Serum fat-soluble vitamins and the menstrual cycle in women of childbearing age†
Yuqing
Zhang
,
Jing
Kong
,
Xiaohong
Jiang
,
Jiangping
Wu
* and
Xiaoli
Wu
 *
*
Department of Obstetrics and Gynecology, Women's Hospital of Nanjing Medical University, Nanjing Maternity and Child Health Care Hospital, Nanjing, 210004, China. E-mail: wuxiaoli@njmu.edu.cn; wujiangping@njmu.edu.cn; Tel: +86-25-52226486
First published on 2nd December 2022
Abstract
Evidence suggests that fat-soluble vitamins are involved in reproduction, but their association with the menstrual cycle, the proxy of female fecundity, remains largely unexplored in women of childbearing age. Serum fat-soluble vitamin levels were measured by HPLC-MS/MS and menstrual cycle data were acquired from 3123 women of reproductive age in Nanjing, China, using standard questionnaires. Irregular and long menstrual cycles occurred in 725 (23.2%) and 604 (19.3%) participants, respectively. Participants with higher levels of vitamins A and K had increased odds of irregular menstrual cycles (vitamin A: OR = 1.39 (95% CI: 1.12, 1.74); vitamin K: OR = 1.41 (95% CI: 1.13, 1.76)) and long menstrual cycles (vitamin A: OR = 1.34 (95% CI: 1.06, 1.69); vitamin K: OR = 1.27 (95% CI: 1.00, 1.61)), and the relationship showed a linear dose–response pattern (P-overall < 0.05, P-nonlinearity > 0.05). Vitamin A was positively associated with the average menstrual cycle length (β: 1.83, 95% CI: 0.28, 3.39). Vitamins A and K were interacted in their associations with irregular menstrual cycles and long cycles. In sensitivity analysis with further exclusion of participants with abnormal thyroid function or a history of polycystic ovary syndrome (PCOS), the association of vitamins A and K with the menstrual cycle remained robust. This study indicates that higher serum vitamin A and K levels in women of childbearing age are significantly associated with higher odds of irregular and long menstrual cycles with a linear dose–response curve. Further investigations are warranted to determine the appropriate fat-soluble vitamin levels for women of childbearing age.
Introduction
Fat-soluble vitamins, including vitamins A, D, E, and K, are usually taken from dietary fat and stored in the liver and fatty tissue for further usage.1 Deficiencies in fat-soluble vitamins lead to adverse health outcomes, such as night blindness, bone fractures, neurological problems, and bleeding.2–5 There is emerging evidence linking fat-soluble vitamins to reproductive functions such as follicular development, sex hormone biosynthesis, and regulation of the cycling endometrium.6–8 Several reproductive tissues express fat-soluble vitamin receptors,9,10 and fat-soluble vitamin deficiency was reported to decrease fertility and increase the risk of pregnancy complications and adverse birth outcomes.11,12 In contrast to water-soluble vitamins, excess lipid-soluble vitamins cannot be discriminated from urine, but they accumulate in the liver and could be built up to the point of causing damage. Some data suggest a connection between overdosing of fat-soluble vitamins and adverse reproductive endpoints.13,14 Dietary reference intake levels of fat-soluble vitamins per day for normal function have been established for different age groups.15,16 However, the standards of fat-soluble vitamin levels for women of reproductive age are not specific. Identifying the appropriate fat-soluble vitamin levels and maintaining relative stability in these levels are of great importance and deserve more attention to ensure the reproductive health of women of childbearing age. Until now, studies regarding fat-soluble vitamins and their association with reproduction in women of childbearing age have been limited, especially in the Chinese population.The menstrual cycle is a proxy for female reproductive health, which depends on the balance of sex hormones and, to some extent, indicates female fecundity. The irregular menstrual cycle is closely connected with infertility.17 Although studies on fat-soluble vitamins and the menstrual cycle characteristics are scarce, there are clues regarding the role of fat-soluble vitamins in regulating menstrual disorders.9,18,19 Lower vitamin D levels have been associated with menstrual disorders and long menstrual cycles.20,21 Vitamin A is inversely associated with premenstrual syndrome.22 The potential toxic effect of fat-soluble vitamins, especially vitamin A, on sex hormone homeostasis has also been suggested in human and animal studies.23,24 The fat-soluble vitamin nutritional status is critical for the normal menstrual cycle. However, previous studies are sparse and limited in sample size and kinds of fat-soluble vitamins. Until now, a full evaluation of all four fat-soluble vitamins with the menstrual cycle is absent, and the relationships between fat-soluble vitamins and menstrual cycle characteristics are largely unknown.
In this study, 3123 women of reproductive age were included, and the serum levels of all four kinds of fat-soluble vitamins were measured. Our primary objective was to examine the association between fat-soluble vitamins and menstrual cycle characteristics as well as the dose–response curve in a population of Chinese reproductive-aged women. We also explored the interactions between fat-soluble vitamins in their association with the menstrual cycle.
