Cu fractionation, isotopic analysis, and data processing via machine learning: new approaches for the diagnosis and follow up of Wilson's disease via ICP-MS†
M. Carmen
García-Poyo
 ab,
Sylvain
Bérail
ab,
Sylvain
Bérail
 a,
Anne Laure
Ronzani
a,
Anne Laure
Ronzani
 a,
Luis
Rello
c,
Elena
García-González
c,
Flávio V.
Nakadi
a,
Luis
Rello
c,
Elena
García-González
c,
Flávio V.
Nakadi
 b,
Maite
Aramendía
b,
Maite
Aramendía
 bd,
Javier
Resano
bd,
Javier
Resano
 e,
Martín
Resano
e,
Martín
Resano
 *b and
Christophe
Pécheyran
*b and
Christophe
Pécheyran
 *a
*a
aInstitut des Sciences Analytiques et de Physico-chimie pour l'Environnement et les Matériaux, UPPA/CNRS 5254, Université de Pau et des Pays de l'Adour, 2 Av Président Angot, Pau, 64000, France. E-mail: christophe.pecheyran@univ-pau.fr
bDepartment of Analytical Chemistry, Aragon Institute of Engineering Research (I3A), University of Zaragoza, Pedro Cerbuna 12, 50009 Zaragoza, Spain. E-mail: mresano@unizar.es
cDepartment of Clinical Biochemistry, IIS Aragón, “Miguel Servet” University Hospital, Paseo Isabel La Católica 1-3, 50009 Zaragoza, Spain
dCentro Universitario de la Defensa de Zaragoza, Carretera de Huesca s/n, 50090, Zaragoza, Spain
eDepartment of Computer Sciences and Systems Engineering (DIIS), Aragon Institute of Engineering Research (I3A), University of Zaragoza, C/Mariano Esquillor SN, 50018, Zaragoza, Spain
First published on 10th November 2022
Abstract
Information about Cu fractionation and Cu isotopic composition can be paramount when investigating Wilson's disease (WD). This information can provide a better understanding of the metabolism of Cu. Most importantly, it may provide an easy way to diagnose and to follow the evolution of WD patients. For such purposes, protocols for Cu determination and Cu isotopic analysis via inductively coupled plasma mass spectrometry were investigated in this work, both in bulk serum and in the exchangeable copper (CuEXC) fractions. The CuEXC protocol provided satisfactory recovery values. Also, no significant mass fractionation during the whole analytical procedure (CuEXC production and/or Cu isolation) was detected. Analyses were carried out in controls (healthy persons), newborns, patients with hepatic disorders, and WD patients. While the results for Cu isotopic analysis are relevant (e.g., δ65Cu values were lower for both WD patients under chelating treatment and patients with hepatic problems in comparison with those values obtained for WD patients under Zn treatments, controls, and newborns) to comprehend Cu metabolism and to follow up the disease, the parameter that can help to better discern between WD patients and the rest of the patients tested (non-WD) was found to be the REC (relative exchangeable Cu). In this study, all the WD patients showed a REC higher than 17%, while the rest showed lower values. However, since establishing a universal threshold is complicated, machine learning was investigated to produce a model that can differentiate between WD and non-WD samples with excellent results (100% accuracy, albeit for a limited sample set). Most importantly, unlike other ML approaches, our model can also provide an uncertainty metric to indicate the reliability of the prediction, overall opening new ways to diagnose WD.
1. Introduction
Wilson's disease (WD) is a recessive genetic disease that affects 1 out of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people. It is produced by a mutation in the ATP7B gene which encodes a transmembrane ATPase protein, which is the central regulator of hepatic copper. This protein plays a role as a Cu ATPase transporter, especially in the liver. It is involved in both the excretion of Cu from the hepatocytes into the bile and the incorporation of Cu into the ceruloplasmin.1–3 In WD patients, a defective ATP7B gene prevents the body from removing excess copper. As a result, Cu levels build up in various organs, mostly the liver and brain, but also the kidneys and eyes (Kayser–Fleischer rings). WD is an irreversible disease, which causes serious clinical injuries and even death, if it is misdiagnosed or the treatment is delayed.3,4 In general, the survival rate depends on the stage of the liver and/or neurological disease, and also on treatment follow-up. At present, there are two kinds of treatments for WD: one is based on the administration of chelators for increasing excretion of Cu that has already accumulated in the organs, such as D-penicillamine and trientine; the other is based on blocking the absorption of Cu by treatment with Zn salts.5,6 The choice of treatment depends on the clinical picture of the patient, but both of them are easy to apply.
000 people. It is produced by a mutation in the ATP7B gene which encodes a transmembrane ATPase protein, which is the central regulator of hepatic copper. This protein plays a role as a Cu ATPase transporter, especially in the liver. It is involved in both the excretion of Cu from the hepatocytes into the bile and the incorporation of Cu into the ceruloplasmin.1–3 In WD patients, a defective ATP7B gene prevents the body from removing excess copper. As a result, Cu levels build up in various organs, mostly the liver and brain, but also the kidneys and eyes (Kayser–Fleischer rings). WD is an irreversible disease, which causes serious clinical injuries and even death, if it is misdiagnosed or the treatment is delayed.3,4 In general, the survival rate depends on the stage of the liver and/or neurological disease, and also on treatment follow-up. At present, there are two kinds of treatments for WD: one is based on the administration of chelators for increasing excretion of Cu that has already accumulated in the organs, such as D-penicillamine and trientine; the other is based on blocking the absorption of Cu by treatment with Zn salts.5,6 The choice of treatment depends on the clinical picture of the patient, but both of them are easy to apply.
Therefore, the main challenge associated with WD is being able to properly diagnose this disease before the onset of symptoms, to avoid the consequences that they entail. Diagnosis can be based on such symptoms as detection of Kayser–Fleischer rings in the eyes, liver damage and/or neurological disorders, which is not ideal, or be based on family screening in the case of asymptomatic patients. Genetic tests could also be useful to diagnose this disease. However, they cannot be routinely undertaken because they are costly and tedious, as there are more than 500 genetic mutations that cause WD.7–9 For this reason, the diagnosis mostly relies on such clinical symptoms and/or on biological tests, such as blood or urine analyses: e.g., total blood/serum Cu, blood/serum ceruloplasmin levels or urine Cu excretion in 24 h, among others.10 Unfortunately, these kinds of tests may provide unreliable and/or inconclusive results.
For instance, if we focus on the serum Cu concentration, this value ranges between 0.7 and 1.5 mg L−1 for healthy people,11 while the typical values found in WD are lower; however, similar values have been found for some controls, particularly for newborns, due to the immaturity of their liver. Values in that range (0.932–0.999 mg L−1) have also been reported for some WD patients, due to the eventual release of Cu from the liver.12
In the case of 24 h urinary Cu excretion, the cutoff is set at an excreted Cu amount of higher than 100 μg in 24 h. However, asymptomatic WD patients sometimes show an excreted Cu amount lower than such a threshold (57–95 μg). These are only some examples of the issues preventing efficient WD diagnosis. As a consequence, it is often necessary to carry out several tests to confirm the disease and, even then, sometimes the diagnosis is not clear.3,13,14
Hence it is important to continue the research on new methods to diagnose WD at an early stage before any symptoms appear. In this regard, investigations focused on Cu isotopic analysis12,15,16 or on parameters related to the Cu bound to ceruloplasmin and/or free Cu13,17–19 have been carried out.
Isotopic analysis has demonstrated its potential for biomedical investigations,20–23 particularly when using multicollector inductively coupled plasma mass spectrometry (MC-ICP-MS).24 In the case of WD, preliminary studies showed that samples from WD patients present a lighter Cu isotopic composition than those obtained from controls, both in serum and in urine samples.15,16 These results have been further supported by recent work, where it was observed that such lighter isotopic compositions seem to be linked to Cu release from the liver, regardless of whether this release is due to the medical treatment with a chelating agent or due to liver damage (cirrhosis).12
On the other hand, besides these studies which focused on isotopic analysis, a few studies have proposed simple approaches for Cu fractionation, differentiating between the amount of Cu that is either strongly bound to some compounds or more freely available. This approach a priori seems interesting in this context, considering that WD affects the binding of Cu to ceruloplasmin. Concepts such as ultrafiltrable Cu (CuUF), which is the Cu bound to low molar mass molecules (amino acids) and exchangeable Cu (CuEXC), which corresponds to the Cu fraction that is not bound to ceruloplasmin and is easily complexed with high-Cu-affinity chelating agents such as ethylenediaminetetraacetic acid (EDTA), have been defined.25,26 The calculation of relative exchangeable Cu (REC) has been proposed as follows:
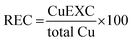 | (1) |
El Balkhi et al. described a method to obtain CuUF and CuEXC and established reference values (4.5–9.9 and 36–71 μg L−1, respectively) for healthy individuals.25 While CuUF was found to be hardly relevant for WD diagnosis, CuEXC and REC were proposed as new biological markers for WD diagnosis.18 In studies with animal models, Schmiltt et al.17 observed that CuEXC values were correlated with acute liver disease and with the dietary Cu intake. Guillaud et al.19 observed in humans that CuEXC levels were higher for WD patients (symptomatic when diagnosed) and for patients that did not follow the treatment correctly with respect to those WD patients that followed the treatments properly or were asymptomatic; and also, CuEXC values were high for patients with other liver disorders, depending on the stage of the disorder. Overall, the results reported indicate that CuEXC is not specific for WD, but it could also be useful in this context.
REC, on the other hand, seems to be a sensitive (which in this context means showing a high true positive rate) and specific (meaning showing a high true negative rate) biomarker for WD diagnostics, providing values close to 100% in terms of sensitivity and specificity. However, a review of the literature reveals that different cutoffs have been proposed by different authors.17–19,27,28 Hence, it is still necessary to continue this line of research in order to clarify this critical parameter.
It has to be also mentioned that fractionation approaches based on ultrafiltration are very simple ones, and that other articles using liquid chromatography coupled to inductively coupled plasma mass spectrometry (ICP-MS) have revealed the more complex nature of the different Cu species present in serum29–31 and the need to keep the ultrafiltration conditions under strict maintenance to maintain the specificity of the CuEXC value.30
This line of work (species fractionation) can also be further reinforced with isotopic analysis, in order to ascertain if some particular fraction contains more specific information to diagnose and follow up the disease. Lauwens et al. used this approach to determine bulk, exchangeable and ultrafiltrable serum copper in healthy and alcoholic cirrhosis subjects.32 However, to the best of the authors’ knowledge, such a strategy has not been investigated any further, neither for WD nor for any other clinical condition.
Besides the need for a more significant number of results, which is always difficult when investigating rare diseases, the use of more advanced chemometrical approaches, such as machine learning (ML),33 combining the results for the different Cu species may help in improving the reliability of the diagnosis. It has already been demonstrated that in complex scenarios where different parameters are monitored, applying the latest ML strategies can help in providing a more accurate classification, although the number of studies reporting on the use of such an approach using trace elemental information of clinical samples is still very limited.34 Even though ideally for building a reliable model it would be better to get access to information from a number of samples as large as possible, it has been recently reported in a similar situation (the use the Cu concentration and isotopic ratios to build a ML model to enhance diagnosis for bladder cancer)33 that a valuable model can be built even from a limited set of samples.
The aim of the present work is to better understand WD and to try to diagnose this disease more efficiently. For this purpose, the two types of diagnostic approaches for WD (Cu fractionation and Cu isotope analysis) will be explored and combined. For this, the Cu concentration and the isotopic composition in both bulk serum (total Cu) and exchangeable Cu fractions from a series of healthy people (controls), patients with liver diseases different from WD, newborns, and WD patients under different treatments (chelators and/or Zn salts) were determined via ICP-MS. Sample ultrafiltration and simple direct microinjection of the samples were preferred over more complex analytical methodologies explored before,12,35 in order to produce protocols that could be replicated in bio analytical labs, while maintaining the minimal sample consumption needed to carry out all the intended analyses.
Moreover, ML has also been applied to these results for the first time, in order to help establish a more efficient way to diagnose WD. We have used ML with two objectives. On the one hand, we want to verify if the collected data contain sufficiently clear and relevant information for the ML models in order to achieve accurate WD diagnosis. On the other hand, we want to enrich the ML output with a metric that reports the uncertainty of the predictions. The importance of both aspects in the context of potentially automated medical diagnosis will be demonstrated.
2. Experimental
2.1. Instrumentation
The development and evaluation of the CuEXC method were carried out with an ELAN DRC II quadrupole ICP-MS and with a NexION 300X ICP-MS, both from PerkinElmer (Waltham, USA). The conventional configuration using a peristaltic pump to deliver the sample to the nebulizer was chosen as the sample introduction system. The conditions are shown in Table 1.| NexION 300X ICP-MS | ELAN DRC II quadrupole ICP-MS | ||
|---|---|---|---|
| Continuous mode | Continuous mode | Time resolved analysis (TRA) | |
| Sample uptake rate (μL min−1) | 300 | 100 | 100 |
| Nebulizer gas, Ar (L min−1) | 1.00 | 0.90 | 0.92 |
| Plasma gas, Ar (L min−1) | 15.00 | 17.00 | 17.00 |
| Auxiliary gas, Ar (L min−1) | 1.20 | 1.00 | 1.00 |
| RF power (W) | 1600 | 1000 | 1000 |
| Dwell time (ms) | 50 | 50 | 10 |
| Gas flow reaction cell gas (mL min−1) | He/1.5 | NH3/0.7 | — |
| RPa | 0 | 0 | 0 |
| RPq | 0.25 | 0.8 | 0.25 |
| Signal acquisition | Continuous | Continuous | TRA |
| Nuclides monitored | 63Cu+, 65Cu+ | 63Cu+, 65Cu+ | 60Ni+, 63Cu+, 65Cu+ |
The determinations of total Cu and CuEXC in the samples were carried out with an ELAN DRC II quadrupole ICP-MS (PerkinElmer). A PFA-ST MicroFlow nebulizer (Elemental Scientific, Nebraska, USA) coupled to a twister cyclonic spray chamber (Glass expansion, Port Melbourne, Australia) was used for this purpose. The instrumental conditions are shown in Table 1.
Cu isotopic analyses were carried out using a Nu 1700 MC-ICP-MS instrument (Nu Instruments, Wrexham, UK), coupled to an Aridus3 (Cetac Teledyne, USA) as a desolvating system, which includes a MicroMist 100 μL min−1 nebulizer. The instrumental parameters are shown in Table 2.
| a The Aridus3 was not connected to any additional nitrogen flow, as in our experience this does not improve the sensitivity for the Nu 1700 instrument. b optimized daily. | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Desolvating Nebulizer System ARIDUS3 | |||||||||||||||||||||||
| N2 gas (mL min−1) | 0a | ||||||||||||||||||||||
| Ar gas (L min−1) | 8 | ||||||||||||||||||||||
| Chamber temperature (°C) | 140 | ||||||||||||||||||||||
| Membrane temperature (°C) | 160 | ||||||||||||||||||||||
| Nu MC-ICP-MS 1700 | |||||||||||||||||||||||
| RF power (W) | 1300 | ||||||||||||||||||||||
| Instrument resolution | Low | ||||||||||||||||||||||
| Integration time (s) | 0.5 | ||||||||||||||||||||||
| Nebuliser pressure (Psi) | 35.10–35.30b | ||||||||||||||||||||||
| Auxiliary gas (L min−1) | 0.8 | ||||||||||||||||||||||
| Coolant gas (L min−1) | 13 | ||||||||||||||||||||||
| Faraday cup configuration | |||||||||||||||||||||||
| Collector | H9 | H8 | H7 | H6 | H5 | H4 | H3 | H2 | H1 | Ax | L1 | L2 | L3 | IC0 | IC1 | IC2 | L4 | IC3 | L5 | IC4 | L6 | ||
| m/z | 66.6 | 66 | 65 | 64 | 63 | 62 | 60 | ||||||||||||||||
For sample digestion, an UltraWAVE microwave system (Milestone Inc., Shelton, USA) was used. An EVAPOCLEAN® unit (Analab, Bischheim, France) was deployed to evaporate samples by sub-boiling in Teflon vessels in a closed environment. A Centrifuge 5810R (Eppendorf, Hamburg, Germany) was used for CuEXC separation.
2.2. Materials and reagents
For CuEXC separation, Amicon® Ultra-4 centrifugal filter devices with a 30 kDa cut-off Ultracel® regenerated cellulose membrane were acquired from Merck Millipore (Cork, Ireland). EDTA purchased from Sigma-Aldrich (St. Louis, USA) and potassium chloride from Merck (Darmstadt, Germany) were also used for this purpose.Poly-prep polypropylene chromatography columns (Bio-Rad, Temse, Belgium) and Cu-specific resin (Triskem, Bruz, France) were used for Cu isolation prior to isotopic analyses. For the preparation of different eluting solutions, HCl (w = 35%) ultratrace® (Scharlau, Barcelona, Spain) was used.
For quantitative analysis, single-element standard solutions of Cu and Ni (1 g L−1) were acquired from SCP SCIENCE (Villebon-Sur-Yvette, France). For Cu isotopic analysis, NIST SRM 3114 (NIST, Gaithersburg, USA) was chosen as a reference in the bracketing sequence. This standard shows a similar composition to NIST SRM 976, which is currently out of stock.36 NIST SRM 986 (Ni) was used as an internal standard for mass bias correction.
To validate the CuEXC protocol, Seronorm Trace Elements Serum level 1 (L-1; Lot: 1309438) and 2 (L-2; Lot: 1309416) (Sero, Billingstad, Norway) were used. These reference materials (RMs) were reconstituted with 3 mL of ultrapure water, according to the manufacturer's instructions.
Instra grade HNO3 (w = 70%) was purchased from JT Baker (New Jersey, USA) and further purified by sub-boiling in a PFA system (DST 1000, Savillex, Eden Prairie, USA). Ultrapure water (18.2 MΩ cm) was obtained from a Direct-Q3 system (Millipore, Molsheim, France).
2.3. Samples and sample preparation
Serum samples from 56 volunteers were obtained from the Hospital Miguel Servet (Zaragoza, Spain). Thirteen of these samples were controls, fourteen originated from patients with hepatic disorders (different from WD), eight came from newborns/infants and twenty-one samples originated from WD patients. The principles outlined in the Declaration of Helsinki regarding all the experimental research involving humans or animals were followed. The experiments were approved by the Clinical Research Ethics Committee of Aragon (CEICA). Informed consents were obtained from human participants of this study.To avoid sample contamination, sample preparation was carried out in an ISO 5 laminar bench flow fitted in an ISO 7 clean lab. Each serum sample was split into two fractions, one for total Cu and the other for CuEXC determination. For each fraction, both Cu determination and Cu isotopic analysis were carried out. This protocol was first validated with serum reference materials (L-1 and L-2), and then applied to real samples.
The first step was to obtain the CuEXC fraction. The procedure proposed by El Balkhi et al.25 was followed but, instead of using NaCl, KCl was selected to avoid the introduction of additional sources of Na, thus preventing spectral overlap (40Ar23Na+) for the monitoring of 63Cu+ in the ICP-MS. 200 μL of serum were mixed in an Eppendorf tube with 200 μL of 3 g L−1 EDTA in 9 g L−1 KCl. The tube was then vortexed for 20 s. After this, the mixture was incubated for at least 1 h to ensure that the equilibrium of CuEXC between proteins and EDTA had been achieved. After this time, the mixture (400 μL) was transferred to the Amicon® Ultra-4 centrifugal filter with a 30 kDa cut-off membrane to be centrifuged for 45 min at 2000 g at 4 °C. For CuEXC determination, 100 μL of the filtrate were transferred to another centrifuge tube and diluted HNO3 (φ = 2%) was added up to 1 mL. The rest of the filtrate was kept for isotopic analysis.
To save the sample (as a limited amount was available), total Cu determination was carried out after sample preparation (Cu isolation) for isotopic analysis, as mentioned below. Total Cu determination in the reference materials was carried out after 30 times dilution of the original material with HNO3 with a volume fraction (φ) of 2%.
For Cu isolation before isotopic analysis, both the total Cu fraction (bulk serum) and the CuEXC fraction (after the ultrafiltration step) were treated in the same way. The first step for Cu isolation was sample digestion. For this purpose, 0.15–0.5 mL of bulk serum and the remaining amount of the CuEXC fraction (around 0.15 mL; exact sample volumes depended on the total sample amount available) were mixed in quartz vials with 3 mL of 14 mol L−1 HNO3 and were digested in a microwave system following the program recommended by the manufacturer for blood. The digest was then transferred to Teflon Savillex® vials to be evaporated until almost dryness at 95 °C using an EVAPOCLEAN® system.
Once the digestion and the evaporation were concluded, Cu isolation was carried out using a Triskem Cu specific resin following the method proposed by Miller et al.,37–39 which was further validated for serum samples by our research group.12 The evaporated fraction was redissolved in 500 μL of 12 mol L−1 HCl and heated at 120 °C in a closed Teflon Savillex® vial for 24 h, to ensure that Cu is found in its chloride form at the highest oxidation state. Then, the sample was evaporated until almost dryness in the EVAPOCLEAN® system and redissolved in 4 mL of 5 mmol L−1 HCl. This process was repeated twice to ensure that any excess of HCl was eliminated. The chromatographic columns were prepared by adding 0.5 mL of Triskem Cu specific resin into the Bio-Rad Poly-Prep columns. A piece of cotton was used as a stopper on the top of the resin. The resin was soaked in EtOH aqueous solution (φ = 20%) overnight before its use, as recommended by the manufacturer. The chromatographic separation was carried out as described elsewhere for serum samples12 and the protocol is summarized in Table S1 (ESI†). The Cu fraction obtained was then evaporated until almost dryness at 95 °C in an EVAPOCLEAN® system and redissolved in 50 μL of diluted HNO3 (φ = 2%).
For total Cu determination using the ICP-MS, the fraction of total Cu was spiked with Ni at a final concentration of 50 μg L−1, as this element was chosen as an internal standard. For isotopic analysis, both fractions (total Cu and CuEXC) for each sample were divided into 6 groups, depending on the total Cu concentration. The Cu concentration was adjusted with diluted HNO3 (φ = 2%) to 50, 100, 150, 200, 350, and 1000 μg L−1, matching with the standard used for the bracketing correction method to avoid additional mass bias. Moreover, the samples were spiked with Ni (NIST SRM 986) at a final concentration of 2 mg L−1, to achieve enough signal, as this element was selected as an internal standard for mass bias correction. A visual schematic of the procedures for sample preparation is shown in Fig. S1 (ESI†).
2.4. Measurement protocol
![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) ± U, where U = (t.s)/√N for a 95% confidence interval (N = 3) and
± U, where U = (t.s)/√N for a 95% confidence interval (N = 3) and ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) is the mean value, t is the t-value, and s is the standard deviation
is the mean value, t is the t-value, and s is the standard deviation
| Laboratory in Pau | Laboratory in Zaragoza | Serum reference material | |||||
|---|---|---|---|---|---|---|---|
| Total Cu (μg L−1) | CuEXC (μg L−1) | REC (%) | Total Cu (μg L−1) | CuEXC (μg L−1) | REC (%) | Total Cu (μg L−1) | |
| RM L-1 | 1141 ± 20 | 362 ± 35 | 32 ± 3 | 1258 ± 37 | 424 ± 10 | 33 ± 1 | 1066 ± 215 |
| RM L-2 | 1901 ± 61 | 1260 ± 51 | 66 ± 3 | 2009 ± 90 | 1419 ± 109 | 71 ± 3 | 1925 ± 387 |
CuEXC determination in real samples was carried out in continuous mode with the ELAN DRC II ICP-MS using the reaction cell with NH3 gas, to minimize spectral overlap. The instrument and data acquisition parameters used during these analyses are gathered in Table 1. For quantification, a calibration curve between 1 and 10 μg L−1 Cu (consisting of one blank solution and four calibration points, prepared using a single-element Cu standard) was used.
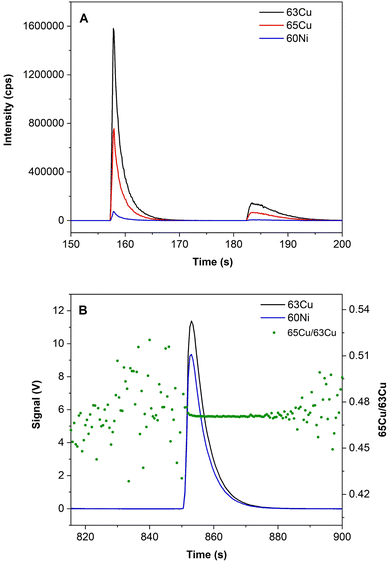
As the sample volume was limited and in order to keep the maximum of this volume for MC-ICP-MS analysis, total Cu determination in real samples was performed using time-resolved analysis (TRA) via the direct μ-injection method developed and evaluated by our research group for total Cu determination.12 In this case, the sample introduction system consisted of a PFA-ST MicroFlow nebulizer with a capillary sampling tube with an internal diameter of 0.25 mm mounted in a peristaltic pump. This capillary was connected to a PVC tubing (orange-blue tube, ID 0.25 mm × OD 2.07 mm from Glass Expansion), which in turn was connected to another additional capillary tube with an ID of 0.25 mm at the other end (external capillary) functioning as a sample probe. During the analysis, except for the time of sample introduction, a diluted HNO3 solution (φ = 2%) was continuously aspirated to rinse. For this purpose, the external capillary was introduced into the vial with the HNO3 solution. For sample injection, the external capillary was disconnected from the peristaltic pump tube for a few seconds and, after that, 1 μL of sample was introduced into the air space formed in the peristaltic pump tube with the help of an electronic micropipette. After the injection, it was necessary to wait for a few seconds before reconnecting the external capillary to the peristaltic pump tube to obtain a well-defined peak (Fig. 1a). For quantification, a Cu calibration curve between 0.1 and 20 mg L−1 (one blank solution and seven calibration points, using a single-element Cu standard for the analysis), spiked with Ni as an IS (at a final concentration of 50 μg L−1), was measured and the quantification was verified by analysing the serum RMs L-1 and L-2 (cf. Section 2.3). The instrument and data acquisition parameters used for total Cu determination are gathered in Table 1. Peak area was used for quantification, and three replicates per sample were carried out.
Before the measurements, the MC-ICP-MS coupled to the desolvating nebulizer system was optimized, and the performance was checked in continuous mode. For this purpose, a mixture of NIST SRM 3114 (Cu) and NIST SRM 986 (Ni) at a final concentration of 2 mg L−1 for each element was used. The 65δ value was calculated using the self-bracketing method, i.e., the standard is considered as the standard and as the sample at the same time, except for the first and the last measured, which are only considered standards. Using this strategy, the expected 65δ value is 0. Moreover, Ni was used for mass bias correction. The average 65δ value obtained was 0.00 ± 0.18‰ (N = 10, 2SD).
All Cu isotopic ratio calculations were carried out with the linear regression slope (LRS) method. With this method, the signal intensities measured for 65Cu were plotted against the signal intensities measured for 63Cu to obtain a linear regression. The Cu isotope ratio is calculated as the slope of this regression.40 For mass bias correction, a combination of internal and external normalization was used. Internal normalization was carried out according to the exponential model described by Maréchal et al.41 using Ni. The factor to correct the Cu ratio was calculated by experimentally determining the 62Ni/60Ni ratio via LRS and using the certified 62Ni/60Ni ratio (62Ni/60Ni = 0.138600 ± 0.000045). External normalization was carried out using the standard-sample-standard bracketing sequence with NIST SRM 3114. More information about the equations used is presented in the ESI.†
Five replicate ratios were obtained, and the mean value was used as a representative result. The uncertainty was calculated as two times the standard deviation.
2.5. Machine learning protocol
To develop the ML models, we have used the Spyder open source scientific environment for python42 and the Keras interface for the TensorFlow open-source machine-learning platform.43 We have selected artificial neural networks (ANNs)44 as the basis for our ML solutions, because, for small problems, they are one of the most powerful ML approaches and they are included in all the ML environments. Moreover, we will use an ensemble of ANNs to reduce the variance of our model and to enrich its output with an additional value that measures the uncertainty of each prediction. As explained by Bhatt et al.,45 measuring the uncertainty of a prediction is a form of transparency that allows for better understanding of predictions and for improved decision-making. However, very few studies to date measured and analyzed the uncertainty of the predictions of the ML models developed. We will carry out such analysis and demonstrate its utility to select the input parameters for our models and to achieve a better understanding of the results finally obtained.ML is based on the “learning from data” paradigm. In our case, the main limitation is that the dataset is very small. As explained before, we have information on only 56 volunteers from the Hospital Miguel Servet (Zaragoza, Spain). When the data set is small, the greatest risk is falling into overfitting. Our goal is to create a system that generalizes well to unseen examples. However, if we use a large network, and we overtrain it, our model may overfit the training data, memorizing all the different inputs and generating the perfect output for them. However, this is not a good solution, since overfitting models are not able to generalize their results to other inputs unseen during the training process.
For model selection, we carried out a grid search exploring four parameters: the number of hidden layers, the number of neurons per layer, the learning rate, and the maximum number of epochs to train.
During the model explorations, we found several hyperparameters that achieve similar, very high accuracy results. This indicates that the data collected contain sufficiently clear and relevant information for the models to use. For our analysis, we could use any of the models that provide equivalent results. We selected the smaller one, which is always a good criterion to prevent overfitting. Regarding the number of epochs, we stopped training as soon as the model achieved stable accuracy results for the training set, even though the loss function that guides the training process indicated that the fit could be improved.
The final selected model is a small ANN classifier with three levels of four neurons each that use rectified linear unit (ReLU) as an activation function and a final output layer with two neurons that use the softmax activation function to generate a probability distribution for the two outputs: WD/Non-WD. These outputs can be interpreted as the probabilities of suffering or not suffering from WD. The model was trained for 2000 epochs with a 0.001 learning rate. As inputs, we started using only the values of the total Cu concentration and the CuEXC concentration for the samples, and after that we explored the effect of including additional inputs. We normalized each value using the standard score to improve the training process. To reduce the variance of the output, we have trained 50 different models with the same setup. Since the training process started from different random initial points, each one of these models is different from the others. Our final model is an ensemble of these 50 NNs, and its classification output is the average of the 50 individual outputs. The internal variations among the 50 models, together with the probabilities generated by the softmax layer (the last layer of the ANN, which generates its final outputs) of the ANN are used to compute the uncertainty values.
The code used to define the model and train it and the code used to generate the uncertainty metric are published in a GitHub repository.46
3. Results and discussion
For the elemental determination of Cu, i.e., non-isotopic analysis, all the blanks (including those with EDTA and KCl) were below 1 μg L−1 Cu. For the isotopic analysis, the δ65Cu was 0.23 ± 0.21‰ for the solutions using diluted HNO3 (φ = 2%) and 0.42 ± 0.22‰ for the CuEXC solution (EDTA + KCl). They were all subtracted when calculating both Cu concentrations and isotope ratios.3.1. Evaluation of the method for CuEXC determination
To determine CuEXC, careful sample preparation is mandatory. To evaluate the method and validate the results before real sample analyses were conducted, an interlaboratory study was performed. The same serum reference materials (L-1 and L-2) were subjected to the CuEXC separation procedure (as explained in Section 2.3), and subsequent analyses in two different laboratories, one in Pau (France) and the other in Zaragoza (Spain), were carried out. In addition to CuEXC, total Cu was also determined, and REC was calculated following eqn (1). The results obtained are shown in Table 3. The total Cu concentration values observed in both laboratories differ (higher levels were obtained in Zaragoza, particularly for RM L-1), but both concentrations are within the concentration range provided by the reference materials. These differences may be due to small variations in the sample preparation because the reference material is freeze-dried and it is necessary to reconstitute it (in principle, Pau lab facilities are less prone to suffer from contamination issues). CuEXC values follow the same trend (a bit higher values are obtained in Zaragoza), but the REC values are rather similar in both cases, which points to minor differences in the reconstitution step, as commented before. The results were overall considered fit-for-purpose, and analysis of the real samples was undertaken next.Once the CuEXC separation protocol was evaluated in terms of Cu recovery, mass fractionation during the whole analytical procedure (including both CuEXC separation and Cu isotopic isolation) was investigated. To verify that no mass fractionation occurs during these sample preparation steps, three aliquots of NIST SRM 3114 were subjected to both procedures consecutively, as detailed in Section 2.3. After the two consecutive protocols, each aliquot was adjusted to a different Cu concentration for analysis: 250, 500, and 1000 μg L−1.
Next, isotopic analyses in TRA mode (cf. Section 2.4 and Fig. 1b) were performed, using a direct μ-injection method. 10 replicates per sample were measured, and the results were expressed as 65δ values, with the standard-sample bracketing method, where the standard is NIST SRM 3114 and the sample is NIST SRM 3114 after undergoing the two consecutive sample preparation procedures (CuEXC separation and Cu isolation). If no isotope fractionation is present, the recorded 65δ value should be 0. The results obtained are shown in Table 4 and external precision is expressed as 2SD in ‰. As can be seen from this table, 65δ values were around 0, meaning that no significant fractionation occurs during both procedures.
| Cu concentration (μg L−1) | δ65Cu “bracketing” ± 2SD (‰), (N = 10) |
|---|---|
| 250 | −0.18 ± 0.24 |
| 500 | −0.05 ± 0.26 |
| 1000 | 0.11 ± 0.19 |
These results are in accordance with those of previous investigations. In fact, a similar method was also validated by Lauwens et al.,32 but in that case, a different procedure was carried out for Cu isolation using the AG-MP-1 resin. The differences between the performances of these two resins (AG-MP-1 and Trisken) are discussed in ref. 12 and will not be further contrasted herein.
3.2. Analysis of the samples for total copper, exchangeable copper, and REC
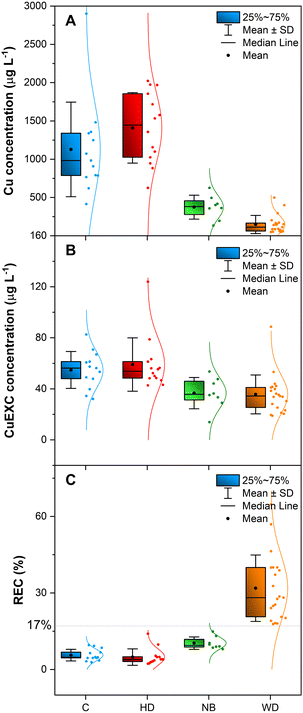
To interpret the results, the samples were divided into four different groups: controls (healthy patients, C), patients with hepatic disorders different from WD (HD), newborns/infants (NB) and WD patients (WD).The Cu concentration obtained for WD patients, regardless of the treatment, ranged between 49 and 498 μg L−1 (mean value = 147 μg L−1). For newborns and infants, the concentration found varied between 134 and 626 μg L−1 (mean value = 373 μg L−1). For controls and patients with other liver disorders, values between 414 and 2901 μg L−1 (mean value = 1128 μg L−1) and between 625 and 2022 μg L−1 (mean value = 1410 μg L−1), respectively, were observed. According to these results, WD patients show lower Cu levels than the rest of the groups tested in this work. However, some overlapping between the Cu levels of WD patients and those of the rest can be observed, particularly with the levels found for newborns and infants, as expected.12,15 This confirms that total Cu determination is useful, but it is not a selective biomarker for WD diagnosis.13
It is necessary to remark that all WD patients analyzed in this study were under treatment, except for one patient. Moreover, a patient who was suspected of not following the treatment correctly presented the highest CuEXC level (88.6 μg L−1), which again agrees well with the report by Guillaud et al.19
On the other hand, the WD patient without any prior treatment shows a CuEXC level of 38.4 μg L−1, which seems to be low for a patient just diagnosed. However, this patient was asymptomatic at the moment of blood collection, which agrees with the results reported by Guillaud et al.19 In view of the results, it is possible to state that CuEXC determination by itself is not enough for reliable WD diagnosis. However, it could be used to follow up the evolution of the disease. In any case, further studies with a larger population are needed to verify these aspects. As stated before, this is a typical problem when dealing with rare diseases, as it is never easy to collect a large number of samples.
Similar conclusions have been drawn in the literature, although the cutoff value is not clearly defined yet. In their first study with REC, El Balkhi et al.18 found a cutoff of 18.5% providing a 100% sensitivity and specificity for distinguishing WD patients from controls, wild-type homozygous and heterozygous. Guillaud et al.,19 on the other hand, could also distinguish WD patients from controls (patients with hepatic diseases different from WD) using the REC. A cutoff of 18.5% was set taking into account only WD patients sampled at the moment of diagnosis or who failed to respond to treatment because of non-compliance. However, if WD patients in stable condition under medical treatment were also included, the cutoff decreased to 14% and provided one false positive. Moreover, they found that the REC was not influenced by the presence of hepatic disorders, as such patients were indistinguishable from healthy controls. Also, no differences in the REC values were observed between WD patients with/without cirrhosis. Trocello et al.27 used the REC for family screening and showed that, by setting a cutoff of 15%, it was possible to differentiate WD patients (carrying 2 mutations in the ATP7B gene) from the subjects without WD (ATP7B heterozygous carriers, and subjects with no identified mutation in the ATP7B gene) with 100% sensitivity and specificity. In a recent study, subjects with alcoholic cirrhosis showed REC values of ≤19%, except for two patients showing REC values of 21 and 25%.32 However, no genetic tests were carried out in that work to investigate the potential disease of such patients.
On the other hand, in studies with animals, where it is possible to control almost all the external parameters much better, Schmitt et al.17 found that it was possible to discriminate Long-Evans Cinnamon rats (LEC, with an ATP7B mutation causing WD) from Long-Evans rats (LE, without any mutation) with a sensitivity of 97.3% and specificity of 100% setting a cutoff of 19%. They also showed that the REC is not influenced by the presence of liver damage or by the Cu intake. In another animal model, Heissat et al.28 set a cutoff at 20% for achieving a sensitivity and specificity of 100% between WD rats and wild-type rats.
To sum up, our results agree well with those of previous studies, further confirming that the REC value can be a sensitive and specific tool for diagnosing WD. However, further studies (with a higher number of both WD and controls) are needed to be able to set a reliable and constant cutoff for the REC (which seems to be in the range between 14 and 20, according to all the literature) and to know the limitations of this biomarker.
3.3. Cu isotopic analysis
In order to further understand the evolution of the disease and to complement the previous biomarkers, Cu isotopic analyses were carried out.To better interpret these analyses, the samples were divided into six different groups in this case: controls (healthy patients, C), patients with hepatic disorders different from WD (HD), healthy infants and newborns (NB), WD under treatment with a chelator (WD-C), WD under treatment with a Zn salt (WD-Zn) and one sample from a WD patient obtained at the moment of diagnosis, without any treatment (WD).
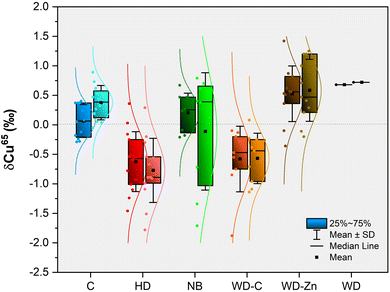
Next, these samples were analyzed for Cu isotopic composition in both bulk serum and the CuEXC fraction. Five replicates per sample were carried out, following the bracketing sequence (cf. Section 2.4.2). Fig. 3 shows the results of δ65Cu (‰) obtained in both bulk serum (dark colors) and CuEXC fraction (light colors) for the samples under investigation.
The isotopic composition of bulk serum for control subjects shows δ65Cu values around 0 with an average δ65Cu value of 0.08 ± 0.27‰ (expressed as x ± SD). Patients with other hepatic disorders show an average δ65Cu of −0.62 ± 0.51‰. Results obtained for newborns show δ65Cu values around 0 or positive, with an average value of 0.21 ± 0.33‰. For WD patients under treatment with a chelator, a negative δ65Cu value was found, with an average value of −0.58 ± 0.56‰, while WD patients under treatment with Zn salts show positive delta values, with an average value of 0.53 ± 0.47‰.
It is interesting to mention that in this latter group there is a patient showing a relatively large negative δ65Cu (−0.36‰). This sample corresponds to the same patient that showed a δ65Cu of −1.88‰, in the group of WD under chelator treatment (value outside the boxplot for WD-C) in a previous sampling when the patient was under treatment with both a chelator and a Zn salt. This means that when the patient stopped the treatment with the chelator keeping only the Zn salt treatment, the δ65Cu value shifted to a heavier value, but it was still negative. The sample of the patient at the moment of the WD diagnosis (she/he was asymptomatic) shows a δ65Cu of 0.68‰.
The results of δ65Cu obtained in bulk serum for controls were consistent with those previously reported in the literature.32,47,48 The results obtained for patients with other hepatic disorders seem to be lighter than the results obtained for the control patients and they agree with previously published results.32,48,49 For newborns, the results obtained are similar to the values obtained for the controls, and they also agree with previously reported data.12 In the same way, the results obtained for WD, regardless of treatment, are in agreement with the results reported before.12 The δ65Cu values are lighter for WD patients treated with a chelator compared to those of WD patients treated with Zn salts, and they are comparable with the δ65Cu values obtained for patients with other hepatic problems. This is coherent with the hypothesis of 63Cu being preferably accumulated in the liver, and the negative delta values being due to Cu being released from the liver, either by the treatment with a chelator or by liver disorders, as postulated in a previous publication.12
On the other hand, the isotopic composition of the CuEXC fraction shows average δ65Cu values of 0.38 ± 0.28‰ for control subjects. For patients with hepatic disorders (non-WD) the average value is −0.70 ± 0.58‰, while the newborns show a mean value of −0.11 ± 0.99‰. For WD patients under chelating treatment, the mean value of δ65Cu is −0.57 ± 0.43‰, while for the patient under Zn salt treatments, this value is 0.58 ± 0.53‰. The WD patient without any treatment (at the moment of WD diagnosis) shows a positive delta value of 0.72‰.
The results of δ65Cu obtained for the CuEXC fractions seem to follow the same trend as those of δ65Cu obtained in bulk serum. This means lighter values were observed for patients with hepatic problems and WD patients under chelating treatment, while heavier values were found for controls, WD patients under Zn salt treatment, and for the only sample of a WD patient without treatment and who was asymptomatic. In the case of newborns/infants, there is a very high variability in comparison with bulk serum.
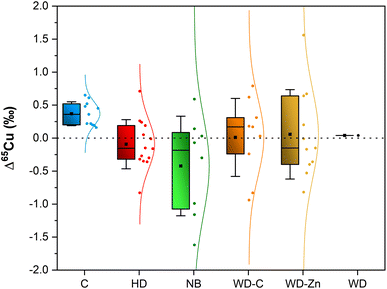
To enable direct comparison of the results obtained in both fractions (bulk serum and CuEXC) for the same sample, the Δ65Cu values were calculated following eqn (2) and are plotted in Fig. 4.
| Δ65Cu = δ65CuEXC − δ65Cubulk serum | (2) |
In this figure, the only group that follows a clear trend is the control group. In this group, it is possible to observe that, in all cases, the isotopic composition of the CuEXC fraction shifts to heavier values compared to the isotopic composition of bulk serum. Meanwhile, for the rest of the samples under investigation, a high variability between them prevents reaching a clear conclusion.
The average Δ65Cu value for control subjects was 0.36 ± 0.18‰. For patients with other hepatic disorders, the average Δ65Cu was −0.08 ± 0.36‰. Newborns and infants show an average value of Δ65Cu −0.32 ± 0.83‰. WD patients show average values of 0.01 ± 0.59‰ and 0.06 ± 0.68‰ for patients under chelator treatment or under treatment with Zn salts, respectively.
The mean value obtained for controls is in agreement with the result obtained by Lauwens et al.32 (0.41 ± 0.15‰, N = 7). On the other hand, Tennant et al.,50via computational calculations, obtained that δ65Cualbumin should be around 60% heavier than δ65Cubulk serum, assuming that serum consists of 10% albumin and 90% ceruloplasmin. In that study, healthy patients (controls) showed an average δ65Cualbumin 37% heavier than that in bulk serum. Our results show the same trend, but the differences between δ65CuEXC and δ65Cubulk serum are not so significant, most likely because not all CuEXC is bound to albumin. Cu may also be loosely bound to other proteins and to other low molar mass molecules, such as amino acids,32 which were not taken into account for the computational calculations referred above. Moreover, the nature of these compounds might be highly variable among subjects belonging to the same group.29,30
The mean value obtained for patients with hepatic disorders (non-WD) is also in agreement with the value reported previously (−0.10 ± 0.23‰, N = 14).32 Despite this fact, it was not possible to find any clear trend, as both positive and negative Δ65Cu were observed. The same occurs for WD independent of the treatment, and for newborns and infants where the variability in the results is high.
To try to explain the variability in the Δ65Cu, it is important to keep in mind that the liver is the principal source of serum proteins. Liver disorders or liver immaturity (in the case of newborns) can affect hepatic metabolism, causing changes in the levels of some proteins and in the coordination environment of Cu, thus affecting the isotopic composition. The isotopic fractionation in general is influenced by the ligand coordination and by the oxidation state.51,52 Generally, heavy isotopes prefer bonding with ligands with stronger electronegativity (O > N > S), particularly, if the metal is in its highest oxidation state. Therefore, it is expected to have a lighter isotopic composition in Cu bound to cysteine or methionine (Cu–S) than in Cu bound to histidine (Cu–N).
In control patients, it is possible to see a clear trend for Δ65Cu, probably because not all healthy bodies are equal, but they tend to work similarly. However, for the rest, since Cu can bond to different molecules depending on the disorder and on its state, it seems unfeasible to appreciate a clear trend above the variability. Information about patients in terms of the levels of the different molecules that can bind Cu (ceruloplasmin, albumin, alpha-2-macroglobulin, etc.) was not available for this study, which could enhance understanding about this topic.
In any case, it becomes clear that while Cu isotopic analysis can provide relevant information related to the evolution of the disease (e.g., once one knows that somebody is affected by WD, Cu isotopic analysis can provide information on how the disease is progressing), it is hardly feasible to use it as the only diagnostic tool, as too many different factors affect the Cu ratios obtained (e.g., treatment, existence of other hepatic disorders, etc.).
3.4. Machine learning
As discussed in the Introduction section, applying ML strategies to our data set can help in developing a more efficient way to diagnose WD. Section 2.5 describes in detail how our model was built and trained. In short, we have followed a cross-validation approach to train our ANN models. Since we have 56 data available, we have divided them into 4 subsets, and we have performed four training runs using 3 subsets for training and one for the test. With this approach, we have obtained the test results for all input data.Fig. 5a presents the results of this approach for the 56 inputs (values of the total Cu concentration and CuEXC concentration for the samples) of the data set. The prediction of the ANN is correct if the output assigns the highest probability value to the correct class. In this case, it is not correct for two of the inputs. This represents a 96.4% accuracy, computed as the number of times the correct class is selected divided by the number of predictions (54/56), multiplied by 100. However, as explained before, accuracy is not our only concern. We also want to measure the uncertainty of the model. This can help us to understand the predictions, identify how to improve them, and to decide whether we can trust them, or else, additional analyses are needed.
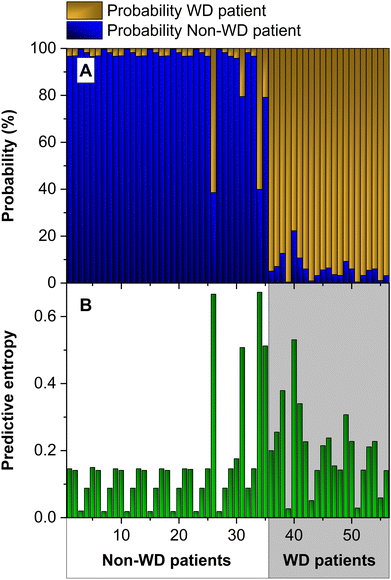 | ||
| Fig. 5 (A) Probability map and (B) predictive entropy obtained with an ensemble of 50 ANNs using the values of total Cu and CuEXC concentrations as inputs. | ||
For this purpose, we use the predictive entropy to measure the uncertainty of the output using eqn (3), where x is the input, y is the output, ck is the ground-truth class label, p(y = ck|x) are the probabilities assigned to each category, K is the number of categories, and T is the number of different outputs (50 in our case, since we have 50 different models working in parallel).
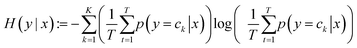 | (3) |
This equation measures the uncertainty inherent in the variable's possible outcomes. If for a given input, the prediction is very clear, then H(x) will be very small. This will happen if all 50 models assign almost 1 to one of the categories and almost 0 to the other one. In contrast, if the probabilities are not clear, or the variance in the predictions of the models is very high, the uncertainty will be high. For example, if some ANNs assign the maximum probability to one category, while others assign it to the other category, or if the models assign similar probabilities to both categories, the predictive entropy will increase.
As can be seen in Fig. 5b, the predictive entropy, uncertainty in the figure, for the two wrong predictions is very high, but it is also high for other inputs for which the predictions were correct. This means that we should not trust the model output for high uncertainty data, even when the model selects the right class. In fact, for a binary classifier like this one, a random model would be correct in half of the cases. To deploy the ML model as an automated diagnostic tool, robust predictions are needed. To this end, in practice, a threshold can be set and, if the uncertainty is above it, the output should be discarded, and an expert should be asked to analyze that particular case. After such data have been analyzed by experts, they can be included in the data set, and iteratively, they can be used to further improve the model.
We have analyzed the data to understand why some inputs exhibit high uncertainty values. The main reason is that some of the newborn samples show total Cu and CuEXC concentration values that are close to those of some of the samples of WD cases used during training. In fact, if we compute the two-dimensions Euclidean distance among all samples, the fact is that for samples 31, 34 and 35, which belong to non-WD patients (newborns), the closest sample originates from a WD patient, and for sample 40, which is a WD sample, the closest one belongs to a newborn.
In one of the cases, sample 26, the reason is different. This patient was affected by a hepatic disorder and showed a very high CuEXC concentration, which is out of range in comparison with the others (highest value in Fig. 2b, above 120 μg L−1). When such data are in the test set, the model has not seen anything like it during training, and for this reason, it generates a very high uncertainty. This is the desired behavior for an automated diagnostic tool. In a medical application, the model should alert for expert review when it encounters data that are very different from those used during training.
Although we have obtained a good accuracy, 96.4% (see Fig. 5), we wanted to further improve our models to reduce their uncertainty. To do so, we added more information to the inputs. Fig. 6 presents the probabilities and the entropy of our model when we train it by adding the REC value to the inputs.
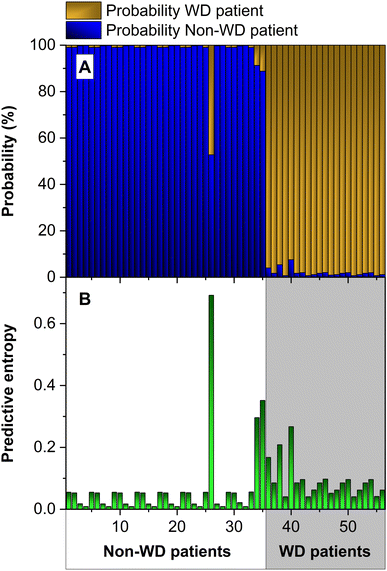 | ||
| Fig. 6 (A) Probability map and (B) predictive entropy obtained with an ensemble of 50 ANNs using the values of the total Cu concentration, CuEXC concentration and REC as inputs. | ||
As can be seen in the figure, the results have clearly improved. In this case the model properly classifies all the cases (100% accuracy), and the probabilities assigned to the correct class are higher than before, being very close to the value 1 in most cases. As for the uncertainty (see Fig. 6b), the average value has been reduced from 0.175 to 0.077. Hence, including the REC value helps to develop a more robust model. This is interesting, since the REC values are calculated from the other two inputs (see eqn (1)), but the model improves when this parameter is also added. The only case with no improvement is number 26, which, as explained before, corresponds to a value outside the range of the others for the CuEXC value. This high uncertainty value is useful to identify this anomaly.
Continuing with this analysis, we added two more input values: δ65Cubulk serum and δ65CuExch. Fig. 7 presents the results. In this case, the additional data increase the uncertainty of the model. Hence, it is not recommendable to use it, because the model output is more robust without it. This can be already anticipated since the δ65Cu values strongly depend on the treatment that the patient is following (see Fig. 3) and, thus, cannot help in differentiating WD patients from the rest.
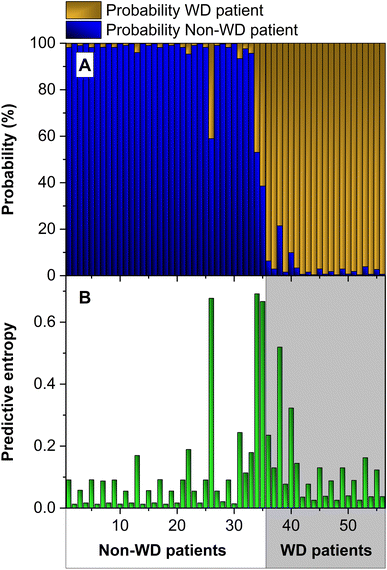 | ||
| Fig. 7 (A) Probability map and (B) predictive entropy obtained with an ensemble of 50 ANNs using the values of the Cu concentration, CuEXC, REC, δ65Cubulk serum and δ65CuEXC as inputs. | ||
The uncertainty metric clearly helps to choose which information should be used as model inputs, but it can also be useful to detect problems with the predictions. As explained before, we have normalized all the inputs before training. To use the model properly, it is important to normalize the inputs using the same normalizing factors always. However, this may be forgotten by mistake. We have analyzed what would happen in that case by entering the test inputs without such normalization. The result is that our classifier fails 30% of the tests. This is normal; if the inputs are not in the correct format, the model cannot do its computations properly. The interesting result is that the uncertainty metric increased by a factor of 12, thus clearly highlighting that these predictions were very unreliable. Therefore, thanks to this metric, it is possible to detect anomalous situations and try to understand and solve them, thus greatly improving the confidence in our results.
Conclusion
This work presents a comprehensive approach to what can be achieved nowadays via ICP-MS analysis of serum samples for the study of WD, combining both isotopic and species-specific Cu information. The results demonstrate that this technique is able to provide key information for diagnosis and follow up of WD.While Cu isotopic analysis via MC-ICP-MS can provide data for better understanding of the disease and for follow-up of WD patients, its contribution to diagnosis seems to be of limited value: Cu delta values for WD patients depend on the treatment followed and overlap with those observed for patients with other hepatic issues, regardless of the Cu fraction subjected to analysis.
On the other hand, it seems that a simple fractionation approach to determine total Cu and the CuEXC fraction, together with the calculations of the REC and the implementation of a ML model can help in providing a more efficient WD diagnosis, which could eventually help in establishing newborn screening programs. Moreover, this information can be obtained in an easy way, enabling hospitals to perform these determinations routinely using a “conventional” (meaning quadrupole-based) ICP-MS, or even other atomic techniques that are sufficiently sensitive (e.g., graphite furnace atomic absorption spectrometry), even though it is hard to compete with the high sample throughput of ICP-MS in this context.
Regarding the application of ML, the analysis of the uncertainty is one of the main contributions of this work. In the literature, one can find one or several ML models for every existing dataset. However, the focus is always on improving accuracy, but, for medical diagnosis, accuracy is not enough. Uncertainty estimation is needed to build a robust automated diagnostic tool. This work shows how the use of an uncertainty metric can help to better understand the results obtained during training, identifying anomalies or ambiguous data. In this way, if the model generates an output with a very small uncertainty, it can be interpreted that the model was trained with similar data and the output is reliable. On the other hand, if the uncertainty is high, it can be anticipated that the prediction is not fully reliable, and an expert should check the data and make the final decision.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the European Regional Development Fund for financial support through the Interreg POCTEFA EFA 176/16/DBS as well as to projects PGC2018-093753-B-I00, PID2021-122455NB-I00 and PID2019-105660RB-C21 funded by MCIN/AEI/10.13039/501100011033 and to the Aragon Government (Construyendo Europa desde Aragón, Groups E43_20R and T58_20R).References
- M. Schaefer and J. D. Gitlin, Am. J. Physiol.: Gastrointest. Liver Physiol., 1999, 276, G311–G314 CrossRef CAS PubMed.
- S. Lutsenko and M. J. Petris, J. Membr. Biol., 2002, 191, 1–12 CrossRef PubMed.
- J. D. Gitlin, Gastroenterology, 2003, 125, 1868–1877 CrossRef PubMed.
- A. Ala, A. P. Walker, K. Ashkan, J. S. Dooley and M. L. Schilsky, Lancet, 2007, 369, 397–408 CrossRef CAS PubMed.
- I. Mohr and K. H. Weiss, Ann. Transl. Med., 2019, 7, S69 CrossRef CAS PubMed.
- E. Roberts and M. L. Schilsky, Hepatology, 2003, 37, 1475–1492 CrossRef PubMed.
- M. Bost, G. Piguet-Lacroix, F. Parant and C. M. R. Wilson, J. Trace Elem. Med. Biol., 2012, 26, 97–101 CrossRef CAS PubMed.
- S. M. Kenny and D. W. Cox, Hum. Mutat., 2007, 12, 1171–1177 CrossRef PubMed.
- I. J. Chang and S. H. Hahn, in Handbook of Clinical Neurology, ed. P. Vinken and G. Bruyn, Elsevier, Amsterdam, 2017, ch. 3, vol. 142, pp. 19–34 Search PubMed.
- E. A. Roberts and M. L. Schilsky, Hepatology, 2008, 47, 2089–2111 CrossRef CAS PubMed.
- C. D. Romero, P. H. Sanchez, F. L. Blanco, E. R. Rodríguez and L. S. Majem, J. Trace Elem. Med. Biol., 2002, 16, 75–81 CrossRef CAS PubMed.
- M. C. García-Poyo, S. Bérail, A. L. Ronzani, L. Rello, E. García-González, B. Lelièvre, P. Cales, F. V. Nakadi, M. Aramendía, M. Resano and C. Pécheyran, J. Anal. At. Spectrom., 2021, 36, 968–980 RSC.
- F. Woimant, N. Djebrani-Oussedik and A. Poujois, Ann. Transl. Med., 2019, 7, S70 CrossRef CAS PubMed.
- T. Müller, S. Koppikar, R. M. Taylor, F. Carragher, B. Schlenck, P. Heinz-Erian, F. Kronenberg, P. Ferenci, S. Tanner, U. Siebert, R. Staudinger, G. Mieli-Vergani and A. Dhawan, J. Hepatol., 2007, 47, 270–276 CrossRef PubMed.
- M. Aramendía, L. Rello, M. Resano and F. Vanhaecke, J. Anal. At. Spectrom., 2013, 28, 675–681 RSC.
- M. Resano, M. Aramendía, L. Rello, M. L. Calvo, S. Bérail and C. Pécheyran, J. Anal. At. Spectrom., 2013, 28, 98–106 RSC.
- F. Schmitt, G. Podevin, J. Poupon, J. Roux, P. Legras, J.-M. Trocello, F. Woimant, O. Laprévote, T. H. NGuyen and S. E. Balkhi, PLoS One, 2013, 8, e82323 CrossRef PubMed.
- S. El Balkhi, J.-M. Trocello, J. Poupon, P. Chappuis, F. Massicot, N. Girardot-Tinant and F. Woimant, Clin. Chim. Acta, 2011, 412, 2254–2260 CrossRef CAS PubMed.
- O. Guillaud, A.-S. Brunet, I. Mallet, J. Dumortier, M. Pelosse, S. Heissat, C. Rivet, A. Lachaux and M. Bost, Liver Int., 2018, 38, 350–357 CrossRef CAS PubMed.
- M. Costas-Rodríguez, J. Delanghe and F. Vanhaecke, TrAC, Trends Anal. Chem., 2016, 76, 182–193 CrossRef.
- F. Albarède, Elements, 2015, 11, 265–269 CrossRef.
- B. Mahan, R. S. Chung, D. L. Pountney, F. Moynier and S. Turner, Cell. Mol. Life Sci., 2020, 77, 3293–3309 CrossRef CAS PubMed.
- F. Vanhaecke and M. Costas-Rodríguez, View, 2021, 2, 20200094 CrossRef CAS.
- F. Vanhaecke and P. Degryse, Isotopic Analysis: Fundamentals and Applications Using ICP-MS, Wiley-VCH, Weinheim, 2012 Search PubMed.
- S. El Balkhi, J. Poupon, J.-M. Trocello, A. Leyendecker, F. Massicot, M. Galliot-Guilley and F. Woimant, Anal. Bioanal. Chem., 2009, 394, 1477–1484 CrossRef CAS PubMed.
- J. M. Walshe, in Advances in Clinical Chemistry, ed. G. S. Makowski, Elsevier, Amsterdam, 2010, ch. 8, vol. 50, pp. 151–163 Search PubMed.
- J.-M. Trocello, S. El Balkhi, F. Woimant, N. Girardot-Tinant, P. Chappuis, C. LLoyd and J. Poupon, Mov. Disord., 2014, 29, 558–562 CrossRef CAS PubMed.
- S. Heissat, A. Harel, K. Um, A.-S. Brunet, V. Hervieu, O. Guillaud, J. Dumortier, A. Lachaux, E. Mintz and M. Bost, J. Trace Elem. Med. Biol., 2018, 50, 652–657 CrossRef CAS PubMed.
- M. E. del Castillo Busto, S. Cuello-Nunez, C. Ward-Deitrich, T. Morley and H. Goenaga-Infante, Anal. Bioanal. Chem., 2022, 414, 561–573 CrossRef CAS PubMed.
- N. Solovyev, A. Ala, M. Schilsky, C. Mills, K. Willis and C. F. Harrington, Anal. Chim. Acta, 2020, 1098, 27–36 CrossRef CAS PubMed.
- C. D. Quarles, M. Macke, B. Michalke, H. Zischka, U. Karst, P. Sullivan and M. P. Field, Metallomics, 2020, 12, 1348–1355 CrossRef CAS PubMed.
- S. Lauwens, M. Costas-Rodríguez, J. Delanghe, H. Van Vlierberghe and F. Vanhaecke, Talanta, 2018, 189, 332–338 CrossRef CAS PubMed.
- W. Wang, X. Liu, C. Zhang, F. Sheng, S. Song, P. Li, S. Dai, B. Wang, D. Lu, L. Zhang, X. Yang, Z. Zhang, S. Liu, A. Zhang, Q. Liu and G. Jiang, Chem. Sci., 2022, 13, 1648–1656 RSC.
- G. L. Donati, in Comprehensive Analytical Chemistry, ed. M. A. Z. Arruda and J. R. de Jesus, Elsevier, Amsterdam, 2022, ch. 2, vol. 97, pp. 53–88 Search PubMed.
- M. C. García-Poyo, A. L. Ronzani, J. Frayret, S. Bérail, L. Rello, E. García-González, B. Lelièvre, F. V. Nakadi, M. Aramendía, M. Resano and C. Pécheyran, Spectrochim. Acta, Part B, 2021, 185, 106306 CrossRef.
- Q. Hou, L. Zhou, S. Gao, T. Zhang, L. Feng and L. Yang, J. Anal. At. Spectrom., 2016, 31, 280–287 RSC.
- K. A. Miller, PhD thesis, University of Calgary, 2018.
- K. A. Miller, C. M. Keenan, G. R. Martin, F. R. Jirik, K. A. Sharkey and M. E. Wieser, J. Anal. At. Spectrom., 2016, 31, 2015–2022 RSC.
- C. Dirks, B. Scholten, S. Happel, A. Zulauf, A. Bombard and H. Jungclas, J. Radioanal. Nucl. Chem., 2010, 286, 671–674 CrossRef CAS.
- J. Fietzke, V. Liebetrau, D. Günther, K. Gürs, K. Hametner, K. Zumholz, T. H. Hansteen and A. Eisenhauer, J. Anal. At. Spectrom., 2008, 23, 955–961 RSC.
- C. N. Maréchal, P. Télouk and F. Albarède, Chem. Geol., 1999, 156, 251–273 CrossRef.
- Spyder, https://www.spyder-ide.org/, accessed July 2022.
- Keras, https://keras.io/, accessed July 2022.
- S. S. Haykin, Neural Networks and Learning Machines, Prentice Hall, New York, 3rd edn, 2009 Search PubMed.
- U. Bhatt, J. Antorán, Y. Zhang, Q. V. Liao, P. Sattigeri, R. Fogliato, G. Melançon, R. Krishnan, J. Stanley, O. Tickoo, L. Nachman, R. Chunara, M. Srikumar, A. Weller and A. Xiang, in Proceedings of the 2021 AAAI/ACM Conference on AI, Ethics, and Society, ACM, Virtual Event USA, 2021, pp. 401–413 Search PubMed.
- https://github.com/JavierResano/ML-for-the-diagnosis-of-Wilson-s-Disease-via-ICP-MS .
- F. Albarède, P. Telouk, A. Lamboux, K. Jaouen and V. Balter, Metallomics, 2011, 3, 926–933 CrossRef PubMed.
- M. Costas-Rodríguez, Y. Anoshkina, S. Lauwens, H. Van Vlierberghe, J. Delanghe and F. Vanhaecke, Metallomics, 2015, 7, 491–498 CrossRef PubMed.
- S. Lauwens, M. Costas-Rodríguez, H. Van Vlierberghe and F. Vanhaecke, Sci. Rep., 2016, 6, 30683 CrossRef CAS PubMed.
- A. Tennant, A. Rauk and M. E. Wieser, Metallomics, 2017, 9, 1809–1819 CrossRef CAS PubMed.
- T. Fujii, F. Moynier, M. Abe, K. Nemoto and F. Albarède, Geochim. Cosmochim. Acta, 2013, 110, 29–44 CrossRef CAS.
- E. A. Schauble, Rev. Mineral. Geochem., 2004, 55, 65–111 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ja00267a |
| This journal is © The Royal Society of Chemistry 2023 |