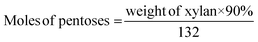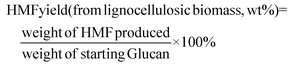The weak interaction between polar aprotic solvent and saline water enables efficient production of furans from lignocellulosic biomass
Tingting
Cai
ab,
Chao
Liu
a,
Jie
Liang
a,
Yan
Ma
a,
Jun
Hu
 c,
Jianchun
Jiang
ab and
Kui
Wang
c,
Jianchun
Jiang
ab and
Kui
Wang
 *ab
*ab
aInstitute of Chemical Industry of Forest Products, Chinese Academy of Forestry; Key Lab. of Biomass Energy and Material, Jiangsu Province; National Engineering Lab. for Biomass Chemical Utilization, Nanjing 210042, China. E-mail: wangkui@caf.ac.cn
bCo-Innovation Center of Efficient Processing and Utilization of Forest Resources, Nanjing Forestry University, Nanjing 210037, China
cSchool of Chemical Engineering, Northwest University, Xi'an 710069, China
First published on 5th December 2022
Abstract
Herein, based on the studies on intermolecular weak interactions, we propose a novel perspective on the design of a biphasic system from a polar aprotic solvent (PAS) and saline water, which plays a vital role in the efficient production of furans from a lignocellulosic biomass. Interestingly, molecular dynamics simulation reveals the formation mechanism of the biphasic system from the original miscible PAS and water. The stronger interaction of Na+ and Cl− with water is proven to be responsible for the dissociation of water from water-miscible γ-valerolactone (GVL) to form the biphasic system. Similarly, the outstanding effect on the extraction and stabilization of furfural (FAL) could also be attributed to the better interaction of PAS with FAL. As a result, a remarkable FAL yield of 80.5 mol% could be obtained in the biphasic system of GVL/NaCl aqueous solution (aq.) with 0.3 mmol AlCl3 at 150 °C for 1 h. Moreover, the synergistic catalytic effect between NaCl and AlCl3 was confirmed by blank experiments and in situ23Na NMR spectroscopy. Besides, this process enables the GVL/NaCl aq. biphasic system and AlCl3 catalyst to exhibit excellent recovery with no significant FAL loss after three times of recycling. This work provides a feasible strategy for efficient biorefinery in biphasic systems.
Introduction
As the most abundant renewable carbon resource, biomass has attracted significant attention due to the excessive consumption of fossil carbon resources and the consequent environmental problems,1,2 among which agricultural and forestry lignocellulosic residues are believed to be potential substitutes of petroleum for the production of fuel and chemicals via the biorefinery process.3–5 Classically, furfural (FAL), obtained via hydrothermal conversion of lignocellulosic biomass such as corncob and straw,6,7 has been identified as one of the top 30 building block chemicals by the US-DOE.8–10 Aldehyde groups and furan rings make FAL a chemical platform for more than 80 value-added chemicals, technically feasible for supplement petroleum-derived commodity chemicals, fuels and materials.11–13 Almost 1 billion dollars in the global market are predicted for FAL,14 more than 70% of which will be produced in China using corncob as a substrate due to its large quantity, low price and high hemicellulose content.15In 1922, Quaker Oats first produced FAL in the industry using oat husks as the raw material and concentrated sulfuric acid as the solvent in a homogeneous hydrothermal system.13 First, oat husks are treated with dilute sulfuric acid to yield FAL, and then FAL is separated by high pressure steam, followed by neutralization and distillation.16 Likewise, Sánchez17et al. hydrolysed corncob in a homogeneous system with 37.06% of FAL yield obtained using sulfuric acid and hydrochloric acid as catalysts at 180 °C. Despite its large-scale application, the acid digestion process still suffers from low FAL yield and high energy consumption, particularly due to serious equipment corrosion and environmental pollution by the application of sulfuric acid and hydrochloric acid.18 In order to solve the problem, solid acid catalysts were introduced in the research of FAL production in homogeneous systems (e.g., SAPO-34/5A, HZSM-5, Sn-MMT, H-SAPO-34, and amorphous Nb2O5),19–26 which were conducive to environmentally benign processes with a higher FAL yield. Moreover, cheap and available metal salts (e.g., FeCl3, Al2(SO4)3, and AlCl3) also exhibited excellent catalytic activity and selectivity in the production of FAL. Zhang27et al. adopted FeCl3 as a catalyst for the conversion of untreated corncob to FAL in a GVL solvent, with 79.6% FAL yield obtained at 185 °C for 100 min. Besides, our group reported similar research for FAL production by metal salt catalysis. Liu28et al. compared the FAL production from xylose in GVL aqueous solutions using different sulfate and chloride salts as catalysts, with the optimized 50.16% FAL yield by Al2(SO4)3 catalysis. However, the difficult separation of FAL from the homogeneous system is still unsolved, leading to a complex purification process with high energy consumption.29 Notably, around 2005, Professor James A. Dumesic30 initially proposed a biphasic solvent system of methyl isobutyl ketone (MIBK) and water for selective production of HMF from fructose. The spontaneous migration of HMF from water to an organic phase was believed to be responsible for its high efficiency and selectivity. Then, in 2007, they conducted research on the production of dimethylfuran from fructose and found that the addition of NaCl could significantly improve the extraction efficiency of the organic phase.31 Similar work for the production of FAL from hemicellulose was also conducted by Dumesic group32 in 2012, based on the design of a biphasic system by addition of 2-sec-butylphenol (SBP), 4-n-hexylphe-nol (NHP) and 4-propyl guaiacol (PG) into an aqueous solution, respectively, which further confirmed the improvement of product separation efficiency by the biphasic system from the incompatible organic solvent and water. Moreover, Hu33,34et al. proposed a novel tetrahydrofuran (THF)/H2O biphasic system for FAL production, from which 51–66% FAL yield can be obtained from various lignocellulosic biomass under microwave heating at 140 °C for 60 min. Despite its excellent performance, THF still suffers from low flash point (−17 °C), high flammability and volatility.