Materials and methods
Study population
The study population was recruited from the preconception care clinic and gynecological clinic of the affiliated hospital of Nanjing Medical University from January 2021 to March 2022. The fat-soluble vitamin status and menstrual cycle characteristics of the patients were retrospectively obtained from the clinical information system. Participants with both information on fat-soluble vitamins and complete menstrual information were included in this study. We excluded those who were pregnant and those aged less than 18 or above 50 years. Those with one ovary removed or a history of ovary surgery, breastfeeding, taking birth control pills or using female hormones within six months before examination were also excluded (ESI Fig. 1†). Information on age, body mass index (BMI), parity, abnormal pregnancy history, abnormal thyroid function, and days since the first day of the last period was extracted from the clinical system. Due to the retrospective observational study design and the anonymous data collection, informed consent was waived25 and this study was approved by the hospital's ethical committees. Finally, 3123 participants were included in this study.Fat-soluble vitamin levels
The concentrations of fat-soluble vitamins A, D, E, and K were measured by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). Five milliliters of blood samples were collected and stored in 4 °C refrigerators for 30 minutes with protection from light to avoid photolytic degradation. After centrifugation at 4000 rpm at 4 °C for 5 min, the supernatant was collected for further detection. Then, 400 μl of supernatant was mixed with 400 μl of standard solution and 100 μl of methanol aqueous solution for 5 min. The mixture was added to 800 μl of 100% hexane and mixed for 5 min and further centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. 500 μl of the supernatant solvent was evaporated to dryness under nitrogen at room temperature. The residue was then reconstituted into 120 μl of methanol solution, of which 100 μl was used for the analyses. Quality control samples were generated with a known amount of vitamin levels (both high and low levels). The accuracy of the method was evaluated by the relative standard deviations (RSDs) of quality control samples analyzed concurrently with the samples and the RSDs ranged from 4.5% to 13.7%. Vitamin D2 was detected in only 3.1% of the participants (>2 ng ml−1) and was excluded from subsequent analysis. The distribution of the concentrations was right skewed and the concentrations were ln-transformed to improve normality. The reference serum vitamin A, 25(OH)D, E, and K levels were determined according to standards.26–28
000 rpm for 5 min. 500 μl of the supernatant solvent was evaporated to dryness under nitrogen at room temperature. The residue was then reconstituted into 120 μl of methanol solution, of which 100 μl was used for the analyses. Quality control samples were generated with a known amount of vitamin levels (both high and low levels). The accuracy of the method was evaluated by the relative standard deviations (RSDs) of quality control samples analyzed concurrently with the samples and the RSDs ranged from 4.5% to 13.7%. Vitamin D2 was detected in only 3.1% of the participants (>2 ng ml−1) and was excluded from subsequent analysis. The distribution of the concentrations was right skewed and the concentrations were ln-transformed to improve normality. The reference serum vitamin A, 25(OH)D, E, and K levels were determined according to standards.26–28
Menstrual cycle characteristics
A standard questionnaire was used to obtain data on menstrual characteristics. The participants were asked if they had regular menstrual cycles in the last year. An irregular menstrual cycle was defined as a variation in menstrual cycle length larger than 7 days between months.29,30 Participants with regular menstrual cycles were asked about their average menstrual cycle length. Those with irregular menstrual cycles were asked about their shortest and longest menstrual cycle lengths in the past year, and the average of these two lengths was the representative length of the irregular menstrual cycle. Participants with a long menstrual cycle were those with an average menstrual cycle length longer than 35 days, while participants with a short menstrual cycle were those with an average length shorter than 21 days.Statistical analysis
The correlation of fat-soluble vitamins was analyzed by Spearman correlation. The distribution of fat-soluble vitamins in different menstrual cycle groups (normal, short, and long menstrual cycles) was analyzed by the Kruskal–Wallis test, and the differences were analyzed in comparison with the normal-menstrual-cycle group.The association between fat-soluble vitamins and menstrual cycle characteristics was analyzed by the generalized linear regression model, restricted cubic spline (RCS) model, and Bayesian kernel machine regression (BKMR) model. In the evaluation of fat-soluble vitamins and long menstrual cycles, participants with short menstrual cycles were excluded from the analysis, and the long-cycle group was compared with the normal-menstrual-cycle group.
In the generalized linear regression model, the concentrations of fat-soluble vitamins were divided into tertile groups, and the association was evaluated by comparing the highest two groups with the lowest one. The association of fat-soluble vitamins with irregular menstrual cycles and long menstrual cycles was assessed using the multivariable logistic regression model, while their association with the average menstrual cycle length was examined by multivariable linear regression, with vitamins being treated as categorical variables. Age, abnormal thyroid function, parity, abnormal pregnancy history, BMI, and the fat-soluble vitamin detection days of menstrual cycles were considered confounding factors as previously reported.8,31,32
The RCS model was used to identify the nonlinear dose–response curve of fat-soluble vitamins and the odds ratio of the irregular menstrual cycle and long menstrual cycle as well as the change in the average menstrual cycle length. The number of knots was chosen based on the smallest Akaike's information criterion (AIC) in the model. The 50th percentile of fat-soluble vitamin concentration was set as the reference level.
The BKMR model has been widely used in chemical mixture exposure.33 By fixing all the other fat-soluble vitamins at their median levels, the dose–response association of each vitamin with the menstrual cycle was examined. To explore whether two of the vitamins interacted with the menstrual cycle, the dose–response curve of one vitamin with the menstrual cycle was evaluated with another vitamin fixed at different quantiles (10th, 50th, and 90th) with all the others fixed at their median levels.
We also conducted sensitivity analysis by further excluding the participants with abnormal thyroid function, as this could disturb menstruation.34 Polycystic ovarian syndrome (PCOS) is characterized by a long menstrual cycle.35 In the sensitivity analysis, we also excluded those previously diagnosed with PCOS or polycystic changes in the ovary. Another sensitivity analysis excluding participants aged 40 years and older was also conducted to reduce the potential bias of reduced fertility of older age. All analyses were performed with the R software (version 3.6.1). A two-tailed P value less than 0.05 was defined as significant.
Results
Characteristics of the study population
The characteristics of the 3123 study participants are shown in Table 1. The median age of the study population was 29 years, and most of them were nulliparous (57.5%). A total of 20.8% of the participants had abnormal pregnancy histories. Most of the participants had normal menstrual cycles (80.3%), and 19.3% of them had long menstrual cycles. In our study, only 0.3% of the participants reported a short menstrual cycle. The participants had a median average menstrual cycle length of 29 days.| Characteristics | Median (IQR) or n (%) |
|---|---|
| IQR: interquartile range; BMI: body mass index; Vit: vitamin; and 25(OH)D: 25-hydroxyvitamin D. | |
| Age (years) | 29 (27, 31) |
| Parity | |
| Nulliparous | 1796 (57.5%) |
| Multiparous | 1327 (42.5%) |
| BMI group | |
| BMI < 18.5 | 1721 (55.1%) |
| 18.5–24.9 | 298 (9.5%) |
| BMI ≥ 25.0 | 371 (11.9%) |
| Missing | 733 (23.5%) |
| Abnormal pregnancy | |
| Yes | 650 (20.8%) |
| No | 2473 (79.2%) |
| Abnormal thyroid function | |
| Yes | 136 (4.4%) |
| No | 2987 (95.6%) |
| Days since the first day of the last period | 14 (9, 24) |
| Irregular menstruation | |
| Yes | 725 (23.2%) |
| No | 2398 (76.8%) |
| Menstrual cycle | |
| Normal | 2509 (80.3%) |
| Short | 10 (0.3%) |
| Long | 604 (19.3%) |
| Average cycle days | 29 (29, 32.5) |
| 25(OH)D (ng ml−1) | 13.40 (10.20, 17.70) |
| Severe deficiency (<10) | 734 (23.5%) |
| Deficiency (10.01–20.00) | 1883 (60.3%) |
| Insufficiency (20.01–30.00) | 451 (14.4%) |
| Normal (30.01–100) | 55 (1.8%) |
| Excess (>100) | 0 |
| Vit A (ng ml−1) | 390 (229, 451) |
| Deficiency (<200) | 8 (0.3) |
| Marginal (200–300) | 318 (10.1) |
| Normal (>300) | 2797 (89.6) |
| Vit D2 (ng ml−1) | <2 (<2, <2) |
| Vit D3 (ng ml−1) | 13.20 (10.10, 17.40) |
| Vit E (μg ml−1) | 6.60 (5.50, 8.10) |
| Deficiency (<5) | 393 (12.6%) |
| Normal (5–18) | 2721 (87.1%) |
| Excess (>18) | 9 (0.3%) |
| Vit K (ng ml−1) | 0.55 (0.34, 0.90) |
| <0.13 | 19 (0.6%) |
| 0.13–1.88 | 2972 (95.2%) |
| >1.88 | 132 (4.2%) |
Fat-soluble vitamin distribution
The percentages of participants deficient in vitamins A, E, and K were 0.3%, 12.6%, and 0.6%, respectively (Table 1). For 25(OH)D, 60.3% of the participants were deficient, and 23.5% were severely deficient. Only a few participants had excess fat-soluble vitamins.The concentrations of 25(OH)D and vitamin D3 were significantly lower in the long-menstrual-cycle group than in the normal group, while vitamin A was significantly higher (Fig. 1). The concentrations of fat-soluble vitamins were positively correlated with each other, and 25(OH)D and vitamin D3 had the highest correlations (ESI Fig. 2,† Spearman's rho = 0.978).
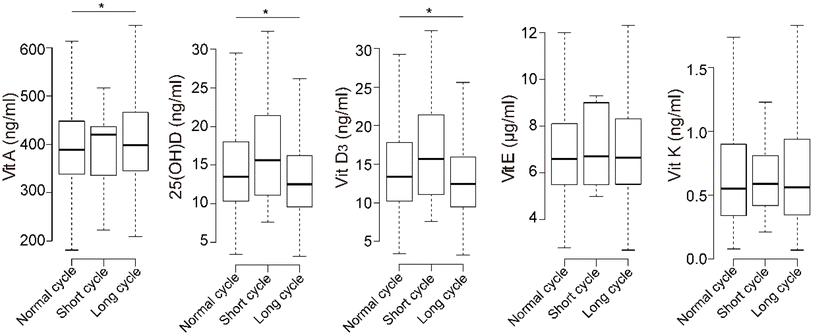 | ||
| Fig. 1 Boxplot of fat-soluble vitamins in different menstrual-cycle groups. * indicates significant differences in the Kruskal–Wallis test and compared with the normal-menstrual-cycle group. | ||
