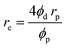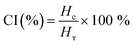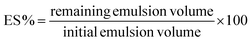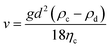Open Access Article
Open Access ArticleInsights into headway in essential oil-based Pickering emulsions for food applications
Reshma
Krishnan
 a,
Kavya
Mohan
ab,
K. V.
Ragavan
ab and
P.
Nisha
a,
Kavya
Mohan
ab,
K. V.
Ragavan
ab and
P.
Nisha
 *ab
*ab
aAgro Processing and Technology Division, CSIR – National Institute for Interdisciplinary Science and Technology (CSIR-NIIST), Thiruvananthapuram 695019, Kerala, India. E-mail: pnisha@niist.res.in; bp.nisha@yahoo.com; Tel: +91 471 2515348
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India
First published on 9th March 2023
Abstract
Essential oils are popular, but the direct application of EOs in food as a preservative is limited as they are highly volatile, and the high flavour of EOs affects the sensory characteristics of the food. The incorporation of EOs into Pickering emulsions (PEs) is explored in detail because of their superlative properties and finds a wide range of applications. The application of PEs as a delivery system for essential oils (EOs) has gained popularity due to the thermal stability, controlled release, and enhanced protection of EOs compared to conventional emulsions. Pickering particles offer higher stability, controllable release properties, and stimuli-responsive behaviour compared to traditional emulsions stabilised by surfactants, making them an area of interest. The presence of Pickering particles at the interface improves emulsion properties and protects the oil properties compared to conventional emulsion systems. This review critically addresses the recent advances in the preparation and characterisation methods pertaining to EO-based PEs, their advantages and disadvantages, their application in the food sector, and their future prospects.
1. Introduction
Essential oils (EOs) are secondary plant metabolites with antibacterial, antifungal, antioxidant, and antiviral properties. These aromatic and volatile liquids are obtained from different parts of plants (leaves, buds, bark, stem, fruits, roots, flowers, etc.). EOs are generally extracted using steam or water distillation, dry distillation, and supercritical or subcritical extraction.1,2 Most of the EOs have been reported as “Generally Recognised as Safe” (GRAS) by USFDA in 21 e-CFR (electronic Code of Federal Regulation) section 182.20.3The global essential oils market is estimated to grow at a compound annual growth rate (CAGR) of 7.4% between 2020 and 2028, with a projected market value of USD 18.6 billion.4 The market is expected to be driven by an increased demand from key industrial sectors such as food, beverage, personal care and cosmetics, and aromatherapy. In contrast to chemical additives and medications, EOs have no detrimental effects. They are hypoallergic and provide health benefits to consumers.5 Another reason for the upsurge in the market value is the shift in consumers' focus from synthetic to natural products. In addition, EOs have been reported to have vast biological activity due to the presence of compounds such as terpenes, terpenoids, sesquiterpenes, phenols, aldehydes, esters, ethers, and alcohols. The broad spectrum of bioactivity of EOs includes antibacterial, antiviral, antioxidant, antifungal, and health benefits like anticancerous and anti-inflammatory activity. Monoterpenes such as α-pinene, β-pinene, 1,8-cineole, camphor, and myrcene disrupt the integrity of bacterial membranes. Rosemary EO has antimicrobial activity against both Gram-positive and Gram-negative bacteria. Compounds responsible for this activity are α-pinene, 1,8-cineole, camphor, and borneol. α-Pinene exhibited an antibacterial effect against most of the microorganisms. Camphor and borneol are accountable for inhibiting Gram-positive bacteria, whereas 1,8-cineole acts against Gram-negative bacteria.6 Carvacrol in oregano EO provides broad-spectrum antibacterial properties against Gram-positive and Gram-negative bacteria. Eugenol, monoterpene ester, phenylpropanoid, and caryophyllene, which are the main components in clove oil, are reported to provide exceptional antibacterial and antioxidant activity.7 Thymol and carvacrol have been reported to have the highest antioxidant activity.8 Eugenol in cloves, cinnamaldehyde in cinnamon, p-cymene in Nigella sativa, β-caryophyllene in pepper, thymol in thyme, etc., are responsible for the antioxidant activity.9 The bioactivity of an EO varies according to the source of the oil used. It is also affected by the type, extraction method, and solvent used.
The bioavailability and efficacy of EOs are dependent on their water/oil solubility, chemical stability and volatile nature. Upon exposure to light, heat, or oxygen, the components in EOs tend to isomerise, dehydrogenate, polymerise, oxidise, or undergo thermal arrangements within them.10 These reactions are prompted either by enzymes or chemicals.10 Moreover, the direct use of EOs in food products is limited due to the interactions between the compounds of EOs and the food ingredients, which will lower the antioxidant and antimicrobial effects as well as sensory attributes of food products.11 Therefore, developing encapsulation systems for protecting EOs is necessary. The general encapsulation method includes spray drying, coacervation, complex coacervation, liposomes, emulsification, emulsion extrusion, molecular inclusion, and ionic gelation.12 Among them, emulsification is one of the commonly used methods. Emulsions are thermodynamically unstable systems containing two immiscible liquids (Fig. 1A). Among different emulsion systems such as conventional emulsions, nanoemulsions, multilayer emulsions, Pickering emulsions, high internal phase emulsions (HIPEs), and solid lipid particle emulsions, Pickering emulsions (PEs) are gaining a lot of interest due to their stability, controllable release properties, and stimuli-responsive behaviour compared to the traditional emulsions stabilised by surfactants (Fig. 1B). In PEs, stabilisation is achieved by the presence of solid particles, which are typically edible natural substances that are irreversibly adsorbed at the oil/water interface.13–15 Various emulsification techniques for developing PEs include high-speed homogenisation, high-pressure homogenisation, and ultrasound.13 These methods are discussed in detail in the upcoming sections. PEs offer better physical and thermal stability to the oil phase and sustained release of the components compared to conventional emulsions.16,18 PEs are one of the best options to improve the efficacy of the active agents present in EOs in the applied products.16 Hence, an EO containing PEs is a better solution to overcome the existing challenges in the utilisation and applicability of EOs and for enhancing their consumer acceptance.17,18
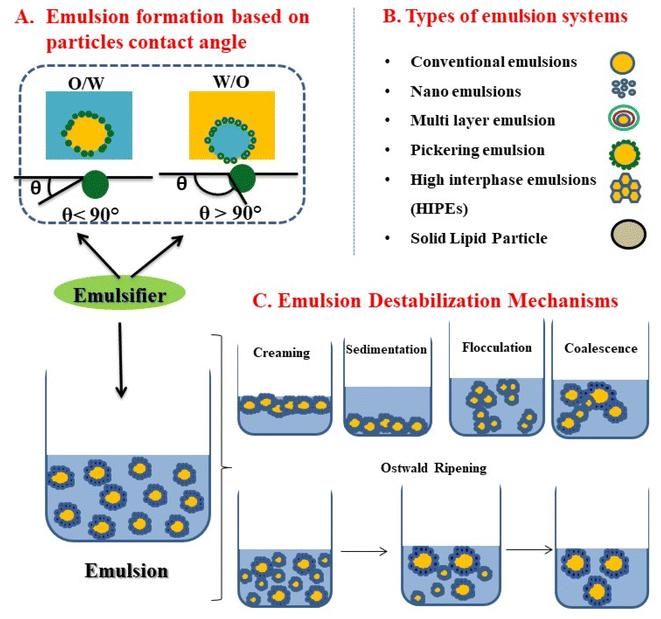 | ||
| Fig. 1 (A) Types of Pickering emulsions based on the contact angle. (B) Types of emulsion systems. (C) Various emulsion destabilization mechanisms. | ||
With this background, the current review covers the details of PEs, existing methods of preparation and characterisation of EO-based PEs, and their application mainly as a preservative or active packaging material. There are no other comprehensive reviews on EO-based PEs. Also, this review critically assesses the limitations of the studies in this field and possible prospects to tackle the current drawbacks. The review is expected to help readers better understand the types of EO-based Pickering emulsions and their characteristics.
