Ag@imidazolium functionalized polymeric yolk–shell hybrid nanoparticles for economical CO2 photoreduction†
Tuanwei
Liu‡
 *ab,
Guodong
Fu‡
a,
Zhongjie
Wu
cd,
Zhongjie
Kang
a,
Jinjian
Wei
a,
Zhide
Zhang
*a,
Xinlin
Yang
*ab,
Guodong
Fu‡
a,
Zhongjie
Wu
cd,
Zhongjie
Kang
a,
Jinjian
Wei
a,
Zhide
Zhang
*a,
Xinlin
Yang
 *b and
Dian-Shun
Guo
*b and
Dian-Shun
Guo
 *a
*a
aCollege of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Shandong Normal University, Jinan 250014, P. R. China. E-mail: twliu2010@163.com; zdzhang@sdnu.edu.cn; chdsguo@sdnu.edu.cn
bKey Laboratory of Functional Polymer Materials, Ministry of Education, Institute of Polymer Chemistry, College of Chemistry, Nankai University, Tianjin 300071, PR China. E-mail: xlyang88@nankai.edu.cn
cShandong Zhongshi Yitong Group Co., Ltd, Jinan 250003, P. R. China
dState Grid Shandong Electric Power Company Electric Power Research Institute, Jinan 250002, P. R. China
First published on 24th November 2022
Abstract
Inspired by green plant photosynthesis, the photocatalytic reduction of CO2 into hydrocarbons is a promising approach to solve the environmental problem of excess CO2 in the atmosphere. In addition to catalyst innovation, it is also necessary to develop a material combining multiple factors for economical CO2 reduction. Herein, we design Ag@P(IVBC-co-DVB) yolk–shell nanoparticles (AIDNPs) that possess an Ag core and an imidazolium functionalized polymeric shell. The Ag nanoparticles transform excellent visible light absorption into hot electron/hole pairs via surface plasmon resonance, pairs which are directly shifted to the adsorbed CO2 on the polymeric shell owing to the suitable core-to-shell diffusion distance of the material. As a result, CO2 is photoreduced to HCOOH with a competitive efficiency of 396 μmol gcat−1 h−1 in the absence of a photosensitizer or a sacrificial reducing agent. The investigation of the factors related to the photocatalytic CO2 production efficiency and the investigation of the photoelectric properties, as well as the proposed possible reduction mechanism provide a comprehensive model for efficient and economic CO2 photoreduction.
Introduction
For the present and foreseeable future, fossil fuels will remain an important energy source. Excessive emission of carbon dioxide (CO2) from fuel combustion results in the green-house effect and acid rain, bringing increasing pressure for sustainable development on Earth. Inspired by photosynthesis in green plants, a promising sustainable development strategy is via the artificial photocatalytic reduction of the excess CO2 in the atmosphere into fuel substances or other energy-rich precursors, such as carbon monoxide, methanol, methane, or formic acid, etc.1–3Currently, the basic components of a photocatalytic CO2 reduction system consists of the catalyst, photo-sensitizer, sacrificial reducing agent, and solvent.4 To achieve a high efficiency for CO2 reduction, researchers have mainly focused on the design and synthesis of the photocatalyst. In particular, metal-containing catalysts are the preferred candidates for their matrix and intermediate activity, recyclability, reduction efficiency, and good selectivity for reduction products.3,5 However, it is still a challenge to develop metal-containing catalysts, especially rare metal catalysts with difficult and high-cost syntheses, that can be recycled and do not result in metal pollution.6,7 Keeping this in mind, a solution that results in new pollution and emissions of carbon source waste would be contrary to the original intention for these catalytic systems of protecting the environment. A possible solution would be to optimize the catalytic system via adjusting the main factors that influence the performance, such as the light absorption, capability for CO2 capture, catalyst efficiency, energy utilization, and the solvents.
Imidazolium ionic liquids, imidazolylidene N-heterocyclic carbenes (NHCs), and zeolitic imidazolium frameworks have been extensively investigated for CO2 adsorption and catalytic conversion.8,9 Utilizing favorable imidazole–CO2 interactions, imidazolium motifs can suppress the undesired hydrogen-evolution reaction in order to further promote CO2 conversion activities.8 during electroreduction, for example, the imidazolium motif in ionic liquids can lower the potential for the formation of a CO2˙− intermediate which can react with H+ at the cathode to produce carbon monoxide.8,10 However, in photocatalysis, ionic liquids have rarely been used due to their low catalytic efficiency. Furthermore, it has not been practical to extract liquid-phase products when ionic liquids were employed as solvents or catalysts.10 Therefore, imidazolium motif functional components should be well designed for efficient CO2 photoreduction.