Owing to its excellent thermo-chemical stability, GVL has attracted more attention than THF for the production of FAL.35–38 More than 70.2–80.4% FAL yield has been reported when xylose was used as the substrate in GVL–H2O.35–37 However, the system exhibited unsatisfactory adaptability of raw materials with lower FAL yields from xylan and lignocellulosic biomass. Luo38et al. conducted an interesting biorefinery work on the separation of hemicellulose from corn stover in the GVL–H2O system with 5%–30% NaCl as a catalyst, which proposed a viable strategy for the solution of substrate adaptability by enhancing the interactions of NaCl with the –OH group in hemicellulose and G units in lignin. However, the facilitated mechanism of the biphasic system on FAL production needs to be further discussed. In order to reveal the promoted effect of the biphasic system, we initially constructed a novel biphasic system with GVL and molten salt hydrates (LiCl·3H2O and LiBr·3H2O) in our previous research work.18 As a molten salt hydrate was used as both the solvent and catalyst, 77.2% of FAL was obtained from xylan reacted at 140 °C for 2 h. Considering the industrial-scale application, NaCl, as a cheap and abundant salt, was adopted to establish the biphasic system instead of molten salt hydrates. Interestingly, GVL is originally miscible with water, which is difficult to form a biphasic system; despite its excellent performance in the biorefinery process,39 stratification began after 10 wt%–25 wt% NaCl was added. Although the solvent effect on FAL in the biphasic system has been well discussed,40 the formation mechanism of the biphasic system has been barely studied yet, particularly for the weak interaction among the solvent, water and solute.
Herein, based on the discussion of intermolecular weak interactions, we propose a novel perspective on the design of a biphasic system from a polar aprotic solvent (PAS) and a sodium chloride solution. A control group experiment was conducted to evaluate the efficiency of the biphasic system from different PAS in the xylan conversion. Notably, the radial distribution function (RDF)40 was introduced to demonstrate the weak interaction effect between PAS and NaCl aq. and verified for the formation of the biphasic system. Besides, the interaction forces between the solute and solvent molecules were discussed to reveal the pathway of FAL extraction from the aqueous phase to the organic solvent. Moreover, in situ27Al NMR, in situ23Na NMR and ESI-MS/MS tests were carried out to elucidate the synergistic catalytic mechanism of NaCl–AlCl3 in the isomerization of xylose in the biphasic system. Furthermore, the recovery of the biphasic system was also involved and applied in the conversion of real biomass substrates (i.e., corncob and moso bamboo) with mass balance, which provides a new perspective for the biorefinery industrial process with efficiency and specificity.
Experimental
Raw materials
Xylan from corncob was obtained from Macklin Co. with 90% pentoses and 10% galactose. Corncob and moso bamboo meals were collected from Jiangsu and Zhejiang Province of China, respectively, which were first chopped and milled, and then passed through a 120-mesh sieve, followed by drying at 105 °C for 24 h, reserved as the real lignocellulosic biomass substrates. All chemicals used in the experiments were of analytical purity and used without further purification. In particular, methyl isobutyl ketone (MIBK) (≥99%), γ-valerolactone (GVL) (98%), tetrahydrofuran (THF) (≥99.5%), ethyl acetate (EA) (≥99.5%), and propylene carbonate (PC) (99%) were purchased from Sinopharm Chemical Reagent Co., LTD. (Shanghai, China).Production of FAL
The production of FAL was carried out in a 316L stainless steel autoclave with quartz lining. First, 0.5 g xylan and metal halide (0.3 mmol Mn+) were initially mixed with 10.0 g of GVL and 20.0 g of NaCl aq. (20 wt% concentration), followed by heating at a programmed rate of 7.5 °C min−1 to the set temperature between 110 and 160 °C. The mixture was stirred at 800 rpm for a certain time between 0.5 and 3.0 h under a nitrogen atmosphere of 2 MPa. After cooling to room temperature, the filtrate was removed to a separation funnel and auto-split into an organic phase and a water phase, which were collected for further analysis, respectively.Product analysis
The obtained FAL was determined by qualitative and quantitative analysis using a gas chromatograph (Shimadzu GC-2010 plus) equipped with a flame ionization detector (FID) and an SH-RTX-5 column (30 m × 0.32 mm × 0.5 um) using 0.43 mL min−1 of gaseous helium as the carrier gas. Classically, the temperature of the injector was set to 260 °C, and the temperature of the detector was maintained at 280 °C. Initially, the column oven was maintained at 100 °C for 2 minutes, and then the temperature was increased to 280 °C at a rate of 8 °C min−1 and kept for 10 minutes.The retention time and peak area of FAL in the chromatographic column were determined by an external standard method, and the FAL yield was calculated using the following equations:
Levulinic acid (LA); 5-Hydroxymethylfurfural (HMF).
Weak interactive calculation
In the molecular dynamics simulation, liquid-phase molecular system was conducted by placing solute and solvent molecules in a nearly 37 Å × 37 Å × 37 Å periodic box using Amorphous cell construction tool, where contains 68 Na+, 68 Cl−, 890 H2O, and 98 organic solvents (i.e., GVL, MIBK, THF, EA, and PC) based on the solution of experiment. When the molecular dynamics simulation was operated, the molecular configurations of each system were equilibrated using a COMPASSII forcefield.41 In order to simulate the actual experiment process more realistically, a Nose thermostat was used to control the temperature at 293 K. When the system was at the minimum energy point, the NVT (constant particle numbers, volume, and temperature) ensemble was used to simulate the dynamics of the system with a duration of 100 ps (time step = 0.1 fs). In the process of dynamic simulation, a Nose–Hoover thermostat was used to control the temperature of the system with a Q ratio of 0.01 (relative to a value corresponding to a relaxation time of 1 ps).42 Meanwhile, the velocity scale was set as 10.0 k temperature difference, and the Andersen method of temperature control was used with a collision ratio of 1.0. The van der Waals interaction was calculated by an atom-based method with a cutoff radius of 18.5 Å to avoid the errors for relatively short-range non-bond interactions, while the electrostatic summation was calculated by the Ewald method with an accuracy of 1.0e−5 kcal mol−1 and a buffer width of 0.5 Å to avoid the errors of long-range non-bond energy in periodic systems. After the kinetic simulation, the radial distribution function (RDF) was calculated based on the trajectory of molecular motion.The interactions of liquid–liquid molecular systems were calculated based on the COMPASSII forcefield and Flory–Huggins model. The energy bin width was 0.02 kcal mol−1, with the selected 1 × 107 pair configurations to find the 100 lowest energy frames, and the reference temperature was set at 423 K. The cluster sample is 1 × 105 and the iterations per cluster is 20.
In the traditional Flory–Huggins model, each component occupies a lattice site. For a lattice with a coordination number Z, the mixing energy (Emix) and interaction parameter (x) can be calculated using eqn (1) and (2):
| Emix = 0.5Z(Ebs + Esb − Ebb − Ess) | (1) |
| x = Emix/RT | (2) |
ESI-MS spectra and NRM spectrometry
The synergistic effect of NaCl aq. and AlCl3 on xylose conversion was investigated using an ESI-Q-TOF-MS (Agilent 6550 iFunnel) and a NMR spectrometer (Bruker Biospin).The ESI-MS spectra were recorded in the positive ion mode at 1.60 kV detector voltage, 1.56 kV ionization voltage, and 100 kPa gas drying pressure. The source temperature and solvent removal temperature were set as 200 °C, and N2 was used as a carrier gas at a flow rate of 1.5 L min−1.18
In situ 27Al NMR spectrum and in situ23Na NMR spectrum were recorded and tested at 156.38 MHz using an Advance III HD 500 MHz NMR spectrometer (Bruker Biospin). The detection conditions were set as follows: 10.50 μs pulse width, 0.1311 s scanning frequency, and 128 times scanning frequency. The data were collected every 10 min and lasted for 2 h at 60 °C.29
Chemical composition and sugar analysis
The chemical components of corncob and bamboo were determined by standard biomass analysis methods provided by the National Renewable Energy Laboratory (NREL).43 Soluble monosaccharides generated by acid hydrolysis of raw materials were qualitatively and quantitatively analyzed using a HPLC (Waters E2695) equipped with a refractive index detector (Waters 2414) and a Biorad Aminex HPX-87H column (300 × 7.8 mm, USA), and the temperature of the detector and column oven was maintained at 40 °C and 55 °C, respectively.2 First, 5 mM H2SO4 was adopted as a mobile phase with a flow rate of 0.6 mL min−1. The content of soluble lignin was determined using a UV-vis spectrophotometer at a wavelength of 205 nm. Notably, corncob powders were detected to be comprising 33.20 wt% of glucan, 37.01 wt% of xylan and 11.61 wt% of lignin. In addition, the component of moso bamboo was determined as 44.46 wt% of glucan, 30.11 wt% of xylan and 25.76 wt% of lignin, respectively.Results and discussion
Effect of organic solvents on xylan conversion to FAL
The effect of different organic solvents on the conversion of xylan to FAL was investigated under the same reaction conditions (0.5 g xylan, 0.0725 g AlCl3·6H2O, 20.0 g 20 wt% NaCl aq., 150 °C, 1 h). As shown in Table 1, the FAL yield was arranged in rank order according to the relative Emix values (Fig. 4) of solvents to FAL shown below: EA (60.41 mol%), MIBK (58.20 mol%), THF (40.55 mol%) and PC (7.79 mol%). Interestingly, despite its larger Emix values of 0.48, GVL still exhibited outstanding performance in the production of FAL with 72.60 mol% yield, which may be due to its stabilized effect on the Al3+ catalytic active center.29 It is worth noting that a PC/NaCl aq. biphasic system transferred to a homogeneous phase after the reaction, leading to the lowest FAL yield of 7.79 mol%, which is ascribed to thermal decomposition of PC and destruction of the biphasic system under 150 °C. Namely, the thermodynamic property of the biphasic system will significantly influence the weak interaction among the solvent and solute, which needs further in-depth discussion.| Entry | Organic solvent | Density [g mL−1] | FAL yield [mol%] | FAL yield [wt%] |
|---|---|---|---|---|
| Reaction conditions: 0.5 g xylan, 0.0725 g AlCl3·6H2O, 0.0979 mol organic solvents, 20.0 g 20 wt% NaCl aq., 150 °C, 1 h. References density values in previous literature.44 | ||||
| 1 | GVL | 1.04 | 72.60 | 52.80 |
| 2 | MIBK | 0.80 | 58.20 | 42.33 |
| 3 | THF | 0.89 | 40.55 | 29.49 |
| 4 | EA | 0.90 | 60.41 | 43.93 |
| 5 | PC | 1.20 | 7.79 | 5.67 |
Effect of the metal halide catalyst on xylan conversion to FAL
A variety of metal halide salt catalysts were selected to investigate the effect on xylan conversion in a GVL/NaCl aq. biphasic system with mineral acid as comparison. As shown in Table 2, 16.14 mol% of FAL could be obtained in the control group without addition of extra metal halide salts as catalysts, which could be attributed to the existence of NaCl. Unexpectedly, the addition of ZnCl2 and CaCl2 has little effects on the improvement of FAL yields. By contrast, significant promotion on xylan conversion could be observed when introducing FeCl3·6H2O and CuCl2 with 54.10 mol% and 57.53 mol% of FAL yield, respectively, which is also consistent with other reported studies.27,45 Notably, AlCl3 exhibited the best catalytic activity for xylan depolymerization, giving 72.60 mol% yield of FAL. The outstanding FAL yield could be ascribed to the synergistic effect of Brønsted and Lewis acid originating from AlCl3 hydrolysis and stabilization process in GVL,46 which was also verified by subsequent NMR and ESI-MS analyses. Comparatively speaking, despite the stronger Brønsted acid center existence in the mineral acid, the absence of Lewis acid significantly affects the key isomerization step of xylose to xylulose,18 which generated FAL yields of 48.98 mol% and 48.09 mol% with H2SO4 and HCl, respectively.| Entry | Catalyst | FAL yield/mol% | FAL yield/wt% |
|---|---|---|---|
| Reaction conditions: 0.5 g xylan, 20.0 g 20 wt% NaCl aq., 10.0 g GVL, catalyst (based on 0.3 mmol Mn+), 150 °C, 1 h. | |||
| 1 | None | 16.14 | 11.74 |
| 2 | ZnCl2 | 16.14 | 11.74 |
| 3 | CaCl2 | 16.70 | 12.15 |
| 4 | FeCl3 | 54.10 | 39.34 |
| 5 | CuCl2 | 57.53 | 41.84 |
| 6 | AlCl3 | 72.60 | 52.80 |
| 7 | H2SO4 | 48.98 | 35.62 |
| 8 | HCl | 48.09 | 34.97 |
Optimization of reaction parameters
The effects of the reaction temperature and time on the conversion of xylan to FAL were investigated, as shown in Fig. 1, with 0.5 g xylan, 20 g 20 wt% NaCl aq., 0.072 g AlCl3·6H2O, and 10 g GVL as the basic conditions. Notably, the FAL yield demonstrated an upward trend accompanied by the temperature variation between 120 °C and 150 °C. An unexpected decrease was detected when the temperature was set as 160 °C, which could be attributed to the repolymerization of FAL with sugar intermediates and the formation of unwanted humins at higher temperatures.47 Interestingly, a similar trend could also be observed along with the time ranging from 0.25 h to 1.5 h. Further prolonging the reaction time generates a delicate increase or a relative decrease in the FAL yield, due to further repolymerization of FAL with other intermediates.47 More than 72.60 mol% of FAL could be obtained at 150 °C for 1 h, which were finally defined as the optimized condition.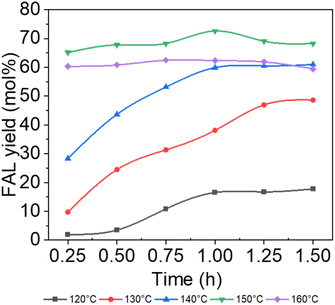 | ||
| Fig. 1 Catalytic conversion of xylan into FAL at different temperatures and time points (reaction conditions: 0.5 g xylan, 0.072 g AlCl3·6H2O, 10.0 g GVL, 20.0 g 20 wt% NaCl aq.). | ||
Fig. 2(a) exhibits that the FAL yield varied with different catalyst loadings. Normally, 72.6 mol% FAL yield can be obtained at a lower AlCl3 loading of 0.072 g under the above-mentioned reaction conditions, accompanied by a spot of sugar intermediates. Notably, more than 80.50 mol% of FAL could be obtained when increasing the AlCl3 loading to 0.080 g. However, the FAL yield slightly decreased with excessive loading of AlCl3. The unnecessary high density of active sites will accelerate the condensation of FAL, which is believed to be responsible for the decrease in FAL yield.7,48
Notably, 0.080 g loading of AlCl3·6H2O was selected for the following study on the influence of mass ratio of GVL and NaCl aq. on FAL production. As shown in Fig. 2(b), a moderate FAL yield of 64.89 mol% could be observed with 25/5 mass ratio of GVL/NaCl aq. The continuous increase in the proportion of NaCl aq. will yield a slight lift in the FAL yield. Notably, as high as 80.50 mol% of FAL yield could be observed when the mass ratio of GVL/NaCl aq. was set as 10/20, which could be attributed to the positive effects on the isomerization of xylose to xylulose by the Lewis acid center yield from NaCl and AlCl3 at a certain concentration. The mechanism of different solvent ratios leading to changes in the activation free energy of xylose isomerization into xylulose has been reported in our previous work.18 Continuously increasing the portion of NaCl aq. to 5/25, the FAL yield visibly decreased to 75.70 mol%, which originated from the reduction of GVL content, followed by the inhibition of extraction of FAL from the aqueous phase. In brief, GVL/NaCl aq. with a mass ratio of 10/20 exhibited the most efficient synergistic effect on the production and stabilization of FAL.
Centre of mass radial distribution functions (RDF) in different biphasic systems
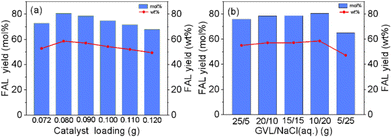
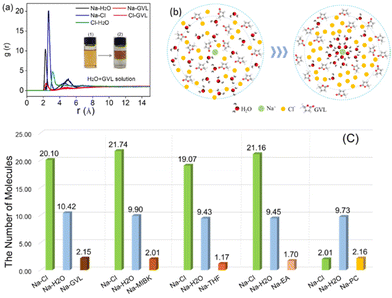
In order to evaluate the weak interaction among organic solvents, NaCl aq. and solute (FAL), further verifying the unclear pathway of biphasic system formation, a series of PAS (i.e., GVL, MIBK, THF, and PC) and EA were introduced to form the biphasic system with NaCl aq., respectively.Notably, molecular dynamics simulation of Na+ in the solvent was introduced to evaluate the weak interaction of solvent molecules, which provides a reliable view for detailed explanation on the formation of the biphasic system. As shown in Fig. 3(a), the local arrangement of solvent molecules around Na+ was revealed by calculating the central of mass radial distribution function (RDF) of ions and molecules. Interestingly, Fig. 3(a) exhibits the different layering process accompanied by the absence (1) and existence (2) of NaCl aq. (20 wt%) in a GVL/H2O solution. Moreover, the first solvation peak for water was 2.25 Å, followed by GVL of 2.33 Å and Cl− of 2.65 Å, and the peak height sorted as Cl− (20.10r) > H2O (10.42r) > GVL (2.15r), namely, the “Na–Cl” and “Na–H2O” curves are much higher than that of “Na–GVL” curves, which indicated a stronger interaction between Na+ and H2O when compared with GVL.
Fig. 3(b) shows more intuitively that the number of H2O around Na+ is far greater than that of GVL molecules in the system of GVL/NaCl aq. In other words, Na+ and Cl− can extract H2O from the miscible H2O–GVL solution by the so-called wrapped effect, yielding the biphasic system. However, Fig. 3(c) provides the number of molecules in the first solvation peak around Na+ of the system in different organic solvents, including GVL, MIBK, THF, EA and PC. It is observed that the amount of “Na–H2O” is much higher than that of “Na–organic Solvent” in the first solvation peak, indicating that the attraction of Na+ to H2O molecules is stronger than that of organic solvents, which is consistent with the observed phenomenon that an initial homogeneous solution exhibited obvious stratification boundaries in a few minutes between organic solutions and NaCl aq.
Table 3 illustrates the permittivity of different solvents and the solubility with water and NaCl aq., which indicated that pure water and organic solutions are miscible or slightly soluble initially. However, it will change after NaCl is added into the solution, which is consistent with computational data. Due to the weak solvation hydration of NaCl in organic solvents with small polarity, it is difficult to ionize free ions. Conversely, the interaction between water and NaCl is strong due to solubility, and NaCl completely ionizes the free hydrated ions to form a stable dispersed solution. Therefore, the smallest water molecule can easily enter the internal solvation peak of Na+. In this case, the imbalance of charge will make Cl− arrange orderly in the outer layer of Na+via electrostatic attraction. In Table 3, the permittivity of GVL and water is 33.2 and 81.0, respectively. The great difference in permittivity between GVL and water manifests that the attraction between GVL and water will be weak. Due to the strong mutual attraction between Na+, Cl− and H2O molecules by solubility and electrostatic attraction but the weak interaction between H2O and GVL, GVL will move from inside to outside, forming a biphasic system.
| Entry | Solvents and solutes | Permittivity | Solubility with water | Solubility with NaCl aq. |
|---|---|---|---|---|
| a References permittivity in previous literature.18 | ||||
| 1 | GVL | 33.2a | miscible | insoluble |
| 2 | MIBK | 13.4a | Slightly Soluble | insoluble |
| 3 | THF | 8.3a | Slightly Soluble | insoluble |
| 4 | EA | 6.1 | Slightly Soluble | insoluble |
| 5 | PC | 69.0 | miscible | insoluble |
| 6 | FAL | 41.9 | miscible | — |
| 7 | H2O | 81.0 | — | — |
In order to understand the selective extraction effect of organic solvents on FAL, the weak interaction between different solvents (GVL, MIBK, THF, EA, PC, H2O) and FAL was also calculated, and is exhibited in Fig. 4. It is well known that the interaction parameter x is a measure of solvent capacity in a solvent system in the lattice theory of Flory–Huggins. As shown in Fig. 4, the x values are in the following order of EA ≈ MIBK < THF ≈ GVL < PC < H2O. Obviously, organic solvents can extract FAL from the aqueous phase due to the relatively stronger binding energy of organic solvents to FAL when compared with that of H2O, which were basically consistent with the following experiment data and characterization results.
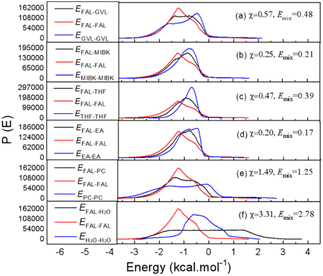 | ||
| Fig. 4 Molecular interactions in the solvent, (a) GVL/FAL, (b) MIBK/FAL, (c) THF/FAL, (d) EA/FAL, (e) PC/FAL, and (f) H2O/FAL. | ||
Determination of the catalytic active centre
According to previous reports,7,29,49 the presence of a [Al(OH)2(H2O)x(Glc)y]+ hexa-coordinated aluminium centre can significantly facilitate the isomerization reaction, which is also consistent with our experimental results. On this basis, in situ27Al NMR spectroscopy and in situ23Na NMR spectroscopy were introduced to demonstrate the coordination forms of AlCl3 and NaCl for xylose conversion in the reaction solution, respectively. As shown in Fig. 5, the presence of a hexa-coordinated aluminium centre in the form of [Al(OH)2(Xylose)(H2O)x]+ could be observed at δ = 3.6 ppm as the resonance characteristic peak, which exhibited outstanding thermostability over the time under reaction conditions. In addition, a strong characteristic peak was also observed at δ = 0.8 ppm, which could be attributed to the interaction between –OH in xylose and NaCl including Na+ and Cl−, benefitting the isomerization process of xylose.38 The in situ NMR result indicated the synergistic effect between AlCl3 and NaCl in the biphasic system.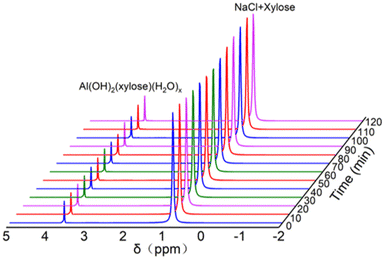 | ||
| Fig. 5 In situ 27Al NMR and in situ23Na NMR spectroscopy of the substrates. Detection conditions: 8.00 g D2O, 0.25 g xylose, 2.0 g NaCl, and 0.04 g AlCl3·6H2O. | ||
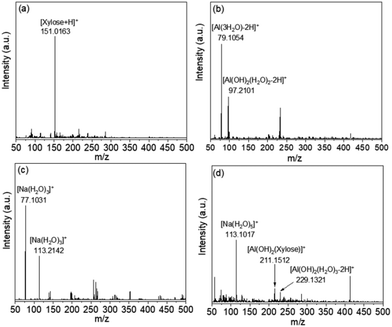
In order to further identify the catalytic active species form of Al3+, the ESI-MS method was adopted. As shown in Fig. 6(a), a xylose peak occurs at m/z = 151. In Fig. 6(b), the two peaks at m/z = 79, 97 belong to [Al(H2O)3 − 2H]+ and [Al(OH)2(H2O)2]+ species, respectively. In Fig. 6(c), the two characteristic peaks at m/z = 77 and 113 could be assigned to [Na(H2O)3]+ and [Na(H2O)5]+ species, respectively. Fig. 6(d) exhibits the ESI-MS spectrum of xylose, AlCl3, and NaCl mixed solution. Notably, the [Na(H2O)5]+ species peak was still observed at m/z = 113, while the [Xylose + H]+ species peak disappeared. Instead, two [Al(OH)2(Xylose)]+ and [Al(OH)2(Xylose) (H2O)]+Al3+ binding peaks were observed at m/z = 211 and 229, which were believed as the catalytic active species form of Al3+ during isomerization of xylose.7
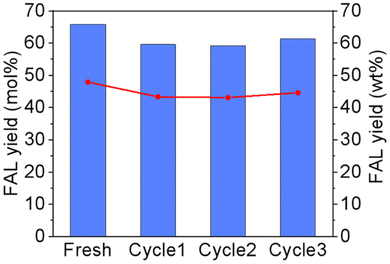
To evaluate the sustainability of the proposed process, the biphasic system with a AlCl3 catalyst was recovered three times. During each run, the upper organic phase containing FAL could be directly separated by a separation funnel, followed by distillation to recover FAL and GVL, which could be reused after facile purification by active carbon adsorption of soluble humins. However, the lower aqueous phase containing NaCl and AlCl3 could be directly reused by addition of a small amount of fresh NaCl solution to make up 20 g. The recovery of the NaCl-AlCl3 system was determined by measuring the yield of FAL in GVL after the reaction. As shown in Fig. 7, the FAL yield in GVL was 66.66% in the original solution. After three times run, 61.34% of FAL yield could still be observed, which indicated that the biphasic system with AlCl3 exhibited favourable stability and catalytic activity during the reaction.
Processing of lignocellulosic biomass feedstocks
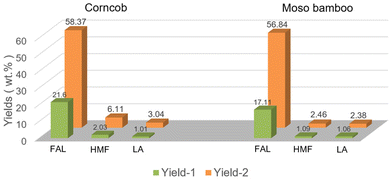
In order to evaluate the optimized FAL production process, we also conducted the conversion of corncob and moso bamboo under the optimal reaction conditions in the biphasic system (i.e., 10.0 g GVL, 20.0 g 20 wt% NaCl aq., 0.08 g AlCl3·6H2O, 150 °C, 1 h), and the product distribution of FAL, HMF and LA are shown in Fig. 8. Due to the higher hemicellulose content, the FAL yield obtained with corncob as the substrate is higher than that of moso bamboo, which is 58.37 wt% and 56.84 wt%, respectively. In addition to FAL, a certain amount of HMF and LA were also detected in the reaction solution. Similarly, the yields of HMF and LA were 6.11 wt% and 3.04 wt% with corncob as the raw material and higher than that of bamboo, which could be ascribed to the relatively low crystallinity of corncob.The mass transfer flow for the conversion of corncob was also involved, and is summarized in Fig. 9. Notably, after the reaction, the system automatically formed into the organic upper layer and aqueous lower layer with 34.14 g of solid residue obtained in the bottom, including 30.24 g glucan and 3.90 g lignin without xylan observed, which indicated that xylan in corncob was completely converted with 27.58 wt% of lignin and 74.69 wt% of glucan remaining. Then, 13.65 g of the dissolved humins in the organic phase could be precipitated by dilution and centrifugation, followed by distillation to separate 18.05 g FAL and other derivates. However, 8.78 g FAL remaining in the aqueous phase could also be extracted by EA. The left GVL and aqueous phase with trace of sugars and metal halide salts could be directly retrieved without further purification. Overall, this process in the biphasic system provides a feasible strategy for the conversion of agricultural and forestry wastes into profitable chemicals.
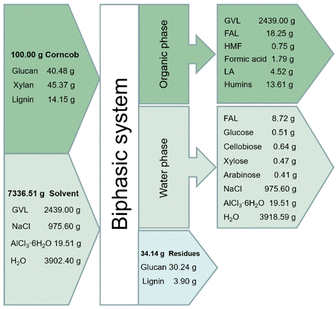 | ||
| Fig. 9 Mass balance of FAL production from corncob (reaction conditions: 100.00 g corncob 2439.00 g GVL, 4878.00 g 20 wt% NaCl aq., 19.51 g AlCl3·6H2O, 150 °C for 1 h). | ||
Conclusions
In this work, we provided novel insights into the weak interaction between PAS, NaCl aq. and FAL for the formation of a biphasic system, which significantly enables the efficient production of FAL from lignocellulosic biomass. More than 80.50 mol% of FAL yield could be obtained in the GVL/NaCl aq. (10/20 wt/wt) biphasic system with 0.3 mmol AlCl3 as a catalyst at 150 °C for 1 h. Moreover, the synergistic catalytic effect between NaCl and AlCl3 was verified by in situ27Al NMR, 23Na NMR and EI-MS/MS. Besides, this process enables the GVL/NaCl aq. biphasic system and AlCl3 catalyst to exhibit excellent recovery with no significant FAL loss after three times of recycling. The evaluation of the process for industrial application was also conducted with corncob and moso bamboo as substrates, which provided a feasible strategy for the efficient production of FAL in the biphasic system.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Nature Science Foundation of China (31870714, 32001274), and the Youth Talent Support Program for Science & Technology Innovation of National Forestry and Grassland (2019132603) for financial support.References
- Z. Wang, Y. Zhou, D. Liu, R. Qi, C. Xia, M. Li, B. You and B. Y. Xia, Angew. Chem., Int. Ed., 2022, 61, e202200552 CAS.
- X. Yin, T. Cai, C. Liu, C. Huang, J. Wang, J. Hu, N. Li, J. Jiang and K. Wang, Chem. Eng. J., 2022, 437, 135408 CrossRef CAS.
- Y. Guo, Y. Jing, Q. Xia and Y. Wang, Acc. Chem. Res., 2022, 55, 1301–1312 CrossRef CAS PubMed.
- W. Lin, J. Yang, Y. Zheng, C. Huang and Q. Yong, Biotechnol. Biofuels, 2021, 14, 143 CrossRef CAS PubMed.
- W. Lin, S. Xing, Y. Jin, X. Lu, C. Huang and Q. Yong, Bioresour. Technol., 2020, 306, 123163 CrossRef CAS PubMed.
- S. Wang, J. Bai, M. T. Innocent, Q. Wang, H. Xiang, J. Tang and M. Zhu, Green Energy Environ., 2021, 4, 578–605 Search PubMed.
- T. Yang, Y. H. Zhou, S. Z. Zhu, H. Pan and Y. B. Huang, ChemSusChem, 2017, 10, 4066–4079 CrossRef CAS PubMed.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 525–728 RSC.
- R. Gerardy, D. P. Debecker, J. Estager, P. Luis and J. M. Monbaliu, Chem. Rev., 2020, 120, 7219–7347 CrossRef CAS PubMed.
- C. Sener, A. H. Motagamwala, D. M. Alonso and J. A. Dumesic, ChemSusChem, 2018, 11, 2266–2266 CrossRef CAS.
- A. Bohre, S. Dutta, B. Saha and M. M. Abu-Omar, ACS Sustainable Chem. Eng., 2015, 3, 1263–1277 CrossRef CAS.
- K. Yan, G. S. Wu, T. Lafleur and C. Jarvis, Renewable Sustainable Energy Rev., 2014, 38, 663–676 CrossRef CAS.
- L. T. Mika, E. Csefalvay and A. Nemeth, Chem. Rev., 2018, 118, 505–613 CrossRef CAS PubMed.
- P. D. Tomke and V. K. Rathod, Int. J. Biol. Macromol., 2018, 117, 366–376 CrossRef CAS PubMed.
- R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sádaba and M. López Granados, Energy Environ. Sci., 2016, 9, 1144–1189 RSC.
- N. Zhou, C. Zhang, Y. Cao, J. Zhan, J. Fan, J. H. Clark and S. Zhang, J. Cleaner Prod., 2021, 311, 127780 CrossRef CAS.
- C. Sánchez, L. Serrano, M. A. Andres and J. Labidi, Ind. Crops Prod., 2013, 42, 513–519 CrossRef.
- C. Liu, L. Wei, X. Yin, X. Pan, J. Hu, N. Li, J. Xu, J. Jiang and K. Wang, Chem. Eng. J., 2021, 425, 130608 CrossRef CAS.
- C. Xu, E. Paone, D. Rodriguez-Padron, R. Luque and F. Mauriello, Chem. Soc. Rev., 2020, 49, 4273–4306 RSC.
- H. Li, J. Ren, L. Zhong, R. Sun and L. Liang, Bioresour. Technol., 2015, 176, 242–248 CrossRef CAS PubMed.
- X. Li, J. Yang, R. Xu, L. Lu, F. Kong, M. Liang, L. Jiang, S. Nie and C. Si, Ind. Crops Prod., 2019, 135, 196–205 CrossRef CAS.
- N. K. Gupta, A. Fukuoka and K. Nakajima, ACS Catal., 2017, 7, 2430–2436 CrossRef CAS.
- T. Zhang, W. Li, S. An, F. Huang, X. Li, J. Liu, G. Pei and Q. Liu, Bioresour. Technol., 2018, 264, 261–267 CrossRef CAS PubMed.
- L. Zhang, G. Xi, J. Zhang, H. Yu and X. Wang, Bioresour. Technol., 2017, 224, 656–661 CrossRef CAS PubMed.
- H. Li, A. Deng, J. Ren, C. Liu, Q. Lu, L. Zhong, F. Peng and R. Sun, Bioresour. Technol., 2014, 158, 313–320 CrossRef CAS PubMed.
- I. Agirrezabal-Telleria, J. Requies, M. B. Güemez and P. L. Arias, Appl. Catal., B, 2014, 145, 34–42 CrossRef CAS.
- L. Zhang, H. Yu, P. Wang and Y. Li, Bioresour. Technol., 2014, 151, 355–360 CrossRef CAS PubMed.
- C. Liu, L. Wei, X. Yin, M. Wei, J. Xu, J. Jiang and K. Wang, Ind. Crops Prod., 2020, 147, 112248 CrossRef CAS.
- C. Liu, M. Wei, J. Wang, J. Xu, J. Jiang and K. Wang, ACS Sustainable Chem. Eng., 2020, 8, 5776–5786 CrossRef CAS.
- G. W. Huber, J. N. Chheda, C. J. Barrett and J. A. Dumesic, Science, 2005, 308, 1446–1450 CrossRef CAS PubMed.
- Y. Roman-Leshkov, C. J. Barrett, Z. Y. Liu and J. A. Dumesic, Nature, 2007, 447, 982–985 CrossRef CAS PubMed.
- E. I. Gurbuz, S. G. Wettstein and J. A. Dumesic, ChemSusChem, 2012, 5, 383–387 CrossRef PubMed.
- Y. Yang, C. W. Hu and M. M. Abu-Omar, ChemSusChem, 2012, 5, 405–410 CrossRef CAS PubMed.
- Y. Yang, C. W. Hu and M. M. Abu-Omar, Green Chem., 2012, 14, 509–513 RSC.
- T. Zhang, W. Li, Z. Xu, Q. Liu, Q. Ma, H. Jameel, H. M. Chang and L. Ma, Bioresour. Technol., 2016, 209, 108–114 CrossRef CAS PubMed.
- Q. Zhang, C. Wang, J. Mao, S. Ramaswamy, X. Zhang and F. Xu, Ind. Crops Prod., 2019, 138, 111454 CrossRef CAS.
- Z. Xu, W. Li, Z. Du, H. Wu, H. Jameel, H. M. Chang and L. Ma, Bioresour. Technol., 2015, 198, 764–771 CrossRef CAS PubMed.
- Y. Luo, Z. Zhao, B. Jiang, M. Wei, Z. Zhang, L. Zeng, J. H. Clark and J. Fan, Green Chem., 2022, 24, 1515–1526 RSC.
- T. Renders, E. Cooreman, S. Van den Bosch, W. Schutyser, S. F. Koelewijn, T. Vangeel, A. Deneyer, G. Van den Bossche, C. M. Courtin and B. F. Sels, Green Chem., 2018, 20, 4607–4619 RSC.
- Q. Lin, Q. Zhan, R. Li, S. Liao, J. Ren, F. Peng and L. Li, Green Chem., 2021, 23, 8510–8518 RSC.
- H. Sun, P. Ren and J. R. Fried, Comput. Theor. Polym. Sci., 1998, 8, 229–246 CrossRef CAS.
- S. Nose, Prog. Theor. Phys. Suppl., 1991, 103, 1–46 CrossRef CAS.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Natl. Renewable Energy Lab. (NREL), 2011, 1–15 Search PubMed.
- D. R. Lide and H. V. Kehiaian, CRC Handbook of Thermophysical and Thermochemical Data, Boca Raton, 2020 Search PubMed.
- X. Lyu and G. G. Botte, Chem. Eng. J., 2021, 403, 126271 CrossRef CAS.
- Y.-B. Huang, T. Yang, Y.-T. Lin, Y.-Z. Zhu, L.-C. Li and H. Pan, Green Chem., 2018, 20, 1323–1334 RSC.
- L. Zhang, H. Yu, P. Wang, H. Dong and X. Peng, Bioresour. Technol., 2013, 130, 110–116 CrossRef CAS PubMed.
- O. Yemis and G. Mazza, Bioresour. Technol., 2011, 102, 7371–7378 CrossRef CAS PubMed.
- J. Tang, X. Guo, L. Zhu and C. Hu, ACS Catal., 2015, 5, 5097–5103 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |