Table S1, ESI†). Meanwhile, as displayed in Fig. 4(c), the a-Ni-Bi/NixB hybrid electrode possesses a Tafel slope of 21.7 mV dec−1, markedly smaller than those of the a-NixB electrode (338.3 mV dec−1) and the a-Ni-Bi electrode (262.7 mV dec−1), suggesting its more favorable electrocatalytic kinetics for the MOR. Furthermore, electrochemical impedance spectroscopy (EIS) was utilized to clarify the charge transfer kinetics during the electrocatalysis. As shown in Fig. 4(d), by virtue of the electrical equivalent circuit (inset), the Nyquist plots of the a-Ni-Bi/NixB hybrid, a-NixB and a-Ni-Bi electrodes can be fitted into two partially overlapped semicircles in high and low frequency regions. Obviously, among these tested electrodes, the a-Ni-Bi/NixB hybrid electrode exhibits the smallest diameter of semicircle in the high frequency region, which demonstrates the lowest charge transfer resistance (Rct) and significantly boosted MOR kinetics on the hybrid electrode since the semicircle at high frequency is closely associated with the Rct during the electrochemical process.
As is known, the electrochemical active surface area (ECSA) and intrinsic reactivity of active sites have imperative influences on the overall electrocatalytic performance, which are deemed as two indispensable descriptors to evaluate high-efficiency MOR catalysts.43,44 In this regard, the electric double-layer capacitance (Cdl) measurement was implemented to assess the ECSA of catalysts by collecting CV curves at different scan rates in a non-faradaic potential range (Fig. S7, ESI†). As shown in Fig. 4(e), the Cdl values for a-NixB, a-Ni-Bi and a-Ni-Bi/NixB hybrid electrodes are calculated to be 280.6, 302.6 and 536.8 μF cm−2, respectively. This result can be indicative of the larger ECSA of the a-Ni-Bi/NixB hybrid catalyst and thus reflects its more exposed active surface derived from the amorphous/amorphous heterojunction structures. As for the inherent activity of catalytic sites towards methanol oxidation, the CV curves for the MOR were normalized by their corresponding Cdl values. As can be seen from Fig. S8 (ESI†), the a-Ni-Bi/NixB hybrid electrode attains a higher normalized MOR current density, which is respectively 19.1 and 5.6 folds larger than those of the a-NixB and a-Ni-Bi electrodes at a potential of 0.6 V vs. Ag/AgCl, demonstrating its markedly increased intrinsic activity for the MOR. Additionally, as another vital parameter to appraise the catalytic performance, the long-term stability of the a-Ni-Bi/NixB hybrid catalyst was further examined using a chronoamperometric curve at a potential of 0.42 V vs. Ag/AgCl. As shown in Fig. 4(f), no evident diminishment in current density can be observed even after 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s of continuous operation in 1 M KOH containing 0.5 M methanol solution. Besides that, the TEM image after the stability test (Fig. S9, ESI†) exhibits negligible variations in the morphology of the a-Ni-Bi/NixB hybrid catalyst, verifying its splendid electrocatalytic endurance as well as structural durability for the MOR under strong alkaline conditions, which makes it more beneficial for the potential application of DMFCs in future. Taken together, these results potently elucidate the exceptional contribution of the amorphous/amorphous heterostructures in promoting the electrocatalytic MOR process.
000 s of continuous operation in 1 M KOH containing 0.5 M methanol solution. Besides that, the TEM image after the stability test (Fig. S9, ESI†) exhibits negligible variations in the morphology of the a-Ni-Bi/NixB hybrid catalyst, verifying its splendid electrocatalytic endurance as well as structural durability for the MOR under strong alkaline conditions, which makes it more beneficial for the potential application of DMFCs in future. Taken together, these results potently elucidate the exceptional contribution of the amorphous/amorphous heterostructures in promoting the electrocatalytic MOR process.
To acquire more insights into the electrochemical process for the MOR, a suite of double-potential-step chronoamperometry tests were carried out by adopting the working potential at 0.6 V vs. Ag/AgCl (first step) and 0 V vs. Ag/AgCl (second step) in 1 M KOH with various methanol concentrations. As displayed in Fig. 5(a), the current density for the a-Ni-Bi/NixB hybrid electrode becomes constant quickly and increases gradually as the methanol concentration increases at the forward potential step, embodying its rapid and stable electrochemical response for methanol oxidation. Obviously, the chronoamperogram of the a-Ni-Bi/NixB hybrid electrode in the case of the absence of methanol exhibits an approximate symmetry between the forward and backward potential steps, which is correlated solely to the reversible electrochemical behavior of the Ni(OH)2/NiOOH redox couple.45 Nevertheless, in the case of the presence of methanol, the corresponding charge density obtained from the forward potential step is strikingly larger than that for the backward potential step, revealing that the methanol oxidation is an irreversible electrocatalytic process. Not only that, taking advantage of the collected chronoamperograms, the catalytic rate constant (k) for the electro-oxidation of methanol can be further determined in accordance with the following equation:46
| IC/IL = γ1/2[π1/2 erf(γ1/2) + exp(−γ)/γ1/2] | (1) |
| IC/IL = π1/2γ1/2 = (πkCt)1/2 | (2) |
According to eqn (2), good linear dependences are obtained by plotting the IC/IL ratio against t1/2 (Fig. 5(c) and Fig. S10, ESI†). Therefore, the k value can be calculated from the slope of linear plots when the methanol concentration is given. As shown in Fig. 5(d), the average k value for the a-Ni-Bi/NixB hybrid electrode is found to be 2.25 × 106 cm3 mol−1 s−1, about 180 and 92 times higher than those for the a-NixB electrode (1.25 × 104 cm3 mol−1 s−1) and the a-Ni-Bi electrode (2.44 × 104 cm3 mol−1 s−1), respectively, emphasizing the noteworthy superiority of the amorphous/amorphous heterostructures in accelerating the electrocatalytic reaction rate of methanol oxidation.
Based on the experimental analysis and results, it can be inferred that the amorphous/amorphous heterointerface in conjunction with the ultrathin 2D structure is predominantly responsible for the significantly enhanced electrochemical MOR performance of the a-Ni-Bi/NixB hybrid catalyst. On one hand, the combination of the advantages of a dominating amorphous structure and an ultrathin 2D configuration allows both the surface and inner atoms of the catalyst to be highly exposed to the electrolyte, which renders the increased electroactive surface area and mass transfer of reactants to speed up the MOR process. On the other hand, the amorphous/amorphous heterojunction structure empowers the catalyst with more exposure of catalytic sites and higher inherent reactivity, which enable the facilitation of charge transfer kinetics and thereby the ultimate optimization of the electrochemical MOR performance.
Conclusions
In summary, an a-Ni-Bi/NixB composite with a dominating amorphous/amorphous heterojunction structure has been successfully synthesized using the liquid-phase reaction method at room temperature, which was subsequently used as a fascinating electrocatalyst for methanol oxidation. Meanwhile, taking the a-Ni-Bi/NixB hybrid system as a satisfactory prototype, the efficacy of the amorphous/amorphous heterojunction structure in the noticeable promotion of the electrocatalytic MOR process is elaborately expounded. It was found that the amorphous/amorphous heterojunction structure is more conducive to the formation and exposure of active NiOOH species and thus achieving accelerated MOR kinetics. Benefiting from the synergistic interactions of the amorphous structure, heterointerface effect and ultrathin 2D configuration, the obtained a-Ni-Bi/NixB hybrid catalyst exhibits impressive electrocatalytic MOR activity, with a smaller onset potential (0.38 V vs. Ag/AgCl), a lower Tafel slope (21.7 mV dec−1) and a higher current density (213 mA cm−2 at 0.6 V vs. Ag/AgCl) relative to the a-Ni-Bi and a-NixB catalysts, as well as remarkable long-term durability in an alkaline electrolyte. This work would offer a promising perspective for the design and construction of low-cost and high-efficiency electrocatalysts for DMFCs.Experimental
Materials
All reagents were of analytical reagent grade and used as received without further purification.Preparation of the a-Ni-Bi/NixB hybrid catalyst
In a typical procedure, 1 mL of ammonia solution (28% wt) was added into 100 mL of 5 mM NiCl2 aqueous solution to form a transparent mixture under constant stirring. After that, 1 mL of 1 M freshly prepared NaBH4 aqueous solution (at about 5 °C maintained using an ice-bath) was added dropwise into the above solution with vigorous stirring and a black precipitate was formed instantaneously. After the termination of bubble generation, the resulting product was collected by centrifugation and washed with deionized water and ethanol several times. Then, it was dried at 60 °C overnight.Preparation of the a-Ni-Bi catalyst
The synthesis of the a-Ni-Bi catalyst is similar to that of the a-Ni-Bi/NixB hybrid catalyst, except that the freshly obtained black precipitate was treated with bubbling oxygen in the primordial mixture solution for another 60 minutes until the color of the mixture solution turned from black to light green.Preparation of the a-NixB catalyst
The a-NixB catalyst was prepared through a liquid-phase reaction in conformity with previous literature.47 Typically, 0.5 mmol NiCl2·6H2O was added into 15 mL of tetraethylene glycol (TEG) and deaerated under Ar gas. Thereafter, 15 mL of TEG solution containing 10 mmol NaBH4 was injected into the above mixture solution and heated at 60 °C for 1 h under an Ar atmosphere. The product was centrifuged and washed with ethanol several times and then dried overnight at 60 °C.Materials characterization
Powder X-ray diffraction (XRD) patterns were obtained using a Japan Rigaku D/max-rA system with Cu Kα radiation (λ = 1.542 Å). Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) were carried out on a JEOL-2100 TEM at an acceleration voltage of 200 kV. X-ray photoelectron spectroscopy (XPS) tests were taken on an ESCALAB MKII X-ray photoelectron spectrometer with an excitation source of Mg Kα = 1253.6 eV. Nitrogen adsorption–desorption tests were performed using a Micromeritics ASAP 2460 system.Electrochemical measurements
Electrochemical tests were carried out on an electrochemical station (CHI660B) in a three-electrode system by adopting a graphite electrode as the counter electrode, a saturated Ag/AgCl electrode as the reference electrode and a glassy carbon electrode (GCE) with different catalysts as the working electrode. Typically, 4 mg of the as-prepared catalysts and 30 μL of Nafion solutions (5 wt%) were dispersed in 1 mL of isopropanol–water solution (volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) by vigorously sonicating for 1 h to obtain a homogeneous ink. Then, 5 μL of the resulting dispersion was evenly loaded onto a GCE (3 mm in diameter). Cyclic voltammetry (CV) was performed in 1 M KOH solution without and with 0.5 M methanol at a scan rate of 50 mV s−1. Electrochemical impedance spectroscopy (EIS) measurements were carried out at a potential of 0.5 V vs. Ag/AgCl. The amplitude of the applied voltage was 5 mV, and the frequency range was 100 kHz to 1 Hz. The chronoamperometric test was implemented in 1 M KOH solution with 0.5 M methanol at a potential of 0.42 V vs. Ag/AgCl. The double layer capacitance (Cdl) of different catalysts was determined from the CV curves at different scan rates in a non-faradaic potential region (0.16–0.20 V vs. Ag/AgCl), which can be evaluated by plotting the ΔJ = (Ja − Jc) at 0.18 V vs. Ag/AgCl against the scan rate. The Cdl value is half of the linear slope.
3) by vigorously sonicating for 1 h to obtain a homogeneous ink. Then, 5 μL of the resulting dispersion was evenly loaded onto a GCE (3 mm in diameter). Cyclic voltammetry (CV) was performed in 1 M KOH solution without and with 0.5 M methanol at a scan rate of 50 mV s−1. Electrochemical impedance spectroscopy (EIS) measurements were carried out at a potential of 0.5 V vs. Ag/AgCl. The amplitude of the applied voltage was 5 mV, and the frequency range was 100 kHz to 1 Hz. The chronoamperometric test was implemented in 1 M KOH solution with 0.5 M methanol at a potential of 0.42 V vs. Ag/AgCl. The double layer capacitance (Cdl) of different catalysts was determined from the CV curves at different scan rates in a non-faradaic potential region (0.16–0.20 V vs. Ag/AgCl), which can be evaluated by plotting the ΔJ = (Ja − Jc) at 0.18 V vs. Ag/AgCl against the scan rate. The Cdl value is half of the linear slope.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21805149, 21675093), the Natural Science Foundation of Shandong Province of China (ZR2018BB012, 2015ZRB01A0D), and the Taishan Scholar Program of Shandong Province of China (ts20110829).Notes and references
- Q. B. Yuan, D. H. Duan, Y. H. Ma, G. Q. Wei, Z. L. Zhang, X. G. Hao and S. B. Liu, Performance of nano-nickel core wrapped with Pt crystalline thin film for methanol electro-oxidation, J. Power Sources, 2014, 245, 886–891 CrossRef CAS.
- N. Kakati, J. Maiti, S. H. Lee, S. H. Jee, B. Viswanathan and Y. S. Yoon, Anode Catalysts for Direct Methanol Fuel Cells in Acidic Media: Do We Have Any Alternative for Pt or Pt–Ru, Chem. Rev., 2014, 114, 12397–12429 CrossRef CAS PubMed.
- A. Badalyan and S. S. Stahl, Cooperative electrocatalytic alcohol oxidation with electron-proton-transfer mediators, Nature, 2016, 535, 406–410 CrossRef CAS PubMed.
- Y. P. Wu, J. W. Tian, S. Liu, B. Li, J. Zhao, L. F. Ma, D. S. Li, Y. Q. Lan and X. H. Bu, A Highly Stable Bi-Microporous MOF with Cubane [Ni4(OH)4] Cluster and Pore Space Partition for Electrocatalytic Methanol Oxidation Reaction, Angew. Chem., Int. Ed., 2019, 58, 12185–12189 CrossRef CAS PubMed.
- R. Ganesan and J. S. Lee, Tungsten Carbide Microspheres as a Noble-Metal-Economic Electrocatalyst for Methanol Oxidation, Angew. Chem., Int. Ed., 2005, 44, 6557–6560 CrossRef CAS PubMed.
- G. G. Liu, W. Zhou, Y. R. Ji, B. Chen, G. T. Fu, Q. B. Yun, S. M. Chen, Y. X. Lin, P. F. Yin, X. Y. Cui, J. W. Liu, F. Q. Meng, Q. H. Zhang, L. Song, L. Gu and H. Zhang, Hydrogen-Intercalation-Induced Lattice Expansion of Pd@Pt Core–Shell Nanoparticles for Highly Efficient Electrocatalytic Alcohol Oxidation, J. Am. Chem. Soc., 2021, 143, 11262–11270 CrossRef CAS PubMed.
- N. Yu, L. Kuai, Q. Wang and B. Y. Geng, Pt nanoparticles residing in the pores of porous LaNiO3 nanocubes as high-efficiency electrocatalyst for direct methanol fuel cells, Nanoscale, 2012, 4, 5386–5393 RSC.
- H. Fang, J. H. Yang, M. Wen and Q. S. Wu, Nanoalloy Materials for Chemical Catalysis, Adv. Mater., 2018, 30, 1705698 CrossRef PubMed.
- L. F. Xiong, B. Wang, H. R. Cai, H. J. Hao, J. Li, T. Yang and S. C. Yang, Understanding the doping effect on hydrogen evolution activity of transition-metal phosphides: Modeled with Ni2P, Appl. Catal., B, 2021, 295, 120283 CrossRef CAS.
- L. Zhao, M. Wen, Y. Tian, Q. Wu and Y. Fu, A novel structure of quasi-monolayered NiCo-bimetal-phosphide for superior electrochemical performance, J. Energy Chem., 2022, 74, 203–211 CrossRef CAS.
- Z. F. Wang, Y. K. Tian, M. Wen, Q. S. Wu, Q. J. Zhu and Y. Q. Fu, Integrating CoNiSe2 Nanorod-arrays onto N-doped Sea-sponge-C spheres for highly efficient electrocatalysis of hydrogen evolution reaction, Chem. Eng. J., 2022, 446, 137335 CrossRef CAS.
- A. Baksi, S. H. Nandam, D. Wang, R. Kruk, M. R. Chellali, J. Ivanisenko, I. Gallino, H. Hahn and S. Bag, Ni60Nb40 Nanoglass for Tunable Magnetism and Methanol Oxidation, ACS Appl. Nano Mater., 2020, 37, 7252–7259 CrossRef.
- A. A. Dubale, Y. Y. Zheng, H. L. Wang, R. Hübner, Y. Li, J. Yang, J. W. Zhang, N. K. Sethi, L. He, Z. K. Zheng and W. Liu, High-performance Bismuth-doped Nickel Aerogel Electrocatalyst for Methanol Oxidation Reaction, Angew. Chem., Int. Ed., 2020, 59, 13891–13899 CrossRef CAS PubMed.
- J. Cheng, X. J. Liu, J. F. Yang, J. Z. Liu, L. B. Zhang and L. X. Zhang, Three-dimensional Ni–MoN nanorod array as active and non-precious metal electrocatalyst for methanol oxidation reaction, J. Electroanal. Chem., 2022, 906, 116001 CrossRef CAS.
- Z. Y. Wang, L. Xu, F. Z. Huang, L. B. Qu, J. T. Li, K. A. Owusu, Z. Liu, Z. F. Lin, B. H. Xiang, X. Liu, K. N. Zhao, X. B. Liao, W. Yang, Y. B. Cheng and L. Q. Mai, Copper–Nickel Nitride Nanosheets as Efficient Bifunctional Catalysts for Hydrazine-Assisted Electrolytic Hydrogen Production, Adv. Energy Mater., 2019, 9, 1900390 CrossRef.
- J. Yin, Q. H. Fan, Y. X. Li, F. Y. Cheng, P. P. Zhou, P. Xi and S. H. Sun, Ni–C–N Nanosheets as Catalyst for Hydrogen Evolution Reaction, J. Am. Chem. Soc., 2016, 138, 14546–14549 CrossRef CAS PubMed.
- X. Sun, H. T. Deng, W. G. Zhu, Z. Yu, C. Z. Wu and Y. Xie, Interface Engineering in Two-Dimensional Heterostructures: Towards an Advanced Catalyst for Ullmann Couplings, Angew. Chem., Int. Ed., 2016, 55, 1704–1709 CrossRef CAS PubMed.
- Q. F. Gong, L. Cheng, C. H. Liu, M. Zhang, Q. L. Feng, H. L. Ye, M. Zeng, L. M. Xie, Z. Liu and Y. G. Li, Ultrathin MoS2(1−x)Se2x Alloy Nanoflakes For Electrocatalytic Hydrogen Evolution Reaction, ACS Catal., 2015, 5, 2213–2219 CrossRef CAS.
- Y. K. Tian, A. J. Huang, Z. G. Wang, M. K. Wang, Q. S. Wu, Y. Shen, Q. J. Zhu, Y. Q. Fu and M. Wen, Two-dimensional hetero-nanostructured electrocatalyst of Ni/NiFe-layered double oxide for highly efficient hydrogen evolution reaction in alkaline medium, Chem. Eng. J., 2021, 426, 131827 CrossRef CAS.
- Y. R. Zheng, P. Wu, M. R. Gao, X. L. Zhang, F. Y. Gao, H. X. Ju, R. Wu, Q. Gao, R. You, W. X. Huang, S. J. Liu, S. W. Hu, J. F. Zhu, Z. Y. Li and S. H. Yu, Doping-induced structural phase transition in cobalt diselenide enables enhanced hydrogen evolution catalysis, Nat. Commun., 2018, 9, 2533 CrossRef PubMed.
- N. L. Yang, H. F. Cheng, X. Z. Liu, Q. B. Yun, Y. Chen, B. Li, B. Chen, Z. C. Zhang, X. P. Chen, Q. P. Lu, J. T. Huang, Y. Zong, Y. H. Yang, L. Gu and H. Zhang, Amorphous/Crystalline Hetero-Phase Pd Nanosheets: One-Pot Synthesis and Highly Selective Hydrogenation Reaction, Adv. Mater., 2018, 30, 1803234 CrossRef PubMed.
- Z. H. Dong, F. Lin, Y. H. Yao and L. F. Jiao, Crystalline Ni(OH)2/Amorphous NiMoOx Mixed-Catalyst with Pt-Like Performance for Hydrogen Production, Adv. Energy Mater., 2019, 9, 1902703 CrossRef CAS.
- J. Hu, S. W. Li, Y. Z. Li, J. Wang, Y. C. Du, Z. H. Li, X. J. Han, J. M. Sun and P. Xu, A crystalline–amorphous Ni–Ni(OH)2 core–shell catalyst for the alkaline hydrogen evolution reaction, J. Mater. Chem. A, 2020, 8, 23323–23329 RSC.
- L. Zhao, W. Zhou, M. Wen, Q. S. Wu, W. Y. Li, Y. Q. Fu, Q. J. Zhu, S. Chen and J. Q. Ran, Trifunctional Cu-Mesh/Cu2O@FeO Nanoarrays for Highly Efficient Degradation of Antibiotic, Inactivation of Antibiotic-Resistant Bacteria, and Damage of Antibiotics Resistance Genes, Energy Environ. Mater., 2023, 6, e12299 CAS.
- H. W. Zhao, F. S. Li, S. X. Wang and L. Guo, Wet Chemical Synthesis of Amorphous Nanomaterials with Well-Defined Morphologies, Acc. Mater. Res., 2021, 2, 804–815 CrossRef CAS.
- F. Z. Zhao, H. C. Liu, H. Y. Zhu, X. Y. Jiang, L. Q. Zhu, W. P. Li and H. N. Chen, Amorphous/amorphous Ni–P/Ni(OH)2 heterostructure nanotubes for an efficient alkaline hydrogen evolution reaction, J. Mater. Chem. A, 2021, 9, 10169–10179 RSC.
- J. H. Li, H. Chen, Y. P. Liu, R. Q. Gao and X. X. Zou, In situ structural evolution of a nickel boride catalyst: synergistic geometric and electronic optimization for the oxygen evolution reaction, J. Mater. Chem. A, 2019, 7, 5288–5294 RSC.
- F. H. Wu, Z. L. Zhang, F. S. Zhang, D. H. Duan, Y. Li, G. Q. Wei, S. B. Liu, Q. B. Yuan, E. Z. Wang and X. G. Hao, Exploring the role of cobalt in promoting the electroactivity of amorphous Ni–B nanoparticles toward methanol oxidation, Electrochim. Acta, 2018, 287, 115–123 CrossRef CAS.
- M. S. Amer, P. Arunachalam, A. M. Alsalman, A. M. Al-Mayouf, Z. A. Almutairi, S. A. Aladeemy and M. Hezam, Facile synthesis of amorphous nickel iron borate grown on carbon paper as stable electrode materials for promoted electrocatalytic urea oxidation, Catal. Today, 2022, 397, 197–205 CrossRef.
- T. He, J. M. V. Nsanzimana, R. J. Qi, J. Y. Zhang, M. Miao, Y. Yan, K. Qi, H. F. Liu and B. Y. Xia, Synthesis of amorphous boride nanosheets by the chemical reduction of Prussian blue analogs for efficient water electrolysis, J. Mater. Chem. A, 2018, 6, 23289–23294 RSC.
- M. Q. Yang, J. D. Dan, S. J. Pennycook, X. Lu, H. Zhu, Q. H. Xu, H. J. Fan and G. W. Ho, Ultrathin nickel boron oxide nanosheets assembled vertically on graphene: a new hybrid 2D material for enhanced photo/electro-catalysis, Mater. Horiz., 2017, 45, 885–894 RSC.
- J. M. V. Nsanzimana, V. Reddu, Y. C. Peng, Z. F. Huang, C. Wang and X. Wang, Ultrathin Amorphous Iron–Nickel Boride Nanosheets for Highly Efficient Electrocatalytic Oxygen Production, Chem. – Eur. J., 2018, 24, 18502–18511 CrossRef CAS PubMed.
- J. Masa, I. Sinev, H. Mistry, E. Ventosa, M. De La Mata, J. Arbiol, M. Muhler, B. Roldan Cuenya and W. Schuhmann, Ultrathin High Surface Area Nickel Boride (NixB) Nanosheets as Highly Efficient Electrocatalyst for Oxygen Evolution, Adv. Energy Mater., 2017, 7, 1700381 CrossRef.
- W. J. Jiang, S. Niu, T. Tang, Q. H. Zhang, X. Z. Liu, Y. Zhang, Y. Y. Chen, J. H. Li, L. Gu, L. J. Wan and J. S. Hu, Reaction Pathway Studies, and Structural Characterization of Crystalline Ni3B Nanoparticles, Angew. Chem., Int. Ed., 2017, 56, 6572–6577 CrossRef CAS PubMed.
- J. H. Ge, Y. C. Lai, M. H. Guan, Y. H. Xiao, J. Kuang and C. Z. Yang, Nickel borate with a 3D hierarchical structure as a robust and efficient electrocatalyst for urea oxidation, Environ. Sci.: Nano, 2021, 8, 1326–1335 RSC.
- F. L. Yang, P. Y. Han, N. Yao, G. Z. Cheng, S. L. Chen and W. Luo, Inter-regulated d-band centers of the Ni3B/Ni heterostructure for boosting hydrogen electrooxidation in alkaline media, Chem. Sci., 2020, 11, 12118–12123 RSC.
- C. Wu, Y. Bai, D. X. Liu, F. Wu, M. L. Pang and B. L. Yi, Ni–Co–B catalyst-promoted hydrogen generation by hydrolyzing NaBH4 solution for in situ hydrogen supply of portable fuel cells, Catal. Today, 2011, 170, 33–39 CrossRef CAS.
- R. Chen, H. Y. Wang, J. W. Miao, H. B. Yang and B. Liu, A flexible high-performance oxygen evolution electrode with three-dimensional NiCo2O4 core–shell nanowires, Nano Energy, 2015, 11, 333–340 CrossRef CAS.
- H. L. Wang, H. S. Casalongue, Y. Y. Liang and H. J. Dai, Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed.
- Y. Z. Chen, T. F. Zhou, L. Li, W. K. Pang, X. M. He, Y. N. Liu and Z. P. Guo, Interfacial Engineering of Nickel Boride/Metaborate and Its Effect on High Energy Density Asymmetric Supercapacitors, ACS Nano, 2019, 13, 9376–9385 CrossRef CAS PubMed.
- X. Cui, P. Xiao, J. Wang, M. Zhou, W. L. Guo, Y. Yang, Y. J. He, Z. W. Wang, Y. K. Yang, Y. H. Zhang and Z. Q. Lin, Highly Branched Metal Alloy Networks with Superior Activities for the Methanol Oxidation Reaction, Angew. Chem., Int. Ed., 2017, 56, 4488–4493 CrossRef CAS PubMed.
- A. A. Dubale, Y. Y. Zheng, H. L. Wang, R. Hübner, Y. Li, J. Yang, J. W. Zhang, N. K. Sethi, L. Q. He, Z. K. Zheng and W. Liu, High-performance Bismuth-doped Nickel Aerogel Electrocatalyst for Methanol Oxidation Reaction, Angew. Chem., Int. Ed., 2020, 59, 13891–13899 CrossRef CAS PubMed.
- J. F. Xie, J. P. Xin, R. X. Wang, X. D. Zhang, F. C. Lei, H. C. Qu, P. Hao, G. W. Cui, B. Tang and Y. Xie, Sub-3 nm pores in two-dimensional nanomesh promoting the generation of electroactive phase for robust water oxidation, Nano Energy, 2018, 53, 74–82 CrossRef CAS.
- W. L. Yang, X. P. Yang, C. M. Hou, B. J. Li, H. T. Gao, J. H. Lin and X. L. Luo, Rapid room-temperature fabrication of ultrathin Ni(OH)2 nanoflakes with abundant edge sites for efficient urea oxidation, Appl. Catal., B, 2019, 259, 11820–11825 Search PubMed.
- R. Ojani, J. B. Raoof, S. Fathi and S. Alami-Valikchali, Electrochemical behavior of Ni(II) incorporated in zeolite Y-modified carbon electrode: application for electrocatalytic oxidation of methanol in alkaline solution, J. Solid State Electrochem., 2011, 15, 1935–1941 CrossRef CAS.
- B. Kaur, R. Srivastava and B. Satpati, Highly Efficient CeO2 Decorated Nano-ZSM-5 Catalyst for Electrochemical Oxidation of Methanol, ACS Catal., 2016, 6, 2654–2663 CrossRef CAS.
- Z. L. Schaefer, X. L. Ke, P. Schiffer and R. E. Schaak, Reaction Pathway Studies, and Structural Characterization of Crystalline Ni3B Nanoparticles, J. Phys. Chem. C, 2008, 112, 19846–19851 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ma01037b |
| This journal is © The Royal Society of Chemistry 2023 |