As visible light constitutes around 43% of solar energy, it is vital to design visible light-responsive photocatalysts.5 Most recently, the photocatalytic CO2 reduction catalyst metals Ag, Au, or Cu nanoparticles have attracted attention for their localized surface plasmon resonance (LSPR) properties in visible wavelength region.11,12 Photoexcitation of the collective electronic oscillations results in generation of hot electron/hole pairs (1e−/1H+ pairs) from the metal nanoparticles surfaces that are transferred to catalyze chemical reactions.11,13 Furthermore, when cationic imidazolium ligands are anchored to Ag surfaces, the contact Ag surfaces are relatively electron poor. Thus, photoexcited electrons are preferably transferred from the Ag surfaces to the ligands, which benefit the catalytic reduction of CO2 absorbed on the imidazolium motifs.1
Keeping these principles in mind, it was necessary to consider all aspects together to achieve high efficiency. Herein, we have designed and synthesized a form of Ag@P(IVBC-co-DVB) yolk–shell nanoparticles (AIDNPs) with a Ag nanoparticle core and an imidazolium functionalized polymeric shell (Fig. 1). This “Ag-core@imidazolium-polymeric-shell” architecture covers the most important aspects for efficient catalysis, including the harvesting of visible light, adsorption and concentration of CO2, as well as the generation of hot electron/hole pairs and energy-transfer via the combination of metallic nanocolloids and imidazolium motifs in one material. First, CO2 is adsorbed and concentrated by the imidazolium functionalized polymeric shell. The Ag nanoparticles then transform the excellent visible light absorption into hot electrons (and holes) due to the presence of surface plasmon resonance, which is then shifted to the adsorbed CO2 over a suitable core-to-shell diffusion distance to lead direct CO2 reduction in the absence of any photosensitizer or sacrificial reducing agent. In terms of structure–function relationships in this work, a core–shell structure should be more advantageous than a yolk–shell structure. However, it was difficult to modify the surface of the Ag nanoparticles for the polymerization of functional monomers and crosslinkers on its surface. In this case, we performed the polymerization on Ag@SiO2 core–shell nanoparticles, and then the silica layer was selectively etched to obtain the yolk–shell structure of the AIDNPs. Besides, due to the swelling effect, the Ag core and the polymeric shell of the AIDNPs would be in contact with each other in the solvent environment, which is similar to the core–shell architecture. After investigating the effects of the solvent, illumination conditions, Ag cores with different diameters, different pHs, photosensitizer and sacrificial reducing agent addition experiments, as well as the photoelectric properties, a possible photoreduction mechanism was proposed for the photocatalytic CO2 reduction using AIDNPs.
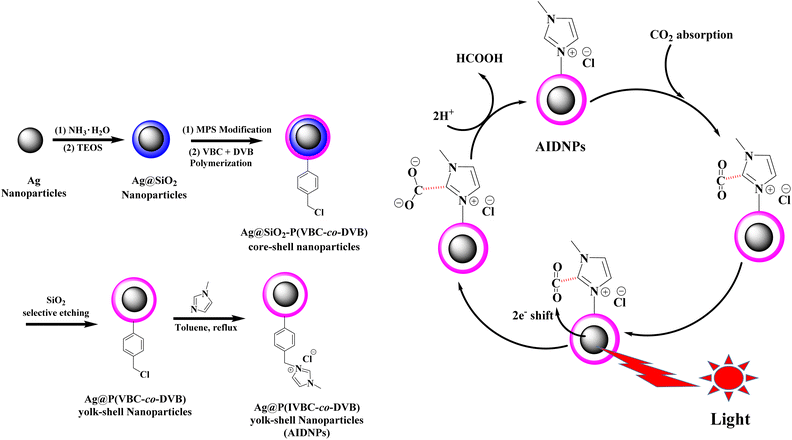 | ||
| Fig. 1 Schematic diagrams of the synthesis of AIDNPs and illustration of the CO2 photoreduction using AIDNPs. | ||
Experimental
Please refer to the detailed experimental information and methods in the ESI.†Results and discussion
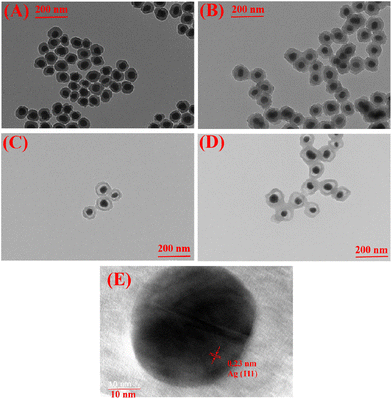
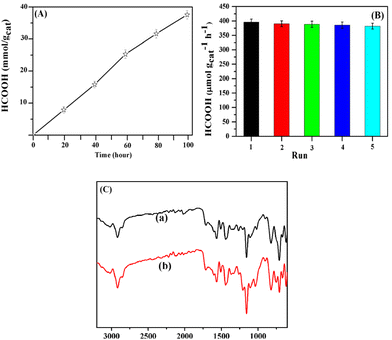
Transmission electron microscope (TEM) images were obtained to analyze the topographical character of the resultant nanoparticles (Table 1 and Fig. 2). A typical core–shell nanoparticle with a deeper contrast Ag core and lighter contrast silica shell revealed the successful synthesis of the Ag@SiO2 core–shell nanoparticles (Dn = 84 nm) via a sol–gel synthesis method with Ag nanoparticles (Dn = 40 nm) as the seeds. Then, distillation precipitation polymerization of 4-vinyl benzyl chloride (VBC, monomer) and divinylbenzene (DVB, crosslinker) was performed on the Ag@SiO2 nanoparticles surfaces. The success of this procedure was confirmed by the characteristic absorption peaks at 2925 cm−1 and 1604 cm−1 indicating a benzyl ring as well as the peak at around 1264 cm−1 contributing to the stretching vibration of the C–H bonds adjacent to the C–Cl bond (Fig. 3B). As a result, the Ag@SiO2-P(VBC-co-DVB) core–shell nanoparticles (Dn = 114 nm) were afforded a polymeric shell of 15 nm. Subsequently, hydrofluoric acid was used to selectively etch the silica mid-layer of the core–shell nanoparticles for the formation of the Ag@P(VBC-co-DVB) nanoparticles with a yolk–shell structure and diameter of 103 nm. The yolk–shell nanoparticles were observed to have a deformed shape when compared to that of the Ag@SiO2-P(VBC-co-DVB) core–shell nanoparticles, which was attributed to the hollow structure without the support from the silica inner shell.14 The TEM image in Fig. 2C shows, from inside to outside, a typical “dark-blank-gray” variation in the changes in contrast, confirming the yolk–shell structure. Moreover, the characteristic Si–O stretching vibration peak at 1075 cm−1 in the Fourier-transform infrared (FTIR) spectrum in Fig. 3C disappeared, reconfirming the successful preparation of the yolk–shell Ag@P(VBC-co-DVB) nanoparticles. Finally, 1-methylimidazole was employed in a replacement reaction with the chlorine groups on the benzyl rings of the poly(VBC-co-DVB) shell to afford the imidazolium functionalized AIDNPs. This was confirmed by the appearance of the stretching vibration peak of the imidazole ring at 1560 cm−1 with the simultaneous disappearance of the stretching vibration of the C–H bonds adjacent to the C–Cl bond at 1264 cm−1 (Fig. 3D). From the N content (4.98%, entry 4 Table 1) of the CHN elemental analysis the amount of imidazolium present was determined to be 11.92%.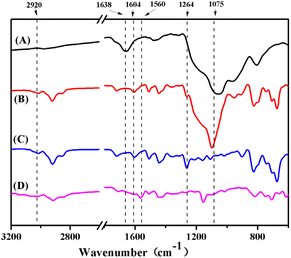 | ||
| Fig. 3 FTIR spectra. (A) Ag@SiO2 core–shell nanoparticles, (B) Ag@SiO2-P(VBC-co-DVB) core–shell nanoparticles, (C) Ag@P(VBC-co-DVB) yolk–shell nanoparticles, and (D) AIDNPs. | ||
| Entry | Sample name | D n (nm) | U | S BET (m2 g−1) | S Langmuir (m2 g−1) | V total (cm3 g−1) | CHN analysis (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | |||||||
| a D n and U refer to the number-average diameters and size distribution of the nanoparticles analysed using TEM. b S BET and SLangmuir refer to the specific surface areas calculated using Brunauer–Emmett–Teller (BET) and Langmuir isotherm methods (Fig. S1†). c V total, p/p0 = 0.97. | |||||||||
| 1 | Ag@SiO2 NPs | 84 | 1.01 | 36.63 | — | — | — | — | — |
| 2 | Ag@SiO2-P(VBC-co-DVB) core–shell nanoparticles | 114 | 1.03 | 22.52 | — | — | — | — | — |
| 3 | Ag@P(VBC-co-DVB) yolk–shell nanoparticles | 103 | 1.04 | 29.39 | — | — | — | — | — |
| 4 | AIDNPs | 105 | 1.03 | 29.73 | 39.89 | 0.16 | 25.34 | 3.73 | 4.98 |
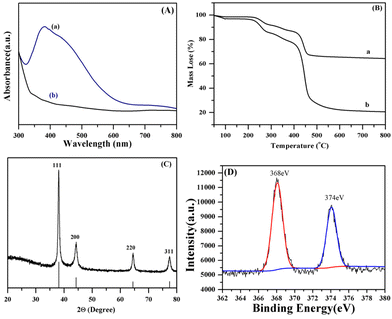
UV-Vis spectrum, thermogravimetric analysis (TGA) thermogram, X-ray photoelectron spectroscopy (XPS) analysis, and X-ray diffraction (XRD) characterization (Fig. 4) were utilized for the investigation of the properties of the AIDNPs, specifically for the phase state and crystallographic structure of the Ag core, which may play an important role in the resulting photoelectric properties. The TGA analysis (Fig. 4B) shows that the AIDNPs keep a 65% residual weight at around 800 °C. Compared to the corresponding hollow P(IVBC-co-DVB) nanoparticles (Fig. S2†), which keep a 20% residual weight at 800 °C, the proportion of the Ag core in the AIDNPs was calculated to be around 45%. This considerable presence of an Ag core in the AIDNPs was also reflected in the UV-Vis spectrum, which shows excellent visible light absorption (Fig. 4A). Furthermore, from the XRD spectrum (Fig. 4C), characteristic peaks at 2θ = 38.17°, 44.14°, 64.64°, and 77.44° were assigned to the (111), (200), (220), and (311) directions, which fit the Joint Committee on Powder Diffraction Standards (JCPDS) Card No. 01-1164.15 Besides, Fig. 2E shows a typical Ag NP HR-TEM image with an interplanar spacing of around 0.23 nm corresponding to the (111) crystal direction of the lattice structure, in line with the XRD data in Fig. 4C. These cubic phase features confirmed the presence of an Ag core with a crystallographic nano-sized structure. Moreover, in the Ag3d XPS spectrum, characteristic peaks at around 374 and 368 eV (Fig. 4D) refer to the Ag3d3/2 and Ag3d5/2 states of the Ag0 configuration,16 revealing the presence of elemental silver in the AIDNPs and their potential capacity for catalysis of photoexcited CO2 reduction. A SPR effect should be based on the excellent light absorption and transformation ability of the Ag NPs inside the AIDNPs. Thus, we investigated the SPR effect by the studying the “light-to-heat” conversion performance of the AIDNPs dispersed in pure water (20 μg mL−1) via the observed temperature variation. Under NIR laser (830 nm, 2.0 W cm−2) illumination for 4 min, the temperature of the suspension gradually increased from 20 °C to around 35 °C (Fig. S3a†), achieving a ΔT value of 15 °C. Compared with the corresponding hollow P(IVBC-co-DVB) nanoparticles without a Ag core (Fig. S3b†), the significant temperature increment observed in the AIDNPs confirmed their “light-to-heat” transformation capacity and surface plasmon resonance properties, which was in good agreement with the literature reported previously.15,17
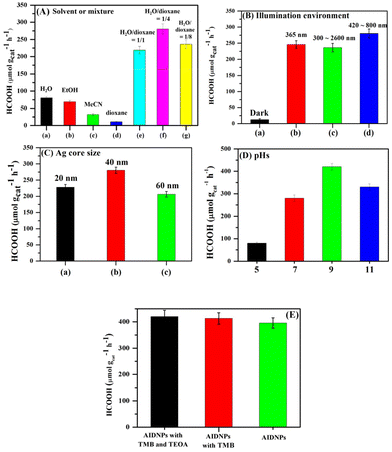
As the photocatalytic CO2 conversion efficiency of the AIDNPs could be affected by its surrounding environment, we intended to investigate the factors related to this efficiency (Fig. 5). To obtain a good efficiency, it was necessary to bind as much CO2 as possible onto the imidazolium functionalized polymeric shell. Since the binding of the imidazolium groups with CO2 occurs in a reversible manner and the equilibrium constant can be regulated by solvent polarity,18 the effect of the solvent on the efficiency was examined first. On the one hand, polar solvents favored CO2 binding with imidazolium groups. On the other hand, non-polar solvents favored CO2 release.19 In other words, polar solvents facilitated CO2 dissolution and its binding with imidazolium groups, while non-polar solvents facilitate the subsequent departure of the CO2 photocatalytic reduction products. Therefore, our strategy was to use a polar solvent as the main environment and adjust it with a weaker polar solvent. In the presence of N,N,N′,N′-tetramethylbenzidine (TMB) as a photo-sensitizer and triethanolamine (TEOA) as a sacrificial reducing agent, when the AIDNPs were dispersed in H2O, EtOH, or MeCN under visible light irradiation (420–800 nm, 2 mW cm−2), the yields of the photocatalytic reduction product, formic acid (HCOOH) (ion chromatograph analysis and 1H NMR analysis, Fig. S4†), were 80, 70, or 26 μmol gcat−1 h−1, respectively (Fig. 5A). In terms of the efficiency and economy, water was the best choice solvent. When further adjusting the polarity of the solvents, according to the previous literature,19 1,4-dioxane mixed with water has worked well in the photocatalytic reduction Indeed, the reduction efficiency rose rapidly via the addition of 1,4-dioxane to the system. When the ratio of water/1,4-dioxane reached 4/1 (v/v), the maximum HCOOH yield reached 289 μmol gcat−1 h−1. To reconfirm the role of the imidazolium groups for CO2 binding as well as how they are affected by the solution polarity, we investigated the CO2 photoreduction efficiency of the yolk–shell Ag@P(VBC-co-DVB) nanoparticles without imidazolium groups. As shown in Fig. S5,† a low HCOOH yield of 60 μmol gcat−1 h−1 was observed due to the low CO2 concentration around the nanoparticles without imidazolium groups. Besides, in order to determine that the 1,4-dioxane only modulated solvent polarity and did not participate in the photoreduction reaction, we investigated the UV-visible absorption curve of the 1,4-dioxane before and after the reaction. The characteristic absorption peak at 230 nm did not show obvious changes after filling up the reaction solution volume by water (Fig. S6†), confirming that 1,4-dioxane did not participate in the photoreduction reaction. Furthermore, the effect of the illumination conditions on the reaction in the H2O/1,4-dioxane (4/1, v/v) mixture were investigated (Fig. 5B). In the dark, only a small amount (8 μmol gcat−1 h−1) of HCOOH was detected (Fig. 5Ba), re-demonstrating that light exposure was indispensable. When different illumination was applied (365 nm UV-light source, simulated sunlight (300–2600 nm), or visible light (420–800 nm)), HCOOH yields of 246, 227, or 289 μmol gcat−1 h−1 were obtained, respectively. These data reveal the efficient utilization of visible light for the photocatalytic reduction, which is consistent with the excellent visible light absorption of the Ag core of the AIDNPs, as shown in Fig. 4A. Although the 365 nm UV-light itself was more powerful, the photoexcited collective electronic oscillations of the visible light on the Ag were more efficient for the generation of hot electron/hole generation.3,11 As for the simulated sunlight from 300–2600 nm, there was some invalid illumination beyond 800 nm.
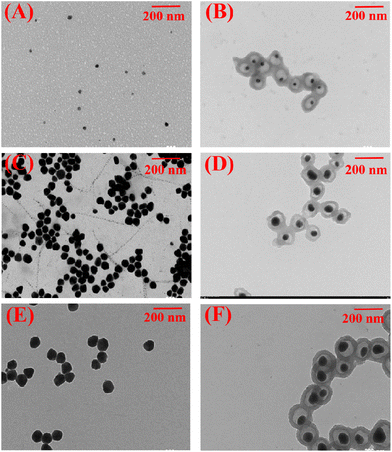
Considering the importance of the Ag core in this photocatalytic reduction system, AIDNPs with different sized Ag cores (Fig. 5C) were subsequently examined. Under visible light in a H2O/1,4-dioxane (4/1, v/v) mixture, the CO2 photocatalytic conversion efficiencies were determined for AIDNP catalysts with Ag core diameters of 20 nm, 40 nm, and 60 nm. The AIDNPs with 40 nm diameter Ag cores showed the best performance. As shown in the TEM pictures in Fig. 6, different sized cores implied a different distance between the Ag core surfaces and the imidazolium functionalized polymeric shell. This is essential for the photoelectrons generated at the Ag surfaces to be effectively transferred to the CO2 adsorbed at the imidazolium groups of the polymeric shell. Although we mentioned above that the Ag core would be in touch with the polymeric shell of the yolk–shell structural AIDNPs in the solvent environment because of the swelling effect, a different distance between the Ag core surfaces to the shell is shown in the TEM pictures which implies that they have different contact areas with each other which would affect the effective transmission distance of the generated photo-electron. From this point of view, AIDNPs containing a larger Ag core with closer core-to-shell distances were superior. However, from the perspective of light energy utilization and photoelectron generation, the smaller sized Ag nanoparticles would be favored.11,20 From the experimental results, medium-sized Ag nanoparticles (40 nm) in the AIDNPs showed the best photocatalytic performance, which balanced the two influencing factors (Fig. 5Cb).
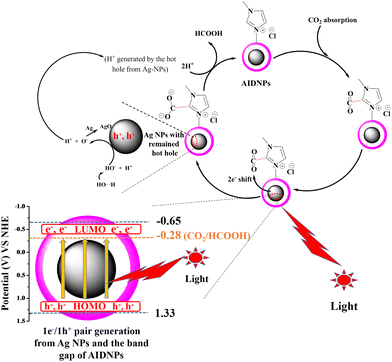
Literature12 reports show that the remaining photoexcited hot holes would generate a proton from the adsorbed H2O on the Ag nanoparticle surfaces. The newly formed proton would transfer from the Ag surface to the electron-rich CO2δ− (CO2 would receive a photoexcited electron) to form an HOCO* intermediate, which can be considered as a bent CO2− derivative stabilized by protonation. In this manner, pH would also be a factor that influences the conversion efficiency. Therefore, we investigated the CO2 photocatalytic performance in acidic (pH = 5), neutral, and basic conditions (pH = 9, or 11), as shown in Fig. 5D. Compared with the performance in neutral conditions, a lower efficiency of 80 μmol gcat−1 h−1 was observed at pH 5 and the highest efficiency of 415 μmol gcat−1 h−1 was observed at pH 9. This revealed that an alkaline environment was of benefit for HCOOH production. This HCOOH yield was high comparing with the heterojunction materials previously reported in the literature.2,21 However, when the pH was further raised from 9 to 11, a downward trend was observed. This implied that a weak alkaline environment was most favorable. The reason for this phenomenon might be attributed from the reactions occurring at the photoexcited Ag nanoparticle surfaces.11 At the beginning, hydroxyl ions are adsorbed on the Ag surface to form an AgnOH species. After acceptance of the photoexcited hot hole, the AgnOH species tends to be transformed into an Agn−1AgO–Hδ+ species. This would generate a proton that is transferred to CO2δ− to obtain the HOCO* intermediate. In this aspect, an alkaline environment is favorable but, when the alkaline environment it too strong CO2 would be transformed into CO32−, which is harmful for the next steps of the reaction.
Gradually, via in-depth research on the mechanism of these “Ag-core@imidazolium-polymeric-shell” nanoparticles for CO2 photocatalytic reduction, the role and necessity of the photosensitizer and sacrificial reducing agent were determined in this system. A photosensitizer such as TMB and a sacrificial reducing agent such as TEOA were employed as they have played important roles in previous reports. In this work, however, AIDNPs displayed a good HCOOH efficiency with a yield of 396 μmol gcat−1 h−1 in the absence of TMB or TEOA (Fig. 5E), which was around 95% of the efficiency in the presence of TMB and TEOA. It is worth mentioning that no obvious CO or methane products were detected when the photochemical reactor headspace was analyzed using gas chromatography GC. Despite the detailed mechanism being unclear, the Ag nanoparticle surfaces with direct photoexcited LSPR properties could serve as a photosensitizer and the water in the solvent mixture could serve as a sacrificial reducing agent. More importantly, a suitable distance to facilitate the efficient movement of the generated electrons from the Ag surfaces to the absorbed CO2 on the imidazolium functionalized polymeric shell has been established, thus avoiding the addition of a photo-sensitizer as an electronic transmission bridge.
To confirm the origin of the HCOOH production, a 13CO2 isotopic labelling experiment was employed and the corresponding products were analyzed using negative electrospray mass spectrometry (Fig. S7†). When CO2 was employed in the photoreduction with AIDNPs, an m/z signal peak at 45 related to HCOO− was observed (Fig. S7A†). For comparison, when the labeled 13CO2 was used in the photoreduction, a H13COO− signal peak at 46 was observed (Fig. S7B†). When CO2 was replaced by nitrogen in the reaction system under similar conditions, no HCOOH was detected (Fig. S7C†) revealing HCOOH was indeed formed via the photoreduction of CO2.
When the continuous HCOOH production efficiency was investigated (Fig. 7A) no significant decay was observed in visible light (420–800 nm) in a H2O/1,4-dioxane mixture (4/1) with a pH of 9 over 96 h, implying that the AIDNPs maintain a high CO2 photoreduction efficiency over a long period. Furthermore, the recycling performance of the AIDNPs was also investigated. After 5 independent consecutive runs, reproducible activities of over 95% were maintained (Fig. 7B). Meanwhile, no obvious differences were observed in the FTIR spectra (Fig. 7C) and TEM images of the catalyst (Fig. 8) after 5 runs, demonstrating the high stability of the AIDNPs for efficient reusability.
To further explore the reaction mechanism, the photoelectric properties of the AIDNPs were characterized. Mott–Schottky plots were obtained to calculate the flat band position. Owing to the positive slope, as shown in Fig. 9A, AIDNPs fit the characteristics of n-type semiconductors. In this manner, the conduction band (CB) potential should be close to the flat band (−0.85 V vs. Ag/AgCl) which could be calculated as −0.65 V (vs. NHE) from the formula:
| EAIDNPs, NHE = EAIDNPs, Ag/AgCl + E0AgCl; |
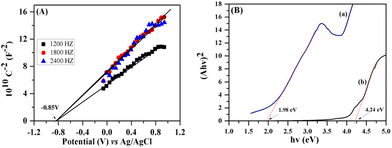 | ||
| Fig. 9 Mott–Schottky plots of the AIDNPs (A); and Tauc plot of the AIDNPs (a) and the corresponding hollow P(IVBC-co-DVB) nanoparticles (b) determined from Kubelka–Munk functions (B). | ||
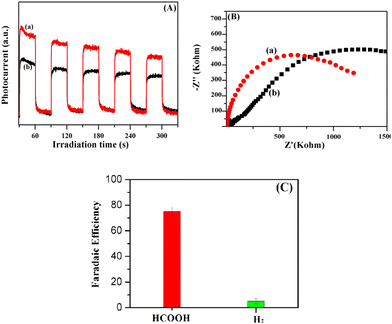
As the CB potential of the AIDNPs was more negative than the CO2/HCOOH reduction potential (ECO2/HCOOH, NHE = −0.28 V),22 the photogenerated electrons will reduce CO2 to HCOOH. Then, since the band gap energy could be calculated as 1.98 eV from a UV-visible diffusion reflectance spectroscopy analysis (Fig. 9B) and the Kubelka–Munk function, the valence band (VB) potential was estimated as 1.33 V. Compared with the corresponding hollow P(IVBC-co-DVB) nanoparticles without Ag cores, the resulting photoelectric data demonstrated the good potential CO2 photocatalytic reduction capacity of the AIDNPs and was consistent with the results obtained from the transient photocurrent response plots. Fig. 10A shows that the transient photocurrent densities of the AIDNPs was almost 50% higher than the corresponding hollow P(IVBC-co-DVB) nanoparticles without an Ag core, revealing good photoinduced electron production and the inhibition of the recombination of the photogenerated electron/hole pairs owing to the LSPR properties of the Ag nanoparticles. To further clarify the above inferences, EIS electrochemical characterization was conducted. As shown in Fig. 10B, a smaller semicircle arc diameter is observed for the AIDNPs, reconfirming a lower electron-transfer resistance as well as a higher efficiency for interfacial charge transfer.
To further characterize the CO2 photoreduction efficiency, we examined the faradaic efficiency of the AIDNPs for H2 and HCOOH production at −0.6 VRHE. In Fig. 10C, a high HCOOH faradaic efficiency (FEHCOOH) of 75% and a low FEH2 of 5% (FEHCOOH/FEH2 = 15) were observed. Compared with some typical reported results in the literature23–26 (Table S1†), the AIDNPs displayed an excellent efficiency and ratio for CO2 photoreduction to HCOOH. Meanwhile, the AIDNPs displayed a good HCOOH conversion efficiency with a yield of 396 μmol gcat−1 h−1 in the absence of TMB or TEOA under visible light (420–800 nm). In comparison, although Li and coworkers27 reported that their system achieved a high HCOOH yield of 488.35 μmol g−1 h−1 under UV-light (200–400 nm), only a low HCOOH generation efficiency of 97.06 μmol g−1 h−1 was achieved under visible light even in the presence of TEOA as a sacrificial reducing agent.
Finally, using the experiments and analysis mentioned above, we propose a possible mechanism for the photocatalytic reduction of CO2 using the AIDNPs (Fig. 11). First, with the functional imidazolium motifs on the polymeric shell CO2 is favorably adsorbed and concentrated around the AIDNPs. Owing to the LSPR properties in the visible wavelength region, the Ag nanoparticle surfaces generate hot electron/hole pairs upon excitation. Then, because of the suitable core-to-shell diffusion distance and because the CB potential (−0.65 V) is more negative than the CO2/HCOOH demanded reduction potential (−0.28 V) provided by the yolk–shell AIDNPs, high interfacial charge transfer ensures that hot electrons are transferred efficiently to CO2, affording CO2δ− without an additional photosensitizer as an electronic transmission bridge. Then, the photoexcited hot hole reacts with the AgnOH species formed by the hydroxyl ions adsorbed at the Ag nanoparticle surfaces, affording an Agn−1AgO–Hδ+ intermediate. Subsequently, the Agn−1AgO–Hδ+ intermediates generate a proton that is transferred to CO2δ− to obtain a HCOO* species which then produces HCOOH after acceptance of another proton from water. During the procedure, there are three rate-limiting reactions: (1) the balance between the CO2 adsorption and the release of the photocatalytic reduction products, which is influenced by the polarity of the solvent; (2) the transfer of the photogenerated hot electrons, which is influenced by the illumination conditions and the core-to-shell diffusion distance which is determined by the size of the Ag nanoparticles core; and (3) the transfer of the photoirradiated holes and the production of a proton, which are highly dependent on the pH of the solution.
Conclusions
In summary, yolk–shell structured nanoparticles (AIDNPs) with an Ag core and an imidazolium functionalized polymeric shell were prepared for the photocatalytic reduction of CO2 for the first time. Without assistance from an additional photosensitizer and sacrificial reducing agent, the AIDNPs give a max HCOOH production efficiency of 396 μmol gcat−1 h−1 in a water/dioxane (4/1, v/v) mixture at pH 9 under visible light irradiation (420–800 nm). To the best of our knowledge, the production efficiency was among the highest in the literature for heterojunction materials. This kind of “Ag-core@imidazolium-polymeric-shell” architecture could not only enhance the photogenerated “electron/hole” pair yield under visible light, but also offer a suitable “core-to-shell” transfer distance, thus effectively catalyzing CO2 reduction at the polymer shell. The investigation of the factors relating to the photocatalytic CO2 production efficiency and the investigation of the photoelectric properties, as well as the proposed possible reduction mechanism provide a comprehensive model to achieve truly efficient green economy CO2 photoreduction in the future.Author contributions
Tuanwei Liu & Guodong Fu: Resources, methodology, data curation and writing – original draft. Zhongjie Wu, Zhongjie Kang & Jinjian Wei: Formal analysis and investigation. Zhide Zhang, Xinlin Yang & Dian-Shun Guo: Conceptualization, validation and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the Shandong Zhongshi Yitong Group and the State Grid Shandong Electric Power Company Electric Power Research Institute.References
- J. R. Pankhurst, Y. T. Guntern, M. Mensi and R. Buonsanti, Chem. Sci., 2019, 10, 10356–10365 RSC.
- K. Guo, X. Zhu, L. Peng, Y. Fu, R. Ma, X. Lu, F. Zhang, W. Zhu and M. Fan, Chem. Eng. J., 2021, 405, 127011 CrossRef CAS.
