Anti-obesity effects of red pepper (Capsicum annuum L.) leaf extract on 3T3-L1 preadipocytes and high fat diet-fed mice†
Mi-Jin
Oh
a,
Hye-Bin
Lee
a,
Guijae
Yoo
a,
Miri
Park
a,
Chang-Hyun
Lee
b,
Inwook
Choi
a and
Ho-Young
Park
 *a
*a
aResearch Division of Food Functionality, Korea Food Research Institute, Jeollabuk-do 55365, Republic of Korea. E-mail: hypark@kfri.re.kr
bDepartment of Anatomy, Woosuk University, Jeollabuk-do 55338, Republic of Korea
First published on 24th November 2022
Abstract
Patients with obesity mostly have metabolic syndrome and this can lead to multiple health problems. In the present study, we evaluated the anti-obesity effect of water-soluble red pepper (Capsicum annuum L.) leaf extract (PLE) on 3T3-L1 adipocytes and high-fat diet (HFD)-fed mice. The adipocyte lipid content was determined using Oil Red O staining, which revealed that 100 μg mL−1 PLE markedly reduced fat accumulation without affecting the cell viability. PLE exhibited high prebiotic activity scores by modulating probiotic strains, contributing to host health improvement. In vivo investigation in HFD-fed mice revealed that PLE supplementation significantly decreased the HFD-induced increases in the body weight, amount of white adipose tissue, and serum triglyceride, total cholesterol, leptin, and insulin levels. Consistent with its effects on reduced lipid droplet formation in the liver, PLE supplementation suppressed the expression of lipid synthesis-related proteins including SREBP-1, FAS, and PPAR-γ in the liver and increased that of PGC-1α, CPT1, and adiponectin in epididymal WAT. PLE treatment improved intestinal barrier function and inflammation and reduced harmful intestinal enzyme activities in the feces. Collectively, these results indicate that PLE inhibits fat accumulation in HFD-fed mice via the suppression of adipogenesis and lipogenesis, suggesting its potential in preventing obesity.
1. Introduction
Obesity, defined as abnormal or excessive fat accumulation, has become a worldwide health concern leading to an increased risk of many diseases, such as hyperlipidemia, hypertension, type 2 diabetes mellitus, coronary heart disease, and cancer, and their complications.1 Consumption of a high-fat diet (HFD) is closely related to multiple metabolic diseases associated with obesity. Adipose tissues are responsible for energy storage, which depends on processes managing the size and number of adipocytes. During adipocyte differentiation, an increase in the number of adipocytes accounts for the adipose tissue expansion observed in obesity.2 Thus, several prevention options are available for the treatment of obesity. Losing weight by restricting calorie intake and engaging in physical exercise are the most common treatments. However, the use of weight-loss medication may have side effects. Therefore, there is a need for identifying suitable methods for the management of obesity for each individual.3In recent years, the demand for numerous natural products, including crude extracts and compounds or phytochemicals isolated from plants, has increased because of their beneficial role in the treatment and prevention of diet-induced obesity.4 Red pepper (Capsicum annuum L.) is the most popular spice in Southeast Asia, Africa, and the Mediterranean region. It has been used as a food coloring pigment and hot flavoring agent and in traditional medicines.5 Pepper is a vegetable containing various carotenoid pigments, including β-carotene with pro-vitamin A activity, and a high level of bioactive compounds including carotenoids (capsanthin, capsorubin, cryptocapsin, and capsaicinoid), quercetin, and luteolin, many of which have been reported to have antioxidant properties.6 Pepper fruits consist of a stem, pericarp, placenta, and seeds. High levels of capsaicin, which has been reported to reduce oxidative damage by activating anti-oxidative defense systems, lowering cholesterol levels, improving blood lipid profiles, and decreasing the serum triacylglycerol content by enhancing energy metabolism, are present in the placenta.7
Numerous studies evaluated the antioxidant, anti-obesity, and anti-diabetic effects of pepper fruits including seed extracts. However, studies on pepper leaves have not yet been reported. Therefore, we hypothesized that water-soluble pepper leaf extract (PLE) might have prebiotic activity and anti-obesity effects and improve obesity and the related metabolic syndrome in a HFD-induced obesity mouse model.
2. Materials and methods
2.1. Preparation of PLE
Dried C. annuum L. leaves were collected from Wanju-gun, Republic of Korea, and ground into a fine powder (≤10 mm). The powder (100 g) was extracted with distilled water (2 L) at 80 °C for 3 h, at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v). Then, it was filtered and concentrated at 40 °C using a vacuum rotary evaporator, and then, lyophilized to obtain PLE. PLE has a color of light brown and highly water-soluble properties up to 200 mg mL−1.
20 (w/v). Then, it was filtered and concentrated at 40 °C using a vacuum rotary evaporator, and then, lyophilized to obtain PLE. PLE has a color of light brown and highly water-soluble properties up to 200 mg mL−1.
2.2. UPLC-qTOF-MS analysis
Chemical profiling of PLE was performed using an ultra-performance liquid chromatograph coupled with a Waters Synapt G2-Si quadrupole time-of-flight mass spectrometer (UPLC–qTOF-MS, Waters, Manchester, UK). Separation was performed by gradient elution using a Waters Acquity® HSS T3 C18 column (2.1 × 50 mm, 1.8 μm), and the column oven temperature was set as 35 °C. The mobile phase was 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) and delivered at 0.5 mL min−1 according to the following linear gradient: 0–1 min, 5% B; 1–20 min, 5–100% B; 20–22.3 min, 100% B; 22.3–22.4 min, 100–5% B; 22.4–25 min, 5% B. The injection volume was 2 μL. The mass spectra were acquired in the negative ion mode by scanning from m/z 55–1100. The optimized MS parameters were as follows: sampling cone, 18 V; collision energy, 5 kV; capillary, 1 kV; source temperature, 110 °C; desolvation gas flow, 780 L h−1; desolvation temperature, 350 °C. Data analysis was performed using Waters UNIFI software.2.3. In vitro assays
The adiponectin contents were analyzed using the Mouse Adiponectin/Acrp30 Quantikine ELISA Kit (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer's protocol.
2.4. Prebiotic activity score
The pH of the fermented broth was determined using a pH meter (Orion Star A211, Thermo Fisher Scientific).
2.5. In vivo assays
At the end of the 8-week treatment period, mice were fasted for 12 h, and then anesthetized with isoflurane and sacrificed. Blood samples were subsequently taken from the abdominal vena cava for serum biomarker analysis. The epididymal white adipose tissue (eWAT) and liver were excised immediately, rinsed with 0.9% saline, and weighed. All samples were stored at −80 °C until analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 5 min, 4 °C). Fluorescence was measured using a microplate reader (Spectra MAX M2) in 96-well plates (excitation: 485 nm, emission: 535 nm). A standard curve was created by diluting FITC-dextran in untreated plasma diluted with phosphate-buffered saline.
000g, 5 min, 4 °C). Fluorescence was measured using a microplate reader (Spectra MAX M2) in 96-well plates (excitation: 485 nm, emission: 535 nm). A standard curve was created by diluting FITC-dextran in untreated plasma diluted with phosphate-buffered saline.
Colon pH was determined using a pH meter (Orion star A211, Thermo Fisher Scientific) after emptying the colon with sterile water.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 15 min, 4 °C). This process was repeated thrice, and 100 μL of the supernatant was mixed with 400 μL of 50 mM sodium phosphate buffer containing 0.129 mg mL−1O-dianisidine and 0.0005% H2O2. The absorbance was measured at 492 nm using a microplate reader (Spectra MAX M2). MPO activity was expressed as optical density per mg protein.
000g, 15 min, 4 °C). This process was repeated thrice, and 100 μL of the supernatant was mixed with 400 μL of 50 mM sodium phosphate buffer containing 0.129 mg mL−1O-dianisidine and 0.0005% H2O2. The absorbance was measured at 492 nm using a microplate reader (Spectra MAX M2). MPO activity was expressed as optical density per mg protein.
The activities of β-glucosidase and β-glucuronidase in the fecal samples were determined based on the rates of p-nitrophenol release. The activity of tryptophanase was measured at 550 nm with indole as a standard.
2.6. Statistical analysis
The results are presented as the mean ± standard deviation (SD). Data were analyzed using one-way analysis of variance with Duncan's multiple range test using SPSS version 20 (SPSS Inc., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.3. Results
3.1. Chemical profiling of PLE
UPLC-qTOF-MS chromatograms of PLE are shown in Fig. 1. The known compounds in the pepper leaf were identified by comparing with reference compounds according to the mass spectrum and retention time. The unknown compounds were identified by analyzing the MS/MS fragmentation and by referring to previous reports. As a result, 24 compounds were unambiguously identified by comparison with the references. The major peaks are summarized in ESI Table 1,† and the most commonly identified compound was flavonoid-O-glycoside. | ||
| Fig. 1 The total ion chromatogram of the water-soluble extract from pepper (Capsicum annuum L.) leaves in negative ion mode ESI-qTOF-MS detection. | ||
3.2. In vitro adipogenesis and the prebiotic effects of PLE
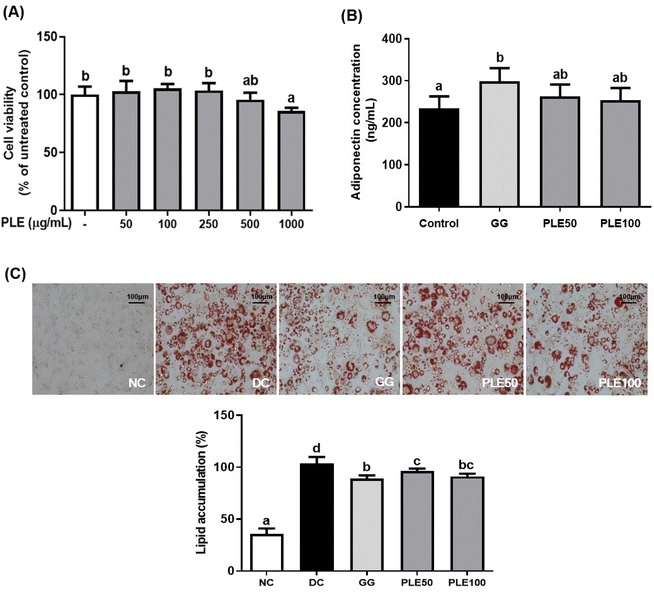
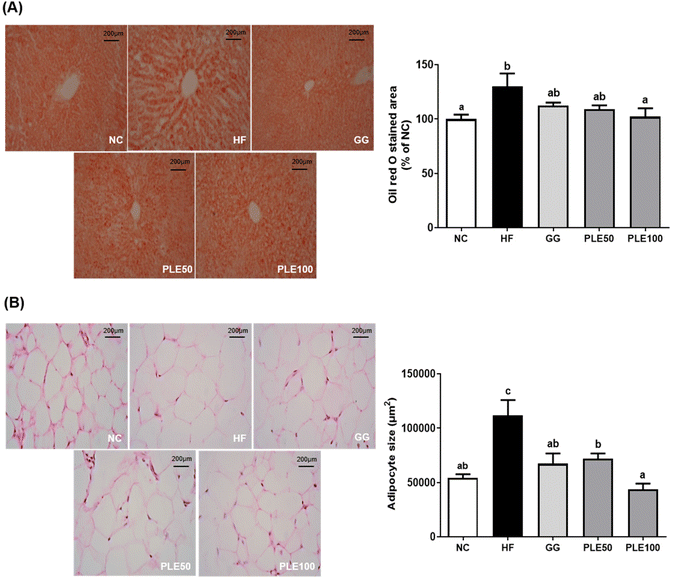
Fig. 2C shows the ORO staining of lipid accumulation in the 3T3-L1 cells. The cells were induced to differentiate and the lipid accumulation levels in the PLE100 group were compared with those in the control group (lipid accumulation set to 100%). Lipid accumulation was significantly (p < 0.05) lower in the PLE100 and GG groups compared with that in the control group. These results showed that PLE suppressed lipid accumulation during differentiation.
3.3. In vivo anti-obesity effects of PLE
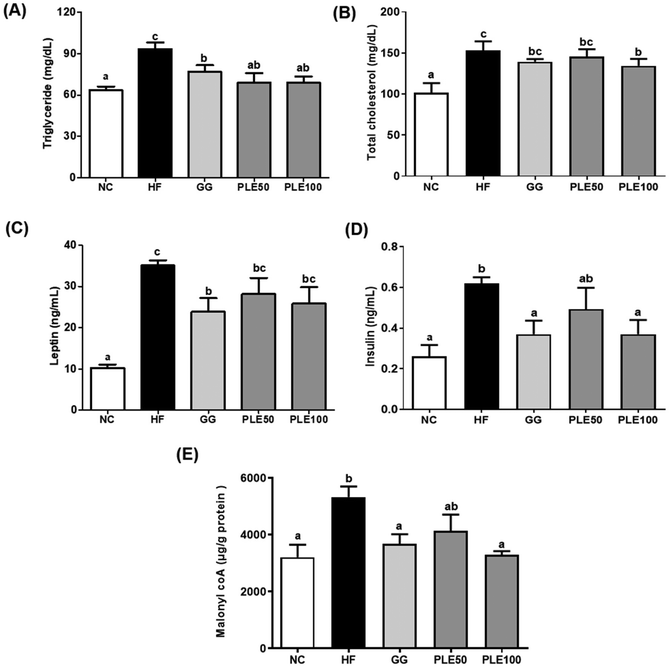
In addition, the hepatic malonyl-CoA levels were measured in the hepatic tissues (Fig. 5E). The PLE50 and PLE100 groups decreased the hepatic malonyl-CoA levels by 22.4 and 38.3%, respectively, as compared with those of the HF groups (p < 0.05).
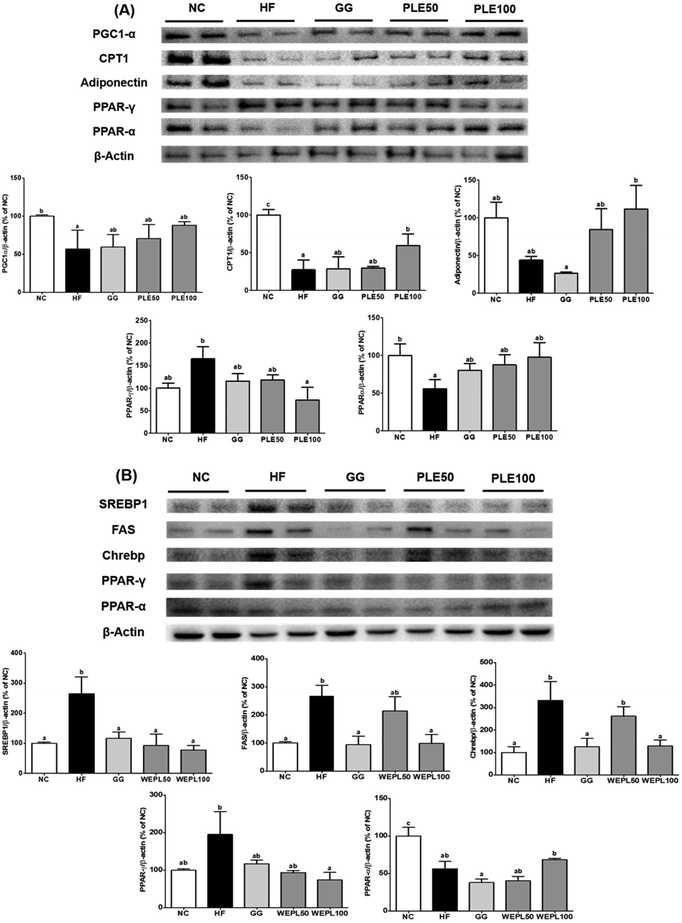
In addition, PPARγ protein expression was reduced by 55.2% in the PLE100 group compared with that in the HF group. In the liver tissues, SREBP1, FAS, ChREBP, and PPAR-γ protein expressions were significantly decreased in the PLE100 group (p < 0.05) by 70.7, 63.2, 61.2, and 61.9%, respectively compared to those in the HF group (Fig. 9B). Furthermore, the PPAR-α protein expression was reduced by 43.6% in the HF group compared to that in the NC group.
4. Discussion
Obesity is a major causative factor of many metabolic diseases associated with numerous health problems, including cancer, diabetes mellitus, vascular disease, and hypertension.11 An HFD induces inflammation, obesity, and other metabolic disorders. Therefore, many studies have been performed on obesity and obesity-induced diseases.12 Consumption of fruits and vegetables has been associated with the prevention of chronic diseases. A constant supply of phytochemical-containing plants with health benefits can reduce the risk of chronic diseases in humans.13C. annuum L., which has various beneficial physiological activities and is characterized by its chemopreventive, anti-inflammatory, antioxidant, hypolipidemic, and weight-reducing effects, has been adopted as a pungent food additive worldwide.14 The present study showed that the water extract of red pepper leaves had anti-obesity effects in both in vitro and in vivo experiments. In particular, its effect on adipogenic differentiation in 3T3-L1 pre-adipocytes cells and body weight and lipid profiles and gene expressions involved in energy metabolism was assessed using HFD-fed C57BL/6J mice.15G. gummi-gutta extract, a commercial anti-obesity ingredient, was used as a positive control to compare the efficacy of PLE.16Adipogenesis, the cellular differentiation of preadipocytes to adipocytes, is a complex process regulated by many factors. The 3T3-L1 preadipocyte cell line is the most popular cell model for adipogenesis and a reliable in vitro model for studying the molecular mechanisms of adipocyte differentiation.17 In this study, we observed that PLE decreases the induction of morphological changes and lipid accumulation in these cells. The cell viability assay during differentiation revealed that the effect of PLE was not due to the effects on 3T3-L1 cell death. The levels of lipid accumulation were significantly decreased following the treatment with 100 μg mL−1 of PLE. Adiponectin is a protein produced by adipocytes. It acts as a hormone and plays an important role in modulating glucose and lipid metabolism as an insulin-sensitizing adipocytokine.18 PLE treatment suppressed the release of adiponectin during differentiation. These results showed that PLE decreased lipid accumulation and the adiponectin concentration.
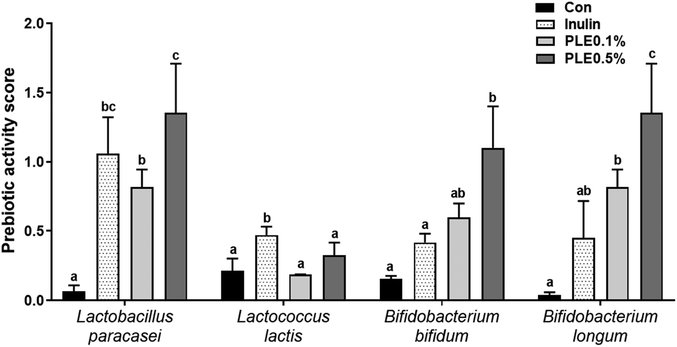
Prebiotics such as fructooligosaccarides, galactooligosaccharides, and inulin selectively enhance the effects of one or a group of beneficial bacteria, directly or indirectly improving the health of the host.19 We quantified the functional stability of inulin and PLE by comparing their prebiotic activity before (0 h) and after (24 h) exposure to four representative probiotic strains. Acidic pH conditions, as might occur in the fermentation of inulin and PLE, influenced the prebiotic activity of all prebiotic strains. Probiotic strains had the highest or similar prebiotic activity score in PLE compared to those in commercial prebiotics, such as inulin.
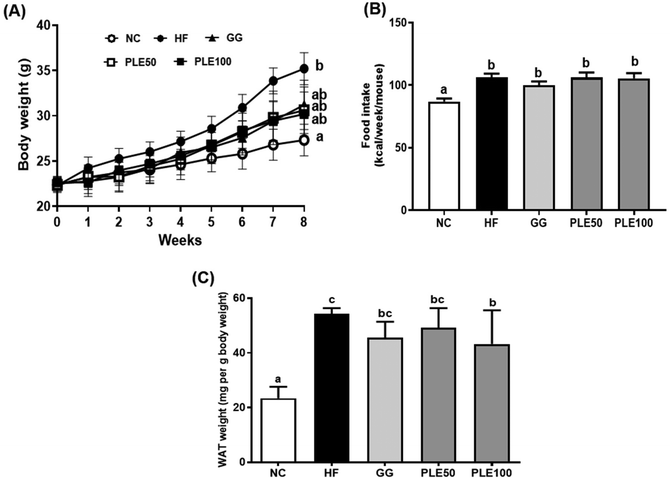
The findings of this study showed that the oral administration of PLE reduced the HFD-induced weight gain in mice without altering food intake. Furthermore, obesity is characterized by an increase in fat tissue mass and adipocyte size. In addition, PLE treatment decreased the serum levels of TG, TC, leptin, and insulin, which are associated with energy expenditure and weigh management, during exposure to a HFD. Leptin also interacts with insulin, impacting glucose and lipid homeostasis.20 PLE dose-dependently decreased lipid accumulation in the liver tissues with lipid droplets and significantly decreased the adipocyte size in the eWAT. Furthermore, recent studies have shown that HFD intake induces lipid droplet formation and leads to increased TG levels, which are associated with lipid accumulation in the liver.21 These results suggest that PLE can regulate body weight gain, serum hormone levels, and lipid droplet production. Malonyl-CoA acutely regulates fatty acid oxidation in the liver in vivo. In the liver, glucose and insulin inhibit fatty acid oxidation by altering the malonyl-CoA levels.22 The concentration of hepatic malonyl-CoA was determined in HFD-induced mice treated with PLE. The results showed that the concentration of malonyl-CoA was higher in the HF group compared with those in the GG and PLE groups.
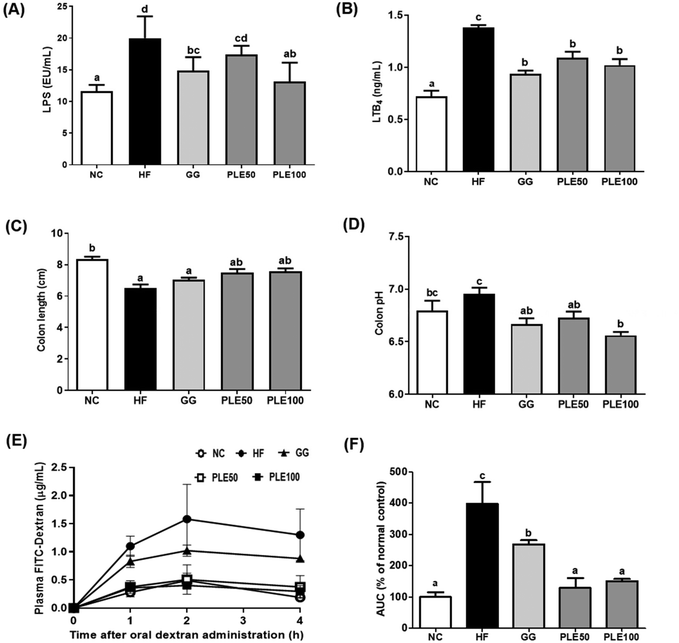
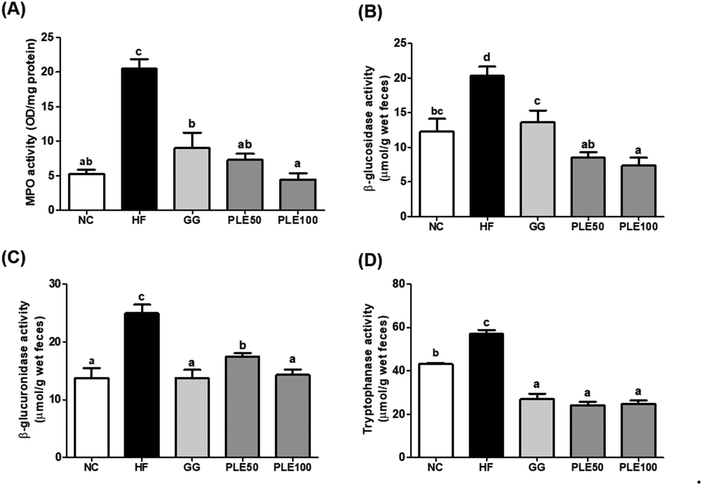
Many studies have reported that HFD-induced metabolic endotoxemia and inflammation are closely related to LPS. LPS, a major component of Gram-negative bacteria, is well known to induce pro-inflammatory responses in macrophages, and an HFD results in a higher serum LPS concentration, known as endotoxemia.23 LTB4 has also been studied as a pro-inflammatory lipid mediator generated from arachidonic acid and it was found that HFD-fed mice exhibit increased LTB4 levels in the liver, muscles, and adipose tissue compared to normal diet-fed mice.24 Our results suggested that PLE-administered mice showed suppressed serum LPS and LTB4 levels compared to those in the HF group. Moreover, obesity-related gut microbiota dysbiosis is directly linked to HFD and characterized by increased gut barrier permeability, known as leaky gut, enhancing the concentration of LPS in the blood stream, which causes inflammation and related metabolic diseases in rodents and humans.25 In agreement with these findings, increased gut permeability and enhanced serum LPS production are associated with an HFD, which were recovered by PLE treatment in vivo. Furthermore, MPO, a highly abundant protein in neutrophils, is considered a marker of inflammation, oxidative stress, lipoprotein oxidation, and atherosclerosis development; MPO may have a regulatory role in diet-induced obesity.26 The activity of MPO in the colon was more potently increased in the HF group compared with that in the PLE groups. Hence, PLE has the potential to prevent intestinal inflammation in rodent models. In addition, fecal enzyme activities such as those of β-glucosidase, β-glucuronidase, and tryptophanase were increased in mice after the consumption of an HFD. These bacterial enzymes exert potentially harmful effects on the host. Fecal enzymes are involved in the conversions of endogenous toxins and the increased growth of harmful E. coli and Enterobacteriaceae species.27 These three bacterial enzyme levels were significantly decreased in the PLE groups compared to those in the HF group. These results showed that PLE ameliorates the gut dysbiosis induced by chronic high fat consumption.
Eight-week administration of PLE significantly downregulated the expressions of PGC1-α, CPT1, and adiponectin in the eWAT of the HFD-fed mice. PGC-1α is a major transcriptional cofactor for mitochondrial biogenesis.28 These proteins can interact in adipose tissues. Increased PPAR-γ expression is observable after HFD treatment. It is well established that PPAR-γ activation decreases adiponectin secretion.29 As expected, PPAR-γ expression was significantly decreased in the PLE group, accompanied by the corresponding increase in adiponectin expression. SREBP1 and ChREBP are central regulators of most hepatic lipid synthesis-related genes, including ACC, FAS, and stearoyl-CoA desaturase 1.30 In the PLE groups, which were on HFD feeding, the expressions of lipogenic genes, such as SREBP1 and ChREBP, were significantly downregulated. PPAR-α is predominantly expressed in the liver, and PPAR-α activation has been shown to be beneficial in hepatic lipid accumulation and protect mice from HFD-induced hepatic steatosis.31 We found increased gene expression of PPAR-α in the PLE100 group compared with that in the HF group. These results suggest that PPAR-γ and PPAR-α regulate the expressions of downstream target genes, such as SREBP1, ChREBP, and FAS.
5. Conclusions
The results of the present work demonstrated that PLE exhibited prebiotic activity with anti-obesity effects. In vitro, PLE increases the growth of beneficial microbes and inhibits intracellular lipid accumulation in 3T3-L1 preadipocytes. In vivo, PLE inhibited HFD-induced body weight gain, decreased the serum endotoxin level and inflammation, and regulated lipid metabolism. Taken together, our findings suggest that PLE possesses the potential to be beneficial for the prevention of obesity and related metabolic diseases.Author contributions
Ho-Young Park designed the research; Mi-Jin Oh, Hye-Bin Lee, and Guijae Yoo conducted the experiments; Miri Park and Inwook Choi analyzed the data and performed the statistical analysis; Chang Hyun Lee collected the experimental pathology data; Mi-Jin Oh and Ho-Young Park wrote the manuscript. All authors discussed the results and approved the final manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by the Main Research Program (E0210602-02) of the Korea Food Research Institute (KFRI) funded by the Ministry of Science and ICT.References
- J.-H. Wang, S. Bose, H.-G. Kim, K.-S. Han and H. Kim, Fermented Rhizoma Atractylodis Macrocephalae alleviates high fat diet-induced obesity in association with regulation of intestinal permeability and microbiota in rats, Sci. Rep., 2015, 5, 1–10 Search PubMed.
- J. Rhyu, M. S. Kim, M.-K. You, M.-A. Bang and H.-A. Kim, Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes, Nutr. Res. Pract., 2014, 8, 33–39 CrossRef PubMed.
- B. O. Cho, J. Choi, H. J. Kang, D. N. Che, J. Y. Shin, J. S. Kim, S. J. Kim and S. I. Jang, Anti-obesity effects of a mixed extract containing Platycodon grandiflorum, Apium graveolens and green tea in high-fat-diet-induced obese mice, Exp. Ther. Med., 2020, 19, 2783–2791 Search PubMed.
- M. L. Bonet, J. A. Canas, J. Ribot and A. Palou, Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity, Arch. Biochem. Biophys., 2015, 572, 112–125 CrossRef PubMed.
- K.-H. Sim and Y.-S. Han, The antimutagenic and antioxidant effects of red pepper seed and red pepper pericarp (Capsicum annuum L.), Prev. Nutr. Food Sci., 2007, 12, 273–278 CrossRef.
- M. Imran, M. S. Butt and H. A. R. Suleria, Capsicum annuum bioactive compounds: health promotion perspectives, in Bioactive Molecules in Food, ed. J. M. Mérillon and K. Ramawat, 2017, pp. 1–22 Search PubMed.
- W.-Y. Song, K.-H. Ku and J.-H. Choi, Effect of ethanol extracts from red pepper seeds on antioxidative defense system and oxidative stress in rats fed high-fat· high-cholesterol diet, Nutr. Res. Pract., 2010, 4, 11–15 CrossRef PubMed.
- S. Zhang, H. Hu, L. Wang, F. Liu and S. Pan, Preparation and prebiotic potential of pectin oligosaccharides obtained from citrus peel pectin, Food Chem., 2018, 244, 232–237 CrossRef PubMed.
- A. Affan, N. Al-Jezani, P. Railton, J. N. Powell and R. J. Krawetz, Multiple mesenchymal progenitor cell subtypes with distinct functional potential are present within the intimal layer of the hip synovium, BMC Musculoskeletal Disord., 2019, 20, 1–12 CrossRef PubMed.
- Y. Xiu, K. Wang, J. Chen, Z. Zhuo and Y. Xiu, Liposomal N-acylethanolamine-hydrolyzing acid amidase (NAAA) inhibitor F96 as a new therapy for colitis, RSC Adv., 2020, 10, 34197–34202 RSC.
- H.-W. Lee and S. Pyo, Acrylamide induces adipocyte differentiation and obesity in mice, Chem.-Biol. Interact., 2019, 298, 24–34 CrossRef CAS PubMed.
- M. Gao, Y. Ma and D. Liu, High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice, PLoS One, 2015, 10, e0119784 CrossRef PubMed.
- S. M. Frank, J. Webster, B. McKenzie, P. Geldsetzer, J. Manne-Goehler, G. Andall-Brereton, C. Houehanou, D. Houinato, M. S. Gurung and B. W. Bicaba, Consumption of fruits and vegetables among individuals 15 years and older in 28 low-and middle-income countries, J. Nutr., 2019, 149, 1252–1259 CrossRef PubMed.
- K. Srinivasan, Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review, Crit. Rev. Food Sci. Nutr., 2016, 56, 1488–1500 CrossRef CAS PubMed.
- M.-C. Kang, N. Kang, S.-C. Ko, Y.-B. Kim and Y.-J. Jeon, Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet, Food Chem. Toxicol., 2016, 90, 36–44 CrossRef.
- C. Guillén-Enríquez, V. Lopez-Teros, U. Martín-Orozco, J. A. López-Díaz, D. Hierro-Ochoa, A. Ramos-Jiménez, H. Astiazarán-García, N. D. R. Martínez-Ruiz and A. Wall-Medrano, Selected physiological effects of a Garcinia gummi-gutta extract in rats fed with different hypercaloric diets, Nutrients, 2018, 10, 565 CrossRef PubMed.
- K. M. de Melo, F. T. B. de Oliveira, R. A. C. Silva, A. L. G. Quinderé, J. D. B. Marinho-Filho, A. J. Araújo, E. D. B. Pereira, A. A. Carvalho, M. H. Chaves and V. S. Rao, α, β-Amyrin, a pentacyclic triterpenoid from Protium heptaphyllum suppresses adipocyte differentiation accompanied by down regulation of PPARγ and C/EBPα in 3T3-L1 cells, Biomed. Pharmacother., 2019, 109, 1860–1866 CrossRef PubMed.
- S. Behl, A. Adem, A. Hussain and J. Singh, Effects of rilpivirine, 17β-estradiol and β-naphthoflavone on the inflammatory status of release of adipocytokines in 3T3-L1 adipocytes in vitro, Mol. Biol. Rep., 2019, 46, 2643–2655 CrossRef PubMed.
- I. Figueroa-Gonzalez, G. Rodriguez-Serrano, L. Gomez-Ruiz, M. Garcia-Garibay and A. Cruz-Guerrero, Prebiotic effect of commercial saccharides on probiotic bacteria isolated from commercial products, Food Sci. Technol., 2019, 39, 747–753 CrossRef.
- K. Mendoza-Herrera, A. A. Florio, M. Moore, A. Marrero, M. Tamez, S. N. Bhupathiraju and J. Mattei, The leptin system and diet: a mini review of the current evidence, Front. Endocrinol., 2021, 12, 749050 CrossRef.
- I. Korovila, A. Höhn, T. Jung, T. Grune and C. Ott, Reduced liver autophagy in high-fat diet induced liver steatosis in new zealand obese Mice, Antioxidants, 2021, 10, 501 CrossRef CAS PubMed.
- S. Rodriguez and M. J. Wolfgang, Targeted chemical-genetic regulation of protein stability in vivo, Chem. Biol., 2012, 19, 391–398 CrossRef CAS PubMed.
- S.-Y. Kwon, M. Ro and J.-H. Kim, Mediatory roles of leukotriene B4 receptors in LPS-induced endotoxic shock, Sci. Rep., 2019, 9, 1–8 CrossRef.
- P. Li, D. Y. Oh, G. Bandyopadhyay, W. S. Lagakos, S. Talukdar, O. Osborn, A. Johnson, H. Chung, M. Maris, J. M. Ofrecio, S. Taguchi, M. Lu and J. M. Olefsky, LTB4 causes macrophage–mediated inflammation and directly induces insulin resistance in obesity, Nat. Med., 2015, 21, 239–247 CrossRef CAS PubMed.
- I. J. Malesza, M. Malesza, J. Walkowiak, N. Mussin, D. Walkowiak, R. Aringazina, J. Bartkowiak-Wieczorek and E. Mądry, High-fat, western-style diet, systemic inflammation, and gut microbiota: a narrative review, Cells, 2021, 10, 3164 CrossRef PubMed.
- M. G. Qaddoumi, M. Alanbaei, M. M. Hammad, I. Al Khairi, P. Cherian, A. Channanath, T. A. Thanaraj, F. Al-Mulla, M. Abu-Farha and J. Abubaker, Investigating the role of myeloperoxidase and angiopoietin-like protein 6 in obesity and diabetes, Sci. Rep., 2020, 10, 1–10 CrossRef PubMed.
- W. Dworzański, E. Cholewińska, B. Fotschki, J. Juśkiewicz and K. Ognik, Oxidative, epigenetic changes and fermentation processes in the intestine of rats fed high-fat diets supplemented with various chromium forms, Sci. Rep., 2022, 12, 9817 CrossRef PubMed.
- M. Kobayashi, Y. Deguchi, Y. Nozaki and Y. Higami, Contribution of pgc-1α to obesity-and caloric restriction-related physiological changes in white adipose tissue, Int. J. Mol. Sci., 2021, 22, 6025 CrossRef PubMed.
- J. F. Landrier, E. Kasiri, E. Karkeni, J. Mihály, G. Béke, K. Weiss, R. Lucas, G. Aydemir, J. Salles and S. Walrand, Reduced adiponectin expression after high–fat diet is associated with selective up–regulation of ALDH1A1 and further retinoic acid receptor signaling in adipose tissue, FASEB J., 2017, 31, 203–211 CrossRef PubMed.
- A. G. Linden, S. Li, H. Y. Choi, F. Fang, M. Fukasawa, K. Uyeda, R. E. Hammer, J. D. Horton, L. J. Engelking and G. Liang, Interplay between ChREBP and SREBP-1c coordinates postprandial glycolysis and lipogenesis in livers of mice, J. Lipid Res., 2018, 59, 475–487 CrossRef PubMed.
- A. Ammazzalorso, I. Bruno, R. Florio, L. De Lellis, A. Laghezza, C. Cerchia, B. De Filippis, M. Fantacuzzi, L. Giampietro and C. Maccallini, Sulfonimide and amide derivatives as novel PPARα antagonists: Synthesis, antiproliferative activity, and docking studies, ACS Med. Chem. Lett., 2020, 11, 624–632 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo03201e |
| This journal is © The Royal Society of Chemistry 2023 |