2. Pickering emulsions
A thermodynamically unstable system containing two immiscible liquids is termed an emulsion. Among the two liquids, one at a lower volume is dispersed (dispersed phase) in the other (dispersion medium or continuous phase). Depending on the nature of dispersed and continuous phases, emulsions are classified into two types: (a) oil-in-water (O/W) emulsions (e.g., milk, mayonnaise, ice cream, dairy drinks, and cream) and (b) water-in-oil (W/O) emulsions (e.g., butter, spreads, and margarine). In an O/W emulsion, the dispersed phase is oil droplets and water is the continuous phase, whereas in a W/O emulsion the dispersed phase is water and the continuous phase is oil. Emulsifiers are amphiphilic in nature that are added to a mixture of oil and water to stabilise the emulsions and delay phase separation kinetically. These emulsifiers get adsorbed at the interface19 and thus stabilise the emulsion by reducing the interfacial tension. Conventional emulsifiers include polysaccharides, proteins (High Molecular Weight Emulsifiers, HMWEs) and low molecular weight emulsifiers (LMWEs).Emulsions stabilised by solid particles (Pickering particles) are termed Pickering emulsions (PEs). This area is an upcoming area of research due to their low cost, reduced toxicity, biocompatibility, and simple recovery in contrast to conventional surfactants.20,21 Ramsden22 and Pickering23 were the first to identify and describe PEs. It is reported that, compared to a conventional emulsion, a PE possesses higher deformation resistance.24 The Pickering particles have partial wetting properties, which causes irreversible particle adsorption at the interface, resulting in higher stability.24,25 Along with wettability, other parameters such as particle size, surface charge, surface coverage, and concentration affect emulsion stability. As the technology advanced, various Pickering particles have been identified, ranging from polysaccharides to inorganic particles. The formation of a monolayer around the dispersed phase prevents contact at the O/W interface, thereby preventing aggregation.20 The amphiphilic behaviour of the particles lowers the surface tension and interfacial tension. The characteristics of the resulting droplets, including concentration, charge, size, and interaction, strongly influence the properties of PEs.26 Gelatin, kafirin nanoparticles, lactoferrin, lactoglobulin, pea protein isolate, soy protein, whey protein, zein particles, etc., come under the category of protein-based Pickering particles.27 Polysaccharide-based particles include cellulose, chitosan, chitin, starch, etc.,27 Pickering emulsions are suitable for delivering bioactives due to their enhanced stability. Octenyl succinic anhydride (OSA) modified quinoa starch granules remained stable for a storage period of more than two years.28 Other Pickering particles that give better stability include soy protein-based microgels, chitosan-based particles, solid-lipid nanoparticles and cellulose-based particles.27 PEs have a myriad of applications in food, drug delivery, oil recovery, and cosmetics, and future research needs to identify other fields of application.20
2.1. Nature and role of Pickering particles
Conventional synthetic emulsifiers influence the gut microbiota, potentially contributing to a pro-inflammatory phenotype and metabolic syndrome.29 This has led to the development of Pickering particles. Pickering particles are the critical components in the development of PEs. A particle to be effective as a Pickering particle must have the following characteristics: (i) the size must be at least one magnitude smaller than the droplets, (ii) it must have a certain degree of wettability, and (iii) surface charge.20 Pickering particles commonly used include polysaccharides, proteins, lipids, and inorganic particles.13 The size of the Pickering particles affects the emulsion droplet size and thus the stability. A factor crucial for emulsion stability is particle concentration which affects interface particle packing. A minimum particle concentration is required to prevent Ostwald ripening.30 Based on relative particle wettability, a PE is categorised as (a) an O/W emulsion: stabilised by hydrophilic particles and (b) a W/O emulsion: stabilised by hydrophobic particles.13,31 O/W emulsions will be formed when the particles have a contact angle (θ) in the range of 15°< θ < 90°, while particles with θ in the range of 90° < θ < 165° will stabilise W/O emulsions.20,31,32 A highly stable emulsion system with suitable viscosity and a small droplet diameter can be obtained by manipulating particle surface wettability.33 Particles with both hydrophilic and hydrophobic hemispheres are called amphiphilic or Janus particles. The amphiphilic Janus particles have a hydrophilic hemisphere submerged in water and a lipophilic hemisphere in oil, and this results in a contact angle θ = 90°.34 These particles significantly improve the interfacial stability and, thus, emulsion stability.34 The shape of the Pickering particle governs its behaviour at the interface. Similar to wettability, the morphology is also reported to influence the development of self-assembled structures on surface-active particles, affecting emulsion stability.31 Various methods have been employed for preparing Janus particles, such as phase separation, selective surface modification, self-assembly of the block copolymer, and microfluidic emulsion templates. Multi-walled carbon nanotubes (MWNTs) with varying hydrophobicity behave as Janus particles. The reduction of the hydrophobicity of MWNTs results in a phase change of the emulsion from W/O to O/W, during which the droplet size decreases initially and then increases.34 Along with the droplet size, the surface charge also plays a vital role in emulsion stability. Surface charge is measured by zeta potential (Zp), which in the range of −30 mV < Zp < +30 mV stabilises the emulsion by reducing the agglomeration of particles.20 The study by Zhou et al.35 points out that with an increase in pH from 4.9–10.6, the value of Zp decreased from −38.3 to −65.2 mV, indicating the production of a stable emulsion. The Zp for a Nutmeg EO-based PE was observed to be in the range of 18.34–21.30 mV.36 The variation in EO concentration leads to a reduction in Zp, upon varying the EO concentration from 0.5% to 1.5% Grammosciadium ptrocarpum Bioss. The EO resulted in a Zp of 7.96 mV and −11.5 mV, respectively.372.2. Thermodynamics of conventional emulsions and PEs
Pickering emulsions are thermodynamically not stable. There is a molecular incompatibility between both phases, so they undergo quick phase separation that reduces the contact area. The equation for free energy is represented as:| ΔG = γΔA |
Phase separation can be delayed by using emulsifiers.26 The use of “thickening agents”, “stabilisers”, or “gelling agents” in foodstuffs prevents the separation of the components and provides a better texture to the food. Polysaccharides and proteins, which are amphiphilic in nature, are the most commonly used stabilisers that stabilise the emulsion by increase the viscosity of the continuous phase.38 “Amphiphilic molecules” have polar and non-polar parts; thus, they adsorb at the O/W interface. Being surface-active molecules, they reduce interfacial tension, thus lowering the free energy of the system. They reduce the creaming or settling velocity, thereby hindering the coalescence and flocculation of droplets. In the case of charged emulsifiers, electrostatic repulsion between the droplets at the interface makes the system stable.19,26 In PEs, the particles irreversibly adsorb at the interface, making the emulsion more stable for an extended period.39 The particle must have a double affinity towards both phases, i.e., amphiphilic, to be partially wetted by both liquids. The energy required to desorb a spherical particle from the interface is given by:
ΔEdesorption of sphere = γ(o/w)πR2(1 − |cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ|)2 θ|)2 |
3. Preparation of PEs
Most of the PE preparation methods reported in the literature use rotor-stator homogenisation; other preparation methods include ultrasound-assisted homogenisation and high-pressure homogenisation.The most popular method for PE preparation is the rotor-stator homogenisation method. Depending upon the homogenisation time as well as rotation speed (rpm), there is a reduction in the emulsion droplet size due to the shear force acting between the rotor and the stator. The disadvantages reported include the lack of uniformity in the emulsion. If the shear rate between the rotor and stator is high, fragile particles/aggregates can be damaged.13,41
Ultrasound-assisted emulsification is one of the recently employed techniques for producing PEs. Ultrasound of frequency above 16 kHz is used in the process. The development of cavitation, turbulence, and shear stress leads to emulsification. The generation of local high temperatures and pressure affects energy consumption and operating cost.42 A PE of marjoram essential oil stabilised by whey protein isolate (WPI) and inulin was prepared by agitation followed by ultrasonication at 20 kHz and 200 W for 20 min, yielding a particle size of 233 nm and Zp of −21.4 mV.43 Fasihi and co-workers employed ultrasonication in the preparation of a cinnamon essential oil-based PE, which was further incorporated in carboxymethyl cellulose (CMC)–polyvinyl alcohol (PVA) films. The prepared films increased the shelf life of bread.44
High-pressure homogenisation is generally used for the continuous process, and the advantage is the development of a uniform emulsion. The emulsification process involves an initial step of preparing a coarse emulsion which is then passed into a high-pressure homogeniser to prepare a fine emulsion. Cavitation, turbulence, and shear are responsible for producing fine emulsion. The emulsion droplet size is less than those produced by the rotor-stator homogeniser.13,45 Pickering particles such as modified longan shell cellulose nanofibers46 and bacterial cellulose from kombucha (KBC) have been created using high-pressure homogenisation. The PE was prepared using the KBC for encapsulating curcumin. The fine emulsion produced initially was subjected to high-pressure homogenisation at 100 MPa 3 times.47 This method is not suitable for the production of a PE with thermally unstable molecules due to the rapid increase in temperature during the process.48
In membrane emulsification technology, the dispersed phase is pressed through a microporous membrane by controlling its injection rate and shear to produce a PE. The main types are direct membrane emulsification (DME) and premixed membrane emulsification (PME). The droplet size of the emulsion is affected by the membrane's pore size and viscosity of dispersed and continuous phases. It is an environment-friendly method requiring less energy to produce an emulsion of uniform size. The disadvantage is that it is time-consuming and is suitable for a low-viscosity system. This has a lower yield, and few studies are available on this technology.13,42
Microfluidic technology, also known as drop-by-drop technology, is reported to yield PEs. The dispersed and continuous phases are allowed to flow perpendicular to each other. When both phases meet, the dispersed phase is converted to spherical droplets under ongoing phase drag. This method lacks shear force and thus does not disturb the agglomerates. Emulsions prepared have superior quality.13,49
4. Characterisation of Pickering emulsions
The parameters analysed in the characterisation of PEs include particle size and distribution, zeta potential, storage stability (creaming index), stability towards shear, rheology, and fluorescence microscopy.18,50–52 Furthermore, for various applications, antimicrobial activity, water vapour permeability (WVP), oxygen permeability, mechanical properties, and visible light transmittance are also analysed by Fourier transform infrared (FTIR) spectroscopy, transmission electron microscopy (TEM), and scanning electron microscopy (SEM).51,524.1. Particle size and PDI
The particle size and distribution of Pickering particles affect the uniformity, stability, and further application of the emulsion system. Particles of smaller size are preferred in PEs. A reduction in particle size implies an increase in stability.20,52 Polydispersity index (PDI) values indicate the homogeneous distribution of oil in the emulsion. PDI values closer to 0 indicate a more uniform distribution and higher emulsion stability without phase separation.50 The size of the emulsion droplet formed depends on the Pickering particle. The correlation between the diameter of the emulsion droplet and the Pickering particle is expressed by:where re, rp, ϕd, and ϕp are the radius of emulsion droplets, Pickering particles, the volume fraction of the dispersed phase, and particles, respectively. At constant ϕd and ϕp, the emulsion droplet size increases with an increase in the size of the Pickering particle. The most suitable size of the Pickering particle needs to be selected to produce the preferred PE. The decrease in emulsion droplet size with particle size has led to a general rule that the selected Pickering particle should be at least one order of magnitude smaller than the desired emulsion size to produce a stable emulsion.20
4.2. Zeta potential
Zeta potential (ζ-potential, Zp) is a critical parameter in determining the stability of PEs. It reflects the potential difference between the dispersed and dispersion media in the emulsion, and more accurately, the electrokinetic potential of the emulsion system. When the Zp value is greater than ± 30 mV, the emulsion is said to be stable due to electrostatic repulsion at the surface, preventing agglomeration of the particles. But a Zp in the range of −30 mV to +30 mV brings about a small degree of aggregation of particles as van der Waals force dominates in such systems. Zp is not only necessary for colloidal properties, but also it plays a crucial role in the adsorption of solid particles at O/W interfaces.204.3. Storage stability
Storage stability is an essential parameter of any product. Emulsions undergo phase separation. Visual evaluation of stability is done by creaming. Creaming is followed by flocculation and coalescence.53 Storage stability is generally assessed using the creaming index (CI). The prepared PE is stored in graduated tubes at room temperature, and the height of the serum layer (HC) and the total height of the emulsion (HT) are observed for a fixed time interval.51CI is expressed as:
4.4. Stability towards shear
The centrifugation method is performed to check the emulsion's stability towards shear. Centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min at 20 °C in graduated tubes is performed. After centrifugation, an oily phase was formed at the top, an emulsion phase at the middle, and an aqueous phase at the bottom.18 In the case of emulsions where coalescence occurred, it can be visually observed, whereas in the case with creaming, the layer can be measured.54 Emulsion stability (ES) is expressed using the equation:
000 rpm for 5 min at 20 °C in graduated tubes is performed. After centrifugation, an oily phase was formed at the top, an emulsion phase at the middle, and an aqueous phase at the bottom.18 In the case of emulsions where coalescence occurred, it can be visually observed, whereas in the case with creaming, the layer can be measured.54 Emulsion stability (ES) is expressed using the equation:4.5. Rheology
Viscoelastic shear flow and hysteresis measurements of the droplet structure allow differentiation between Newtonian and non-Newtonian flows. Thixotropic behaviour can be observed when the initial shear stress at the time of increase in the shear rate is more prominent than measured during the progressive decrease. This is attributable to the partial breakdown of the emulsion microstructure under a shear flow.184.6. Fluorescence microscopy
The microstructure of the emulsion can be identified using this technique. A stained PE is pipetted on a glass slide and observed under a microscope. Stains such as Nile red and Nile blue are commonly used.52 Nile red is a lipophilic stain and fluoresces in a lipid-rich environment,55 whereas Nile blue and calcofluor white stains hydrophilic molecules.5. Challenges in Pickering emulsions
5.1. Stability
Emulsions are highly unstable colloidal systems. The term “emulsion stability” refers to an emulsion's ability to withstand changes, i.e., to keep its properties unaffected over time.20,56 The major instability issues associated with emulsion are creaming/sedimentation (gravitational separation), Ostwald ripening, flocculation, phase separation, and coalescence (Fig. 1C). The density differences between the two immiscible liquids will also lead to phase separation after emulsion formation. If the dispersed phase has a less density, it will move up (creaming) and vice versa (moving down is sedimentation). The simplest way to measure creaming is by using the creaming index (CI) (as discussed). The CI value is also supported by Stokes' law, which calculates the gravitational separation rate of the PE:where v is the gravitational separation rate, g is the acceleration due to gravity, d is the emulsion droplet diameter, ρc and ρd are the densities of continuous (water) and dispersed (oil) phases, respectively, and ηc is the viscosity of the continuous phase.20,57
Emulsion coalescence occurs by the collision of emulsion droplets to produce a single large droplet.58 Ostwald ripening occurs when there is a steady growth of large droplets at the expense of small droplets, in which the tiny droplets formed initially will disappear in time.59 This phenomenon is analogous to coalescence and flocculation, in which the emulsion droplets collide and aggregate.60 After coalescence, the flocs or the large droplets either settle down (sedimentation) or float (creaming) because of gravitational separation; ultimately, phase separation occurs.61
5.2. Oxidation
Chemical degradation of emulsions takes place mainly by oxidation. Environmental changes during the production of food-grade PEs will also cause lipid oxidation leading to the loss of bioactive substances and changes in flavour and quality. The interface composition can inhibit the lipid oxidation of PEs, and the chemical oxidation can be retarded by the interface membrane's steric effect.13 The PE of flaxseed oil (FSO) stabilised by flaxseed protein and polysaccharides as Pickering particles increased the oxidative stability of FSO, and the integration of thymol into the oil fraction further increased the oxidation stability of FSO.62 E. Hosseini et al. (2020)63 found that chitosan-myristic acid (CH-MA) nanogels, as well as clove essential oil (CEO), could stabilise FSO-PE.13 Lipid oxidation was inhibited by the steric hindrance offered by CH-MA nanogels and the interfacial adsorption of CEO. As reported by Atarian et al. (2019)64 an O/W emulsion of sunflower oil stabilised by chitosan (CH)-stearic acid (SA) nano-gels had higher oxidative stability than the emulsion stabilised by Tween 80. The production of hydroperoxides was inhibited in mayonnaise incorporated with fish oil encapsulated in CH-SA nanogel. The incorporation of clove EO improved the stability of the mayonnaise. The formation of a thick layer of CH-SA nano-gel over the oil has reduced oxidation by shielding the oil from oxidants.656. Essential oil-based Pickering emulsions
EOs comprise a highly volatile and lipophilic fraction with a molecular weight lower than 300. The components of EOs vary according to the plant material, health, climate, harvest, growth stage, etc. These components get converted to each other by oxidation, cyclisation, dehydrogenation, and isomerisation, which is chemically or enzymatically triggered.10 EOs are highly susceptible to damage, leading to quality loss. Therefore, the protection of EOs is important.In the case of edible or vegetable oils, triacylglycerides are the major constituents (96%). Free fatty acids (FFA), phospholipids, antioxidants, phytosterols, tocopherols and waxes are also found.66 Oxidation is a phenomenon that occurs in all fats and oils. But the rate of oxidation depends upon FFA, moisture, impurities, temperature, oxygen, light and other processing parameters. Lipid oxidation is an undesired phenomenon not just because of the rancid odour and unpleasant flavour but also because of the safety parameters. These components can be harmful to human health. Oils with a higher concentration of unsaturated fats are more prone to oxidation.67 A challenge with EOs is that the components are smaller, volatile, and less viscous and get easily polymerised. Therefore, Pickering particles must be less porous and compact in EO-based PEs than in edible oil-based PEs.
The presence of diverse bioactives in EOs helps in wide application in food systems. Lipid oxidation and microbial contamination are responsible for reducing the shelf-life of food products and enhancing food quality. The use of EOs in meat, fish, and vegetables has been established to provide shelf-life enhancement. EOs from cloves, rosemary, cinnamon, oregano, and thyme have been applied to a variety of food products to extend their shelf-life.68 The intense flavour and the high volatility limit the direct use of EOs in food. PEs based on various EOs using different Pickering particles are proposed to overcome these limitations and to enhance their application in food. PEs can also be used for sustained release by selecting appropriate Pickering particle. PEs of clove, cinnamon, oregano, tea tree oil, marjoram oil, peppermint oil, cedarwood, cardamom, and rosemary oil are mostly reported and are discussed in detail in the following sections (Table 1) and detailed in the subsequent sections.
| Essential oil | Pickering particle | Fabrication method | Particle size and zeta potential | Application | References |
|---|---|---|---|---|---|
| Cinnamon | Zein-pectin composite nanoparticles | High speed homogenization | 660.8 ± 8.1 nm | Antimicrobial agent in apple slices | 89 |
| −31.23 ± 0.70 mV | |||||
| Cinnamon oil and corn oil | Sodium starch octenyl succinate (SSOS) | Homogenization | 314 to 389 nm | Active biodegradable films with improved antimicrobial and antioxidant activities | 50 |
| −23 mV to −28 mV | |||||
| At various EO concentrations | |||||
| Marjoram | Whey protein isolate (WPI)/inulin | Ultrasonication | 233.3 nm | Active pectin film as a new active packaging system | 43 |
| −21.4 mV | |||||
| Clove | Zein colloidal particles | High speed homogenization and ultrasonication | 1.40 μm | Chitosan based edible film with antimicrobial properties | 52 |
| −51.73 mV | |||||
| At 3% zein concentration | |||||
| Clove | Cellulose nanofiber (CNF) | High speed homogenization | 342 ± 6.8 nm | Gelatin/agar active film with improved antioxidant activity, application as active food packaging | 7 |
| −51.8 ± 0.9 mV | |||||
| Peppermint | Chitosan decorated silica nanoparticles | Homogenization | 118.12–152.5 nm | PE based composite microcapsules using HPMC, a promising strategy for antibacterial applications | 107 |
| 42.5 mV | |||||
| Oregano | Zein-pectin nanoparticle | High speed homogenization | 571.48 ± 8.03 nm | Strawberries preserved in konjac glucomannan active packaging films | 94 |
| −30.74 ± 0.60 mV | |||||
| Oregano | ZnO nanoparticle | High-shear homogenization | 26.85 μm (1.5 wt% ZnO and 20% oil) | Cellulose nanofibril film with antioxidant and antimicrobial activity | 51 |
The activity of the bioactive compounds present in EOs is reported to enhance in PEs, which offers improved application in end products.16 As PEs of EOs possess high stability and less tendency of evaporation and oxidation, they are suitable for delivering active agents and can act as a vehicle for delivering antimicrobial, antioxidant and active packaging agents. The improved properties of EOs in PEs are due to the higher solubility in aqueous solution, better stability and controlled release properties. The applications of EO-PE, ranging from food preservatives to active packaging, have been discussed in the subsequent sections.
6.1. Clove oil
Cloves are used as a flavouring and preservative agent in various food products. Clove oil is an EO obtained from the buds of Syzygium aromaticum.7,69 The constituents of cloves, such as eugenol, caryophyllene, phenylpropanoid, and monoterpene esters, are responsible for the excellent antibacterial and antioxidant properties. They are reported to be non-toxic and safe. Even though the antibacterial and antioxidant activities make them a good food preservative, their application is limited owing to their volatility, oxidation sensitivity, and low water solubility.7,70,71 PEs could be a promising solution to overcome these disadvantages by selecting an appropriate composition of the emulsion and Pickering particles.A highly stable nanosized PE containing clove essential oil (CO) was prepared using the rotor-stator homogenisation method with nano-cellulose fibre as Pickering particles.7 The encapsulation efficiency was 77.7 ± 1.3%, and the light-yellow colour of the PE indicates the oil loading. The emulsion's particle size, PDI, and Zp value are reported to be 342 ± 6.8 nm, 0.45 ± 0.08, and −51.8 ± 0.9 mV, respectively. The developed PE was incorporated (0, 1, 5, and 10%wt based on polymer) in gelatin/agar functional films for active food packaging applications (Fig. 2A). The film integrated with the PE had a pale-yellow colour compared to the colourless control film. The film showed exceptional antioxidant and UV-barrier properties. The film's enhanced physical and functional properties make it appropriate for active food packaging applications through a marginal reduction in mechanical and water vapour barrier properties.
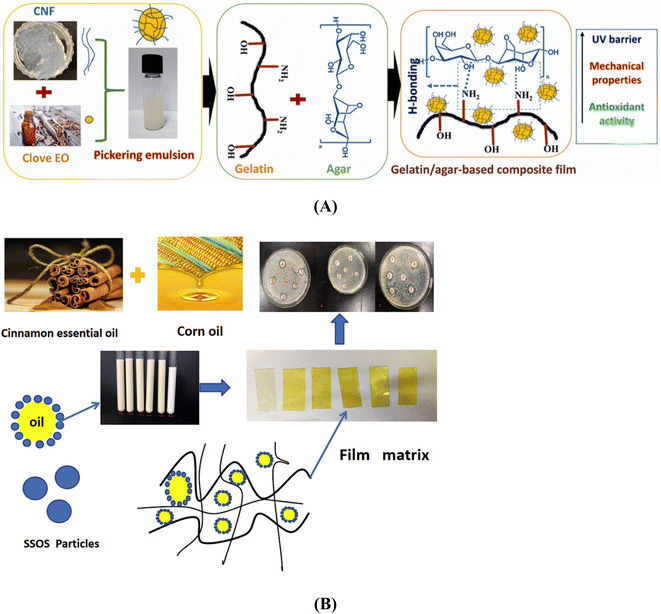 | ||
| Fig. 2 Graphical representation of (A) the Pickering emulsion added gelatin/agar-based films. Adapted with permission from (Roy & Rhim, 2021).7 Copyright 2021 Elsevier. (B) SSOS-based Pickering emulsion films incorporated with cinnamon essential oil. Adapted with permission from (H. Sun et al., 2020).50 Copyright 2020 Elsevier. | ||
Another study aimed to develop an antibacterial system containing a CO and carboxymethyl cellulose sodium-modified cellulose nanocrystal (CNC) Pickering emulsion (PE) and compared it with submicron emulsions (SE) prepared using d-α-tocopheryl polyethylene glycol succinate (TPGS).72 A piston-gap high-pressure homogeniser was used for PE preparation. The antibacterial efficacy of the PE against Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive) was determined using the minimum inhibitory concentration (MIC) method. The PE stabilised with 1% CNC had better stability and a rough surface, which improved the antimicrobial properties and exhibited better stability at elevated pH values or salt concentrations because of the presence of CNCs. CO-SE (submicron emulsion) had a droplet size of 550 nm and Zp of −35 mV. The CO-PE had a MIC value of 1.25 μl ml−1 against E. coli when compared to CO-SE and clove oil with MIC values of 2.5 μl ml−1 and 6.25 μl ml−1, respectively. Thus, CNC-stabilised CO-PE demonstrated better antimicrobial activity due to the adherence of the rough surface of CNCs to the bacterial membrane leading to better antimicrobial efficacy in contrast to CO-SE (smooth surface).
Edible films have attracted research interest as they improve quality and food safety and prevent environmental pollution.73 The direct addition of EOs negatively impacts the physio-chemical properties of edible films; the active components of the EOs quickly escape from the film.52,74 But adding a PE to edible films improves the mechanical, structural, barrier, and antimicrobial properties of the edible as studied by Xu et al. (2020).52 Zein stabilised a clove oil PE and was used to prepare chitosan-based edible films. The PE was prepared using a high-speed homogeniser and ultrasonication with an ultrasonic disperser. The emulsion with 3% w/v zein and 50% clove oil had a lower particle size (1.40 μm) and better PDI. Water vapour permeability (WVP) and PE-based film's tensile strength were reported to be lower than those of the control. The fracture resistance of the film was estimated through elongation at break (EAB); it increased up to a PE concentration of 0.4% w/v and decreased to 0.6% w/v. With the increasing concentration of PE in the film, antimicrobial properties were boosted. Shen et al. (2021)74 developed pullulan/gelatin edible films incorporating a nanoemulsion (NE) and PE of CO. The PE showed a better release profile when compared to the NE. The PE incorporated film showed excellent mechanical properties, water barrier properties, low water content, high density, and antioxidant activities, making it desirable in active packaging applications.
In the work by Bangar et al. (2022),75 CNCs were extracted from Kudzu (Pueraria montana) vine and starch from pearl millet. Kudzu CNCs were dispersed in distilled water, and the clove bud oil was added to the dispersion and stirred to prepare the PE. The prepared PE was incorporated into starch-based films. Pearl millet starch (PMS) films, films incorporated with CNCs, and films incorporated with the PE were compared for their mechanical properties, water barrier properties, and biodegradability. The fastest biodegradability was observed in pure PMS films, followed by PMS with CNCs, and lowest in PMS with the PE (5% Kudzu CNCs and 1.5% cove bud oil). All the films degraded between 15 and 21 days. The incorporation of the PE increased the tensile strength and reduced the water vapour permeability of the films. In another study by Punia Bangar et al. (2022),76 the same films were used in the packaging of red grapes to improve their shelf-life. The grapes' weight, firmness, and soluble solid content were unaffected up to 15 days of storage at 5 °C. Therefore, this result suggests that the nanocomposite films with clove oil can be used for the shelf-life extension of fruits.
6.2. Cinnamon oil
Cinnamon (Cinnamomum zeylanicum) barks and leaves are used as a spice and flavouring agent in foods and have applications in medicine.77 Cinnamon essential oil (CEO) is a GRAS additive by Food and Drug Administration (FDA) 21 CFR part 172.515.78,79 It has antioxidant and antimicrobial effects; the major active components include cinnamaldehyde and eugenol. These phenolic compounds delay microbial growth and neutralise free radicals.50,80The PE of CEO and corn oil with sodium starch octenyl succinate (SSOS) was incorporated into biodegradable films to improve antimicrobial and antioxidant activities.50 The schematic representation of the work is shown in Fig. 2B. Ultrasonication and homogenisation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm were done to prepare the PE. The OSA starch has suitable properties as a stabiliser independent of the ionic strength and pH of the medium. It also has excellent film-forming and emulsifying capabilities.50,81–83 The study observed that with an increase in the proportion of EO in the emulsion, the particle size decreased, the Zp increased, and a more stable PE was produced. The PE was incorporated into the film-forming solution (FFS) of SSOS, glycerol (plasticiser), and sodium alginate (reinforcing agent) and cast in a plexiglass application and subsequently, oven-dried to obtain the film. The antimicrobial effects of the developed film were assessed against Staphylococcus aureus, Bacillus subtilis (Gram-positive), and Escherichia coli (Gram-negative). An increase in the concentration of EO improved the antimicrobial effects. A very high concentration of EO in the mixed oil film lowered the inhibitory effect. As a result of the addition of the PE to the film, it had a lower tensile strength (TS), and the elongation at break (EAB) increased. The addition of the PE improved water barrier and oxygen barrier properties. The films had excellent antibacterial and antioxidant activities, with promising applications in active packaging films for food applications.
000 rpm were done to prepare the PE. The OSA starch has suitable properties as a stabiliser independent of the ionic strength and pH of the medium. It also has excellent film-forming and emulsifying capabilities.50,81–83 The study observed that with an increase in the proportion of EO in the emulsion, the particle size decreased, the Zp increased, and a more stable PE was produced. The PE was incorporated into the film-forming solution (FFS) of SSOS, glycerol (plasticiser), and sodium alginate (reinforcing agent) and cast in a plexiglass application and subsequently, oven-dried to obtain the film. The antimicrobial effects of the developed film were assessed against Staphylococcus aureus, Bacillus subtilis (Gram-positive), and Escherichia coli (Gram-negative). An increase in the concentration of EO improved the antimicrobial effects. A very high concentration of EO in the mixed oil film lowered the inhibitory effect. As a result of the addition of the PE to the film, it had a lower tensile strength (TS), and the elongation at break (EAB) increased. The addition of the PE improved water barrier and oxygen barrier properties. The films had excellent antibacterial and antioxidant activities, with promising applications in active packaging films for food applications.
