 Open Access Article
Open Access ArticleBack-to-cyclic monomers: chemical recycling of silicone waste using a [polydentate ligand–potassium silanolate] complex†
Nam Duc
Vu
 a,
Aurélie
Boulègue-Mondière
b,
Nicolas
Durand
b,
Jean
Raynaud
a,
Aurélie
Boulègue-Mondière
b,
Nicolas
Durand
b,
Jean
Raynaud
 *a and
Vincent
Monteil
*a and
Vincent
Monteil
 *a
*a
aLaboratory of Catalysis, Polymerization, Processes and Materials, CP2M (UMR 5128, CNRS/Université de Lyon 1- Claude Bernard/CPE Lyon), Université de Lyon, 43 Bd du 11 Nov. 1918, 69616, Villeurbanne cedex, France. E-mail: jean.raynaud@univ-lyon1.fr; vincent.monteil@univ-lyon1.fr
bElkem Silicones, R&D Chemistry, R&I Centre “ATRiON”, 9 rue Spécia, 69190, Saint-Fons, France
First published on 14th March 2023
Abstract
Silicones are ubiquitous materials owing to their exceptional mechanical and thermal stability as well as low toxicity. Recycling them has become a relevant target for circular economy purposes. Conventional processes of chemically recycling polysiloxanes allow for the recovery of valuable cyclic monomers. Unfortunately, they lack efficiency and still require high operating temperatures, thus yielding detrimental by-products. Herein, we introduce an efficient method for the solvent-free depolymerisation of linear polydimethylsiloxanes using a [polydentate ligand–potassium silanolate] complex as a catalyst that promotes the chemical recycling of silicones into cyclic monomers from many industrial substrates including actual waste materials. Our method only requires a small amount of catalyst (0.1 mol%) and proceeds over a wide range of temperatures (60 °C–170 °C) to efficiently yield a mixture of cyclosiloxanes (up to 98–99% yield) from up to a 100 g scale of waste silicone oils. Moreover, the recyclability of this catalyst was demonstrated over five runs without loss of activity.
Introduction
Silicone polymers are widely used in applications ranging from lubricants to materials for construction and automotive parts, electronics, medical formulations and engineered products.1,2 In 2020, the world's production of silicones was over 8 million tons, representing a market value of 15.1 billion USD.3 Silicone polymers consist of Si–O–Si units bound in highly resistant backbones. Si–O bonds are longer than C–O bonds and the bond angle of Si–O–Si is wider than that of carbon analogues. These materials are thus thermally stable, mostly chemically inert (except to strong acids and bases) and highly flexible.3 Recycling these high-value inorganic polymers has become strategically important for both environmental and economic reasons.Polydimethylsiloxanes (PDMS) are one of the most important silicone products. Most of them are produced through the hydrolysis of chlorosilanes that are prepared via the “Rochow direct process”,4–6 from which the associated produced cyclic monomers (mainly D3 and D4) are subsequently polymerized in the presence of a base catalyst.7 Although silicon (Si) is an abundant element in the Earth's crust, the production of metal grade Si necessary for the “direct process” is highly energy-intensive requiring an extremely high temperature (>1400 °C).8 Moreover, the process also releases a substantial amount of greenhouse gases detrimental to our environment.9 The recycling of out-of-specification or end-of-life polydimethylsiloxanes to value-added compounds, such as cyclic monomers or functional oligomers, has thus emerged as a highly desirable and potentially sustainable solution.
The recycling of silicone polymers and materials remains limited and principally involves downcycling through mechanical processing (powdered waste elastomers, for instance) or limited chemical recycling.10,11 Chemical recycling methods relying on halogenated reagents exist but are limited to out-of-the-loop products regarding the overall silicone process and are thus outside the scope of silicone circularity.12 Most of the chemical recycling back-to-monomer methods and their associated industrial processes rely on a rather high “catalytic” amount (thus questioning the efficient catalysis) of alkaline hydroxides,11 or strong organic and inorganic acids,13,14 and the reaction conditions as well as separation steps of the processes mandate high temperatures and low pressures to recover valuable cyclic monomers.11 There is thus a dire need for catalysis upgrading to allow for a wider implementation of the chemical recycling of silicones in the framework of silicone circularity.
The chemical recycling of waste silicones has been widely studied for over 80 years.10 Initially, the decomposition of polysiloxanes was carried out at a very high temperature (600 °C) under inert conditions or in air (300 °C).15 On the one hand, the reaction at 600 °C gives a mixture of cyclosiloxanes (D3–D8) favouring small cyclic monomers. On the other hand, CO2 and silica are obtained as major products during thermal cracking in air at 300 °C. Besides, steam could be used as an agent for the conversion of polysiloxanes at high temperature to (oligo)silanols.16 However, most of these methods require high temperature and give a moderate yield of a mixture of products, such as cyclic siloxanes, but also silanols, silica, etc. Recycling to recover PDMS-based cyclic monomers, the shortest cyclic monomers within the silicone industry, remains highly desirable, but lacks very competitive catalysts for it to become viable on very large scales.