- K. M. Choi, D. Kim, B. Rungtaweevoranit, C. A. Trickett, J. T. Barmanbek, A. S. Alshammari, P. Yang and O. M. Yaghi, J. Am. Chem. Soc., 2017, 139, 356–362 CrossRef CAS PubMed.
- A. U. Pawar, U. Pal, J. Y. Zheng, C. W. Kimd and Y. S. Kang, Appl. Catal., B, 2022, 303, 120921 CrossRef CAS.
- H. Q. Xu, J. Hu, D. Wang, Z. Li, Q. Zhang, Y. Luo, S. H. Yu and H. L. Jiang, J. Am. Chem. Soc., 2015, 137, 13440–13443 CrossRef CAS.
- K. Song, S. Liang, X. Zhong, M. Wang, X. Mo, X. Lei and Z. Lin, Appl. Catal., B, 2022, 309, 121232 CrossRef CAS.
- Z. Tang, W. He, Y. Wang, Y. Wei, X. Yu, J. Xiong, X. Wang, X. Zhang, Z. Zhao and J. Liu, Appl. Catal., B, 2022, 311, 121371 CrossRef CAS.
- W. Zhao, D. Zhai, C. Liu, D. Zheng, H. Wu, L. Sun, Z. Li, T. Yu, W. Zhou, X. Fang, S. Zhai, K. Han, Z. He and W. Q. Deng, Appl. Catal., B, 2022, 300, 120719 CrossRef CAS.
- S. Wang and X. Wang, Angew. Chem., Int. Ed., 2016, 55, 2308–2320 CrossRef CAS.
- B. A. Rosen and I. Hod, Adv. Mater., 2018, 30, 1706238 CrossRef PubMed.
- G. Kumari, X. Zhang, D. Devasia, J. Heo and P. K. Jain, ACS Nano, 2018, 12, 8330–8340 CrossRef CAS.
- A. Naldoni, V. M. Shalaev and M. L. Brongersma, Science, 2017, 356, 908 CrossRef CAS.
- Y. Kim, J. G. Smith and P. K. Jain, Nat. Chem., 2018, 10, 763–769 CrossRef CAS.
- T. W. Liu, J. J. Wei, G. D. Fu, P. Zhang, Z. D. Zhang, D. S. Guo and X. L. Yang, Polym. Chem., 2021, 12, 1023–1029 RSC.
- T. W. Liu, X. Li, J. J. Wang, P. Zhang, X. Y. Huang, Z. D. Zhang, D. S. Guo and X. L. Yang, J. Mater. Chem. B, 2020, 8, 5483–5490 RSC.
- Y. Y. Cui, J. Yang, Q. F. Zhou, P. Liang, Y. L. Wang, X. Y. Gao and Y. T. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 5900–5906 CrossRef CAS PubMed.
- J. H. Byeon and Y. W. Kim, ACS Macro Lett., 2014, 3, 205–210 CrossRef CAS PubMed.
- D. M. Denning and D. E. Falvey, J. Org. Chem., 2014, 79, 4293–4299 CrossRef CAS.
- D. M. Denning, M. D. Thum and D. E. Falvey, Org. Lett., 2015, 17, 4152–4155 CrossRef CAS.
- S. Yu, A. J. Wilson, G. Kumari, X. Zhang and P. K. Jain, ACS Energy Lett., 2017, 2, 2058–2070 CrossRef CAS.
- A. Wagner, C. D. Sahm and E. Reisner, Nat. Catal., 2020, 3, 775–786 CrossRef CAS.
- Z. Liu, Y. Huang, S. Chang, X. Zhu, Y. Fu, R. Ma, X. Lu, F. Zhang, W. Zhu and M. Fan, Sustainable Energy Fuels, 2021, 5, 2871–2876 RSC.
- B. Shan, S. Vanka, T. T. Li, L. Gautier, M. K. Brennaman, Z. Mi and T. J. Meye, Nat. Energy, 2019, 4, 290–299 CrossRef CAS.
- W. Dong, I. A. Navid, Y. Xiao, J. W. Lim, J. L. Lee and Z. Mi, J. Am. Chem. Soc., 2021, 143, 10099–10107 CrossRef CAS PubMed.
- A. C. Ghosh, A. Legrand, R. Rajapaksha, G. A. Craig, C. Sassoye, G. Balázs, D. Farrusseng, S. Furukawa, J. Canivet and F. M. Wisser, J. Am. Chem. Soc., 2022, 144(8), 3626–3636 CrossRef CAS.
- Z. H. Zhu, B. H. Zhao, S. L. Hou, X. L. Jiang, Z. L. Liang, B. Zhang and B. Zhao, Angew. Chem., Int. Ed., 2021, 60, 23394–23402 CrossRef CAS PubMed.
- N. Li, J. J. Liu, J. W. Sun, B. X. Dong, L. Z. Dong, S. J. Yao, Z. Xin, S. L. Li and Y. Q. Lan, Green Chem., 2020, 22, 5325–5332 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: For experimental details and analyses. See DOI: https://doi.org/10.1039/d2gc04028j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |