Open Access Article
Open Access ArticleCellulose processing in ionic liquids from a materials science perspective: turning a versatile biopolymer into the cornerstone of our sustainable future
László
Szabó
 *ab,
Romain
Milotskyi
*ab,
Romain
Milotskyi
 c,
Gyanendra
Sharma
c,
Gyanendra
Sharma
 c and
Kenji
Takahashi
c and
Kenji
Takahashi
 *c
*c
aForestry and Forest Products Research Institute, 1 Matsunosato, Tsukuba, Ibaraki 305-8687, Japan. E-mail: szabo@affrc.go.jp
bInternational Center for Young Scientists (ICYS), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba 305-0044, Japan
cInstitute of Science and Engineering, Kanazawa University, Kakuma-machi, Kanazawa 920-1192, Japan. E-mail: ktkenji@staff.kanazawa-u.ac.jp
First published on 26th June 2023
Abstract
Shaping cellulose into functional materials entered a new era with the introduction of ionic liquids as novel, green solvents about 20 years ago. As non-volatile solvents with high thermal and chemical stability, ionic liquids can provide an environmentally more benign tunable platform for cellulose processing, compared to existing technologies. The past decades have seen fruitful efforts devoted to the development of materials based on ionic liquid/cellulose processing systems. In this review we discuss the experiences gained, and highlight the emerging applications. In particular, coatings and thin film applications for structural materials (e.g., for packaging), thin film filtration membranes, immobilisation of enzymes, and catalytically active nanoparticles, separator membranes and conductive composites for energy storage and other electronics applications, cellulose/biopolymer green biocomposites, cellulose-based ionogels, hydrogels and aerogels, and cellulose-based or composite fibres will be discussed in detail. We will also take a critical look at the perspectives of this field. The use of a certain grade of technical cellulose, and the purity of the prepared materials should be more carefully justified in the future, as they have an influence not only on the properties of the fabricated material, but also on the economics of the process. Furthermore, to make ionic liquids truly green solvents, and competitive alternatives to existing technologies, more studies are needed on recyclability after material fabrication, and on ways to minimise the energy consumption of such processes, among other issues.
1. Introduction
Cellulose has been with us in various forms since ancient times, as it is the most abundant biopolymer available on Earth. Materials made from wood, cotton and cellulose fibers have shaped our way of surviving, thriving and living (to generate heat and energy, to construct buildings and tools); and determined our cultural and societal development (e.g., think about the use of paper, clothing/fashion). In fact, thinking of the modern age – the first thermoplastic polymer ever made was also derived from cellulose (called celluloid, a nitrocellulose-based plastic containing camphor). Unfortunately, the momentum that cellulose once had in our life has been greatly suppressed by the emerging synthetic polymer industry utilising fossil fuels. Our society became heavily dependent on these finite resources, and our anthropogenic impact grew to its extremities. This brought about irreversible changes on Earth, such as environmental pollution by microplastics, and climate change among other unusual ecological phenomena. While many may think that humanity is already doomed to fail, we argue that it is still not too late to change our way of living: this means turning away from fossil-fuel centred life and shifting to a material-based society centred around biobased solutions. In this regard, cellulose may come again to our rescue, as it shaped our history time and time again.Material scientists have long been interested in shaping cellulose into functional materials. The processing and shaping of cellulose entered a new era with the introduction of ionic liquids as novel solvents, first reported in the work of Swatloski et al.1 20 years ago. Ionic liquids (IL) are a type of molten salts, defined here as those with melting point below 100 °C.2 They are extensively recognised as green solvents mainly due to their non-volatile nature together with thermal and chemical stability.3 These solvents gained popularity partly due to their exceptional solvating power and unprecedented tunability, as a number of cation–anion combinations exist leading to various physicochemical properties. The past two decades have seen a number of valuable works devoted to ionic liquid/cellulose processing systems to develop materials for a variety of applications. Nevertheless, there has been no systematic review published so far that would discuss material fabrication and structure in detail with a clear focus on applications. Therefore, it is time to summarise the experiences gained and highlight valuable discoveries in this field. Our aim is to discuss the emerging applications of cellulose-based advanced materials fabricated using ionic liquids from a materials science perspective, in order to give guidance in this field, and stimulate more research on these green cellulose processing systems. We really hope that this review will be helpful also for those interested in materials, but have not yet necessarily worked with ionic liquids before, to promote this topic among a wider readership and thereby facilitate industrial implementations.
2. Solid-state structure of cellulose
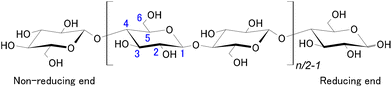
Cellulose is a linear-chain polymer composed of repeating β-D-glucopyranose units linked through glycosidic bonds connecting the C4 and C1 carbon atoms of neighbouring groups (Fig. 1). Due to these β-1,4 linkages, adjacent anhydroglucose (AGU) units are rotated 180° in plane along the chain, preferring a 4C1 chair conformation. Two rings connected through β-1,4 linkages give the repeating disaccharide cellobiose units. The number of glucopyranose units is defined as the degree of polymerisation (DP), which is used to describe the chain length of cellulose that can vary depending on the source and extraction/treatment method.There are three –OH groups per glucopyranose units, which partly determines the reactivity of the polymer. These free hydroxyl groups provide exceptional tunability to the molecule, a wide variety of cellulose derivatives have been synthesised with various properties.4 The amount of substituents on the hydroxyl groups is described with the degree of substitution (DS), which takes a value of 3 for a fully substituted derivative (all hydroxyl groups are occupied). Chain degradation can take place under acidic or enzymatic hydrolysis of the glycosidic bonds, which can eventually lead to cellulose decomposition into monomeric sugars.5 In addition, one end of the polymer chain is terminated with a monomeric unit that has the original C1–OH group. This part of the molecule is in equilibrium with the aldehyde form, a phenomenon known as mutarotation, and therefore, it is called the reducing end. Although reactions targeting the reducing end has been known for quite long,6end-wise modification has recently gained interest for nanocelluloses that can enable novel routes to their directed assembly.7
Cellulose is the most abundant optically active natural macromolecule, the stereoregular sequences in its structure enables chiral recognition. Several cellulose derivatives have found success in enantiomeric separation, for example as chiral separation phases for gas and liquid chromatography.8
The hydroxyl groups form complex hydrogen bonding network, determining the supramolecular arrangement of cellulose chains with various identified polymorphs and amorphous domains in the solid state. Cellulose polymorphs are grouped into 4 types: cellulose I (there are two subtypes which are present concomitantly: Iα and Iβ) which can be found in native cellulose, and thermodynamically more favoured cellulose II, III and IV crystal structures can be obtained irreversibly under certain conditions (Fig. 2A). Cellulose II allomorph is the most stable, and it is also the most important for materials science. It forms from cellulose I after mercerisation (treatment with aqueous NaOH), or after dissolving native cellulose followed by precipitation in an antisolvent – a process often called regeneration. The latter method is widely used to shape cellulose into various materials, such as regenerated films, fibres and numerous composites. As notable difference, in the cellulose I allomorph the chains are oriented parallel in the unit cell, while cellulose II has antiparallel chain polarity.9,10 Furthermore, while for cellulose I the hydroxymethyl groups (–CH2OH) adopt a trans–gauche (tg) conformation (Fig. 2B), they have a gauche–trans (gt) conformation in cellulose II.11,12 The hydrogen bonding network also differs in these crystal structures. It was reported that the intramolecular hydrogen bonds in cellulose Iβ are well defined, while the intermolecular hydrogen bonds connecting sheets are rather disorganised.11 In cellulose II a more ordered, single hydrogen bonding system exists that forms a three-dimensional network.12 In contrast, cellulose III is obtained by treatment of cellulose I or II with liquid ammonia. Cellulose III prepared from cellulose I is referred to as cellulose IIII in contrast to the allomorph cellulose IIIII prepared from cellulose II. The detailed structural differences between cellulose IIII and cellulose IIIII have not been determined.13 It was reported that cellulose IV is formed by heat treatment of both cellulose IIII and IIIII in glycerol,14 although some studies debate the existence of this allomorph.10Fig. 3 highlights a schematic representation of the allomorphs of cellulose. It was previously reported that cellulose III exhibits enhanced hydrolysis by cellobiohydrolase I, producing cellobiose at rates more than five times higher than from cellulose I.15 It was also shown that the surface hydrophilicity/hydrophobicity of the regenerated cellulose films can be controlled by post-treatment with liquid ammonia, or hot glycerol, due to the inherent structural anisotropy of the AGU. These results were supported by contact angle measurements as well.16
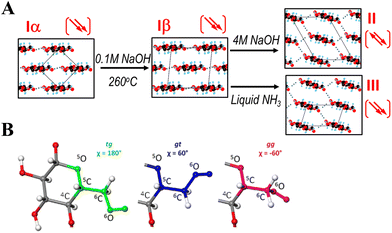 | ||
| Fig. 2 (A) Formation of polymorphs under certain conditions. Arrows indicate the chain polarity, which is antiparallel for cellulose II. Chain orientation is shown on the a–b plane of the unit cell. (B) Possible conformations of the hydroxymethyl groups (–CH2–OH), tg: trans–gauche, gt: gauche–trans, gg: gauche–gauche. χ indicates angles between the C5–O5 and C6–O6 bonds. Reproduced from ref. 10 with permission from Springer Nature, copyright 2019. | ||
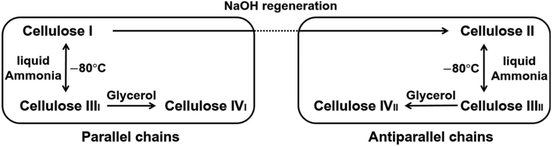 | ||
| Fig. 3 Phase transition between various crystalline allomorphs of cellulose (cellulose I, II, III, and IV). Reproduced from ref. 17, copyright 2021 The Authors. | ||
Cellulose can be available as raw material from numerous sources, with varying degree of polymerisation and crystallinity. Plant-based cellulose in its purest form can be found in cotton fibres (nearly 90%), this type of cellulose has high degree of polymerisation (DP) (plant fibres have DP values about 800–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 depending on the treatment) and crystallinity (crystallinity index18(CI) ≈ 50–80%).19,20 Cellulose can be produced as an exopolysaccharide in very pure form by some bacteria. This type of cellulose is called bacterial cellulose or microbial cellulose, it can have very high degree of polymerisation (DP > 10
000 depending on the treatment) and crystallinity (crystallinity index18(CI) ≈ 50–80%).19,20 Cellulose can be produced as an exopolysaccharide in very pure form by some bacteria. This type of cellulose is called bacterial cellulose or microbial cellulose, it can have very high degree of polymerisation (DP > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and crystallinity (with CI > 80%).21,22 Wood has about 40–50% cellulose content. After removing lignins and the hemicelluloses via chemical or mechanical means (pulping), wood pulp can be obtained. A specialty pulp called dissolving pulp is produced after chemical refining (e.g., sulphite pulping or kraft pulping processes) and bleaching.23 Dissolving pulp is characterised by high cellulose content (>90%) and a relatively uniform molecular weight distribution. It is an important form of technical cellulose used commercially in large quantities to prepare cellulose derivatives, regenerated cellulose and other materials. By acidic hydrolysis, microcrystalline cellulose can be produced from dissolving pulp, this material has relatively low molecular weight (DP 25–300), and gained interest for numerous applications (e.g., pharmaceutical, food industry, etc.).
000) and crystallinity (with CI > 80%).21,22 Wood has about 40–50% cellulose content. After removing lignins and the hemicelluloses via chemical or mechanical means (pulping), wood pulp can be obtained. A specialty pulp called dissolving pulp is produced after chemical refining (e.g., sulphite pulping or kraft pulping processes) and bleaching.23 Dissolving pulp is characterised by high cellulose content (>90%) and a relatively uniform molecular weight distribution. It is an important form of technical cellulose used commercially in large quantities to prepare cellulose derivatives, regenerated cellulose and other materials. By acidic hydrolysis, microcrystalline cellulose can be produced from dissolving pulp, this material has relatively low molecular weight (DP 25–300), and gained interest for numerous applications (e.g., pharmaceutical, food industry, etc.).
Another intriguing class of cellulose materials can be isolated after disintegrating the cellulose microfibrils into nanosized domains via chemical, mechanical or enzymatic means. The obtained cellulose nanocrystals (CNCs) or nanofibers (CNFs) have found great scientific and industrial interest. Ionic liquids can be also used for the fabrication of these materials from cellulose microfibrils. This topic has been summarised in a recent work,24 and is excluded from this review.
This review will focus on material fabrication processes that are mostly based on the dissolution of cellulose in ionic liquids, except some special cases (ionogels based on gel-forming cellulose derivatives, bacterial cellulose and nanocellulose in combination with non-dissolving ionic liquids, see section 4.7.1).
3. Dissolution of cellulose in ionic liquids
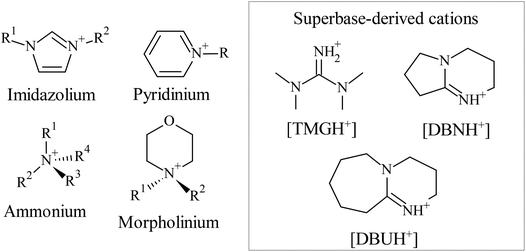
On account of its high molecular weight, and the existence of strong intra- and intermolecular hydrogen bonding network with highly ordered crystalline domains, cellulose has low solubility in common single-component solvents, and is not melt processable. These features posed considerable obstacles for materials scientists aimed at shaping cellulose into functional structures. For long time, heterogeneous systems have been applied to chemically modify cellulose without complete solubilisation,4 in order to prepare readily soluble and melt-processable derivatives (such as thermoplastic cellulose esters soluble in a range of organic solvents). There have been great efforts towards developing solvent systems able to directly dissolve cellulose, facilitating its direct shaping into various materials (fibres, films, composites, etc.), and enabling greener and controllable homogeneous chemical modification. Several aqueous (aqueous inorganic metal complexes, such as cuprammonium hydroxide; molten inorganic salt hydrates, such as LiCl·5H2O; aqueous solution of alkali hydroxides, such as NaOH/urea/H2O; N-methylmorpholine N-oxide (NMMO) monohydrate), non-aqueous (e.g., N,N-dimethylacetamide (DMA)/LiCl; dimethyl sulfoxide (DMSO)/tetrabutylammonium fluoride (TBAF)), and derivatising (e.g., the viscose process through cellulose xanthate; N,N-dimethylformamide (DMF) or DMSO with N2O4) solvent systems have been studied. These solvent systems all have some demerits (e.g., toxicity/non-recyclability/instability), which have been extensively recognised and critically evaluated in the literature.4,25,26 The introduction of ionic liquids as direct, tunable solvents for cellulose opened new horizons in this field, leading to exciting material discoveries and properties that have not been seen before (e.g., see section 4.7.1).The first group of ionic liquids to dissolve cellulose with melting points below 100 °C were based on dialkylimidazolium cations (see Fig. 4) in combination with chloride, bromide and thiocyanate anions.1 Among the studied ionic liquids, 1-butyl-3-methylimidazolium chloride ([Bmim][Cl]) was the most powerful solvent able to dissolve cellulose at 10 wt% concentration when heated to 100 °C, and up to 25 wt% with the help of microwave heating. Furthermore, 1-allyl-3-methylimidazolium chloride ([Amim][Cl]) and 1-ethyl-3-methylimidazolium acetate ([Emim][Ac]) were also reported to dissolve cellulose even at high loading around 100 °C (up to 30 wt%).27–29 These ionic liquids have lower melting points and lower viscosities after cellulose dissolution, making them more attractive for industrial application compared to [Bmim][Cl]. Following these initial reports, the effect of the structure of the anion and cation on cellulose solubility was comprehensively studied.30 It appeared that ionic liquids with strong dissolution power contain anions with strong hydrogen bond basicity, such as chloride,1 carboxylate (e.g., formate, acetate, lactate),31–35 amino acid,36 phosphate or phosphonate anions.37 Likewise, ionic liquids containing anions with poor ability to form hydrogen bonds with the hydroxyl groups of cellulose can be classified as non-solvents, anions of hexafluorophosphate ([PF6]), tetrafluoroborate ([BF4]), dicyanamide ([N(CN)2]) and bis(trifluoromethylsulfonyl)imide ([NTf2]) are some of the examples. Some non-solvents of cellulose containing [MeSO4] and [HSO4] anions can dissolve hemicellulose and lignin, these ionic liquids gained appreciable interest for the pretreatment of lignocellulosic biomass.38–40 Effect of the structure of imidazolium cations on the solubility of cellulose was also addressed in several works.41,42 Long alkyl chain substituents, and moieties that increase the hydrogen bond acidity, have in general a negative effect on cellulose dissolution. It should be noted that, although dialkylimidazolium carboxylate ionic liquids, such as [Emim][Ac], may be favourable for industrial application (low melting point and viscosity compared to other cellulose dissolving ionic liquids), they are not inert media for cellulose solubilisation as reaction can take place at the reducing end of the polymer.43 Nevertheless, such side reaction does not take place in [Bmim][Cl].
Pyridinium-based ionic liquids (Fig. 4) that are capable of dissolving cellulose, such as 1-butyl-3-methylpyridinium chloride ([BmPyr][Cl]),44 along with other analogues,45 were also reported. Depolymerisation of cellulose, and other side reactions were recognised in pyridinium-based ionic liquids, e.g., in 1-ethylpyridinium chloride ([EtPyr][Cl]),46 limiting their applications.
Some quaternary ammonium-based ionic liquids (Fig. 4) containing carboxylate anions were also reported as efficient solvents of cellulose. Ammonium ionic liquids with cyclohexyl substituent,47 with dialkoxy functional groups,48 with PEG side chains,49 and derivatives with long alkyl chains50 are some of the reported examples. Benzyl alkylammonium ionic liquids containing acetate anion were also reported to dissolve cellulose.51 The aromatic structure in the latter work was derived from lignin monomers.
Ionic liquids based on the morpholinium cation (Fig. 4) proved also efficient to dissolve cellulose. It was reported that 4-benzyl-4-methylmorpholinium formate ([BMmorf][HCOO]) or acetate ([BMmorf][Ac]) can dissolve cellulose; the advantage of this type of ionic liquid is the moderate to low toxicity as evaluated by Pernak et al.52 Later, N-allyl-N-methylmorpholinium acetate ([AMMorp][Ac]) was introduced as an excellent solvent able to dissolve up to 30 wt% of cellulose at 120 °C without degradation of the polymer.53,54
An intriguing class of ionic liquids to dissolve cellulose are derived from superbases (Fig. 4). First reported examples are based on cations derived from polycyclic amidine bases,55 such as 1,5-diazabicyclo-[4.3.0]non-5-ene ([DBN]) and 1,8-diazabicyclo[5.4.0]undec-7-ene ([DBU]). When paired with carboxylic acids, such as acetate or propionate, cellulose solutions with relatively low viscosities could be obtained.56 Another intriguing example is based on 1,1,3,3-tetramethylguanidine ([TMG]) organic superbase in combination with short-chain carboxylic acids (formic, acetic, propionic).57 The advantage of superbase-derived ionic liquids, besides their relatively low cost and simple preparation, is that they can be readily distilled and recycled with high purity (e.g., in some cases at much higher pressures than [Emim][Ac], affording more cost-effective recovery of the ionic liquid).56
It should be noted that cellulose solubility in ionic liquids greatly depends on the degree of polymerisation of the starting material, the temperature and method of heating (e.g., microwave-assisted heating can greatly enhance solubility1), the amount of water and other impurities (e.g., for tetrabutylammonium acetate/DMSO system some alkali metal ion impurities have negative effect on the dissolution process58) present in the solvent.59 Furthermore, the addition of aprotic co-solvent to ionic liquids, such as DMSO, DMF, etc., can enhance their dissolution power for cellulose.59–61 This phenomenon might be explained by the decreasing viscosity of the system, which increases the mass transport while not affecting the forming hydrogen bonding network in the solution involving the ionic liquid and the polymer.60 Furthermore, other additives, such as solid acids (e.g., Amberlyst® 15 and CsxH3−xPW12O40 in [Bmim][Cl])62 and metal chlorides (e.g., ZnCl2, LiCl, or NaCl in [Amim][Cl])63 were reported to enhance cellulose dissolution (i.e., higher cellulose loading/shorter dissolution time/lower temperature). In a report by Yang et al.,64 the addition of an amino acid, L-arginine could prevent cellulose degradation in [Bmim][Cl], [Emim][Cl] and [Amim][Cl] ionic liquids when dissolution was performed at 130 °C for long time (24 h).
The dissolution mechanism of cellulose in ionic liquids has been the subject of intense discussions and debates over the years. Interested readers are directed to the comprehensive review of Li et al.65 It may be of interest for the reader to understand some of the selection criteria to obtain suitable ionic liquid for dissolving cellulose. It has been known that the difficulties of cellulose dissolution in common organic solvents resides in the strongly concentrated hydrogen bonds present in cellulose, as we mentioned above. There are two major factors that most of the studies consider: a solvent with high hydrogen bond basicity is needed to break down the hydrogen bonding network, and it should be considered that water impurities can negatively affect the dissolution ability of such systems.30,66 Nevertheless, this is not the case for organic onium/inium hydroxide aqueous solutions (OHAS), an intriguing class of ionic liquids that needs the presence of water (upon drying they would decompose). For example, tetrabutylphosphonium hydroxide ([P4444][OH]) and tetrabutylammonium hydroxide ([N4444][OH]) can dissolve cellulose with presence of 40 wt% water.67 It was reported that a weaker hydrogen bonding network around the H2O and [OH] is preferred in these cases (i.e., strong self-associating behaviour of the IL is unfavourable), as followed by the chemical shift of the protons of water molecules.68 It is of utmost importance to develop ionic liquids that can tolerate water content, which is naturally present in biomass.
4. Cellulose-based materials using ionic liquids as processing media
4.1. Coatings and thin films
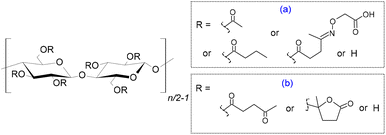 | ||
| Fig. 5 Mixed cellulose ester obtained by using levulinic acid and acyl anhydrides as reactants, followed by the reaction of the corresponding mixed ester with (aminooxy)acetic acid to afford the water-dispersible derivative (a);76 and obtained thorough alpha-angelica lactone in 1.8-diazabicyclo[5.4.0]undec-7-ene ([DBU])/dimethyl sulfoxide/carbon dioxide organocatalytic system (b).77 | ||
Thin cellulose ester films have found applications in a range of devices (e.g., in liquid crystal displays (LCDs)) as protective optical films, based on their high transparency, unique optical characteristics, together with excellent heat resistance, and mechanical properties.73,74,78 Advanced polariser protective films and optical retardation films can be achieved using cellulose esters by chemical modification or by specific additives.78
Various cellulosic films were developed for food packaging applications. The most notable example is cellophane, which is produced through cellulose xanthate (viscose). The viscose process, however, requires high-quality pulp and suffers from its large environmental footprint due to the use and generation of toxic chemicals and byproducts (e.g., sulphur byproducts,79 heavy metals80). Several alternative methods have been developed using cellulose derivatives (cellulose acetate, methyl cellulose, carboxymethyl cellulose81), nanocellulose films,82 and environmentally more friendly processing systems for cellulose dissolution and regeneration (through cellulose carbamate in the CarbaCell process, and using N-methylmorpholine N-oxide (NMMO)83).
Cellulosic coatings have gained importance in controlled-release applications as well. Cellulose esters,74 cellulose ethers,84 different forms of nanocelluloses,85 and cellulose-based hydrogels86 have found applications in large variety of drug delivery systems in the biomedical field. Cellulosic materials can be conveniently modified for targeted applications and can be readily used to create intrinsically biocompatible systems, which greatly contributed to their success in this field. Furthermore, based on their environmentally benign character, several cellulosic materials are used also for controlled release in agrochemical formulations.87
Table 1 shows a list of selected works88–100 devoted to the fabrication of regenerated cellulose films for e.g., packaging application using various ionic liquids such as [Amim][Cl],88–90,97 [Bmim][Cl],91–95,97 [Bmim][Ac]92 and [Emim][Ac].88,96–100 Transparent films could be obtained (Fig. 6) with optical transmittance values in some cases greater than that of commercial cellophane (optical transmittance: 85%).92,97,98 The barrier properties (oxygen and water vapor permeability) of the regenerated cellulose films can be controlled by additives such as plasticisers, as demonstrated by Jin et al.91 Furthermore, in the cellulose/ionic liquid solution active agents can be dissolved such as curcumin (using [Amim][Cl]) to prepare antibacterial regenerated cellulose films,101 or the regenerated cellulose film can be post-modified to obtain films with antibacterial102 or antioxidant103 properties for state-of-the-art active food packaging applications.
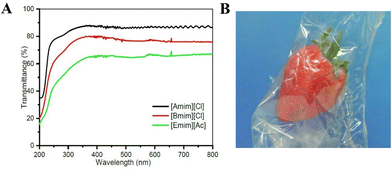 | ||
| Fig. 6 (A) Transmittance of cellulose films regenerated from ionic liquids in water as coagulant (the film from [Amim][Cl] has transparency of about 90% at 550 nm). Reproduced from ref. 97 with permission from Elsevier, copyright 2018. (B) Transparent regenerated cellulose film for food packaging application (cotton linter cellulose dissolved in [Emim][Ac], containing 25% glycerol as plasticiser). Reproduced from ref. 98 with permission from Springer Nature, copyright 2013. | ||
| Type of cellulose | DPa | CIa | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | DPb | CIb | Mechanical properties | Note | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Corn husk-derived | 509 | 59.6 | [Emim][Ac] | 4 wt% | 80 °C (2 h) | 370 | 47.9 | 47c (4.11)d | — | Water | 88 |
| [Amim][Cl] | 4 wt% | 80 °C (4 h) | 410 | 49.5 | 112c (6.01)d | Water | |||||
| Agave-derived cellulose microfibrils | — | 64.4 | [Amim][Cl] | 4 wt% | 80 °C (−) | — | 55.9 | 135c (8.15)d | Remaining undissolved cellulose microfibrils as “self-reinforcing” agents | Water | 89 |
| Cotton linter fiber | 920 | 54 | [Amim][Cl] | 5 wt% | 90 °C (30 min) | — | 38 | ∼50c (−)d | Addition of plasticisers (glycerol, sorbitol, carboxymethyl cellulose). | Water | 90 |
| No plasticiser | |||||||||||
| Softwood pulp | 1460 | 77.3 | [Bmim][Cl] | 6 wt% | 80 °C (8 h) with static conditions, then 95 °C (3 h) with stirring | — | 32.9 | 52.8c (−)d | Effect of plasticiser (glycerol). | 1. 10 vol% [Bmim][Cl] in water; 2. film immersed in glycerol/H2O | 91 |
| No plasticiser | |||||||||||
| Microcrystalline | — | — | [Bmim][Cl] | 4 wt% | 85 °C (7 h) | — | — | 1.3c (−)d | Fluorescent sensing composite | Water | 92 and 93 |
| [Bmim][Ac] | 14 wt% | 50 °C (7 h) | — | — | 12.51c (−)d | Adsorption study | Water | 92 | |||
| Natural luffa fiber-derived | — | — | [Bmim][Cl] | 15 wt% | 80 °C (10 h), then 100 °C (5 h, under vacuum) | — | — | — | Nanoporous film | Water | 94 |
| Type of cellulose | DPa | CIa | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | DPb | CIb | Mechanical properties | Note | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Degree of polymerisation (DP) and crystallinity index (CI in %) of the starting materials. b Degree of polymerisation (DP) and crystallinity index (CI in %) of the regenerated films. c Tensile strength in MPa. d Elastic modulus in GPa. | |||||||||||
| Wheat straw-derived | 580 | — | [Bmim][Cl] | 5 wt% | 90 °C (12 h) | 470 | — | 170c (−)d | Effect of dissolution time and temperature | Water | 95 |
| Microcrystalline | 240 | 51.36 | [Emim][Ac] | 5 wt% | Stirring at 80 °C (0.5 h); then standing at 80 °C for 1 h (for degassing) | 145 | 40.58 | 69c (−)d | — | Water | 96 |
| Cotton linter | 831 | 54.01 | 570 | 34.04 | ∼105c (−)d | ||||||
| Pine-derived | 819 | 49.02 | 559 | 39.58 | 120c (−)d | ||||||
| Bamboo-derived | 625 | 46.69 | 460 | 32.93 | ∼80c (−)d | ||||||
| Coniferous dissolving pulp | 500 | 73.8 | [Amim][Cl] | 3 wt% | 90 °C (0.5 h) | 354 | 52.4 | 152c (−)d | — | Water | 97 |
| [Bmim][Cl] | 400 | 49.2 | 75c (−)d | ||||||||
| [Emim][Ac] | 451 | 44.8 | 50c (−)d | ||||||||
| Cotton linter | 920 | — | [Emim][Ac] | ∼5 wt% | 80 °C (5 min) | — | 34 (no plasticiser) | 84.5c (−)d | Addition of plasticiser (sorbitol, glycerol and CMC) | Water | 98 |
| Never dried commercial 96α Eucalyptus dissolving pulp | — | — | [Emim][Ac] | 4 wt% | Drying overnight at 60 °C, followed by continuous stirring at 60 °C for 2 h | — | — | — | Effect of the coagulation medium | Water/ethanol/1-propanol | 99 |
| Microcrystalline cellulose | — | 65 | [Emim][Ac] | 0.1–2 wt% | 50 °C (overnight) | — | — | — | Ultrathin 10 nm thick film | Ethanol | 100 |
The degree of polymerisation (DP) and crystallinity index was strongly correlated with the mechanical properties of the regenerated cellulose films in several works.88,95–98 It was suggested that above a certain level of DP (given as ∼450), the mechanical properties are dominated mostly by the crystallinity.96 In addition, other secondary interactions such as intra- and intermolecular hydrogen bonding, and hydrophobic stacking interactions are also important in shaping the physical properties of the material. In general, the structure of cellulose changes from cellulose I to cellulose II as a result of the regeneration process (Fig. 7). The crystallinity index also decreases compared to the native cellulose, a more amorphous material is usually obtained. Cellulose II is known to be thermodynamically favoured, its stability is attributed to ring-stacking via hydrophobic interactions and to additional hydrogen bonding interactions between sheets (inter-molecular), forming a three-dimensional structure (see section 2).99
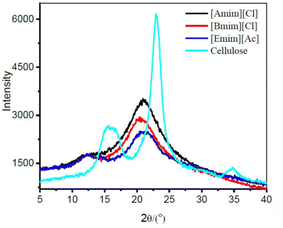 | ||
| Fig. 7 X-ray diffraction (XRD) patterns of native cellulose (coniferous dissolving pulp), exhibiting typical cellulose I structure, and that of the films regenerated from different ionic liquids in water with XRD profiles typical for a cellulose II structure. Crystallinity index: [Amim][Cl] > [Bmim][Cl] > [Emim][Ac]. Reproduced from ref. 97 with permission from Elsevier, copyright 2018. | ||
The crystallinity of the regenerated cellulose can be influenced by several factors, such as the coagulation process. Östlund et al.99 dissolved pulp in [Emim][Ac], and studied the effect of the coagulation media (water, ethanol and 1-propanol) on the structure of the regenerated cellulose. When water was used as coagulation medium, mainly cellulose II structure was obtained, while other solvents yielded mostly amorphous films. This was explained by the slow diffusion of [Emim][Ac] to ethanol/1-propanol compared to water on account of their lower solubility for [Emim][Ac]. The hydroxymethyl group in cellulose dissolved in [Emim][Ac] is in gauche–trans (gt) conformation, which can be trapped in this stage during fast solvent exchange (in water). This conformation is beneficial for the formation of the cellulose II structure (hydroxymethyl group is in gt conformation in contrast to native cellulose I with trans–gauche conformation, see section 2, Fig. 2). Furthermore, molecular mobility was suggested to be another factor that can have an effect on the crystallinity of the regenerated films. Zheng et al.97 studied the effect of ionic liquids with increasing dissolution power ([Bmim][Cl] < [Amim][Cl] < [Emim][Ac]) on the properties of the regenerated cellulose. They found that the cellulose regenerated from [Amim][Cl] solution had the highest tensile strength, which was attributed to its highest crystallinity among the samples. They argued that the higher crystallinity was due to the lower molecular weight of the sample compared to the others, resulting in enhanced molecular mobility aiding molecular arrangement during the regeneration process. The regenerated cellulose film from [Amim][Cl] showed excellent transparency (90% at 550 nm) according to their study. Pang et al.96 compared the properties of regenerated films from different cellulose starting materials (microcrystalline, cotton linter, pine, bamboo celluloses) dissolved in [Emim][Ac]. They found that pine-derived cellulose sample with high DP (559) and crystallinity index (39.58%) gave the highest mechanical performance (tensile strength of 120 MPa), compared to the poorly performing microcrystalline cellulose that had although high crystallinity index (40.58%), its DP was the lowest (145) among the samples.
Several studies point out the importance of dissolution conditions, such as time and temperature on the structure of the regenerated cellulose, and thus on its mechanical properties.88,95 Cao et al.88 prepared regenerated cellulose films from cornhusk cellulose using [Amim][Cl] and [Emim][Ac] ionic liquids. They realised that the DP decreases with increasing dissolution time and temperature, and the latter had greater effect on cellulose degradation (Fig. 8). Chen et al.95 reached a similar conclusion when dissolving wheat straw cellulose in [Bmim][Cl]. By optimising the dissolution time and temperature to avoid substantial cellulose degradation (i.e., decrease in DP), a regenerated cellulose film with high tensile strength could be obtained (170 MPa).
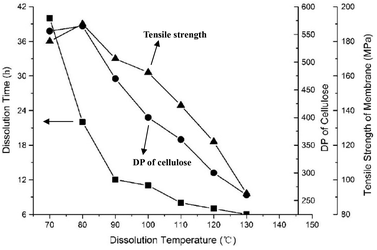 | ||
| Fig. 8 Effect of dissolution time and temperature on the tensile strength and degree of polymerisation (DP) of regenerated cellulose (dissolved in [Bmim][Cl], regenerated in water). Reproduced from ref. 95 with permission from Wiley, copyright 2012. | ||
Regenerated cellulose films can show brittle characteristics, which can be disadvantageous for packaging applications. To overcome this limitation, several studies focused on including plasticisers (sorbitol, glycerol and carboxymethyl cellulose (CMC)) in the ionic liquid/cellulose solution before the regeneration process using [Amim][Cl]90 and [Emim][Ac] solvent systems.98 Interestingly, the crystallinity indices of the regenerated cellulose films with plasticisers decreased when [Amim][Cl] was used, meanwhile an increase was observed for [Emim][Ac] compared to a control regenerated cellulose film without plasticiser. In all cases, an increase in the tensile strength was observed by the addition of the plasticiser, which was attributed to secondary interactions forming between cellulose and plasticiser molecules. Somewhat different result was obtained when the regenerated film (using [Bmim][Cl]) was immersed in a plasticiser solution as a post-treatment process instead of its direct inclusion in the ionic liquid solution.91 In this case, tensile strength decreased, while elongation at break increased as an effect of the glycerol plasticiser.
The hydrophilicity of the surface is another important parameter for applications, such as for packaging films or thin film substrates. It is known that amorphous regions in regenerated cellulose are prone to swelling, these regions provide sufficient mobility that is advantageous for the orientational requirement of hydrogen bond formation with water molecules.104,105 Since the crystallinity of the films regenerated from ionic liquids can be controlled to some extent depending on experimental conditions, this provides an opportunity to prepare films with various wettability. Amorphous cellulose film regenerated from [Emim][Ac] solution (DMSO co-solvent, ethanol coagulant) has low static water contact angle (∼30°), similarly to amorphous thin films regenerated using other solvent systems.100 Pang et al.96 prepared regenerated cellulose films using [Emim][Ac] (water coagulant) with various crystallinity depending on the starting cellulose material. They observed high water contact angles (∼73° for regenerated microcrystalline cellulose; ∼77° for pine-derived regenerated cellulose) for the films with high crystallinity indices (see Table 1). This phenomenon was attributed to the different hydrogen bonding network in crystalline/amorphous samples. It was also demonstrated that plasticisers can also have an effect on the wettability of the surface. Pang et al.90 showed that the presence of sorbitol/glycerol/CMC in the regenerated cellulose film ([Amim][Cl] solvent, water coagulation medium) leads to a significant increase in the recorded water contact angles (59°/74°/75°, respectively). They argued that surface morphology of the films, and interference of the plasticiser molecules with the hydrogen bonding network of cellulose contribute to a reduced surface free energy. Similar result was observed by Hameed et al.106 with poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (abbreviated as PHBV, a microbial biopolymer) as additive in regenerated cellulose blends using [Bmim][Cl] (water coagulant). In this case, however, it should be noted that PHBV regenerated from the ionic liquid has already hydrophobic character (with water contact angle of about 97°).
Several studies reported on the formation of a nanoporous film upon regeneration from ionic liquid solutions. Wang et al.94 dissolved natural luffa-derived cellulose in [Bmim][Cl], and found that the regenerated film has nanopores with an average pore size of about 18 nm. Östlund et al.99 studied the porosity of regenerated cellulose films ([Emim][Ac] solvent) through NMR cryoporometry, using various coagulation media (water, ethanol and 1-propanol). In all cases, pores with radius below 15 nm were observed (spherical pore geometry model). It was also possible to obtain uniform porous structure with pore radius centred around 3 nm, using 1-propanol as coagulant, through repeated drying cycles.
Ultrathin regenerated cellulose films could also be prepared from an [Emim][Ac] solution containing 0.1 wt% cellulose (microcrystalline) that was spin-coated on a silicon wafer substrate, and regenerated using ethanol as antisolvent.100 AFM image of the ultrathin film indicated the presence of fine nanofibrillar cellulose structure on the surface (Fig. 9).
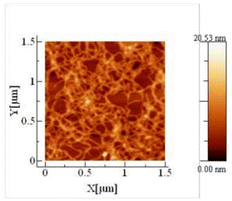 | ||
| Fig. 9 Atomic force microscopy (AFM) image of cellulose ultrathin film regenerated from [Emim][Ac] solution (0.1 wt% cellulose), using ethanol as coagulant. Reproduced from ref. 100 with permission from Walter de Gruyter GmbH, copyright 2018. | ||
Radiation-curable coating was also developed using ionic liquid monomers that can dissolve cellulose, this approach is considered a green alternative to coating systems that apply volatile organic solvents. Isik et al.107,108 synthesised 2-cholinium lactate methacrylate monomer that dissolved 5–10 wt% cellulose. Photopolymerisation at 368 nm in the presence of 2,2-dimethoxy-2-phenylacetophenon photoinitiator afforded a transparent coating film from the 5 wt% cellulose/ionic liquid solution (Fig. 10). Cholinium-based cellulose-dissolving ionic liquids are advantageous due to their low toxicity, biodegradability, and low cost.109
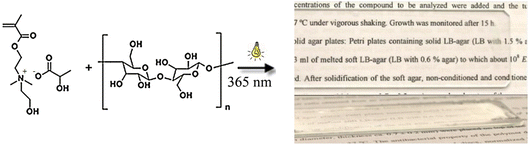 | ||
| Fig. 10 Photopolymerisable cellulose coating using a cholinium-based ionic liquid methacrylic monomer as both solvent and crosslinker. Reproduced from ref. 107 with permission from the American Chemical Society, copyright 2013. A part reproduced from ref. 108 with permission from Wiley, copyright 2014. | ||
The cellulose/ionic liquid system can be used to form regenerated cellulose coating also for controlled drug release. Song et al.110 fabricated chitosan hydrogel beads that could be coated with regenerated cellulose by dropping the hydrogels in the cellulose/[Emim][Ac] solution. Regenerated cellulose coating was formed by the diffusion of water from the hydrogel. The fabricated material showed good properties for controlled drug release applications.
4.2. Thin film filtration membranes
Cellulosic materials have long history in separation science dating back to ancient times (e.g., just think about cotton cloth for filtering particles). Both natural and physically/chemically modified cellulose were shown to be efficient as adsorbent for water, organic solvents, metal ions and organic substances (such as dyes).111 Furthermore, cellulose can be converted to various forms of activated carbons with high surface area, extending its efficiency and application to other adsorbates (e.g., gases). Among the chemically modified cellulose derivatives, cellulose esters proved particularly successful as membranes in the separation field, basically covering all range of the filtration spectrum including micro-, ultra- and nanofiltration, reverse osmosis and dialysis applications.74The introduction of ionic liquids as solvent of cellulose opened the gates for the preparation of novel thin film membranes directly from the natural polymer.
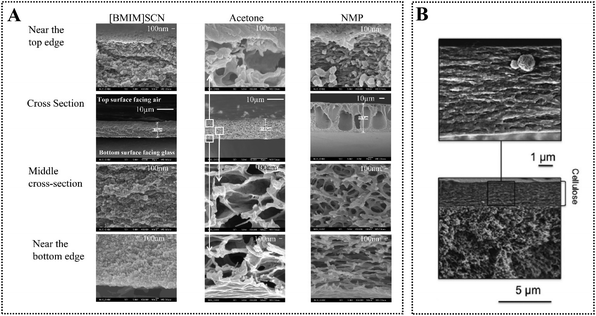 | ||
| Fig. 11 (A) Morphology of cellulose acetate membranes regenerated from different solvents (10 wt% cellulose acetate solution). Reproduced from ref. 113 with permission from the American Chemical Society, copyright 2010. (B) Morphology of cellulose membrane on porous polysulphone support regenerated from 10 wt% cellulose solution in [Emim][Ac]. Reproduced from ref. 116 with permission from Elsevier, copyright 2015. | ||
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Membrane properties | Membrane thickness | Membrane performance | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cellulose acetate (39.8% acetyl content) | [Bmim][SCN] | 10 wt% | 50 °C (5 h) | Dense, macrovoid-free, self-standing membrane with low porosity (∼6%) | 8.72 μm | Pure water permeability: 114.14 L m−2 h−1 bar−1 (at 0.15 MPa). Mean pore size ∼39 nm based on neutral solute rejection experiment (PEG/PEO solute) | Water | 113 |
| α-Cellulose (DP 300) | [Amim][Cl] | 8 wt% | 90 °C (25 min) | Dense, macrovoid-free, layered structure. Film casted on PET non-woven fabric | 10.5 μm | Pure water permeability: 128.5 L m−2 h−1 at 0.4 MPa. Antifouling membrane could be developed for filtration of organic dyes with a MWCO less than 700 Da, and for BSA in PBS | Water | 114 |
| Wheat straw-derived (DP 580) | [Bmim][Cl] | 5 wt% | 90 °C (12 h) | Self-supporting asymmetric porous membrane. Dense thin surface layer with small holes, inner layer with bigger holes. Tensile strength of 170 MPa (6.4% breaking elongation) | ∼15 μm | Pure water flux of 238.9 L m−2 h−1 at 0.3 MPa. Rejection of BSA ∼97% | Water | 95 |
| Bamboo fiber-derived | [Bmim][Cl] | 3, 5, 10 wt% | 80 °C (8 h) | Self-supporting membranes. Dense, nodular, macrovoid-free structure. Denser membrane fabricated from concentrated solution (10 wt%), showing higher rejection towards organic dyes (best performance) | — | Best performance: pure water-flux: 150 L m−2 h−1 (0.4 MPa); about 100% flux recovery ratio, good antifouling performance; maximum organic dye rejection: 89% (for crystal violet) | Water | 115 |
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Membrane properties | Membrane thickness | Membrane performance | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: PEG – polyethylene glycol; PEO – polyethylene oxide; MWCO – molecular weight cut-off; BSA – bovine serum albumin; PBS – phosphate-buffered saline; THF – tetrahydrofuran; DMF – N,N-dimethylformamide; NMP – N-methyl-2-pyrrolidone; DMA – dimethylacetamide. | ||||||||
| Microcrystalline cellulose (Mw 160–560 kDa) | [Emim][Ac] | 2, 5, 10 wt% | 80 °C (24 h) | Regenerated cellulose membranes on various porous substrates. Self-supporting membrane showing good solvent-tolerance (THF, hexane, DMF, NMP, DMA) | 0.4–6.8 μm | Lowest MWCO for the membrane prepared from 10 wt% cellulose solution. MWCO (using PEG) as low as 3000 g mol−1 with 13.8 L m−2 h−1 bar−1 at 0.5 MPa (polysulphone (PSU) support) | Water | 116 |
| Microcrystalline cellulose (Mw 160–560 kDa) | [Emim][Ac] | 2, 5 wt% | 60 °C (−) | Dense, selective coating on porous PSU substrate | 0.4 μm, 0.9 μm | Oil could be selectively removed from the oil-in-water emulsions. The thin 0.4 μm coating gave the highest permeance and flux recovery – pure water permeance: 150 L m−2 h−1 at 0.5 MPa (90 L m−2 h−1 flux recovery for oil-in-water emulsion) | Water | 117 |
| Cotton linter | [Emim][Ac] (acetone as cosolvent for some samples) | 8, 12, 20 wt% | 70 °C or RT, samples with cosolvent | Self-supporting membranes. Dye rejection was correlated with membrane affinity (sorption), attributed to hydrogen-bonding and electrostatic interactions | — | Highest rejection (regenerated from 20 wt% cellulose solution) - 94% for bromothymol blue, with permeance of 0.3 L m−2 h−1 in ethanol at 0.4 MPa | Water | 118 |
| Cotton linter | [Emim][Ac] (DMSO as cosolvent for some samples) | 8 wt% | 70 °C or RT, samples with cosolvent | Self-supporting membranes. Dense, nodular, macrovoid-free structure. Symmetric morphology | — | Although cellulose membranes with different morphologies could be obtained by using cosolvent and different coagulation baths, the dried membranes performed similarly with high organic dye rejection (over 80%), water permeance 0.5–1 L m−2 h−1, and ethanol permeance 0.1–0.3 L m−2 h−1 at 0.4 MPa | Water/ethanol | 119 |
4.3. Enzyme immobilisation using cellulose/ionic liquid systems
Cellulose, owing to its biologically compatible and nontoxic nature, has received significant interest as a support material for enzyme immobilisation. Table 3 shows selected works on enzyme immobilisation using regenerated cellulose films/beads through ionic liquid processing media. Turner et al.120 was the first who studied enzyme encapsulation in regenerated cellulose films using [Bmim][Cl] solvent (via a cold processing method to avoid thermal denaturation), and laccase from Rhus vernificera as a model enzyme (an oxidoreductase responsible for the biodegradation of e.g., lignin121). They observed a significant decrease in laccase activity (18% of the activity compared to the free form) for the enzyme-encapsulated regenerated film. They also realised that a hydrophobic ionic liquid ([Bmim][NTf2]) “precoating” enables the formation of a more stable microenvironment in the regenerated cellulose film that results in an enhanced enzymatic activity (29% compared to the native form in aqueous solution). It is known that Cl− in neat ionic liquids can form H-bonds with proteins, which can disturb their secondary structure, leading to denaturation. In addition, several enzymes show higher activity in more hydrophobic ionic liquids.122 Following the initial experiences on entrapping enzymes in a regenerated cellulose film, Turner et al.123 investigated enzyme immobilisation on the surface of the substrate, a strategy known to increase enzyme stability. They prepared regenerated cellulose/poly(oxyalkeneamine) composites by directly mixing the amine-containing polymer with the cellulose/[Bmim][Cl] solution. When laccase was attached to the surface using glutaraldehyde crosslinker, for one of the examined primary amine-containing polymers the enzyme retained about 50% of its activity. This increase was attributed to the improvement in the flexibility of the enzyme attached to the surface. Further improvement was achieved with regenerated cellulose films by using polyamidoamine (PAMAM) dendrimers dissolved together with cellulose in [Bmim][Cl].124 Interestingly, the highest enzyme activity was observed when the cellulose-PAMAM matrix was crosslinked with 1,3-phenylene diisocyanate, and surface attachment was done by incubating the enzyme with the regenerated film. This strategy outperformed even the frequently applied method of surface attachment via glutaraldehyde crosslinker. Several works prepared bioactive films through similar strategies by directly including horseradish peroxidase/soybean peroxidase in the cellulose/[Bmim][Cl] solution,125 or by surface attachment of β-galactosidase on regenerated polyamine/cellulose composite films (hydrophobic [Bmim][NTf2] as solvent – note that cellulose acetate was used here instead of cellulose which does not dissolve in this ionic liquid) postmodified using glutaraldehyde as crosslinker.126 Regenerated cellulose beads proved also efficient in laccase immobilisation as demonstrated by Gu et al.127 They fabricated crosslinked cellulose beads by dropping a cellulose/[Emim][Ac] solution containing crosslinkers and reactive functions (epoxy and catechol) into water by means of a syringe, and immobilised laccase by simply incubating the enzyme with the prepared beads. Furthermore, magnetic micro/nanoparticle composites gained interest in enzyme immobilisation as they can be separated easily from the reaction media using a magnet. Liu et al.128 prepared cellulose-chitosan magnetic microspheres (as hydrogels with about 92% water content) using Fe3O4 magnetic support in [Bmim][Cl]. The regeneration procedure was different from others in that the [Bmim][Cl] solution was added to an oil-surfactant mixture, followed by the addition of ethanol. The enzyme glucose oxidase (from Aspergillus niger) was anchored on the surface of the microspheres through the amine group of chitosan using glutaraldehyde. Enhanced thermal and storage stability, and wider pH tolerance window was reported as a result of the enzyme immobilisation. Recently, Suo et al.129 modified carboxymethyl cellulose-coated magnetic nanoparticles with an imidazolium ionic liquid containing carboxyl function and PF6− counterion (Fig. 12). The porcine pancreatic lipase enzyme was immobilised via the pendant carboxyl group on the surface of the nanoparticles by applying EDC/NHS coupling protocol. By investigating the structure of the immobilised enzyme, they proposed that the lid of the enzyme active site is open to a higher degree (low α-helix content), and the enzyme has a more rigid structure (high β-sheet content) compared to the free enzyme or enzyme immobilised without the ionic liquid anchor. They concluded that these features can be responsible for the high enzyme activity and stability.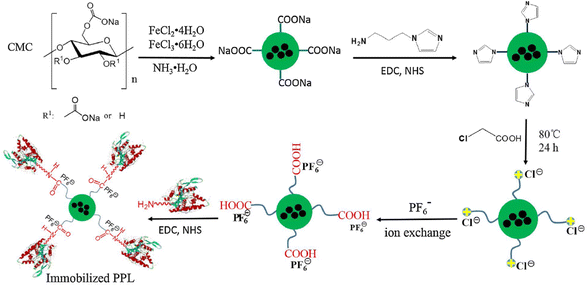 | ||
| Fig. 12 Enzyme (porcine pancreatic lipase (PPL)) immobilisation on carboxymethylcellulose-coated magnetic nanoparticles via an ionic liquid-derived anchor. Reproduced with some modification from ref. 129 with permission from Elsevier, copyright 2020. | ||
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Immobilised enzyme | Immobilisation strategy | Enzyme activity | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|---|
| Microcrystalline cellulose | [Bmim][Cl] | 4.75 wt% | Microwave pulse heating | Rhus vernificera laccase | Dispersion in the ionic liquid/cellulose solution (at R.T.). A “precoating” step using a hydrophobic ionic liquid improved enzyme activity in the regenerated film | 29% (“precoating” strategy) compared to the native form in aqueous solution | Water | 120 |
| Microcrystalline cellulose | [Bmim][Cl] | 5 wt% | Microwave pulse heating | Rhus vernificera laccase | Immobilisation on the surface using pendant amine groups present in the regenerated cellulose/primary-amine polymer composite film. Glutaraldehyde crosslinker | Close to 50% activity retained compared to the native form in aqueous solution | Water | 123 |
| Microcrystalline cellulose | [Bmim][Cl] | 6.5 wt% | Microwave pulse heating | Rhus vernificera laccase | Regenerated cellulose film with polyamidoamine (PAMAM) dendrimer. The crosslinked (using 1,3-phenylene diisocyanate) generation 1 PAMAM dendrimer (lowest amount of amino groups) provided the highest enzyme activity. Covalent attachment via crosslinker using glutaraldehyde resulted in a lower enzyme activity | Enhanced enzyme activity compared to the free form | Water | 124 |
| Microcrystalline cellulose | [Bmim][Cl] | 3.5 wt% | 85 °C (6 h) | Horseradish peroxidase and soybean peroxidase | Co-dissolution of the protein with cellulose and the ionic liquid at 85 °C. Immobilised in the regenerated film | Retained more than 50% of their original activity in the regenerated films | Water | 125 |
| Dissolving pulp | [Bmim][Cl] | 7 wt% | 100 °C (30 min) | Aspergillus niger glucose oxidase | Cellulose-chitosan microspheres as hydrogels coated on magnetic nanoparticles. Enzyme immobilisation by covalent attachment (via chitosan amine group) using glutaraldehyde | Enhanced thermal and storage stability, wider pH optima | Oil with surfactant, addition of ethanol | 128 |
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Immobilised enzyme | Immobilisation strategy | Enzyme activity | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: EDC: 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide; NHS: N-hydroxysuccinimide | ||||||||
| Cellulose acetate | [Bmim][NTf2] | Around 6 wt% cellulose acetate in acetone; addition of the ionic liquid (around 20 wt% in the final solution) | No heating | Kluveromyces lactis β-galactosidase | Immobilisation of β-galactosidase via triethylenetetramine anchors on the film using glutaraldehyde crosslinker. Improved flexibility and formability compared to cellulose acetate | 60% of the initial enzyme activity could be retained after immobilisation | No antisolvent, acetone was evaporated | 126 |
| Microcrystalline cellulose | [Emim][Ac] | 6 wt% | 70–80 °C (3 h) | Trametes versiclor laccase | Crosslinked regenerated cellulose beads. Covalent surface attachment of laccase via epoxy groups or catechol moieties (dopamine) | Improved thermal stability and pH tolerance compared to the free form | Water | 127 |
| Carboxymethyl cellulose | — | — | — | Porcine pancreatic lipase | Magnetic carboxymethyl cellulose nanoparticles functionalised with an imidazolium ionic liquid bearing carboxyl function (PF6− counterion). Enzyme attachment using EDC/NHS activator | Enzyme activity 2.83 folds higher than free lipase (1.43 folds higher without ionic liquid functionalisation) | — | 129 |
The structure of ionic liquids has a profound impact on enzyme activity, the dominating factors have been comprehensively studied.122 In case of cellulose/ionic liquid systems, the ionic liquid needs to be both a good solvent of cellulose, and should have ideally a positive effect on enzyme activity and stability.130,131 There has been great interest in designing novel biocompatible, cellulose-dissolving ionic liquids.132,133
4.4. Catalytically active cellulose-based composite materials using ionic liquids
Ionic liquids received great interest as tunable platform for the synthesis of various metal nanoparticles with controlled size and shape, sometimes with superior properties compared to those fabricated via conventional methods.134,135 Furthermore, ionic liquids are flexible media in catalysis in that they can not only act as solvents, but also as stabilisers, supports and ligands in various multiphase catalytic transformations.134–137The first work that described the use of an ionic liquid/metal nanoparticle/cellulose system was from Li et al.138 They studied the thermally-induced formation of gold microparticles from an Au(III) salt in BmimCl using cellulose as the reducing agent. Gold microparticles with various sizes and shapes could be obtained by modulating the temperature, owing to the cellulose template and the ionic liquid.
It was Gelesky et al.139 who first recognised the use of cellulose to prepare catalytic polymeric membranes through the immobilisation of metal nanoparticles in combination with an ionic liquid. The polymeric support can reduce the loss of the nanoparticles in the course of the process, provides porous contact in gas/liquid multiphase reactions, and prevents agglomeration of the nanoparticles. The latter phenomenon is a well-known limitation when ionic liquids are used in transition metal nanoparticle catalysis.134,137 Gelesky et al.139 dispersed Pt(0) and Rh(0) nanoparticles in cellulose acetate substrate using [Bmim][NTf2]/acetone solvent system, and obtained films of various thicknesses (10–40 μm) by evaporating acetone. The cellulose/ionic liquid/Pt(0) 20 μm thick composite film showed much higher catalytic activity (turnover frequency (TOF): 7353 h−1) for the hydrogenation of cyclohexane than the nanoparticles alone in the ionic liquid (TOF: 329 h−1). This phenomenon was attributed to the stabilisation of the nanoparticles in the cellulose substrate, and to the presence of porous regions conducive to the effective gas/liquid contact. They also noted that the presence of ionic liquid confers higher flexibility and formability to the membrane. Since this initial work, several studies have been reported on the fabrication of cellulose/metal nanoparticle composite films/spheres for catalytic applications, a list of selected works can be found in Table 4.
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Nanoparticle | Film properties, observations | Catalytic activity | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cellulose acetate | [Bmim][NTf2] | Around 6 wt% cellulose acetate in acetone; addition of the ionic liquid (around 20 wt% in the final solution) | No heating | Pt(0)/Rh(0) | Metal nanoparticle catalyst immobilisation inside the cellulose acetate-IL composite. Synergistic effect was observed on the catalytic activity of Rh(0) and Pt(0) nanoparticles | Higher catalytic activity of IL/cellulose/nanoparticle composite (TOF 7353 h−1; 20 μm thick film, Pt(0)) than IL/nanoparticle system (TOF 329 h−1, Pt(0)) in hydrogenation of cyclohexane | No antisolvent, acetone was evaporated | 139 |
| Microcrystalline cellulose (DP 200) | [Bmim][Ac] | 8, 10, 12, 14 wt% | 70 °C (1 h) | TiO2 | Higher viscosity results in a more compact film. Dispersion of nanoparticles is negatively affected by increasing viscosity | Photocatalytic activity demonstrated on films impregnated with methylene blue/rhodamine B dye solutions (UV irradiation at 365 nm). Less effective in degradation of dye in solution | Water | 140 |
| [Bmim][Cl] | 90 °C (1 h) | |||||||
| Microcrystalline cellulose (DP 210–230) | [Bmim][Ac] (DMSO co-solvent) | 8 wt% | 70 °C (5 h) | TiO2 | Porous film could be fabricated via freeze-drying. Macrospheres via drop-wise addition to the coagulation medium | Wet porous films showed the best photocatalytic activity in dye degradation. Dye adsorbed on porous film could be eliminated by sunlight irradiation in 15 min – self-cleaning surfaces | Water | 141 |
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Nanoparticle | Film properties, observations | Catalytic activity | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|---|
| Abbreviations: TOF: turnover frequency. | ||||||||
| Microcrystalline cellulose (DP 180) | [Bmim][Ac] (IL/DMSO 3/1) | 8 wt% | 70 °C (5 h) | TiO2 + Fe2O3 | Magnetite nanoparticles are more stable in [Emim][Ac]/DMSO system compared to [Bmim][Ac]/DMSO system. Agglomerate size is smaller in the latter one | Films have lower photocatalytic activity (about one order of magnitude) compared to TiO2 powder. Addition of magnetite did not have negative effect on the photocatalytic activity. Degradation of organic dyes | Water | 142 |
| [Emim][Ac] (IL/DMSO 3/1) | Less macrovoids, denser film | |||||||
| Microcrystalline cellulose (DP 180) | [Bmim][Ac] (IL/DMSO 3/1) | 6 wt% | 70 °C (−) | TiO2 + Fe2O3 | Porous macrospheres. Superior TiO2 dispersion, higher porosity of the resulting spheres in [Bmim][Ac]/DMSO | Higher photocatalytic activity of the spheres from [Bmim][Ac] solutions. Additional adsorption property for heavy metals (demonstrated on Cu2+) | Water | 143 |
| [Emim][Ac] (IL/DMSO 3/1) | ||||||||
| Microcrystalline cellulose (DP 180) | [Emim][Ac] | 7 wt% | 80 °C (−) | TiO2 | Cellulose/carrageenan/TiO2 composite film | The negatively charged composite has high adsorption capacity for a cationic dye. Photodegradation efficiency is higher than TiO2/cellulose composite | Ethanol, water | 144 |
| Waste cotton fabric | [Amim][Cl] | ∼2 wt% | 65 °C (1 h) | Bi2WO6 | IL/cellulose solution added to Bi2WO6/DMA solution prior to the regeneration. Good compatibility between cellulose and the nanoparticle. Layered porous film | Good adsorption and photocatalytic activity to remove organic dyes under visible light irradiation | Water | 145 |
Wittmar et al.140 prepared TiO2-doped regenerated cellulose films using [Bmim][Ac]/[Bmim][Cl] ionic liquids (Fig. 13A). The prepared film showed good photocatalytic efficiency in degradation of organic dye when impregnated on the cellulosic substrate. However, the process was less effective in bulk solution of the organic dye, which the authors have attributed to the poor solute diffusion owing to the low porosity of the film. In order to improve the performance of the material, in another study from the same group, TiO2-doped porous films were prepared via a freeze-drying process using [Bmim][Ac] solvent141 (Fig. 13B). They noted the beneficial effect of DMSO as co-solvent to decrease the viscosity of the ionic liquid, and thereby improve the processability. Interestingly, when irradiated with sunlight, dye decomposition was efficient in case of dye-impregnated films. This feature is promising for application as “self-cleaning” material under sunlight. Furthermore, Fe2O3–TiO2 co-doped regenerated porous cellulose film could be also prepared.142 In this case, it was noted that the presence of magnetite nanoparticle did not affect the photocatalytic activity of TiO2 (although the activity decreased an order of magnitude compared to TiO2 powder). The magnetic, photocatalytically active film can be easily separated from the reaction solution using a magnet (Fig. 13C), and can be also readily heated by alternating magnetic field (AMF, Fig. 13D). Furthermore, in order to aid the recyclability of the photocatalytic regenerated cellulose material, it was further shaped into porous macrospheres in a recent study.143 In this case, the superiority of [Bmim][Ac]/DMSO system over [Emim][Ac]/DMSO medium was reported, as it provided a material with higher porosity, and it also dispersed TiO2 nanoparticles better. In another work by Jo et al.,144 carrageenan, a polysaccharide with sulphate moieties, was added to the TiO2-doped regenerated films ([Emim][Ac] solvent) that resulted in increased adsorption capacity for the cationic organic dye, and lead to an improved degradation efficiency in the photocatalytic reaction. The compatibility of the cellulose/ionic liquid system for other photocatalytically active nanoparticles, such as Bi2WO6, was also demonstrated.145
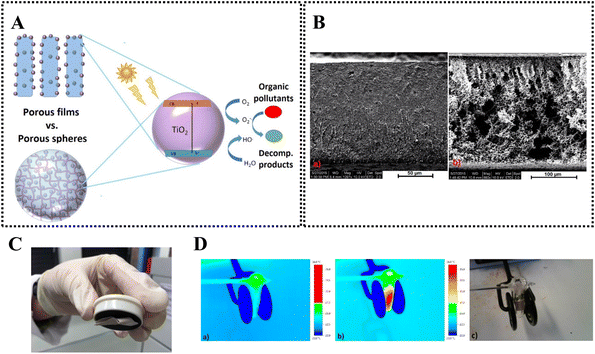 | ||
| Fig. 13 (A) Preparation of TiO2-doped regenerated cellulose films for photocatalytic degradation of organic pollutants. (B) Effect of the freeze-drying process on the resulting regenerated cellulose film (left: air drying; right: freeze-drying). (A) and (B) reproduced from ref. 141 with permission from the American Chemical Society, copyright 2017. (C) Magnetic properties of the Fe2O3–TiO2 co-doped regenerated cellulose film. (D) AMF-induced temperature increase of the Fe2O3–TiO2 co-doped regenerated cellulose film. (C) and (D) reproduced from ref. 142 with permission from the American Chemical Society, copyright 2017. | ||
These studies clearly show that ionic liquids provide versatile platform for the fabrication of catalytically active cellulose-based composites with tailored structures and properties.
4.5. Cellulose-based separator membranes and conductive composite films for electronic devices, using ionic liquid processing media
Ionic liquids provide a versatile platform to prepare nanoporous membranes for energy storage devices from cellulose via the phase inversion method (which we have already mentioned in section 4.2.1). Zhao et al.148 prepared regenerated cellulose film (22 μm-thick) from cellulose/[Bmim][Cl] solution (4 wt% cellulose content) using water as coagulant. They obtained a film (Fig. 14A) with good flexibility and mechanical properties (tensile strength of 171.5 MPa, elastic modulus of 8.93 GPa). The tensile strength is among the highest reported in the literature (see Tables 1 and 2), this may be attributed partly to the starting cellulose that had a high DP of 1484, and to the processing conditions. The regenerated membrane exhibited high porosity (71.78%) with uniformly distributed mesopores having diameters in the range of 5–30 nm (Fig. 14B). The thermal stability, which is an important parameter in separator applications, remained comparable (thermal degradation starts around 275 °C) to commercial cellulose-based separators. The thermal stability of cellulosic membrane is superior compared to commercial polyolefin-based separators (structural changes above 100 °C).146 Furthermore, high ionic conductivity (0.325 S cm−1 after soaking in 6 M KOH) and good electrolyte retention was observed. In line with these excellent characteristics, the assembled solid state supercapacitor (sandwich-like configuration, cellulose separator impregnated with 6 M KOH placed between the activated carbon electrodes) device outperformed other devices based on commercial separators in terms of specific capacitance (110 F g−1 at 1 A g−1 current density), rate capability (i.e., high capacitance retention at high current density), capacitance retention (84.7% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles), equivalent series resistance, energy density and power density (energy density more than 1.5-times higher than the performance achieved with commercial cellulose-based separators). Furthermore, the authors also prepared interdigitated finger-type micro-supercapacitors via a novel method (Fig. 14C): patterns (PTFE mask and activated carbon powder) were deposited on the cellulose/IL substrate prior to the regeneration step, which represents a simple, cost-effective method without using additives and special tools applied for common patterning methods (photolithography, in-printing, etc.). The micro-supercapacitor device showed excellent formability without decrease in capacitance or change in the electric double layer behaviour (see Fig. 14D, similar cyclic voltammograms). The device gave superior energy density and power density compared to the performance of other micro-supercapacitors in the literature (Fig. 14E). In addition, it was demonstrated that the micro-supercapacitor can be connected in series (giving twice the operating voltage) or parallel (giving about twice the current and discharge time), and can drive a digital watch in plane or bent state (Fig. 14F and G).
000 cycles), equivalent series resistance, energy density and power density (energy density more than 1.5-times higher than the performance achieved with commercial cellulose-based separators). Furthermore, the authors also prepared interdigitated finger-type micro-supercapacitors via a novel method (Fig. 14C): patterns (PTFE mask and activated carbon powder) were deposited on the cellulose/IL substrate prior to the regeneration step, which represents a simple, cost-effective method without using additives and special tools applied for common patterning methods (photolithography, in-printing, etc.). The micro-supercapacitor device showed excellent formability without decrease in capacitance or change in the electric double layer behaviour (see Fig. 14D, similar cyclic voltammograms). The device gave superior energy density and power density compared to the performance of other micro-supercapacitors in the literature (Fig. 14E). In addition, it was demonstrated that the micro-supercapacitor can be connected in series (giving twice the operating voltage) or parallel (giving about twice the current and discharge time), and can drive a digital watch in plane or bent state (Fig. 14F and G).
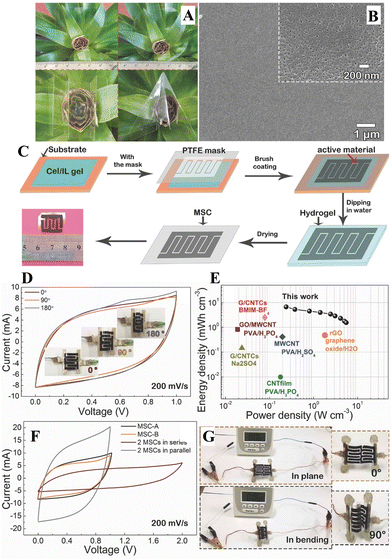 | ||
| Fig. 14 (A) Photograph showing the regenerated cellulose film with high transparency and flexibility. (B) Scanning electron microscopy (SEM) image about the pore structure. (C) Processing steps for fabricating the interdigitated finger-type electrode. (D) Cyclic voltammograms recorded for different bent states of the flexible micro-supercapacitor. (E) Ragone-plot that compares the performance of the device with others from the literature. (F) Cyclic voltammogram recorded for devices connected in parallel or in series. (G) Micro-capacitor device powering a digital watch. Reproduced from ref. 148 with permission from Wiley, copyright 2017. | ||
In another work from the same group,149 a regenerated cellulose/glass microfiber composite film was prepared and used as a flexible separator for lithium-ion batteries (Fig. 15A). The composite film was prepared by carefully choosing the concentration (1 wt% as optimal), and thus adjusting the viscoelastic property of the cellulose/[Bmim][Cl] solution in order to make it penetrate into the microfiber structure. The presence of cellulose conferred exceptional flexibility and structural stability to the microfiber separator, which could be shaped into various forms without fracture even when wetted with electrolyte (Fig. 15B and C). Furthermore, the composite separator exhibited good thermal stability (200 °C), electrolyte wettability; superior electrolyte retention, ionic conductivity and Li+ transference number compared to the glass microfiber film as reference. The assembled LiFePO4–Li battery cell showed lower charge transfer resistance (102.2 Ω), higher discharge specific capacitance (160.6 mA h g−1 at 0.2 C), better rate capability (145.9 mA h g−1 at 2 C) and cycling stability (108.5 mA h g−1 after 1000 cycles at 2 C) compared to the reference (Fig. 15D). A pouch cell was also assembled to demonstrate a flexible Li battery application. It was concluded that the inhibition of Li dendrite growth and rapid ion diffusion through the composite membrane contributed to the excellent rate performance and cycling stability.
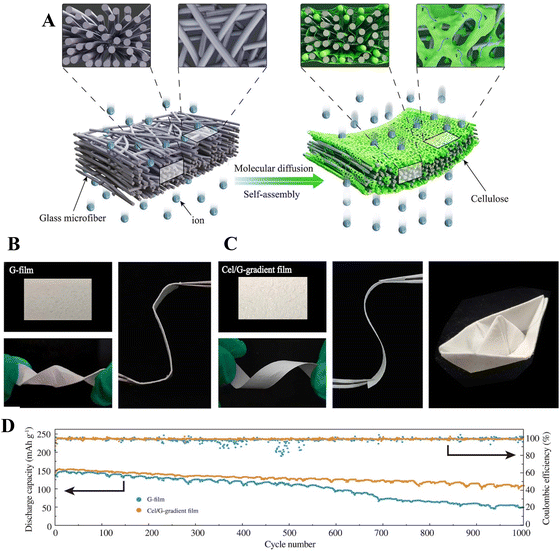 | ||
| Fig. 15 (A) Fabrication of cellulose/glass microfiber composite film for application as separator in lithium-batteries. (B) Glass microfiber film with poor flexibility. (C) Cellulose/glass microfiber composite film with excellent flexibility and formability. (D) Cycling stability of a LiFePO4/Li cell using glass microfiber (blue line) vs. cellulose/glass microfiber composite (orange line) separators. Reproduced from ref. 149, copyright 2021 The Authors. | ||
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Conductive filler | Conductivity | Film properties, observations | Application | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Unmodified plant cellulose | [Bmim][Cl] | — | — | Vertically aligned multiwalled carbon nanotubes (MWCNTs) | — | Few tens of microns thick film | Ultrathin flexible supercapacitor, Li-ion battery, hybrid supercapacitor-battery | Ethanol | 150 |
| Cotton linter pulp | [Bmim][Cl] | ∼4 wt% | 100 °C (−) | Graphite powder in the film. Polypyrrole nanoparticle coating on the film surface | 55 S m−1 at 200% graphite loading. Conductivity attributed to the polypyrrole coating (graphite forms discontinuous segments) | 10–100 μm thick film. Graphene and polypyrrole both improve electromagnetic shielding synergistically | Lightweight electromagnetic interference shielding material | Water | 154 |
| Cotton linter pulp | [Amim][Cl] | ∼5 wt% | PBS (poly(butylene succinate)) dispersed (melt) first at 130 °C (1 h); cellulose added at 90 °C (2 h) | MWCNTs | 1.3 × 10−5 S m−1 (4 wt% MWCNTs) | PBS particles and MWCNTs uniformly dispersed in the matrix | Presence of PBS could improve toughness and ductility. Elastic modulus and tensile strength increase due to MWCNT | Water | 151 |
| α-Cellulose (DP 650) | [Amim][Cl] | — | 90 °C (2 h) | Exfoliated graphite (single and multilayers of graphene) | 2.5 S m−1 (0.3 wt% graphene loading) | Regenerated cellulose nanofibers in the film | Increasing tensile strength and toughness by addition of graphene (0.1–1 wt%) | Water | 155 |
| Coniferous dissolving pulp (DP 500) | [Amim][Cl] | 3 wt% | 90 °C (0.5 h) | Ag nanofiber deposited on the surface | Sheet resistance of 29 Ω □−1 | HNO3 treatment improved the transmittance. Optimal treatment gave 80% transmittance | Perovskite solar cells | Water | 156 |
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Conductive filler | Conductivity | Film properties, observations | Application | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Microcrystalline cellulose | [Emim][Cl] | 6 wt% | 90 °C (24 h) | Single-walled carbon nanotubes (SWCNTs) | Maximum electrical conductivity at 2 wt% SWCNT loading (0.3 S m−1) | Thickness about 30 μm. Uniformly dispersed SWCNTs | 1 wt% SWCNT increases elastic modulus by 188%, tensile strength by 129%, elongation at break by 51%. Improved oxygen barrier and water absorption resistance | Water | 152 |
| Wood pulp cellulose (DP 900) | [Emim][DEP] | ∼1 wt% | 90 °C (3 h) | Graphene nanoplatelets (GN) + MWCNTs | Highest: 1124 S m−1 (GN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MWCNT MWCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cellulose 7 Cellulose 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by weight) 2 by weight) |
14–83 μm | After carbonisation, application as electrode in Li-ion battery | Water | 153 |
The first work on conductive regenerated cellulose films was done by Pushparaj et al.150 They impregnated vertically aligned multiwalled carbon nanotubes (MWCNTs; grown on a silicon substrate via thermal chemical vapor deposition (CVD) technique) with a cellulose/[Bmim][Cl] solution. After immersing in ethanol, the regenerated cellulose/MWCNT film could be peeled off from the substrate. Symmetric supercapacitor device was fabricated using the composite film, affording a flexible, thin and lightweight design. An extra thin cellulose layer was used as the separator, and [Bmim][Cl] as electrolyte (see Fig. 16). The supercapacitor showed good performance (≈13 W h kg−1 energy density, 1.5 kW kg−1 power density), with excellent operating temperature window (195–423 K). The device could be operated with various body fluids as electrolytes (such as body sweat, blood), which is promising for biomedical applications (e.g., biomedical microelectromechanical systems). The authors fabricated flexible Li-ion battery using the cellulose/MWCNT composite as cathode. Furthermore, the supercapacitor and battery device were also combined to make hybrid devices.
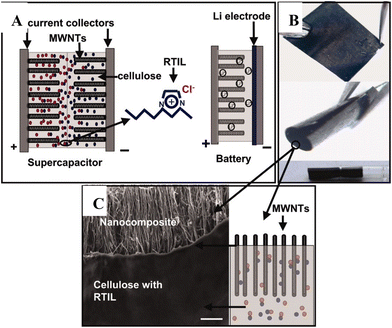 | ||
| Fig. 16 (A) Supercapacitor and Li-ion battery device fabricated using the regenerated cellulose/MWCNT composite. (B) Demonstration on the flexibility of the composite film. (C) Cross-sectional SEM image of the nanocomposite film. Reproduced from ref. 150 with permission from the National Academy of Sciences, copyright 2007. | ||
Several works prepared conductive filler/regenerated cellulose composite by including the filler in the ionic liquid/cellulose solution prior to the coagulation process. Interestingly, it was observed by Ye et al.157 that graphite can be exfoliated directly in a [Bmim][Cl]/cellulose solution under ultrasonication. They recognised that cellulose can help the exfoliation process, probably through hydrogen bonding between –OH groups of cellulose and the π electrons of graphene. Similar phenomenon was noted later by Zhang et al.155 using [Amim][Cl]. They also observed the positive effect of the presence of small amount of single/multilayer graphene (0.1–1 wt%) on the mechanical properties of the regenerated cellulose film. In the regenerated cellulose film, cellulose nanofibers were observed, the diameter of these fibres were affected by the amount of graphene in the composite. At the lowest graphene loading, graphene was nicely dispersed resulting in small cellulose nanofibers, meanwhile increasing the graphene content resulted in graphene aggregation and larger cellulose nanofibers. Toughness and tensile strength were the highest when cellulose nanofibers had the smallest diameter. Furthermore, Chen et al.154 studied relatively high amount of graphite loading (10–200 wt%) in regenerated cellulose films, which had negative effect on the tensile properties. In this case, graphite flakes were present in the film, exfoliation by e.g., ultrasonication was not done, graphite flakes were only stirred in the ionic liquid. The prepared graphite/regenerated cellulose film coated with a polypyrrole layer showed good properties as an electromagnetic interference shielding material (electromagnetic shielding effectiveness: 30 dB). Relatively small loading of single walled carbon nanotubes (SWCNT) had also positive effect on the tensile strength and elongation at break, with an increase in the elastic modulus.152 Furthermore, Wang et al.151 prepared regenerated cellulose/poly(butylene succinate) composite with improved toughness and ductility (compared to regenerated cellulose), the presence of multiwalled carbon nanotubes (0.5–4 wt%) could further improve the tensile strength of the material. In respect to electronics applications of the films, it was noted in several cases that electrical conductivity is limited by the discontinuity of the conductive filler at low concentrations. At high concentrations, agglomeration limits the continuity, and thus the electric contact between filler segments. In order to prevent graphene restacking, and improve the contact points throughout the cellulose matrix, Zhou et al.153 prepared graphene/MWCNT/cellulose composite using [Emim][DEP] ionic liquid. At optimum graphene/MWCNT/cellulose loading the regenerated film reached a conductivity of 1124 S m−1, which is the highest reported to the best of our knowledge for a conductive regenerated cellulose film prepared via ionic liquid processing.
4.6. Regenerated cellulose/biopolymer green composites using ionic liquids
Since a synthetic petroleum-derived polymer additive would compromise the green nature of cellulose-based materials, in order to tailor their properties, several works focused on blending cellulose with other biopolymers to obtain tailor-made biocomposites with improved properties (Tables 6–8). Some ionic liquids that can dissolve cellulose show good ability to dissolve other biopolymers as well, among them are a range of polysaccharides: chitin/chitosan, glycosaminoglycans (e.g., heparin), starch, agarose, carrageenan, etc.; proteins: silk, wool keratin, collagen; and lignocellulosics: xylan and lignins, etc.158 This provides unique opportunity to prepare homogeneous blends of cellulose with biopolymers, which lead in several cases to advantageous film properties.| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Biopolymer additive | Film properties, observations | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|
| Microcrystalline cellulose | [Bmim][Cl] | 10 wt% | 100 °C (24 h) | Chitin; 5 wt% [Amim][Br] solution, same dissolution procedure as cellulose | Elasticity of the film decreases with increasing chitin content | Water | 159 |
| Microcrystalline cellulose (DP 124) | [Bmim][Ac]/γ-valerolactone 8/1 | 8 wt% | 90 °C (3 h) | Chitin; 2 wt% solution prior to mixing with cellulose/solvent system | Chitin contributes to the elasticity of the composite; hydrophobicity increases with increasing chitin content | Ethanol, soaking for 4 h, then Soxhlet extraction for 24 h | 162 |
| Microcrystalline cellulose | [Dmim][Cl] | 4 wt% | 75 °C (15 min) | Chitosan; 4 wt% using [Dmim][Cl]/[Hmim][Cl] 9/1 | Composite has no porous structure. Chitosan makes the composite brittle; tensile strength improves with cellulose content | Methanol | 164 |
| Cellulose pulp (DP 670) | [Bmim][Ac] | 6 wt% | 85–95 °C (12 h) | Chitosan; 6 wt% 85–95 °C (3–4 days) | Good miscibility without phase separation | Methanol/water 50/50 | 165 |
| Cellulose pulp (DP 630) | [Amim][Cl] with 1 wt% Gly·HCl | 3–5 wt% | 80 °C (12 h) | Chitosan; 2–35 wt% relative to cellulose, simultaneous dissolution of the two polymers | Antibacterial films could be obtained | Water | 166 |
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Biopolymer additive | Film properties, observations | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|
| Microcrystalline cellulose | [Emim][Ac] | 1 wt% | 100 °C (1 h) | Xyloglycan | At certain composition nanostructuration was observed, which resulted in increased tensile strength and strain at break | Water | 169 |
| Cellulose from spruce via chlorite delignification | [Emim][Ac] | ∼4 wt% | 80 °C (4 days) | Arabinoglucuronoxylan | Addition of xylan increased the stiffness of the composite at relatively low humidity conditions | Ethanol | 170 |
| Cellulose solution in [Emim][Ac] (Cellionic™); DP ∼ 680 | [Emim][Ac] | 5 wt% | 90 °C (3 h) | Xylan from birch wood; softwood kraft lignin from pine | Synthetic wood composite with enhanced hydrophobicity, thermal stability, tensile strength and UV shielding properties | Water | 172 |
| Microcrystalline cellulose | [Emim][Ac] | 5 wt% | 80 °C (3 h) | Xylan from birch wood; Alkali lignin; 80 °C (2 h) mixing with the cellulose/[Emim][Ac] solution | Synthetic wood composite. Biodegradability (cellulase) was increased with xylan content whereas decreased with lignin addition. Water vapor solubility was positively correlated with xylan content | Water | 173 |
| Cellulose bamboo pulp (DP 650) | [Amim][Cl] | ∼2–5 wt% | 80 °C (30 min) | Starch, lignin and cellulose dissolved at a 6 wt% total concentration in [Amim][Cl] | By optimising the ratio of cellulose/lignin/starch, the mechanical properties could be maximised. The biopolymers exert synergistic effects. Gas barrier properties promising for food packaging applications | Water | 175 |
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Biopolymer additive | Film properties, observations | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|
| Eucalyptus cellulose (via chlorite delignification) | [Bmim][Ac] | 5 wt% | 100 °C (3 h) | Gutta percha (natural rubber) from Eucommia ulmoides Oliver leaves. 5–15 wt% relative to cellulose. Dissolved in chloroform | Enhanced tensile strength and elongation at break. Decreasing oxygen permeability. Increasing hydrophobicity. Food packaging applications | Water | 192 |
| Cellulose (not specified) | [Bmim][Cl] | 10 wt% | Preheating at 70 °C, then microwave heating | Heparin imidazolium salt dissolved in 1-ethyl-3-methylimidazolium benzoate | Nanoporous, blood compatible membrane for renal dialysis | Ethanol | 193 |
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Biopolymer additive | Film properties, observations | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|
| Microcrystalline cellulose | [Bmim][Cl] | 2 wt% | 90 °C (−) | Silk fibroin from Bombix mori | Enhanced mechanical properties (tensile strength, elongation at break), thermal stability and water resistance. Conformational transition from random coil to β-sheet | Methanol | 178 |
| Cotton linter pulp | [Bmim][Cl] | 4 wt% | 90 °C (−) | Silk fibroin from Bombix mori | Detailed solid-state NMR study revealing hydrogen bonding between the biopolymers, cellulose structure and secondary protein structure in the composite | Methanol | 180 |
| Microcrystalline cellulose | [Amim][Cl] | 10 wt% | 85 °C (−) | Silk fibroin from Bombix mori | Intra- and intermolecular interactions between the biopolymers that shape the supramolecular structures (studied by small- and wide-angle X-ray scattering) and determine the thermal/physical properties of the composite. These features can be tuned by changing the composition | Water | 179 |
| Microcrystalline cellulose | [Bmim][Cl] | 10 wt% | 100 °C (−) | Silk fibroin from Bombix mori (silk fibroin/cellulose fixed at 1/9 weight ratio) | The bulkiness of the anion strongly affects the fraction of β-sheet structure, the highest amount was obtained for [Bmim][Br] and [Bmim][MeSO3]. The highest level of β-sheet configuration also meant the highest thermal stability | Water | 181 |
| [Emim][Cl] | |||||||
| [Amim][Cl] | |||||||
| [Emim][Ac] | |||||||
| [Bmim][Br] | |||||||
| [Bmim][MeSO3] | |||||||
| Microcrystalline cellulose | [Amim][Cl] | 5 wt% | 103 °C (48 h) | Silk fibroin from Bombix mori | Theoretical model to predict the crystallinity, thermal stability and Young's modulus of silk fibroin/cellulose blends | Water | 182 |
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Biopolymer additive | Film properties, observations | Antisolvent | Ref. |
|---|---|---|---|---|---|---|---|
| Microcrystalline cellulose | [Emim][Ac] | 9 wt% | 85 °C (24 h) | Silk fibroin from Bombix mori (silk fibroin/cellulose fixed at 1/9 weight ratio; 10 wt% total polymer concentration) | Positive correlation between cellulose crystallite size (cellulose II) and H2O2 concentration. Protein structure is not much affected by the coagulation process | 0–25% aqueous H2O2 solution | 183 |
| Microcrystalline cellulose | [Emim][Ac] or [Emim][Cl] | 5 wt% | 85 °C (24 h) | Silk fibroin from Bombix mori (silk fibroin/cellulose fixed at 1/1 weight ratio; 10 wt% total polymer concentration) | The anion of the ionic liquid has an effect on the protein structure (a more amorphous structure is obtained when [Emim][Ac] is used) | 0–25% aqueous H2O2 solution | 184 |
| Microgranular cellulose | [Bmim][Cl] | 5 wt% | 100 °C (−) | Wool keratin. 5 wt% dissolved at 100 °C for 10 h. The cellulose and keratin solutions added at 100 °C, stirred for 12 h | Improved thermal stability and elongation at break compared to the individual biopolymers. Hydrogen bonding interactions between the biopolymers | Water | 186 |
| Microcrystalline cellulose | [Emim][Ac] | 10 wt% (total polymer) | 80 °C (24 h) | Wool keratin (Keratin Azure) | The secondary structure of the protein can be influenced by the coagulation medium. Cellulose crystallinity increased when aqueous 25% H2O2 was used as coagulation bath | Aqueous 1% or 25% EtOH and 1% or 25% H2O2 | 187 |
| Cotton cellulose | [Bmim][Cl] | — | 100 °C (6 h) | Native skin collagen | — | Water/methanol/ethanol/acetone; at 4 °C | 188 |
| Microcrystalline cellulose | [Emim][Ac] | ∼6 wt% | 60 °C (4 h) | Calf skin collagen. Collagen dissolution at 25 °C (6 h) | Hydrogen bonding between the biopolymers. Denaturation temperature increases in the composites (except at high collagen loading, 5/1 cellulose/collagen). High collagen content leads to phase separation and inhomogeneity | Water; at 4 °C | 190 |
| Calf skin collagen. Collagen dissolution at 25 °C (36 h) | 10% aqueous ethanol | 191 |
 | ||
| Fig. 17 (A) Normalised tensile properties of the xyloglycan/cellulose blends. Visual models for composites with (B) continuous cellulose matrix (xyloglycan/cellulose <40%), (C) continuous xyloglycan and cellulose matrix – nanostructured composite (xyloglycan/cellulose ∼40–50%), (D) continuous xyloglycan matrix (xyloglycan/cellulose >50%). Red line represents amorphous xyloglycan domains, black line indicates cellulose semicrystalline domains. (E) AFM phase contrast images of the composites. Reproduced from ref. 169 with permission from Elsevier, copyright 2017. | ||
Several works prepared so called “synthetic wood” composites that contain lignin besides cellulose and hemicellulose, and thus have all the major constituents of lignocellulosic biomass (Table 7). Lignin is the most abundant aromatic biopolymer in nature, it is a heteropolymer made of phenylpropanoid units.171 Synthetic wood composites based on the tricomponent biopolymer system was studied first by Simmons et al.172 using [Emim][Ac] as solvent, and water as coagulant. The ionic liquid solution contained 5 wt% cellulose, 3 wt% xylan from birch wood and 2 wt% softwood kraft lignin prior to the regeneration process, mimicking wood composition. The regenerated synthetic wood composite was more hydrophobic (water contact angle of 72°) than regenerated cellulose (45°) or cellulose/lignin (54°) composite, which was attributed to the smooth surface of the film (with an average roughness of about 50 nm). In addition, the synthetic wood composite had superior tensile strength (∼62 MPa), the synergistic effect may have arisen from the strong hydrogen bonding interactions between the biopolymers. The authors observed also better UV shielding properties and thermal stability compared to the regenerated cellulose film. Furthermore, synthetic wood composites with PEG, chitosan and multiwalled carbon nanotubes were prepared to endow the film with enhanced hydrophilicity, antibacterial properties and with increased dielectric constant, respectively. Kim et al.173 studied later the biodegradability and water vapor solubility of similar synthetic wood films prepared using [Emim][Ac] and water coagulation bath. The authors found that the biodegradability (cellulase test) increased with increasing xylan content, however, decreased with increasing lignin content. The water vapor solubility also increased with the addition of xylan, which may be a reason for the enhanced cellulase activity. The synthetic wood composite was studied further by Lee et al.174 for biomimetic artificial photosynthesis through incorporating light harvesting porphyrins in the composite film (Fig. 18A and B). The porphyrins were solubilised in [Emim][Ac], and then mixed with the synthetic wood solution using the same solvent, prior to the regeneration step in water upon which the porphyrins could be entrapped in the matrix. The synthetic wood composite showed higher level of NADH generation (Fig. 18C), higher photo-to-dark current ratio, and faster response compared to that of the composite based on cellulose only (Fig. 18D). The authors attributed the enhanced charge transfer efficiency to the presence of lignin, which has redox active phenol groups.
 | ||
| Fig. 18 (A) Light harvesting apparatus of green plants (left) and the concept of using a synthetic wood composite for artificial photosynthesis. (B) The structure of the natural photosynthetic pigment chlorophyll and the studied porphyrins as additives. (C) Photochemical NADH generation using the synthetic wood composite/cellulose with porphyrins, [Cp*Rh(bpy)H2O]2+ as hydride transfer mediator, and triethanolamine as sacrificial electron donor. (D) Photocurrent generation of the respective composites. Reproduced from ref. 174 with permission from Wiley, copyright 2011. | ||
Starch (a complex carbohydrate consisting of two types of polysaccharides: amylose (mainly α-1,4-linked D-glucopyranose units) and amylopectin (highly branched polymer, α-1,6-linkages besides the α-1,4-linked D-glucopyranose residues)) has also been considered as a biopolymer additive. Wu et al.175 prepared cellulose/lignin/starch ternary composites via simultaneously dissolving the polysaccharides in [Amim][Cl], followed by regeneration in water. By optimising the amount of each biopolymer in the composite, the mechanical properties (tensile strength, elongation at break) could be greatly enhanced. It appeared that cellulose contributes to the flexibility of the material. The synergistic interaction of the biopolymers could be explained by destruction of the hydrogen bonds between each biopolymer upon dissolving in [Amim][Cl], followed by reconstitution of a hydrogen boding network between the homogeneously dispersed components during the regeneration step. In addition, good gas barrier properties and CO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 permeation ratios (1.05–1.67) indicated the applicability of the film for food packaging purposes.
O2 permeation ratios (1.05–1.67) indicated the applicability of the film for food packaging purposes.
 | ||
| Fig. 19 (A) Structure of cellulose and silk fibroin with numbering for peak assignment (below). (B) 13C CP/MAS NMR spectra of regenerated silk fibroin (SF), cellulose and cellulose/silk fibroin composite (3/1). Asterix indicates spinning sideband. (C) 13C NMR spectra of regenerated SF and cellulose/SF composite in the Ala 13Cβ region for comparison. Reproduced from ref. 180 with permission from the American Chemical Society, copyright 2017. | ||
Wool keratin is another interesting natural protein to consider as additive in cellulose composites. It is an underutilised, low-cost material generated in large quantities as waste by the wool textile industry, and by the food industry through the operation of butcheries. The dissolution of wool keratin in an ionic liquid was first reported by Xie et al.185 using [Bmim][Cl], and regenerated wool keratin and cellulose/wool keratin blends were also fabricated in this work using methanol as coagulation medium. The properties of the cellulose/wool keratin regenerated films were studied later in detail as a function of the composition by Hameed and Guo.186 The blends showed higher thermal stability and elongation at break compared to the individual components, these properties reached maximum value at a certain composition (cellulose/wool keratin 60/40). It was also observed through FTIR measurements that hydrogen bonding interactions take place between the biopolymers. In a recent study,187 the effect of the coagulation bath (1% or 25% EtOH and 1% or 25% H2O2 aqueous solution) on the structure of cellulose and wool keratin was studied using [Emim][Ac] as solvent. It appeared that the amount of β-sheet and α-helical protein structures can be manipulated by the composition, coagulation bath additive and its concentration. The highest amount of α-helices (34.3%) was obtained for 75/25 wool keratin/cellulose composites using 25% H2O2 aqueous solution as coagulation bath, this value is much higher than that of the initial wool keratin sample (8.7%). The highest amount of β-sheets (33.6%) were observed when the coagulation bath was 25% aqueous EtOH solution, this value is close to the initial keratin sample (33.0%). Furthermore, an increase in cellulose crystallinity was observed when higher concentration of H2O2 was used.
Type I collagen is an abundant protein found in vertebrates, it is widely used for biomedical applications, and in the food and cosmetic industry, owing to its biocompatible, biodegradable and edible character. The processing of collagen in an ionic liquid was reported using [Bmim][Cl] for the first time,188 the dissolution was done at 100 °C (for 6 h). It was recognised through FTIR and XRD analysis that the triple helical structure of collagen is partly destroyed, random coils are formed and possible degradation may also take place. It was also observed that the secondary structure can be significantly influenced by the type of the coagulant (they used water, ethanol, methanol and acetone). Collagen/cellulose blends could also be prepared and shaped into various forms during the coagulation step (films, gels, fibres). Using this processing system ([Bmim][Cl], 100 °C), Wang et al.189 prepared hydrogel beads by dropping the collagen/cellulose binary solution into water. These beads were used for the adsorption of Cu(II), this process showed a positive correlation with collagen content until a limiting value (a mass ratio of 2/1 collage/cellulose). Zhang et al.190 used [Emim][Ac] as solvent to dissolve first cellulose at 60 °C (for 4 h), followed by the addition of collagen at room temperature, in order to operate below the denaturation temperature of the protein. They found that the denaturation temperature of the protein increased in the regenerated cellulose/collagen films. Their FTIR results indicated the formation of hydrogen bonds between the polymers. While the addition of relatively small amount of collagen (cellulose/collagen 30/1) increased the thermal stability (maximum decomposition temperature) and mechanical properties (elastic moduli from dynamic mechanical analysis (DMA)), these parameters declined at higher collagen loading (10/1 cellulose/collagen). Cellulose crystallinity also decreased significantly for this higher collagen content. In another study from the same group,191 it was confirmed that at high collagen loading aggregates exist in the solution (at 10/1 cellulose/collagen; based on dynamic rheology experiments). The lack of homogeneity and phase separation was also observed at high collagen content in the composite films (5/1 cellulose/collagen).
Heparin, is a glycosaminoglycan, a negatively charged polysaccharide that can be found in animals, and is an important medication owing to its anticoagulant properties. Murugesan et al.193 aimed at fabricating blood-compatible dialysis membranes using regenerated cellulose/heparin composites. The imidazolium salt of heparin was first prepared and dissolved in 1-ethyl-3-methylimidazolium benzoate ([Emim][Ba]). Cellulose was separately dissolved in [Bmim][Cl] (10 wt% concentration), and the two solutions were mixed to prepare the binary composite film using ethanol coagulation bath. The successful entrapment of heparin in the matrix was confirmed by leaching test, showing small amount of loss. The excellent blood compatibility of the membrane was demonstrated via Activated Partial Thromboplastin Time (APTT) and Thromboelastography (TEG) tests. The composite exhibited a nanoporous structure; in an equilibrium dialysis test urea could diffuse through the membrane while bovine serum albumin (BSA) was mostly retained. This biocompatible membrane is promising for renal dialysis application while avoiding systemic heparinisation and the complications associated with that process.
Park et al.194 in a comprehensive work studied agar, agarose, arabic gum, bacterial cellulose, chitosan, collagen, β-cyclodextrin, dextran, gelatine, κ-carrageenan, lignin, silk, starch, tragacanth gum, xanthan gum and xylan biopolymers as additives to cellulose, using [Emim][Ac] solvent (dissolution at 60 °C, stirring with cellulose for 3 h), and ethanol as coagulant (cellulose/biopolymer 4/1). The composites had diverse physicochemical properties. The water contact angle varied between 90.1° (cellulose/chitosan) and 33.0° (cellulose/κ-carrageenan), thus more hydrophobic and hydrophilic films could be obtained, respectively, compared to cellulose (water contact angle of 52.8°). The high light barrier properties of the lignin/cellulose blend were noted below 400 nm, indicating good UV-shielding properties (Fig. 20A). The chitosan/cellulose film exhibited excellent adsorption capacity towards organic dyes (9.1-fold and 7.9-fold higher adsorption capacity, respectively, compared to cellulose film). The biopolymer composite films showed enhanced adsorption for the enzyme lysozyme compared to cellulose films, and for the enzyme pepsin some films showed excellent adsorption behaviour exceeding that of cellulose (e.g., for the cellulose/β-cyclodextrin film). Furthermore, outstanding cell viability (TCMK-1 cells) could be observed for some of the films (superior to cellulose), the highest was achieved for the cellulose/gelatine films (Fig. 20B).
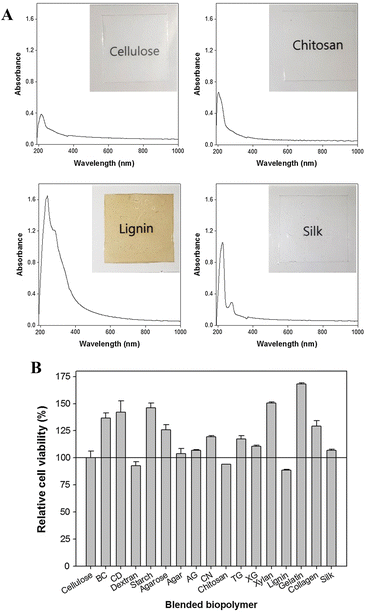 | ||
| Fig. 20 (A) UV-Vis spectra of regenerated cellulose, cellulose/chitosan, cellulose/lignin and cellulose/silk films with good light barrier properties below 400 nm. (B) Cell viability of TCMK-1 cells cultured on the composite films. Reproduced from ref. 194 with permission from Springer Nature, copyright 2020. | ||
4.7. Cellulose-based ionogels, hydrogels and aerogels using ionic liquids
The first cellulose-based physical ionogel was reported by Kadokawa et al.196 in 2008. They prepared a concentrated cellulose solution (15 wt%) in [Bmim][Cl] (at 100 °C for 24 h), which was kept between glass slides for 7 days. The obtained gel was washed with ethanol to remove the excluded ionic liquid. Besides the ionic liquid and cellulose, the ionogel contained considerable amount of water as well (in their work water amount was 8.69–16.0 eq. per glucose unit, whereas ionic liquid content was 2.48–4.68 eq. per glucose unit). According to their explanation, the ionogel forms as water slowly diffuses into the ionic liquid/cellulose solution, creating non-crystalline aggregates as physical crosslinking points. Such materials can be classified as physical ionogels, as they are generated through reversible non-covalent interactions, in contrast to chemical ionogels that contain covalent crosslinks between the polymer chains. The physical ionogel obtained this way can be easily reversed to its original fluid form by heating to 150 °C. Please note that a gel material can be easily identified, and its physical/chemical gel character judged most commonly through oscillatory rheometry or dynamic mechanical analysis (DMA) measurements;197,198 unfortunately, such characterisation is not always present in the literature. The physical ionogels that were prepared by others through similar strategy to that of the original work of Kadokawa et al.196 by dissolving cellulose, are summarised in Table 9.
| Type of cellulose | Ionic liquid | Cellulose concentration | Dissolution temperature (time) | Properties, observations | Application | Ref. |
|---|---|---|---|---|---|---|
| Microcrystalline cellulose | [Bmim][Cl] | 15 wt% | 100 °C (24 h) | Solution kept between glass slides for 7 days. Finally washing with ethanol | — | 196 |
| Microcrystalline cellulose | [Bmim][Cl] | 5 wt% (and 10 wt% chitin solution in [Amim][Br]) | 100 °C (24 h) | Cellulose/chitin composite ionogel at 3/1 weight ratio (solutions mixed at 100 °C (1 h)) | Supercapacitor | 199 and 200 |
| Microcrystalline cellulose | [Bmim][Cl] | 13 wt% (DMA as plasticiser and co-solvent) | 100 °C (15 min) | Performance superior to common dielectric elastomers | Actuator | 201 |
| Microcellulose (ARBOCEL® MF 40-7, J. Rettenmaier & Sons) | Phosphonate-based ionic liquid with [Emim] cation/[Emim][NTf2] | — | — | [NTf2] anion helps improving ionic mobility, while the phosphonate anion based ionic liquids form gels with cellulose | Flexible paper-based electronics; electrolyte-gated field-effect transistors (FETs) | 202 |
| Cellulose powder (Sigma Aldrich) | 1-methyl-3-propylimidazolium iodide ([Mpim][I])/[Emim][SCN] 1/1 | 5 wt% | 90 °C (24 h) | A maximum photoconversion efficiency of 3.33% | Gel electrolyte for dye-sensitised solar cells | 203 |
| Cellulose source not defined | [Bmim][Cl] | 8 wt% | Not defined | Dynamic ionogel with tunable properties by adjusting the water content | Demonstrated application as electronic skin (sensing). Other possible applications such as flexible electronics, soft robotics, energy storage and other intelligent devices | 205 |
Cellulose-based physical ionogels are promising materials for solid electrolytes in electrochemical applications. The first study towards this direction was done by Yamazaki et al.199,200 on hybrid cellulose/chitin ionogels for supercapacitor devices. Cellulose was dissolved in [Bmim][Cl], while chitin was dissolved in [Amim][Br]. The two solutions were mixed (to reach a cellulose/chitin weight ratio of 3/1), and ionogel was obtained after leaving the sample at room temperature for 4 days. Additionally, the obtained gel was immersed in H2SO4 solution in order to have an acidic cellulose/chitin ionogel. Symmetric two-electrode device was assembled using the ionogel sandwiched between activated carbon fiber cloths as electrodes. The supercapacitor device exhibited a specific capacitance somewhat higher than the device with H2SO4 electrolyte, together with good cycling stability (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, 80% capacitance retention, performed at 5 A g−1 current density).199 The device showed good stability for operating at wide temperature window (−10–60 °C).200 Furthermore, Kunchornsup and Sirivat201 fabricated cellulose/[Bmim][Cl]/DMA ionogels as electro-active paper for actuator applications. According to their results, several electromechanical parameters of the ionogel were superior to other studied dielectric elastomers at room temperature. However, they identified the slow response speed of the ionogel as a limiting parameter, which should be improved in the future. Fig. 21A shows the experimental setup to study the response of the ionogel towards an applied electric field. According to the authors’ explanation, ionic polarisation of the imidazolium cations, together with polarisation of the hydroxyl groups of the cellulose structure cause the movement (Fig. 21B). Images of the bending and swinging responses are shown in Fig. 21C–F.
000 cycles, 80% capacitance retention, performed at 5 A g−1 current density).199 The device showed good stability for operating at wide temperature window (−10–60 °C).200 Furthermore, Kunchornsup and Sirivat201 fabricated cellulose/[Bmim][Cl]/DMA ionogels as electro-active paper for actuator applications. According to their results, several electromechanical parameters of the ionogel were superior to other studied dielectric elastomers at room temperature. However, they identified the slow response speed of the ionogel as a limiting parameter, which should be improved in the future. Fig. 21A shows the experimental setup to study the response of the ionogel towards an applied electric field. According to the authors’ explanation, ionic polarisation of the imidazolium cations, together with polarisation of the hydroxyl groups of the cellulose structure cause the movement (Fig. 21B). Images of the bending and swinging responses are shown in Fig. 21C–F.
 | ||
| Fig. 21 (A) Experimental setup for studying the bending response of the ionogel under an applied electric field. (B) Schematic representation of the proposed mechanisms of the bending response. (C and D) Deflection and (E and F) swinging movement of the ionogel in response to a ground and positive electric field at room temperature. Reproduced from ref. 201 with permission from Elsevier, copyright 2012. | ||
Thiemann et al.202 developed cellulose ionogels for flexible electronics application. They fabricated electrolyte gated field-effect transistors (FETs), using multilayer coated flexible paper substrate in one of the devices (Fig. 22A and B). In their work, microcellulose (ARBOCEL® MF 40-7, from J. Rettenmaier & Sons) was casted on a glass slide first, followed by dropping ionic liquid on the film to obtain an ionogel (with cellulose/ionic liquid mass ratio of 3/70). They used ionic liquids that are based on [Emim] cations and methylphosphonate anions or their mixture with [Emim][NTf2] (Fig. 22C, note that TFSI = [NTf2]). [Emim] cations with methylphosphonate anions show good ability to dissolve/gelate cellulose, while [Emim][NTf2] is a non-dissolving ionic liquid that does not interact with cellulose, and its main role in the system is to increase ionic mobility. It was found that the highest specific capacitance (18.4 μF cm−2 determined via impedance spectroscopy measurement) was obtained for a binary system with [Emim][MeAc(Me)PO3] (where [MeAc(Me)PO3] is 2-methoxy-2-oxoethyl methylphosphonate anion)/[Emim][NTf2] at 9/1 ratio. These ionogels with high ionic mobility performed well for side-gated FET devices. The authors noted that by increasing the bulkiness of the anion (increasing ethylene glycol chain length) of the ionic liquid, the specific capacitance of the ionogel decreases. These ionogels with lower ionic mobility gave rise to higher electron mobility in spray-coated ZnO FETs.
 | ||
| Fig. 22 (A) The structure of the ionic liquids used to fabricate the ionogels for electrolyte-gated field-effect transistor (FET) devices. (B) Schematic description of the flexible FET device with ZnO nanorods (NRs; an SEM image is shown above the device) and cellulose ionogel. (C) Photograph of the flexible paper-based FET. Reproduced from ref. 202 with permission from Wiley, copyright 2013. | ||
Salvador et al.203 studied the application of cellulose-based ionogels as electrolytes for dye-sensitised solar cells. They used a mixture of two ionic liquids: 1-methyl-3-propylimidazolium iodide ([Mpim][I]) that can supply ions for the iodide/triiodide redox couple, a mediator in mesoscopic dye-sensitised solar cells;204 and [Emim][SCN] which helps decreasing the high viscosity of the [Mpim][I]/cellulose system, thereby increasing ionic mobility. They found that a 50/50 mixture of the two ionic liquids provides the best photovoltaic performance. Increasing cellulose concentration had a positive effect on the photocurrent and photovoltage, reaching a plateau at 5 wt% cellulose loading. Dissolution of cellulose was done in the ionic liquid mixture together with other electrolyte components (LiI and I2) at 90 °C (24 h), followed by gelation at room temperature for one day. 4-tert-Butylpyridine, another important component for the electrolytes of mesoscopic dye-sensitised solar cells, was added after the gelation process due to its volatile nature. A maximum photoconversion efficiency of 3.33% could be achieved with the fabricated dye-sensitised solar cell containing the quasi-solid electrolyte. Interestingly, the authors observed that the photovoltaic performance increases in the beginning of the operation, and reaches a plateau at a later time. According to their explanation, the movement of ions are hindered by the forming hydrogen bonds with the hydroxyl groups of cellulose. During operation, through the energy input, the energy barrier of these interactions can be overcome, releasing free iodide ions that can contribute to the improvement of the photovoltaic performance.
Zhao et al.205 developed a dynamic gel based on the [Bmim][Cl]/cellulose type ionogel. They realised that the ionogel exhibits tunable, and reversible dynamic properties based on the water content (Fig. 23A). At low water content (6 wt%), hydrogen bonds are generated between water and the cellulose molecules leading to a loosely packed Turing type topological network. In this system, ions exhibit low mobility, leading to low ionic conductivity. In addition, this ionogel exhibits excellent self-healing properties owing to the reversibility of the hydrogen bonds, and can adhere strongly to various substrates, e.g., glass, plastics and metals (it can lift more than 30 times its weight ones adhered to the metal substrate). At high water content (32 wt%), more hydrogen bonds are formed and a denser Turing microstructure is generated that exhibits robust toughness with excellent strength and formability (e.g., withstanding repeated rolling and folding). This system contains more hydrated ions with enhanced mobility, leading to a high ionic conductivity (a maximum of 40 mS cm−1). Switching between the two topological networks can be reversibly done by adjusting the water content of the ionogel (drying or increasing the relative humidity), with arriving at the original properties as demonstrated by the authors. Such dynamic ionogel can be useful for a range of applications such as soft robotics and flexible electronics. The authors demonstrated the application for electronic skin (e-skin, see Fig. 23B). The e-skin device was fabricated by sandwiching conductors between two ionogels (by taking advantage of the self-healing property of the material). The e-skin device can be applied for sensing various external stimuli such as breathing patterns, air flow and touching.
 | ||
| Fig. 23 (A) Transition between two topological networks by adjusting the water content of a cellulose/[Bmim][Cl] ionogel. The ionogel thus exhibits tunable dynamic properties as shown in the bottom of the figure. (B) Application of the dynamic ionogel in an electronic skin device that can be used for sensing e.g., breathing pattern, airflow and touching. Reproduced from ref. 205 with permission from Elsevier, copyright 2019. | ||
We would like to note that the ionic diffusion process that determines the conductivity in [Bmim][Cl]/cellulose type ionogels has been studied in detail by NMR spectroscopy.206 It was revealed that two cation diffusion processes can be identified in the matrix: (1) a fast translational diffusion in the pores where the ionic liquid behaves similarly to its bulk structure, and (2) a slow ion diffusion close to the surface of cellulose owing to secondary interactions. The authors noted that the small difference between the ionic conductivity of the ionogel and the bulk ionic liquid can be explained by the presence of the fast, unrestricted diffusion process supplying ions through the channels within the matrix.
Besides common unmodified cellulose starting materials, several works used bacterial cellulose,207methylcellulose208–210 and nanocellulose211 that have already been proven excellent for the preparation of other types of hydrogels (Table 10). The preparation of the physical ionogel is different in these works compared to previous studies discussed above, in that they do not follow the usual dissolution – gelation strategy. Bacterial cellulose is interesting in that it has high water absorption capacity, a hydrogel can have as high as 99 wt% water content. In the work of Smith et al.,207 a bacterial cellulose hydrogel was first prepared, followed by a solvent exchange process with ethanol to obtain an alcogel. Ionic liquid/ethanol mixture was added to this alcogel, and after evaporating ethanol, an ionogel could form. In a similar fashion, through co-solvent assisted diffusion (using ethanol, which is then evaporated), chemosensory molecules could also be incorporated in the ionogels for NH3 sensing and H2S detection. This bacterial cellulose ionogel was capable of hosting 99 wt% ionic liquid, which is an outstanding performance compared to other ionogels. The authors selected ionic liquids with [NTf2] anion and [Emim], 1-butyl-1-methylpyrrolidinium ([Bmpy]) and trihexyltetradecylphosphonium ([P14,6,6,6]) cations, which are non-dissolving ionic liquids for cellulose. Methylcellulose is another intriguing gelator for the preparation of physical ionogels. It is known that fibrillar methylcellulose can dissolve in water, and upon a heating–cooling cycle it reforms the nanofibrils which eventually generate an interconnected fibrillar network leading to gelation.212 The same phenomenon occurs when methylcellulose is dissolved in DMF.213 In the work of Mantravadi et al.,208 [Bmpy][NTf2] was used as ionic liquid to fabricate ionogels using methylcellulose. [Bmpy][NTf2] does not dissolve methylcellulose, the ionic liquid and methylcellulose was first dissolved separately in DMF. After mixing these solutions at certain ratios, gel formation was achieved by a thermal heating–cooling cycle (heating to 90 °C, cooling to room temperature; gelation occurs due to the presence of DMF). After removing DMF, an ionogel could be obtained with high 97 wt% ionic liquid content. This ionogel did not show thermoreversible sol–gel transition anymore, it remains solid until decomposition occurs (at around 300 °C). The authors highlighted that the film is self-standing and flexible with high moduli (>100 MPa) and high ionic conductivity (>10−3 S cm−1, room temperature). This procedure was later adapted to prepare [Emim][NTf2]/methyl cellulose ionogels with 95 wt% ionic liquid content.209 This ionogel was used as solid electrolyte for symmetric supercapacitors with activated porous carbon nanofiber electrodes. The supercapacitor device exhibited a wide operation window (3.5 V) owing to the ionic liquid, and high gravimetric capacitance (153 F g−1), energy density (around 65 W h kg−1 at 1150 W kg−1 power density) and cycling stability (4% capacitance loss after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles at 1 A g−1 current density) were reported. In the same way of preparation, a solvate (or chelate) ionic liquid/methylcellulose ionogel was also prepared, where the ionic liquid was [G4Li][NTf2] (G4 means tetraglyme here, with 1/1 molar ratio in respect to the Li+; 90 wt% ionic liquid content).210 Glymes can wrap around the Li+ ions, resulting in a stable complex; the resulting ionic liquid when combined with [NTf2] anion has high anodic stability (4.5 V), and exhibits high lithium ion transference number (tLi+ ≈ 0.5; fraction of electric current derived from lithium ions in respect to the total current, it indicates the mobility of lithium ions in the electrolyte). Interestingly, the electrochemical working window of the [G4Li][NTf2]/methylcellulose ionogel is about 5 V, somewhat higher than the liquid electrolyte [G4Li][NTf2]. The authors attributed this phenomenon to the forming hydrogen bonds between the oxygen groups of glyme and cellulose, thereby protecting them against oxidation. In addition, the ionogel showed high ionic conductivity (4 × 10−4 S cm−1, 30 °C), storage modulus (60 MPa) and thermal stability (>200 °C). The assembled Li0/ionogel/LiFePO4 coin cell showed comparable performance to that of the cell with the pure [G4Li][NTf2] ionic liquid.
000 charge–discharge cycles at 1 A g−1 current density) were reported. In the same way of preparation, a solvate (or chelate) ionic liquid/methylcellulose ionogel was also prepared, where the ionic liquid was [G4Li][NTf2] (G4 means tetraglyme here, with 1/1 molar ratio in respect to the Li+; 90 wt% ionic liquid content).210 Glymes can wrap around the Li+ ions, resulting in a stable complex; the resulting ionic liquid when combined with [NTf2] anion has high anodic stability (4.5 V), and exhibits high lithium ion transference number (tLi+ ≈ 0.5; fraction of electric current derived from lithium ions in respect to the total current, it indicates the mobility of lithium ions in the electrolyte). Interestingly, the electrochemical working window of the [G4Li][NTf2]/methylcellulose ionogel is about 5 V, somewhat higher than the liquid electrolyte [G4Li][NTf2]. The authors attributed this phenomenon to the forming hydrogen bonds between the oxygen groups of glyme and cellulose, thereby protecting them against oxidation. In addition, the ionogel showed high ionic conductivity (4 × 10−4 S cm−1, 30 °C), storage modulus (60 MPa) and thermal stability (>200 °C). The assembled Li0/ionogel/LiFePO4 coin cell showed comparable performance to that of the cell with the pure [G4Li][NTf2] ionic liquid.
| Type of cellulose | Ionic liquid | Maximum ionic liquid content | Preparation method | Properties, observations | Application | Ref. |
|---|---|---|---|---|---|---|
| Bacterial cellulose | [Emim][NTf2] or [Bmpy][NTf2] or [P14,6,6,6][NTf2] | 99 wt% | First hydrogel, then alcogel after solvent exchange with ethanol. Ionic liquid incorporation via co-solvent assisted diffusion and then co-solvent evaporation gives the ionogel | Ionogels of various shapes and thicknesses can be fabricated by using a template during culturing | Incorporation of chemosensory molecules (via co-solvent assisted diffusion) for ammonia and H2S detection | 207 |
| Methylcellulose | [Bmpy][NTf2] | 97 wt% | Methylcellulose and [Bmpy][NTf2] dissolved in DMF, then mixed. Gelation occurs after a heating–cooling (up to 90 °C) cycle. DMF removed by vacuum drying | Flexible ionogel with high ionic conductivity (3.3 mS cm−1 at 30 °C), wide electrochemical stability window (5.6 V). Thermally stable (no thermoreversible sol–gel transition) until degradation (around 300 °C) | Solid state electrolyte | 208 |
| Methylcellulose | [Emim][NTf2] | 95 wt% | Same as previous procedure, using DMF as co-solvent | Soft, but mechanically strong (5 MPa storage modulus) gel with good compatibility for the porous carbon nanofiber electrode. High ionic conductivity (5.7 mS cm−1 at 25 °C) | Self-standing solid-state supercapacitor | 209 |
| Methylcellulose | [G4Li][NTf2] | 90 wt% | Same as previous procedure, using DMF as co-solvent | High ionic conductivity (0.4 mS cm−1 at 30 °C) and storage modulus (60 MPa) at R.T. High thermal (up to 200 °C) and anodic stability (up to 5 V). Li0/iongel/LiFePO4 coin cell with performance comparable to that of the liquid electrolyte | Lithium metal battery | 210 |
| Cellulose nanocrystals (CNC) | Hyperbranched polymeric ionic liquid (PIL) + [Emim][NTf2] | 95 wt% [Emim][NTf2] | CNC/PIL aqueous suspension first, then solvent exchange with ethanol. [Emim][NTf2]/ethanol diffusion into gel, followed by evaporation of ethanol | High compressive elastic modulus (5.6 MPa) and ionic conductivity (7.8 mS cm−1 at 30 °C) | — | 211 |
Cellulose nanocrystals (CNC) have also been used to prepare ionogels. Lee et al.211 developed a system that contains a polymeric hyperbranched ionic liquid (PIL; see Fig. 24) and cellulose nanocrystals in order to create a mechanically persistent (high compressive modulus of 5.6 MPa) ionogel that can hold 95 wt% [Emim][NTf2]. The ionogel exhibited also high ionic conductivity owing to the continuous nanoporous network structure that enables efficient transport of ions throughout the structure. For preparing the ionogel, the authors used similar solvent exchange strategy we discussed above for bacterial cellulose. First, a CNC and PIL binary suspension was prepared in aqueous medium, followed by a solvent exchange process with ethanol. An ionic liquid/ethanol solution was then added, which slowly diffused into the gel. After evaporating the co-solvent ethanol, an ionogel was obtained (4.5 wt% CNC content, 0–2 wt% PIL content).
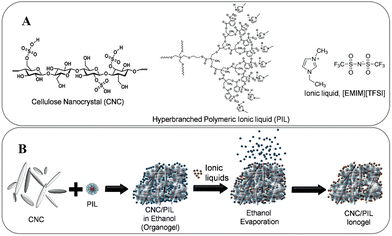 | ||
| Fig. 24 (A) Ionogel containing cellulose nanocrystals, hyperbranched polymeric ionic liquid and [Emim][NTf2]. (B) Method to prepared the CNC/PIL ionogel containing [Emim][NTf2]. Note that TFSI = NTf2. Reproduced from ref. 211 with permission from Wiley, copyright 2021. | ||
Chemical ionogels were prepared for example by crosslinking the cellulose solution in ionic liquids using glutaraldehyde (HCl catalyst) crosslinker (in [Bmim][Cl]);214via acrylic acid monomer (AIBN initiator) grafting/in situ polymerisation in cellulose/[Bmim][I] solution (for dye-sensitised solar cell with quasi-solid-state electrolyte);215 and through ionising radiation (γ-rays) induced crosslinking of cellulose/[Emim][Ac] or cellulose/N,N-diethyl-N-methyl-N-(2-methoxyethyl)ammonium (DEMA)-formate in the presence of water.216 Furthermore, as another strategy, dual network structure ionogel can also be prepared by polymerising a monomer in the presence of cellulose as it was shown by Rana et al.217 In their work, cellulose was dissolved in the ionic liquid 1,3-dimethylimidazolium methyl phosphite [DMIM][MeO(H)PO3] containing small amount of [Bmim][NTf2] (9/1 weight ratio). During the dissolution process, the hydroxyl groups connected to the C6 carbon atoms get phosphorylated in [DMIM][MeO(H)PO3] (Fig. 25A). While the role of [DMIM][MeO(H)PO3] is to dissolve cellulose, [Bmim][NTf2] increases the conductivity of the ionogel due to the presence of [NTf2] anion that does not interact with polar groups and exhibits high mobility in the system. The use of [NTf2] anion is a common strategy seen in the literature to ensure the good ionic conductivity of the ionogels. After the dissolution/modification process, 2-hydroxyethyl methacrylate (HEMA) monomer was polymerised using N,N′-methylenebisacrylamide crosslinker and benzoyl peroxide as initiator (Fig. 25B). The ionogel was used to fabricate flexible symmetric supercapacitors by sandwiching one between two activated carbon electrodes. The flexible supercapacitor device could be operated at 2.5 V within wide temperature range (30–120 °C), exhibiting good performance.
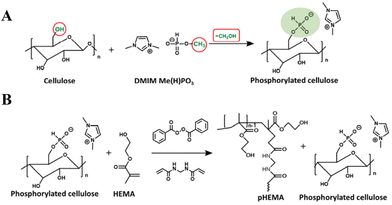 | ||
| Fig. 25 (A) Cellulose dissolution and modification in [DMIM][MeO(H)PO3] ionic liquid. (B) Polymerisation of HEMA in the presence of phosphorylated cellulose in order to obtain a dual network ionogel. Reproduced from ref. 217 with permission from Elsevier, copyright 2019. | ||
Cellulose/biopolymer binary hydrogels have also been prepared using polysaccharide additives such as κ-carrageenan, chitosan, guar gum and starch.223 In the latter work, cellulose sample was extracted from spent tea leaves, and [Amim][Cl] was used as solvent to dissolve cellulose together with the other polysaccharide additives. All of the composites showed cell compatibility without cytotoxicity, and some biopolymer-specific functional properties (such as specific mechanical and physicochemical characteristics) could be also observed. Furthermore, okara-derived (food residue from soybean industry) cellulose hydrogels containing hemicellulose (remained after the extraction process) were prepared by Wu et al.224 They reported on an improvement in the performance of the hydrogel (e.g., gelling behaviour, mechanical properties) upon TEMPO oxidation of the extracted okara cellulose.
Ionic liquids can be also useful for dissolving drugs and cellulose together in order to obtain drug-loaded hydrogels after the gelation and solvent exchange process. The use of ionic liquids can be especially advantageous for drugs with poor solubility profile in other common solvents. As an example, Santra and Sen225 dissolved cellulose and selenourea in [Emim][Ac]/DMSO solvent (1/2) system, in some cases together with tannic acid or L-methionine, to obtain drug-loaded hydrogels with promising sustained release profile.
Hydrogels obtained via ionic liquid processing can be hosts for catalytic nanoparticles. Su et al.226 prepared wheat straw cellulose/feather protein physical hydrogel after dropping their [Bmim][Cl] solution into water. Magnetic hydrogel was then obtained by a co-precipitation technique using mixed solution of Fe2+ and Fe3+. Subsequently, the hydrogels were immersed in a solution of copper salt in order to obtain a magnetic hydrogel loaded with Cu nanoparticles after in situ reduction with NaBH4. The magnetic hydrogel showed good catalytic activity for the reduction of 2-nitrobenzoic acid. The hydrophilic functional groups of cellulose and the feather protein (e.g., –OH, –NH2, –COOH) may have helped the immobilisation of the metal nanoparticles in this case. FTIR results indicated for example the presence of coordination between –COO− groups and Cu2+. In addition, Li et al.227 dissolved cellulose (cotton pulp) in the 1,1,3,3-tetramethyl guanidine [TMG]/DMSO/CO2 superbase solvent system. Subsequently, cellulose was acylated with cyclic anhydrides (succinic anhydride and pyromellitic dianhydride), in this process 1,1,3,3-tetramethyl guanidine acted as a catalyst and provided the positive counter ion for the forming carboxylate group (Fig. 26). The addition of bicyclic anhydrides was essential to the formation of a chemically crosslinked 3D network leading to a chemical hydrogel. The guanidine moiety is known to exhibit good coordination ability with transition metals. Pd nanoparticles were immobilised in the hydrogel by soaking in the solution of the metal salt (PdCl2), followed by a reduction step (NaBH4). The hydrogel showed good catalytic activity and reusability in the reduction of 4-nitrophenol. The authors attributed the absorption, dispersion, and stabilisation ability of the hydrogel for hosting Pd nanoparticles to the presence of the guanidine moiety.
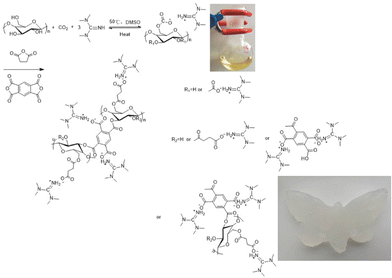 | ||
| Fig. 26 Synthesis of hydrogel with 1,1,3,3-tetramethylguanidinium-based protic ionic liquid. Reproduced from ref. 227 with permission from Elsevier, copyright 2019. | ||
Conductive chemical hydrogels were fabricated by Liang et al.228 using [Bmim][Cl]. Cellulose/[Bmim][Cl] solution was crosslinked with N,N-methylene bisarcylamide (benzoyl peroxide initiator), followed by solvent exchange with water to form the chemical hydrogel. Polypyrrole was in situ polymerised in the hydrogel that was soaked in ferric chloride/sodium p-toluenesulfonate solution (TsONa) first, followed by the addition of the monomer pyrrole. The hydrogel doped with TsONa exhibited good electrical conductivity (∼8 mS cm−1, RT).
Highly tough cellulose/reduced graphene oxide physical hydrogels were prepared by Xu et al.229 In their work, cellulose was dissolved in [Bmim][Cl], while in a separate experiment graphene oxide was reduced in [Bmim][Cl] using ascorbic acid. The two liquids were mixed and then regenerated in water in order to obtain the hydrogel by complete solvent exchange (Fig. 27A). At a certain reduced graphene oxide content (0.5 wt%), high compression modulus was observed (∼19 MPa) compared to the reference cellulose hydrogel (∼5 MPa). After freeze-drying process, mechanically strong cellulose/reduced graphene oxide aerogels could be obtained (Fig. 27B and C).
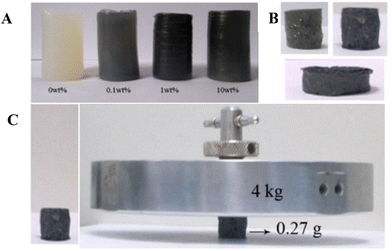 | ||
| Fig. 27 (A) Cellulose/reduced graphene oxide (rGO) hydrogels with varying rGO content. (B) Aerogels obtained after freeze-drying process. (C) Demonstration of the mechanical strength of the aerogel. Reproduced from ref. 229 with permission from Elsevier, copyright 2015. | ||
The prepared cellulose aerogels have usually no directional ordering, they possess an isotropic structure. Hierarchically assembled, ordered structures are omnipresent in nature, such features are known to give extraordinary functionality and mechanical properties to these materials, a good example for this is bamboo.234 Design of anisotropic gels and aerogels is of great interest as it could allow the directional control on e.g., cell growth, mechanical properties, and other physical phenomena (e.g., diffusion of molecules, propagation of light, etc.). Efforts towards anisotropic cellulose gels/aerogels regenerated from ionic liquid solution have been made by Plappert et al.235 They dissolved cellulose in the superbase-derived ionic liquid 1,1,3,3-tetramethylguanidinium acetate ([TMGH][Ac]). Cellulose was dissolved in [TMGH][Ac] at 100 °C, and poured into porous molds to allow the homogeneous diffusion of ethanol into the [TMGH][Ac]/cellulose system at 20 °C. Under this condition, the ionic liquid is in a “supercooled” state (melting point of [TMGH][Ac] is ∼90–97 °C), exhibiting highly viscous glassy state. Therefore, the diffusion of the antisolvent ethanol is significantly decelerated compared to other systems. Furthermore, [TMGH][Ac] has poor miscibility with ethanol, which also contributes to the slow decelerated de-mixing of cellulose and the ionic liquid. There is a diffusion zone that slowly progresses as antisolvent induced de-mixing occurs at the interface, where cellulose supramolecular assembly takes place as it slowly coagulates. This process results in onion-like concentrical cellulose layers with higher degree of orientation of the fibrillar network structure from the core towards the skin layer (Fig. 28). The obtained aerogels after the supercritical CO2 drying process have high crystallinity index (∼72%; cellulose II polymorph), with a fibrillar strut size of about 2.5 nm. The orientation-dependent compression properties of the obtained aerogels have been also studied.236 When compression test was done parallel to network orientation, the aerogel exhibited more resilience compared to the perpendicular direction.
 | ||
| Fig. 28 Cellulose gel with concentric onion-like layers that have different level of ordering as shown with the S parameter, which indicates higher degree of ordering from the core towards the skin layer. Reproduced from ref. 236 with permission from The Royal Society of Chemistry, copyright 2019. | ||
Addition of a surfactant to the cellulose/ionic liquid solution can help the formation of large μm-sized pores, as reported by An et al.237 In their work, cellulose was dissolved in [Amim][Cl], followed by the addition of sodium dodecyl sulphate (SDS) and vigorous stirring to facilitate foaming. By adjusting the cellulose concentration and the amount of the air phase, a stable biphasic system could be obtained. After the regeneration process with water and freeze-drying, ionic liquid was added to the system again (to a final concentration of 50.1 wt%). The presence of the ionic liquid imparted exceptional superelasticity and ionic conductivity to the system. The authors demonstrated the application of this flexible cellulose/ionic liquid aerogel as a wearable sensing device monitoring motions such as pulse, walking, speaking, etc.
Lignocellulose aerogels have also been fabricated using ionic liquids, these composites contain lignin and hemicellulose fraction in addition to cellulose.238–240 Please note that in other works such composition is referred as “synthetic wood”,165,166 there is a discussion about these composites above in section 4.6 (regenerated cellulose/biopolymer green composites using ionic liquids). The first work in this topic was done by Aaltonen and Jauhiainen.238 They prepared cellulose, sodalignin, cellulose/sodalignin, cellulose/sodalignin/xylan and spruce wood aerogels using [Bmim][Cl] as solvent, and aqueous ethanol coagulation bath. They pointed out that gels could be obtained at specific biopolymer compositions, and the dissolution time and ethanol concentration in the bath appeared to be also important for the gelation process. The aerogels were then produced after solvent exchange with ethanol, followed by drying with supercritical CO2. The aerogels had an open pore structure with various morphologies (macropore size depending on the composition), and contained also mesopores. In an interesting work by Li et al.,239 a cyclic freezing-thawing process (from −20 °C to 20 °C) was applied on the wood/[Amim][Cl] solution, followed by coagulation in water bath, and solvent exchange with acetone prior to a supercritical CO2 drying process. They noted that the freezing-thawing process was important to obtain a gel that can keep its form. According to their explanation, the cyclic freezing–thawing process acts as a physical crosslinker to form a continuous 3D network. Later, an ultralow freezing-thawing process was also developed using liquid nitrogen (from −196 °C to 20 °C), in this way a mesoporous lignocellulose aerogel could be obtained.240 Th authors argued that the formation of smaller ionic liquid crystals during the fast ultralow freezing process compared to the other freezing process (from −20 °C to 20 °C) facilitates the development of the 3D fibrillar network with mesopores. The fabricated lignocellulose aerogel showed good thermal insulating properties, and exhibited also good performance for noise reduction.
Cellulose aerogels have found application as adsorbent materials.232 In order to develop an aerogel with selective adsorption to specific contaminants, the surface of the aerogel can be further modified. As an example, Zhang et al.241 developed superhydrophobic and superoelophilic aerogel by plasma treatment followed by silanisation of the cellulose hydroxyl groups.
Various nanomaterials can be also incorporated into the cellulose/ionic liquid system to impart additional functions to the aerogels, such as improved thermal insulation by developing cellulose/nanosilica composite aerogels,242 or incorporating quantum dots to obtain gels/aerogels with photoluminescence properties.243 In the latter case, the photoluminescence wavelength could be controlled in the visible light spectrum, thus gels/aerogels with colour-tuned photoluminescence could be developed.
It should be noted that the cellulose aerogels can be converted to carbon aerogels through thermal treatment.233 Carbon aerogels have various applications in energy storage, catalysis, water treatment, gas storage/separation and thermal management.244
4.8. Cellulose-based fibres from ionic liquid solutions
Fibres fulfil important functions in living systems,234 and have been used by humans for creating structural materials since prehistoric times. These high aspect ratio structures possess intriguing properties for materials science, such as a combination of flexibility with strength (exceptional formability), high surface area, network formation possibilities, and small defect size, just to mention a few.245 Recently, smart textiles comprising of fibres with functional properties came into the forefront, which may change our approach towards personalised healthcare,246 and the way we communicate with electronic devices247 in the future.Textile fibre filaments used in cloths and for households have a diameter of about 5–50 μm, although finer fibres with diameters down to about 3 μm have been also achieved with common spinning techniques, they are referred to as microfibers in the literature.245 Nanofibers with sub-micrometer diameters can be produced through electrospinning technique (some common spinning techniques are illustrated in Fig. 29), which usually yields nonwoven fabrics (random web structure with intertwined filaments).
Synthetic and natural cellulose fibres make up a large share of the textile market. The textile industry is continuously growing, and so is the demand for cellulosic fabrics. Since the production of cotton has several limitations, man-made synthetic cellulosic fibres are expected to fill the gap to meet the market demand in the future.248,249
Thus far, the most successful ionic liquid for the production of synthetic cellulose fibres has been 1,5-diazabicyclo[4.3.0]non-5-enium acetate ([DBNH][Ac]), a non-imidazolium superbase-derived ionic liquid introduced by researchers from Aalto University and Helsinki University.259–261 The process, denoted as Ioncell-F, produces fibres via a dry-jet wet spinning process with properties better or comparable to that of Lyocell fibres. [DBNH][Ac] is excellent solvent of cellulose allowing low processing temperatures to avoid cellulose and solvent degradation. Furthermore, due to the low viscosity of the system, relatively high spinning dope concentrations can be applied (e.g., 10–17 wt% (ref. 254)). This technique has been studied in detail over the years to optimise the spinning parameters.262 The influence of cellulose structure (molecular weight, polydispersity) on the spinnability was also investigated,263 and it was demonstrated that even waste papers and cardboards can be upcycled into textiles (Fig. 30).264 Recycled newspapers could be also spun into Ioncell-F fibres after deinking and alkaline glycerol pulping processes.265 As a notable achievement, even coloured textile waste could be recycled using the Ioncell-F process, the colour of the dyes could be sufficiently retained in the new textile products in most of the cases.266 Since recycling of the ionic liquid is crucial for the industrialisation of the technique in order to compete with the Lyocell process (with a closed-loop system, recycling almost 100% of the solvent), recyclability of the solvent has been studied,267 and a spinning bath setup for a closed loop operation has also been suggested.268 Ioncell-F fibres were demonstrated to be suitable for the production of printed fabrics. The technology entered the upscaling phase for commercialisation.
 | ||
| Fig. 30 (Left) Ioncell-F fibres produced using bleached pulp (white), cardboard (beige) and pulp with lignin as colorant additive (brown). (Right) Fabrics produced from the upcycled Ioncell-F fibres. Reproduced from ref. 264 with permission, copyright 2016 The Authors. Design: Marjaana Tanttu; photos: Eeva Suorlahti. | ||
As we have discussed earlier, several other biopolymers can be dissolved in ionic liquids (polysaccharides, proteins, etc.), which enables the preparation of cellulose composite fibres by properly choosing the ionic liquid system (see section 4.6). Sun et al.269 directly dissolved lignocellulosic biomass (southern yellow pine and bagasse) in [Emim][Ac] and prepared all-wood composite fibres via a dry-jet wet spinning process. The authors highlighted the importance of high temperature and short dissolution time (175 °C, 30 min) as a prerequisite for the preparation of continuous filaments from dissolved wood. Furthermore, Nypelö et al.270 prepared cellulose/lignin, cellulose/xylan and cellulose/lignin/xylan all-wood composite fibres based on the Ioncell-F method. Chitosan/cellulose fibres could be also prepared via the Ioncell-F process.271Chitin/cellulose fibres were obtained via a wet and dry-jet wet spinning method using [Emim][OPr] ([OPr] – propionate) as solvent.272Cellulose/lignin composite fibres have also gained interest, they have been fabricated using the Ioncell-F process,273 or using [Emim][Ac] as solvent.274
Cellulose/lignin fibres gained interest as precursors for the preparation of carbon fibres, in order to improve the carbon yield which is generally low for carbon fibres prepared using only cellulose as precursor.275 Cellulose/lignin filaments from the Ioncell-F process,276–281 and from a process that uses [Emim][Ac],282–286 were studied in detail for this purpose. Interestingly, cellulose/chitosan fibres also gave higher carbon yield compared to pure cellulose precursors due to the intimate connection of the two biopolymers.271,287
Various organic and inorganic additives can be included in the cellulose/ionic liquid system to prepare functional fibres. By loading magnetite into cellulose/[Emim][Ac] solution magnetic fibres were produced via a dry-jet wet spinning process.288 In a similar manner, cellulose/TiO2composite fibres could also be fabricated from [Emim][Cl] solution.289 Fibres with improved tensile properties could be prepared via dry-jet wet spinning using nano-SiO2additive included in the cellulose/[Amim][Cl] solution.290Cellulose/multiwalled carbon nanotube composite fibres fabricated through a dry-jet wet spinning process from [Amim][Cl] showed also better mechanical and thermal properties compared to pure synthetic cellulose fibres.291 Similar results were reported by Rahatekar et al.292 using [Emim][Ac]. The Ioncell-F process was also used to fabricate functional fibres. Silver and gold nanoparticles could be incorporated into the [DBNH][Ac] solution to obtain fibres with promising UV-shielding properties.293 Furthermore, by adding the nature-derived plant-based water repellents betulin and betulinic acid to the spinning dope, hydrophobic fabrics could be obtained (Fig. 31).294 Ioncell-F fibres with carbon nanotube and graphene oxide additives were also reported.295
 | ||
| Fig. 31 Hydrophobic Ioncel-F fibres with 10 wt% (A and B) betulin and betulinic acid (C and D). Left pictures are nonwovens, right pictures are hand loomed/knitted fabrics. Reproduced from ref. 294, copyright 2021 The Authors. | ||
Electrospinning technology has also been applied to prepare cellulose nanofibers using ionic liquids. This topic has been comprehensively reviewed in recent works.296,297 The use of pure ionic liquid/cellulose solutions is rather difficult for this process on account of the low volatility of the solvent (usually the solvent evaporates during electrospinning), together with its high ionic strength. Xu et al.298 for example used the co-solvent DMSO for [Amim][Cl] to decrease surface tension and viscosity, and employed a high humidity chamber for electrospinning in order to solidify the fibres. The electrospun fibres were washed with ethanol to remove the residual solvent. Furthermore, a wet electrospinning process was also introduced by Quan et al.,299 in this case fibres were directly electrospun from [Bmim][Cl] solution at 100 °C into a water bath as “collector”.
5. Critical outlook on the future of cellulose processing in ionic liquids for material fabrication
Ionic liquids are considered green solvents based on their non-volatile character, with chemical and thermal stability. They gained popularity as designer solvents – their physicochemical properties can be tuned with the cation–anion combination. These features have nevertheless sparked significant interest into their environmental fate, biodegradability and toxicity profile, with several comprehensive reviews published to date.3,300–304 As a result of continuing efforts, several ways to produce non-toxic and biodegradable ionic liquids have been introduced.3 It became apparent that not only the ionic liquid itself but the preparation method also needs to be placed under scrutiny for a more holistic picture (cradle-to-gate assessment), if they are to be considered green solvents.305 We expect to see more research on using non-toxic, biodegradable ionic liquids for cellulose processing and material fabrication in the future. We would like to highlight here that machine learning approaches could provide time- and cost-effective ways to navigate within the large chemical space of ionic liquids,306 in order to design optimal processing media for material fabrication. We believe that designing ionic liquids that can tolerate high water content (present typically in biomass) while dissolving cellulose is an important direction for practical applications.307Furthermore, for the economic viability of the technology, the cost of the ionic liquid and its recyclability need to be considered. In particular, the ionic liquid needs to be recovered and recycled with favourable energy consumption that will not compromise the economics of the technology. For the recyclability, possible side reactions with cellulose (which has been seen for e.g., [Emim][Ac]43) need to be avoided. In cellulose processing with ionic liquids, some efforts have been made to recycle the solvent, for example during the production of Ioncel-F fibres using the distillable non-derivatising ionic liquid [DBNH][Ac].267,268 We hope to see more development in this field. Furthermore, to understand the real cost of ionic liquids, it is important to perform life cycle assessment (LCA). LCA considers the cradle, production, uses, disposal, and environmental impact of the chemicals.308 IL have been seen as expensive chemicals partly owing to the lack of comprehensive LCA data. The LCA data of IL can help understand the process improvement when these solvents are used. There are only few LCA reports on IL, based on these works, IL can be very competitive with common organic solvents. Baaqel et al.309 for example applied monetisation method which combines LCA and process simulation on protic ionic liquids used for lignocellulose pretreatment (delignification, cellulose non-dissolving ionic liquids). They found that triethylammonium hydrogen sulfate ([TEA][HSO4]) had the lowest cost among the studied systems (four systems were taken into consideration: [TEA][HSO4], 1-methylimidazolium hydrogen sulfate ([Hmim][HSO4]), acetone, and glycerol for cost comparison) for pretreatment of lignocellulose. In a previous report,39 technoeconomic assessment indicated that the cost of these ionic liquids could be below that of common organic solvents (about 1.24 $ per kg, compared to the price of acetone/ethyl acetate being around 1.3–1.4 $ per kg). It was also found that in biomass pretreatment, imidazole-based IL ([Emim][Ac]) for example have higher cost mainly due to their multistep synthesis process.310,311 However, on bulk scale the regeneration and recycling process of IL can eventually make them cost effective, thus emphasising the importance of this issue for the economic viability of the technique. At last, we should mention that although ionic liquids are sometimes denoted as expensive solvents of cellulose, when we consider alternative petroleum-derived materials with large carbon footprint – in light of the carbon pricing, advocating the “high” cost of ionic liquids for cellulose processing may be misguiding. When comparing different technologies and materials, a more holistic assessment is necessary, as we pointed out on using LCA.
In addition, the energetics of cellulose dissolution should not be neglected either – it must be minimised. Ionic liquids with low melting point and viscosity may be preferred, to perform the mixing with low energy input at low temperatures. Alternative heating methods, such as microwave-heating1 and ultrasonication312,313 may facilitate the dissolution process (decrease dissolution time), and lower the energy requirements. Cellulose degradation under such conditions must be avoided. There may be other ways to decrease the energy consumption of the dissolution step, this issue needs to be addressed more in detail in the future. Recently, reactive extrusion (REX) has proven to be a fast and efficient technology for cellulose dissolution and modification using ionic liquids.314,315 Cellulose chemical modification in ionic liquids via REX can outpace the conventional heterogeneous batch process due to the continuous flow, scalability and higher cellulose concentration. Moreover, the combination of homogeneous dissolution of cellulose in ionic liquids with efficient mixing in an extruder can yield a desired product within minutes of reaction time.316,317
Regarding the choice and structure of the ionic liquid, the most popular ones for material fabrication are first generation ionic liquids based on dialkylimidazolium cations, such as [Emim][Ac], [Amim][Cl] and [Bmim][Cl]. While in many cases an ionic liquid with good dissolution power proved sufficient to fulfill the target purpose (e.g., for thin film and coating applications), there are cases where the structure of ionic liquid could substantially influence the performance for the application. We showed that the choice of the ionic liquid can have a substantial impact on the mechanical properties of regenerated cellulose films (section 4.1), by influencing the crystallinity index and degree of polymerisation of the final material. Some studies suggested that [Amim][Cl] is superior compared to other ionic liquids (e.g., [Emim][Ac], [Bmim][Cl]).88,97 More studies are needed involving other types of ionic liquids as well, to have a clear picture on the influence of ionic liquid structure on the mechanical properties. The dissolution conditions (temperature and time) also need to be considered to avoid cellulose degradation. Furthermore, we think that by tuning the structure of the ionic liquid, the structure of the obtained membrane (section 4.2), such as its porosity, could be tailored during the phase inversion process.116 It is needless to say that the porosity of the membrane is crucial for filtration performance. We expect to see more progress in this field. Furthermore, it is also important to consider the structure of ionic liquid when adding enzymes to the system (section 4.3): in order to maintain the activity of the enzyme the interactions between the ionic liquid and the protein must be considered. It was shown for example that the addition of a hydrophobic ionic liquid ([Bmim][NTf2]) to [Bmim][Cl]/cellulose system can have a beneficial effect on enzyme activity, by providing a better environment to accommodate the protein.120 More understanding is needed to tailor enzyme/ionic liquid/cellulose ternary systems. When cellulose composites are prepared with other biopolymers (section 4.4), the solubility of the other biopolymer in the ionic liquid needs to be considered, and binary ionic liquid systems might be necessary, as we could see with cellulose/chitin composites.161 We would like to highlight here the nice work done on cellulose/natural protein composites, with valuable studies focusing on how the structure of the ionic liquid can be used to tailor the nanostructure of the material.181 Ionogels gained substantial interest as solid state electrolytes (section 4.7). It was shown that addition of ionic liquid, which does not interact with cellulose ([Bmim][NTf2]), can improve ionic mobility within the ionogel, beneficial for various electronic applications. Thiemann et al.202 showed that increasing bulkiness of the anion can lead to a decrease in ionic mobility, and pointed out the superiority of [NTf2] to improve this property. We think that more research could be done to tailor the ionic mobility in these materials. For fiber fabrication (section 4.8), the viscosity of the system and processing temperature are crucial parameters with a focus on avoiding cellulose and solvent degradation, and thereby enabling solvent recycling with advantageous energetics. In this field, the superbase ionic liquid [DBNH][Ac] came to the forefront. It is clear that more research is needed to understand the influence of ionic liquid structure on the properties of the fabricated materials for the target applications. Studies should go beyond the use of common first generation dialkylimidazolium cation based ionic liquids, and should more eagerly involve the large chemical space of ionic liquids. Computational methods may be involved here to replace time and resource consuming screening processes, as discussed in a recent comprehensive review by Koutsoukos et al.306 Machine learning can be a very helpful tool in various aspects. It could be used to develop ionic liquids with good ability to dissolve cellulose, and together with life cycle assessment, aid the realisation of cost-effective systems. Furthermore, it could also help designing ionic liquids for achieving specific material properties, an approach sometimes referred to as machine learning-assisted materials design. Recent advancement can be taken as example from ongoing efforts on tailoring organic molecule and polymer properties via machine learning algorithms.318
As of similarities in selecting an ionic liquid for material fabrication, when mechanical properties are important, ionic liquids that can process cellulose without appreciable degradation at low temperature with acceptable viscosities are preferred. Furthermore, the recyclability of the system necessitates the use of non-derivatising ionic liquids that can be recycled with favourable energetics. The latter is especially important when studies go beyond laboratory experiments, and industrial scale realisation is the target. This issue came to the fore especially for fiber formation processes. So far, the dialkylimidazolium cation based ionic liquid [Amim][Cl], and the superbase derived ionic liquid [DBNH][Ac] are the most promising choices. We need to emphasise here that more studies are needed since our knowledge on other types of ionic liquids is very limited. As of differences between ionic liquid systems, we would like to draw attention to physical ionogels as promising materials for solid state electrolytes. In this specific case, the use of non-dissolving ionic liquids, with anions that would interact with cellulose the least ([Bmim][NTf2]), are preferred. This is due to the high ionic mobility required within the gelous network for achieving high ionic conductivity. This is in contrast to the requirements when cellulose solvents are considered.
The purity of the final regenerated cellulose material is not examined in most of the studies, although the purity of the ionic liquid itself has been the focus of more intense investigation among works that study solvent recyclability. It can be expected that remaining ionic liquid in the final product could have a considerable impact on mechanical as well as other properties (e.g., ionic liquids have been reported as plasticiser additives,319,320 and other functions have also also been revealed when added to various polymer matrices321) important for the target application. Therefore, we highly recommend future studies to address this issue. It is clear that even small amount of impurities from the ionic liquid could have a devastating effect on the recycling step on an industrial scale, compromising the economics of the whole process. Therefore, it is crucial to optimise the regeneration step. We have to note here that purity assessment solely based on 1H-NMR analysis may not be sufficient enough in some cases, and more sensitive analysis (e.g., more sensitive HPLC techniques) may be required to claim the purity of the final material, especially when the target application is e.g., food packaging, biomedical sensing (i.e., artificial skin), etc.
Another issue is related to the prudent choice of the technical cellulose. Many studies focus on the commercially available microcrystalline cellulose. From a technological point of view, however, the use of industrially relevant technical celluloses such as dissolving pulp would be preferred (e.g., if we think about packaging application). This should be considered in the first place, and the use of a certain grade of technical cellulose should be justified (to be necessary for the material to fulfill its function/property). Dissolving pulp has high degree of polymerisation and therefore, a viscose solution may be obtained in some cases that can be more difficult to process. Handling of microcrystalline cellulose is although easier, it has higher cost that may jeopardise the economics of the technology, and its conversion from the raw cellulose will certainly increase the ecological footprint of the process.
We would also like to raise attention to the source of cellulose, in particular to the importance of upcycling agri-food losses and wastes into value-added materials for a circular economy. This topic has been comprehensively addressed in a recent review.322 These resources can represent sustainable alternatives, as they can be readily available. A good example is banana stem – banana can grow fast, and once yielding fruits, the stalk is cut back and the stem is disposed, representing an untapped source of agri-food residue derived cellulose.323,324 There are many more examples of agri-food wastes produced annually at millions of tons of quantities,322 such as the sugar beet pulp, which contains about 22–30% cellulose (on a dry weight basis). Furthermore, sugar beet pulp has a comparatively low lignin content (about 3 wt%) with respect to other lignocellulosic materials (e.g., wheat straw has about 15 wt% lignin), making it an exciting biomass to study for cellulose valorisation. Sugarcane bagasse is considered the most abundant biomass waste on Earth and it consists of 40–50 wt% cellulose – making it a material with a huge potential for obtaining cellulosic pulp for industrial applications. Recently, brewer's spent grain from the brewing industry (cellulose content around 16–25 wt%) has also emerged as a new source of cellulose. Upcycling agri-food waste could also make local communities and industries more prosperous and resilient. We believe that technological innovations in the near future will provide farmers and related businesses with both economic and social incentives to fully take advantage of the chemical diversity present in the biomass they grow.
Finally, as a goal of a tutorial review, we would really like to encourage those who have not necessarily worked with ionic liquids before, to explore this promising area of green chemistry. Although we do not really see any problems in preparing these materials, for starting practitioners, there may be some difficulties in the dissolution step of cellulose, due to the high viscosity of the solution in some cases. Handling of such solution may need some practice, and proper machinery. Furthermore, we need to note that most of the ionic liquid systems available at present do not tolerate water impurities present in raw cellulose materials or in the ionic liquid itself, so the starting materials must be dried before any processing. As we have noted above, development of ionic liquid systems that can tolerate high water content is underway. We hope to see many more interesting cellulose-based functional materials created via ionic liquid processing systems in the near future.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
L. Szabó would like to thank the support from the International Center for Young Scientists (ICYS) in the National Institute for Materials Science (NIMS; budget code: QN3360, ICYS25). K. Takahashi would like to acknowledge the Center of Innovation (COI) program on open innovation platform for industry academia co-creation (COI-NEXT) supported by the Japan Science and Technology Agency (JST, JPMJPF2102).References
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, Dissolution of cellulose with ionic liquids, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- F. Endres and S. Z. El Abedin, Air and water stable ionic liquids in physical chemistry, Phys. Chem. Chem. Phys., 2006, 8, 2101–2116 RSC.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Green and sustainable solvents in chemical processes, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed.
- K. N. Onwukamike, S. Grelier, E. Grau, H. Cramail and M. A. R. Meier, Critical review on sustainable homogeneous cellulose modification: why renewability is not enough, ACS Sustainable Chem. Eng., 2019, 7, 1826 CrossRef CAS.
- A. Cabiac, E. Guillon, F. Chambon, C. Pinel, F. Rataboul and N. Essayem, Cellulose reactivity and glycosidic bond cleavage in aqueous phase by catalytic and non catalytic transformations, Appl. Catal., A, 2011, 402, 1–10 CrossRef CAS.
- M. Koyama, W. Helbert, T. Imai, J. Sugiyama and B. Henrissat, Parallel-up structure evidences the molecular directionality during biosynthesis of bacterial cellulose, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 9091–9095 CrossRef CAS PubMed.
- K. Heise, G. Delepierre, A. W. T. King, M. A. Kostiainen, J. Zoppe, C. Weder and E. Kontturi, Chemical modification of reducing end-groups in cellulose nanocrystal, Angew. Chem., Int. Ed., 2021, 60, 66–87 CrossRef CAS PubMed.
- J. Shen and Y. Okamoto, Efficient separation of enantiomers using stereoregular chiral polymers, Chem. Rev., 2016, 116, 1094–1138 CrossRef CAS PubMed.
- P. Zugenmeier, Order in cellulosics: a historical review of crystal structure research on cellulose, Carbohydr. Polym., 2021, 254, 117417 CrossRef PubMed.
- M. Makarem, C. M. Lee, K. Kafle, S. Huang, I. Chae, H. Yang, J. D. Kubicki and S. H. Kim, Probing cellulose structures with vibrational spectroscopy, Cellulose, 2019, 26, 35–79 CrossRef CAS.
- Y. Nishiyama, P. Langan and H. Chanzy, Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction, J. Am. Chem. Soc., 2002, 124, 9074–9082 CrossRef CAS PubMed.
- P. Langan, Y. Nihsiyama and H. Chanzy, A revised structure and hydrogen-bonding system in cellulose II from a neutron fiber diffraction analysis, J. Am. Chem. Soc., 1999, 121, 9940–9946 CrossRef CAS.
- R. N. Goldberg, J. Schliesser, A. Mittal, S. R. Decker, A. F. L. O. M. Santos, V. L. S. Freitas, A. Urbas, B. E. Lang, C. Heiss, M. D. M. C. Ribeiro da Silva, B. F. Woodfield, R. Katahira, W. Wang and D. K. Johnson, A thermodynamic investigation of the cellulose allomorphs: cellulose (am), cellulose Iβ(cr), cellulose II(cr), and cellulose III(cr), J. Chem. Thermodyn., 2015, 81, 184–226 CrossRef CAS.
- M. Wada, L. Heux and J. Sugiyama, Polymorphism of cellulose I family: reinvestigation of cellulose IVI, Biomacromolecules, 2004, 5, 1385–1391 CrossRef CAS PubMed.
- K. Igarashi, M. Wada and M. Samejima, Activation of crystalline cellulose to cellulose IIII results in efficient hydrolysis by cellobiohydrolase, FEBS J., 2007, 274, 1785–1792 CrossRef CAS PubMed.
- C. Yamane, T. Aoyagi, M. Ago, K. Sato, K. Okajima and T. Takahashi, Two different surface properties of regenerated cellulose due to structural anisotropy, Polym. J., 2006, 38, 819–826 CrossRef CAS.
- H. Seddiqi, E. Oliaei, H. Honarkar, J. Jin, L. C. Geonzon, R. G. Bacabac and J. Klein-Nulend, Cellulose and its derivatives: towards biomedical applications, Cellulose, 2021, 28, 1893–1931 CrossRef CAS.
- N. Terinte, R. Ibbet and K. C. Schuster, Overview on native cellulose and microcrystalline cellulose structure studied by X-ray diffraction (WAXD): comparison between measurement techniques, Lenzinger Ber., 2011, 89, 118–131 CAS.
- Y.-L. Hsieh, Chemical structure and properties of cotton, in Cotton: Science and Technology, ed. S. Gordon and Y.-L. Hsieh, Woodhead Publishing Series in Textiles, Woodhead Publishing Limited, Cambridge, England, 2007, pp. 3–34 Search PubMed.
- L. E. Hessler, G. V. Merola and E. E. Berkley, Degree of polymerization of cellulose in cotton fibers, Text. Res. J., 1948, 18, 628–634 CrossRef.
- L. Urbina, M.Á Corcuera, N. Gabilondo, A. Eceiza and A. Retegi, A review of bacterial cellulose: sustainable production from agricultural waste and applications in various fields, Cellulose, 2021, 28, 8229–8253 CrossRef CAS.
- N. Tahara, M. Tabuchi, K. Watanabe, H. Yano, Y. Morinaga and F. Yoshinaga, Degree of polymerization of cellulose from Acetohacter xylinum BPR2001 decreased by cellulase produced by the strain, Biosci., Biotechnol., Biochem., 1997, 61, 1862–1865 CrossRef CAS PubMed.
- T. Heinze and T. Liebert, Celluloses and polyoses/hemicelluloses, in Polymer Science: A Comprehensive Reference, Volume 10: Polymers for a Sustainable Environment and Green Energy, 2012, 83–152 Search PubMed.
- G. A. S. Haron, H. Mahmood, M. H. Noh, Z. Alam and M. Moniruzzaman, Ionic liquids as a sustainable platform for nanocellulose processing from bioresources: overview and current status, ACS Sustainable Chem. Eng., 2021, 9, 1008–1034 CrossRef CAS.
- H. Wang, G. Gurau and R. D. Rogers, Ionic liquid processing of cellulose, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- K. Jedvert and T. Heinze, Cellulose modification and shaping – a review, J. Polym. Eng., 2017, 37, 845–860 CrossRef CAS.
- J. Zhang, Q. Ren and J. S. He, CN Patent, ZL02155945, 2002 Search PubMed.
- H. Zhang, J. Wu, J. Zhang and J. He, 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose, Macromolecules, 2005, 38, 8272–8277 CrossRef CAS.
- J. Zhang, J. Wu, J. Yu, X. Zhang, J. He and J. Zhang, Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: state of the art and future trends, Mater. Chem. Front., 2017, 1, 1273–1290 RSC.
- A. Brandt, J. Gräsvik, J. P. Hallett and T. Welton, Deconstruction of lignocellulosic biomass with ionic liquids, Green Chem., 2013, 15, 550–583 RSC.
- Y. Fukaya, A. Sugimoto and H. Ohno, Superior solubility of polysaccharides in low viscosity, polar, and halogen-free 1,3-dialkylimidazolium formates, Biomacromolecules, 2006, 7, 3295–3297 CrossRef CAS PubMed.
- A. Xu, J. Wang and H. Wang, Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems, Green Chem., 2010, 12, 268–275 RSC.
- B. Zhao, L. Greiner and W. Leitner, Cellulose solubilities in carboxylate-based ionic liquids, RSC Adv., 2012, 2, 2476–2479 RSC.
- Y. Zhang, A. Xu, B. Lu, Z. Li and J. Wang, Dissolution of cellulose in 1-allyl-3-methylimizodalium carboxylates at room temperature: A structure-property relationship study, Carbohydr. Polym., 2015, 117, 666–672 CrossRef CAS PubMed.
- K. Ohira, Y. Abe, M. Kawatsura, K. Suzuki, M. Mizuno, Y. Amano and T. Itoh, Design of cellulose dissolving ionic liquids inspired by nature, ChemSusChem, 2012, 5, 388–391 CrossRef CAS PubMed.
- Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Cellulose dissolution with polar ionic liquids under mild conditions: required factors for anions, Green Chem., 2008, 10, 44–46 RSC.
- A. Brandt, M. J. Ray, T. Q. To, D. J. Leak, R. J. Murphy and T. Welton, Ionic liquid pretreatment of lignocellulosic biomass with ionic liquid-water mixtures, Green Chem., 2011, 13, 2489–2499 RSC.
- P. Verdía, A. Brandt, J. P. Hallett, M. J. Ray and T. Welton, Fractionation of lignocellulosic biomass with the ionic liquid 1-butylimidazolium hydrogen sulfate, Green Chem., 2014, 16, 1617–1627 RSC.
- L. Chen, M. Sharifzadeh, N. M. Dowell, T. Welton, N. Shah and J. P. Hallett, Inexpensive ionic liquids: HSO
![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) -based solvent production at bulk scale, Green Chem., 2014, 16, 3098–3106 RSC.
-based solvent production at bulk scale, Green Chem., 2014, 16, 3098–3106 RSC. - A. George, A. Brandt, K. Tran, S. M. S. N. S. Zahari, D. Klein-Marcuschamer, N. Sun, N. Sathitsuksanoh, J. Shi, V. Stavila, R. Parthasarathi, S. Singh, B. M. Holmes, T. Welton, B. A. Simmons and J. P. Hallett, Design of low-cost ionic liquids for lignocellulosic biomass pretreatment, Green Chem., 2015, 17, 1728–1734 RSC.
- H. Zhao, G. A. Baker, Z. Song, O. Olubajo, T. Crittle and D. Peters, Designing enzyme-compatible ionic liquids that can dissolve carbohydrates, Green Chem., 2008, 10, 696–705 RSC.
- A. Pinkert, K. M. Marsh and S. Pang, Reflections on the solubility of cellulose, Ind. Eng. Chem. Res., 2010, 49, 11121–11130 CrossRef CAS.
- M. T. Clough, K. Geyer, P. A. Hunt, S. Son, U. Vagt and T. Welton, Ionic liquids: not always innocent solvents for cellulose, Green Chem., 2015, 17, 231–243 RSC.
- T. Heinze, K. Schwikal and S. Barthel, Ionic liquids as reaction medium in cellulose functionalization, Macromol. Biosci., 2005, 5, 520–525 CrossRef CAS PubMed.
- E. S. Sashina, D. A. Kashirskii, M. Zaborski and S. Jankowski, Synthesis and dissolving power of 1-alkyl-3-methylpyridinium-based ionic liquids, Russ. J. Gen. Chem., 2012, 82, 2040–2045 Search PubMed.
- A. Miyata and H. Miyafuji, Reaction behavior of cellulose in various pyridinium-based ionic liquids, J. Wood Sci., 2014, 60, 438–445 CrossRef CAS.
- J. Pernak, R. Kordala, B. Markiewicz, F. Walkiewicz, M. Popławski, A. Fabiańska, S. Jankowski and M. Łożyński, Synthesis and properties of ammonium ionic liquids with cyclohexyl substituent and dissolution of cellulose, RSC Adv., 2012, 2, 8429–8438 RSC.
- Z. Chen, S. Liu, Z. Li, Q. Zhang and Y. Deng, Dialkoxy functionalized quaternary ammonium ionic liquids as potential electrolytes and cellulose solvents, New J. Chem., 2011, 35, 1596–1606 RSC.
- S. Tang, G. A. Baker, S. Ravula, J. E. Jones and H. Zhao, PEG-functionalized ionic liquids for cellulose dissolution and saccharification, Green Chem., 2012, 14, 2922–2932 RSC.
- Y.-H. Tseng, Y.-Y. Lee and S.-H. Chen, Synthesis of quaternary ammonium room-temperature ionic liquids and their application in the dissolution of cellulose, Appl. Sci., 2019, 9, 1750 CrossRef CAS.
- V. Diez, A. DeWeese, R. S. Kalb, D. N. Blauch and A. M. Socha, Cellulose dissolution and biomass pretreatment using quaternary ammonium ionic liquids prepared from H-, G-, and S–type lignin derived benzaldehydes and dimethyl carbonate, Ind. Eng. Chem. Res., 2019, 58, 16009–16017 CrossRef CAS.
- J. Pernak, N. Borucka, F. F. Walkiewicz, B. Markiewicz, P. Fochtman, S. Stolte, S. Steudte and P. Stepnowski, Synthesis, toxicity, biodegradability and physicochemical properties of 4-benzyl-4-methylmorpholinium-based ionic liquids, Green Chem., 2011, 13, 2901–2910 RSC.
- D. G. Raut, O. Sundman, W. Su, P. Virtanen, Y. Sugano, K. Kordas and J.-P. Mikkola, A morpholinium ionic liquid for cellulose dissolution, Carbohydr. Polym., 2015, 130, 18–25 CrossRef CAS PubMed.
- H.-W. Ren, Q.-H. Wang, S.-H. Guo, D.-S. Zhao and C.-M. Chen, The role and potential of morpholinium-based ionic liquids in dissolution of cellulose, Eur. Polym. J., 2017, 92, 204–212 CrossRef CAS.
- D. Giovanni, S. Laszlo, M. Klemens and S. Veit, Ionic liquids for solubilizing polymers, PCT Patent, WO2008043837, 2006 Search PubMed.
- A. Parviainen, A. W. T. King, I. Mutikainen, M. Hummel, C. Selg, L. K. J. Hauru, H. Sixta and I. Kilpeläinen, Predicting cellulose solvating capabilities of acid-base conjugate ionic liquids, ChemSusChem, 2013, 6, 2161–2169 CrossRef CAS PubMed.
- A. W. T. King, J. Asikkala, I. Mutikainen, P. Järvi and I. Kilpeläinen, Distillable acid-base ionic liquids for cellulose dissolution and processing, Angew. Chem., Int. Ed., 2011, 50, 6301–6305 CrossRef CAS PubMed.
- J. Miao, H. Sun, Y. Yu, X. Song and L. Zhang, Quaternary ammonium acetate: an efficient ionic liquid for the dissolution and regeneration of cellulose, RSC Adv., 2014, 4, 36721–36724 RSC.
- D. L. Minnick, R. A. Flores, M. R. DeStefano and A. M. Scurto, Cellulose solubility in ionic liquid mixtures: temperature, cosolvent and antisolvent effects, J. Phys. Chem. B, 2016, 120, 7906–7919 CrossRef CAS PubMed.
- J.-M. Andanson, E. Bordes, J. Devémy, F. Leroux, A. A. H. Pádua and M. F. C. Gomes, Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids, Green Chem., 2014, 16, 2528–2538 RSC.
- L. Zhang, C. Huang, C. Zhang and H. Pan, Swelling and dissolution of cellulose in binary systems of three ionic liquids and three co-solvents, Cellulose, 2021, 28, 4643–4653 CrossRef CAS.
- Y. Meng, Z. Pang and C. Dong, Enhancing cellulose dissolution in ionic liquid by solid acid addition, Carbohydr. Polym., 2017, 163, 317–323 CrossRef CAS PubMed.
- X. Li, H. Li, T. You, X. Chen, S. Ramaswamy, Y.-Y. Wu and F. Xu, Enhanced dissolution of cotton cellulose in 1-allyl-3-methylimidazolium chloride by the addition of metal chlorides, ACS Sustainable Chem. Eng., 2019, 7, 19176–19184 CrossRef CAS.
- J. Yang, X. Lu, X. Yao, Y. Li, Y. Yang, Q. Zhou and S. Zhang, Inhibiting degradation of cellulose dissolved in ionic liquid via amino acids, Green Chem., 2019, 21, 2777–2787 RSC.
- Y. Li, J. Wang, X. Liu and S. Zhang, Towards a molecular understanding of cellulose dissolution in ionic liquids: anion/cation effect, synergistic mechanism and physicochemical aspects, Chem. Sci., 2018, 9, 4027–4043 RSC.
- L. Berga, I. Bruce, T. W. J. Nicol, A. J. Holding, N. Isobe, S. Shimizu, A. J. Walker and J. E. S. J. Reid, Cellulose dissolution and regeneration using a non-aqueous, non-stoichiometric protic ionic liquid system, Cellulose, 2020, 27, 9593–9603 CrossRef CAS.
- M. Abe, Y. Fukaya and H. Ohno, Fast and facile dissolution of cellulose with tetrabutylphosphonium hydroxide containing 40 wt% water, Chem. Commun., 2012, 48, 1808–1810 RSC.
- A. Tsurumaki, M. Tajima, M. Abe, D. Sato and H. Ohno, Effect of cation structure on cellulose dissolution in aqueous solutions of organic onium hydroxides, Phys. Chem. Chem. Phys., 2020, 22, 22602–22608 RSC.
- Eastman Corporate Headquarters, Eastman cellulose esters for formulated products – enhancing performance, productivity, and appearance, https://www.eastman.com/Literature_Center/E/E325.pdf (last accessed on 16/03/2022).
- European Commission, The VOC solvents emissions directive, https://ec.europa.eu/environment/archives/air/stationary/solvents/legislation.htm (last accessed on 16/03/2022).
- US Environmental Protection Agency, National volatile organic compound emission standards for consumer and commercial products, https://www.govinfo.gov/content/pkg/CFR-2019-title40-vol6/xml/CFR-2019-title40-vol6-part59.xml (last accessed on 16/03/2022).
- D. Klemm, B. Philipp, T. Heinze, U. Heinze and W. Wagenknecht, Comprehensive Cellulose Chemistry, Wiley-VCH, Weinheim, 1st edn, 1998, vol. 1, pp. 17–27 Search PubMed.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Cellulose: fascinating biopolymer and sustainable raw material, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- K. J. Edgar, C. M. Buchanan, J. S. Debenham, P. A. Rundquist, B. D. Seiler, M. C. Shelton and D. Tindall, Advances in cellulose ester performance and application, Prog. Polym. Sci., 2001, 26, 1605–1688 CrossRef CAS.
- J. D. Posey-Dowty, K. S. Seo, K. R. Walker and A. K. Wilson, Carboxymethylcellulose acetate butyrate in water-based automotive paints, Surf. Coat. Int., Part B, 2002, 85, 203–208 CrossRef CAS.
- C. J. Tristram, J. M. Mason, D. B. G. Williams and S. F. R. Hinkley, Doubly renewable cellulose polymer for water-based coatings, ChemSusChem, 2015, 8, 63–66 CrossRef CAS PubMed.
- M. Pei, X. Peng, Y. Shen, Y. Yang, Y. Guo, Q. Zheng, H. Xie and H. Sun, Synthesis of water-soluble, fully biobased cellulose levulinate esters through the reaction of cellulose and alpha-angelica lactone in a DBU/CO2/DMSO solvent system, Green Chem., 2020, 22, 707–717 RSC.
- M. Yamaguchi, K. Songsurang, H. Shimada, S. Nobukawa and M. E. A. Manaf, Novel methods to control the optical anisotropy of cellulose esters, in Pulp Production and Processing - High-Tech Applications, ed. V. I. Popa, De Gruyter, 2020 Search PubMed.
- S. C. Gondhalekar, P. J. Pawar, S. S. Dhumal and S. Thakre, Fate of CS2 in viscose process: a chemistry perspective, Cellulose, 2022, 9, 1451–1461 CrossRef.
- D. M. Rock and A. Grady, Zinc Precipitation and Recovery from Viscose Rayon Waste Water, Water Pollution Control Research Series, US Environmental Protection Agency, Water Quality Office, Washington, 1971 Search PubMed.
- S. Paunonen, Strength and barrier enhancement of cellophane and cellulose derivative films: a review, BioResources, 2013, 8, 3098–3121 CrossRef.
- S. S. Ahankari, A. R. Subhedar, S. S. Bhadauria and A. Dufresne, Nanocellulose in food packaging: a review, Carbohydr. Polym., 2021, 255, 117479 CrossRef CAS PubMed.
- H.-P. Fink, J. Ganster and A. Lehmann, Progress in cellulose shaping: 20 years industrial case studies at Fraunhofer IAO, Cellulose, 2014, 21, 31–51 CrossRef.
- H. C. Arca, L. I. Mosquera-Giraldo, V. Bi, D. Xu, L. S. Taylor and K. J. Edgar, Pharmaceutical applications of cellulose ethers and cellulose ether esters, Biomacromolecules, 2018, 19, 2351–2376 CrossRef CAS PubMed.
- A. Sheikhi, J. Hayashi, J. Eichenbaum, M. Gutin, N. Kuntjoro, D. Khorsandi and A. Khademhosseini, Recent advances in nanoengineering cellulose for cargo delivery, J. Controlled Release, 2019, 294, 53–76 CrossRef CAS PubMed.
- S. Bhaladhare and D. Das, Cellulose: a fascinating biopolymer for hydrogel synthesis, J. Mater. Chem. B, 2022, 10, 1923–1945 RSC.
- L. Pang, Z. Gao, H. Feng, S. Wang and Q. Wang, Cellulose based materials for controlled release formulations of agrochemicals: a review of modifications and applications, J. Controlled Release, 2019, 316, 105–115 CrossRef CAS PubMed.
- Y. Cao, H. Li, Y. Zhang, J. Zhang and J. He, Structure and properties of novel regenerated cellulose films prepared from cornhusk cellulose in room temperature ionic liquids, J. Appl. Polym. Sci., 2010, 116, 547–554 CrossRef CAS.
- K. O. Reddy, J. Zhang, J. Zhang and A. V. Rajulu, Preparation and properties of self-reinforced cellulose composite films from Agave microfibrils using an ionic liquid, Carbohydr. Polym., 2014, 114, 537–545 CrossRef CAS PubMed.
- J. Pang, X. Liu, X. Zhang, Y. Wu and R. C. Sun, Fabrication of cellulose film with enhanced mechanical properties in ionic liquid 1-allyl-3-methylimidaxolium chloride (AmimCl), Materials, 2013, 6, 1270–1284 CrossRef PubMed.
- Z. Jin, S. Wang, J. Wang and M. Zhao, Effect of plasticization conditions on the structures and properties of cellulose packaging films from ionic liquid BMIMCl, J. Appl. Polym. Sci., 2012, 125, 704–709 CrossRef CAS.
- S. V. Muginova, D. A. Myasnikova, S. G. Kazarian and T. N. Shekhovtsova, Evaluation of novel applications of cellulose hydrogel films reconstituted from acetate and chloride of 1-butyl-3-methylimidazolium by comparing their optical, mechanical, and adsorption properties, Mater. Today Commun., 2016, 8, 108–117 CrossRef CAS.
- S. V. Muginova, E. S. Vakhranyova, D. A. Myasnikova, S. G. Kazarian and T. N. Shekhovtsova, Fluorescence-based artemisinin sensing using a pyronin B-doped cellulose film reconstituted from ionic liquid, Anal. Lett., 2018, 51, 870–891 CrossRef CAS.
- Y. Wang, Y.-C. Ji, L. Wei, Q.-G. Wang and H. Li, Dissolution and regeneration in films of natural luffa in 1-butyl-3-methylimidazolium chloride, J. Macromol. Sci. Phys., 2015, 54, 571–580 CrossRef CAS.
- H.-Z. Chen, N. Wang and L.-Y. Liu, Regenerated cellulose membrane prepared with ionic liquid 1-butyl-3-methylimidazolium chloride as solvent using wheat straw, J. Chem. Technol. Biotechnol., 2012, 87, 1634–1640 CrossRef CAS.
- J. H. Pang, M. Wu, Q. H. Zhang, X. Tan, F. Xu, X. M. Zhang and R. C. Sun, Comparison of physical properties of regenerated cellulose films fabricated with different cellulose feedstocks in ionic liquid, Carbohydr. Polym., 2015, 121, 71–78 CrossRef CAS PubMed.
- X. Zheng, F. Huang, L. Chen, L. Huang, S. Cao and X. Ma, Preparation of transparent film via cellulose regeneration: correlations between ionic liquid and film properties, Carbohydr. Polym., 2019, 203, 214–218 CrossRef CAS PubMed.
- X. Liu, J. Pang, X. Zhang, Y. Wu and R. C. Sun, Regenerated cellulose film with enhanced tensile strength prepared with ionic liquid 1-ethyl-3-methylimidazolium acetate (EMIMAc), Cellulose, 2013, 20, 1391–1399 CrossRef CAS.
- A. Östlund, A. Idström, C. Olsson, P. T. Larsson and L. Nordstierna, Modification of crystallinity and pore size distribution in coagulated cellulose films, Cellulose, 2013, 20, 1657–1667 CrossRef.
- R. Kargl, T. Mohan, V. Ribitsch, B. Saake, J. Puls and K. S. Kleinschek, Cellulose thin films from ionic liquid solutions, Nord. Pulp Pap. Res. J., 2015, 30, 6–13 CrossRef CAS.
- N. Luo, K. Varaprasad, G. V. S. Reddy, A. V. Rajulu and J. Zhang, Preparation and characterization of cellulose/curcumin composite films, RSC Adv., 2012, 2, 8483–8488 RSC.
- X. Huang, Y. Ji, L. Guo, Q. Xu, L. Jin, Y. Fu and Y. Wang, Incorporating tannin onto regenerated cellulose film towards sustainable active packaging, Ind. Crops Prod., 2022, 180, 114710 CrossRef CAS.
- Y. Hu, Q. Guo, P. Liu, R. Zhu, F. Lu, S. Ramaswamy, Y. Wu, F. Xu and X. Zhang, Fabrication of novel cellulose-based antibacterial film loaded with poacic acid against Staphylococcus aureus, J. Polym. Environ., 2021, 29, 745–754 CrossRef CAS.
- S. M. Notley, M. Eriksson, L. Wågberg, S. Beck and D. G. Gray, Surface forces measurements of spin-coated cellulose thin films with different crystallinity, Langmuir, 2006, 22, 3154–3160 CrossRef CAS PubMed.
- C. Aulin, S. Ahola, P. Josefsson, T. Nishino, Y. Hirose, M. Österberg and L. Wågberg, Nanoscale cellulose films with different crystallinities and mesostructures – their surface properties and interaction with water, Langmuir, 2009, 25, 7675–7685 CrossRef CAS PubMed.
- N. Hameed, Q. Guo, F. H. Tay and S. G. Kazarian, Blends of cellulose and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) prepared from the ionic liquid 1-butyl-3-methylimidazolium chloride, Carbohydr. Polym., 2011, 86, 94–104 CrossRef CAS.
- M. Isik, R. Garcia, L. C. Kollnus, L. C. Tomé, I. M. Marrucho and D. Mecerreyes, Cholinium-based poly(ionic liquid)s: synthesis, characterization, and application as biocompatible ion gels and cellulose coatings, ACS Macro Lett., 2013, 2, 975–979 CrossRef CAS PubMed.
- M. Isik, R. Garcia, L. C. Kollnus, L. C. Tomé, I. M. Marrucho and D. Mecerreyes, Cholinium lactate methacrylate: ionic liquid monomer for cellulose composites and biocompatible ion gels, Macromol. Symp., 2014, 342, 21–24 CrossRef CAS.
- Q. Zhang, M. Benoit, K. D. O. Vigier, J. Barrault and F. Jérôme, Green and inexpensive choline-derived solvents for cellulose decrystallization, Chem. – Eur. J., 2012, 18, 1043–1046 CrossRef CAS PubMed.
- M.-H. Song, T. P. T. Pham and Y.-S. Yun, Ionic liquid-assisted cellulose coating of chitosan hydrogel beads and their application as drug carriers, Sci. Rep., 2020, 10, 13905 CrossRef CAS PubMed.
- Suhas, V. K. Gupta, P. J. M. Carrott, R. Singh, M. Chaudhary and S. Kushwaha, Cellulose: a review as natural, modified and activated carbon adsorbent, Bioresour. Technol., 2016, 216, 1066–1076 CrossRef CAS PubMed.
- A. K. Hołda and I. F. J. Vankelecom, Understanding and guiding the phase inversion process for synthesis of solvent resistant nanofiltration membranes, J. Appl. Polym. Sci., 2015, 132, 42130 CrossRef.
- D. Y. Xing, N. Peng and T.-S. Chung, Formation of cellulose acetate membranes via phase inversion using ionic liquid, BMIMSCN, as the solvent, Ind. Eng. Chem. Res., 2010, 49, 8761–8769 CrossRef CAS.
- X.-L. Li, L.-P. Zhu, B.-K. Zhu and Y. Y. Xu, High-flux and anti-fouling cellulose nanofiltration membranes prepared via phase inversion with ionic liquid as solvent, Sep. Purif. Technol., 2011, 83, 66–73 CrossRef.
- M. R. Esfahani, A. Taylor, N. Serwinowski, Z. J. Parkerson, M. P. Confer, I. Kammakakam, J. E. Bara, A. R. Esfahani, S. N. Mahmoodi, N. Koutahzadeh and M. Z. Hu, Sustainable novel bamboo-based membranes for water treatment fabricated by regeneration of bamboo waste fibers, ACS Sustainable Chem. Eng., 2020, 8, 4225–4235 CrossRef CAS.
- S. Livazovic, Z. Li, A. R. Behzad, K.-V. Peinemann and S. P. Nunes, Cellulose multilayer membranes manufactured with ionic liquid, J. Membr. Sci., 2015, 490, 282–293 CrossRef CAS.
- D. Kim, S. Livazovic, G. Falca and S. P. Nunes, Oil-water separation using membranes manufactures from cellulose/ionic liquid solutions, ACS Sustainable Chem. Eng., 2019, 7, 5649–5659 CrossRef CAS.
- F. M. Sukma and P. Z. Culfaz-Emecen, Cellulose membranes for organic solvent nanofiltration, J. Membr. Sci., 2018, 545, 329–336 CrossRef CAS.
- E. N. Durmaz and P. Z. Culfaz-Emecen, Cellulose-based membranes via phase inversion using EMIMOAc-DMSO mixtures as solvent, Chem. Eng. Sci., 2018, 178, 93–103 CrossRef CAS.
- M. B. Turner, S. K. Spear, J. D. Holbrey and R. D. Rogers, Production of bioactive cellulose films reconstituted from ionic liquids, Biomacromolecules, 2004, 5, 1379–1384 CrossRef CAS PubMed.
- R. Mehra, J. Muschiol, A. S. Meyer and K. P. Kepp, A structural-chemical explanation of fungal laccase activity, Sci. Rep., 2018, 8, 17285 CrossRef PubMed.
- H. Zhao, Protein stabilization and enzyme activation in ionic liquids: specific ion effects, J. Chem. Technol. Biotechnol., 2016, 91, 25–50 CrossRef CAS PubMed.
- M. B. Turner, S. K. Spear, J. D. Holbrey, D. T. Daly and R. D. Rogers, Ionic liquid-reconstituted cellulose composites as solid support matrices for biocatalyst immobilization, Biomacromolecules, 2005, 6, 2497–2502 CrossRef CAS PubMed.
- M. Bagheri, H. Rodríguez, R. P. Swatloski, S. K. Spear, D. T. Daly and R. D. Rogers, Ionic liquid-based preparation of cellulose-dendrimer films as solid supports for enzyme immobilization, Biomacromolecules, 2008, 9, 381–387 CrossRef CAS PubMed.
- S. V. Muginova, D. A. Myasnikova, A. E. Polyakov and T. N. Shekhovtsova, Immobilization of plant peroxidases in cellulose-ionic liquid films, Mendeleev Commun., 2013, 23, 74–75 CrossRef CAS.
- M. P. Klein, C. W. Scheeren, A. S. G. Lorenzoni, J. Dupont, J. Frazzon and P. F. Hertz, Ionic liquid-cellulose film for enzyme immobilization, Process Biochem., 2011, 46, 1375–1379 CrossRef CAS.
- Y. Gu, P. Xue and K. Shi, A novel support of sponge-like cellulose composite polymer for immobilizing laccase and its application in nitrogenous organics biodegradation, J. Porous Mater., 2020, 27, 73–82 CrossRef CAS.
- Z. Liu, H. Wang, B. Li, C. Liu, Y. Jiang, G. Yu and X. Mu, Biocompatible magnetic cellulose-chitosan hybrid gel microspheres reconstituted from ionic liquids for enzyme immobilization, J. Mater. Chem., 2012, 22, 15085–15091 RSC.
- H. Suo, L. Xu, Y. Xue, X. Qiu, H. Huang and Y. Hu, Ionic liquids-modified cellulose coated magnetic nanoparticles for enzyme immobilization: improvement of catalytic performance, Carbohydr. Polym., 2020, 234, 115914 CrossRef CAS PubMed.
- H. Zhao, G. A. Baker, Z. Song, O. Olubajo, T. Crittle and D. Peters, Designing enzyme-compatible ionic liquids that can dissolve carbohydrates, Green Chem., 2008, 10, 696–705 RSC.
- R. A. Sheldon, Biocatalysis in ionic liquids: state-of-the-union, Green Chem., 2021, 23, 8406–8427 RSC.
- K. Kuroda, H. Satria, K. Miyamura, Y. Tsuge, K. Ninomiya and K. Takahashi, Design of wall-desctructive but membrane-compatible solvents, J. Am. Chem. Soc., 2017, 139(45), 16052–16055 CrossRef CAS PubMed.
- G. Sharma, Y. Kato, A. Hachisu, K. Ishibashi, K. Ninomiya, K. Takahashi, E. Hirata and K. Kuroda, Synthesis of a cellulose dissolving liquid zwitterion from general and low-cost reagents, Cellulose, 2022, 29, 3017–3024 CrossRef CAS.
- P. Migowski and J. Dupont, Catalytic applications of metal nanoparticles in imidazolium ionic liquids, Chem. – Eur. J., 2007, 13, 32–39 CrossRef CAS PubMed.
- M. M. Seitkalieva, D. E. Samoylenko, K. A. Lotsman, K. S. Rodygin and V. P. Ananikov, Metal nanoparticles in ionic liquids: synthesis and catalytic applications, Coord. Chem. Rev., 2021, 445, 213982 CrossRef CAS.
- J. Dupont, G. S. Fonseca, A. P. Umpierre, P. F. P. Fichtner and S. R. Teixeira, Transition-metal nanoparticles in imidazolium ionic liquids: recyclable catalysts for biphasic hydrogenation reactions, J. Am. Chem. Soc., 2002, 124, 4228–4229 CrossRef CAS PubMed.
- J. D. Scholten, B. C. Leal and J. Dupont, Transition metal nanoparticle catalysis in ionic liquids, ACS Catal., 2012, 2, 184–200 CrossRef CAS.
- Z. Li, A. Friedrich and A. Taubert, Gold microcrystal synthesis via reduction of HAuCl4 by cellulose in the ionic liquid 1-butyl-3-methyl imidazolium chloride, J. Mater. Chem., 2008, 18, 1008–1014 RSC.
- M. A. Gelesky, C. W. Scheeren, L. Foppa, F. A. Pavan, S. L. P. Dias and J. Dupont, Metal nanoparticle/ionic liquid/cellulose: new catalytically active membrane materials for hydrogenation reactions, Biomacromolecules, 2009, 10, 1888–1893 CrossRef CAS PubMed.
- A. Wittmar, H. Thierfeld, S. Köcher and M. Ulbricht, Routes towards catalytically active TiO2 doped porous cellulose, RSC Adv., 2015, 5, 35866–35873 RSC.
- A. S. M. Wittmar and M. Ulbricht, Ionic liquid-based route for the preparation of catalytically active cellulose-TiO2 porous films and spheres, Ind. Eng. Chem. Res., 2017, 56, 2967–2975 CrossRef CAS.
- A. S. M. Wittmar, Q. Fu and M. Ulbricht, Photocatalytic and magnetic porous cellulose-based nanocomposite films prepared by a green method, ACS Sustainable Chem. Eng., 2017, 5, 9858–9868 CrossRef CAS.
- A. S. M. Wittmar, Q. Fu and M. Ulbricht, Photocatalytic and magnetic porous cellulose macrospheres for water purification, Cellulose, 2019, 26, 4563–4578 CrossRef CAS.
- S. Jo, Y. Oh, S. Park, E. Kan and S. H. Lee, Cellulose/carrageenan/TiO2 nanocomposite for adsorption and photodegradation of cationic dye, Biotechnol. Bioprocess Eng., 2017, 22, 734–738 CrossRef CAS.
- Q. Qin, R. Guo, S. Lin, S. Jiang, J. Lan, X. Lai, C. Cui, H. Xiao and Y. Zhang, Waste cotton fiber/Bi2WO6 composite film for dye removal, Cellulose, 2019, 26, 3909–3922 CrossRef CAS.
- L. Zhang, Z. Liu, G. Cui and L. Chen, Biomass-derived materials for electrochemical energy storages, Prog. Polym. Sci., 2015, 43, 136–164 CrossRef CAS.
- M. M. Pérez-Madrigal, M. G. Edo and C. Alamán, Powering the future: application of cellulose-based materials for supercapacitors, Green Chem., 2016, 18, 5930–5956 RSC.
- D. Zhao, C. Chen, Q. Zhang, W. Chen, S. Liu, Q. Wang, Y. Liu, J. Li and H. Yu, High performance, flexible, solid-state supercapacitor based on a renewable and biodegradable mesoporous cellulose membrane, Adv. Energy Mater., 2017, 7, 1700739 CrossRef.
- Y. Zhu, K. Cao, W. Cheng, S. Zeng, S. Dou, W. Chen, D. Zhao and H. Yu, A non-Newtonian fluidic cellulose-modified glass microfiber separator for flexible lithium-ion batteries, EcoMat, 2021, 3, e12126 CAS.
- V. L. Pushparaj, M. M. Shaijumon, A. Kumar, S. Murugesan, L. Ci, R. Vajtai, R. J. Linhardt, O. Nalamasu and P. M. Ajayan, Flexible energy storage devices based on nanocomposite paper, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13574–13577 CrossRef CAS PubMed.
- H. Wang, P.-G. Ren, C.-Y. Liu, L. Xu and Z.-M. Li, Enhanced toughness and strength of conductive cellulose-poly(butylene succinate) films filled with multiwalled carbon nanotubes, Cellulose, 2014, 21, 1803–1812 CrossRef CAS.
- M. Soheilmoghaddam, H. Adelnia, G. Sharifzadeh, M. U. Wahit, T. W. Wong and A. A. Yussuf, Bionanocomposite regenerated cellulose/single-walled carbon nanotube films prepared using ionic liquid solvent, Cellulose, 2017, 24, 811–822 CrossRef CAS.
- L. Zhou, F. Pan, S. Zeng, Q. Li, L. Bai, Y. Liu and Y. Nie, Ionic liquid assisted fabrication of cellulose-based conductive films for Li-ion battery, J. Appl. Polym. Sci., 2020, 137, e49430 CrossRef.
- J. Chen, J. Xu, K. Wang, X. Qian and R. C. Sun, Highly thermostable, flexible, and conductive films prepared from cellulose, graphite, and polypyrrole nanoparticles, ACS Appl. Mater. Interfaces, 2015, 7, 15641–15648 CrossRef CAS PubMed.
- T. Zhang, X. Zhang, Y. Chen, Y. Duan and J. Zhang, Green fabrication of regenerated cellulose/graphene films with simultaneous improvement of strength and toughness by tailoring the nanofiber diameter, ACS Sustainable Chem. Eng., 2018, 6, 1271–1278 CrossRef CAS.
- X. Ma, Q. Deng, L. Wang, X. Zheng, S. Wang, Q. Wang, L. Chen, L. Huang, X. Ouyang and S. Cao, Cellulose transparent conductive film and its feasible use in perovskite solar cells, RSC Adv., 2019, 9, 9348–9353 RSC.
- W. Ye, X. Li, H. Zhu, X. Wang, S. Wang, H. Wang and R. C. Sun, Green fabrication of cellulose/graphene composite in ionic liquid and its electrochemical and photothermal properties, Chem. Eng. J., 2016, 299, 45–55 CrossRef CAS.
- S. Park, K. K. Oh and S. H. Lee, Biopolymer-based composite materials prepared using ionic liquids, Adv. Biochem. Eng./Biotechnol., 2019, 168, 133–176 CrossRef CAS PubMed.
- A. Takegawa, M. Murakami, Y. Kaneko and J. Kadokawa, Preparation of chitin/cellulose composite gels and films with ionic liquids, Carbohydr. Polym., 2010, 79, 85–90 CrossRef CAS.
- K. Prasad, M. Murakami, Y. Kaneko, A. Takada, Y. Nakamura and J. Kadokawa, Weak gel of chitin with ionic liquid, 1-allyl-3-methylimidazolium bromide, Int. J. Biol. Macromol., 2009, 45, 221–225 CrossRef CAS PubMed.
- J. Kadokawa, K. Hirohama, S. Mine, T. Kato and K. Yamamoto, Facile preparation of chitin/cellulose composite films using ionic liquids, J. Polym. Environ., 2012, 20, 37–42 CrossRef CAS.
- Y. Duan, A. Freyburger, W. Kunz and C. Zollfrank, Cellulose and chitin composite materials from an ionic liquid and a green solvent, Carbohydr. Polym., 2018, 192, 159–165 CrossRef CAS PubMed.
- Y. Wu, T. Sasaki, S. Irie and K. Sakurai, A novel biomass-ionic liquid platform for the utilization of native chitin, Polymer, 2008, 49, 2321–2327 CrossRef CAS.
- W. Xiao, Q. Chen, Y. Wu, T. Wu and L. Dai, Dissolution and blending of chitosan using 1,3-dimethylimidazolium chloride and 1-H-3-methylimidazolium chloride binary ionic liquid solvent, Carbohydr. Polym., 2011, 83, 233–238 CrossRef CAS.
- C. Stefanescu, W. H. Daly and I. I. Negulescu, Biocomposite films prepared from ionic liquid solutions of chitosan and cellulose, Carbohydr. Polym., 2012, 87, 435–443 CrossRef CAS PubMed.
- R. Fu, X. Ji, Y. Ren, G. Wang and B. Cheng, Antibacterial blend films of cellulose and chitosan prepared from binary ionic liquid system, Fibers Polym., 2017, 18, 852–858 CrossRef CAS.
- C. Ardean, C. M. Davidescu, N. S. Nemeş, A. Negrea, M. Ciopec, N. Duteanu, P. Negrea, D. Duda-Seiman and V. Musta, Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization, Int. J. Mol. Sci., 2021, 22, 7449 CrossRef CAS PubMed.
- T. Hayashi and R. Kaida, Functions of xyloglucan in plant cells, Mol. Plant, 2011, 4, 17–24 CrossRef CAS PubMed.
- A. Bendaoud, R. Kehrbusch, A. Baranov, B. Duchemin, J. E. Maigret, X. Falourd, M. P. Staiger, B. Cathala, D. Lourdin and E. Leroy, Nanostructured cellulose-xyloglycan blends via ionic liquid/water processing, Carbohydr. Polym., 2017, 168, 163–172 CrossRef CAS PubMed.
- J. Sundberg, G. Toriz and P. Gatenholm, Effect of xylan content on mechanical properties in regenerated cellulose/xylan blend films from ionic liquid, Cellulose, 2015, 22, 1943–1953 CrossRef CAS.
- B. M. Upton and A. M. Kasko, Strategies for the conversion of lignin to high-value polymeric materials: review and perspective, Chem. Rev., 2016, 116, 2275–2306 CrossRef CAS PubMed.
- T. J. Simmons, S. H. Lee, J. Miao, M. Miyauchi, T.-J. Park, S. S. Bale, R. Pangule, J. Bult, J. G. Martin, J. S. Dordick and R. J. Linhardt, Preparation of synthetic wood composites using ionic liquids, Wood Sci. Technol., 2011, 45, 719–733 CrossRef CAS.
- S. H. Kim, M. H. Kim, J. H. Kim, S. Park, H. Kim, K. Won and S. H. Lee, Preparation of artificial wood films with controlled biodegradability, J. Appl. Polym. Sci., 2015, 132, 42109 CrossRef.
- M. Lee, J. H. Kim, S. H. Lee, S. H. Lee and C. B. Park, Biomimetic artificial photosynthesis by light-harvesting synthetic wood, ChemSusChem, 2011, 4, 581–586 CrossRef CAS PubMed.
- R.-L. Wu, X.-L. Wang, F. Li, H.-Z. Li and Y.-Z. Wang, Green composite films prepared from cellulose, starch, and lignin in room-temperature ionic liquid, Bioresour. Technol., 2009, 100, 2569–2574 CrossRef CAS PubMed.
- L.-D. Koh, Y. Cheng, C.-P. Teng, Y.-W. Khin, X.-J. Loh, S.-Y. Tee, M. Low, E. Ye, H.-D. Yu, Y.-W. Zhang and M.-Y. Han, Structures, mechanical properties and applications of silk fibroin materials, Prog. Polym. Sci., 2015, 46, 86–110 CrossRef CAS.
- D. M. Philips, L. F. Drummy, D. G. Conrady, D. M. Fox, R. R. Naik, M. O. Stone, P. C. Trulove, H. C. De Long and R. A. Mantz, Dissolution and regeneration of Bombyx mori silk fibroin using ionic liquids, J. Am. Chem. Soc., 2004, 126, 14350–14351 CrossRef PubMed.
- S. Shang, L. Zhu and J. Fan, Physical properties of silk fibroin/cellulose blend films regenerated from the hydrophilic ionic liquid, Carbohydr. Polym., 2011, 86, 462–468 CrossRef CAS.
- J. Stanton, Y. Xue, J. C. Waters, A. Lewis, D. Cowan, X. Hu and D. Salas-de la Cruz, Structure-property relationships of blended polysaccharide and protein biomaterials in ionic liquid, Cellulose, 2017, 24, 1775–1789 CrossRef CAS.
- D. Tian, T. Li, R. Zhang, Q. Wu, T. Chen, P. Sun and A. Ramamoorthy, Conformations and intermolecular interactions in cellulose/silk fibroin blend films: a solid-state NMR perspective, J. Phys. Chem. B, 2017, 121, 6108–6116 CrossRef CAS PubMed.
- J. Stanton, Y. Xue, P. Pandher, L. Malek, T. Brown, X. Hu and D. Salas-de la Cruz, Impact of ionic liquid type on the structure, morphology and properties of silk-cellulose biocomposite materials, Int. J. Biol. Macromol., 2018, 108, 333–341 CrossRef CAS PubMed.
- A. Hadadi, J. W. Whittaker, D. E. Verrill, X. Hu, L. Larini and D. Salas-de la Cruz, A hierarchical model to understand the processing of polysaccharides/protein-based films in ionic liquids, Biomacromolecules, 2018, 19, 3970–3982 CrossRef CAS PubMed.
- S. A. Love, E. Popov, K. Rybacki, X. Hu and D. Salas-de la Cruz, Facile treatment to fine-tune cellulose crystals in cellulose-silk biocomposites through hydrogen peroxide, Int. J. Biol. Macromol., 2020, 147, 569–575 CrossRef CAS PubMed.
- S. A. Love, X. Hu and D. Salas-de la Cruz, Controlling the structure and properties of semi-crystalline cellulose/silk fibroin biocomposites by ionic liquid type and hydrogen peroxide concentration, Carbohydr. Polym. Technol. Appl., 2022, 3, 100193 CAS.
- H. Xie, S. Li and S. Zhang, Ionic liquids as novel solvents for the dissolution and blending of wool keratin fibers, Green Chem., 2005, 7, 606–608 RSC.
- N. Hameed and Q. Guo, Blend films of natural wool and cellulose prepared from an ionic liquid, Cellulose, 2010, 17, 803–813 CrossRef CAS.
- K. Rybacki, S. A. Love, B. Blessing, A. Morales, E. McDermott, K. Cai, X. Hu and D. Salas-de la Cruz, Structural and morphological properties of wool keratin and cellulose biocomposites fabricated using ionic liquids, ACS Mater. Au, 2022, 2, 21–32 CrossRef CAS PubMed.
- Z. Meng, X. Zheng, K. Tang, J. Liu, Z. Ma and Q. Zhao, Dissolution and regeneration of collagen fibers using ionic liquid, Int. J. Biol. Macromol., 2012, 51, 440–448 CrossRef CAS PubMed.
- J. Wang, L. Wei, Y. Ma, K. Li, M. Li, Y. Yu, L. Wang and H. Qiu, Collagen/cellulose beads reconstituted from ionic liquid solution for Cu(II) adsorption, Carbohydr. Polym., 2013, 98, 736–743 CrossRef CAS PubMed.
- M. Zhang, C. Ding, L. Chen and L. Huang, The preparation of cellulose/collagen composite films using 1-ethyl-3-methylimidazolium acetate as a solvent, BioResources, 2014, 9, 756–771 Search PubMed.
- M. Zhang, C. Ding, L. Huang, L. Chen and H. Yang, Interactions of collagen and cellulose in their blends with 1-ethyl-3-methylimidazolium acetate as solvent, Cellulose, 2014, 21, 3311–3322 CrossRef CAS.
- J. Xu, B. Liu, J. Hu and H. Hou, Ionic liquid mediated technology for fabrication of cellulose film using gutta percha as an additive, Ind. Crops Prod., 2017, 108, 140–148 CrossRef CAS.
- S. Murugesan, S. Mousa, A. Vijayaraghavan, P. M. Ajayan and R. J. Linhardt, Ionic liquid-derived blood-compatible composite membranes for kidney dialysis, J. Biomed. Mater. Res., Part B, 2006, 79, 298–304 CrossRef PubMed.
- S. Park, Y. Oh, J. Yun, E. Yoo, D. Jung, K. S. Park, K. K. Oh and S. H. Lee, Characterization of blended cellulose/biopolymer films prepared using ionic liquid, Cellulose, 2020, 27, 5101–5119 CrossRef CAS.
- C. Hopson, M. M. Villar-Chavero, J. C. Domínguez, M. V. Alonso, M. Oliet and F. Rodriguez, Cellulose ionogels, a perspective of the last decade: a review, Carbohydr. Polym., 2021, 274, 118663 CrossRef CAS PubMed.
- J. Kadokawa, M. Murakami and Y. Kaneko, A facile preparation of gel materials from a solution of cellulose in ionic liquid, Carbohydr. Res., 2008, 343, 769–772 CrossRef CAS PubMed.
- G. M. Kavanagh and S. B. Ross-Murphy, Rheological characterisation of polymer gels, Prog. Polym. Sci., 1998, 23, 533–562 CrossRef CAS.
- T. K. L. Meyvis, B. G. Stubbe, M. J. Van Steenbergen, W. E. Hennink, S. C. De Smedt and J. Demeester, A comparison between the use of dynamic mechanical analysis and oscillatory shear rheometry for the characterisation of hydrogels, Int. J. Pharm., 2002, 244, 163–168 CrossRef CAS PubMed.
- S. Yamazaki, A. Takegawa, Y. Kaneko, J. Kadokawa, M. Yamagata and M. Ishikawa, An acidic cellulose-chitin hybrid gel as novel electrolyte for an electric double layer capacitor, Electrochem. Commun., 2009, 11, 68–70 CrossRef CAS.
- S. Yamazaki, A. Takegawa, Y. Kaneko, J. Kadokawa, M. Yamagata and M. Ishikawa, High/low temperature operation of electric double layer capacitor utilizing acidic cellulose-chitin hybrid gel electrolyte, J. Power Sources, 2010, 195, 6245–6249 CrossRef CAS.
- W. Kunchornsup and A. Sirivat, Physically cross-linked cellulosic gel via 1-butyl-3-methylimidazolium chloride ionic liquid and its electrochemical responses, Sens. Actuators, A, 2012, 175, 155–164 CrossRef CAS.
- S. Thiemann, S. J. Sachnov, F. Pettersson, R. Bollström, R. Österbacka, P. Wasserscheid and J. Zaumseil, Cellulose-based ionogels for paper electronics, Adv. Funct. Mater., 2014, 24, 625–634 CrossRef CAS.
- G. P. Salvador, D. Pugliese, F. Bella, A. Chiappone, A. Sacco, S. Bianco and M. Quaglio, New insights in long-term photovoltaic performance characterization of cellulose-based gel electrolytes for stable dye-sensitized solar cells, Electrochim. Acta, 2014, 146, 44–51 CrossRef CAS.
- Y. Cao, J. Zhang, Y. Bai, R. Li, S. M. Zakeeruddin, M. Grätzel and P. Wang, Dye-sensitized solar cells with solvent-free ionic liquid electrolytes, J. Phys. Chem. C, 2008, 112, 13775–12781 CrossRef CAS.
- D. Zhao, Y. Zhu, W. Cheng, G. Xu, Q. Wang, S. Liu, J. Li, C. Chen, H. Yu and L. Hu, A dynamic gel with reversible and tunable topological networks and performances, Matter, 2020, 2, 390–403 CrossRef CAS.
- J. Kaszyńska, A. Rachocki, M. Bielejewski and J. Tritt-Goc, Influence of cellulose gel matrix on BMIMCl ionic liquid dynamics and conductivity, Cellulose, 2017, 24, 1641–1655 CrossRef.
- C. J. Smith II, D. V. Wagle, H. M. O'Neill, B. R. Evans, S. N. Baker and G. A. Baker, Bacterial cellulose ionogels as chemosensory support, ACS Appl. Mater. Interfaces, 2017, 9, 38042–38051 CrossRef PubMed.
- R. Mantravadi, P. R. Chinnam, D. A. Dikin and S. L. Wunder, High conductivity, high strength solid electrolytes formed by in situ encapsulation of ionic liquids in nanofibrillar methyl cellulose networks, ACS Appl. Mater. Interfaces, 2016, 8, 13426–13436 CrossRef CAS PubMed.
- S. K. Simotwo, P. R. Chinnam, S. L. Wunder and V. Kalra, Highly durable, self-standing solid-state supercapacitor based on an ionic liquid-rich ionogel and porous carbon nanofiber electrodes, ACS Appl. Mater. Interfaces, 2017, 9, 33749–33757 CrossRef CAS.
- S. Chereddy, J. Aguirre, D. Dikin, S. L. Wunder and P. R. Chinnam, Gel electrolyte comprising solvate ionic liquid and methyl cellulose, ACS Appl. Energy Mater., 2020, 3, 279–289 CrossRef CAS.
- H. Lee, A. Erwin, M. L. Buxton, M. Kim, A. V. Stryutsky, V. V. Shevchenko, A. P. Sokolov and V. V. Tsukruk, Shape persistent, highly conductive ionigels from ionic liquids reinforced with cellulose nanocrystal network, Adv. Funct. Mater., 2021, 31, 2103083 CrossRef CAS.
- P. W. Schmidt, S. Morozova, S. P. Ertem, M. L. Coughlin, I. Davidovich, Y. Talmon, T. M. Reineke, F. S. Bates and T. P. Lodge, Internal structure of methylcellulose fibrils, Macromolecules, 2020, 53, 398–405 CrossRef CAS.
- R. P. Singh and P. P. Kundu, DSC and micro structural studies of methylcellulose gels in N,N-dimethylformamide, J. Polym. Res., 2013, 20, 226 CrossRef.
- W. Kunchornsup and A. Sirivat, Effects of crosslinking ratio and aging time on properties of physical and chemical cellulose gels via 1-butyl-3-methylimidazolium chloride solvent, J. Sol-Gel Sci. Technol., 2010, 56, 19–26 CrossRef CAS.
- P. Li, Y. Zhang, W. Fa, Y. Zhang and B. Huang, Synthesis of a grafted cellulose gel electrolyte in an ionic liquid (BmimI) for dye-sensitized solar cells, Carbohydr. Polym., 2011, 86, 1216–1220 CrossRef CAS.
- A. Kimura, N. Nagasawa and M. Taguchi, Cellulose gels produced in room temperature ionic liquids by ionizing radiation, Radiat. Phys. Chem., 2014, 103, 216–221 CrossRef CAS.
- H. H. Rana, J. H. Park, G. S. Gund and H. S. Park, Highly conducting, extremely durable, phosphorylated cellulose-based ionogels for renewable flexible supercapacitors, Energy Storage Mater., 2020, 25, 70–75 CrossRef.
- L. Li, Z. B. Lin, X. Yang, Z. Z. Wan and S. X. Cui, A novel cellulose hydrogel prepared from its ionic liquid solution, Chin. Sci. Bull., 2009, 54, 1622–1625 CAS.
- H. Satani, M. Kuwata and A. Shimizu, Simple and environmentally friendly preparation of cellulose hydrogels using an ionic liquid, Carbohydr. Res., 2020, 494, 108054 CrossRef CAS PubMed.
- P. Berton, X. Shen, R. D. Rogers and J. L. Shamshina, 110th anniversary: high-molecular-weight chitin and cellulose hydrogels from biomass in ionic liquids without chemical crosslinking, Ind. Eng. Chem. Res., 2019, 58, 19862–19876 CrossRef CAS.
- H. Peng, S. Wang, H. Xu and G. Dai, Preparations, properties, and formation mechanism of novel cellulose hydrogel membrane based on ionic liquid, J. Appl. Polym. Sci., 2018, 135, 45488 CrossRef.
- M. Kimura, Y. Shinohara, J. Takizawa, S. Ren, K. Sagisaka, Y. Lin, Y. Hattori and J. P. Hinestroza, Versatile molding process for tough cellulose hydrogel materials, Sci. Rep., 2015, 5, 16266 CrossRef PubMed.
- Z. Liu and H. Huang, Preparation and characterization of cellulose composite hydrogels from tea residue and carbohydrate additives, Carbohydr. Polym., 2016, 147, 226–233 CrossRef CAS PubMed.
- C. Wu, D. J. McClements, M. He, Z. Fan, Y. Li and F. Teng, Preparation of okara cellulose hydrogels using ionic liquids: structure, properties, and performance, J. Mol. Liq., 2021, 331, 115744 CrossRef.
- D. Santra and K. Sen, Ionic liquid-modified cellulosic hydrogels or loading and sustained release of selenourea: an ensuing inhibition of tyrosinase activity, Mater. Today Chem., 2020, 18, 100365 CrossRef CAS.
- R. Su, F. Wang, J. Ding, Q. Li, W. Zhou, Y. Liu, B. Gao and Q. Yue, Magnetic hydrogel derived from wheat straw cellulose/feather protein in ionic liquids as copper nanoparticles carrier for catalytic reduction, Carbohydr. Polym., 2019, 220, 202–210 CrossRef CAS PubMed.
- X. Li, F. Dong, L. Zhang, Q. Xu, X. Zhu, S. Liang, L. Hu and H. Xie, Cellulosic protic ionic liquids hydrogel: a green and efficient catalyst carrier for Pd nanoparticles in reduction of 4-nitrophenol in water, Chem. Eng. J., 2019, 372, 516–525 CrossRef CAS.
- X. Liang, B. Qu, J. Li, H. Xiao, B. He and L. Qian, Preparation of cellulose-based conductive hydrogels with ionic liquid, React. Funct. Polym., 2015, 86, 1–6 CrossRef CAS.
- M. Xu, Q. Huang, X. Wang and R. Sun, Highly tough cellulose/graphene composite hydrogels prepared from ionic liquids, Ind. Crops Prod., 2015, 70, 56–63 CrossRef CAS.
- C. Tsioptsias, A. Stefopoulos, I. Kokkinomalis, L. Papadopoulou and C. Panayiotou, Development of micro- and nano-porous composite materials by processing cellulose with ionic liquids and supercritical CO2, Green Chem., 2008, 10, 965–971 RSC.
- M. Deng, Q. Zhou, A. Du, J. van Kasteren and Y. Wang, Preparation of nanoporous cellulose foams from cellulose-ionic liquid solutions, Mater. Lett., 2009, 63, 1851–1854 CrossRef CAS.
- M. Xu, W. Bao, S. Xu, X. Wang and R. Sun, Porous cellulose aerogels with high mechanical performance and their absorption behaviours, BioResources, 2016, 11, 8–20 CAS.
- L.-Y. Long, Y.-X. Weng and Y.-Z. Wang, Cellulose aerogels: synthesis, applications, and prospects, Polymers, 2018, 10, 623 CrossRef PubMed.
- U. G. K. Wegst, H. Bai, E. Saiz, A. P. Tomsia and R. O. Ritchie, Bioinspired structural materials, Nat. Mater., 2015, 14, 23–36 CrossRef CAS PubMed.
- S. F. Plappert, J.-M. Nedelec, H. Rennhofer, H. C. Lichtenegger, S. Bernstorff and F. W. Liebner, Self-assembly of cellulose in super-cooled ionic liquid under the impact of decelerated antisolvent diffusion: an approach towards anisotropic gels and aerogels, Biomacromolecules, 2018, 19, 4411–4422 CrossRef CAS PubMed.
- H. Rennhofer, S. F. Plappert, H. C. Lichtenegger, S. Bernstorff, M. Fitzka, J.-M. Nedelec and F. W. Liebner, Insight into the nanostructure of anisotropic cellulose aerogels upon compression, Soft Matter, 2019, 15, 8372–8380 RSC.
- X. An, X. Zhang, M. Li, D. Pei, X. Ma and C. Li, Bubble-templated design of superelastic cellulose foam as a durable ionotropic sensor, ACS Sustainable Chem. Eng., 2022, 10, 1714–1721 CrossRef CAS.
- O. Aaltonen and O. Jauhiainen, The preparation of lignocellulosic aerogels from ionic liquid solutions, Carbohydr. Polym., 2009, 75, 125–129 CrossRef CAS.
- J. Li, Y. Lu, D. Yang, Q. Sun, Y. Liu and H. Zhao, Lignocellulose aerogel from wood-ionic liquid solution (1-allyl-3-methylimidazolium chloride) under freezing and thawing conditions, Biomacromolecules, 2011, 12, 1860–1867 CrossRef CAS PubMed.
- Y. Lu, Q. Sun, D. Yang, X. She, X. Yao, G. Zhu, Y. liu, H. Zhao and J. Li, Fabrication of mesoporous lignocellulose aerogels from wood via cyclic liquid nitrogen freezing-thawing in ionic liquid solution, J. Mater. Chem., 2012, 22, 13548–13557 RSC.
- H. Zhang, Y. Li, Y. Xu, Z. Lu, L. Chen, L. Huang and M. Fan, Versatile fabrication of a superhydrophobic and ultralight cellulose-based aerogel for oil spillage clean-up, Phys. Chem. Chem. Phys., 2016, 18, 28297–28306 RSC.
- A. Demilecamps, C. Beauger, C. Hildebrand, A. Rigacci and T. Budtova, Cellulose-silica aerogels, Carbohydr. Polym., 2015, 122, 293–300 CrossRef CAS PubMed.
- H. Wang, Z. Shao, M. Bacher, F. Liebner and T. Rosenau, Fluorescent cellulose aerogels containing covalently immobilized (ZnS)x(CuInS2)1-x/ZnS (core/shell) quantum dots, Cellulose, 2013, 20, 3007–3024 CrossRef CAS PubMed.
- J.-H. Lee and S.-J. Park, Recent advances in preparations and applications of carbon aerogels: a review, Carbon, 2020, 163, 1–18 CrossRef CAS.
- J. W. S. Hearle, Textile fibers: a comparative overview, in Encyclopedia of Materials: Science and Technology, ed. K. H. J. Buschow, R. W. Cahn, M. C. Flemings, B. Ilschner, E. J. Kramer, S. Mahajan and P. Veyssière, Elsevier, Oxford, UK, 2001, pp. 9100–9116, ISBN 978-0-08-043152-9 Search PubMed.
- A. Libanori, G. Chen, X. Zhao, Y. Zhou and J. Chen, Smart textiles for personalized healthcare, Nat. Electron., 2022, 5, 142–156 CrossRef CAS.
- X. Shi, Y. Zou, P. Zai, J. Shen, Y. Yang, Z. Gao, M. Liao, J. Wu, J. Wang, X. Xu, Q. Tong, B. Zhang, B. Wang, X. Sun, L. Zhang, Q. Pei, D. Jin, P. Chen and H. Peng, Large-area display textiles integrated with functional systems, Nature, 2021, 591, 240–245 CrossRef CAS PubMed.
- F. M. Häemmerle, The cellulose gap (the future of cellulose fibres), Lenzinger Ber., 2011, 89, 12–21 Search PubMed.
- C. Felgueiras, N. G. Azoia, C. Gonçalves, M. Gama and F. Dourado, Trends on the cellulose-based textiles: raw materials and technologies, Front. Bioeng. Biotechnol., 2021, 9, 608826 CrossRef PubMed.
- M. Hummel, A. Michud, M. Tanttu, S. Asaadi, Y. Ma, L. K. J. Hauru, A. Parviainen, A. W. T. King, I. Kilpeläinen and H. Sixta, Ionic liquids for the production of man-made cellulosic fibers: opportunities and challenges, Adv. Polym. Sci., 2016, 271, 133–168 CrossRef CAS.
- F. Hermanutz, M. P. Vocht, N. Panzier and M. R. Buchmeiser, Processing of cellulose using ionic liquids, Macromol. Mater. Eng., 2019, 304, 1800450 CrossRef.
- G. Laus, G. Bentivoglio, H. Schottenberger, V. Kahlenberg, H. Kopacka, T. Röder and H. Sixta, Ionic liquids: current developments, potential and drawbacks for industrial applications, Lenzinger Ber., 2005, 84, 71–85 CAS.
- G. Bentivoglio, T. Röder, M. Fasching, M. Buchberger, H. Schottenberger and H. Sixta, Cellulose processing with chloride-based ionic liquids, Lenzinger Ber., 2006, 86, 154–161 CAS.
- B. Kosan, C. Michels and F. Meister, Dissolution and forming of cellulose with ionic liquids, Cellulose, 2008, 15, 59–66 CrossRef CAS.
- D. Ingildeev, F. Effenberger, K. Bredereck and F. Hermanutz, Comparison of direct solvents for regenerated cellulosic fibers via the Lyocell process and by means of ionic liquids, J. Appl. Polym. Sci., 2013, 128, 4141–4150 CrossRef CAS.
- C. Zhu, R. M. Richardson, K. D. Potter, A. F. Koutsomitopoulou, J. S. van Duijneveldt, S. R. Vincent, N. D. Wanasekara, S. J. Eichhorn and S. S. Rahatekar, High modulus regenerated cellulose fibers spun from a low molecular weight microcrystalline cellulose solution, ACS Sustainable Chem. Eng., 2016, 4, 4545–4553 CrossRef CAS.
- L. Zhou, Z. Kang, Y. Nie and L. Li, Fabrication of regenerated cellulose fibers with good strength and biocompatibility from green spinning process of ionic liquid, Macromol. Mater. Eng., 2021, 306, 2000741 CrossRef CAS.
- J. Zhang, K. Tominaga, N. Yamagishi and Y. Gotoh, Comparison of regenerated cellulose fibers spun from ionic liquid solutions with Lyocell fiber, J. Fiber Sci. Technol., 2020, 76, 257–266 CrossRef.
- A. Michud, A. King, A. Parviainen, H. Sixta, L. Hauru, M. Hummel and I. Kilpeläinen, Process for the production of shaped cellulose articles, International Patent, 2014/162062A1, 2014 Search PubMed.
- L. K. J. Hauru, M. Hummel, A. Michud and H. Sixta, Dry jet-wet spinning of strong cellulose filaments from ionic liquid solution, Cellulose, 2014, 21, 4471–4481 CrossRef CAS.
- H. Sixta, A. Michud, L. Hauru, S. Asaadi, Y. Ma, A. W. T. King, I. Kilpeläinen and M. Hummel, Ioncell-F: a high-strength regenerated cellulose fibre, Nord. Pulp Pap. Res. J., 2015, 30, 43–57 CrossRef CAS.
- A. Michud, M. Hummel and H. Sixta, Influence of process parameters on the structure formation of man-made cellulosic fibers from ionic liquid solution, J. Appl. Polym. Sci., 2016, 133, 43718 CrossRef.
- A. Michud, M. Hummel and H. Sixta, Influence of molar mass distribution on the final properties of fibers regenerated from cellulose dissolved in ionic liquid by dry-jet wet spinning, Polymer, 2015, 75, 1–9 CrossRef CAS.
- Y. Ma, H. Hummel, M. Määttänen, A. Särkilahti, A. Harlin and H. Sixtaa, Upcycling of waste paper and cardboard to textiles, Green Chem., 2016, 18, 858–866 RSC.
- Y. Ma, M. Hummel, I. Kontro and H. Sixta, High-performance man-made cellulosic fibres from recycled newsprint, Green Chem., 2018, 20, 160–169 RSC.
- S. Haslinger, Y. Wang, M. Rissanen, M. B. Lossa, M. Tanttu, E. Ilen, M. Määttänen, A. Harlin, M. Hummel and H. Sixta, Recycling of vat and reactive dyed textile waste to new colored man-made cellulose fibers, Green Chem., 2019, 21, 5598–5610 RSC.
- S. Elsayed, B. Viard, C. Guizani, S. Hellsten, J. Witos and H. Sixta, Limitations of cellulose dissolution and fiber spinning in the Lyocell process using mTBDHOAc and DBNHOAc solvents, Ind. Eng. Chem. Res., 2020, 59, 20211–20220 CrossRef CAS.
- C. Guizani, S. Larkiala, K. Moriam, D. Sawada, S. Elsayed, S. Rantasalo, M. Hummel and H. Sixta, Air gap spinning of a cellulose solution in DBNHOAc ionic liquid with a novel vertically arranged spinning bath to simulate a closed loop operation in the Ioncell® process, J. Appl. Polym. Sci., 2021, 138, e49787 CrossRef.
- N. Sun, W. Li, B. Stoner, X. Jiang, X. Lu and R. D. Rogers, Composite fibers spun directly from solutions of raw lignocellulosic biomass, Green Chem., 2011, 13, 1158–1161 RSC.
- T. Nypelö, S. Asaadi, G. Kneidinger, H. Sixta and J. Konnerth, Conversion of wood-biopolymers into macrofibers with tunable surface anergy via dry-jet wet spinning, Cellulose, 2018, 25, 5297–5307 CrossRef PubMed.
- H. Zahra, D. Sawada, C. Guizani, Y. Ma, S. Kumagai, T. Yoshioka, H. Sixta and M. Hummel, Close packing of cellulose and chitosan in regenerated cellulose fibers improves carbon yield and structural properties of respective carbon fibers, Biomacromolecules, 2020, 21, 4326–4335 CrossRef CAS PubMed.
- A. Ota, R. Beyer, U. Hageroth, A. Müller, P. Tomasic, F. Hermanutz and M. R. Buchmeiser, Chitin/cellulose blend fibers prepared by wet and dry-wet spinning, Polym. Adv. Technol., 2021, 32, 335–342 CrossRef CAS.
- Y. Ma, S. Asaadi, L.-S. Johansson, P. Ahvenainen, M. Reza, M. Alekhina, L. Rautkari, A. Michud, L. Hauru, M. Hummel and H. Sixta, High-strength composite fibers from cellulose-lignin blends regenerated from ionic liquid solution, ChemSusChem, 2015, 8, 4030–4039 CrossRef CAS PubMed.
- C. Olsson, B. Hangström, E. Sjöholm and A. Reimann, in 18th International Symposium on Wood, Fibre, Pulp Chemistry (ISWFPC), Vienna, Austria, 2015, Proceeding vol. II, pp. 126–129 Search PubMed.
- E. Frank, L. M. Steudle, D. Ingildeev, J. M. Spörl and M. R. Buchmeiser, Carbon fibers: precursor systems, processing, structure and properties, Angew. Chem., Int. Ed., 2014, 53, 5262–5298 CrossRef CAS PubMed.
- N. Byrne, R. D. Silva, Y. Ma, H. Sixta and M. Hummel, Enhanced stabilization of cellulose-lignin hybrid filaments for carbon fiber production, Cellulose, 2018, 25, 723–733 CrossRef CAS PubMed.
- N.-D. Le, M. Trogen, Y. Ma, R. J. Varley, M. Hummel and N. Byrne, Cellulose-lignin composite fibers as precursors for carbon fibers: Part 2 - The impact of precursor properties on carbon fibers, Carbohydr. Polym., 2020, 250, 116918 CrossRef CAS PubMed.
- M. Trogen, N.-D. Le, D. Sawada, C. Guizani, T. V. Lourençon, L. Pitkänen, H. Sixta, R. Shah, H. O'Neill, M. Balakshin, N. Byrne and M. Hummel, Cellulose-lignin composite fibres as precursors for carbon fibres. Part 1 - Manufacturing and properties of precursor fibres, Carbohydr. Polym., 2021, 252, 117133 CrossRef CAS PubMed.
- N.-D. Le, M. Trogen, R. J. Varley, M. Hummel and N. Byrne, Effect of boric acid on the stabilisation of cellulose-lignin filaments as precursors for carbon fibres, Cellulose, 2021, 28, 729–739 CrossRef CAS.
- N.-D. Le, M. Trogen, Y. Ma, R. J. Varley, M. Hummel and N. Byrne, Understanding the influence of key parameters on the stabilisation of cellulose-lignin composite fibres, Cellulose, 2021, 28, 911–919 CrossRef CAS.
- N.-D. Le, M. Trogen, R. J. Varley, M. Hummel and N. Byrne, Chemically accelerated stabilization of a cellulose-lignin precursor as a route to high yield carbon fiber production, Biomacromolecules, 2022, 23, 839–846 CrossRef CAS PubMed.
- C. Olsson, E. Sjöholm and A. Reimann, Carbon fibres from precursors produced by dry-jet wet-spinning of kraft lignin blended with kraft pulps, Holzforschung, 2017, 71, 275–283 CrossRef CAS.
- A. Bengtsson, J. Bengtsson, C. Olsson, M. Sedin, K. Jedvert, H. Theliander and E. Sjöholm, Improved yield of carbon fibres from cellulose and kraft lignin, Holzforschung, 2018, 72, 1007–1016 CrossRef CAS.
- A. Bengtsson, J. Bengtsson, M. Sedin and E. Sjöholm, Carbon fibers from lignin-cellulose precursors: effect of stabilization conditions, ACS Sustainable Chem. Eng., 2019, 7, 8440–8448 CrossRef CAS.
- A. Bengtsson, P. Hecht, J. Sommertune, M. Ek, M. Sedin and E. Sjöholm, Carbon fibers from lignin-cellulose precursors: effect of carbonization conditions, ACS Sustainable Chem. Eng., 2020, 8, 6826–6833 CrossRef CAS.
- A. Bengtsson, J. Bengtsson, K. Jedvert, M. Kakkonen, O. Tanhuanpää, E. Brännvall and M. Sedin, Continuous stabilization and carbonization of a lignin-cellulose precursor to carbon fiber, ACS Omega, 2022, 7, 16793–16802 CrossRef CAS PubMed.
- H. Zahra, D. Sawada, S. Kumagai, Y. Ogawa, L.-S. Johansson, Y. Ge, C. Guizani, T. Yoshioka and M. Hummel, Evolution of carbon nanostructure during pyrolysis of homogeneous chitosan-cellulose composite fibers, Carbon, 2021, 185, 27–38 CrossRef CAS.
- N. Sun, R. P. Swatloski, M. L. Maxim, M. Rahman, A. G. Harland, A. Haque, S. K. Spear, D. T. Daly and R. D. Rogers, Magnetite-embedded cellulose fibers prepared from ionic liquid, J. Mater. Chem., 2008, 18, 283–290 RSC.
- M. L. Maxim, N. Sun, R. P. Swatloski, M. Rahman, A. G. Harland, A. Haque, S. K. Spear, D. T. Daly and R. D. Rogers, Properties of cellulose/TiO2 fibers processed from ionic liquids, ACS Symp. Ser., 2010, 1033, 261–274 CrossRef CAS.
- H.-Z. Song, Z.-Q. Luo, C.-Z. Wang, X.-F. Hao and J.-G. Gao, Preparation and characterization of bionanocomposite fiber based on cellulose and nano-SiO2 using ionic liquid, Carbohydr. Polym., 2013, 98, 161–167 CrossRef CAS PubMed.
- H. Zhang, Z. Wang, Z. Zhang, J. Wu, J. Zhang and J. He, Regenerated-cellulose/multiwalled-carbon-nanotube composite fibers with enhanced mechanical properties prepared with the ionic liquid 1-allyl-3-methylimidazolium chloride, Adv. Mater., 2007, 19, 698–704 CrossRef CAS.
- S. S. Rahatekar, A. Rasheed, R. Jain, M. Zammarano, K. K. Koziol, A. H. Windle, J. W. Gilman and S. Kumar, Solution spinning of cellulose carbon nanotube composites using room temperature ionic liquids, Polymer, 2009, 50, 4577–4583 CrossRef CAS.
- S. Haslinger, Y. Ye, M. Rissanen, M. Hummel and H. Sixta, Cellulose fibers for high-performance textiles functionalized with incorporated gold and silver nanoparticles, ACS Sustainable Chem. Eng., 2020, 8, 649–658 CrossRef CAS.
- K. Moriam, M. Rissanen, D. Sawada, M. Altgen, L.-S. Johansson, D. V. Evtyugin, C. Guizani, M. Hummel and H. Sixta, Hydrophobization of the man-made cellulosic fibers by incorporating plant-derived hydrophobic compounds, ACS Sustainable Chem. Eng., 2021, 9, 4915–4925 CrossRef CAS.
- M. J. Lundahl, D. Sawada, M. Merilä and M. Hummel, Effect of graphitic additives on the rheology of cellulose solutions for the preparation of templated carbon fiber precursors, J. Appl. Polym. Sci., 2022, e52670 CAS.
- B. A. da Silva, R. D. S. Cunha, A. Valério, A. D. N. Junior, D. Hotza and S. Y. G. González, Electrospinning of cellulose using ionic liquids: an overview on processing and applications, Eur. Polym. J., 2021, 147, 110283 CrossRef.
- A. Kramar and F. J. González-Benito, Cellulose-based nanofibers processing techniques and methods based on bottom-up approach – a review, Polymers, 2022, 14, 1–35 CrossRef PubMed.
- S. Xu, J. Zhang, A. He, J. Li, H. Zhang and C. C. Han, Electropinning of native cellulose from nonvolatile solvent system, Polymer, 2008, 49, 2911–2917 CrossRef CAS.
- S.-L. Quan, S.-G. Kang and I.-J. Chin, Characterization of cellulose fibers electrospun using ionic liquid, Cellulose, 2010, 17, 223–230 CrossRef CAS.
- T. P. T. Pham, C.-W. Cho and Y.-S. Yun, Environmental fate and toxicity of ionic liquids: a review, Water Res., 2010, 44, 352–372 CrossRef CAS PubMed.
- M. Petkovic, K. R. Seddon, L. P. Rebelo and C. S. Pereira, Ionic liquids: a pathway to environmental acceptability, Chem. Soc. Rev., 2011, 40, 1383–1403 RSC.
- K. S. Egorova and V. P. Ananikov, Toxicity of ionic liquids: eco(cyto)activity as complicated, but unavoidable parameter for task-specific optimization, ChemSusChem, 2014, 7, 336–360 CrossRef CAS PubMed.
- J. Flieger and M. Flieger, Ionic liquids toxicity – benefits and threats, Int. J. Mol. Sci., 2020, 21, 6267 CrossRef CAS PubMed.
- C.-W. Cho, T. P. T. Pham, Y. Zhao, S. Stolte and Y.-S. Yun, Review of the toxic effects of ionic liquids, Sci. Total Environ., 2021, 786, 147309 CrossRef CAS PubMed.
- A. Mehrkesh and A. T. Karunanithi, Life-cycle perspectives on aquatic ecotoxicity of common ionic liquids, Environ. Sci. Technol., 2016, 50, 6814–6821 CrossRef CAS PubMed.
- S. Koutsoukos, F. Philippi, F. Malaret and T. Welton, A review on machine learning algorithms for the ionic liquid chemical space, Chem. Sci., 2021, 12, 6820–6843 RSC.
- E. R. D. Seiler, Y. Takeoka, M. Rikukawa and M. Yoshizawa-Fujita, Development of a novel cellulose solvent based on pyrrolidinium hydroxide and reliable solubility analysis, RSC Adv., 2020, 10, 11475–11480 RSC.
- J. B. Guinée, R. Heijungs, G. Huppes, A. Zamagni, P. Masoni, R. Buonamici, T. Ekvall and T. Rydberg, Life cycle assessment: past, present, and future, Environ. Sci. Technol., 2011, 45, 90–96 CrossRef PubMed.
- H. Baaqel, I. Díaz, V. Tulus, B. Chachuat, G. Guillén-Gosálbez and J. P. Hallett, Role of life-cycle externalities in the valuation of protic ionic liquids – a case study in biomass pretreatment solvents, Green Chem., 2020, 22, 3132–3140 RSC.
- L. Liang, J. Yan, Q. He, T. Luong, T. R. Pray, B. A. Simmons and N. Sun, Scale-up of biomass conversion using 1-ethyl-3-methylimidazolium acetate as the solvent, Green Energy Environ., 2019, 4, 432–438 CrossRef.
- X. Liang and H. Liu, High-efficiency recovery of 1-ethyl-3-methylimidazolium acetate for sugarcane bagasse pretreatment with industrialized technology, Sep. Purif. Technol., 2020, 238, 116437 CrossRef CAS.
- W. Lan, C.-F. Liu, F.-X. Yue, R.-C. Sun and J. F. Kennedy, Ultrasound-assisted dissolution of cellulose in ionic liquid, Carbohydr. Polym., 2011, 86, 672–677 CrossRef CAS.
- J.-P. Mikkola, A. Kirilin, J.-C. Tuuf, A. Pranovich, B. Holmbom, L. M. Kustov, D. Y. Murzin and T. Salmi, Ultrasound enhancement of cellulose processing in ionic liquids: from dissolution towards functionalization, Green Chem., 2007, 9, 1229–1237 RSC.
- M. E. Gibril, L. Huan, L. X. Da, Z. Yue, K. Han and Y. Muhuo, Reactive extrusion process for the preparation of a high concentration solution of cellulose in ionic liquid for in situ chemical modification, RSC Adv., 2013, 3, 1021–1024 RSC.
- R. Milotskyi, L. Szabó, T. Fujie, K. Sakata, N. Wada and K. Takahashi, Low waste process of rapid cellulose transesterification using ionic liquid/DMSO mixed solvent: towards more sustainable reaction systems, Carbohydr. Polym., 2021, 256, 117560 CrossRef CAS PubMed.
- S. C. Hernandez, R. Milotskyi, S. Takagi, E. R. D. Ito, S. Suzuki, N. Wada and K. Takahashi, Continuous production of cellulose mixed esters via homogeneous reactive twin-screw extrusion catalyzed by ionic liquid, Cellulose, 2023, 30, 2873–2882 CrossRef CAS.
- R. Milotskyi, G. Sharma, T. Fujie, D. Hirose, N. Wada and K. Takahashi, Continuous process of cellulose dissolution and transesterification reaction catalysed by ionic liquid in twin screw extruder, React. Chem. Eng., 2023, 1–8 Search PubMed.
- G. Chen, Z. Shen, A. Iyer, U. F. Ghumman, S. Tang, J. Bi, W. Chen and Y. Li, Machine-learning-assisted de novo design of organic molecules and polymers: opportunities and challenges, Polymers, 2020, 12, 163 CrossRef CAS PubMed.
- A. Bendaoud and Y. Chalamet, Plasticizing effect of ionic liquid on cellulose acetate obtained by melt processing, Carbohydr. Polym., 2014, 108, 75–82 CrossRef CAS PubMed.
- L. Li, Y. Zhang, Y. Sun, S. Sun, G. Shen, P. Zhao, J. Cui, H. Qiao, Y. Wang and H. Zhou, Manufacturing pure cellulose films by recycling ionic liquids as plasticizers, Green Chem., 2020, 22, 3835–3841 RSC.
- S. Livi, J. Duchet-Rumeau, J.-F. Gérard and T. N. Pham, Polymers and ionic liquids: a successful wedding, Macromol. Chem. Phys., 2015, 216, 359–368 CrossRef CAS.
- C. G. Otoni, H. M. C. Azeredo, B. D. Mattos, M. Beaumont, D. S. Correa and O. J. Rojas, The food-materials nexus: next generation bioplastics and advanced materials from agri-food residues, Adv. Mater., 2021, 33, 2102520 CrossRef CAS PubMed.
- https://www.japan.go.jp/tomodachi/2020/summer2020/innovating_paper.html (last accessed on 28/09/2022).
- https://www.japantimes.co.jp/life/2022/09/05/people/banana-stem-one-planet-cafe-grows-change (last accessed on 28/09/2022).
| This journal is © The Royal Society of Chemistry 2023 |