Association of fat-soluble vitamins with menstrual cycle characteristics
In the generalized linear regression model, vitamins A and K were positively associated with the irregular menstrual cycle (vitamin A: OR: 1.39, 95% CI: 1.12, 1.74; vitamin K: OR: 1.41, 95% CI: 1.13, 1.76) and long menstrual cycle (vitamin A: OR: 1.34, 95% CI: 1.06, 1.69; vitamin K: OR: 1.27, 95% CI: 1.00, 1.61) when comparing the highest vitamin levels with the lowest levels. Additionally, vitamin A had a positive association with the average menstrual cycle length (β: 1.83; 95% CI: 0.28, 3.39). We did not find association between the menstrual cycles and 25(OH)D, vitamin D3, or vitamin E (Table 2).| Vitamin | Irregular cycle OR (95% CI) | Long cycle OR (95% CI) | Average length β (95% CI) |
|---|---|---|---|
| OR: odds ratio; CI: confidence interval; T: tertile; and Ref: reference. The concentrations of fat-soluble vitamins were treated as categorical variables with the lowest group as references. Models were adjusted for age, parity, abnormal thyroid function, abnormal pregnancy, BMI, and the number of days since the first day of the last period. Bold font indicates statistical significance. | |||
| Vit A | |||
| T1 | Ref. | Ref. | Ref. |
| T2 | 1.14 (0.91, 1.43) | 1.13 (0.89, 1.43) | 1.24 (−0.30, 2.78) |
| T3 | 1.39 (1.12, 1.74) | 1.34 (1.06, 1.69) | 1.83 (0.28, 3.39) |
| 25(OH)D | |||
| T1 | Ref. | Ref. | Ref. |
| T2 | 0.88 (0.71, 1.09) | 0.96 (0.77, 1.20) | 0.11 (−1.42, 1.64) |
| T3 | 0.94 (0.75, 1.17) | 0.82 (0.65, 1.05) | −0.93 (−2.49, 0.64) |
| Vit D3 | |||
| T1 | Ref. | Ref. | Ref. |
| T2 | 0.85 (0.69, 1.06) | 0.97 (0.77, 1.21) | −0.23 (−1.77, 1.31) |
| T3 | 0.94 (0.75, 1.17) | 0.83 (0.65, 1.06) | −0.60 (−2.17, 0.96) |
| Vit E | |||
| T1 | Ref. | Ref. | Ref. |
| T2 | 0.97 (0.78, 1.20) | 1.00 (0.79, 1.26) | 0.46 (−1.06, 1.98) |
| T3 | 1.23 (0.99, 1.54) | 1.24 (0.98, 1.58) | 0.77 (−0.80, 2.34) |
| Vit K | |||
| T1 | Ref. | Ref. | Ref. |
| T2 | 1.20 (0.96, 1.49) | 1.11 (0.88, 1.40) | −0.81 (−2.35, 0.73) |
| T3 | 1.41 (1.13, 1.76) | 1.27 (1.00, 1.61) | −0.18 (−1.73, 1.37) |
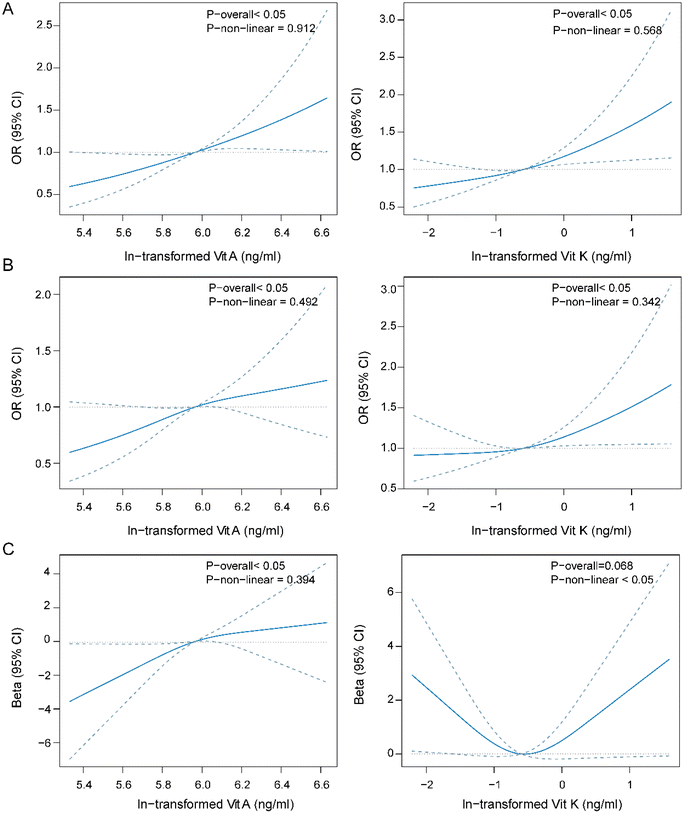
The dose–response curve of fat-soluble vitamins and the menstrual cycle
To explore the nonlinear relationship between fat-soluble vitamins and the menstrual cycle, we employed a restricted cubic spline regression model. Vitamins A and K were positively associated with irregular and long menstrual cycles in a linear manner (P-overall < 0.05, P-nonlinear > 0.05, Fig. 2A and B). Significant positive linear association was also found between vitamin E and irregular menstrual cycle (ESI Fig. 3†).With the average menstrual cycle length, vitamin A had a positive linear association. Vitamin K had a borderline significant association with the average menstrual cycle length, and the dose–response curve was U-shaped (Fig. 2C).
Association between fat-soluble vitamin combined levels and interactions with menstrual cycle characteristics
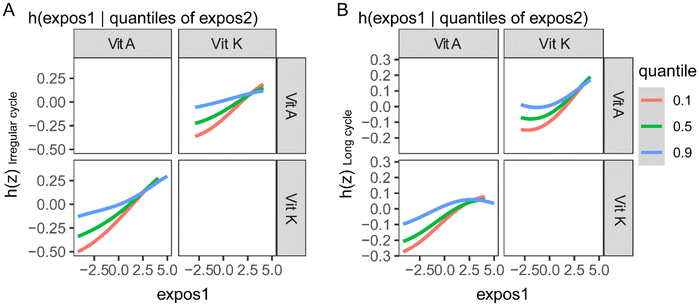
The BKMR model was also used to explore the association between fat-soluble vitamins and menstrual cycle characteristics by controlling the serum levels of other vitamins. After all the other fat-soluble vitamins were fixed at their median levels, vitamin A and vitamin K were found to be positively associated with irregular menstrual cycles and long menstrual cycles (ESI Fig. 4A and B†). In the analysis of the association between fat-soluble vitamins and the menstrual cycle length, vitamin A was found to be positively associated with it (ESI Fig. 4C†). 25(OH)D showed inverse trends with irregular menstrual cycles and long menstrual cycles (ESI Fig. 4†). In the exploration of the interactions between fat-soluble vitamins in their association with menstrual cycle characteristics, vitamin A and vitamin K showed evident interactions (Fig. 3).Sensitivity analysis
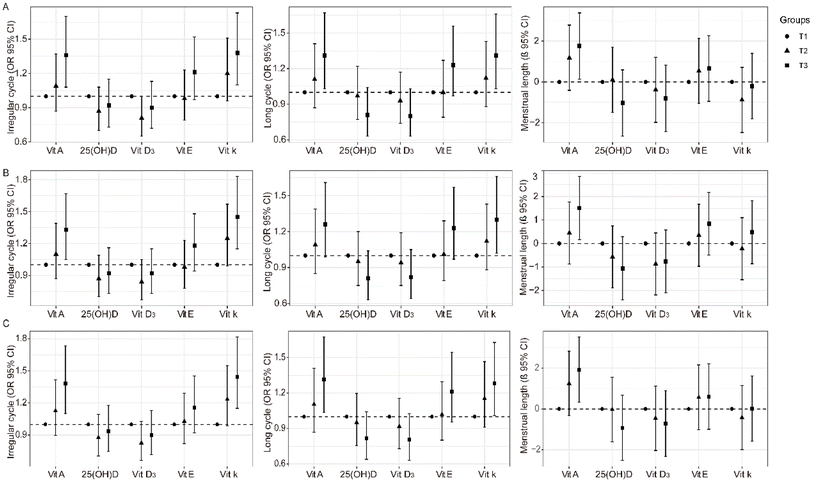
As thyroid plays a substantial role in the reproductive system and affects menstrual characteristics, we further excluded participants with abnormal thyroid function. Positive association of vitamin A or vitamin K with the irregular menstrual cycle and long menstrual cycle was also evident (Fig. 4A and Table S1†). Vitamin A was found to be associated with the average menstrual cycle length (Fig. 4A and Table S1†).PCOS is characterized by a long menstrual cycle, and the causes of this disease are complex. After the exclusion of patients with PCOS or PCO, fat-soluble vitamins A and K were still associated with irregular menstrual cycles and long menstrual cycles (Fig. 4B and Table S2†).
To reduce the bias of decreased fertility induced by older age, we further excluded participants aged 40 years and older. The association of vitamins A and K with the irregular menstrual cycle and long menstrual cycle remained stable (Fig. 4C and Table S3†).
Discussion
Fat-soluble vitamins are necessary nutrients for human beings but their association with the female reproductive system is largely unexplored. Here, the relationships between fat-soluble vitamins and menstrual cycles were evaluated. Vitamin A was found to be positively associated with the irregular menstrual cycle, long menstrual cycle, and average menstrual cycle length in a linear dose–response manner. We also found that vitamin K had positive association with irregular menstrual cycles and long menstrual cycles. Vitamins A and K had interactions in their association with menstrual cycles. The positive association of vitamin A and vitamin K with the menstrual cycle was independent of the thyroid function, PCOS status, and older age of the participants.Vitamin A with its three forms, namely, retinol, retinal, and retinoic acid, was reported to take part in immune function, night vision, and skin health.36 In this study, we found that vitamin A was positively associated with an irregular menstrual cycle, especially a long menstrual cycle. The results were robust considering the thyroid function, PCOS status, and older age of the participants. The association between vitamin A and menstrual cycle characteristics has rarely been studied. There is some evidence of the physiological role of vitamin A in reducing the presence of premenstrual syndrome as well as the maintenance and regulation of the cycling endometrium.22 However, studies have also shown that retinol augments androgen production in PCOS cells and is positively related to insulin resistance biomarkers,23,37,38 which indicates the role of vitamin A in menstrual disorders. A serious concern regarding vitamin A has also been raised, as studies have found that excessive vitamin A intake in the first trimester is associated with miscarriage and congenital birth defects in the eye, skull, lung, and heart.14,39 Despite the potentially toxic effects, the upper intake levels (ULs) of vitamin A are still not clear. Routine vitamin A supplementation is not recommended except in regions with vitamin A deficiency. Although vitamin A deficiency is still a public health concern in some developing countries and is closely related to maternal and child health,40,41 the effect of clinical deficiency of vitamin A on the menstrual cycle has not raised enough concern and should be further explored in vitamin A deficient countries. In our study, only 8 participants (0.3%) were deficient in vitamin A (<200 ng ml−1) and most of the participants had serum levels above 300 ng ml−1, which means that vitamin A deficiency was not a health concern in this study and that supplementation with vitamin A should be done with caution in our reproductive-age population of women.
Vitamin K, known to be a key element in the synthesis of active coagulation factors and involved in blood clotting, was also reported to have a biological function in reproduction.10 In our study, vitamin K was found to be positively associated with the irregular menstrual cycle, especially the long menstrual cycle, which has not been reported before. In the context of the female reproductive system, vitamin K antagonists were reported to increase the risk of heavy menstrual bleeding.42 In addition, vitamin K was reported to reduce menstrual pain and relieve nausea during pregnancy.43 The main molecular pathway of vitamin K, PXR transcriptional activation, is present in the tissue involved in the reproductive system.10 Vitamin K also regulates the HPG axis and induces the synthesis of steroids in ovaries, and dietary vitamin K supplementation was reported to increase plasma testosterone.10 The disorder of sex hormones could induce an irregular menstrual cycle. The daily intake of vitamin K is usually from green leafy vegetables, vegetable oil, and grains, which are adequate for the recommended intake. In our study, only 0.6% of participants had serum vitamin K levels <0.13 ng ml−1. Vitamin K deficiency contributes to severe bleeding, osteoporosis, and cardiovascular disease, but its association with the menstrual cycle was not explored before.44 Vitamin K is considered to have low potential toxicity as no obvious adverse effects of vitamin K have been reported in animal and human studies. Although there are no tolerable ULs for vitamin K, the potential adverse influence of excessive vitamin K on menstrual cycles should be considered with caution and further explored.
We found that serum 25(OH)D and vitamin D3 were significantly lower in the long-menstrual-cycle group than in the normal group, but only a negative trend was found between 25(OH)D and the menstrual cycles after adjusting for other covariates. To the best of our knowledge, this study is the first investigation conducted in a representative Chinese female population with a large sample size. The relationship between vitamin D and menstrual cycles has been explored in Polish and U.S. populations, but the findings were not consistent.20,21,45 The heterogeneity might be explained by the populations of disparate ethnicities and ages, different confounding factors, and variations in vitamin D levels. In our study, 83.8% of the childbearing-aged women were deficient in serum vitamin D. However, in previous studies, the prevalence of vitamin D deficiency was much lower.20,21,45 On the condition that most of the study population is deficient in vitamin D levels, the association between sufficient vitamin D and menstrual cycles could be underestimated. Further studies are warranted to explore the relationship between sufficient vitamin D or vitamin D supplementation and menstrual cycle characteristics.
This study has several strengths. Our study provides evidence of connections between fat-soluble vitamins and menstrual cycles, which have not been comprehensively explored before. The sample size was large, and the study was conducted in a representative population of Chinese women of childbearing age. We excluded participants with one-sided ovary removal, who were breastfeeding, and using female hormones, which could greatly alter menstrual cycle characteristics. The potential bias from the thyroid function, PCOS, and older age of participants was considered in the sensitivity analysis, and the results remained robust. We also included an evaluation of the dose–response curve and interactions between vitamins, to address the limitations of previous studies.
One of the limitations of this study is that other factors, including exercise, supplements, medicine usage, dietary patterns, and psychological factors which have been reported to be associated with vitamin levels or the menstrual cycle,46–50 were not considered and should be fully evaluated in future research. Menstrual characteristics were obtained using questionnaires, which could lead to inaccurate estimation and induce recall bias. Additionally, menstrual bleeding is an important source of fat-soluble vitamin discharge. Although we included days since the first day of the last period, the basic serum vitamin D levels with consistent timing of sampling were important. In our study, the most common type of irregular menstrual cycle among women of childbearing age was the long menstrual cycle, which is consistent with other studies.29 The sample size of short menstrual cycles was small in our study, and its relationship with fat-soluble vitamins was not further explored. The menstrual blood volume was not precisely estimated and was not considered in our study. In addition, this study was cross-sectionally designed and not informative regarding the temporal relationships between fat-soluble vitamins and menstrual cycle characteristics.
Conclusion
In conclusion, vitamin A and vitamin K have significantly positive association with irregular menstrual cycles and long cycles in a linear dose–response manner, and there are interactions between these two vitamins. Vitamin A is also positively associated with the average menstrual cycle length. Given the exploratory nature of this study, more investigations are warranted to identify their causal relationships and elucidate the underlying mechanisms.Author contributions
Yuqing Zhang: data curation, formal analysis, writing – original draft, and writing – review & editing. Jing Kong and Xiaohong Jiang: data curation and writing – review & editing. Jiangping Wu: supervision and writing – review & editing. Xiaoli Wu: funding acquisition, project administration, supervision, and writing – review & editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant no. 82103793 and 81801413).References
- S. L. Stevens, Fat-Soluble Vitamins, Nurs. Clin. North Am., 2021, 56, 33–45 CrossRef PubMed.
- N. Martin Ask, M. Leung, R. Radhakrishnan and G. P. Lobo, Vitamin A Transporters in Visual Function: A Mini Review on Membrane Receptors for Dietary Vitamin A Uptake, Storage, and Transport to the Eye, Nutrients, 2021, 13, 3987 CrossRef CAS PubMed.
- I. R. Reid, M. J. Bolland and A. Grey, Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis, Lancet, 2014, 383, 146–155 CrossRef CAS PubMed.
- R. Ricciarelli, F. Argellati, M. A. Pronzato and C. Domenicotti, Vitamin E and neurodegenerative diseases, Mol. Aspects Med., 2007, 28, 591–606 CrossRef CAS PubMed.
- M. J. Shearer, X. Fu and S. L. Booth, Vitamin K nutrition, metabolism, and requirements: current concepts and future research, Adv. Nutr., 2012, 3, 182–195 CrossRef CAS PubMed.
- Y. Jiang, L. Chen, R. N. Taylor, C. Li and X. Zhou, Physiological and pathological implications of retinoid action in the endometrium, J. Endocrinol., 2018, 236, R169–R188 CAS.
- Z. Merhi, A. Doswell, K. Krebs and M. Cipolla, Vitamin D Alters Genes Involved in Follicular Development and Steroidogenesis in Human Cumulus Granulosa Cells, J. Clin. Endocrinol. Metab., 2014, 99, E1137–E1145 CrossRef CAS PubMed.
- S. L. Mumford, R. W. Browne, K. C. Schliep, J. Schmelzer, T. C. Plowden, K. A. Michels, L. A. Sjaarda, S. M. Zarek, N. J. Perkins, L. C. Messer, R. G. Radin, J. Wactawski-Wende and E. F. Schisterman, Serum Antioxidants Are Associated with Serum Reproductive Hormones and Ovulation among Healthy Women, J. Nutr., 2016, 146, 98–106 CrossRef PubMed.
- E. Lerchbaum and B. Obermayer-Pietsch, Vitamin D and fertility: a systematic review, Eur. J. Endocrinol., 2012, 166, 765–778 CrossRef CAS PubMed.
- S. Beato, F. J. Toledo-Solís and I. Fernández, Vitamin K in Vertebrates’ Reproduction: Further Puzzling Pieces of Evidence from Teleost Fish Species, Biomolecules, 2020, 10, 1303 CrossRef CAS PubMed.
- M. Azar, A. Basu, A. J. Jenkins, A. J. Nankervis, K. F. Hanssen, H. Scholz, T. Henriksen, S. K. Garg, S. M. Hammad, J. A. Scardo, C. E. Aston and T. J. Lyons, Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: a longitudinal study, Diabetes care, 2011, 34, 1258–1264 CrossRef CAS PubMed.
- S. Agarwal, O. Kovilam and D. K. Agrawal, Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review, Crit. Rev. Food Sci. Nutr., 2018, 58, 755–769 CrossRef CAS PubMed.
- H. Ma, Z. Qiao, N. Li, Y. Zhao and S. Zhang, The relationship between changes in vitamin A, vitamin E, and oxidative stress levels, and pregnancy outcomes in patients with gestational diabetes mellitus, Ann. Palliat. Med., 2021, 10, 6630–6636 CrossRef PubMed.
- A. H. Piersma, E. V. Hessel and Y. C. Staal, Retinoic acid in developmental toxicology: teratogen, morphogen and biomarker, Reprod. Toxicol., 2017, 72, 53–61 CrossRef CAS PubMed.
- S. P. Murphy, Dietary reference intakes for the US and Canada: Update on implications for nutrient databases, J. Food Compos. Anal., 2002, 15, 411–417 CrossRef CAS.
- C. N. Society, Dietary reference intakes for Chinese, 2013 Search PubMed.
- C. M. Small, A. K. Manatunga, M. Klein, H. S. Feigelson, C. E. Dominguez, R. McChesney and M. J. E. Marcus, Menstrual cycle characteristics: associations with fertility and spontaneous abortion, Epidemiology, 2006, 52–60 CrossRef PubMed.
- M. Clagett-Dame and H. F. DeLuca, The role of vitamin A in mammalian reproduction and embryonic development, Annu. Rev. Nutr., 2002, 22, 347–381 CrossRef CAS PubMed.
- S. S. Mohd Mutalip, S. Ab-Rahim and M. H. Rajikin, Vitamin E as an Antioxidant in Female Reproductive Health, Antioxidants, 2018, 7, 22 CrossRef PubMed.
- K. Łagowska, The Relationship between Vitamin D Status and the Menstrual Cycle in Young Women: A Preliminary Study, Nutrients, 2018, 10, 1729 CrossRef PubMed.
- A. M. Jukic, A. Z. Steiner and D. D. Baird, Lower plasma 25-hydroxyvitamin D is associated with irregular menstrual cycles in a cross-sectional study, Reprod. Biol. Endocrinol., 2015, 13, 20 CrossRef PubMed.
- A. Bahrami, H. Bahrami-Taghanaki, Z. Khorasanchi, A. Timar, N. Jaberi, E. Azaryan, M. Tayefi, G. A. Ferns, H. R. Sadeghnia and M. Ghayour-Mobarhan, Menstrual problems in adolescence: relationship to serum vitamins A and E, and systemic inflammation, Arch. Gynecol. Obstet., 2020, 301, 189–197 CrossRef CAS PubMed.
- J. K. Wickenheisser, V. L. Nelson-DeGrave, K. L. Hendricks, R. S. Legro, J. F. Strauss 3rd and J. M. McAllister, Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome, J. Clin. Endocrinol. Metab., 2005, 90, 4858–4865 CrossRef CAS PubMed.
- S. A. Blondin, E. H. Yeung, S. L. Mumford, C. Zhang, R. W. Browne, J. Wactawski-Wende and E. F. Schisterman, Serum Retinol and Carotenoids in Association with Biomarkers of Insulin Resistance among Premenopausal Women, ISRN Nutr., 2013, 2013, 619516 Search PubMed.
- L. Nijhawan, M. Janodia, B. Muddukrishna, K. Bhat, K. Bairy, N. Udupa and P. Musmade, Informed consent: Issues and challenges, J. Adv. Pharm. Technol. Res., 2013, 4, 134–140 CrossRef PubMed.
- W. H. Organization, Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations, World Health Organization, 2011 Search PubMed.
- K. Amrein, M. Scherkl, M. Hoffmann, S. Neuwersch-Sommeregger, M. Köstenberger, A. Tmava Berisha, G. Martucci, S. Pilz and O. Malle, Vitamin D deficiency 2.0: an update on the current status worldwide, Eur. J. Clin. Nutr., 2020, 74, 1498–1513 CrossRef CAS PubMed.
- M. G. Traber, Vitamin E inadequacy in humans: causes and consequences, Adv. Nutr., 2014, 5, 503–514 CrossRef CAS PubMed.
- W. Zhou, L. Zhang, C. Tong, F. Fang, S. Zhao, Y. Tian, Y. Tao and J. Zhang, Plasma Perfluoroalkyl and Polyfluoroalkyl Substances Concentration and Menstrual Cycle Characteristics in Preconception Women, Environ. Health Perspect., 2017, 125, 067012 CrossRef PubMed.
- I. S. Fraser, H. O. Critchley, M. Broder and M. G. Munro, The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding, Semin. Reprod. Med., 2011, 29, 383–390 CrossRef PubMed.
- C. M. Small, A. K. Manatunga, M. Klein, H. S. Feigelson, C. E. Dominguez, R. McChesney and M. Marcus, Menstrual cycle characteristics: associations with fertility and spontaneous abortion, Epidemiology, 2006, 17, 52–60 CrossRef PubMed.
- D. A. Koutras, Disturbances of menstruation in thyroid disease, Ann. N. Y. Acad. Sci., 1997, 816, 280–284 CrossRef CAS PubMed.
- J. F. Bobb, L. Valeri, B. Claus Henn, D. C. Christiani, R. O. Wright, M. Mazumdar, J. J. Godleski and B. A. Coull, Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures, Biostatistics, 2015, 16, 493–508 CrossRef PubMed.
- D. A. Koutras, Disturbances of menstruation in thyroid disease, Ann. N. Y. Acad. Sci., 1997, 816, 280–284 CrossRef CAS PubMed.
- K. Walker, A. H. Decherney and R. Saunders, Menstrual dysfunction in PCOS, Clin. Obstet. Gynecol., 2021, 64, 119–125 CrossRef PubMed.
- D. S. Goodman, Vitamin A and retinoids in health and disease, N. Engl. J. Med., 1984, 310, 1023–1031 CrossRef CAS PubMed.
- X. Pang, S. Yang, X. Guo, H. Li, Y. Zhang, C. Wei, Y. Wang, C. Sun and Y. Li, The Association and Mediating Biomarkers of Serum Retinol in Influencing the Development of Type 2 Diabetes: A Prospective Cohort Study in Middle-Aged and Elderly Population, Front. Nutr., 2022, 9, 831950 CrossRef PubMed.
- S. K. Hutchison, C. Harrison, N. Stepto, C. Meyer and H. J. Teede, Retinol-binding protein 4 and insulin resistance in polycystic ovary syndrome, Diabetes care, 2008, 31, 1427–1432 CrossRef CAS PubMed.
- R. K. Miller, A. G. Hendrickx, J. L. Mills, H. Hummler and U. W. Wiegand, Periconceptional vitamin A use: how much is teratogenic?, Reprod. Toxicol., 1998, 12, 75–88 CrossRef CAS PubMed.
- M. S. Radhika, P. Bhaskaram, N. Balakrishna, B. A. Ramalakshmi, S. Devi and B. S. Kumar, Effects of vitamin A deficiency during pregnancy on maternal and child health, BJOG, 2002, 109, 689–693 CrossRef CAS PubMed.
- S. Akhtar, A. Ahmed, M. A. Randhawa, S. Atukorala, N. Arlappa, T. Ismail and Z. Ali, Prevalence of vitamin A deficiency in South Asia: causes, outcomes, and possible remedies, J. Health. Popul. Nutr., 2013, 31, 413–423 Search PubMed.
- J. Beyer-Westendorf and S. Marten, Reproductive issues in women on direct oral anticoagulants, Res. Pract. Thromb. Haemostasis, 2021, 5, e12512 CAS.
- F. Tarkesh, B. Namavar Jahromi, N. Hejazi and H. Tabatabaee, Beneficial health effects of Menaquinone-7 on body composition, glycemic indices, lipid profile, and endocrine markers in polycystic ovary syndrome patients, Food Sci. Nutr., 2020, 8, 5612–5621 CrossRef CAS PubMed.
- R. E. Eden and J. M. Coviello, StatPearls, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island (FL), 2022 Search PubMed.
- A. M. Z. Jukic, K. Upson, Q. E. Harmon and D. D. Baird, Increasing serum 25-hydroxyvitamin D is associated with reduced odds of long menstrual cycles in a cross-sectional study of African American women, Fertil. Steril., 2016, 106, 172–179 CrossRef CAS PubMed.
- J. D. Prescott, V. J. Drake and J. F. Stevens, Medications and Micronutrients: Identifying Clinically Relevant Interactions and Addressing Nutritional Needs, J. Pharm. Technol., 2018, 34, 216–230 CrossRef CAS PubMed.
- V. Ganji, B. Martineau and W. E. Van Fleit, Association of serum vitamin D concentrations with dietary patterns in children and adolescents, Nutr. J., 2018, 17, 58 CrossRef PubMed.
- K. P. Dzik, T. Grzywacz, M. Łuszczyk, S. Kujach, D. J. Flis and J. J. Kaczor, Single bout of exercise triggers the increase of vitamin D blood concentration in adolescent trained boys: a pilot study, Sci. Rep., 2022, 12, 1825 CrossRef CAS PubMed.
- J. Bae, S. Park and J. W. Kwon, Factors associated with menstrual cycle irregularity and menopause, BMC women's health, 2018, 18, 36 CrossRef PubMed.
- C. L. Rock, D. W. Gorenflo, A. Drewnowski and M. A. Demitrack, Nutritional characteristics, eating pathology, and hormonal status in young women, Am. J. Clin. Nutr., 1996, 64, 566–571 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo02765h |
| This journal is © The Royal Society of Chemistry 2023 |