Towards greener and sustainable ionic liquids using naturally occurring and nature-inspired pyridinium structures†
Morten
Suk
 and
Klaus
Kümmerer
*
and
Klaus
Kümmerer
*
Institute of Sustainable Chemistry, Leuphana University of Lüneburg, 21335 Lüneburg, Germany. E-mail: klaus.kuemmerer@leuphana.de; Tel: +49 4131 677 2893
First published on 14th December 2022
Abstract
The present study investigated the ready biodegradability of naturally occurring and nature-inspired pyridinium cations for the design of greener or sustainable ionic liquids (ILs). Our results showed that trigonelline ([C1COOHPy][Cl]) and 1-methylnicotinamide ([C1CONH2Py][I]) were completely mineralizable and non-toxic under the conditions of the Closed Bottle Test (CBT), while [C1CONH2Py][I] was readily biodegradable. In contrast, the nicotinium structures, 1-methylnicotinium and 1,1′-dimethylnicotinium, were biologically persistent but non-toxic to the inoculum, whereas S-nicotine itself was identified as readily biodegradable. The biodegradability of these pyridinium structures was compared with that of the commercial pyridinium ILs [C1Py][PF6] and [C2Py][Br] having short alkyl residues. While [C2Py][Br] demonstrated only partial but not ultimate biodegradation, [C1Py][PF6] was shown to be readily biodegradable in the CBT. HRMS confirmed the biodegradation results. The results also showed the negative influence of short alkyl residues on biodegradability. Based on the results, new highly biodegradable and non-toxic pyridinium ILs can be designed to limit the accumulation of persistent ILs in the aquatic environment. Moreover, since some of our pyridinium structures and all their pyridine precursors can be isolated either from renewable sources or waste materials, ILs based on [C1COOHPy][Cl], [C1CONH2Py][I] and [C1Py][PF6] as cationic scaffolds can also be referred to as sustainable ILs if the anion fulfills the criteria too. Besides their physicochemical and environmental properties, the use of such methylated pyridinium blocks offers the opportunity for biotechnological synthesis using S-adenosyl methionine as the methyl donor catalyzed by N-methyltransferases and therefore the avoidance of commonly used carcinogenic methylating agents such as iodomethane or dimethyl sulfate. Thus, the synthesis of these ILs can be improved towards sustainability and the principles of Green Chemistry (e.g., 3rd less hazardous chemical synthesis, 9th catalysis, and 12th inherently safer chemistry for accident prevention) and an inherently safer pathway to ILs can be created.
1. Introduction
Ionic liquids (ILs) are often promoted as green or even sustainable chemicals, since they show negligible vapor pressure, have catalytic properties, are non-flammable and support efficient recovery.1,2 However, these classifications can be misleading as they assume the generality of these terms for all ILs. In fact, many ILs have already been shown to be highly ecotoxic, cytotoxic, biologically persistent and able to inhibit human acetylcholinesterase, demonstrating the discrepancy between the advertised greenness and the actual greenness.3–7 Moreover, recent studies reported the proliferation and dissemination of antibiotic resistance genes by the persistent IL [C4mIm][PF6] due to an enhanced horizontal gene transfer, while similar structures such as benzalkonium chloride, cetylpyridinium chloride and other biocidal quaternary ammonium compounds have long been known to increase antibiotic resistance.8–15 To reduce or even avoid these negative effects of ILs, environmentally mineralizing ILs would be preferable, especially if the molecules are emitted into the environment within their life cycle or are introduced directly by their intended use.One way to reduce accumulation in the environment and thereby detrimental effects is the use of pyridinium ILs as more environmentally benign cationic scaffolds compared to 3-methylimidazolium ILs or ammonium scaffolds.16,17 However, many of these pyridinium ILs are derived from non-renewable sources and even if they are based on renewable materials, they commonly require extensive synthetic modifications to obtain the final pyridinium structure.18–21 An essential step in many of these syntheses is the alkylation of the pyridine nitrogen using volatile, toxic and carcinogenic chemicals such as alkyl halides.19,20 As a result, these structures can still be referred to as greener ILs in some respect, yet not in general and they lack the sustainability character. A potentially greener and more sustainable pathway to ILs would be the application of biobased pyridinium ions which already have a pyridinium core or could be synthesized from biobased pyridine structures by enzymatic alkylation. The utilization of naturally present pyridinium structures could not only avoid additional synthetic steps and waste to synthesize the pyridinium scaffold, but also ensure the needs of the present generation for chemical compounds without compromising the needs of future generations by using less non-renewable resources.
The enzymatic methylation of pyridines, in particular by S-adenosyl methionine (SAM), a naturally occurring sulfonium structure, and N-methyltransferases, is a common pathway to synthesize pyridinium compounds in nature.22,23 An important natural pyridinium structure is nicotinamide adenine dinucleotide and its corresponding phosphate which are essential to life. A precursor and degradation product of these compounds is nicotinamide (NA) that can be further metabolized by nicotinamide N-methyltransferase to obtain the pyridinium structure 1-methylnicotinamide ([C1CONH2Py][I]). Analogously, the carboxylic acid trigonelline ([C1COOHPy][Cl]) is formed via enzymatic methylation of nicotinic acid and can be isolated from many plants.24,25 Since the pyridinium derivatives of nicotinic acid have already been demonstrated to catalyze acetalization reactions (3,5-bis(methoxycarbonyl)-1-phenylpyridinium bromide) and to be an effective solvent for the Diels–Alder reactions (3-(butoxycarbonyl)-1-methyl-pyridinium bistriflimide), [C1COOHPy][Cl] and its amide, [C1CONH2Py][I], are interesting scaffolds for the design of potentially sustainable ILs.26,27
S-Nicotine (NIC) is a naturally occurring pyridine alkaloid and agonist of nicotinic acetylcholine receptors (nAChR) and can be found in tobacco plants. Inspired by NIC, alkylated derivatives such as 1-methylnicotinium ([C1mPyrPy][I]) and 1-ethylnicotinium have already been incorporated in the design of ILs as chiral solvating agents.28 Among these nicotinium structures, a similar 1-isopropylnicotinium salt was prepared by Albrecht et al. and used as a precursor of a palladium-pyridylidene N-heterocyclic carbene complex for Suzuki-type cross-coupling.29 However, instead of using NIC directly from the tobacco plant, a green source of NIC could be the valorization of tobacco waste from the production of cigarettes which has a common NIC content of 18 g kg−1 dry weight, but is therefore also classified as toxic and hazardous waste according to European law.30 In Italy, an annual production of about 3000 t of this waste is estimated, corresponding to approximately 54 t of NIC.30 Utilization of this harmful waste material for ILs would not only reduce the toxicity of the remaining waste, but also improve the sustainability of tobacco production and ILs by using renewable resources and waste materials. Moreover, while the non-methylated form of NIC has a high affinity for nAChR, the 1 and 1,1′ methylated compounds do not, thereby lowering the acute toxicity of these molecules.31 Similarly, ester-based nicotinium ILs with surface active properties do not have cytotoxic effects on C6-Glioma cells.32 NIC and 1,1′-dimethylnicotinium diiodide ([C1mC1PyrPy][I]2) are also non-ecotoxic (MIC ≥ 500 μM) against several bacterial strains, thereby making the nicotinium cation an interesting scaffold for chiral, biobased and non-toxic ILs.33 Although many pyridinium and pyridine structures occur naturally in the environment, it is still important for industrial applications to understand their environmental fate as their natural occurrence does not automatically ensure high biodegradability or low toxicity.
Therefore, our study was focused on the evaluation of such naturally occurring and nature-inspired pyridinium cations for the design of sustainable ILs in accordance with the 4th (designing safer chemicals), 7th (use of renewable sources) and 10th (design for degradation) principles of Green Chemistry (Fig. 1). As for pyridinium cations, the biodegradability of [C1COOHPy][Cl], its corresponding amide, [C1CONH2Py][I] and the nicotine-based structures (S-enantiomers only) [C1mPyrPy][I] and [C1mC1PyrPy][I]2 was investigated by employing a modified Closed Bottle Test (CBT). In addition to the pyridinium structures, the corresponding non-charged pyridine forms, NA and NIC, were studied too to understand the influence of methylation on biodegradability. [C1Py][PF6] and [C2Py][Br] were used as reference compounds. The CBT was further accompanied by HRMS to identify the possibly formed degradation products of incomplete mineralization and to monitor the primary elimination.
 | ||
| Fig. 1 Structures of the studied pyridine (NA and NIC) and alkylated pyridinium molecules ([C1COOHPy][Cl], [C1CONH2Py][I], [C1mPyrPy][I], [C1mC1PyrPy][I]2, [C1Py][PF6] and [C2Py][Br]). | ||
2. Materials and methods
2.1. Synthesis of the methylated pyridinium structures
The synthesis of pyridinium ILs was carried out according to the literature with minor modifications in the case of [C1mPyrPy][I] and by methylation with iodomethane in the cases of [C1CONH2Py][I] and [C1mC1PyrPy][I]2.28 Structural confirmation was done by NMR (Advance DRX 400 spectrometer, Bruker, Billerica, MA, USA) and HRMS analysis (LTQ-Orbitrap-XL, Thermo Scientific, Dreieich, Germany).2.2. Evaluation of the ready biodegradability by CBT
The ready biodegradability of the pyridinium and pyridine structures was investigated by a modified CBT using optode-based BOD determination (Fibox 3, PreSens, Regensburg, Germany).34 As the inoculum source, two drops per L of filtered secondary effluent derived from a municipal sewage treatment plant (Abwasser, Grün & Lüneburg Services GmbH, Lüneburg, Germany, 325![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 population equivalent) was used. The test consisted of a series of vessels in parallel (blank, quality control, test compound and toxicity control) to monitor the general background, the activity of the inoculum, the biodegradability of the test compounds and the possible toxic effects on the microorganisms. The test concentrations of each compound corresponded to a theoretical oxygen demand of 5 mg of O2 per L without considering nitrification (ThODNH3). However, since some molecules showed nitrification, the biodegradability results were corrected to the evaluation of the test results by including nitrification (ThODNO3) in the cases of [C1COOHPy][Cl], NA and NIC. All vessels were incubated for 28 days at 20 ± 1 °C in the dark to prevent photosynthesis and photolysis. A sampling of the CBT was done on day 0 and day 28 without any additional filtration steps.
000 population equivalent) was used. The test consisted of a series of vessels in parallel (blank, quality control, test compound and toxicity control) to monitor the general background, the activity of the inoculum, the biodegradability of the test compounds and the possible toxic effects on the microorganisms. The test concentrations of each compound corresponded to a theoretical oxygen demand of 5 mg of O2 per L without considering nitrification (ThODNH3). However, since some molecules showed nitrification, the biodegradability results were corrected to the evaluation of the test results by including nitrification (ThODNO3) in the cases of [C1COOHPy][Cl], NA and NIC. All vessels were incubated for 28 days at 20 ± 1 °C in the dark to prevent photosynthesis and photolysis. A sampling of the CBT was done on day 0 and day 28 without any additional filtration steps.
2.3. Identification of the degradation products by HRMS analysis
The primary elimination and identification of the possible degradation products were done by a UHPLC (Ultimate 3000, Thermo Scientific, Dreieich, Germany) coupled to an LTQ-Orbitrap-XL (Thermo Scientific, Dreieich, Germany) equipped with a HESI source (Thermo Scientific, Dreieich, Germany). All spectra were recorded in the positive mode at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 FWHM (m/z 400). The chromatographic separation of the pyridinium and pyridine structures was carried out on a CN Hypersil Gold column (150 × 2.1 mm, 1.9 μm, Thermo Fisher Scientific, Germany) in the hydrophilic interaction chromatography mode. The eluents consisted of 50 mM ammonium acetate (A) and acetonitrile (B). The gradient program started with 99% B and was held for 1.5 min, then B decreased to 10% at 10 min, held for 6 min, increased again to 99% B within 0.5 min, and was held for 3.5 min to re-equilibrate the column. The column temperature and the flow rate were set to 40 °C and 0.3 mL min−1, respectively. The injection volume was 25 μL.
000 FWHM (m/z 400). The chromatographic separation of the pyridinium and pyridine structures was carried out on a CN Hypersil Gold column (150 × 2.1 mm, 1.9 μm, Thermo Fisher Scientific, Germany) in the hydrophilic interaction chromatography mode. The eluents consisted of 50 mM ammonium acetate (A) and acetonitrile (B). The gradient program started with 99% B and was held for 1.5 min, then B decreased to 10% at 10 min, held for 6 min, increased again to 99% B within 0.5 min, and was held for 3.5 min to re-equilibrate the column. The column temperature and the flow rate were set to 40 °C and 0.3 mL min−1, respectively. The injection volume was 25 μL.
3. Results and discussion
3.1. Synthesis of the pyridinium structures
The NMR and HRMS spectra confirmed that the structures were consistent with the literature where available (Fig. S1–S21†).28,35 The NOESY spectra of [C1mPyrPy][I] and [C1mC1PyrPy][I]2 showed several cross peaks which indicated the presence of rotamers (Fig. S13 and S21†). The first cross peaks revealed the exchange between the stereogenic protons at 3.41–3.46 ppm ([C1mPyrPy][I]) and 4.97–5.02 ppm ([C1mC1PyrPy][I]2) and the protons of the pyridinium ring at C2 (8.63 ppm, [C1mPyrPy][I]; 9.14 ppm [C1mC1PyrPy][I]2) and C4 (8.37–8.39 ppm [C1mPyrPy][I]; 8.76–8.78 ppm [C1mC1PyrPy][I]2). Another cross peak indicated the spatial proximity of the methyl protons of the pyrrolidine ring (2.10 ppm, [C1mPyrPy][I]; 2.84 and 3.15 ppm [C1mC1PyrPy][I]2) to the protons of the pyridinium ring at C2 and C4.3.2. Biodegradability of [C1CONH2Py][I] and [C1COOHPy][Cl]
The ready biodegradability of the ILs was evaluated based on the CBT. According to the results of the CBT, both [C1CONH2Py][I] and [C1COOHPy][Cl] classify as ultimately biodegradable in accordance with the OECD guideline 301D reaching 78 ± 0.4% ThODNH3 and 84 ± 4.3% ThODNO3, respectively (Fig. 2a and b).36 Yet, [C1COOHPy][Cl] cannot be classified as readily biodegradable if nitrification is included in the equations as the required 10-day window was violated, whereas [C1CONH2Py][I] was also readily biodegradable but did not exhibit any nitrification phase (Table 1). These findings are supported by the biodegradation results of a trigonelline butyl ester being completely mineralizable in the CO2 headspace test.27 The biodegradation level of NA was 79 ± 2.0% ThODNO3 and a nitrification phase was observed after 18 days (Fig. 2c). Hence, NA is fully mineralizable but not readily degradable as the 10-day window was also violated due to the inclusion of nitrification in the biodegradation calculations. These findings are consistent with the HRMS results showing complete primary elimination and detection of no degradation products. Due to the low α- and β-diversities of microorganisms, the CBT addresses biodegradation under environmental conditions. Hence, the ILs are expected to be completely biodegradable in surface waters (low bacterial density and diversity) too. The high biodegradability of the compounds also implies that none of these ILs will bioaccumulate and cause chronic toxic effects. Moreover, all compounds showed no apparent acute toxic effects on the inoculum as indicated by the absence of a lag phase in the toxicity controls. The chloride analogue of [C1CONH2Py][I] was also shown to be non-genotoxic in the Ames test and micronucleus test as well as non-toxic in rats.37 However, while the biodegradation rate of [C1COOHPy][Cl] was comparable to the kinetics of sodium acetate, rendering [C1COOHPy][Cl] an attractive scaffold for environmentally biodegradable ILs, the kinetics of [C1CONH2Py][I] were significantly slower as demonstrated by a lag phase of 8 days (Fig. 2a and b). This prolongation of the lag phase indicates that the carboxylic acid is preferred over the amide moiety when rapid biodegradation is desired, which is consistent with common knowledge. Similar effects were observed by comparing the lag phases of [C1CONH2Py][I] and NA and the influence of the methyl group. It caused a prolongation of the lag phase from 4 days to 8 days, while no difference was observed regarding the final biodegradation levels of NA and [C1CONH2Py][I] (Fig. 2a and c). The prolonged lag phase of [C1CONH2Py][I] may be associated with its increased polarity and overall positive charge, leading to an expected lower membrane permeability at the applied pH of the CBT compared to the neutrally charged NA and [C1COOHPy][Cl], which exhibited only short lag phases and were then rapidly degraded.| Substance | Structure | ThOD [%] | Results |
|---|---|---|---|
| a Calculations based on ThODNH3. b Readily biodegradable: ≥60% ThOD within the required 10-day window.36 c Calculations based on ThODNO3. d Ultimately biodegradable: ≥60% ThOD outside the required 10-day window.38,39 | |||
| [C1CONH2Py][I] |

|
78 ± 0.4a | Readily biodegradableb |
| [C1COOHPy][Cl] |

|
84 ± 4.3c | Ultimately biodegradabled |
| NA |

|
79 ± 2.0c | Ultimately biodegradable |
3.3. Biodegradability of [C1mPyrPy][I]and [C1mC1PyrPy][I]2
The next series that was evaluated were methylated nicotinium structures. Although [C1mPyrPy][I] and [C1mC1PyrPy][I]2 were inspired by the natural compound NIC, the pyridinium salts did not exhibit significant biodegradation (Table 2 and Fig. 3a, b). In contrast, NIC itself was identified as readily biodegradable, reaching 79 ± 0.2% ThODNO3, indicating the interference of the methylated nicotinium ions with the biodegradation pathway of NIC (Fig. 3c). Bacterial degradation of NIC has been reported to occur by oxidation at the pyridine and pyrrolidine rings to either 6-hydroxy-N-methylmyosmine or N-methylmyosmine, depending on the strain, followed by hydrolysis of the enamine intermediate.40 The interference of this pathway can be attributed to either steric hindrance, since both oxidation sites are in proximity to the methyl groups, or to the pyridinium fragment in general, and the associated change in electron density and redox potential. In addition, the bioavailability and membrane permeability of the methylated nicotinium ions might be reduced under CBT conditions (pH 7.4 ± 0.2) due to the double positive charge of the ions compared to the mostly single positive charged and partially unionized NIC. The toxic effects of NIC and the methylated structures on the inoculum were not observed and are consistent with the reports in the literature for NIC and [C1mC1PyrPy][I]2.33 The accompanying HRMS results confirmed the biodegradation test results, as no primary elimination was detected for the nicotinium ILs.3.4. Biodegradability of [C1Py][PF6] and [C2Py][Br]
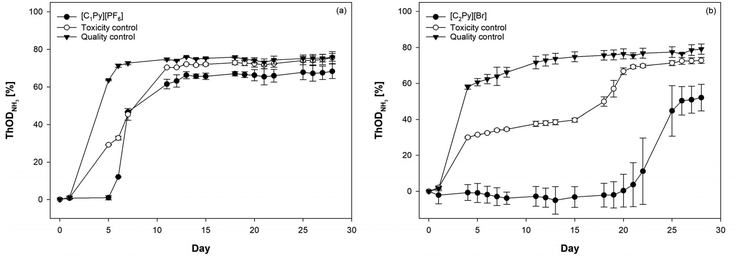
In addition to the biobased ILs, the ready biodegradability of [C1Py][PF6] and [C2Py][Br] was investigated in order to compare the results of the naturally occurring and nature-inspired ILs with commercially popular short alkylated pyridinium ILs. Only [C1Py][PF6] could be classified as readily biodegradable showing 68 ± 3.9% ThODNH3, whereas [C2Py][Br] reached 55 ± 7.4% ThODNH3 and has therefore been classified as partially biodegradable (Table 3). Moreover, in contrast to the natural pyridinium structures, neither [C1Py][PF6] nor [C2Py][Br] showed nitrification. The degradation result obtained in our study for [C2Py][Br] is in contradiction to the results of Stolte et al. who reported no biodegradation for the chloride analogue in the CBT.41 A possible intermediate in the biodegradation of [C2Py][Br], 1-carboxymethylpyridinium bromide, was tested in a previous study and showed a similar lag phase of 18 days in the CBT too but fulfilled the OECD criteria for ready biodegradability.16 Due to this high lag phase and the general variability of lag phases, the final degradation level may vary significantly between tests depending on the composition of the inoculum, which might be responsible for the negative results reported in the literature. The lag phase also indicates that an essential adaptation of the microorganism to [C2Py][Br] is required in order to mineralize the IL. Once this adaptation took place, the biodegradation rate was similar to that of [C1Py][PF6]. This lag phase, however, might be reduced by using higher inoculum concentrations, which would also go along with higher metabolic diversity, in biodegradation assays (e.g., OECD 301F) and thus also enhance biodegradability. On the other hand, the prolongation in the lag phase demonstrates the negative influence of long alkyl chains on biodegradability in the CBT starting already at an ethyl residue. Albeit some long alkylated imidazolium ILs show primary elimination under CBT conditions too, they do not exhibit high mineralization levels.41,42 This effect might therefore be responsible for the non-mineralizable character of many pyridinium ILs, since they commonly have alkyl chains longer than C2.41 Similar effects have been reported for aprotic ammonium ILs, however, their low biodegradability can also be attributed to the increased bacterial toxicity along with the increasing alkyl length.7 For [C1Py][PF6] and the analogues, no biodegradation results were reported in the literature so far. However, while the degradation process of [C1Py][PF6] occurred rapidly after a lag phase of 5 days, [C2Py][Br] exhibited a significantly longer lag phase of 21 days, which was reduced by the presence of sodium acetate as the readily biodegradable co-substrate for 15 days (Fig. 4a and b). The general toxic effects of both ILs on the inoculum were not observed, which is in accordance with the literature reporting high ecotoxicity typically only for long alkyl chains.433.5. Design of greener and more sustainable ILs by the application of natural molecules
Developing greener and more sustainable ILs, and chemicals in general, is a challenging process, but is urgently needed and goes hand in hand with the goal of sustainable development.44 The call for such sustainable development was published in the Brundtland report, which defines sustainable development as a development “that meets the needs of the present without compromising the ability of future generations to meet their own needs”.45 However, Green Chemistry alone will not be able to achieve a sustainable development, as Green Chemistry and its principles focus only on the synthesis and some design aspects (environmental dimension of sustainability) but mostly disregard the social and economic aspects. In order to include these dimensions and achieve sustainability, Sustainable Chemistry and its holistic perspectives are required. Although there is still no uniform definition of Sustainable Chemistry, there is a common understanding that Sustainable Chemistry should help society towards sustainable development as defined in the Brundtland report. A sustainable chemical should therefore bring about improvements in the social, economical and ecological dimensions. However, it is also agreed that Sustainable Chemistry cannot be conducted in the absence of Green Chemistry.46 To do justice to these understandings, Böschen et al. have proposed a definition of Sustainable Chemistry that comprises two elements.47 The first element focuses on the optimization of processes and products (e.g., toxicity, efficiency, degradability), which requires interdisciplinarity, whereas the second element deals with the interaction with non-scientific actors and transdisciplinary aspects.47 Accordingly, sustainable ILs have to meet technological as well as toxicological and environmental requirements that take into account all life stages (raw materials, synthesis, usage, and disposal) of the chemicals and have to provide an improvement for society.48–50 As our results show, naturally occurring and nature-inspired molecules could play an important role in such a development, especially in the optimization of processes and products not only because of their potentially low toxicity and high degradability but also because of a possible more environmentally friendly production process due to enzymatic methylation and the use of renewable resources (Table 4).Another example towards a more sustainable production of ILs, and chemicals in general, is the use of biocatalysis.54 This biosynthesis can either be done in vitro or in vivo by genetically modified microorganisms and was previously demonstrated for [C1COOHPy] by Mizuno et al., who isolated the genes encoding nicotinic acid methylation in coffee plants and expressed them in E. coli to produce [C1COOHPy].51 Although other alkyl chains than methyl groups could be donated by synthesizing SAM analogues through alkylation reactions, methylation is preferred as it avoids additional synthetic steps and the use of toxic chemicals.54,55 Therefore, the application of biotechnological synthesis can provide an inherently safer route to ILs (the 3rd and 12th principles of Green Chemistry). Furthermore, as these reactions are catalyzed by enzymes, the 9th principle is fulfilled too. Nevertheless, the limitations of biotechnological synthesis in terms of greenness must be taken into account.
Although the tested nicotinium derivatives are less preferable for the design of environmentally degrading ILs for many applications due to their persistence, this persistence may even be desirable if the IL is intended to be circulated within a closed system and is never released into the environment. Hence, due to the persistence of the molecule, the ILs are able to be reintegrated through a circular economy and therefore save resources and energy. This is particularly desirable for complex chemicals or compounds which require an energy-intensive synthesis process. Furthermore, it is unlikely that a green or sustainable chemical fulfills all the dimensions of sustainability (economical, environmental and social dimensions) equally or completely. Accordingly, tradeoffs between these dimensions have to be made to ensure functionality while taking the environmental and toxicological considerations into account.
Considering these compromises, the persistent but non-toxic [C1mPyrPy][I] might be applied as a green and inexpensive precursor for Suzuki-type catalysts substituting the often applied Pd(PPh3)4. The use of such scaffolds for Suzuki-type coupling was demonstrated for the 1-isopropylnicotinium analogue.29 Moreover, [C1mPyrPy][I] and [C1mC1PyrPy][I]2 could also be used in other N-heterocyclic carbene mediated cross-coupling reactions such as the Heck reaction and Sonogashira coupling.58 Another possible use as a catalyst could be in the synthesis of acetals, ketals or Friedel–Crafts acetylation as shown by the literature for other simple pyridinium molecules instead of common Lewis acids such as AlCl3.26,59 The persistence of such catalysts to biodegradation and hydrolysis, as well as their low toxicity, may therefore be desirable for synthesis in general and represent a necessary compromise between functionality and environmental concerns.
Similarly, NIC itself can still be used as a biodegradable cation in protic ILs. While its high acute toxicity to humans and animals remains problematic for most applications, certain chemicals, i.e., insecticides, require high acute toxicity for their function and a tradeoff between functionality and toxicity has to be made. NIC could therefore be used as a cation in biodegradable insecticidal ILs for agricultural usage or in other ILs that require high acute toxicity as a technicophore. A possible anion for its agricultural use could be pelargonate, a naturally occurring herbicidal fatty acid, which has already been used in designing herbicidal ILs.60
4. Conclusion
Designing ILs with green and sustainable properties is challenging but urgently needed. The aim of our study was to evaluate the potential of pyridinium cations derived from (the 7th principle of Green Chemistry) or inspired by naturally available chemicals for the design of greener and more sustainable ILs. Our results demonstrate that [C1COOHPy][Cl], [C1CONH2Py][I] and [C1Py][PF6] are fully mineralizable and non-ecotoxic pyridinium scaffolds (the 4th and 10th principle of Green Chemistry) and can be used as cations for the design of greener or more sustainable aprotic ILs. Since these molecular features occur naturally in the environment or can be isolated from natural resources or their treatment including waste, no additional modifications to obtain the cationic pyridinium structure are required. Moreover, since the synthesis of methylated pyridinium building blocks can be achieved via biochemical reactions involving SAM as the methyl donor and N-methyltransferases as the catalyst, common carcinogenic agents such as iodomethane or dimethyl sulfate can also be avoided, improving the synthesis process in accordance with the 3rd, 9th and 12th principles of Green Chemistry, avoiding additional waste and creating safer synthetic steps. However, the results also demonstrate that even nature-inspired chemicals require investigations of their environmental fate as the presumption that naturally inspired or occurring compounds are in general environmentally biodegradable or mineralizing is not true. All in all, the use of these naturally occurring pyridinium building blocks can fulfill 6 out of the 12 principles of Green Chemistry when used for enzymatic methylation in the synthesis, while in theory other principles such as the use of safer solvents during the reaction could also be fulfilled when water is used during biotechnological synthesis. Furthermore, the results of this study also allow for first design rules for such biodegradable and greener ILs, which are of importance for the design and development of greener ILs of the future:■ Using renewable [C1COOHPy][Cl] or [C1CONH2Py][I] as cationic cores. To generate new derivatives, [C1COOHPy][Cl] can be linked via an ester bond to other biodegradable structures in order to tune its properties (e.g., fatty acid esters). [C1CONH2Py][I] can be used as an aprotic IL without any modifications of the cationic core and could be an important cation in pharmaceutically active ILs.
■ Using [C1Py] as a green and sustainable cationic building block utilizing waste materials e.g., from coffee production.
■ Avoid [C1mPyrPy][I] and [C1mC1PyrPy][I]2 as pyridinium elements if the IL does not circulate in a closed system but is released into the environment. NIC could be applied in protic ILs with the required high acute toxicity i.e., insecticides or rodenticides.
■ Use only methyl functionality in the linear alkyl chain attached to the pyridine ring to design biodegradable ILs, as an ethyl chain already has a negative impact on biodegradability. Moreover, methyl residues offer biotechnological production too and therefore would avoid the use of volatile alkylating agents.
Nevertheless, it should be noted that these naturally occurring pyridinium cations still require effort, processes and additional chemicals for the isolation and work-up process which may shift the problems of Green Chemistry and more sustainability to other chemicals and spheres or into the future and should therefore not be disregarded. Moreover, to be defined as fully sustainable ILs, the impact on the social and economic dimensions of sustainability has to be evaluated for the final ILs (cation and anion) including their application and possible shortcomings or improvements in the respective sectors. Hence, in order to ensure the sustainability of the molecules and their applications, i.e., the service and function they provide, only biodegradable, ideally fully environmentally mineralizing anions from renewable sources and non-toxic inorganic ions should be applied. Furthermore, inorganic anions containing metals can contribute to the dissipative loss of these which again is non-sustainable. The same holds for phosphorus which is a limited and critical resource and indispensable for life too. Otherwise, the ILs can only be claimed as greener ILs if they meet one or a few criteria for greenness and sustainability.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank Karina Witte (Albert-Ludwigs-University Freiburg, Germany) for performing the NMR measurements. Morten Suk would like to thank the Leuphana University Lüneburg for providing a scholarship.References
- R. Sheldon, Chem. Commun., 2001, 2399–2407 RSC.
- C. M. Gordon, Appl. Catal., A, 2001, 222, 101–117 CrossRef CAS.
- T. P. T. Pham, C.-W. Cho and Y.-S. Yun, Water Res., 2010, 44, 352–372 CrossRef CAS PubMed.
- F. Stock, J. Hoffmann, J. Ranke, R. Störmann, B. Ondruschka and B. Jastorff, Green Chem., 2004, 6, 286–290 CAS.
- J. Arning, S. Stolte, A. Böschen, F. Stock, W. R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2008, 10, 47–58 RSC.
- A. Jordan and N. Gathergood, Chem. Soc. Rev., 2015, 44, 8200–8237 CAS.
- K. Erfurt, M. Markiewicz, A. Siewniak, D. Lisicki, M. Zalewski, S. Stolte and A. Chrobok, ACS Sustainable Chem. Eng., 2020, 8, 10911–10919 CAS.
- Y. Luo, Q. Wang, Q. Lu, Q. Mu and D. Mao, Environ. Sci. Technol. Lett., 2014, 1, 266–270 CrossRef CAS.
- Q. Wang, D. Mao, Q. Mu and Y. Luo, PLoS One, 2015, 10, 1–14 Search PubMed.
- S. Langsrud, G. Sundheim and A. L. Holck, J. Appl. Microbiol., 2004, 96, 201–208 CrossRef CAS PubMed.
- G. Mcdonnell and A. D. Russell, Clin. Microbiol. Rev., 1999, 12, 147–179 CrossRef CAS.
- Y. Han, Z. C. Zhou, L. Zhu, Y. Y. Wei, W. Q. Feng, L. Xu, Y. Liu, Z. J. Lin, X. Y. Shuai, Z. J. Zhang and H. Chen, Environ. Sci. Pollut. Res., 2019, 26, 28352–28360 CrossRef CAS PubMed.
- B. Chen, J. Han, H. Dai and P. Jia, Environ. Pollut., 2021, 283, 117074 CrossRef CAS.
- U. Tattawasart, J.-Y. Maillard, J. R. Furr and A. D. Russell, J. Hosp. Infect., 1999, 42, 219–229 CrossRef CAS PubMed.
- H. Maseda, Y. Hashida, R. Konaka, A. Shirai and H. Kourai, Antimicrob. Agents Chemother., 2009, 53, 5230–5235 CrossRef CAS.
- M. Suk, A. Haiß, J. Westphal, A. Jordan, A. Kellett, I. V. Kapitanov, Y. Karpichev, N. Gathergood and K. Kümmerer, Green Chem., 2020, 22, 4498–4508 RSC.
- S. Stolte, S. Steudte, A. Igartua and P. Stepnowski, Curr. Org. Chem., 2011, 15, 1946–1973 CrossRef CAS.
- S. Kirchhecker, S. Tröger-Müller, S. Bake, M. Antonietti, A. Taubert and D. Esposito, Green Chem., 2015, 17, 4151–4156 CAS.
- S. P. M. Ventura, M. Gurbisz, M. Ghavre, F. M. M. Ferreira, F. Gonçalves, I. Beadham, B. Quilty, J. A. P. Coutinho and N. Gathergood, ACS Sustainable Chem. Eng., 2013, 1, 393–402 CrossRef CAS.
- I. V. Kapitanov, A. Jordan, Y. Karpichev, M. Spulak, L. Perez, A. Kellett, K. Kümmerer and N. Gathergood, Green Chem., 2019, 21, 1777–1794 RSC.
- H. Prydderch, A. Haiβ, M. Spulak, B. Quilty, K. Kümmerer, A. Heise and N. Gathergood, RSC Adv., 2017, 7, 2115–2126 CAS.
- M. Fontecave, M. Atta and E. Mulliez, Trends Biochem. Sci., 2004, 29, 243–249 CrossRef CAS PubMed.
- M. Richter, Nat. Prod. Rep., 2013, 30, 1324–1345 RSC.
- C. Campa, J. F. Ballester, S. Doulbeau, S. Dussert, S. Hamon and M. Noirot, Food Chem., 2004, 88, 39–43 CrossRef CAS.
- R. Kuroda, K. Kazumura, M. Ushikata, Y. Minami and K. Kajiya, J. Agric. Food Chem., 2018, 66, 8714–8721 CrossRef CAS PubMed.
- B. Procuranti and S. J. Connon, Org. Lett., 2008, 10, 4935–4938 CrossRef CAS PubMed.
- J. R. Harjani, R. D. Singer, M. T. Garcia and P. J. Scammells, Green Chem., 2009, 11, 83–90 RSC.
- T. Heckel, A. Winkel and R. Wilhelm, Tetrahedron: Asymmetry, 2013, 24, 1127–1133 CrossRef CAS.
- M. Albrecht and H. Stoeckli-Evans, Chem. Commun., 2005, 5, 4705–4707 RSC.
- M. Civilini, C. Domenis, N. Sebastianutto and M. De Bertoldi, Waste Manage. Res., 1997, 15, 349–358 CrossRef CAS.
- L. H. Wilkins, V. P. Grinevich, J. T. Ayers, P. A. Crooks and L. P. Dwoskin, J. Pharmacol. Exp. Ther., 2003, 304, 400–410 CrossRef CAS PubMed.
- G. Singh, R. Kamboj, V. Singh Mithu, V. Chauhan, T. Kaur, G. Kaur, S. Singh and T. Singh Kang, J. Colloid Interface Sci., 2017, 496, 278–289 CrossRef CAS PubMed.
- M. D. Joyce, M. C. Jennings, C. N. Santiago, M. H. Fletcher, W. M. Wuest and K. P. Minbiole, J. Antibiot., 2016, 69, 344–347 CrossRef CAS.
- J. Friedrich, A. Längin and K. Kümmerer, Clean: Soil, Air, Water, 2012, 41, 251–257 Search PubMed.
- R. Lavilla, T. Gotsens, F. Gullón and J. Bosch, Tetrahedron, 1994, 50, 5233–5244 CAS.
- OECD, OECD 301 - Ready Biodegradability, 1992 Search PubMed.
- D. Turck, J. Bresson, B. Burlingame, T. Dean, S. Fairweather-Tait, M. Heinonen, K. I. Hirsch-Ernst, I. Mangelsdorf, H. J. McArdle, A. Naska, M. Neuhäuser-Berthold, G. Nowicka, K. Pentieva, Y. Sanz, A. Siani, A. Sjödin, M. Stern, D. Tomé, M. Vinceti, P. Willatts, K. Engel, R. Marchelli, A. Pöting, M. Poulsen, J. R. Schlatter, W. Gelbmann, E. Ververis and H. van Loveren, EFSA J., 2017, 15, 1–16 Search PubMed.
- ECHA, Guidance on information requirements and chemical safety assessment - Chapter R.7b: Endpoint specific guidance, 2017 Search PubMed.
- ECHA, Guidance on information requirements and chemical safety assessment - Chapter R.11: PBT/vPvB assessment, 2017 Search PubMed.
- J. P. Kaiser, Y. Feng and J. M. Bollag, Microbiol. Rev., 1996, 60, 483–498 CrossRef CAS PubMed.
- S. Stolte, S. Abdulkarim, J. Arning, A. K. Blomeyer-Nienstedt, U. Bottin-Weber, M. Matzke, J. Ranke, B. Jastorff and J. Thöming, Green Chem., 2008, 10, 214–224 RSC.
- J. R. Harjani, J. Farrell, M. T. Garcia, R. D. Singer and P. J. Scammells, Green Chem., 2009, 11, 821–882 RSC.
- S. Viboud, N. Papaiconomou, A. Cortesi, G. Chatel, M. Draye and D. Fontvieille, J. Hazard. Mater., 2012, 215–216, 40–48 CrossRef CAS.
- J. B. Zimmerman, P. T. Anastas, H. C. Erythropel and W. Leitner, Science, 2020, 367, 397–400 CrossRef CAS PubMed.
- World Commission on Environment and Development, Our Common Future, Oxford University Press, 1987 Search PubMed.
- P. T. Anastas and J. B. Zimmerman, Curr. Opin. Green Sustainable Chem., 2018, 13, 150–153 CrossRef.
- S. Böschen, D. Lenoir and M. Scheringer, Naturwissenschaften, 2003, 90, 93–102 CrossRef PubMed.
- B. Jastorff, R. Störmann, J. Ranke, K. Mölter, F. Stock, B. Oberheitmann, W. Hoffmann, J. Hoffmann, M. Nüchter, B. Ondruschka and J. Filser, Green Chem., 2003, 5, 136–142 RSC.
- J. Ranke, S. Stolte, R. Störmann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183–2206 CrossRef CAS PubMed.
- D. Kralisch, D. Reinhardt and G. Kreisel, Green Chem., 2007, 9, 1308–1318 RSC.
- K. Mizuno, M. Matsuzaki, S. Kanazawa, T. Tokiwano, Y. Yoshizawa and M. Kato, Biochem. Biophys. Res. Commun., 2014, 452, 1060–1066 CrossRef CAS PubMed.
- H. Taguchi, M. Sakaguchi and Y. Shimabayashi, Vitamins, 1986, 60, 537–546 CAS.
- R. H. Stadler, N. Varga, J. Hau, F. A. Vera and D. H. Welti, J. Agric. Food Chem., 2002, 50, 1192–1199 CrossRef CAS PubMed.
- S. Mordhorst, J. Siegrist, M. Müller, M. Richter and J. N. Andexer, Angew. Chem., Int. Ed., 2017, 56, 4037–4041 CrossRef CAS PubMed.
- H. Stecher, M. Tengg, B. J. Ueberbacher, P. Remler, H. Schwab, H. Griengl and M. Gruber-Khadjawi, Angew. Chem., Int. Ed., 2009, 48, 9546–9548 CrossRef CAS PubMed.
- J. R. Harjani, T. J. Abraham, A. T. Gomez, M. T. Garcia, R. D. Singer and P. J. Scammells, Green Chem., 2010, 12, 650–665 RSC.
- S. Chlopicki, J. Swies, A. Mogielnicki, W. Buczko, M. Bartus, M. Lomnicka, J. Adamus and J. Gebicki, Br. J. Pharmacol., 2007, 152, 230–239 CAS.
- G. C. Fortman and S. P. Nolan, Chem. Soc. Rev., 2011, 40, 5151–5169 RSC.
- Y. Xiao and S. V. Malhotra, J. Organomet. Chem., 2005, 690, 3609–3613 CrossRef CAS.
- J. Pernak, K. Czerniak, M. Niemczak, Ł. Ławniczak, D. K. Kaczmarek, A. Borkowski and T. Praczyk, ACS Sustainable Chem. Eng., 2018, 6, 2741–2750 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc03178g |
| This journal is © The Royal Society of Chemistry 2023 |