Open Access Article
Open Access ArticleSimultaneous production of proteases and antioxidant biopeptides by solid-state fermentation†
Luciane Maria
Colla
 *a,
Christian Oliveira
Reinehr
a,
Paola Gouvêa
Manfredini
a,
Vítor Augusto
Farina Cavanhi
b and
Jorge Alberto
Vieira Costa
*a,
Christian Oliveira
Reinehr
a,
Paola Gouvêa
Manfredini
a,
Vítor Augusto
Farina Cavanhi
b and
Jorge Alberto
Vieira Costa
 a
a
aGraduation Program in Food Science and Technology, University of Passo Fundo (UPF), Passo Fundo, Brazil. E-mail: lmcolla@upf.br
bChemical Engineering Course, University of Passo Fundo (UPF), Passo Fundo, Brazil
First published on 29th August 2023
Abstract
Usually, biopeptides and proteases are bioproducts produced in separate processes. However, through solid-state fermentation, they can be produced simultaneously, using agroindustrial by-products as substrates, reducing the environmental pollution and contributing to a circular bioeconomy. We aimed at the simultaneous production of proteases and biopeptides, using Aspergillus niger grown in soy bran and soy husk (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). The variables pH, moisture and nitrogen concentration were studied using Full Factorial Design 23. The best and simultaneous production of the biocompounds was obtained at pH 7, 50% moisture and 0.5% sodium nitrate as a nitrogen source, with 677 U g−1 proteases and 4154 μg mL−1 soluble proteins in 48 h, and an antioxidant activity of 2875.6 (mg mL−1 of Trolox equivalents) in 96 h of fermentation, with productivity confirmed in a double production scale. The production of these two biocompounds simultaneously in SSF has never been reported in the literature, thus highlighting the novelty and importance of this work.
30). The variables pH, moisture and nitrogen concentration were studied using Full Factorial Design 23. The best and simultaneous production of the biocompounds was obtained at pH 7, 50% moisture and 0.5% sodium nitrate as a nitrogen source, with 677 U g−1 proteases and 4154 μg mL−1 soluble proteins in 48 h, and an antioxidant activity of 2875.6 (mg mL−1 of Trolox equivalents) in 96 h of fermentation, with productivity confirmed in a double production scale. The production of these two biocompounds simultaneously in SSF has never been reported in the literature, thus highlighting the novelty and importance of this work.
Sustainability spotlightThis article presents sustainable progress by using raw materials that are agro-industrial waste to obtain high-value products, which have application in the food and pharmaceutical industry, connecting with the circular bioeconomy. The production of antioxidant enzymes and biopeptides via Solid State Fermentation allows the valuation of these agro-industrial residues. It relates to SDG 2 (zero hunger and sustainable agriculture) and 3 (health and well-being), using agriculture wastes and producing compounds that can be used in the development of functional foods. |
Introduction
In the last few decades many foods have been documented to have antioxidant, antihypertensive, antimicrobial and immunomodulatory properties, with potential application for food preservation, microbial inhibition and medicinal applications.1 These bioactive compounds found in foods have been considered a trend in the development of new foods and nutraceuticals. Bioactive peptides are examples of these compounds, derived from proteins.2 These biopeptides could be obtained from the hydrolysis or fermentation of proteins of vegetables such as cereals or plant wastes,3,4 animal proteins such as whey5 or proteins of microbial origin, such as algae.6–8Bioactive peptides are organic molecules usually formed by 2 to 20 amino acid residues, attached by covalent bonds known as peptide bonds,2,9 with molecular masses usually lower than 6000 Da.10 They can be released by enzymatic hydrolysis of proteins and have several physiological activities in the body, such as antioxidant, antihypertensive, antitumor, antimicrobial, and antithrombotic activities, as well as in gastrointestinal health, among others. Their activity depends on the chain length, composition, and amino acid sequence, and they can be used to improve food functional properties.1–3,7,11 Currently, a database called the ‘BIOPEP-UWM Virtual database’ reported 4474 bioactive peptides, 533 sensory peptides and 163 virtual peptides, and this database is being frequently updated.12,13
Bioactive peptides are formed by hydrolysis catalysed by proteases,14,15 which are important enzymes used and produced by the biotechnology industry accounting for about 60% of the global market, with applications in detergents, leather processing, food and feed processing, pharmaceuticals, chemicals, and waste treatment.16 In foods, proteases have two main applications: in the processing of traditional food products and in the processing of new protein-based ingredients, which due to their characteristics can be called functional foods.17
Proteases can be isolated from plants (papain and bromelain),16 animals (pepsin, trypsin and chymotrypsin) and microorganisms.3,18 The use of microorganisms to produce proteases or to promote the fermentation of foods with the aim of obtaining biopeptides has advantages because of the wide and diverse types of proteases that can be obtained in large quantities, with the possibility of genetic manipulation for obtaining enzymes with specific characteristics.3,19
Microbial fermentation of bran substrates is an efficient, environmentally friendly bioprocess for increasing the release of bioactive compounds. The microorganisms involved in the bioprocess generate new compounds during fermentation.20 Zheng et al.18 and Pokora et al.15 mentioned the production of proteases by fungi and yeasts, but it is well known that the bioprocesses can produce a wide range of bioproducts, using submerged fermentation (SmF) or solid-state fermentation (SSF). Solid-state fermentation has been used to produce several enzymes, such as amylases,21 lipases,22 cellulases and xylanases,23,24 and proteases.24,25 Besides, other bioproducts, such as organic acids,26 phenolic compounds,27,28 dyes,29 and biopeptides,30,31 can be obtained. Studies show that it can also be used to improve the functional properties of foods and reduce their anti-nutritional components, such as increasing solubility and digestibility and reducing the amount of trypsin inhibitors and allergens, mainly from soy.32,33
In solid-state fermentation (SSF) the microorganisms grow on the surface of wet solid substrates in the absence of free flow of water, in which the components of the substrates are hydrolysed by enzymes excreted by the microbial population.34,35 SSF is an efficient process that has as its main advantages the use of agro-food and by-products as substrates for the action of microorganisms35 and the production of proteases, biopeptides and other metabolites in the same process.30,36,37 With this, there is a reduction in costs and process time, valuation of these residues and by-products and, consequently, greater possibility of acceptance by the industry.
According to FAO38 the food loss from post-harvest to distribution in 2016 was 8–9% for cereals and pulses, ∼23% for fruits and vegetables and up to 25% for roots, tubers and oil-crops. This document explains that reducing food loss and waste is seen as a way to lower production costs, improve food security and nutrition, and contribute towards environmental sustainability, notably by easing the pressure on natural resources and decreasing greenhouse gas (GHG) emissions. Considering that there was a challenge of sustainably feeding a world population projected to reach almost 10 billion in 2050, it is very important to minimize food loss and waste. Food loss and waste has become a major global issue and is enshrined in SDG 12 (responsible consumption and production), which even sets a specific target related to the reduction of food loss and waste. Thus, the search for alternatives for reuse and generation of new products is very important to reduce waste in the food industry and minimize environmental impacts, contributing to the development of the circular bioeconomy.39
We aim to evaluate the simultaneous production of proteases and biopeptides by Aspergillus niger in solid-state fermentation. Soy husks and soy bran were used as substrates, in order to induce the production of hydrolytic fungal enzymes, making the process less expensive and more attractive, in accordance with the precepts of the circular bioeconomy. Proteases can be applied in several types of industries, and biopeptides can be used in the production of functional foods or nutraceuticals, in the substitution of synthetic drugs, and in the development of smart packaging. Although solid-state fermentation using fungi for enzyme production is extensively reported in the literature, the concomitant production of proteases and bioactive peptides by these microorganisms in a single process remains unreported.
2 Materials and methods
2.1 Agroindustrial by-products
The substrates, soy husk and soy bran, were supplied by the company BSBios, located in Passo Fundo and kept frozen at −10 °C until their use. The chemical composition of the substrates was evaluated for carbohydrate, protein, lipid, ash and moisture content, using the methodology described by the AOAC.40 The determination of proteins was accomplished using the micro-Kjeldahl total nitrogen determination method with a conversion factor of 6.25; the ashes were determined by a gravimetric method in a muffle furnace (550–600 °C) and the moisture content was determined using a gravimetric method in an oven (105 °C). The lipids were extracted using a Soxhlet method, and carbohydrates were determined by difference. The particle size was determined by the AOAC 965.22 method by sieving using sieves in the sizes: 10 mesh (1.70 mm), 20 mesh (0.850 mm), 28 mesh (0.600 mm), 48 mesh (0.300 mm), 80 mesh (0.180 mm), 140 mesh (0.160 mm) and 170 mesh (0.090 mm).2.2 Microorganism and inoculum preparation
The fungus Aspergillus niger O4, identified as Aspergillus niger DAOM (100% identity, GenBank accession number: KC545858.1) by Colla et al. (2015), was used.41 The fungus was stored and kept in a Potato Dextrose Agar (PDA) medium, under refrigeration at 4 °C. This fungus is considered a microorganism producer of non-toxic and safe bioproduct, designated as GRAS (generally recognized as safe), and can therefore be used to obtain products for human and animal nutrition. The inoculum was prepared in 100 mL sterile PDA culture medium and incubated for 5 days at 30 °C. After this period, 50 mL of a 0.01% Tween 80 solution and 3 glass beads were added to it and mixed in order to obtain a spore suspension, which was filtered through sterile cotton gauze and used to inoculate the substrates for the solid-state fermentation.2.3 Experimental design and substrates preparation
The work was carried out in two stages. In the first stage, pH, moisture and initial nitrogen concentration were studied as variables. In the second stage, a scale-up was performed, and the pH was varied to evaluate the influence on the production of metabolites. For the preparation of the culture medium for the SSF, the substrates were the agro-industrial by-products soy husk and soy bran, in a proportion of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30%, determined by previous studies (data not shown).
30%, determined by previous studies (data not shown).
For the first stage, the cultivation variables studied were pH (5, 6 and 7), moisture (50%, 60% and 70%) and sodium nitrate concentration (0.5%, 1.25% and 2%), through a Full-Factorial Design – FFD 23 with central points, as shown in Table 1, totaling 11 experiments. The pH was adjusted in the culture media with solutions of 1 M NaOH or 1.5 M H2SO4. To every 40 g of cultivation medium was added 30 mL of saline solution42 and the final moisture was adjusted with distilled water.
| Experiment | X 1 (initial pH) | X 2 (moisture %) | X 3 (sodium nitrate %) |
|---|---|---|---|
| 1 | −1 (5) | −1 (50) | −1 (0.5) |
| 2 | +1 (7) | −1 (50) | −1 (0.5) |
| 3 | −1 (5) | +1 (70) | −1 (0.5) |
| 4 | +1 (7) | +1 (70) | −1 (0.5) |
| 5 | −1 (5) | −1 (50) | +1 (2) |
| 6 | +1 (7) | −1 (50) | +1 (2) |
| 7 | −1 (5) | +1 (70) | +1 (2) |
| 8 | +1 (7) | +1 (70) | +1 (2) |
| 9 | 0 (6) | 0 (60) | 0 (1.25) |
| 10 | 0 (6) | 0 (60) | 0 (1.25) |
| 11 | 0 (6) | 0 (60) | 0 (1.25) |
The substrates were inoculated with the spore suspension in order to obtain 106 spores per g. The experiments were carried out in beakers of 250 mL and incubated at 30 °C for 7 days in an incubator. Samples of 5 g of each cultivation medium were taken every 24 h and frozen at −20 °C for later extraction of bio-compounds and analytical determinations.
The second stage of the experiment was accomplished using 80 g of media prepared in the best condition obtained in stage I, with pH variation (6, 7 and 8) to assess if this factor could still increase the production of proteases and biopeptides. The experiments were carried out in 500 mL beakers and incubated at 30 °C for 4 days in an incubator. Samples of 14 g from each cultivation medium were taken at 0 and 96 hours and frozen at −20 °C until being used for the analytical determinations. The same relation of saline solution of the first stage was used in the second stage.
2.4 Proteases and biopeptides extraction
The method described by Ortiz et al.43 with modifications was used for the extraction of proteases and biopeptides. 1 g of each sample was collected and added to 10 mL of distilled water, mixed in a thermostatic bath with agitation at 28 °C and 180 rpm for 1 hour. Afterwards, the contents were filtered through cotton gauze and centrifuged at 5000 rpm for 30 min. The supernatant was transferred to a sample bottle, identified and frozen at −20 °C until being used in the determination of protease activity and antioxidant potentials.2.5 Protease activity
The protease activity was measured using azocasein according to Charney and Tomarelli,44 with modifications. The reaction was carried out with incubation at 37 °C of the mixture containing 500 μL of the extract with 500 μL of azocasein 0.5% (w/v), in pH 7 buffer, and it was interrupted by adding 500 μL of 10% trichloroacetic acid (TCA). The test tubes were centrifuged at 3000 rpm for 10 min. An aliquot of 1.0 mL of the supernatant was neutralized with 1.0 mL of 5 M KOH. The absorbance at 430 nm was measured with a spectrophotometer. One unit of enzymatic activity (U) is defined as the amount of enzyme required to increase in 0.01 min−1 the absorbance at 430 nm, under the test conditions described. The reaction blank was tested using a pH 7 buffer solution replacing the enzymatic extract. In addition, for each sample, a control tube was made, adding 10% TCA before the enzymatic extract.2.6 Antioxidant activity
The ABTS 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) method described by Re et al.45 was used in the determination of the total antioxidant activity, by the capture of the free radical ABTS+. The cationic radical ABTS+ was prepared from the reaction of an ABTS stock solution (7 mmol L−1) with a potassium persulfate solution (2.45 mmol L−1), at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v. This solution was kept in the dark, at room temperature, for at least 12 to 16 h before use.
1 v/v. This solution was kept in the dark, at room temperature, for at least 12 to 16 h before use.
The UV-vis spectrophotometer was standardized with ethanol at 734 nm to read the samples. Thereafter, the ABTS+ solution was diluted in 96% ethanol to obtain an absorbance of 0.70 nm (+0.005 nm). In an environment shielded from light, 1 mL of ABTS radical diluted in 10 μL of the extract sample was added (10 μL of distilled water for the blank tube), followed by stirring for homogenization for 5 seconds, and the solution was kept in the dark for 6 minutes to react until performing the absorbance measurements of the samples.
The results were described in two ways: from a standard curve (Trolox equivalents) and by the percentage of inhibition of the ABTS radical, calculated using eqn (1).
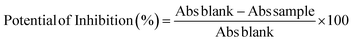 | (1) |
The yield (Y) of each bioreactor was calculated using eqn (2).
| Y = Pf − P0 | (2) |
2.7 Proteins soluble in acid medium
The determination was made with aliquots of 1 mL of the extracts adding 9 mL of a 6.25% trichloroacetic acid solution (TCA), being left at rest for 10 min to inactivate the protease. After this procedure, the samples were centrifuged for 5 min at 5000 rpm to remove the insoluble material precipitated by TCA. The soluble protein content of the supernatant was determined using the Folin–Lowry method,46 with the standard curve using albumin.2.8 Data treatment and statistical analysis
Data were tabulated to the calculations of media and standard deviations and analysis of variance (ANOVA) was used to determine significant differences (p < 0.05). Differences between means were determined by the Tukey test.3 Results and discussion
3.1 Centesimal composition of substrates
The centesimal compositions of the agro-industrial residues and by-products used as substrates in the SSF for the concomitant production of proteases and biopeptides are presented in Table 2.| Chemical component | Soy bran | Soy husk |
|---|---|---|
| a Mean values ± standard deviation. Means followed by the same letter, in the same line, do not differ from each other, by the Tukey test, at 5% significance. * Carbohydrate was calculated from the difference. | ||
| Moisture | 11.76 ± 0.75a | 6.16 ± 0.07b |
| Ashes | 5.72 ± 0.05a | 4.27 ± 0.06b |
| Proteins | 40.68 ± 1.81a | 9.53 ± 0.21c |
| Lipids | 2.52 ± 0.27b | 2.83 ± 0.14b |
| Carbohydrates | 39.32 ± 2.74c* | 77.21 ± 0.21a* |
The composition of substrates used in solid-state fermentation is one of the main factors affecting the production of enzymes and metabolites by microorganisms. In the production of proteases, a rich source of protein is important to induce enzyme secretion of proteases by the microorganism. On the other hand, the carbon and nitrogen (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N) ratio of the substrate must be adequate for this fermentation to occur.47
N) ratio of the substrate must be adequate for this fermentation to occur.47
Soy bran was the substrate with the highest protein content and soy husk had a high carbohydrate content, being composed of 32.3% of fibres. Similar results were found in the supplier's data sheet. The proportion of 70% soy bran and 30% soy husk was used due to being chosen in previous experiments (data not shown) presenting 34.8% proteins (dry basis).
Another important parameter to be considered in SSF is the particle size of the substrates, as it is directly related to the porosity and compaction of the medium. A particle size that does not compromise the process is required, allowing for better aeration and the largest possible microbial growth surface. In a study on the production of protease by A. oryzae using coffee by-products as the substrate, among the variables observed, the particle size (0.5, 1 and 2 mm) influenced the synthesis of the enzyme. The highest protease activity (7998 U g−1 dry extract) was verified in the medium with a particle size of 1 mm and the worst being observed at 0.5 mm. The authors attributed this result to bed aeration.48 De Castro and Sato49 observed that a larger particle size made the production of protease difficult when they used soybean meal with 72.9% of its granulometric distribution larger than 1.68 mm.
In our study, we used the proportion of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 of soy husk and soy bran to prepare a medium similar to those in the studies mentioned regarding particle distribution. The first substrate presented a particle distribution with sizes between 0.180 and 0.850 mm, with a predominance of particles between 0.300 and 0.600 mm (33.35%). This granulometric distribution of soy husk allows a good surface for microbial growth. Soy bran presented a predominance of particles larger than 1.70 mm (33.58%) and 0.850 mm (43.16%), but this substrate was used with just 30% of the total medium. While the soy husk contributed with a surface for microbial growth, the soy bran contributed to the media aeration.
30 of soy husk and soy bran to prepare a medium similar to those in the studies mentioned regarding particle distribution. The first substrate presented a particle distribution with sizes between 0.180 and 0.850 mm, with a predominance of particles between 0.300 and 0.600 mm (33.35%). This granulometric distribution of soy husk allows a good surface for microbial growth. Soy bran presented a predominance of particles larger than 1.70 mm (33.58%) and 0.850 mm (43.16%), but this substrate was used with just 30% of the total medium. While the soy husk contributed with a surface for microbial growth, the soy bran contributed to the media aeration.
3.2 Production of proteases and biopeptides – stage I
| Experiment/time (h) | 48 | 72 | 96 |
|---|---|---|---|
| a In the times 0 h and 24 h no protease activity was detected. Results presented in mean values ± standard deviation. Means followed by the same lowercase letter, in the same column, do not differ from each other, by the Tukey test, at 5% significance. E1: pH 5, moisture 50%, sodium nitrate 0.5%; E2: pH 7, moisture 50%, sodium nitrate 0.5%; E3: pH 5, moisture 70%, sodium nitrate 0.5%; E4: pH 7, moisture 70%, sodium nitrate 0.5%; E5: pH 5, moisture 50%, sodium nitrate 2%; E6: pH 7, moisture 50%, sodium nitrate 2%; E7: pH 5, moisture 70%, sodium nitrate 2%; E8: pH 7, moisture 60%, sodium nitrate 1.25%. | |||
| E1 | 597 ± 4.24b | 462 ± 11.31b | 315 ± 4.24bc |
| E2 | 677 ± 11.31a | 529 ± 1.41a | 426 ± 5.52a |
| E3 | 214 ± 5.66f | 136 ± 2.83f | 264 ± 8.49cde |
| E4 | 143 ± 4.24g | 115 ± 1.41f | 365 ± 41.01ab |
| E5 | 420 ± 3.50 cd | 310 ± 5.66d | 233 ± 4.24de |
| E6 | 466 ± 19.8c | 379 ± 1.41c | 286 ± 11.31cde |
| E7 | 100 ± 8.49g | 143 ± 12.73f | 116 ± 11.31f |
| E8 | 143 ± 1.41g | 195 ± 21.21e | 229 ± 18.38e |
| E9 | 406 ± 22.63d | 408 ± 8.49c | 298 ± 16.97cd |
| E10 | 334 ± 16.97e | 410 ± 25.46c | 301 ± 9.9bc |
| E11 | 392 ± 14.14d | 372 ± 2.27c | 275 ± 1.41cde |
The protease activities in 48 h of fermentation were higher than those obtained in 72 or 96 h cultivation times for most of the assays. Experiment E2 (50% moisture, pH 7 and 0.5% available nitrogen) presented the best result for this response variable, with 677 U g−1 protease activity. The same assay showed higher protease activity at 72 and 96 hours (p < 0.05 in comparison with the results of 24 h), when comparing the means of all assays (Table 3). The protease production reduced over time for most of the experiments. Possible causes are the depletion of nutrients of the medium, alteration of pH that can inhibit the enzyme's synthesis, and the increase in the temperature of the medium due to the fermentation process, which can affect the microbial metabolism.50
The lowest values of protease activity, in 48 h, were observed in experiments E4, E7 and E8, with values of 143 U g−1, 100 U g−1 and 143 U g−1, respectively. All these experiments presented an initial moisture content of 70%. Moisture in solid state fermentation is a crucial factor for the development of microorganisms. However, it is impacted by several factors, including the aeration of the medium, the water retention capacity of the solid substrates, and the temperature, in addition to the configuration of the bioreactor. Very low initial moisture will result in low water activity (aw), slowing the growth of fungi, even though they have the capacity to grow at lower aw than bacteria and yeasts. If the solid substrates are not able to absorb the water added at the beginning of the bioprocess, this water will be available in the pores and spaces between particles, making oxygen transfer difficult, which also decreases microbial growth rates, since fungi are aerobic. In addition, the aeration intensity can cause moisture evaporation and the bioreactor configuration can favor or hinder mass transfer, impacting moisture and therefore microbial growth and metabolite production.24,51,52
Protease activity obtained by SSF can range from 20 to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1.53 Many studies report the production of proteases by A. niger via SSF. Purushothaman et al.54 produced a protease by A. niger using wheat bran and obtained 38.36 U g−1 of protease activity after purification processes with ammonium sulfate, gel filtration and chromatographic techniques. De Castro et al.19 obtained a maximum protease activity of 262.78 U g−1 when using wheat bran and soy bran as substrates, in SSF with 50% moisture and 30 °C.
000 U g−1.53 Many studies report the production of proteases by A. niger via SSF. Purushothaman et al.54 produced a protease by A. niger using wheat bran and obtained 38.36 U g−1 of protease activity after purification processes with ammonium sulfate, gel filtration and chromatographic techniques. De Castro et al.19 obtained a maximum protease activity of 262.78 U g−1 when using wheat bran and soy bran as substrates, in SSF with 50% moisture and 30 °C.
The analysis of the results of protease activity at 48 h and 96 h of fermentation through analysis of variance resulted in the average interaction graphs presented in Fig. 1a and b.
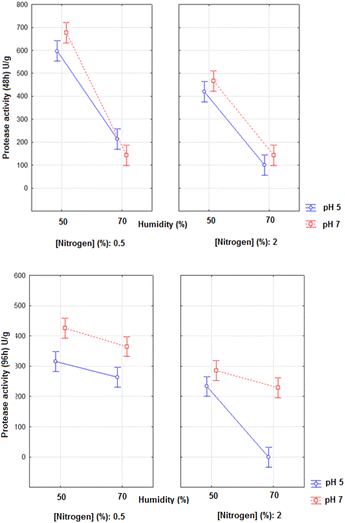 | ||
| Fig. 1 Protease activity as a function of the variables pH, moisture and nitrogen concentration during 48 h (a) and 96 h (b) of solid-state fermentation with A. niger (p < 0.05). | ||
The Anova of the regression model obtained in 48 h resulted in R2 = 0.9831, F value of the model = 116, and F critical = 2.19. The moisture and sodium nitrate concentration presented significant estimated effects on the protease activities in 48 h (p < 0.05; estimated effects of −389.75 and −125.25, respectively). The third order interaction effects were still significant (36.75, p = 0.025), demonstrating that the highest pH (7), lowest moisture (50%) and lowest nitrogen concentration (0.5%) were more favourable for the production of protease in 48 h, as is shown in Fig. 1a. The same behaviour can be observed in the effect of the interaction of the variables on the protease production in Fig. 1b.
El-Braky et al.53 cited studies that reveal conditions for the protease production process with pH from 6 to 8.5, moisture of 50% and mesophilic temperature (30 °C) to thermophilic (50 °C), conditions similar to those found to be ideal in our experiment.
The pH of the medium can affect the growth and metabolic regulation of microorganisms, since they are sensitive to the hydrogen ion concentration of the medium.50 The type of protease secreted is directly dictated by the pH of the medium.55 Protease production using residues from different rice varieties as substrates for SSF by A. niger revealed a higher protease activity of 67.7 U g−1 under optimized conditions for maximum enzyme production (34 °C, pH 7 and 96 h of fermentation).56
The lowest nitrogen concentration (0.5%) was favourable for the production of protease, with the addition of 2% of this nutrient source not being necessary, possibly because the complex medium used, composed of soy husk and soy bran, is enough to support the production of proteases by the fungi, not requiring a large supplementation, since the by-products used have a high concentration of proteins, especially soy bran (40.68% proteins).
| Experiment | Time of fermentation (h) | ||||
|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | |
| a Mean values ± standard deviation. Means followed by the same lowercase letter, in the same column, do not differ from each other, by the Tukey test, at 5% significance. E1: pH 5, moisture 50%, sodium nitrate 0.5%; E2: pH 7, moisture 50%, sodium nitrate 0.5%; E3: pH 5, moisture 70%, sodium nitrate 0.5%; E4: pH 7, moisture 70%, sodium nitrate 0.5%; E5: pH 5, moisture 50%, sodium nitrate 2%; E6: pH 7, moisture 50%, sodium nitrate 2%; E7: pH 5, moisture 70%, sodium nitrate 2%; E8: pH 7, moisture 60%, sodium nitrate 1.25%. | |||||
| E1 | 886.29 ± 26.65 | 949.11 ± 17.77 | 1692.97 ± 33.32c | 1589.28 ± 6.66cd | 2607.03 ± 6.66bc |
| E2 | 806.19 ± 19.99 | 1201.97 ± 33.31 | 1952.11 ± 39.98ab | 2178.26 ± 53.31b | 2875.6 ± 13.33ab |
| E3 | 672.69 ± 48.86 | 949.11 ± 35.54 | 1259.49 ± 33.31d | 1221.8 ± 33.32f | 1768.35 ± 33.32f |
| E4 | 851.53 ± 26.65 | 1203.54 ± 17.77 | 1377.29 ± 53.31d | 1410.27 ± 19.99e | 2263.08 ± 26.65de |
| E5 | 933.4 ± 26.65 | 1302.48 ± 46.64 | 1787.2 ± 59.97bc | 1678.83 ± 39.98c | 2738.95 ± 86.62abc |
| E6 | 1134.46 ± 48.86 | 1299.34 ± 2.22 | 2013.36 ± 19.99a | 2371.44 ± 6.66a | 2988.67 ± 93.29a |
| E7 | 660.13 ± 17.76 | 1018.21 ± 4.44 | 1047.47 ± 79.96e | 1207.67 ± 66.63f | 1895.57 ± 66.63f |
| E8 | 919.27 ± 19.98 | 1190.97 ± 22.21 | 1410.27 ± 46.64d | 1462.1 ± 66.63de | 2215.96 ± 39.98e |
| E9 | 1082.6 ± 11.11 | 1381 ± 11.11 | 1711.81 ± 59.97c | 1537.48 ± 13.33cde | 2541.06 ± 15.32cd |
| E10 | 1076.32 ± 33.31 | 1376.29 ± 4.44 | 1716.52 ± 66.63c | 1561.04 ± 59.97cde | 2483.47 ± 13.32c |
| E11 | 1136 ± 2.22 | 1376.16 ± 31.1 | 1711.81 ± 46.64c | 1565.75 ± 26.65cde | 2771.93 ± 10.66abc |
The fermented media exhibited different antioxidant activities and the best potential was found in 96 h of fermentation, revealing that the process increased the concentration of antioxidant compounds during cultivation (Table 4). The experiments that presented the best results of antioxidant activity were E2, E5 and E6 that have in common the low moisture of the medium. However, when analysing the yield of antioxidant activity, subtracting the antioxidant activity of the initial time, it was found that the E2 assay (pH 7, 50% moisture and 0.5% nitrogen) showed the best result, with an increase of 256.69%. These same conditions were also considered ideal when analysing the protease activity and formation of soluble proteins (Table 5), correlating the enzymatic hydrolysis with the formation of antioxidant peptides.
| Exp | 0 h | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|---|
| a Mean values ± standard deviation. Means followed by the same lowercase letter, in the same column, do not differ from each other, by the Tukey test, at 5% significance. E1: pH 5, moisture 50%, sodium nitrate 0.5%; E2: pH 7, moisture 50%, sodium nitrate 0.5%; E3: pH 5, moisture 70%, sodium nitrate 0.5%; E4: pH 7, moisture 70%, sodium nitrate 0.5%; E5: pH 5, moisture 50%, sodium nitrate 2%; E6: pH 7, moisture 50%, sodium nitrate 2%; E7: pH 5, moisture 70%, sodium nitrate 2%; E8: pH 7, moisture 60%, sodium nitrate 1.25%. | |||||
| E1 | 1926.49 ± 6.03 | 2511.26 ± 5.76 | 3685.07 ± 78.47c | 3249.7 ± 6.03cd | 3898.49 ± 6.03b |
| E2 | 1559.41 ± 18.11 | 2455.77 ± 6.03 | 4154.60 ± 30.18a | 3723.49 ± 7.03b | 4090.57 ± 07.87a |
| E3 | 1320.38 ± 18.11 | 2216.74 ± 7.23 | 2515.53 ± 18.11g | 2489.92 ± 9.54f | 2191.13 ± 6.54i |
| E4 | 1102.69 ± 72.43 | 2156.99 ± 30.18 | 2643.58 ± 6.04g | 2331.99 ± 60.36f | 1666.12 ± 60.36j |
| E5 | 1495.38 ± 63.36 | 2067.35 ± 36.22 | 3023.47 ± 24.15e | 2763.09 ± 6.03e | 3642.19 ± 30.18c |
| E6 | 1696.00 ± 6.04 | 2293.57 ± 6.04 | 3313.72 ± 12.07d | 3975.32 ± 8.11a | 2711.88 ± 18.11g |
| E7 | 1410.02 ± 12.07 | 1999.05 ± 60.36 | 2865.54 ± 54.33f | 2831.39 ± 12.76e | 2370.41 ± 30.18h |
| E8 | 1277.7 ± 42.26 | 1981.98 ± 22.16 | 3219.82 ± 24.15d | 3177.13 ± 14.56d | 2707.61 ± 61.25g |
| E9 | 1589.29 ± 30.36 | 2972.25 ± 34.87 | 3945.44 ± 12.07b | 3207.01 ± 18.11d | 3441.07 ± 72.44d |
| E10 | 1529.53 ± 12.07 | 2831.29 ± 42.25 | 3843.04 ± 63.54b | 3403.36 ± 6.04c | 3040.81 ± 5.24f |
| E11 | 1529.53 ± 48.29 | 2566.75 ± 30.18 | 3633.00 ± 6.03c | 3219.82 ± 12.07d | 3228.35 ± 14.32e |
It is difficult to compare the antioxidant activity with literature data due to differences in substrates, microorganisms, extraction methods and variables presented. Janiszewska et al.27 evaluated the fermentation of quinoa by R. oligosporus, N. intermedia and A. oryzae and observed that in the first stage of fermentation, R. oligosporus and N. intermedia were more efficient in the production of antioxidants. After 4 days, R. oligosporus still increased its antioxidant activity by 66%, corresponding to the increase in peptides and amino acids, since it reduced the phenolic compounds during this time of fermentation. Chi and Cho32 verified in soy bran fermentation that B. amyloliquefaciens U304 exerted better antioxidant activity in relation to ABTS+ removal when compared to the unfermented substrate and other studied microorganisms. El-Braky et al.53 cited studies that reveal conditions for the protease production process with pH from 6 to 8.5, moisture of 50% and mesophilic temperature (30 °C) to thermophilic (50 °C), conditions similar to those found to be ideal in our experiment.
The pH of the medium can affect the growth and metabolic regulation of microorganisms, since they are sensitive to the hydrogen ion concentration of the medium.50 The type of protease secreted is directly dictated by the pH of the medium.55 Protease production using residues from different rice varieties as substrates for SSF by A. niger revealed a higher protease activity of 67.7 U g−1 under optimized conditions for maximum enzyme production (34 °C, pH 7 and 96 h of fermentation).56
The lowest nitrogen concentration (0.5%) was favorable for the production of protease, with the addition of 2% of this nutrient source not being necessary, possibly because the complex medium used, composed of soy husk and soy bran, is enough to support the production of proteases by the fungi, not requiring a large supplementation, since the by-products used have a high concentration of proteins, especially soy bran (40.68% proteins).
Czelej et al.58 mention that the properties of the substrate, as well as the conditions under which the hydrolysis reaction is carried out, affect the final antioxidant potential of the peptides obtained from hydrolyzed animal or vegetable proteins, according to the structural properties of the compounds obtained, such as size or amino acid sequences. Thus, there is no consensus on which would be the best method for the determination of the antioxidant capacities, with the need for standardization of methodologies for each type of matrix. Several references mentioned the use of the ABTS radical for the antioxidant activity of biopeptides and because of that, we standardized the use of this methodology in our study. As can be seen in the results obtained for antioxidant capacity, proteolytic activity and soluble proteins obtained, they presented coherence, so we considered it a good choice for monitoring the results presented. In addition, Oliveira59 mentions that the DPPH method is indicated for the antioxidant potential of phenolic compounds and there are some related to the need for standardization, since there are several existing protocols, with interference from the type of solvent used to dissolve or radical. The radical also does not seem to be appropriate to mediate the antioxidant capacity in reaction media that become proteins. Farias et al.60 achieved the characterization of peptides obtained from soybean (a substrate also used in our study) using several methods. The higher molecular weight peptide fractions showed the highest antioxidant activities by three of the methods used, including ABTS, and did not show a good antioxidant activity result for DPPH, which worked better for the lower molecular weight fractions. In this work, peptides of low molecular mass were 481.5 to 2763.21 Da. High molecular weight peptides ranged from 2996.60 to 6230.25 Da.
The Anova of the results of antioxidant activity in 96 h of fermentation resulted in the average interaction graphs presented in Fig. 2. The Anova of the regression model obtained in 96 h resulted in R2 = 0.9002, F value of the model = 18.05, and F critical = 2.19. The variables pH and moisture presented significant effects on the antioxidant activities in 96 h (p < 0.05; effects of 333.35 and −766.82 for pH and moisture, respectively).
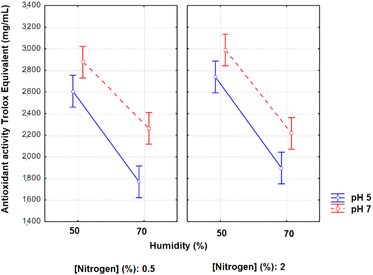 | ||
| Fig. 2 Antioxidant activity in Trolox equivalents (mg mL−1) as a function of the variables pH, moisture and nitrogen concentration in 96 h of solid-state fermentation with A. niger. | ||
The lower the moisture and the higher the pH, the better the antioxidant activity is.
The protease produced by A. niger during SSF led to the breakdown of complex proteins into more simple and soluble forms. The hydrolysates of fermented soy bran and soy husk showed an increase in TCA-soluble protein during fermentation, which reflects the hydrolysis of soy protein during the process (Table 5). In fermentation, proteins are degraded to peptides by endoproteases, which are then degraded to free amino acids by exoproteases.61
The best results were observed at 48 h and 96 h (Table 5). In some experiments, there was a reduction in the amount of soluble proteins at the time of 72 h. Our hypothesis is that the fungus could use amino acids and small peptides as a form of nutrition. Later, by the action of enzymatic activity, small peptides were formed again.
The best result at 48 and 96 hours was observed in experiment E2 composed of pH 7, 50% moisture and 0.5% nitrogen, the same experiment that yielded the best result in protease and antioxidant activities. In 48 hours, the increase in soluble proteins was 166.49% and in 96 hours it was 162.32%. In 72 hours, the experiment with the best performance was E6, with an increase of 134.39%, and differs from E2 only in the amount of nitrogen (2%). Thus, the low moisture and a more neutral pH were shown to be favourable characteristics for the hydrolysis of proteins.
Peptide size is a significant factor and is directly related to antioxidant activity and other functional properties of hydrolysates. Given this fact, proteolysis levels are often assessed by quantifying soluble peptides in a medium with trichloroacetic acid (TCA).47
This parameter indicates the formation of small peptides and amino acids, in addition to having a significant and positive correlation with the degree of hydrolysis (p < 0.05).62
Furthermore, the analysis of soluble protein in acid medium is used to confirm that the antioxidant potential is due to the increase in soluble proteins and not due to the increment of other compounds. Sanjukta et al.63 studied the SSF of soy by B. subtilis and confirmed the increase of both free phenolic compounds through the production of β-glucosidase and biopeptides through the production of proteases, during fermentation. Weng and Chen64 reported an increase in the protein hydrolysis degree, via SSF by R. oligosporus and B. subtilis, and Dey and Kuhad28 found an increase in phenolic compounds through SSF, by R. oryzae, using wheat as a substrate.
Chi and Cho32 showed that the concentration of soluble proteins of soy bran fermented by B. amyloliquefaciens U304 increased significantly, suggesting that this strain improves the hydrophilic property of the compound through the hydrolysis process. Rai et al.36 performed SSF with soy, using different strains of B. subtilis for the production of antioxidant compounds. The fermented soy hydrolysates showed high TCA-soluble protein during fermentation, which reflects the hydrolysis of soy protein during fermentation. The hydrolysis also resulted in increased DPPH radical elimination activity (antioxidant activity).
The evaluation of the soluble proteins in 96 hours of fermentation by Anova gave the following results: R2 = 0.9432, F value of the model = 33.208, and F critical = 2.19. The moisture and pH presented significant estimated effects on the soluble proteins in 96 h (p < 0.05; effects of −231.56 and −1352.02, respectively). The third order interaction effects were still significant (496.20, p = 0.0003), demonstrating that when the lowest amount of nitrogen (0.5%), highest pH (7) and lowest moisture (50%) were used in the fermentation, higher concentrations of final soluble proteins were obtained (Fig. 3).
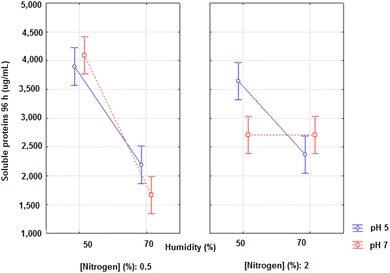 | ||
| Fig. 3 Soluble proteins (μg mL−1) as a function of the variables pH, moisture and nitrogen concentration in 96 h of solid-state fermentation with A. niger. | ||
It is suggested that the same conditions that favour protease activity allow greater hydrolysis and, consequently, the formation of small peptides, which in turn contributed to the increase of the antioxidant activity.
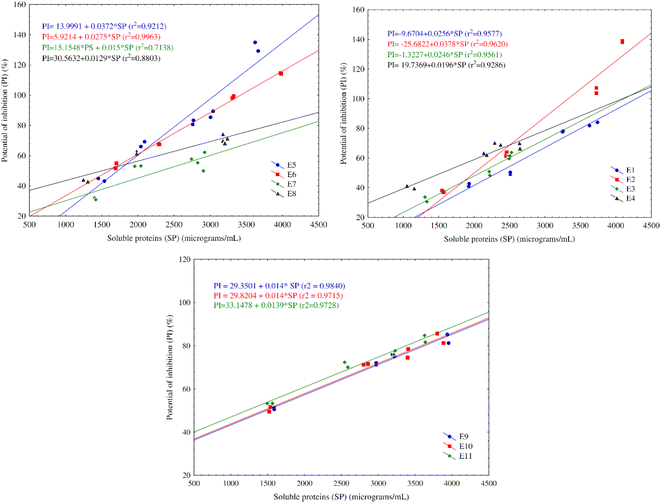
A significant, high and positive correlation for the majority of the experiments is found between the concentration of soluble proteins and antioxidant potential, as shown in Fig. 4. This relation between soluble proteins and percentage of inhibition of the oxidation of reactive species demonstrates that the increase of smaller peptides led to the increase of the antioxidant activity, proving the production of antioxidant biopeptides throughout the fermentation.
3.3 Stage II – study of scale up
In the second stage, the pH did not influence the protease activity during the fermentation, and in all assays a high and statistically similar protease activity was obtained (Table 6). In the previous stage of this work, we observed that in 96 hours of fermentation, experiment E2, composed of 50% moisture, nitrogen concentration of 0.5% and pH 7, resulted in 426 U g−1 of protease activity, a result slightly lower than that observed in this stage (479 U g−1), when the same conditions were used, however, with a greater amount of substrate. In this case, there is a positive effect on the scale-up of the medium.| Parameter | pH 6 | pH 7 | pH 8 |
|---|---|---|---|
| a Mean values ± standard deviation. Means followed by the same lowercase letter, in the same line, do not differ from each other, by the Tukey test, at 5% significance. | |||
| Protease activity (U g−1) 0 h | 0 | 0 | 0 |
| Protease activity (U g−1) 96 h | 467.5 ± 10.6a | 479.00 ± 18.4a | 433.00 ± 18.4a |
| Soluble proteins (μg mL−1) 0 h | 1064.28 ± 30.2c | 1320.38 ± 18.1a | 1205.13 ± 12.1b |
| Soluble proteins (μg mL−1) 96 h | 2865.54 ± 66.4b | 3138.72 ± 18.1a | 3014.93 ± 56.2ab |
| Yield soluble proteins (μg mL−1) | 1801.26 ± 36.2a | 1818.34 ± 35.2a | 1809.8 ± 12.1a |
| Antioxidant activity Trolox equivalents (mg mL−1) 0 h | 332.11 ± 2.5c | 417.63 ± 2.8b | 461.78 ± 2.3a |
| Antioxidant activity Trolox equivalents (mg mL−1) 96 h | 1495.12 ± 3.4b | 1678.88 ± 3.4a | 1714.68 ± 3.4a |
| Yield of antioxidant activity (final–initial) Trolox equivalents | 1163.01 ± 3.38b | 1261.25 ± 0.56a | 1252.9 ± 3.26a |
| Potential of inhibition (%) 0 h | 31.31 ± 0.20c | 39.34 ± 0.30b | 43.47 ± 0.20a |
| Potential of inhibition (%) 96 h | 142.73 ± 0.3b | 159.97 ± 0.3a | 163.33 ± 2.8a |
Comparing experiment E1, from stage 1, composed of pH 5, 50% moisture and nitrogen concentration of 0.5%, with 315 U g−1 of protease activity, it was verified that the higher pH (6, 7 and 8) and greater amount of substrate yielded greater enzymatic activity with the same time of fermentation.
The fermentation in 96 hours resulted in different concentrations of soluble proteins between the assays of the second stage (Table 6), showing that pH 7 during fermentation allows a greater amount of soluble proteins. However, when observing the amount of initial soluble protein, all assays obtained the same yield and, therefore, they are statistically similar (p > 0.05), a fact that can be explained by the protease activity verified previously. When comparing these results with experiments 1 (pH 5, moisture of 50% and 0.5% nitrogen) and 2 (pH 7, moisture of 50% and 0.5% nitrogen) of the first stage, with 3898.49 μg mL−1 and 4090.57 μg mL−1, respectively, we observed a lower formation of soluble proteins, in 96 hours of fermentation. This result reveals that a greater protease activity does not necessarily lead to greater hydrolysis and formation of smaller peptides in the second stage. A factor that can influence the action of enzymes on proteins is the aeration, which was lower at this stage of the process, which led to agglomeration of the medium, increased temperature and less enzymatic hydrolysis.
However, at the beginning of the process, the amount of soluble proteins in the medium was lower than that found in stage 1, a fact that can be explained by the differences in medium in the initial time.
The analysis of antioxidant activity showed that the increase of the pH contributed to the obtention of higher antioxidant activity. The same result was confirmed by the yield analysis, when the final and initial values are taken into account (Table 6).
4 Conclusions
In the study of the variables: pH (5, 6 and 7), moisture (50, 60 and 70%) and initial nitrogen concentration (0.5, 1.25 and 2%), the best conditions are neutral pH, lowest moisture and lowest nitrogen concentration, yielding higher protease activity at 48 hours of fermentation (677 U g−1) and higher antioxidant activity at 96 hours (2875.6 Trolox equiv. mg mL−1).The results found in this study are very important, since proteases are used in different types of industries, such as textiles, detergents, leather, and food and beverages, and biopeptides can be added to foods, intelligent packaging and as substitutes in synthetic drugs. The production of the two metabolites in the same fermentation using soy husk and soy bran as substrates reduces time and allows the valuation of the by-products, especially soy husk, a compound little used in SSF. This work still demonstrates than the proteases produced during the solid-state fermentation accomplish the hydrolysis of proteins, producing active biopeptides, showing the dependence between the two processes and the simultaneous production of both these biocompounds. This has not been reported in the literature using fungal strains until now.
Author contributions
Luciane Maria Colla: conceptualization, supervision, visualization, writing – review & editing. Christian Oliveira Reinehr: conceptualization, writing – review & editing. Paola Gouvêa Manfredini: investigation, methodology, writing – original draft. Vítor Augusto Farina Cavanhi: investigation, methodology, writing – original draft. Jorge Alberto Vieira Costa: conceptualization, supervision, visualization, writing – review & editing.Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.Acknowledgements
The authors would like to thank the University of Passo Fundo for providing a scholarship for the master's course student and for CNPq for providing a scholarship for the undergraduate student involved, and also for the research productivity grants for the professors.References
- S. Saadi, N. Saari, F. Anwar, A. A. Hamid and H. M. Ghazali, Recents advances in food biopeptides: Production, biological functionalities and therapeutic applications, Biotechnol. Adv., 2015, 33(1), 80–116, DOI:10.1016/j.biotechadv.2014.12.003.
- P. G. Manfredini, V. A. F. Cavanhi, J. A. V. Costa and L. M. Colla, Bioactive peptides and proteases: characteristics, applications and the simultaneous production in solid-state fermentation, Biocatal. Biotransform., 2020, 39, 360–377, DOI:10.1080/10242422.2020.1849151.
- R. Nasri, O. Abdelhedi, M. Nasri and M. Jrid, Fermented protein hydrolysates: biological activities and applications, Curr. Opin. Food Sci., 2022, 43, 120–127, DOI:10.1016/j.cofs.2021.11.006.
- D. Montesano, M. Gallo, F. Blasi and L. Cossignani, Biopeptides from vegetable proteins: new scientific evidences, Curr. Opin. Food Sci., 2020, 31, 31–37, DOI:10.1016/j.cofs.2019.10.008.
- L. B. Olvera-Rosales, A. E. Cruz-Guerrero, J. M. García-Garibay, L. C. Gómez-Ruíz, E. Contreras-López, F. Guzmán-Rodríguez and L. G. González-Olivares, Bioactive peptides of whey: obtaining, activity, mechanism of action, and further applications, Crit. Rev. Food Sci. Nutr., 2022, 25, 1–31, DOI:10.1080/10408398.2022.2079113.
- R. Bai, T. T. Nguyen, Y. Zhou, Y. Diao and W. Zhang, Identification of Antioxidative Peptides Derived from Arthrospira maxima in the Biorefinery Process after Extraction of C-Phycocyanin and Lipids, Mar. Drugs, 2023, 21(3), 146, DOI:10.3390/md21030146.
- S. Allur Subramaniyan, N. Begum, S. J. Kim, Y. H. Choi and T. J. Nam, Biopeptides of Pyropia yezoensis and their potential health benefits: A review, Asian Pac. J. Trop. Biomed., 2021, 11(9), 375–384, DOI:10.4103/2221-1691.321127.
- A. L. Astolfi, A. Rempel and V. A. F. Cavanhi, et al., Simultaneous saccharification and fermentation of Spirulina sp. and corn starch for the production of bioethanol and obtaining biopeptides with high antioxidant activity, Bioresour. Technol., 2020, 301, 122698, DOI:10.1016/j.biortech.2019.122698.
- A. Sánchez and A. Vázquez, Bioactive peptides: a review, Food Qual. Saf., 2017, 1, 29–46, DOI:10.1093/fqsafe/fyx006.
- J. Sun, H. He and B. J. Xie, Novel antioxidant peptides from fermented mushroom Ganoderma lucidum, J. Agric. Food Chem., 2004, 52, 6646–6652, DOI:10.1021/jf0495136.
- M. F. Juárez-Chairez, M. S. Cid-Gallegos, O. G. Meza-Márquez and C. Jiménez-Martínez, Biological functions of peptides from legumes in gastrointestinal health. A review legume peptides with gastrointestinal protection, J. Food Biochem., 2022, 46, e14308, DOI:10.1111/jfbc.14308.
- P. Minkiewicz, A. Iwaniak and M. Darewicz, BIOPEP-UWM Database of bioactive peptides: Current opportunities, Int. J. Mol. Sci., 2019, 20(23), 5978, DOI:10.3390/ijms20235978.
- P. Minkiewicz, A. Iwaniak and M. Darewicz, Communication BIOPEP-UWM Virtual—A Novel Database of Food-Derived Peptides with In Silico-Predicted Biological Activity, Appl. Sci., 2022, 12, 7204, DOI:10.3390/app12147204.
- J. Mamo and F. Assefa, The role of microbial aspartic protease enzyme in food and beverage industries, J. Food Qual., 2018, 7957269, DOI:10.1155/2018/7957269.
- M. Pokora, A. Zambrowicz, A. Zabłocka, A. Dąbrowska, M. Szołtysik, K. Babij, E. Eckert, T. Trziszka and J. Chrzanowska, The use of serine protease from Yarrowia lipolytica yeast in the production of biopeptides from denatured egg white proteins, Acta Biochim. Pol., 2017, 64(2), 245–253, DOI:10.18388/abp.2016_1316.
- F. D. Troncoso, D. A. Sánchez and M. L. Ferreira, Production of Plant Proteases and New Biotechnological Applications: An Updated Review, ChemistryOpen, 2022, 11, e202200017, DOI:10.1002/open.202200017.
- R. C. S. Ribeiro, T. R. S. Ribeiro, C. M. Souza-Motta, E. V. Medeiros and K. A. Moreira, Production and partial characterizion of proteases from Mucor hiemalis URM 3773, Acta Sci. Biol. Sci., 2015, 37, 71–79, DOI:10.4025/actascibiolsci.v37i1.21016.
- L. Zheng, X. Yu, C. Wei, L. Qiu, C. Yu, Q. Xing, Y. Fan and Z. Deng, Production and characterization of a novel alkaline protease from a newly isolated Neurospora crassa through solid-state fermentation, LWT–Food Sci. Technol., 2020, 122, 108990, DOI:10.1016/j.lwt.2019.108990.
- R. J. S. De Castro, A. Ohara, T. G. Nishide, M. P. Bagali, F. F. G. Dias and H. H. Sato, A versatile system based on substrate formulation using agroindustrial wastes for protease production by Aspergillus niger under solid state fermentation, Biocatal. Agric. Biotechnol., 2015, 4, 678–684, DOI:10.1016/j.bcab.2015.08.010.
- S. A. Nemes, L. F. Călinoiu, F. V. Dulf, A. C. Fărcas and D. C. Vodnar, Integrated Technology for Cereal Bran Valorization: Perspectives for a Sustainable Industrial Approach, Antioxidants, 2022, 11, 2159, DOI:10.3390/antiox11112159.
- S. Afrisham, A. Badoei-Dalfard, A. Namaki-Shoushtari and Z. Karami, Characterization of a thermostable, CaCl2-activated and raw-starch hydrolyzing alpha-amylase from Bacillus licheniformis AT70: production under solid-state fermentation by utilizing agricultural wastes, J. Mol. Catal. B: Enzym., 2016, 132, 98–106, DOI:10.1016/j.molcatb.2016.07.002.
- L. M. Colla, A. M. Ficanha, J. Rizzardi, T. E. Bertolin, C. O. Reinehr and J. A. V. Costa, Production and Characterization of Lipases by two new isolates of Aspergillus trough solid-state and submerged fermentation, BioMed Res. Int., 2015, 2015, 725959, DOI:10.1155/2015/725959.
- S. S. Behera and R. C. Ray, Solid state fermentation for production of microbial cellulases: recent advances and improvement strategies, Int. J. Biol. Macromol., 2016, 86, 656–669, DOI:10.1016/j.ijbiomac.2015.10.090.
- A. M. Castro, L. R. Castilho and D. M. G. Freire, Performance of a fixed-bed solid-state fermentation bioreactor with forced aeration for the production of hydrolases by Aspergillus awamori, Biochem. Eng. J., 2015, 93, 303–308, DOI:10.1016/j.bej.2014.10.016.
- I. Amadou, G. W. Le, Y. H. Shi, O. S. Gbadamisi, M. T. Kamara and S. Jin, Optimized Lactobacillus plantarum LP6 solid-state fermentation and proteolytic hydrolysis improve some nutritional Attributes of soybean protein meal, J. Food Biochem., 2011, 35, 1686–1694, DOI:10.1111/j.1745-4514.2010.00493.x.
- G. S. Dhillon, G. M. L. Rosine, S. Kaur, K. Hegde, S. K. Brar, P. Drogui and M. Verma, Novel biomaterials from citric acid fermentation as biosorbents for removal of metals from waste chromated cooper arsenate wood leachates, Int. Biodeterior. Biodegrad., 2017, 119, 147–154, DOI:10.1016/j.ibiod.2016.09.014.
- A. S. Janiszewska, B. Stodolak, A. M. Gomez-Caravaca, B. Mickowska, B. Martin-Garcia and L. Byczynski, Mould starter selection for extended solid-state fermentation of quinoa, LWT – Food Sci. Technol., 2019, 99, 231–237, DOI:10.1016/j.lwt.2018.09.055.
- T. B. Dey and R. C. Kuhad, Enhanced production and extraction of phenolic compounds from wheat by solid state fermentation with Rhizopus oryzae RCK2012, Biotechnol. Rep., 2014, 4, 120–127, DOI:10.1016/j.btre.2014.09.006.
- E. B. Eryilmaz, D. Dursun and A. C. Dalgiç, Multiple optimization and statistical evaluation of astaxanthin production utilizing olive pomace, Biocatal. Agric. Biotechnol., 2016, 7, 224–227, DOI:10.1016/j.bcab.2016.06.012.
- M. Ayyash, S. K. Johnson, S. Q. Liu, N. Mesmari, S. Dahmani, A. S. Al Dhaheri and J. Kizhakkayil, In vitro investigation of bioactivities of solid-state fermented lupin, quinoa and wheat using Lactobacillus spp, Food Chem., 2019, 275, 50–58, DOI:10.1016/j.foodchem.2018.09.031.
- A. C. Cheng, H. L. Lin, Y. L. Shiu, Y. C. Tyan and C. H. Liu, Isolation and characterization of antimicrobial peptides derived from Bacillus subtilis E20-fermented soybean meal and its use for preventing Vibrio infection in shrimp aquaculture, Fish Shellfish Immunol., 2017, 67, 270–279, DOI:10.1016/j.fsi.2017.06.006.
- C. H. Chi and S. J. Cho, Improvement of bioactivity of soybean meal by solid-state fermentation with Bacillus amyloliquefaciens versus Lactobacillus spp. And Saccharomyces cerevisiae, LWT – Food Sci. Technol., 2016, 68, 619–625, DOI:10.1016/j.lwt.2015.12.002.
- H. G. Zheng, X. Q. Yang, I. Ahmad, W. Min, J. H. Zhu and D. B. Yuan, Soybean β-conglycinin constituent subunits: Isolation, solubility and amino acid composition, Food Res. Int., 2009, 42, 998–1003, DOI:10.1016/j.foodres.2009.04.018.
- J. A. V. Costa, H. Treichel, V. Kumar and A. Pandey, Chapter 1 – Advances in Solid-State Fermentation, Current Developments in Biotechnology and Bioengineering, Elsevier, 2018, pp. 1–17, DOI:10.1016/B978-0-444-63990-5.00001-3.
- C. R. Soccol, E. S. F. Costa, L. A. J. Letti, S. G. Karp, A. L. Woiciechowski and L. P. S. Vandenberghe, Recent developments and innovations in solid state fermentation, Biotechnol. Res. Innov., 2017, 1, 52–71, DOI:10.1016/j.biori.2017.01.002.
- A. K. Rai, S. Sanjukta, R. Chourasia, I. Bhat, P. K. Bhardwaj and D. Sahoo, Production of bioactive hydrolysate using protease, β-glucosidase and α-amylase of Bacillus spp. Isolated from kinema, Bioresour. Technol., 2017, 235, 358–365, DOI:10.1016/j.biortech.2017.03.139.
- E. J. Park, C. V. Garcia, S. J. Youn, C. D. Park and S. P. Lee, Fortification of γ-aminobutyric acid and bioactive compounds in Cucurbit moschata by novel two-step fermentation using Bacillus subtilis and Lactobacillus plantarum, LWT – Food Sci. Technol., 2019, 102, 22–29, DOI:10.1016/j.lwt.2018.07.065.
- FAO, The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction, Rome, 2019, Licence: CC BY-NC-SA 3.0 IGO Search PubMed.
- P. Sharma, V. K. Gaur, R. Sirohi, S. Varjani, S. H. Kim and J. W. C. Wong, Sustainable processing of food waste for production of bio-based products for circular bioeconomy, Bioresour. Technol., 2021, 325, 124684, DOI:10.1016/j.biortech.2021.124684.
- ASSOCIATION OF OFFICIAL ANALYTICAL CHEMISTRY – AOAC, Official Methods of Analysis of the AOAC International, AOAC, Arlington, 16 edn, 2005, p. 1025p Search PubMed.
- L. M. Colla, A. M. M. Ficanha, J. Rizzardi, T. E. Bertolin, C. O. Reinehr and J. A. V. Costa, Production and Characterization of Lipases by Two New Isolates of Aspergillus through Solid-State and Submerged Fermentation, BioMed Res. Int., 2015, 2015, 725959, DOI:10.1155/2015/725959.
- T. E. Bertolin, J. A. V. Costa and G. D. L. Pasquali, Glucoamylase production in batch and fed-batch solid state fermentation: effect of maltose or starch addition, J. Microbiol. Biotechnol., 2001, 11(1), 13–16 CAS.
- G. E. Ortiz, D. G. Noseda, M. C. Poncemora, M. N. Recuper, M. Blasco and E. Albertó, A comparative study of new Aspergillus strains for proteolytic enzymes production by solid state fermentation, Enzyme Res., 2016, 3016149, DOI:10.1155/2016/3016149.
- J. Charney and L. M. Tomarelli, A colorimetric method for the determination of the proteolytic activity of duodenal juice, J. Biol. Chem., 1947, 171, 501–505 CrossRef CAS PubMed.
- R. Re, N. Pellegrini, A. Proteggente, A. Panalla, M. Yang and C. Rice-Evans, Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radical Biol. Med., 1999, 26(9–10), 1231–1237, DOI:10.1016/S0891-5849(98)00315-3.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, Protein measurement with the Folin-phenol reagent, J. Biol. Chem., 1951, 193, 265–275 CrossRef CAS PubMed.
- R. J. S. De Castro and H. H. Sato, Advantages of an acid protease from Aspergillus oryzae over commercial preparations for production of whey protein hydrolysates with antioxidant activities, Biocatal. Agric. Biotechnol., 2014, 3, 58–65, DOI:10.1016/j.bcab.2013.11.012.
- P. S. Murthy and M. M. Naidu, Protease production by Aspergillus oryzae in solid-state fermentation utilizing coffee by-products, World Appl. Sci. J., 2010, 8(2), 199–205 CAS.
- R. J. S. De Castro and H. H. Sato, Synergistic effects of agroindustrial wastes on simultaneous production of protease and α-amylase under solid state fermentation using a simplex centroid mixture design, Ind. Crops Prod., 2013, 49, 813–821, DOI:10.1016/j.indcrop.2013.07.002.
- H. Sattar, Z. Bibi, A. Kamran, A. Aman and S. A. U. Qader, Degradation of complex casein polymer: Production and optimization of a novel serine metalloprotease from Aspergillus niger KIBGE-IB36, Biocatal. Agric. Biotechnol., 2019, 21, 101256, DOI:10.1016/j.bcab.2019.101256.
- A. Pandey, Solid-state fermentation, Biochem. Eng. J., 2003, 13, 81–84 CrossRef CAS.
- Z. Hamidi-Esfahani, S. A. Shojaosadati and A. Rinzema, Modelling of simultaneous effect of moisture and temperature on A.niger growth in solid-state fermentation, Biochem. Eng. J., 2004, 21, 265–272, DOI:10.1016/j.bej.2004.07.007.
- M. El-Bakry, J. Abraham, A. Cerda, R. Barrena, S. Ponsá, T. Gea and A. Sánchez, From wastes to high value added products: Novel aspects of SSF in the production of enzymes, Crit. Rev. Environ. Sci. Technol., 2015, 45, 1999–2042, DOI:10.1080/10643389.2015.1010423.
- K. Purushothaman, S. K. Bhat, S. A. Singh, G. K. Marathe and A. R. G. A. Rao, Aspartic protease from Aspergillus niger: Molecular characterization and interaction with pepstatin A, Int. J. Biol. Macromol., 2019, 139, 199–212, DOI:10.1016/j.ijbiomac.2019.07.133.
- C. Snyman, L. W. Theron and B. Divol, Understanding the regulation of extracellular protease gene expression in fungi: a key step towards their biotechnological applications, Appl. Microbiol. Biotechnol., 2019, 103, 5517–5532, DOI:10.1007/s00253-019-09902-z.
- R. Paranthaman, K. Alagusundaram and J. Indhumathi, Production of protease from rice mill wastes by Aspergillus niger in solid state fermentantion, World J. Agric. Sci., 2009, 5(3), 308–312 CAS.
- J. H. Lee, S. R. Hwang, Y. H. Lee, K. Kim, K. Cho and Y. B. Lee, Changes occurring in compositions and antioxidant properties of healthy soybean seeds [Glycine max (L.) Merr.] and soybean seeds diseased by Phomopsis longicolla and Cercospora kikuchii fungal pathogens, Food Chem., 2015, 185, 205–211, DOI:10.1016/j.foodchem.2015.03.139.
- M. Czelej, K. Garbacz, T. Czernecki, J. Wawrzykowski and A. Wásko, Protein Hydrolysates Derived from Animals and Plants—A Review of Production Methods and Antioxidant Activity, Foods, 2022, 11, 1953, DOI:10.3390/foods11131953.
- G. L. S. Oliveira, Determinação da capacidade antioxidante de produtos naturais in vitro pelo método do DPPH˙: estudo de revisão, Rev. Bras. Plant. Med., 2015, 17(1), 36–44, DOI:10.1590/1983-084X/12_165.
- T. C. Farias, J. P. Abreu, J. P. S. Oliveira, A. F. Macedo, A. Rodríguez-Veja, A. P. Tonin, F. S. N. Cardoso, E. C. Meurer and M. G. B. Koblitz, Bioactive properties of peptide fractions from Brazilian soy protein hydrolysates: In silico evaluation and experimental evidence, Food Hydrocolloids Health, 2023, 3, 100112, DOI:10.1016/j.fhfh.2022.100112.
- F. Liu, Z. Chen, J. Shao, C. Wang and C. Zhan, Effect of fermentation on the peptide content, phenolics and antioxidant activity of defatted wheat germ, Food Biosci., 2017, 20, 141–148, DOI:10.1016/j.fbio.2017.10.002.
- D. Zhou, L. Qin, B. Zhu, D. Li, J. Yang, X. Dong and Y. Murata, Optimisation of hydrolysis of purple sea urchin (Strongylocentrotus nudus) gonad by response surface methodology and evaluation of in vitro antioxidant activity of the hydrolysate, J. Sci. Food Agric., 2012, 92, 1694–1701, DOI:10.1002/jsfa.5534.
- S. Sanjukta, A. K. Rai, A. Muhammed, K. Jeyaram and N. C. Talukdar, Enhancement of antioxidant properties of two soybean varieties of Sikkim Himalayan region by proteolytic Bacillus subtilis fermentation, J. Funct. Foods, 2015, 14, 650–658, DOI:10.1016/j.jff.2015.02.033.
- T. M. Weng and M. T. Chen, Effect of two-steps fermentation by Rhizopus oligosporus and Bacillus subtilis on protein of fermented soybean, Food Sci. Technol. Res., 2011, 17, 393–400, DOI:10.3136/fstr.17.393.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fb00077j |
| This journal is © The Royal Society of Chemistry 2023 |