In 1997, Allandrieu and Cardinaud found that strong alkaline hydroxides or quaternary ammonium-based hydroxide could be used as an efficient catalyst for the depolymerisation of silanol-terminated PDMS.11 The reaction was carried out at 140 °C to yield approximately 90% of a mixture of cyclosiloxanes. Later, GE Bayer Silicones claimed that the monoester or diester of phosphoric acid (RO)x(HO)3−xPO (x = 1 or 2) could be used as an activator for the depolymerisation of both uncrosslinked and crosslinked polysiloxanes.17 After 77–184 hours at room temperature, the viscosity of siloxane oils decreases from 2.7 to 4.3 times in comparison with that in the absence of an activator, indicating the potential applications of the functionalization of polysiloxanes into oligosiloxane derivatives. Interestingly, when anionic processes are employed cyclic monomers from D3 to D5 are obtained, whereas when acid-catalysed cationic processes are utilized, larger cyclic monmers (up to D6 or even larger) devoid of D3 are produced.10
Additionally, Tremco, Inc. reported a method for the depolymerisation of silicone rubber wastes to produce cyclosiloxanes. This process occurs in diethylene glycol monobutyl ether at 150–180 °C in the presence of 2.5 wt% of H2SO4 and then 3.5 wt% of KOH at 80–115 °C under a reduced pressure of 16–24 mbar.18
Furthermore, numerous reports have focused on thermal cracking of polysiloxanes. For example, alcoholysis of siloxane polymers has been extensively studied.19,20 Okamoto and Petrus used dimethyl carbonate and methanol in the presence of KF at 150 °C or an heterobimetallic aryloxide of Mg–K with the support of a methylsalicylato ligand at 220 °C as the catalyst. The produced alkoxy(oligo)siloxanes could be further derivatised to polysiloxanes by hydrolysis.
More recently, Hoge and his colleagues have reported the first example of isolated silanol–silanolated anions [Si–O⋯H–O–Si].21,22 These counter-anions exist in combination with weakly coordinating phosphazenium counter-cations. These anions are thus strong nucleophiles and could be utilised as catalysts for the depolymerisation of silicone oils. However, only 38% yield of cyclosiloxanes was obtained under the reaction conditions (90 °C, 7 mbar). Moreover, these silanolate salts start to decompose at 90 °C in vacuo and rapid decomposition occurs above 100 °C, therefore limiting their application as chemical recycling catalysts. As such, there is still a dire need for more robust and very efficient catalysts for the chemical depolymerization of PDMS-based oils into valuable cyclic monomers and industrial intermediates (such as D5).
In this study, we developed an efficient and straightforward method for thermal cracking of linear PDMS (both virgin and waste oils of various viscosities and functionalities) to a mixture of cyclosiloxanes: D3, D4 and D5, all desirable industrial monomers/raw materials. Potassium silanolate in combination with a polydentate complexing agent of K+, such as crown ether or polyethylene glycol dimethyl ether, was efficiently used as a catalyst for this reaction.
Results and discussion
Catalyst synthesis and corresponding characterisation
As mentioned above, alkaline hydroxides such as KOH, CsOH, etc. can be used as efficient catalysts for the depolymerisation of silanol-terminated (Si–OH) PDMS into mainly cyclic monomers.11,18,23 It is a relatively efficient method that suffers from the unavoidable introduction of water into the recycling process, lowering the overall efficiency and limiting the types of waste that this chemistry can recycle. Si–H containing waste, for instance, becomes a hazard in the presence of water and base. Moreover, the use of an alkaline hydroxide can lead to the demethylation of a dimethylsiloxane moiety ((CH3)2SiO), which subsequently leads to the formation of T (or even Q) moieties, which are “non-recyclable”, accumulating inside the reaction media.24 This detrimental demethylation reaction preferentially occurs at a terminal –SiMe2–OH moiety and further condensation reactions then promote other T and Q moieties (respectively tertiary Si with respect to the Si–O bonds and quaternary, when standard D moieties feature two Si–O bonds), when further demethylation proceeds. Terminal –Si–OH moieties are linked to the overall water content of the reaction medium as well as initial functionalities of the silicone waste materials to be recycled.24 Thus one should minimize the [OH] content to favour maximal yields of cyclic monomers.In order to solve this problem and increase the effectiveness of the thermal cracking process, a new “OH-free” catalyst system comprising potassium silanolate and a complexing agent (such as crown ether-18–6 and PEG derivatives), could prove already to be more efficient than the existing KOH-catalyzed cracking process. We hope to limit the side reactions, thus affording improved yields. We envisioned that the benefit could be threefold: (1) the ligand could dissociate the ion pair and improve the nucleophilicity of counter-anions and then enhance the kinetics of the depolymerisation reaction (or lower the required catalytic amount); (2) the [crown ether–potassium cation] could act as a mild Lewis acid to activate the Si–O–Si bond, resulting in the same effect (Fig. 1); (3) this complex could additionally limit the demethylation pathway, which involves the K+ cation with its key intermediate.24
 | ||
| Fig. 1 Postulated mechanism for polydentate ligand–silanolate-catalyzed chemical recycling of silicone oil. | ||
We reckoned that the more dissociated silanolate anions could behave as enhanced active species to effectively cleave the siloxane chains. The cyclosiloxanes would be released through more efficient back-biting reactions. We synthesized this new catalyst following a procedure described by Schultz et al.25 In the 1H-NMR spectrum (Fig. S1a†), one can easily determine the chemical shift of characteristic protons of the crown ether in the complex at 3.60 ppm, which is a similar chemical shift compared to that in the free crown ether (δ = 3.57 ppm). Moreover, a new signal ascribed to silanolate appears at −0.13 ppm that is different to those in HMDSO (hexamethyldisiloxane) or KOSiMe3 (δ = 0.07 ppm), indicating the formation of the complex [KOSiMe3–crown-ether-18-C-6]. In addition, the 29Si-NMR and 13C-NMR spectra of [KOSiMe3–crown-ether-18-C-6] show a singlet at −13.6 ppm and 4.8 ppm that are different from those displayed in HMDSO (δ = 7.6 ppm and 1.8 ppm) (Fig. S1b and c†), further evidencing the formation of the desired complex.
Depolymerization of silicone oil
| Entry | Catalyst (mol%) | Yield of cyclosiloxanesb (%) | Ratioc of D3/D4/D5/othersd | Mass balancee (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (10 mL, 120 mmol SiMe2-O unit), catalyst (0.1 or 4 mol%), solvent-free. b The yield of siloxanes was determined by the ratio between the mass of the distilled fraction and the mass of silicone oil. c The ratio of cyclic siloxanes was determined by the ratio of each compound in 29Si-NMR spectra. d Others = HMDSO + D6. e Mass balance was calculated by the ratio between the mass of residue and the distilled fraction and the mass of silicone oil. f A mixture of 9.5 g of silicone oil 1 and 1 g of cyclic siloxanes Dn was used, as described in US Patent 5670689. | ||||
| 1 | KOH (0.1) | 16 | 7/79/13/1 | 98 |
| 2 | KOSiMe3 (0.1) | 15 | 8/81/11/2 | 98 |
| 3f | KOH (4) | 16 | 7/78/13/2 | 98 |
| 4 | KOSiMe3 (0.1) + 18C6 (0.1) | 97 | 6/80/11/3 | 98 |
| 5 | 18C6 (0.1) | 0 | Nd | 100 |
| Entry | T (°C) | P (mbar) | Yield of cyclosiloxanesb (%) | Ratioc of D3/D4/D5/othersd | Mass balancee (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (10 mL, 120 mmol Si–O), KOSiMe3 (0.1 mol%), solvent-free. b The yield of siloxanes was determined by the ratio between the mass of the distilled fraction and the mass of silicone oil. c The ratio of cyclic siloxanes was determined by the ratio of each compound in 29Si-NMR spectra. d Others = HMDSO + D6. e Mass balance was calculated by the ratio between the mass of residue and the distilled fraction and the mass of silicone oil. | |||||
| 1 | 60 | 0.1 | 91 | 2/68/27/3 | 93 |
| 2 | 70 | 0.1 | 95 | 2/74/22/2 | 97 |
| 3 | 90 | 0.1 | 94 | 2/68/28/2 | 95 |
| 4 | 110 | 0.1 | 94 | 2/66/28/4 | 95 |
| 5 | 140 | 10 | 97 | 6/80/11/3 | 98 |
| 6 | 170 | 35 | 96 | 8/76/14/2 | 98 |
Next, a series of salts in combination with 18C6 were investigated for the depolymerisation of silicone oil 1. When potassium trimethylsilanolate and potassium dimethylvinyl silanolate were used, a yield of ∼96% of the cyclic siloxanes was observed with an excellent mass balance (98%), indicating that minimal volatile products (D3, HMDSO) were lost during the distillation process (traces on glassware and a vacuum trap, Table 3, entries 1 and 2). Similar results were also observed when KOH and t-BuOK were evaluated for this reaction, giving high yields of cyclosiloxanes at 95% and 97%, respectively (Table 3, entries 3 and 4). More importantly, this experiment shows a far superior yield compared to the use of KOH as a sole catalyst for the depolymerisation reaction/reactive distillation (Table 1, entry 1). Moreover, the composition of the siloxane mixture seems independent of the initially chosen counter-anions, suggesting that once the active chain end, or silanolate, is obtained, it drives the depolymerization. D4 is always the major component of the cyclic siloxane mixture, at approximately 80%, while D5, D3 and others often represent around 11%, 7% and 2%, respectively. Otherwise, potassium acetate or halogenated potassium salts K–X (X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) F, Cl, Br, I) were also tested under the same conditions (Table 3, entry 5). However, no reactivity was observed, due to the lack of nucleophilicity of the anions; thus these salts were unable to generate the required active silanolate.
F, Cl, Br, I) were also tested under the same conditions (Table 3, entry 5). However, no reactivity was observed, due to the lack of nucleophilicity of the anions; thus these salts were unable to generate the required active silanolate.
| Entry | Anion | Yield of cyclosiloxanesb (%) | Ratioc of D3/D4/D5/othersd | Mass balancee (%) |
|---|---|---|---|---|
| a Reaction conditions: 1 (10 mL, 120 mmol Si–O), catalyst (K–X, 0.1 mol%), solvent-free. b The yield of siloxanes was determined by the ratio between the mass of the distilled fraction and the mass of silicone oil. c The ratio of cyclic siloxanes was determined by the ratio of each compound in 29Si-NMR spectra. d Others = HMDSO + D6. e Mass balance was calculated by the ratio between the mass of residue and the distilled fraction and the mass of silicone oil. | ||||
| 1 | OSiMe3 | 97 | 6/80/11/3 | 98 |
| 2 | OSiMe2Vi | 96 | 8/83/8/1 | 98 |
| 3 | OH | 95 | 7/78/14/1 | 97 |
| 4 | O-tBu | 97 | 5/78/14/3 | 98 |
| 5 | OAc, F, Cl, Br, I | 0 | n.d. | n.d. |
Next, we turned our attention to the role of the cation. First, a series of silanolate salts were examined as the catalyst for the depolymerisation of PDMS (Scheme 1). If Li salts failed to display the reactivity (even with 12C4), Na salts provided <20% yields of cyclic monomers (with 15C5). We thus selected K and larger alkali metal cations (Rb, Cs) to maximize charge separation for the anion/cation pair and thus enhance the reactivity of the corresponding counter-anions.26 The potassium salts provided a good yield of the desired products (97%). Moreover, caesium silanolate and rubidium silanolate were also tested under standard conditions and the desired products were afforded at 95% yield. Additionally, a particular siloxane catalyst, oligomer-based potassium trimethylsilanolate, which is used as a catalyst for the industrial R.O.P. of cyclic siloxanes,4,7 was tested as the active species for a depolymerisation reaction, giving 92% yield of desired products. A series of alkaline hydroxides were also evaluated as standard catalysts for the base cracking of PDMS for a better comparison. KOH and CsOH also gave excellent yields of cyclic siloxanes. Finally, we selected a mixture of KOSiMe3 and 18C6 as the optimised catalyst for this reaction, combining the best yield and enhanced solubility without resorting to additional diluent (in particular, water).
Scope for depolymerisation of in-chain and chain-end functionalized silicone oils and even crosslinked elastomers
After optimising the conditions for thermal cracking of the linear PDMS (140 °C, 0.1 mol% KOSiMe3 + 18C6), we applied these conditions to the depolymerization of a series of silicone oils. Several trimethylsilyl-terminated polysiloxanes with different viscosities from 100 cSt, 350 cSt to 1000 cSt silicone oil 1, 2 (waste from a thermostatic-oil bath) and 3 were first investigated (Table 4, entries 1–3). Interestingly, all of these gave an excellent yield of cyclosiloxanes (95–97%), indicating that the molar masses of the linear PDMS do not affect the efficiency of the depolymerization method. More interestingly, depolymerization of waste oil 2 afforded similar chemical recycling yields as virgin oils. Moreover, silicone gum 4, which is an extremely high molar-mass polysiloxane (Mn = 0.5–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106) with very high viscosity, was also evaluated under optimised conditions but almost no cyclic products were obtained, due to the extremely high viscosity of the medium, which rendered stirring impossible during the depolymerisation process. To circumvent this issue, a mixture of silicone gum 4 and silicone oil 1 (mass ratio 1
106) with very high viscosity, was also evaluated under optimised conditions but almost no cyclic products were obtained, due to the extremely high viscosity of the medium, which rendered stirring impossible during the depolymerisation process. To circumvent this issue, a mixture of silicone gum 4 and silicone oil 1 (mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
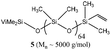
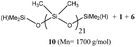
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was considered. Interestingly, a homogeneous mixture of manageable viscosity was obtained after 30 minutes of stirring (Table 4, entry 4). The depolymerisation then proceeded straightforwardly, giving 96% of cyclic siloxane monomers. D4 remains the major product, accounting for 80% of the cyclic monomer mixture. Then, polysiloxanes containing a vinyl moiety (5) were also investigated under optimised reaction conditions (Table 4, entry 5). Once again, a good yield (94%) of cyclosiloxanes was isolated after the distillation. Additionally, OH-terminated silicone oil (6) was tested under this set of conditions (Table 4, entry 6). Unlike the previous studies suggesting that Si–OH-terminated PDMS degraded faster than trimethylsilyl-ended PDMS under thermal/environmental decomposition,10,29 cyclic siloxanes were isolated in lower yield (75%). This lower efficiency could be explained by the reversible condensation reaction. At an elevated temperature (140 °C), silanol condensation is more favoured than the depolymerisation reaction.30 In order to solve this issue, silicone oil 1 (10–90% mass) was introduced to change the relative kinetics between back-biting and condensation reactions (Table 4, entry 7). Notably, the overall yield of cyclic siloxane was increased up to 98%. Next to those simple silicone oils, in-chain and chain-end functionalized silicone oils or even crosslinked silicone elastomers were also subjected to depolymerization under standard conditions (Table 4, entries 8–10). On the one hand, the reaction with vinylmethyl-siloxane-co-PDMS (7), phenylmethyl-siloxane-co-PDMS (8) and amino-pendent polysiloxanes (9) gave 93%, 79% and 68% yield of cyclosiloxanes, respectively. More interestingly, a small amount of Dvi4 – which is also a valuable monomer to recover – was observed (indicated by a singlet peak at −18.6 ppm) since copolymer 7 was used as a substrate for the depolymerisation reaction. On the other hand, up to 95% of cyclic products were afforded when a low Si–H content silicone oil, such as 11, or the part containing Si–H moieties of an RTV2 formulation was used as the model substrate (Table 4, entries 11 and 12). We suspect a prerequisite reaction of the terminal Si–H with Si–OH (combined use of oils 1 and 6 as diluents) is needed, while retaining manageable viscosity, and then conventional back-biting. Additionally, the recycling of RTV2 crosslinked elastomer itself (the product of platinum-catalysed crosslinking), which is much more difficult to reprocess than silicone oils, was tested under optimised conditions, providing 95% of the desired product, from the long linear PDMS chains in between the crosslinking knots (Table 4, entry 13). This is a big step forward in the quest for circularity of the silicone industry where waste elastomers are to be addressed. Finally, fluorosilicone oil 12 was depolymerized (Table 4, entry 14). Interestingly, 92% yield of fluorinated cyclosiloxanes was afforded. All of these results indicate a high robustness and chemo-compatibility of this catalyst for the depolymerisation of linear PDMS chains.