Though chitosan-based films exhibit exceptional oxygen barrier properties, being hydrophilic, they have inadequate moisture barrier properties.11 A chitosan (CH) based active film of CEO-PE was developed by J. Liu et al. (2022).84 CEO-CNC-PE was incorporated into the CH film forming a matrix to improve the antimicrobial activity of the film. The prepared emulsion was stable for a period of one month. The developed film had improved water barrier and antibacterial properties, but the mechanical strength was reduced. The C3E30 film (3% w/v CNC and 30% v/v CEO) prolonged the shelf life of pork, while maintaining film stability and becomes a suitable material for food packaging. The study by K. Yu et al. (2021)85 dealt with the development of a chitosan (CH) coating incorporated with a CNC-CEO PE to enhance the shelf-life of mangoes (Mangifera indica Linn.), thereby reducing their post-harvest losses. Films of different compositions, such as CH-PE coatings, pure CH coatings, and a coating made with a CH-CEO emulsion (CH-E) (developed using Tween 80) coating, were prepared. Their efficacy was evaluated during the storage of mangoes. It was figured out that the CH-PE coating effectively maintained the mango's visual appearance stored at 25 °C for 12 days by slowing down water loss and reducing yellowing and dark spots. The CH-PE coating ensured a sustained release of CEO compared to CH-E. The weight loss was lowest in mangoes coated with CH-PE as it had the lowest water vapour permeability (WVP). The hardness of control mangoes after 12 days of storage reduced sharply, implying that they have lost their edible value, reducing consumer acceptance. CH-PE coatings had low WVP and water solubility (WS) and maintained the highest hardness values. The CH-PE coating was the perfect alternative for extending the shelf-life of mangos because: (a) enzymatic browning was reduced by inhibiting polyphenol oxidase (PPO) activity, (b) pectin methyl esterase (PME) as well as polygalacturonase (PG) activities were inhibited, thus delaying the decrease of hardness, (c) enhanced nutrient quality by increasing the ascorbic acid content, total soluble solids (TSS), and titratable acidity (TA), (d) lowered the formation of malondialdehyde (MDA) by enhancing the peroxidase (POD) activity and (e) lowered disease incidence by activating disease-resistant enzyme phenylalanine ammonia-lyase (PAL).
It is reported that prolamin colloidal particles can be used as Pickering particles. These include kafirin, gliadin, and zein. Among them, zein is a low-cost prolamin consisting of hydrophobic and hydrophilic amino acids.86,87 Zein colloidal nanoparticle (ZNS) stabilised CEO-PE is used as a butter substitute in pound cake.86 The PE was prepared by high shear homogenisation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. Pound cake is a snack and dessert, popular for its flavour and mouthfeel. Pound cake is also susceptible to microbial growth, particularly mould.88 The PE of 20 g ZNS + 15 g oil + 5 g CEO was found to be the best substitute 20% butter in pound cake with a shelf-life extension of four days without changing the colour and texture. Jiang et al. (2020)89 reported zein-pectin nanoparticles as an emulsifier for CEO-based PEs. Zein-pectin nanoparticles are ideal to be used as Pickering particles due to their stability, sustainability, and low cost. Fresh-cut apple slices were dipped into the diluted PE and stored at 20 °C for six days. The emulsion had improved antibacterial properties compared to the pure EO due to sustained-release properties and appropriate dispersibility, thus becoming a promising alternative for antimicrobial applications. The developed PE showed inhibitory effects on Alternaria alternata and Botrytis cinerea.
000 rpm for 10 min. Pound cake is a snack and dessert, popular for its flavour and mouthfeel. Pound cake is also susceptible to microbial growth, particularly mould.88 The PE of 20 g ZNS + 15 g oil + 5 g CEO was found to be the best substitute 20% butter in pound cake with a shelf-life extension of four days without changing the colour and texture. Jiang et al. (2020)89 reported zein-pectin nanoparticles as an emulsifier for CEO-based PEs. Zein-pectin nanoparticles are ideal to be used as Pickering particles due to their stability, sustainability, and low cost. Fresh-cut apple slices were dipped into the diluted PE and stored at 20 °C for six days. The emulsion had improved antibacterial properties compared to the pure EO due to sustained-release properties and appropriate dispersibility, thus becoming a promising alternative for antimicrobial applications. The developed PE showed inhibitory effects on Alternaria alternata and Botrytis cinerea.
The study by Fasihi et al. (2019)44 focussed on the development of active films of carboxymethyl cellulose (CMC)–polyvinyl alcohol (PVA) integrating CEO as a PE. The in situ hydrophobisation method using oleic acid (OL) was used for preparing the PE. The films displayed 100% antifungal activity (against Penicillium digitatum) at a concentration of 1.5% and 3% CEO, and superior anti-UV properties, and therefore proposed as a promising material for bread preservation.
6.3. Oregano oil
Oregano (Origanum vulgare) is an important herb grown mainly in the Mediterranean and Asia regions, rich in phenolic compounds.90 The significant compounds in oregano essential oil (OEO) are thymol, carvacrol, and γ-terpinene, which have strong anti-inflammatory, antioxidant and antibacterial properties.90–92 OEO is reported to have broad-spectrum antibacterial properties.93In the research by Zhang et al. (2022),94 the production of OEO-PE stabilised by zein-pectin composite (ZPEO) was attempted. Konjac glucomannan (KGM) is a renewable water-soluble polysaccharide abundant in tubers of Amorphophallus konjac. KGM is composed of β-D-glucose and β-D-mannose.95,96 KGM is applied as an edible and biodegradable packaging material mainly because of its biodegradability, non-toxicity, biocompatibility, and film-forming ability.94,95 An edible film of KGM and a KGM film comprising ZPEO at various concentrations were prepared, and their suitability as edible films for food applications was evaluated and compared. The ZPEO–KGM films were uniform and compatible with potent antioxidant and antibacterial properties. They also had fruit preservation properties, as observed in strawberries. On increasing the emulsion content in the films, the antioxidant and antibacterial properties increased, and EAB also increased, whereas WVP decreased. This study validated the plasticising effect of ZPEO along with the potential of ZPEO–KGM films for food preservation or packaging applications.
ZnO nanoparticles (1–100 nm) can be used as a food antimicrobial agent; they have activity against Escherichia coli, Listeria monocytogenes, and Vibrio cholerae.97–99 The preparation of a PE using ZnO-NPs and OEO with improved antimicrobial efficacy and better release properties was reported,51 where OEO was encapsulated entirely, and microcapsules were formed. The developed PE was blended with a cellulose nanofibril (CNF) film-forming matrix. The antioxidant and antimicrobial activity of the film was high, with barrier properties against oxygen, water vapour, and visible light, thereby enhancing the shelf life of foods.
A CNC-stabilised PE of OEO was developed by Zhou et al. (2018)35 (Fig. 3A). The factors that influence the formation of the PE were also studied. CNCs were prepared from microcrystalline cellulose (MCC) using ammonium persulfate (APS) hydrolysis. The emulsions with higher CNC concentration and pH values or a lower oil/water ratio and salt concentration were stable because they had a large absolute magnitude of Zp. The average droplet size decreased with increasing particle concentration in the emulsions. Increasing the volume fraction of oil with fixed particle concentration increased the average drop diameter. The PE inhibited the growth of microorganisms, but the MIC indicated that the antimicrobial efficiency of the PE was marginally lower than that of pure OEO. Further studies must be conducted to enhance antimicrobial activity.
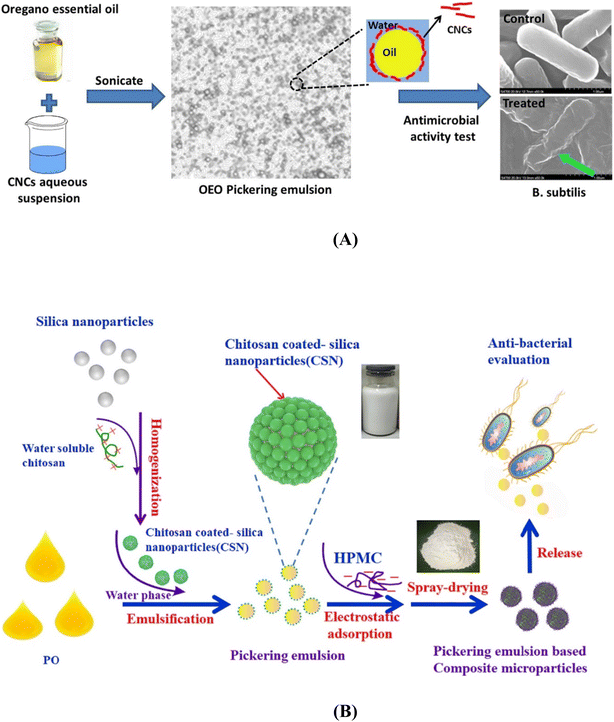 | ||
| Fig. 3 Schematic image of (A) oregano essential oil Pickering emulsion stabilized by CNCs. Adapted with permission from (Zhou et al., 2018).35 Copyright 2018 Elsevier. (B) Peppermint oil-loaded composite microcapsule based on chitosan-decorated silica nanoparticle stabilized Pickering emulsion templating. Adapted with permission from (Lai et al., 2021).107 Copyright 2021 Elsevier. | ||
6.4. Other essential oils
Tea tree oil (TTO) from Melaleuca alternifolia is an efficient antioxidant and a broad-spectrum antibacterial agent but is one of the least explored EOs. It contains mainly cyclic monoterpenes, sesquiterpenes, and accompanying tertiary alcohols.100,101 Compounds found in TTO include p-cymene, terpinen-4-ol, terpinolene, 1, 8-cineole, α-pinene, and γ-terpinene. The main active constituent of the oil is terpinene-4-ol.102Zinc sulfide nanoparticles (ZnSNPs) have antimicrobial properties and low toxicity.103 Roy & Rhim's (2021)104 work aimed at developing a carrageenan/agar-based film incorporated with ZnSNPs and TTO-PE stabilised with nanocellulose fibres. The loading efficiency of TTO in the nanocellulose-based PE was greater than 95%. Incorporating ZnSNPs and PET, alone/in combination, slightly improved the film's water vapour barrier, water resistance, and thermal stability. The film exhibited moderate antimicrobial and antioxidant activity.
The peppermint oil (PO) extracted from Peppermint Mentha piperita is utilised in food, cosmetic and pharmaceutical industries.105 The major components identified in the oil were menthone, menthol, menthofuran, β-phellandrene, isomenthone, menthol acetate, etc.106 These components are responsible for the antibacterial, antifungal, anti-inflammatory, and antiviral activity of PO.107 Surface modification of chitosan to produce chitosan-decorated silica nanoparticles for stabilising the PO PE is reported (Fig. 3B).107 The hydrophobicity of silica nanoparticles was enhanced by surface modification of chitosan facilitating adsorption at the O/W interface. An in vitro study revealed the sustained release of PO from the emulsion. The PE was coated with hydroxypropyl methylcellulose (HPMC) as a wall material and then spray-dried to produce microcapsules (PO-CM). PO-CM turns out to be a promising food antibacterial agent as it exhibited antibacterial activity, even after 60 days of storage.
Marjoram essential oil (MEO) extracted from Origanum majorana L. has been reported to exhibit antimicrobial, anticancer, antioxidant, and anti-inflammatory properties.108,109 The major components are terpinen-4-ol, (+)-cis-sabinene hydrate, α- and γ-terpinene and terpinolene, thymol, and carvacrol.110 The EO is used as a preservative and a flavouring agent. Whey protein isolate (WPI) is a better Pickering particle for encapsulating hydrophobic bioactives.111 The use of inulin as a secondary encapsulating material is of research interest. Inulin needs to be combined with other wall materials as it lacks emulsifying properties.112,113 In the study by Almasi et al. (2020),43 the NE and PE of MEO were incorporated to develop an active film of pectin. The Pickering particle used for the study was WPI/inulin. The particle size for the NE and PE was 97.5 and 233.3 nm, respectively. Films incorporated with the PE were dense, had lower permeability, and exhibited good mechanical and water barrier properties. The antioxidant activity was lower than that of NE films. Films with the PE, when compared to the NE, had a slow-release profile for the bioactive. The films with the PE had unique features compared to the NE, thus paving the way for pectin-based active food packaging systems.
Cedarwood essential oil is extracted from the root, stem, leaves, and flowers of Cedrus deodara. It has antibacterial, insect-repellent, anti-inflammatory, and analgesic effects.15,114 The compounds responsible for these properties include α-cedarene, β-cedarene, and thujopsene.115 The PE was prepared using octenyl succinic anhydride (OSA)-modified starch and the NE using Tween 80 and Span 80. Both emulsions were studied for their antioxidant and antibacterial effects.115 The NE and PE particle sizes were 135.14 ± 1.1 nm and 626.21 ± 6.05 nm, respectively. The highest stability of the NE was observed at an emulsifier concentration of 5%, and that of the PE was at 1% concentration of OSA-modified starch, and the stability and antioxidant activity of the NE were greater than those of the PE, whereas the PE was better in terms of antibacterial activity. The application and stability of cedarwood oil are reported to be improved by emulsification.
Rosemary essential oil (REO) extracted from Rosmarinus officinalis L. is used as a flavouring agent and is a GRAS additive according to FDA 21 CFR (Code of Federal Regulation) part 182.20. The oil has antioxidant and antimicrobial properties. The major components in REO are 1,8-Cineole, α, and β-Pinene, Camphor, and Camphene.56,116 A biodegradable film was made from CMC and PVA, emulsified with oleic acid (OL), and combined with REO.56 The physico-chemical properties of the film and its antifungal properties were determined. The films had an increased UV absorbance, and EAB reduced tensile strength and thermal stability and exhibited antioxidant and antimicrobial properties. Antifungal tests performed in vitro against Penicillium digitatum displayed 100% inhibition in films containing 3% oil. Similarly, no fungal growth was observed in bread slices stored within films containing 3% REO after 60 days of storage at 25 °C, indicating a slow release of oil. The study suggested that using a PE is a promising method to improve the properties of active films.
The role of CNFs as a Pickering particle for the encapsulation of EOs from Cinnamon cassia (Cinnamomum cassia), cardamom (Elettaria cardamomum), and Ho wood (Cinnamomum camphora) and the stability of the PE were studied by A. G. Souza et al. (2020).18 The freshly prepared PE had a particle size of 12–14 μm. An increase in the size of CNF-Ho wood emulsion was observed, suggesting low emulsion stability, whereas the other two maintained similar sizes. The volume of emulsions decreased during storage, indicating the evaporation of non-stabilised EO. The cardamom EO emulsion preserved a homogeneous nature without phase separation, even if there was a reduction in the volume of CNF-Cardamom. The chemical structures of cardamom oil and cinnamon oil are stable, due to the presence of electron pairs in resonance which favour the electronic attraction between the components, i.e., the aldehyde group and the cellulose hydroxyls in the case of CNF-cassia and CNF-cardamom electronic attraction takes place between esters and cellulose hydroxyls, and there is steric stabilisation forming a CNF 3D network. Ho wood oil has the most reactive structure; therefore, the addition of CNFs leads to the development of hydrogen bonding between the alcohol group of linalool and cellulose hydroxyls, consequently creating a monolayer around the oil and inducing coalescence. These results suggests that this PE paves for multiple applications in various fields like active food packaging, biocide on surfaces, cosmetics, antibacterial cleaning products, or as a wound-healing agent.
7. Prospects and conclusion
Pickering emulsions have overcome many of the critical drawbacks of conventional emulsions, and it is expected to be a potential alternative in providing stability to encapsulates and their sustained release. Its environment friendliness, low cost, and better quality and stability over conventional emulsion make it an area of increasing interest. The antimicrobial and antioxidant properties of EOs are not fully exploited for food applications mainly due to their pungency, volatility, and chemical instability. The application of a PE in EOs for food applications was thus found promising. Studies suggest that EO-based PEs can directly act as a preservative in food formulations and products and can be incorporated into active and edible packaging applications. Most of the studies on films incorporated with PEs with EOs reported improved film barrier properties. Further studies need to focus on improving the tensile and other mechanical properties of films loaded with EO-based PEs.In conclusion, essential oil-based Pickering emulsions are non-toxic and biodegradable and can be fabricated using natural Pickering particles and therefore have applications in food, cosmetics, biomedicine, etc. They can find applications in food as a preservative, antioxidant and antimicrobial agent. The biodegradability issues with conventional packaging materials pave the way for incorporating an EO-loaded PE into biodegradable packaging films to generate active and edible films. The application of an EO-loaded PE as a natural preservative for improved shelf stability of food products needs to be exploited further. The applications of EO-loaded PEs in biodegradable packaging applications need to be explored further with a focus on improving the barrier properties and mechanical strength. The challenges associated with PEs for EOs include the selection of appropriate Pickering particles, large-scale production, delivery of desired attributes in the food product, stability under food processing conditions and controlled or sustained release of the encapsulated material. More studies are required to identify the properties of Pickering particles. Pickering emulsions of spice essential oil need to be exploited further as a green process to enhance the shelf life of agriproducts, processed foods, and other applications such as drug delivery, oil recovery, and cosmetics.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Reshma Krishnan and Dr P Nisha acknowledge the Spices Board, India for funding (MKT-PD&R/0002/2020-Marketing.), and the authors acknowledge CSIR, India for the facilities.References
- R. Becerril, C. Nerín and F. Silva, Molecules, 2020, 25, 1134 CrossRef CAS PubMed
.
- A. G. de Souza, R. R. Ferreira, E. S. F. Aguilar, L. Zanata and D. dos S. Rosa, Polysaccharides, 2021, 2, 608–625 CrossRef CAS
.
- USFDA, CFR – Code of Federal Regulations Title 21, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=182%26showFR=1, accessed 15 February 2022.
-
Grand View Research, Essential Oils Market Size|Industry Report, 2021-2028, 2020 Search PubMed
.
-
E. Chivandi, R. Dangarembizi, T. T. Nyakudya and K. H. Erlwanger, in Essential Oils in Food Preservation, Flavor and Safety, Elsevier, 2016, pp. 85–91 Search PubMed
.
- A. M. Ojeda-Sana, C. M. van Baren, M. A. Elechosa, M. A. Juárez and S. Moreno, Food Control, 2013, 31, 189–195 CrossRef CAS
.
- S. Roy and J.-W. Rhim, Colloids Surf., A, 2021, 627, 127220 CrossRef CAS
.
- P. Tongnuanchan and S. Benjakul, J. Food Sci., 2014, 79, R1231–R1249 CrossRef CAS PubMed
.
- A. Mutlu-Ingok, D. Devecioglu, D. N. Dikmetas, F. Karbancioglu-Guler and E. Capanoglu, Molecules, 2020, 25, 4711 CrossRef CAS PubMed
.
- C. Turek and F. C. Stintzing, Compr. Rev. Food Sci. Food Saf., 2013, 12, 40–53 CrossRef CAS
.
- S. M. Ojagh, M. Rezaei, S. H. Razavi and S. M. H. Hosseini, Food Chem., 2010, 122, 161–166 CrossRef CAS
.
- H. Majeed, Y.-Y. Bian, B. Ali, A. Jamil, U. Majeed, Q. F. Khan, K. J. Iqbal, C. F. Shoemaker and Z. Fang, RSC Adv., 2015, 5, 58449–58463 RSC
.
- L. Chen, F. Ao, X. Ge and W. Shen, Molecules, 2020, 25, 3202 CrossRef CAS PubMed
.
- F. Zhu, Trends Food Sci. Technol., 2019, 85, 129–137 CrossRef CAS
.
- S. Zrira and M. Ghanmi, J. Essent. Oil-Bear. Plants, 2016, 19, 1267–1272 CrossRef CAS
.
- Y. Cahyana, Y. S. E. Putri, D. S. Solihah, F. S. Lutfi, R. M. Alqurashi and H. Marta, Molecules, 2022, 27, 7872 CrossRef CAS PubMed
.
- S. Zhao, G. Tian, C. Zhao, C. Li, Y. Bao, C. DiMarco-Crook, Z. Tang, C. Li, D. Julian McClements, H. Xiao and J. Zheng, Food Chem., 2018, 269, 577–587 CrossRef CAS PubMed
.
- A. G. Souza, R. R. Ferreira, L. C. Paula, L. F. G. Setz and D. S. Rosa, J. Mol. Liq., 2020, 320, 114458 CrossRef CAS
.
- C. C. Berton-Carabin and K. Schroën, Annu. Rev. Food Sci. Technol., 2015, 6, 263–297 CrossRef CAS PubMed
.
- L. E. Low, S. P. Siva, Y. K. Ho, E. S. Chan and B. T. Tey, Adv. Colloid Interface Sci., 2020, 277, 102117 CrossRef CAS PubMed
.
- J. Tang, P. J. Quinlan and K. C. Tam, Soft Matter, 2015, 11, 3512–3529 RSC
.
- W. Ramsden, Proc. R. Soc. London, 1904, 72, 156–164 Search PubMed
.
- S. U. Pickering, J. Chem. Soc. Trans., 1907, 91, 2001–2021 RSC
.
- T. Xia, C. Xue and Z. Wei, Trends Food Sci. Technol., 2021, 107, 1–15 CrossRef CAS
.
- I. Kalashnikova, H. Bizot, B. Cathala and I. Capron, Langmuir, 2011, 27, 7471–7479 CrossRef CAS PubMed
.
-
D. J. McClements, Food Emulsions: Principles, Practices, and Techniques, CRC Press, 2nd Edition, 2004 Search PubMed
.
- W. W. Mwangi, H. P. Lim, L. E. Low, B. T. Tey and E. S. Chan, Trends Food Sci. Technol., 2020, 100, 320–332 CrossRef CAS
.
- M. Matos, A. Timgren, M. Sjöö, P. Dejmek and M. Rayner, Colloids Surf., A, 2013, 423, 147–153 CrossRef CAS
.
- L. Miclotte, K. De Paepe, L. Rymenans, C. Callewaert, J. Raes, A. Rajkovic, J. Van Camp and T. Van de Wiele, Front. Microbiol., 2020, 11 DOI:10.3389/fmicb.2020.577474
.
- C. Linke and S. Drusch, Crit. Rev. Food Sci. Nutr., 2018, 58, 1971–1985 CrossRef CAS PubMed
.
- D. Gonzalez Ortiz, C. Pochat-Bohatier, J. Cambedouzou, M. Bechelany and P. Miele, Engineering, 2020, 6, 468–482 CrossRef
.
- G. Kaptay, Colloids Surf., A, 2006, 282–283, 387–401 CrossRef
.
- J.-X. Liu, H.-J. Zhu, P. Wang and J.-M. Pan, Pet. Sci., 2021, 18, 1551–1563 CrossRef CAS
.
- Z. Sun, X. Yan, Y. Xiao, L. Hu, M. Eggersdorfer, D. Chen, Z. Yang and D. A. Weitz, Particuology, 2022, 64, 153–163 CrossRef CAS
.
- Y. Zhou, S. Sun, W. Bei, M. R. Zahi, Q. Yuan and H. Liang, Int. J. Biol. Macromol., 2018, 112, 7–13 CrossRef CAS PubMed
.
- B. Yudhistira, A. S. Sulaimana, F. Punthi, C.-K. Chang, C. T. Lung, S. P. Santoso, M. Gavahian and C.-W. Hsieh, SSRN Electron. J., 2022, 14, 1618 CAS
.
- A. Ghadetaj, H. Almasi and L. Mehryar, Food Packag. Shelf Life, 2018, 16, 31–40 CrossRef
.
-
T. A. M. Msagati, in Chemistry of Food Additives and Preservatives, Wiley, 2012, pp. 67–82 Search PubMed
.
- B. P. Binks, Adv. Mater., 2002, 14, 1824–1827 CrossRef CAS
.
- G. Gauthier and I. Capron, JCIS Open, 2021, 4, 100036 CrossRef
.
- M. V. Kempin, M. Kraume and A. Drews, J. Colloid Interface Sci., 2020, 573, 135–149 CrossRef CAS PubMed
.
- C. Albert, M. Beladjine, N. Tsapis, E. Fattal, F. Agnely and N. Huang, J. Controlled Release, 2019, 309, 302–332 CrossRef CAS PubMed
.
- H. Almasi, S. Azizi and S. Amjadi, Food Hydrocolloids, 2020, 99, 105338 CrossRef CAS
.
- H. Fasihi, N. Noshirvani, M. Hashemi, M. Fazilati, H. Salavati and V. Coma, Food Packag. Shelf Life, 2019, 19, 147–154 CrossRef
.
- A. Sharkawy, M. F. Barreiro and A. E. Rodrigues, Carbohydr. Polym., 2020, 250, 116885 CrossRef CAS PubMed
.
- D. Wang, K. Wang, L. Zhao, X. Liu and Z. Hu, J. Food Eng., 2023, 339, 111264 CrossRef CAS
.
- Z. Li, W. Hu, J. Dong, F. Azi, X. Xu, C. Tu, S. Tang and M. Dong, Food Sci. Hum. Wellness, 2023, 12, 669–679 CrossRef CAS
.
- H. Dai, J. Wu, H. Zhang, Y. Chen, L. Ma, H. Huang, Y. Huang and Y. Zhang, Trends Food Sci. Technol., 2020, 102, 16–29 CrossRef CAS
.
- J. Wu and G. H. Ma, Small, 2016, 12, 4633–4648 CrossRef CAS PubMed
.
- H. Sun, S. Li, S. Chen, C. Wang, D. Liu and X. Li, Int. J. Biol. Macromol., 2020, 159, 696–703 CrossRef CAS PubMed
.
- M. Wu, Z. Zhou, J. Yang, M. Zhang, F. Cai and P. Lu, Int. J. Biol. Macromol., 2021, 190, 433–440 CrossRef CAS PubMed
.
- Y. Xu, Y. Chu, X. Feng, C. Gao, D. Wu, W. Cheng, L. Meng, Y. Zhang and X. Tang, Int. J. Biol. Macromol., 2020, 156, 111–119 CrossRef CAS PubMed
.
- Y.-Q. Hu, S.-W. Yin, J.-H. Zhu, J.-R. Qi, J. Guo, L.-Y. Wu, C.-H. Tang and X.-Q. Yang, Food Hydrocolloids, 2016, 61, 300–310 CrossRef CAS
.
- M. Gestranius, P. Stenius, E. Kontturi, J. Sjöblom and T. Tammelin, Colloids Surf., A, 2017, 519, 60–70 CrossRef CAS
.
- V. Martinez and M. Henary, Chem.–Eur. J., 2016, 22, 13764–13782 CrossRef CAS PubMed
.
- H. Fasihi, M. Fazilati, M. Hashemi and N. Noshirvani, Carbohydr. Polym., 2017, 167, 79–89 CrossRef CAS PubMed
.
- D. J. Mcclements, Crit. Rev. Food Sci. Nutr., 2007, 47, 611–649 CrossRef CAS PubMed
.
- G. Marrucci, Chem. Eng. Sci., 1969, 24, 975–985 CrossRef CAS
.
- S. Tcholakova, N. D. Denkov and A. Lips, Phys. Chem. Chem. Phys., 2008, 10, 1608 RSC
.
- D. Guzey and D. J. McClements, Adv. Colloid Interface Sci., 2006, 128–130, 227–248 CrossRef CAS PubMed
.
- S. J. Choi, J. W. Won, K. M. Park and P.-S. Chang, J. Food Process Eng., 2014, 37, 229–236 CrossRef CAS
.
- M. Nikbakht Nasrabadi, A. Sedaghat Doost, S. A. H. Goli and P. Van der Meeren, Food Chem., 2020, 311, 125872 CrossRef CAS PubMed
.
- E. Hosseini, A. Rajaei, M. Tabatabaei, A. Mohsenifar and K. Jahanbin, Food Biophys., 2020, 15, 216–228 CrossRef
.
- M. Atarian, A. Rajaei, M. Tabatabaei, A. Mohsenifar and H. Bodaghi, Carbohydr. Polym., 2019, 210, 47–55 CrossRef CAS PubMed
.
- R. S. Hosseini and A. Rajaei, Carbohydr. Polym., 2020, 241, 116340 CrossRef CAS PubMed
.
-
B. Matthäus, in Oxidation in Foods and Beverages and Antioxidant Applications, Elsevier, 2010, pp. 183–238 Search PubMed
.
-
A. Syed, in Oxidative Stability and Shelf Life of Foods Containing Oils and Fats, Elsevier, 2016, pp. 187–207 Search PubMed
.
- R. Ribeiro-Santos, M. Andrade, A. Sanches-Silva and N. R. de Melo, Food Bioprocess Technol., 2018, 11, 43–71 CrossRef CAS
.
- W. Wang, Y. Zhang, Z. Yang and Q. He, Int. J. Biol. Macromol., 2021, 166, 578–586 CrossRef CAS PubMed
.
- R. de Souza Silva, B. M. M. Santos, G. G. Fonseca, C. Prentice and W. R. Cortez-Vega, J. Polym. Environ., 2020, 28, 421–432 CrossRef CAS
.
- N. Hasheminejad, F. Khodaiyan and M. Safari, Food Chem., 2019, 275, 113–122 CrossRef CAS PubMed
.
- H. Yu, G. Huang, Y. Ma, Y. Liu, X. Huang, Q. Zheng, P. Yue and M. Yang, Int. J. Biol. Macromol., 2021, 170, 24–32 CrossRef CAS PubMed
.
- S. Dehghani, S. V. Hosseini and J. M. Regenstein, Food Chem., 2018, 240, 505–513 CrossRef CAS PubMed
.
- Y. Shen, Z. J. Ni, K. Thakur, J. G. Zhang, F. Hu and Z. J. Wei, Int. J. Biol. Macromol., 2021, 181, 528–539 CrossRef CAS PubMed
.
- S. P. Bangar, W. S. Whiteside, K. D. Dunno, G. A. Cavender and P. Dawson, Food Res. Int., 2022, 157, 111384 CrossRef CAS PubMed
.
- S. Punia Bangar, W. S. Whiteside, F. Ozogul, K. D. Dunno, G. A. Cavender and P. Dawson, Food Biosci., 2022, 47, 101621 CrossRef CAS
.
- Y. C. Wong, M. Y. Ahmad-Mudzaqqir and W. A. Wan-Nurdiyana, Orient. J. Chem., 2014, 30, 37–47 CrossRef CAS
.
- M. Ghaderi-Ghahfarokhi, M. Barzegar, M. A. Sahari, H. Ahmadi Gavlighi and F. Gardini, Int. J. Biol. Macromol., 2017, 102, 19–28 CrossRef CAS PubMed
.
- J. Hu, X. Wang, Z. Xiao and W. Bi, LWT–Food Sci. Technol., 2015, 63, 519–526 CrossRef CAS
.
- Y. Han, M. Yu and L. Wang, Food Packag. Shelf Life, 2018, 15, 35–42 CrossRef
.
- E. Dickinson, Food Hydrocolloids, 2018, 78, 2–14 CrossRef CAS
.
- Q. Lin, R. Liang, F. Zhong, A. Ye and H. Singh, Food Hydrocolloids, 2018, 77, 549–556 CrossRef CAS
.
- C. Yan, D. J. McClements, L. Zou and W. Liu, Food Funct., 2019, 10, 5446–5460 RSC
.
- J. Liu, F. Song, R. Chen, G. Deng, Y. Chao, Z. Yang, H. Wu, M. Bai, P. Zhang and Y. Hu, Carbohydr. Polym., 2022, 275, 118704 CrossRef CAS PubMed
.
- K. Yu, J. Xu, L. Zhou, L. Zou and W. Liu, Foods, 2021, 10, 3003 CrossRef CAS PubMed
.
- X. Feng, Y. Sun, Y. Yang, X. Zhou, K. Cen, C. Yu, T. Xu and X. Tang, LWT, 2020, 122, 109025 CrossRef CAS
.
- J. Xiao, Y. Li and Q. Huang, Trends Food Sci. Technol., 2016, 55, 48–60 CrossRef CAS
.
- J. Ju, X. Xu, Y. Xie, Y. Guo, Y. Cheng, H. Qian and W. Yao, Food Chem., 2018, 240, 850–855 CrossRef CAS PubMed
.
- Y. Jiang, D. Wang, F. Li, D. Li and Q. Huang, Int. J. Biol. Macromol., 2020, 148, 1280–1289 CrossRef CAS PubMed
.
- A. Figiel, A. Szumny, A. Gutiérrez-Ortíz and Á. A. Carbonell-Barrachina, J. Food Eng., 2010, 98, 240–247 CrossRef CAS
.
- H. Cui, C. Zhang, C. Li and L. Lin, Ind. Crops Prod., 2019, 139, 111498 CrossRef CAS
.
- A. Govaris, N. Solomakos, A. Pexara and P. S. Chatzopoulou, Int. J. Food Microbiol., 2010, 137, 175–180 CrossRef CAS PubMed
.
- M. Gonçalves Cattelan, M. Bonatto Machado de Castilhos, P. Juliana Pinsetta Sales and F. Leite Hoffmann, Nutr. Food Sci., 2013, 43, 169–174 CrossRef
.
- S. Zhang, Z. He, F. Xu, Y. Cheng, G. I. N. Waterhouse, D. Sun-Waterhouse and P. Wu, Food Hydrocolloids, 2022, 124, 107222 CrossRef CAS
.
- J. Wang, X. Chen, C. Zhang, A. R. Akbar, Z. Shi, Q. Yang and C. Xiong, Cellulose, 2019, 26, 3155–3165 CrossRef CAS
.
- C. Wu, Y. Li, Y. Du, L. Wang, C. Tong, Y. Hu, J. Pang and Z. Yan, Food Hydrocolloids, 2019, 89, 682–690 CrossRef CAS
.
- R. M. Al-Mosawi and R. M. Al-Badr, IOSR J. Dent. Med. Sci., 2017, 16, 49–55 CrossRef
.
- P. J. P. Espitia, N. d. F. F. Soares, J. S. d. R. Coimbra, N. J. de Andrade, R. S. Cruz and E. A. A. Medeiros, Food Bioprocess Technol., 2012, 5, 1447–1464 CrossRef CAS
.
- S. Sheik Mydeen, R. Raj Kumar, M. Kottaisamy and V. S. Vasantha, J. Saudi Chem. Soc., 2020, 24, 393–406 CrossRef CAS
.
-
S. D. Cox, C. M. Mann, J. L. Markham, J. E. Gustafson, J. R. Warmington and S. G. Wyllie, in Molecules, 2001, vol. 6, pp. 87–91 Search PubMed
.
- I. Tarach, E. Olewnik-Kruszkowska, A. Richert, M. Gierszewska and A. Rudawska, Materials, 2020, 13, 1–16 CrossRef PubMed
.
- E. Yadav, S. Kumar, S. Mahant, S. Khatkar and R. Rao, J. Essent. Oil Res., 2017, 29, 201–213 CrossRef CAS
.
- S. Kannan, N. P. Subiramaniyam and M. Sathishkumar, J. Mater. Sci.: Mater. Electron., 2020, 31, 9846–9859 CrossRef CAS
.
- S. Roy and J. W. Rhim, Int. J. Biol. Macromol., 2021, 193, 2038–2046 CrossRef CAS PubMed
.
- M. Moghaddam, M. Pourbaige, H. K. Tabar, N. Farhadi and S. M. A. Hosseini, J. Essent. Oil-Bear. Plants, 2013, 16, 506–512 CrossRef CAS
.
- J. B. Cannon, C. L. Cantrell, T. Astatkie and V. D. Zheljazkov, Ind. Crops Prod., 2013, 41, 214–220 CrossRef CAS
.
- H. Lai, Y. Liu, G. Huang, Y. Chen, Y. Song, Y. Q. Ma and P. Yue, Int. J. Biol. Macromol., 2021, 183, 2314–2325 CrossRef CAS PubMed
.
- E. Arranz, L. Jaime, M. C. López de las Hazas, G. Reglero and S. Santoyo, Ind. Crops Prod., 2015, 67, 121–129 CrossRef CAS
.
- H. Hajlaoui, H. Mighri, M. Aouni, N. Gharsallah and A. Kadri, Microb. Pathog., 2016, 95, 86–94 CrossRef CAS PubMed
.
- E. Vági, B. Simándi, Á. Suhajda and É. Héthelyi, Food Res. Int., 2005, 38, 51–57 CrossRef
.
- P. N. Ezhilarasi, D. Indrani, B. S. Jena and C. Anandharamakrishnan, J. Food Eng., 2013, 117, 513–520 CrossRef CAS
.
- R. V. de B. Fernandes, S. V. Borges, D. A. Botrel and C. R. de Oliveira, Int. J. Food Sci. Technol., 2014, 49, 1522–1529 CrossRef CAS
.
- S. Ronkart, C. Blecker, C. Fougnies, J. C. Van Herck, J. Wouters and M. Paquot, Carbohydr. Polym., 2006, 63, 210–217 CrossRef CAS
.
- F. J. Eller, R. K. V. Meer, R. W. Behle, L. B. Flor-Weiler and D. E. Palmquist, Environ. Entomol., 2014, 43, 762–766 CrossRef CAS PubMed
.
- K. Huang, R. Liu, Y. Zhang and X. Guan, Food Chem., 2021, 346, 128970 CrossRef CAS PubMed
.
- Y. Jiang, N. Wu, Y.-J. Fu, W. Wang, M. Luo, C.-J. Zhao, Y.-G. Zu and X.-L. Liu, Environ. Toxicol. Pharmacol., 2011, 32, 63–68 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |