LM-19 lawsonite: a potential reference material for in situ oxygen isotope determination in lawsonite by ion microprobe†
Aleksey E.
Melnik
 *a,
Qiu-Li
Li
*a,
Qiu-Li
Li
 ab,
Jiao
Li
a,
Guo-Qiang
Tang
a,
Lian-Jun
Feng
a,
Jin-Hua
Li
bc,
Qian
Mao
a,
Benita
Putlitz
d and
Xian-Hua
Li
ab,
Jiao
Li
a,
Guo-Qiang
Tang
a,
Lian-Jun
Feng
a,
Jin-Hua
Li
bc,
Qian
Mao
a,
Benita
Putlitz
d and
Xian-Hua
Li
 *ab
*ab
aState Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing 100029, China. E-mail: aleksei@mail.iggcas.ac.cn; lixh@gig.ac.cn
bCollege of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing 100049, China
cKey Laboratory of Earth and Planetary Physics, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing 100029, China
dInstitute of Earth Sciences, University of Lausanne, Lausanne CH-1015, Switzerland
First published on 1st December 2022
Abstract
Lawsonite is a water-rich mineral that has occurred in the geological record since the late Neoproterozoic era. This mineral forms at low-temperature and high- to ultrahigh-pressure conditions in cold subduction zones and has been predicted to be stable down to ca. 300 km. Therefore, lawsonite has been discussed as an essential carrier of water to mantle depths, and its oxygen isotopic composition has been considered a promising tool in characterizing subduction-related fluids down to the deeper upper mantle. In this study, we characterize LM-19 lawsonite in detail as a potential working reference material for oxygen isotopic microanalysis. Multiple LM-19 measurements by secondary ion mass spectrometry reveal that it is homogeneous in oxygen isotopes at the micro-scale. Precise oxygen isotopic analysis by isotope ratio mass spectrometry yields a mean δ18O of 8.98 ± 0.10‰ (1SD) that we recommend as the reference value for the LM-19 lawsonite. This reference material is most suitable for determination of oxygen isotopes in unknown lawsonite samples with low Cr (Cr2O3 <0.3 wt%) and low to moderate total Ti and Fe concentrations (i.e., TiO2 + Fe2O3* <1.36 wt%). The main limitation is that the chemical variability of LM-19 in Ti and Fe may cause a minor matrix effect resulting in the average δ18O bias of −0.30‰ for the regions enriched in total Ti and Fe contents. However, these enriched regions most likely compose only a minor portion of the LM-19 lawsonite (i.e., ca. 20%), meaning that the matrix effect should not significantly affect the accuracy of in situ oxygen isotopic analysis.
1 Introduction
Lawsonite, CaAl2Si2O7(OH)2·H2O, is a characteristic metamorphic mineral of cold subduction zones, which has occurred in the geological record since the late Neoproterozoic era.1,2 Lawsonite-bearing metamafic, metasedimentary, and metasomatic rocks form at low-temperature and, mainly, high- to ultrahigh-pressure (HP-UHP) conditions, corresponding to blueschist (HP) and eclogite (HP-UHP) facies.1,3 However, lawsonite growth at moderate pressures (i.e., low-grade conditions of blueschist facies) has also been identified.2,3Lawsonite has been assumed to be the most essential component of cold subduction zones in water transfer into the upper sub-lithospheric mantle (references in Whitney et al.3). That is owing to its amazingly high-water content (the total of H2O and OH is ∼11.5 wt% (ref. 2 and 3)) and the predicted stability within subducting slabs down to more than 300 km.3–6 Moreover, the significance of lawsonite in delivering some elements (e.g., Th, U, Sr, Pb, rare earth elements) to mantle depths has been widely recognized.3,7–11 Furthermore, applying stable isotopes, particularly oxygen, in lawsonite is thought to be potentially helpful in deciphering the sources of subduction-zone fluids.3,12 Such an investigation of lawsonite is also potentially crucial for assessing the oxygen isotopic compositions of slab-derived fluid influx into the upper mantle. Therefore, demand for developing methods to measure oxygen isotopes in lawsonite currently exists.3
Lawsonite crystals commonly contain mineral and/or fluid inclusions,3 multiple voids (see Section 2), and their growth history and zoning may be quite complex.3,11,13,14 Moreover, being frequently replaced by other mineral phases at subduction and especially exhumation stages, lawsonite is sparsely preserved in the geological record compared to other HP minerals,3 and it also occurs as small (dozens to hundreds of μm in size) inclusions only in some rock samples (e.g., ref. 3, 10, 15 and 16). Due to all these factors, bulk methods are largely unsuitable for routine oxygen isotopic analysis of natural lawsonite samples, and therefore in situ methods are essentially required. High spatial resolution (10–20 μm) and internal precision (2SE, standard error, between 0.1 and 0.3‰) of single analyses17,18 make secondary ion mass spectrometry (SIMS) an effective technique for in situ analysis of oxygen isotopes. However, the main limitation of this analysis is the matrix effect, meaning that actual oxygen isotopes bias from the measured values upon sample chemistry.19 Hence, accurate SIMS oxygen isotopic analysis requires the standardization employing well-characterized matrix-matched reference materials. However, although in situ studies of oxygen isotopes in lawsonite have recently been under development,12,20 to our knowledge, no corresponding lawsonite standards have been made available to the scientific community so far. Therefore, we report here compositional and oxygen isotopic data for lawsonite sample LM-19. Our results indicate that the LM-19 could be a suitable reference material for in situ oxygen isotope analysis in most natural lawsonite samples.
2 Sample information and preparation
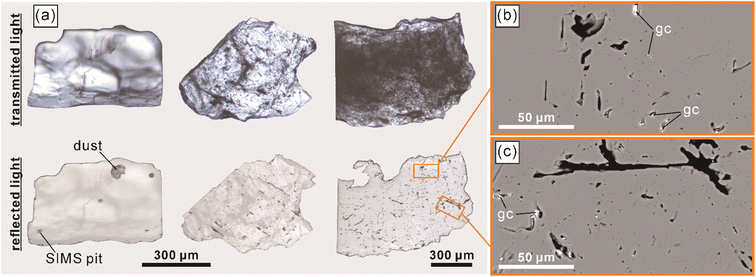
The LM-19 lawsonite represents multiple crystals up to ca. 2 cm in the rock matrix dominated by white mica (Fig. 1). The sample (3.8 × 4.4 cm) was purchased from a mineral trader in 2019 as a lawsonite-rich rock sample from Marin County, California, USA. Within Marin County, the sample was probably collected at the type locality of Reed Station, Tiburon Peninsula.21 The LM-19 lawsonite is white to pale blue and is mainly translucent at macro-scale, with only rare subtransparent regions. The major part of the sample was crushed, and lawsonite with a total weight of 5.0 g was isolated. The separated lawsonite includes 4.7 g of small (0.1–1 mm) fragments and six chips (Fig. S1†) of 2–5 mm in size, weighing 0.3 g. In terms of transparency, the small fragments fall into two groups: (1) translucent to subtransparent and (2) completely transparent. The first group predominates. As it is clearly visible in transmitted and reflected light microphotographs (Fig. 2a) and back-scattered electron (BSE) images (Fig. 2b and c), the low transparency, characteristic of the majority of fragments of lawsonite LM-19, is most likely related to the presence of multiple voids and cracks. As opposed, either no or a minor number of cracks and voids is present within the transparent fragments. White to pale blue are characteristic colors of the first group, while the second group fragments are either bluish or colorless. The remaining part of the sample (Fig. S1†) weighs ca. 5.4 g and contains at least 1 to 2 g of pure lawsonite. Therefore, the total weight of the LM-19 lawsonite is estimated at 6–7 g at a minimum, which makes this material sufficient for sharing within the scientific community.During this study, we tested more than a hundred 0.1–1.0 mm lawsonite fragments of variable color and transparency (see Section 4.2.1 for details) for oxygen isotope homogeneity. These fragments were embedded into epoxy mounts with silicate glass NIST Standard Reference Material (SRM) 610 (hereafter, NIST610). Each of those lawsonite fragments that had been analyzed in situ for oxygen isotopes was verified as lawsonite by Raman spectroscopy (Fig. 3) and semi-quantitative energy dispersive X-ray spectroscopy (the analytical details are provided in Section 3.1). Mineral inclusions and veins of secondary mineralization were identified within less than 10% of the studied lawsonite fragments. The minerals include amphibole (actinolite and minor glaucophane), clinopyroxene (omphacite), pumpellyite, titanite, and minor zircon and K-feldspar (Fig. S2†). The inclusions and mineral veins are commonly up to several dozen μm in diameter and thickness, respectively. Owing to the rusty color visible within the whole sample (Fig. 1) and some lawsonite fragments, the presence of iron hydroxides is also likely.
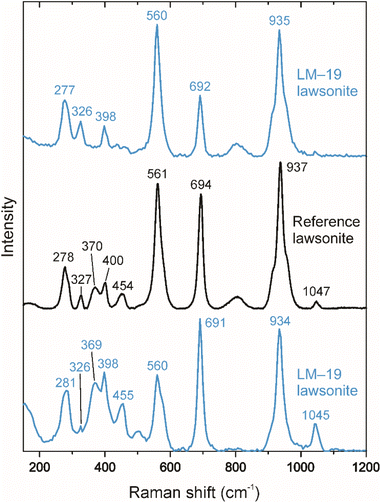 | ||
| Fig. 3 Representative Raman spectra (the top and bottom spectra) of LM-19 lawsonite. The identified peaks are typical of lawsonite.22 The reference spectrum for lawsonite (the middle spectrum) from the RRUFF database (https://rruff.info; ID = R050042) is shown for comparison. | ||
3 Analytical methods
All analytical works for this study, but a part of bulk oxygen isotope analyses at the University of Lausanne (UNIL, Lausanne, Switzerland), were conducted at the Institute of Geology and Geophysics, Chinese Academy of Sciences (IGGCAS, Beijing, China).3.1 Mineral characterization and chemistry
Mineralogical, morphological, and chemical features of LM-19 fragments were characterized by Raman spectroscopy, scanning electron microscopy (SEM), conventional optical microscopy, and electron probe microanalysis (EPMA).Raman spectroscopic measurements were carried out using a confocal Raman microscope (WITec Alpha 300R) and a 532 nm laser. A Zeiss 100× objective (numerical aperture = 0.9) was employed to focus the laser, and the spatial resolution was <1 μm. The settings of measurements were either 10 or 20 acquisitions of 4 s each, the laser power of ∼10 mW, and a 300 grooves per mm grating. A silicon reference material was used for calibration.
BSE imaging was performed by SEM on a Hitachi tabletop microscope TM4000 and a Thermo Scientific Apreo S field-emission scanning electron microscope. The former instrument is equipped with an Oxford AZtecOne energy dispersive X-ray spectrometer and was used to identify mineral inclusions and secondary mineralization hosted by the LM-19 lawsonite and to verify all the studied lawsonite fragments.
EPMA experiments were conducted for representative LM-19 lawsonite fragments by a CAMECA SXFive, employing wavelength-dispersive spectrometers (WDS) with an acceleration voltage of 20 keV, a beam current of ∼30 nA, and a defocused beam of 10 μm. The following standards were employed: rhodonite (Si, Ca, and Mn), metal Al (Al), rutile (Ti), albite (Na), K-feldspar (K), periclase (Mg), metal V (V), metal Fe (Fe), metal Cr (Cr), metal Sr (Sr). The peak counting times were 20 s for Si, Al, Ca, Fe, and Mn; 30 s for Ti, Cr, Mg, V, K, Na, and Sr. The PAP (ZAF) method was used for matrix correction. The average detection limits (for elements) ranged from 0.01 to 0.04 wt% (3σ). Qualitative X-ray mapping was conducted at the following conditions: 20 kV accelerate voltage, 100 nA beam current, a focused beam, and a dwell time of 50 ms per pixel. The step size was 1 μm. The X-ray maps and spot analyses were obtained for some of the fragments previously analyzed for oxygen isotopes by ion microprobe.
3.2 Secondary ion mass spectrometry oxygen isotope analysis
In situ oxygen isotope measurements were carried out on a CAMECA IMS-1280 ion microprobe. Analytical procedure and conditions were mainly similar to those described by Li et al.17 and Tang et al.18,23 A 20 keV primary Cs+ beam (1.3–2.6 nA) was focused to a spot of ∼10–15 μm in size. Each measurement consisted of 16 cycles with a total acquisition time of ∼3 minutes. The measured 18O/16O ratios were normalized to the Vienna Standard Mean Ocean Water (VSMOW, 18O/16O = 0.0020052 (ref. 24)). The oxygen isotopic data are given in the conventional delta notation (δ18O in ‰). The measured δ18O values from every analytical session were corrected to the LM-19 lawsonite value defined by isotope ratio mass spectrometry (IRMS). Analyses of reference glass NIST610 (ref. 25) were interspersed with the LM-19 lawsonite to monitor ion microprobe conditions in analytical sessions. Internal uncertainties on single analyses are between 0.1 and 0.3‰ (2SE).3.3 Isotope ratio mass spectrometry oxygen isotope analysis
Bulk IRMS oxygen isotope analysis of the LM-19 lawsonite was performed employing the CO2 laser fluorination technique at the stable isotope laboratories at IGGCAS and UNIL. Separate fractions of (1) translucent to subtransparent and (2) completely transparent groups of small (0.1–1 mm) fragments were handpicked from the 4.7 g portion of the LM-19 lawsonite under a binocular microscope for this analysis, monitoring the absence of inclusions and secondary mineralization. The measured 18O/16O ratios were normalized to the standard value of Vienna Standard Mean Ocean Water and provided in the conventional delta notation (δ18O in ‰).Laser fluorination oxygen isotope analysis at IGGCAS was conducted using a New Wave Research MIR10-30 laser coupled to a vacuum extraction system. We mainly followed the protocol described by Feng et al.26 Samples (each of ca. 1.5 mg) reacted with purified BrF5 in the sample chamber to liberate oxygen. Several cryogenic traps at liquid nitrogen temperature purified the generated gases. A MAT252 mass spectrometer analyzed the purified O2. The Penglai zircon17 (δ18O = 5.17‰) and the 04BXL07 garnet27 (δ18O = 3.70‰) were employed as reference materials. A long-term reproducibility (1SD) of these reference zircon and garnet corresponds to ±0.08‰ and ±0.11‰, respectively.
Laser fluorination oxygen isotope analysis at UNIL was carried out over three sessions, following the method primary reported by Sharp.28 The details on the analytical conditions and procedure at UNIL can be found in Lacroix and Vennemann.29 The NBS-28 quartz reference material (δ18O = 9.64‰ (ref. 30)) was analyzed together with lawsonite aliquots of 1.0–2.0 mg. Primary oxygen isotope results for the LM-19 lawsonite from the analytical sessions were corrected to the corresponding mean values of the reference quartz. Each session included 4–5 analyses of the NBS-28 quartz, and the reproducibility (1SD) per session varied within ±0.02–0.11‰.
4 Results and discussion
4.1 Major and minor element compositions
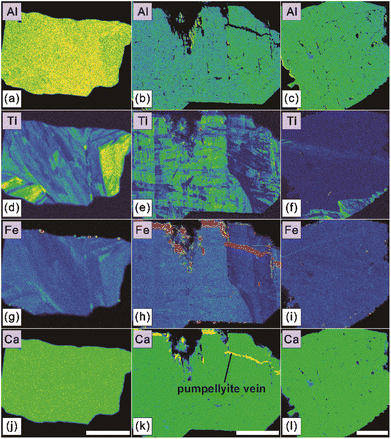
In this study, we performed 105 individual chemical composition measurements on seventy-seven LM-19 lawsonite fragments (one to three analyses per fragment). These fragments were previously investigated for oxygen isotopes (Section 4.2.1). All obtained EPMA data are provided in ESI Table S1.† Besides CaO (17.01–17.68 wt%), Al2O3 (29.73–31.25 wt%), and SiO2 (36.91–38.76 wt%) that collectively constitute the major components of lawsonite, the measured LM-19 fragments contain much lower concentrations of V2O3 (0.03–0.10 wt%), Cr2O3 (<0.02–0.22 wt%), TiO2 (<0.01–0.77 wt%), and Fe2O3* (0.42–1.45 wt%). No detectable concentrations of K2O and SrO were determined in the lawsonite. As for the vast majority of obtained analyses, Na2O, MgO, and MnO contents were also below the detection limits. Only four individual analyses yield barely detectable concentrations of Na2O, MgO, or MnO that vary in a range of 0.02–0.03 wt%.High-resolution X-ray maps (Fig. 4 and S3†) reveal complex zoning in Fe and Ti for most mapped LM-19 fragments, reflecting the compositional variations identified for these elements. Because Fe (as Fe3+), Ti, and Cr substitute for Al in lawsonite structure,3,13 it is also visible from the maps (Fig. 4 and S3†) that Al zoning correlates with zoning in Fe and Ti.
Lawsonite typically has significant amounts of Fe, Ti, and Cr.3,10,13 Notably, this mineral from different lithologies contains <1 wt% of TiO2, and, in most cases, the concentrations of Cr2O3 and Fe2O3* are <0.5 wt% and <1.5–2 wt%, respectively,3,9–11,13,15,16,31,32 which all are similar to the LM-19 compositions. This means that the LM-19 lawsonite is compositionally similar (i.e., matrix-matched) to most natural lawsonite samples, excluding those occasional samples that are rich in either Fe (Fe2O3* of 2–8 wt% (ref. 11, 13, 33 and 34)) or Cr (Cr2O3 of 2–11 wt% (ref. 3, 11 and 13)).
4.2 Oxygen isotope analysis
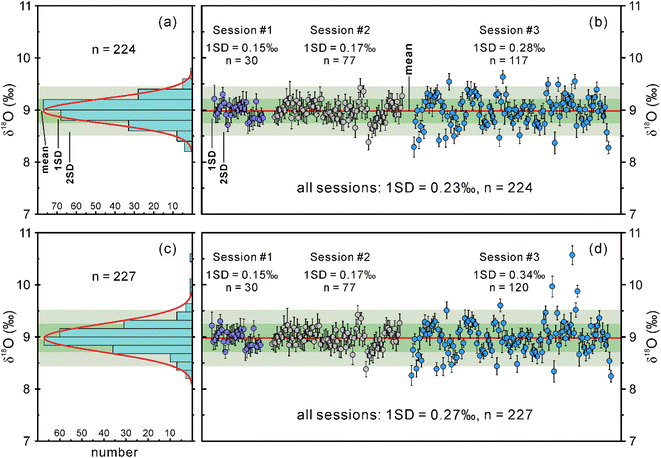
The SIMS and IRMS oxygen isotope data obtained for the LM-19 lawsonite during this study, and the corresponding details are summarized in ESI Tables S2 and S3,† respectively.Excluding three mixed analyses within two lawsonite fragments that probed both the host mineral and its inclusions (Table S2†), we collected oxygen isotope data for ninety-nine LM-19 fragments in session 3. These fragments are 0.1–1 mm in size and were selected from the main crushed portion of the LM-19 lawsonite to represent variable characteristics of color and transparency. One to three SIMS oxygen isotope measurements were performed within each of the ninety-nine fragments. In session 3, the measured δ18O values for both LM-19 and NIST610 tend to slightly increase with time (Fig. S4a†), which indicates a minor instrumental drift. Therefore, a correction to this drift was applied to the measured δ18O values from this session (Fig. S4b†). The drift-corrected δ18O values yield a 1SD of 0.34‰ (n = 120). Such repeatability of δ18O values is noticeably lower than those in sessions 1 and 2. However, the δ18O repeatability obtained for the NIST610 in this session is even lower (1SD = 0.43‰, n = 36), meaning that either the analyzed reference material was heterogeneous or the machine might have deviated from the ideal conditions during the analysis. If the latter is the case, we can filter the obtained dataset of δ18O values by removing some anomalous data. For this reason, we excluded three measurements with abnormally high δ18O values (yellow in Fig. S4†) of the NIST610 from session 3, which led to considerable improvement of oxygen isotope reproducibility for this reference material (1SD = 0.19‰, n = 33). The three highest δ18O values (yellow in Fig. S4†) were accordingly filtered out from the dataset obtained for LM-19 lawsonite in the same session, resulting in much better δ18O reproducibility with 1SD of 0.28‰ (n = 117).
Overall, the filtered dataset of 224 δ18O measurements for the lawsonite from three sessions forms a Gaussian distribution (Fig. 5a; Z-scores of skewness and kurtosis are within the range between −3.29 and 3.29, see Table S2†) with a 1SD of 0.23‰ (Fig. 5b), meaning that the LM-19 is fairly homogeneous in oxygen isotope composition. Even the unfiltered lawsonite dataset of 227 δ18O measurements (Fig. 5c and d) yields quite a homogeneous composition in oxygen isotopes with a 1SD of 0.27‰ (Fig. 5d).
4.3 Assessment of possible matrix effects
Thirty-two EPMA measurements of the LM-19 lawsonite were placed near (30 μm or less) the pits of SIMS oxygen isotope analyses from session 3 to assess possible compositional matrix effects. Correlation analysis (Table S4†) revealed that the drift-corrected δ18O has moderate negative correlations with concentrations of TiO2 (Pearson's r [hereafter r] = −0.54), Fe2O3* (r = −0.49), and TiO2 + Fe2O3* (r = −0.60; Fig. S5a†), meaning that the variation in Ti and Fe contents identified within the LM-19 lawsonite likely causes a detectable matrix effect. We also determined a moderate positive correlation between Al2O3 content and δ18O (r = 0.60) and a strong negative correlation between Al2O3 and TiO2 + Fe2O3* (r = −0.88); both are due to Ti and Fe collectively substitute for Al in lawsonite structure (see Section 4.1).Further correlation analysis identified no statistically significant relationship between the oxygen isotopic and total Ti + Fe compositions for TiO2 + Fe2O3* concentrations of <1.36 wt% (Table S4; Fig. S5b†), indicating a negligible or no matrix effect caused by these low total Ti + Fe contents in the lawsonite. The mean of drift-corrected δ18O values of lawsonite regions with regular Ti + Fe contents (i.e., TiO2 + Fe2O3* = 0.50–1.28 wt%), which cause no detectable matrix effect, is 14.81‰ (n = 20; 1SD = 0.22‰). The corresponding mean δ18O value for regions enriched in Ti + Fe (i.e., TiO2 + Fe2O3* = 1.36–2.22 wt%) is 14.51‰ (n = 12; 1SD = 0.23‰). Hence, the matrix effect likely leads to an average δ18O bias of −0.30‰ at the lawsonite regions with higher (i.e., ≥1.36 wt%) concentrations of TiO2 + Fe2O3*. However, such a bias is similar to the upper limit of typical standard errors (2SE) of single SIMS analyses (i.e., 0.1–0.3‰). In addition, the proportion of EPMA measurements showing enriched Ti + Fe compositions (i.e., TiO2 + Fe2O3* ≥1.36 wt%) is ca. 20% (Table S1†), meaning that the matrix effect will likely insignificantly contribute to the long-term δ18O reproducibility of the LM-19 lawsonite.
The matrix effect might have been another reason contributing to lower δ18O repeatability of the LM-19 in session 3 compared with those in sessions 1 and 2 (Section 4.2.1); the differences in δ18O repeatability are visible even for the filtered data (Fig. 5b). This suggestion is supported by a higher variability of total Ti + Fe contents (Table S1†) in the lawsonite fragments analyzed by SIMS in session 3 (TiO2 + Fe2O3* = 0.46–2.22 wt%; A5692) as opposed to those analyzed in first two sessions (TiO2 + Fe2O3* = 0.64–1.69 wt%; A5358).
5 Conclusions
Results of this study indicate that the LM-19 is sufficiently homogeneous in its oxygen isotope composition to become a working reference material for the in situ analysis of unknown lawsonite samples. We recommend the δ18O value of 8.98 ± 0.10‰ (1SD) as the reference value for the LM-19 lawsonite. This standard will be especially suitable for analyzing lawsonite samples with low Cr (Cr2O3 <0.3 wt%) and low to moderate total Ti and Fe concentrations (i.e., TiO2 + Fe2O3* <1.36 wt%).Owing to the presence of voids and mineral inclusions in the LM-19 lawsonite, we suggest guiding the microbeam analysis of this reference material by employing BSE-SEM and reflected light images. Another limitation is that regions of LM-19 that are enriched in Ti and Fe (i.e., TiO2 + Fe2O3* = 1.36–2.22 wt%) may cause a minor matrix effect resulting in the average δ18O bias of −0.30‰. However, considering the relatively small value of this bias and that the enriched regions (based on the available EPMA data) compose only ca. 20% of the standard, the matrix effect should not significantly affect the accuracy of in situ oxygen isotopic analysis. We also recommend to use several fragments in every analysis to minimize influence of the matrix effect and to further monitor homogeneity of the LM-19 standard material.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Hong-Xia Ma for help with the preparation of epoxy mounts. The authors are grateful to Yu Liu for helpful comments on the SIMS data. This paper benefited significantly from the insightful and constructive reviews by Axel Schmitt and an anonymous referee. This research was supported by the National Natural Science Foundation of China (grants 41890831, 42150410391, and 42003012). A.E.M. acknowledges the awarding of a postdoctoral fellowship (grant 2021PD03) under the IGG International Fellowship Initiative (IIFI) of the Institute of Geology and Geophysics, Chinese Academy of Sciences (IGGCAS).References
- T. Tsujimori, V. B. Sisson, J. G. Liou, G. E. Harlow and S. S. Sorensen, Very-low-temperature record of the subduction process: A review of worldwide lawsonite eclogites, Lithos, 2006, 92(3–4), 609–624 CrossRef CAS.
- T. Tsujimori and W. G. Ernst, Lawsonite blueschists and lawsonite eclogites as proxies for palaeo-subduction zone processes: a review, J. Metamorph. Geol., 2014, 32, 437–454 CrossRef CAS.
- D. L. Whitney, K. F. Fornash, P. Kang, E. D. Ghent, L. Martin, A. I. Okay and A. Vitale Brovarone, Lawsonite composition and zoning as tracers of subduction processes: A global review, Lithos, 2020, 370–371, 105636 CrossRef CAS.
- M. W. Schmidt and S. Poli, The stability of lawsonite and zoisite at high pressures: Experiments in CASH to 92 kbar and implications for the presence of hydrous phases in subducted lithosphere, Earth Planet. Sci. Lett., 1994, 124(1–4), 105–118 CrossRef CAS.
- K. Okamoto and S. Maruyama, The high-pressure synthesis of lawsonite in the MORB+H2O system, Am. Mineral., 1999, 84, 362–373 CrossRef CAS.
- E. O'Bannon III, C. M. Beavers, M. Kunz and Q. Williams, The high-pressure phase of lawsonite: A single crystal study of a key mantle hydrous phase, J. Geophys. Res.: Solid Earth, 2017, 122(8), 6294–6305 CrossRef.
- R. Tribuzio, B. Messiga, R. Vannucci and P. Bottazzi, Rare earth element redistribution during high-pressure–low-temperature metamorphism in ophiolitic Fe-gabbros (Liguria, northwestern Italy): Implications for light REE mobility in subduction zones, Geology, 1996, 24(8), 711–714 CrossRef CAS.
- T. Ueno, REE-bearing sector-zoned lawsonite in the Sanbagawa pelitic schists of the eastern Kii Peninsula, central Japan, Eur. J. Mineral., 1999, 11(6), 993–998 CrossRef CAS.
- C. Spandler, J. Hermann, R. Arculus and J. Mavrogenes, Redistribution of trace elements during prograde metamorphism from lawsonite blueschist to eclogite facies; implications for deep subduction-zone processes, Contrib. Mineral. Petrol., 2003, 146(2), 205–222 CrossRef CAS.
- L. A. J. Martin, J. Hermann, L. Gauthiez-Putallaz, D. L. Whitney, A. Vitale Brovarone, K. F. Fornash and N. J. Evans, Lawsonite geochemistry and stability - implication for trace element and water cycles in subduction zones, J. Metamorph. Geol., 2014, 32, 455–478 CrossRef CAS.
- A. Vitale Brovarone, O. Alard, O. Beyssac, L. Martin and M. Picatto, Lawsonite metasomatism and trace element recycling in subduction zones, J. Metamorph. Geol., 2014, 32, 489–514 CrossRef CAS.
- P. Kang, D. L. Whitney, L. Martin, A. Vitale Brovarone, E. D. Ghent and K. F. Fornash, Lawsonite oxygen isotope and trace element records of subduction fluids, in American Geophysical Union Fall Meeting Abstracts, 2019. p. #V43E-0143 Search PubMed.
- K. F. Fornash, D. L. Whitney and N. C. A. Seaton, Lawsonite composition and zoning as an archive of metamorphic processes in subduction zones, Geosphere, 2019, 15(1), 24–46 CrossRef.
- R. Tamblyn, M. Hand, L. Morrissey, T. Zack, G. Phillips and D. Och, Resubduction of lawsonite eclogite within a serpentinite-filled subduction channel, Contrib. Mineral. Petrol., 2020, 175(8), 74 CrossRef CAS.
- R. Altherr, G. Topuz, H. Marschall, T. Zack and T. Ludwig, Evolution of a tourmaline-bearing lawsonite eclogite from the Elekdaǧ area (Central Pontides, N Turkey): evidence for infiltration of slab-derived B-rich fluids during exhumation, Contrib. Mineral. Petrol., 2004, 148(4), 409–425 CrossRef CAS.
- T. Usui, E. Nakamura and H. Helmstaedt, Petrology and geochemistry of eclogite xenoliths from the Colorado Plateau: Implications for the evolution of subducted oceanic crust, J. Petrol., 2006, 47(5), 929–964 CrossRef CAS.
- X.-H. Li, W.-G. Long, Q.-L. Li, Y. Liu, Y.-F. Zheng, Y.-H. Yang, K. R. Chamberlain, D.-F. Wan, C.-H. Guo, X.-C. Wang and H. Tao, Penglai Zircon Megacrysts: A Potential New Working Reference Material for Microbeam Determination of Hf-O Isotopes and U-Pb Age, Geostand. Geoanal. Res., 2010, 34(2), 117–134 CrossRef CAS.
- G.-Q. Tang, X.-H. Li, Q.-L. Li, Y. Liu, X.-X. Ling and Q.-Z. Yin, Deciphering the physical mechanism of the topography effect for oxygen isotope measurements using a Cameca IMS-1280 SIMS, J. Anal. At. Spectrom., 2015, 30(4), 950–956 RSC.
- J. M. Eiler, C. Graham and J. W. Valley, SIMS analysis of oxygen isotopes: matrix effects in complex minerals and glasses, Chem. Geol., 1997, 138(3–4), 221–244 CrossRef CAS.
- P. Kang, D. L. Whitney, L. Martin and E. D. Ghent, Lawsonite oxygen isotope and trace element records of subduction fluids, in Goldschmidt Abstracts, 2018, p. 1225 Search PubMed.
- F. L. Ransome, On lawsonite, a new rock-forming mineral from the Tiburon Peninsula, Marin County, California, Dep. Geol. Sci. Bull., 1895, 1, 301–312 CAS.
- A. Le Cléac’h and P. Gillet, IR and Raman spectroscopic study of natural lawsonite, Eur. J. Mineral., 1990, 2(1), 43–54 CrossRef.
- G.-Q. Tang, Y. Liu, Q.-L. Li, L.-J. Feng, G.-J. Wei, W. Su, Y. Li, G.-H. Ren and X.-H. Li, New natural and fused quartz reference materials for oxygen isotope microanalysis, At. Spectrosc., 2020, 41(5), 188–193 CrossRef CAS.
- P. Baertschi, Absolute 18O content of standard mean ocean water, Earth Planet. Sci. Lett., 1976, 31(3), 341–344 CrossRef CAS.
- S. Kasemann, A. Meixner, A. Rocholl, T. Vennemann, M. Rosner, A. K. Schmitt and M. Wiedenbeck, Boron and oxygen isotope composition of certified reference materials NIST SRM 610/612 and reference materials JB-2 and JR-2, Geostand. Newsl., 2001, 25(2–3), 405–416 CrossRef CAS.
- L. Feng, H. Li and T. Li, Potential reference materials for hematite oxygen isotope analysis, Minerals, 2020, 10(11), 987 CrossRef CAS.
- B. Gong, Y.-F. Zheng and R.-X. Chen, TC/EA-MS online determination of hydrogen isotope composition and water concentration in eclogitic garnet, Phys. Chem. Miner., 2007, 34(10), 687–698 CrossRef CAS.
- Z. D. Sharp, A laser-based microanalytical method for the in situ determination of oxygen isotope ratios of silicates and oxides, Geochim. Cosmochim. Acta, 1990, 54(5), 1353–1357 CrossRef CAS.
- B. Lacroix and T. Vennemann, Empirical calibration of the oxygen isotope fractionation between quartz and Fe-Mg-chlorite, Geochim. Cosmochim. Acta, 2015, 149, 21–31 CrossRef CAS.
- T. B. Coplen, C. Kendall and J. Hopple, Comparison of stable isotope reference samples, Nature, 1983, 302(5905), 236–238 CrossRef CAS.
- M. Çetinkaplan, O. Candan, R. Oberhänsli and R. Bousquet, Pressure-temperature evolution of lawsonite eclogite in Sivrihisar; Tavşanlı Zone–Turkey, Lithos, 2008, 104(1–4), 12–32 CrossRef.
- T. Usui, K. Kobayashi, E. Nakamura and H. Helmstaedt, Trace element fractionation in deep subduction zones inferred from a lawsonite-eclogite xenolith from the Colorado Plateau, Chem. Geol., 2007, 239(3–4), 336–351 CrossRef CAS.
- R. L. Sedlock, Metamorphic petrology of a high-pressure, low-temperature subduction complex in west-central Baja California, Mexico, J. Metamorph. Geol., 1988, 6, 205–233 CrossRef CAS.
- H. Maekawa, M. Shozul, T. Ishll, P. Fryer and J. A. Pearce, Blueschist metamorphism in an active subduction zone, Nature, 1993, 364(6437), 520–523 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S5 and Tables S1–S4. See DOI: https://doi.org/10.1039/d2ja00301e |
| This journal is © The Royal Society of Chemistry 2023 |