1) was considered. Interestingly, a homogeneous mixture of manageable viscosity was obtained after 30 minutes of stirring (Table 4, entry 4). The depolymerisation then proceeded straightforwardly, giving 96% of cyclic siloxane monomers. D4 remains the major product, accounting for 80% of the cyclic monomer mixture. Then, polysiloxanes containing a vinyl moiety (5) were also investigated under optimised reaction conditions (Table 4, entry 5). Once again, a good yield (94%) of cyclosiloxanes was isolated after the distillation. Additionally, OH-terminated silicone oil (6) was tested under this set of conditions (Table 4, entry 6). Unlike the previous studies suggesting that Si–OH-terminated PDMS degraded faster than trimethylsilyl-ended PDMS under thermal/environmental decomposition,10,29 cyclic siloxanes were isolated in lower yield (75%). This lower efficiency could be explained by the reversible condensation reaction. At an elevated temperature (140 °C), silanol condensation is more favoured than the depolymerisation reaction.30 In order to solve this issue, silicone oil 1 (10–90% mass) was introduced to change the relative kinetics between back-biting and condensation reactions (Table 4, entry 7). Notably, the overall yield of cyclic siloxane was increased up to 98%. Next to those simple silicone oils, in-chain and chain-end functionalized silicone oils or even crosslinked silicone elastomers were also subjected to depolymerization under standard conditions (Table 4, entries 8–10). On the one hand, the reaction with vinylmethyl-siloxane-co-PDMS (7), phenylmethyl-siloxane-co-PDMS (8) and amino-pendent polysiloxanes (9) gave 93%, 79% and 68% yield of cyclosiloxanes, respectively. More interestingly, a small amount of Dvi4 – which is also a valuable monomer to recover – was observed (indicated by a singlet peak at −18.6 ppm) since copolymer 7 was used as a substrate for the depolymerisation reaction. On the other hand, up to 95% of cyclic products were afforded when a low Si–H content silicone oil, such as 11, or the part containing Si–H moieties of an RTV2 formulation was used as the model substrate (Table 4, entries 11 and 12). We suspect a prerequisite reaction of the terminal Si–H with Si–OH (combined use of oils 1 and 6 as diluents) is needed, while retaining manageable viscosity, and then conventional back-biting. Additionally, the recycling of RTV2 crosslinked elastomer itself (the product of platinum-catalysed crosslinking), which is much more difficult to reprocess than silicone oils, was tested under optimised conditions, providing 95% of the desired product, from the long linear PDMS chains in between the crosslinking knots (Table 4, entry 13). This is a big step forward in the quest for circularity of the silicone industry where waste elastomers are to be addressed. Finally, fluorosilicone oil 12 was depolymerized (Table 4, entry 14). Interestingly, 92% yield of fluorinated cyclosiloxanes was afforded. All of these results indicate a high robustness and chemo-compatibility of this catalyst for the depolymerisation of linear PDMS chains.
| Entry | Substrate | Yield of cyclosiloxanesb (%) | Ratioc of D3/D4/D5/othersd |
|---|---|---|---|
a Reaction conditions: silicone oil (10 mL, 120 mmol Si–O), catalyst (KOSiMe3 + 18C6) (0.1 mol%), solvent-free, 140 °C, 10 mbar.
b The yield of siloxanes was determined by the ratio between the mass of the distilled fraction and the mass of silicone oil.
c The ratio of cyclic siloxanes was determined by the ratio of each compound in 29Si-NMR spectra.
d Others = HMDSO + D6.
e A mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of silicone gum and silicone oil was used.
f A mixture of 6 and 1 at a mass ratio of 1 1 of silicone gum and silicone oil was used.
f A mixture of 6 and 1 at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used.
g A mixture of 8 and 1 at a mass ratio of 1 1 was used.
g A mixture of 8 and 1 at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used.
h A mixture of 10 (6 g), 1 (3 g) and 6 (3 g) at a mass ratio of 2 1 was used.
h A mixture of 10 (6 g), 1 (3 g) and 6 (3 g) at a mass ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used.
i RTV2 formulation is a mixture containing 94% vinyl-terminated silicone oil (with viscosity around 40 1 was used.
i RTV2 formulation is a mixture containing 94% vinyl-terminated silicone oil (with viscosity around 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cSt) and 6% of short-chain Si–H oil.
j A mixture of crosslinked gel RTV2 (3 g) and 1 (9 g) was used.
k Distillation conditions: 160 °C, 0.1 mbar.
l The main products obtained are D4F and D5F.
m Traces of Dvi4 were observed at −18.6 ppm by 29Si NMR spectroscopy (see ESI, Fig. S14†). 000 cSt) and 6% of short-chain Si–H oil.
j A mixture of crosslinked gel RTV2 (3 g) and 1 (9 g) was used.
k Distillation conditions: 160 °C, 0.1 mbar.
l The main products obtained are D4F and D5F.
m Traces of Dvi4 were observed at −18.6 ppm by 29Si NMR spectroscopy (see ESI, Fig. S14†).
|
|||
| 1 |

|
97 | 6/80/11/3 |
| 2 |

|
97 | 7/81/11/1 |
| 3 |

|
95 | 9/80/10/1 |
| 4e |

|
96 | 7/78/13/1 |
| 5 |
|
94 | 10/74/13/3 |
| 6 |

|
75 | 10/82/8/0 |
| 7f |

|
98 | 4/82/14/0 |
| 8 |

|
93 | 1/73/22/4m |
| 9g |

|
79 | 8/77/13/2 |
| 10 |

|
68 | 7/78/14/1 |
| 11h |
|
95 | 13/79/8/0 |
| 12i | The part containing Si–H moieties (6% mol) of an RTV2 formulation (11) | 96 | 10/80/10/0 |
| 13j | Crosslinked gel RTV2 + 1 | 95 | 8/79/11/2 |
| 14k |

|
92l | n.d. |
Depolymerisation of industrial silicone waste
An actual supply of high volume industrial silicone waste was provided by Elkem Silicones©. It comprised poly(dimethysiloxanes) with a viscosity of 100 cSt, containing mainly the D moiety with a variety of impurities, such as Si–H, H2O, HCl, AlCl3, etc. First, we studied the depolymerisation of this industrial waste under our standard reaction conditions (150 °C, 5 mbar) in the presence of a low amount of the catalytic system (0.1 mol%) (Table 5, entry 1). Unfortunately, no cyclic siloxanes were obtained after a certain time of distillation. Moreover, the desired products (Dn) could only be obtained in 10% yield when the catalyst loading was increased to 0.5 mol% (Table 5, entry 2). However, the rate of distillation was very slow and a viscous gel residue was afforded after the distillation was completed. This phenomenon could be explained by the formation of T moieties through dehydrogeno-condensation reactions between Si–H and Si–OH (arising from the former, in the presence of H2O) in the presence of a basic catalytic system. In order to avoid the formation of these detrimental T moieties, which contribute to an unmanageable viscosity, either a diluent is required to reduce the viscosity of the reaction medium or improved kinetics has to be targeted in order to favour cyclisation over the formation of the T moiety. First, the reaction was performed in the presence of KOSiMe3 (0.5 mol%) and PEG-DME 500 (2 mol%), which were used as a ligand and a diluent for the depolymerisation reaction. Interestingly, the yield of cyclic siloxanes increased to 75% (Table 5, entry 3). Second, other high-boiling-point diluents (with low mass ratio) have been used to facilitate the depolymerisation and the best result was obtained in the presence of n-octadecanol (10 wt%), giving 75% yield of cyclosiloxanes (ESI, Table S1† and Table 5, entry 4). It is likely that the preferential T moieties formed from the condensation of this long-chain alcohol and the residual Si–H moieties yield manageable viscosities. Further study at 170 °C and 5 mbar provided a better 84% overall yield (Table 5, entry 5), in which D4 mainly accounted for 80% of this mixture. This result is very promising for conversion of industrial waste back to valuable monomer products.| Entry | Catalyst (mol%) | Diluent | Yield of cyclosiloxanesb (%) | Ratioc of D3/D4/D5/othersd |
|---|---|---|---|---|
| a Reaction conditions: industrial silicone waste (10 mL, 9.5 g), catalyst (xx mol%), and diluent (10 wt%, if necessary). b The yield of siloxanes was determined by the ratio between the mass of the distilled fraction and the mass of silicone oil. c The ratio of cyclic siloxanes was determined by the ratio of each compound in 29Si-NMR spectra. d Others = HMDSO + D6. e The distillation conditions were 170 °C, 5 mbar. | ||||
| 1 | KOSiMe3 (0.1) 18C6 (0.1) | –– | 0 | n.d. |
| 2 | KOSiMe3 (0.5) 18C6 (0.5) | –– | 10 | 19/78/2/1 |
| 3 | KOSiMe3 (0.5) PEG-DME 500 (2) | –– | 75 | 13/79/8/0 |
| 4 | KOSiMe3 (0.5) 18C6 (0.5) | C18H37OH (10 wt%) | 75 | 7/80/12/1 |
| 5e | KOSiMe3 (0.5) 18C6 (0.5) | C18H37OH (10 wt%) | 84 | 8/80/11/1 |
Recyclability of the catalyst
The recyclability of the catalyst has been studied using our optimised conditions (140 °C, 1 hour and then distilled under a pressure of 10 mbar) (Fig. 2). The first run was performed using KOSiMe3 + 18C6 (0.1 mol%), and an excellent yield (96%) of a mixture of cyclosiloxanes was obtained. This result is very similar to the one previously obtained (Table 5, entry 1), indicating the reproducibility of this method. After removing the volatile products, the “catalyst” remains as a residue within a small amount of the PDMS (to avoid the thermal decomposition of the catalyst). It is very likely that the catalyst is an altered version of the original silanolate: after each back-biting reaction, a new silanolate is generated, thus regenerating the active species (Fig. 1). Then, the flask with catalyst was refilled with silicone oil and was stirred continuously at 140 °C for 1 hour before being distilled again under a reduced pressure. Interestingly, no reactivity was lost over the next 4 runs, showing the recyclability of the catalyst (or at least of the active silanolate species) under our reaction conditions. Moreover, a 29Si-NMR spectrum of the residue after 5 runs was also analysed and only the signal of the remaining polysiloxanes (or oligosiloxanes) was detected (ESI, Fig. S3†).Depolymerization–repolymerization coupling using a single catalyst (KOSiMe3 + 18C6)
After optimising the reaction conditions for the depolymerisation of silicone oils, we carried out the scale-up of this reaction using waste silicone oil 2 (100 g) as a standard substrate (Scheme 3). First, waste silicone oil 2 that was used over a several-year period in the laboratory for heating a thermostatic bath, was introduced into a flask in the presence of KOSiMe3 (0.1 mol%) and 18C6 (0.1 mol%). This mixture was heated to 140 °C for 1 hour. Then, the volatile product was continuously distilled off (140 °C, 10 mbar). After 3 hours, 99 g of the siloxanes mixture (D3, D4, D5, others) was collected, highlighting the high efficiency of our catalytic system. Inside this mixture, D4 accounts for 82%, whereas D3 and D5 account for 7% and 8%, respectively.With this mixture in hand, we then performed the ring-opening polymerization of cyclosiloxanes (D3, D4, and D5) following a modified procedure described by Fleury et al. (Scheme 3).7 The obtained cyclic siloxanes (99 g) with HMDSO (0.6 g) were introduced into the flask and heated to 160 °C. Then, KOSiMe3 (50 ppm) and 18C6 (4 ppm) were added to this solution at this temperature and the reaction mixture was kept at 160 °C for 3 hours. After that, the reaction medium was cooled down to room temperature and a neutralizing agent was introduced. Then, the resulting liquid was evaporated under reduced pressure (160 °C, 1 mbar, 5 hours) to give a colourless silicone oil (87 g, 87% yield). The measurement of the level of volatile cyclic siloxanes was thus assessed at between 12.6 and 13.5%, which suggests that the thermodynamic equilibrium had been reached. The resulting silicone oil was characterised by SEC (toluene) analysis (Mn = 8000 g mol−1, D ∼ 2.0; see Fig. S27 and S28, ESI†) as well as 1H and 29Si-NMR analyses, indicating the effectiveness of the depolymerisation–repolymerization strategy using a “single-catalyst” (see Fig. S23–S25, ESI†).
Conclusion
We have developed a new protocol for the depolymerisation of silicone oil using a mixture of KOSiMe3 and an adequate polydentate complexing agent (such as 18C6 or the cheaper PEG500–DME) as the original catalytic species. The reaction was performed over a wide range of temperatures (60–170 °C). After testing, 140 °C was selected as the optimal temperature to achieve an efficient compromise of fast kinetics for reactive distillation without overheating. A low loading of the catalyst (typically 0.1 mol%) provides an excellent yield of cyclosiloxanes (up to 99%), with excellent purity, and the strategy is extremely robust and tolerant to a wide range of functionalities. Additionally, the catalyst could be recycled over 5 runs without losing any reactivity. Moreover, the reaction was scaled up (100 g) and the concept of depolymerisation–repolymerization using a “single” catalyst was established, indicating the potential of this process for industrial application towards circularity for silicones.Experimental section
General procedure for the depolymerisation of siloxane oil 1 (10 g scale)
Into a flask (50 mL) connected with a short Vigreux column, siloxane oil (9.5 g, 100 mmol of siloxane units), KOSiMe3 (14 mg, 0.1 mol%) and 18C6 (30 mg, 0.1 mol%) were introduced and then this mixture was heated at 140 °C for 1 hour. Then, the reaction mixture was distilled under reduced pressure (140 °C, 10 mbar) to give a mixture of cyclosiloxanes D3, D4 and D5 (9.2 g, 97% yield) as a colourless liquid.General procedure for the depolymerisation of siloxane oil 1 (100 g scale)
Into a flask (250 mL) connected with a long Vigreux column, siloxane oil (100 g, 1 mol of siloxane units), KOSiMe3 (142 mg, 0.1 mol%) and 18C6 (300 mg, 0.1 mol%) were introduced and then this mixture was heated at 140 °C for 1 hour. Then, the reaction mixture was distilled under reduced pressure (140 °C, 10 mbar) to give a mixture of cyclosiloxanes D3, D4 and D5 (99 g, 99% yield) as a colourless liquid.General procedure for the polymerisation of an oil mixture of cyclic siloxanes (100 g scale)
An obtained mixture of siloxanes from the depolymerisation of silicone oil 1 (99 g) and HMDSO (0.6 g) was introduced into a three-neck round bottom flask and then this mixture was heated to 160 °C. At this temperature, KOSiMe3 (50 ppm) and 18C6 (4 ppm) were added and the polymerisation experiment was performed at 160 °C for 3 hours. Then, the reaction was cooled down to room temperature and a neutralizing solution containing phosphoric acid was introduced. Finally, the resulting mixture was evaporated under reduced pressure (170 °C, 1 mbar) for 3 hours to give a silicone oil (87 g, 87% yield) as a colourless liquid.Author contributions
All of the experiments were conducted by N. D. V. All the authors contributed to the preparation of this manuscript. Conceptualization: J. R. and V. M. Resources: A. B.-M. and N. D. Writing – original draft, review and editing: N. D. V., J. R., and V. M.Conflicts of interest
N. D. V., A. B.-M., N. D., J. R. and V. M. are also the inventors of a patent application not yet published.Acknowledgements
The authors gratefully acknowledge the financial support from BPI France and Région Auvergne-Rhône-Alpes (project PSPC Régions REPOS). The authors would like to thank Dr Winnie Raynaud (INSA-Lyon) for her help for the characterization of repolymerized silicone oil (SEC analysis and volatile content). The authors are also grateful to Dr Louis Vovelle and Joséphine Munsch for stimulating discussions on circular economy and particularly on the circularity of silicones.References
- H.-H. Moretto, M. Schulze and G. Wagner, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000, 33, 9–12 Search PubMed.
- J. E. Mark, Acc. Chem. Res., 2004, 37, 946–953 CrossRef CAS PubMed.
- Global Silicones Market (2021 to 2026) – Industry Trends, Share, Size, Growth, Opportunity and Forecasts, https://www.globenewswire.com/news-release/2021/06/02/2240181/28124/en/Global-Silicones-Market-2021-to-2026-Industry-Trends-Share-Size-Growth-Opportunity-and-Forecasts.html.
- T. Köhler, A. Gutacker and E. Mejía, Org. Chem. Front., 2020, 7, 4108–4120 RSC.
- E. G. Rochow, J. Am. Chem. Soc., 1945, 67, 963–965 CrossRef CAS.
- E. G. Rochow, US Pat., US2380995A, 1941.
- E. Fleury, J.-M. Mas and K. Ramdani, US Pat., US7776988, 2010.
- M. Takla, N. E. Kamfjord, H. Tveit and S. Kjelstrup, Energy, 2013, 58, 138–146 CrossRef CAS.
- G. Saevarsdottir, T. Magnusson and H. Kvande, J. Sustain. Metall., 2021, 7, 848–857 CrossRef.
- B. Rupasinghe and J. C. Furgal, Polym. Int., 2022, 71, 521–531, DOI:10.1002/pi.6340.
- C. Allandrieu and D. Cardinaud, US Pat, US5670689, 1997 Search PubMed.
- See more details in Enthaler's work: (a) S. Enthaler, Angew. Chem., Int. Ed., 2014, 53, 2716–2721 CrossRef CAS PubMed; (b) S. Enthaler, J. Appl. Polym. Sci., 2015, 132, 41287 CrossRef; (c) P. Döhlert, J. Pfrommer and S. Enthaler, ACS Sustainable Chem. Eng., 2015, 3(1), 163–169 CrossRef.
- N. Okui and J. H. Magill, Polymer, 1977, 18, 845–850 CrossRef CAS.
- M. A. Brook, Z. Shigui, L. Lihua and C. Yang, Can. J. Chem., 2012, 90, 153–160 CrossRef CAS.
- W. Patnode and D. F. Wilcock, Methylpolysiloxanes, J. Am. Chem. Soc., 1946, 68, 358–363 CrossRef CAS.
- W. Noll, Chemistry and Technology of Silicone Oligomers, 1968 Search PubMed.
- R. Friebe, W. Weber and K.-H. Sockel, US Pat, US6001888, 1999 Search PubMed.
- T. W. Greenlee, US Pat, US5110972, 1992 Search PubMed.
- M. Okamoto, S. Suzuki and E. Suzuki, Appl. Catal., A, 2004, 261, 239–245 CrossRef CAS.
- R. Petrus, J. Utko, R. Gniłka, M. G. Fleszar, T. Lis and P. Sobota, Macromolecules, 2021, 54(5), 2449–2465 CrossRef CAS.
- R. F. Weitkamp, B. Neumann, H.-G. Stammler and B. Hoge, Angew. Chem., Int. Ed., 2020, 59, 5494–5499 CrossRef CAS PubMed.
- R. F. Weitkamp, B. Neumann, H.-G. Stammler and B. Hoge, Chem. – Eur. J., 2021, 27, 915–920 CrossRef CAS PubMed.
- A. Oku, W. Huang and Y. Ikeda, Polymer, 2002, 43, 7289–7293 CrossRef CAS.
- For more details on demethylation of silanol-terminated polydimethylsiloxanes, see: (a) D. Yun-qiao, L. Hai-feng, M. Qiu-hong and L. Xiao, Polym. Degrad. Stab., 2020, 182, 109367 CrossRef; (b) T. H. Thomas and T. C. Kendrick, J. Polym. Sci., Part A, 1970, 2(8), 1823–1830 CrossRef; (c) K. Chenoweth, S. Cheung, A. C. T. van Duin, W. A. Goddard III and E. M. Kober, J. Am. Chem. Soc., 2005, 127, 7192–7202 CrossRef CAS PubMed.
- K. Bläsing, R. Labbow, D. Michalik, F. Reiß, A. Schulz, A. Villinger and S. Walker, Chem. – Eur. J., 2020, 26, 1640–1652 CrossRef PubMed.
- K. Bürglová and J. Hlaváč, Synthesis, 2018, 1199–1208 Search PubMed.
- E. Weber, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2007, 10, 471–480 Search PubMed.
- Y. Hu, J. Steinbauer, V. Stefanow, A. Spannenberg and T. Werner, ACS Sustainable Chem. Eng., 2019, 7, 13257–13269 CrossRef CAS.
- (a) S. Xu, R. G. Lehmann, J. R. Miller and G. Chandra, Environ. Sci. Technol., 1998, 32, 1199–1206 CrossRef CAS; (b) M. Cypryk and Y. Apeloig, Organometallics, 2002, 21, 2165–2175 CrossRef CAS.
- M. E. Belowich, J. M. Roberts, T. H. Peterson, E. Bellinger, K. Syverud and T. Sidle, Macromolecules, 2020, 53, 7487–7495 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc00293d |
| This journal is © The Royal Society of Chemistry 2023 |









