Open Access Article
Open Access ArticleSingle-cell protein production by Pleurotus ostreatus in submerged fermentation†
Georgios
Bakratsas
 a,
Angeliki
Polydera
a,
Oskar
Nilson‡
b,
Lalie
Kossatz
b,
Charilaos
Xiros
b,
Petros
Katapodis
*a and
Haralambos
Stamatis
a,
Angeliki
Polydera
a,
Oskar
Nilson‡
b,
Lalie
Kossatz
b,
Charilaos
Xiros
b,
Petros
Katapodis
*a and
Haralambos
Stamatis
 *a
*a
aBiotechnology Laboratory, Department of Biological Applications and Technologies, University of Ioannina, 45110 Ioannina, Greece. E-mail: pkatapo@uoi.gr; hstamati@uoi.gr
bRISE Processum AB, SE-891 22 Örnsköldsvik, Sweden
First published on 21st February 2023
Abstract
Agricultural land shrinkage, decreasing global water resources, population increase and malnutrition highlight the need for new food sources. Single-cell protein derived from microorganisms could be a solution to high protein demand. The aim of this work was to optimize the cultivation conditions for single-cell protein production by Pleurotus ostreatus LGAM 1123 in submerged cultures and valorize fiber sludge, a low cost industrial side stream from the pulp and paper industry, as a substrate for single-cell protein (SCP) production. A study on the effect of different cultivation conditions on fungal growth and protein production has been conducted. Response surface methodology was used to investigate the combined effect of the most important factors (glucose and yeast extract medium concentrations) and optimize the process. A maximum protein production of 10.0 ± 0.9 g L−1 was found for the submerged cultivation of the fungus in a 3.5 L stirred-tank bioreactor, while the biomass produced and its total protein content were 26.0 ± 2.0 g L−1 and 44.8 ± 0.8%, respectively. As an industrial application, a cellulosic hydrolysate obtained after enzymatic hydrolysis of fibre sludge in the optimized medium composition was used. Fibre sludge was shown to be an excellent feedstock for SCP production achieving productivity and protein content very similar to glucose fermentations. Single-cell protein of P. ostreatus presented higher amino acid scores compared to the recommended ones for valine, leucine, and aromatic amino acids in human nutrition. Therefore, P. ostreatus biomass could stand as an alternative vegan protein source due to its high protein content and amino acid composition.
Introduction
A deficit of protein sources observed in the market worldwide is a problem that could be expanded in the next few decades. The predicted increase in population up to 9 billion until 2050, as well as the increase in the number of malnourished people, especially in developing countries, could have a negative impact on this situation resulting in an increase in the demand for food in general.1 Moreover, the ongoing shrinking of agricultural land and decreasing global water resources could hinder both cultivation of rich-in-protein crops (e.g. soybeans) and animal/fish farming in the future, leading to a decrease in the main protein sources used in human nutrition. In addition, the use of high protein wild fish for the production of fishmeal used in aquaculture or animal breeding instead of human nutrition is also a matter of concern.1 Research towards alternative protein sources could therefore be an answer to the problem of high protein demand. The usage of, especially, vegan protein sources is gaining attention due to advantages such as low environmental cost and reduced animal product consumption and carbon footprint.2Searching for alternative vegan protein sources should lead us to the exploitation of high protein biomass derived from microorganisms such as algae, yeasts, fungi or bacteria, also known as single-cell proteins (SCPs).3 Mycoprotein was first discovered in the 1960s and is referred to as a sustainable protein derived from fungi. The first related food product approved for sale, first in the UK, then in the European Union, and finally around the globe, was Quorn™.4 In addition, attempts to produce food products from mycoproteins have been made by different biotechnology companies.5
Although in the last few decades many small and medium-sized enterprises (SMEs) dealing with SCP production have been founded, the use of pure sugars or starch-originated glucose as the feedstock raises sustainability concerns. The next target for the SCP industry is to use inexpensive, sustainable feedstocks as the source of glucose. Using an industrial side stream for such a purpose would improve process economics as well as the ecological impact of SCP production. To this end, side streams with high carbohydrate content derived from the forestry industry, the food sector, the pulp and paper industry, or agriculture residues could be excellent feedstock candidates for SCP production. The successful implementation of such sugar sources in SCP value chains would establish microbial SCP production as a rapidly developing technology that could play an important role in the alternative protein market, supplying cheap and environmentally clean proteins with a virtually unlimited production scale.6–9
Apart from SCP, mushrooms are also potential candidates to stand as a vegan-protein source next to other plant-based proteins due to their high protein content, texture and aroma.10 One of the members of Basidiomycota that is consumed worldwide in large quantities is genus Pleurotus and more specifically Pleurotus ostreatus, which is an edible mushroom with a specific taste and aroma, low calories and also nutraceutical properties.11 Except for its high protein content, P. ostreatus should be considered a vegan protein source due to its high protein quality and the ability to provide us with essential amino acids and nitrogen useful for different body functions.12 Among different protein sources such as mushrooms, beef jerky, whole milk and black beans, P. ostreatus seems to have the highest protein to energy ratio.12,13 Furthermore, P. ostreatus prevails due to its increased umami taste, which is the taste that meat amino acids have. Umami is closely related to the monosodium glutamate content, which is estimated from aspartic acid, glutamic acid and some 5′-nucleotides.11,14P. ostreatus has shown the highest equivalent umami concentration and best umami taste among 17 edible mushrooms, according to results from a trained human panel.14,15
Except for traditional cultivation of mushrooms, submerged cultivation of mycelia has gained attention in the last few decades. The main reasons for this were the faster growth, the safe biomass production, the control of cultivation factors, and the reproducibility of cultivation.16,17 Submerged cultivation is the growth of mycelia in liquid media containing carbon and nitrogen sources, as well as micronutrients, while oxygen supply is reinforced with agitation.18 The cultivation of P. ostreatus in a liquid medium, to be used as a source of enzymes, bioactive metabolites, fatty acids, glucans, dietary fibres, and anticancer exopolysaccharides, has already been reported.19–23 In addition, P. ostreatus seems to present low RNA levels (0.443 ± 0.031 mg g−1 biomass), something crucial for human consumption of single-cell protein produced by submerged cultivation of the strain.17 However, there are not a lot of studies in the scientific literature concerning protein content and amino acid analysis in submerged cultivation of P. ostreatus. A comparative study between submerged cultivated mycelia and fruiting bodies has shown higher protein content in the case of mycelial cultivation (29.76%) compared to that of fruiting bodies (24.69%).24 Regarding amino acid analysis, a study conducted for P. ostreatus “Florida” has shown that the mycelium contained higher levels of aspartic acid, cysteine, phenylalanine, and leucine, while fruiting bodies contained more valine and isoleucine.25 Manu Tawiah et al. have reported higher amino acid content in submerged cultivation of P. ostreatus in a waste medium rather than in a basal medium of glucose. Moreover, the essential amino acid composition in the case of a waste medium was similar to that of chicken eggs, as well as to the reference amino acid pattern provided by the FAO/WHO.26 The higher content of essential amino acids in mycelia rather than fruiting bodies, eggs and FAO/WHO references has also been confirmed in submerged cultivation of Cordyceps militaris.27
Protein quality and amino acid composition could be influenced by protein extraction methods. Protein extraction methods are separated into mechanical (ultrasound, microwave, bead milling, high-pressure homogenization, and pulsed electric field (PEF) technology), physical (osmotic shock and thermolysis), chemical (acid or alkali extraction, chelating agents, detergents, and solvents) and enzymatic (cellulases and proteases) ones or a combination of them.28,29 Conventional chemical extraction methods are not ideal for food applications, as they can influence their protein nutritional value. For example, alkali extraction activates a number of unhealthy reactions including denaturation, racemization and lysinoalanine formation, resulting in poor protein functionality and reduced protein nutritional value.30 In contrast, physical extraction techniques seem to prevail as being suitable for extraction of bioactive compounds such as proteins. Among them, ultrasound extraction is an inexpensive and simple method with shorter extraction times and lower operating temperatures.31
In the present work, aiming at the optimization of protein production in submerged cultivation of P. ostreatus LGAM 1123, the effect of different cultivation conditions on protein production was investigated. After a preliminary factor screening experimental design at two-levels, response surface methodology (RSM) was used to model the effect and interaction of the two most significant factors found, i.e., concentrations of carbon and nitrogen sources in the culture medium, on protein production. Among the different sources studied, glucose and yeast extract were selected to be used as a carbon and nitrogen source, respectively. Optimization of protein production has been conducted based on a central composite circumscribed (CCC) design composed of a factorial design and star points. The optimal glucose and yeast extract concentration values estimated by RSM have been used for the cultivation of P. ostreatus LGAM 1123 in a lab scale stirred-tank bioreactor to maximize protein production. An ultrasound technique has been used for protein extraction. Amino acid analysis has been conducted to estimate the protein quality and explore further the use of macrofungal biomass as a vegan protein source. Finally, investigating the potential of an industrial application of the process with a reduced total cost, SCP was produced in a lab scale bioreactor by submerged cultivation of P. ostreatus LGAM 1123 in a cellulosic hydrolysate obtained after enzymatic hydrolysis of fibre sludge, a side stream from the pulp and paper industry.
Experimental
Chemicals and reagents
All chemicals used in this study were of analytical grade. Potato dextrose agar (PDA) and yeast extract were purchased from Neogen Europe Ltd (UK). Glucose, zinc sulphate heptahydrate (ZnSO4·7H2O), manganese(II) sulfate heptahydrate (*7H2O) (MnSO4·H2O), ethylenediaminetetraacetic acid disodium salt dihydrate (*2H2O) (EDTA-Na2) (sodium EDTA), thiamine hydrochloride (vitamin B1), xylose, fructose, maltose, peptone, 4-(dimethylamino)azobenzene-4′-sulfonyl chloride, (dabsyl chloride), bovine serum albumin (BSA), and phenol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium nitrate (NaNO3), di-potassium hydrogen phosphate anhydrous (dibasic) (K2HPO4), potassium chloride (KCl), sodium nitrate (NaNO3), potassium nitrate (KNO3), ammonium chloride (NH4Cl) and ammonium sulfate ((NH4)2SO4) were purchased from AppliChem (Darmstadt, Germany). Magnesium sulfate anhydrous (MgSO4), calcium chloride dihydrate (CaCl2·2H2O), ferrous sulfate heptahydrate (*7H2O) (FeSO4·7H2O), ammonium molybdate (*4H2O) ((NH4)Mo7O2·4H2O), sucrose, and urea were purchased from Fluka (Switzerland). Sodium hydroxide (NaOH) was obtained from Panreac (Barcelona), chloroform, methanol, acetonitrile and Pierce™ BCA protein assay kit were purchased from Thermo Fisher Scientific (Waltham, USA), and sulfuric acid was purchased from Honeywell Riedel-de Haën. Hydrochloric acid (HCl) was purchased from Merck (KGaA Darmstadt, Germany).Microorganism
P. ostreatus LGAM 1123 from the fungal culture collection of the Laboratory of General and Agricultural Microbiology (Agricultural University of Athens, Athens, Greece) was used in this study. The strain was maintained on potato dextrose agar (PDA) and was preserved in Petri dishes at 4 °C.Media and growth conditions in Erlenmeyer flasks
For inoculum preparation, 1 cm of the PDA agar cultures was transferred with a sterilized cutter into a 250 mL Erlenmeyer flask containing 100 mL of the basal medium (g L−1): glucose, 30, yeast extract, 10, NaNO3, 0.4, MgSO4, 1.15, K2HPO4, 0.7, KCl, 0.75, ZnSO4·7H2O, 0.0114, CaCl2·2H2O, 0.52, MnSO4·H2O, 0.03, FeSO4·7H2O, 0.03, (NH4)Mo7O2·4H2O, 0.01, sodium EDTA, 0.75, and vitamin B1, 0.015. After twelve days of incubation in a rotary shaker at 150 rpm at 28 °C, 5 mL of culture was transferred into a new flask containing the basal medium slightly modified depending on the experiment. Before heat sterilization at 121 °C for 20 min, the initial pH of the medium was adjusted to 5.0 with the addition of 1 M NaOH. At certain time intervals, samples were withdrawn to measure the biomass produced as well as its protein content.Experimental design for optimization of protein production
With an objective to optimize protein production in submerged cultivation of P. ostreatus LGAM 1123, an initial screening of different parameters affecting protein production has been conducted. Concentrations of carbon and nitrogen sources in the culture medium were found to be the two most significant factors. Among the different sources studied, glucose and yeast extract were selected to be used as a carbon and nitrogen source, respectively. Response surface methodology (RSM) was used to model the effect and interaction of the concentration of these two sources on protein production and maximize the process. The suitable range of parameter values used in RSM was estimated based on experiments studying the effect of each one of the parameters as a single factor, varying its values in a specific range while keeping the other parameter at a constant value. To validate the model obtained by RSM, the optimal glucose and yeast extract concentration values estimated for maximum protein production were applied in a lab scale bioreactor for the cultivation of P. ostreatus LGAM 1123 in either a glucose-containing medium or in a cellulosic hydrolysate obtained after enzymatic hydrolysis of fibre sludge (Fig. 1).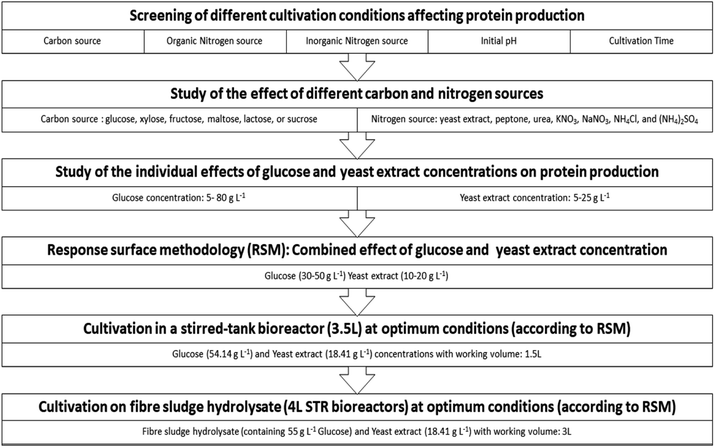 | ||
| Fig. 1 Flowchart of experimental design for SCP production by submerged cultivation of Pleurotus ostreatus LGAM 1123. | ||
Screening of different cultivation conditions affecting protein production
A regular two-level five-factor factorial design (25-1) with 3 central points was used (MODDE 7.0, Umetrics AB, Umeå, Sweden). The factors tested were the carbon source (glucose) concentration, organic nitrogen source (yeast extract) concentration, inorganic nitrogen source (NaNO3) concentration, initial pH, and cultivation time. The central point was run in triplicate to allow the estimation of experimental error. The range and levels of factors tested are given in Table 1.| Factor tested | Units | Type | Low (−1) | High (+1) |
|---|---|---|---|---|
| Glucose | g L−1 | Numeric | 4 | 40 |
| Yeast extract | g L−1 | Numeric | 1 | 10 |
| NaNO3 | g L−1 | Numeric | 0.4 | 4 |
| pH | — | Numeric | 5 | 7 |
| Cultivation time | Days | Numeric | 8 | 16 |
Study of the effect of different carbon and nitrogen sources
The effect of carbon source type on fungus growth and protein production was studied by adding different carbon sources (glucose, xylose, fructose, maltose, lactose, or sucrose) into the basal medium at a concentration of 20 g L−1. Samples were withdrawn every two days of cultivation to measure the biomass produced, as well as its protein content.To investigate the effect of nitrogen source type on growth of P. ostreatus LGAM 1123, as well as on protein production, different organic (yeast extract, peptone and urea) and inorganic (KNO3, NaNO3, NH4Cl, and (NH4)2SO4) nitrogen sources were tested. Therefore, 10 g L−1 of each nitrogen sources was added into the basal culture medium containing 20 g L−1 glucose as the carbon source. Samples were withdrawn every two days of cultivation to measure the biomass produced, as well as its protein content.
Study of the individual effects of glucose and yeast extract concentrations on protein production
The effect of glucose concentration was tested by adding different glucose concentrations (5, 10, 20, 30, 40, 50, 60, 70, and 80 g L−1) in Erlenmeyer flasks with the basal medium and pH adjusted to 5.0 before autoclaving. Yeast extract was supplemented at 10 g L−1. After 8 days of cultivation, flasks were withdrawn to measure the biomass produced as well as its protein content.The effect of yeast extract concentration on protein production was tested by adding different concentrations of yeast extract (5, 10, 15, 20, and 25 g L−1) in Erlenmeyer flasks containing the basal medium with 40 g L−1 glucose. The pH value was adjusted to 5.0 before autoclaving. After 8 days of cultivation, flasks were withdrawn to measure the biomass produced as well as its protein content.
Response surface methodology (RSM)
Based on the above experiments, glucose and yeast extract were chosen for further optimization through RSM using a central composite circumscribed design composed of a factorial design with 3 center points and star points at a distance of ±1.41 from the central point, to generate 11 treatment combinations (MODDE 7.0, Umetrics AB, Umeå, Sweden). Multiple linear regression analysis was performed (MODDE 7.0, Umetrics AB, Umeå, Sweden) to fit a quadratic polynomial model. A multiple linear regression analysis was performed as described by eqn (1):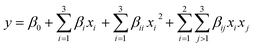 | (1) |
| Factor tested | Units | Low (−1) | High (+1) | Step | −Alpha | +Alpha |
|---|---|---|---|---|---|---|
| Glucose | g L−1 | 30 | 50 | 10 | 25.86 | 54.14 |
| Yeast extract | g L−1 | 10 | 20 | 5 | 7.93 | 22.07 |
| Non-center points: 8 | Center point: 3 | Alpha = 1.41 | Total runs: 11 | |||
Cultivation in a stirred-tank bioreactor
The bioreactor used was a 3.5 L stirred tank bioreactor (Ralph, Bioengineering). The optimal glucose (54.14 g L−1) and yeast extract concentrations (18.41 g L−1) found by RSM were used instead of the respective ones in the basal medium (section Media and growth conditions in Erlenmeyer flasks) for the cultivation of P. ostreatus LGAM 1123. The working volume was 1.5 L. The inoculation of the bioreactor was performed with the addition of 5% (v/v) of the total volume of fermentation from a well-grown culture of 12 days of the above medium in Erlenmeyer flasks. The initial growth conditions were pH adjusted to 5.0, agitation of 200 rpm (controlled), and aeration of 1 vvm (controlled). The temperature was controlled automatically at 28 °C. At specific time intervals, samples were withdrawn and biomass was separated from the supernatant, and kept for further analysis.Cultivation on fibre sludge hydrolysate
The fibre sludge hydrolysate was generated after enzymatic hydrolysis of the fibre sludge (provided by Domsjö Fabriker, Örnsköldsvik, Sweden) using commercial cellulases (Celic Ctec II, Novozymes A/S, Bagsværd, Denmark). After hydrolysis, the hydrolysate contained ∼100 g L−1 glucose and was diluted before the experiments to achieve an initial glucose concentration of 55 g L−1 in the culture . Minerals and yeast extract were added to the cultivation medium to match the growth conditions of P. ostreatus in previous experiments (section Cultivation in a stirred-tank bioreactor). The cultivation was performed in 4 L STR bioreactors (Belach Bioteknik, Sweden). The working volume was 3 L. Inoculum was added at 5% (v/v) of the working volume. T and pH were adjusted to 28 °C and 5 respectively, while the agitation speed varied from 200 to 800 rpm depending on the oxygen demand and viscosity increase of the culture. At specific time intervals, samples were withdrawn and biomass was separated from the supernatant, and kept for further analysis.Biomass determination
Five millilitres of culture samples were used for the quantification of biomass. Biomass was determined using filtration, under vacuum, of a known volume through a 0.45 μm Millipore cellulose filter. The cells were washed with distilled water three times and then placed in an oven at 60 °C until they achieved a constant weight. The filters were weighted using an analytical scale (Ohaus PX323 Pioneer Analytical Balance). The rest of the sample was centrifuged (4000 rpm, 10 min) and the supernatant was removed. The biomass was washed three times and kept at −20 °C for further analysis.Protein estimation
Total protein content was estimated after total nitrogen content had been measured by the Dumas method. For N2 determination a CN628 carbon/nitrogen elemental analyzer (LECO) was used, equipped with a non-dispersive infrared (NDIR) cell for the detection of carbon (as carbon dioxide) and a thermal conductivity cell (TC) to detect nitrogen (N2). Moisture content was analyzed using a TGA 701 thermogravimetric analyzer. The protein content was estimated by multiplying the N values by a factor of 6.25.Protein production was estimated according to eqn (2):
 | (2) |
For intracellular protein estimation, 2 mg of lyophilized biomass were disrupted via ultrasonication at 40% intensity (8 kHz) and 80% pulse for 6 min. Cell debris was removed via centrifugation at 4000 rpm for 10 min to obtain intracellular substances. Proteins were quantified by the BCA method (Pierce™ BCA protein assay kit) according to manufacturer’s instructions.32,33 More specifically, 25 μL of sample or standard were added to 200 μL of BCA reagent (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reagent A
1 reagent A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) reagent B) in a 96-well microplate. Samples were incubated at 37 °C for 30 min and then absorbance was measured at 562 nm on a microplate spectrophotometer (Multiskan Spectrum, Thermo Fisher Scientific, Waltham, USA). Protein content was estimated according to a bovine serum albumin (BSA) standard curve.
reagent B) in a 96-well microplate. Samples were incubated at 37 °C for 30 min and then absorbance was measured at 562 nm on a microplate spectrophotometer (Multiskan Spectrum, Thermo Fisher Scientific, Waltham, USA). Protein content was estimated according to a bovine serum albumin (BSA) standard curve.
Total lipid estimation
For total lipid extraction, a modified Folch method was used.34 One hundred milligrams of lyophilized biomass were mixed with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) chloroform/methanol for overnight extraction. After overnight incubation, Milli-Q water was added and centrifuged at 4000 rpm for 5 min. The chloroform layer which contained lipids was recovered and the solvent was evaporated. Lipids were quantified gravimetrically using an analytical scale (Ohaus PX323 Pioneer analytical balance).35
1 (v/v) chloroform/methanol for overnight extraction. After overnight incubation, Milli-Q water was added and centrifuged at 4000 rpm for 5 min. The chloroform layer which contained lipids was recovered and the solvent was evaporated. Lipids were quantified gravimetrically using an analytical scale (Ohaus PX323 Pioneer analytical balance).35
Carbohydrate estimation
For total carbohydrate estimation, a modified Visca et al. protocol was used.36 Specifically, 1 mg of lyophilized biomass was added in Pyrex tubes with 125 μL of 72% sulfuric acid and incubated at 30 °C for 1 h. Afterwards, 3.5 mL of Milli-Q water was added to the vials (to achieve a final concentration of sulfuric acid of 4%) and kept for 1 hour at 120 °C in an autoclave. A sample of 1 mL was withdrawn from the vial and centrifuged at 4000 rpm for 5 min. Total carbohydrates, as well as intracellular polysaccharides (IPSs) obtained as described in section protein estimation for intracellular substances, were determined by phenol-sulfuric acid assay according to Dubois et al., using glucose as a standard.37 Specifically, 50 μL of samples were added to a 96-well microplate. One hundred and fifty milliliters of sulfuric acid (98% w/w) were added and mixed. Thirty milliliters of 5% phenol in water were added and the plate was incubated for 5 min at 90 °C. After incubation, the microplate was left for 5 min at room temperature and the absorbance was measured at 490 nm in a microplate spectrophotometer (Multiskan Spectrum, Thermo Fisher Scientific, Waltham, USA).Amino acid analysis
For protein hydrolysis into amino acids, 20 mg of lyophilized biomass were transferred into pyrex vials (20 mL). Four ml of 6 M HCl were added to the vials and hydrolysed at 110 °C for 24 h. After incubation, the solvent was removed using a rotary evaporator under vacuum and the resulting extract was then lyophilized using a freeze drying lyophilizer (MRC). The lyophilized sample was kept at −20 °C for amino acid analysis.38,39For amino acid analysis, a derivatization procedure with dabsyl chloride was performed according to Ribeiro et al.40 The above-lyophilized samples were resuspended with 0.1 M HCl. Twenty microliters of sample were diluted with 180 μL of reaction buffer (0.15 mol L−1 NaHCO3, pH 8.6) and mixed by vortexing. Then, 200 μL of dabsyl chloride (12.4 mM, diluted in acetone) was added and the vials were incubated at 70 °C for 15 min. The reaction was stopped with an ice bath incubation for 5 min. Four hundred microliters of dilution buffer (50 mL of acetonitrile, 25 mL of ethanol, and 25 mL of elution buffer) were added, mixed well, and centrifugated at 5000 rpm for 5 min. The supernatant was kept at −20 °C until HPLC analysis.
After the above derivatization of amino acids, the dabsyl derivatives were separated on an HPLC unit (Shimadzu, Kyoto, Japan) with a photodiode array detector. Specifically, a reversed-phase C18 column (μBondapack, Waters Ireland) with dimensions of 3.9 × 300 mm, 10 μm particle size, and 125 Å pore size, was used. Twenty microliters of derivatized samples were injected. The solvent system was composed of two eluents: acetonitrile 80% (A) and elution buffer (B). Elution was performed at a flow rate of 1 mL min−1, starting with 20% A until 7 min and installing a gradient to obtain 35% A at 35 min, 50% A at 45 min, and 100% A at 66 min, maintaining 100% A until 76 min. Detection was achieved at 461 nm. Amino acid quantification was accomplished by estimating the peak areas in the chromatograms in comparison to the respective ones of the external amino acid standards.40
Statistical analysis
All experiments were conducted in triplicate. The data were expressed as mean ± standard deviation and were analysed using one-way analysis of variance (ANOVA) with Tukey's multiple range test, with p values < 0.05 being regarded as significant using IBM SPSS statistics (version 28.0.1.0, IBM Corporation, NY, USA). Screening and response surface methodology (RSM) were performed using MODDE 7.0, Umetrics AB, Umeå, Sweden.Results and discussion
Effect of different cultivation conditions on protein production
The effect of the carbon source (glucose), organic nitrogen source (yeast extract), inorganic nitrogen source (NaNO3), pH and cultivation time on biomass and protein production in submerged cultivation of P. ostreatus LGAM 1123 was studied, using a regular two-level factorial design (25-1) for a preliminary factor screening test. Based on the experimental design, 19 individual runs were executed as shown in ESI Table 1.† The model was tested for adequacy by the analysis of variance (ESI Table 2†). The significance of the model (at a 95% confidence level) was confirmed using the computed regression F-value (230), as well as its low P-value (P = 0.00). No lack of fit was observed as indicated by the respective P-value. The coefficient of variation (R2 = 0.99) estimated for the regression indicates a high correlation between the experimentally observed and predicted values, whereas Q2 = 0.91 indicates how well the model predicts new data.Carbon and nitrogen sources were found to play the most important role among the tested factors for protein production (ESI Fig. 1†). The initial pH had a negative effect indicating that low pH was better for protein production. The NaNO3 concentration played a positive role. As an individual factor, cultivation time did not have a statistically significant effect on protein production (p > 0.05), whereas its combination with either the glucose (Glu*Time) or yeast extract concentration (Yea*Time) had a positive or negative effect, respectively. The combination effect of glucose and yeast extract (Glu*Yea) was in accordance with the individual effects of glucose and yeast extract concentrations. According to the above results, pH 5 and CNaNO3 = 0.4 g L−1 were selected for the next experiments in this study. Moreover, the effect of cultivation time was also taken into account in the experiments studying the effect of carbon and nitrogen source types.
According to Cueva et al. 2017, a high impact of the carbon and nitrogen ratio on protein production was confirmed for P. ostreatus.41 Our results are in accordance with those described in a study on Tuber sinense submerged cultivation showing that biomass production is positively affected by glucose and yeast extract concentrations.42 A study on the influence of different cultivation conditions, such as carbon and nitrogen sources, pH, temperature and period of cultivation, for the submerged cultivation of Lentinus citrinus on mycelial biomass and protease production, confirmed the positive effect of nitrogen source on biomass production.43 Our results have shown that the protein production by cultivation of P. ostreatus was enhanced at pH 5.0, whereas Choi et al. 2011 have shown that pH between 6.0 and 6.5 favors mycelial production in Mycoleptodonoides aitchisonii submerged cultivation in an air-lift bioreactor.44
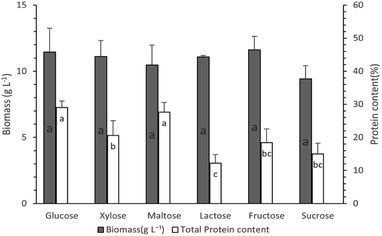
Study of the effect of carbon source type on protein production
Different carbon sources (glucose, xylose, fructose, maltose, lactose, and sucrose) were tested in order to study the effect of carbon source type on fungus growth and protein production, according to Experimental “Study of the effect of different carbon and nitrogen sources”. In all cases, the exponential phase lasted until the 8th day of cultivation, while, after that, the stationary and death phases followed (data not shown). Biomass and protein content reached their maximum values on the 8th day of cultivation.Protein content was maximum when glucose was used (29.0 ± 2.0%) as a carbon source, while maltose followed (27.6 ± 3.0%) with no statistically significant difference (p > 0.05) (Fig. 2). However, as far as protein production is concerned the use of glucose led to a higher value (3.32 ± 0.20 g L−1) compared to that in the case of maltose (2.89 ± 0.14 g L−1). Xylose was the third best carbon source leading to a protein production of 2.29 ± 0.11 g L−1. Based on the higher protein production value, glucose has been chosen and used as a carbon source in the next experiments. Our results concerning protein production are in accordance with others published in the scientific literature. A study of Pleurotus pulmonarius submerged cultivation has shown that glucose was the best carbon source for protein production (1.26 ± 0.015 g L−1), whereas a maximum protein content (26.1 ± 0.5%) of the biomass produced was observed in the case of arabinose. However, the respective protein production was rather low, due to a decreased biomass production (0.9 g L−1).39 In another study, concerning cultivation of Morchella fluvialis for optimization of protein production, it has been concluded that the use of a glucose-medium could lead to a biomass with high protein content.45
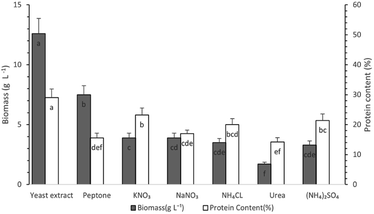
Study of the effect of nitrogen source type on protein production
Different organic (yeast extract, peptone and urea) and inorganic (KNO3, NaNO3, NH4Cl, and (NH4)2SO4) nitrogen sources were tested in order to study the effect of nitrogen source type on fungus growth and protein production, according to Experimental “Study of the effect of different carbon and nitrogen sources”. Biomass production and protein content reached their maximum values on the 8th day of cultivation similarly to our previous experiments on the effect of carbon source type.As can be seen in Fig. 3, the highest protein content was observed in the case of a yeast extract-containing medium (29.9 ± 2.9%), followed by the respective one with KNO3 (23.2 ± 2.3%). Similarly, biomass production reached its maximum value when yeast extract was used as a nitrogen source. Peptone was found to lead to the second higher value, with KNO3 following (Fig. 3). Based on the above results, a similar rank order was observed for the effect of different nitrogen sources on protein production (as estimated using eqn (2)). The values of 3.76 ± 0.04 g L−1, 1.17 ± 0.01 g L−1 and 0.9 ± 0.01 g L−1 were found for yeast extract, peptone and KNO3 containing media, respectively. Therefore, yeast extract has been selected as the optimum nitrogen source for protein production in submerged cultivation of P. ostreatus LGAM 1123 for further investigation.
A study on submerged cultivation of Morchella fluvialis in media containing different nitrogen sources confirmed that organic nitrogen sources seem to favor protein production compared to inorganic ones with yeast extract leading to a biomass of higher protein content compared to ammonium nitrate and urea.45
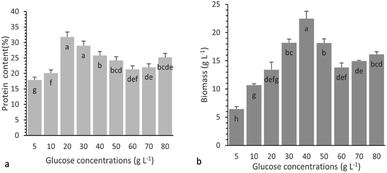
Study of the individual effect of glucose concentration on protein production
In order to study the effect of glucose concentration on protein production, submerged cultivation of P. ostreatus LGAM 1123 in various flasks with the basal medium containing different glucose concentrations was conducted, according to Experimental “Study of the individual effects of glucose and yeast extract concentrations on protein production”. Fig. 4a and b show the protein content and biomass production, respectively, on the 8th day of cultivation, as a function of the concentration of glucose in the medium.It seems that an initial increase in the glucose concentration up to a specific level had a positive effect on both the % protein content and biomass production, whereas a further increase in the glucose concentration had a negative effect leading to lower values of both responses. The maximum protein content was observed for a 20 g L−1 glucose concentration, while a not statistically different protein content was found for a concentration of 30 g L−1 (Fig. 4a). However, as can be seen from Fig. 4b, the maximum biomass production was reached when the glucose concentration was 40 g L−1 (p < 0.05). After estimating the protein production using eqn (2), a similar effect of glucose concentration on protein production was also found, reaching its highest value of 5.79 ± 0.07 g L−1 at a glucose concentration of 40 g L−1. According to the above results, a glucose concentration ranging from 30 g L−1 to 50 g L−1 was selected for further investigation of the combined effect of glucose and yeast extract concentrations on protein production in submerged cultivation of P. ostreatus LGAM 1123 using RSM.
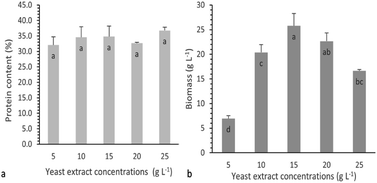
Study of the individual effect of yeast extract concentration on protein production
The effect of yeast extract concentration on protein production was tested by adding different yeast extract concentrations into the basal medium according to Experimental “Study of the individual effects of glucose and yeast extract concentrations on protein production”. Fig. 5a and b show the % protein content and biomass production on the 8th day of cultivation of P. ostreatus LGAM 1123, at different yeast extract concentrations.As can be seen, all cultivation conditions led to similar protein contents of the produced biomass, indicating that changing the yeast extract concentration in the culture medium had no effect on the % protein content. Concerning the effect of yeast extract concentration on biomass production (Fig. 5b), an initial increase in the biomass concentration was observed with the increase in the yeast extract concentration up to a certain value, while a further increase in the nitrogen source concentration led to a decrease in the biomass produced. Taking into account eqn (2) for protein production estimation, the effect of yeast extract concentration on protein production followed a similar trend. A maximum value equal to 9.0 ± 0.3 g L−1 was obtained at a yeast extract concentration of 15 g L−1, while lower values (7.4 ± 0.02 g L−1 and 6.1 ± 0.06 g L−1) were achieved at higher yeast extract concentrations of 20 g L−1 and 25 g L−1, respectively. Based on the above results, a yeast extract concentration ranging from 10 to 20 g L−1 was chosen as a suitable range for a further study of the combined effect of glucose and yeast extract concentrations on protein production using response surface methodology.
Optimization of protein production using response surface methodology
The combined effect of glucose and yeast extract concentrations on protein production in submerged cultivation of P. ostreatus LGAM 1123 was investigated by a RSM using a two variable central composite circumscribed design as described in Experimental section “Response surface methodology (RSM)”. The range of concentrations tested was 30–50 g L−1 for glucose and 10–20 g L−1 for yeast extract according to previous experiments. The levels for each factor tested (indicated as −1.41, −1, 0, 1, and 1.41), their ranges, as well as the experimental response values for biomass, protein content and protein production, used for response surface analysis, are shown in Table 3.| Exp no. | Glucose (g L−1) | Yeast extract (g L−1) | Biomass (g L−1) | Protein content (%) | Protein production (g L−1) |
|---|---|---|---|---|---|
| 1 | 30 (−1) | 10 (−1) | 22.5 | 41.7 | 9.4 |
| 2 | 50 (1) | 10 (−1) | 25.1 | 43.2 | 10.8 |
| 3 | 30 (−1) | 20 (1) | 27.5 | 43.7 | 12.0 |
| 4 | 50 (1) | 20 (1) | 28.3 | 47.7 | 13.5 |
| 5 | 25.86 (−1.41) | 15 (0) | 23.0 | 45.0 | 10.4 |
| 6 | 54.14 (1.41) | 15 (0) | 28.4 | 47.0 | 13.3 |
| 7 | 40 (0) | 7.93 (−1.41) | 19.6 | 46.1 | 9.0 |
| 8 | 40 (0) | 22.07 (1.41) | 24.1 | 48.8 | 11.8 |
| 9 | 40 (0) | 15 (0) | 24.3 | 49.2 | 12.0 |
| 10 | 40 (0) | 15 (0) | 26.2 | 47.9 | 12.5 |
| 11 | 40 (0) | 15 (0) | 25.8 | 48.5 | 12.5 |
With an objective to optimize the protein production, the analysis of RSM is extensively described only for the specific response variable, while the models obtained for the description of biomass production and protein content are not shown. The respective contour and surface plots are depicted in the ESI section.† The optimum biomass production achieved was 28.9 g L−1 when concentrations of glucose and yeast extract were 54.14 g L−1 and 17 g L−1 respectively (ESI Fig. 3 and 4†). Regarding protein content, a maximum of 49.0% was estimated at 42.7 g L−1 glucose and 17.8 g L−1 yeast extract (ESI Fig. 5 and 6†).
The quadratic polynomial model obtained for the description of the protein production (response y) as a function of the factors used in the experimental design, after estimating the coefficient values through multiple linear regression analysis, is given by eqn (3). The term glucose*yeast was omitted from the model since it was found to have no significant effect (p > 0.05).
| y = −5.3 + 0.2 × glucose + 1.3 × yeast − 0.002 × glucose2 − 0.04 × yeast2 | (3) |
This model was tested for adequacy by the analysis of variance (ESI Table 3†). The computed F-value (F = 28), together with the low probability P-value (P = 0.00), indicate the significance of the model at a high confidence level. No lack of fit was estimated according to the large P-value found. The coefficient of variation (R2 = 0.96) indicates a high correlation between the experimentally observed and predicted values, whereas Q2 = 0.80 indicates how well the model predicts new data. As shown in ESI Fig. 2†, a very good correlation was observed between experimental values and predicted values by the model for protein production.
To investigate the combined effect of glucose and yeast extract concentrations on protein production, a contour plot as well as a 3D surface plot were drawn (Fig. 6). In both plots, a maximum point for protein production could be observed. As can be seen, at all glucose concentration levels studied, an increase in the yeast extract concentration up to a specific value had a positive effect on the protein production, as calculated using the software's numerical optimization function and depicted in contour and surface plots, was 13.6 g L−1 and was achieved when glucose and yeast extract concentrations were 54.14 g L−1 and 18.25 g L−1, respectively.
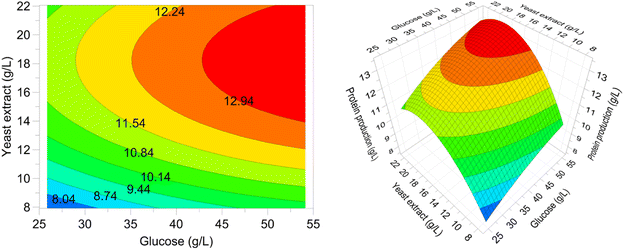 | ||
| Fig. 6 Contour and 3D surface plots of protein production as a function of glucose and yeast extract concentrations in submerged cultivation of P. ostreatus LGAM 1123. | ||
To validate the model obtained by RSM, the optimal glucose and yeast extract concentrations estimated for maximum protein production were used for the cultivation of P. ostreatus in Erlenmeyer flasks. After eight days of cultivation, biomass production and protein content were found to be 24.3 g L−1 and 42.7%, respectively, leading to a maximum protein production of 10.4 g L−1, an experimental value similar to the one predicted by the model. As far as we know, other studies concerning the optimization of protein production in submerged cultivation of P. ostreatus as a function of carbon and nitrogen sources have not been reported. However, there are similar studies for the effect of glucose and yeast extract on biomass production in submerged cultivation of different fungi. P. ostreatus cultivation has led to a maximum biomass production of 18 g L−1 at a combination of 45 g L−1 glucose and 15 g L−1 yeast extract.46 In another study using a lower range of yeast extract concentrations (1.0 to 5.0 g L−1) in the culture medium for P. ostreatus growth, a low value (1.72 g L−1) of biomass production was achieved at 40 g L−1 glucose and 3.0 g L−1 yeast extract.47 Concerning the submerged cultivation of Tuber sinense, the maximum biomass production (24.8 g L−1) was found when glucose and yeast extract concentrations were 60 g L−1 and 30 g L−1, respectively.42 In addition, in the case of Ganoderma australe cultivation, the estimated optimal values of glucose and yeast extract concentrations were 13.7 g L−1 and 30 g L−1 respectively, reaching a maximum biomass production of 11.8 g L−1.48
Amino acid analysis of the protein produced by submerged cultivation of P. ostreatus LGAM 1123
To determine the dietary value of the produced protein in submerged cultivation of P. ostreatus LGAM 1123 in flasks, an amino acid analysis was conducted. The amino acid composition found, in comparison with the recommended amino acid scoring patterns for infants, children, older children, adolescents and adults, according to the report of FAO Expert Consultation for Dietary Protein Quality Evaluation in Human Nutrition (2013), is presented in Table 4.| Amino acids | % Total amino acids | mg g−1 protein | Recommended amino acid scoring (FAO) (mg g−1 protein) | ||
|---|---|---|---|---|---|
| Infant (birth to 6 months) | Child (6 months to 3 years) | Older child, adolescent, and adult | |||
| Asp | 4.9 ± 0.2 | 49.0 ± 2.3 | — | — | — |
| Glu | 4.3 ± 0.3 | 42.8 ± 3.1 | — | — | — |
| Asn | 0.4 ± 0.1 | 3.6 ± 0.8 | — | — | — |
| Gln | 3.8 ± 0.1 | 37.7 ± 0.9 | — | — | — |
| Ser | 1.0 ± 0.1 | 10.2 ± 1.5 | — | — | — |
| Gly | 28.2 ± 0.4 | 282.2 ± 4.2 | — | — | — |
| Val | 8.2 ± 0.3 | 82.4 ± 2.6 | 55 | 43 | 40 |
| Pro | 14.4 ± 0.5 | 143.5 ± 5.1 | — | — | — |
| Arg | 6.0 ± 1.0 | 59.9 ± 10.2 | — | — | — |
| Met | 0.3 ± 0.01 | 2.5 ± 0.5 | — | — | — |
| Ile | 4.7 ± 0.2 | 47.1 ± 2.3 | 55 | 32 | 30 |
| Leu | 10.4 ± 0.4 | 103.5 ± 3.5 | 17 | 8.5 | 6.6 |
| Trp | 4.6 ± 0.2 | 46.5 ± 1.5 | |||
| Phe | 6.7 ± 0.01 | 66.7 ± 0.1 | — | — | — |
| Cys | 0.6 ± 0.1 | 5.7 ± 0.7 | — | — | — |
| Lys | 0.3 ± 0.03 | 2.9 ± 0.3 | 69 | 57 | 48 |
| Tyr | 0.3 ± 0.03 | 3.4 ± 0.3 | — | — | — |
| AAA (Phe + Tyr) | 7.0 ± 0.2 | 70.1 ± 2.3 | 33 | 27 | 23 |
| SAA (Met + Cys) | 0.8 ± 0.1 | 8.3 ± 1.1 | 94 | 52 | 41 |
| Total | 98.9 | 989 | |||
As can be seen, 17 amino acids were detected in P. ostreatus LGAM 1123 biomass. The most abundant amino acids were glycine and proline reaching a percentage of 28.2% and 14.4% respectively, whereas leucine, valine, phenylalanine, and arginine were also detected in large amounts. More specifically, an amount of 82.4 mg of valine per gram of protein was measured, a value much higher than the recommended amino acid score for all the age groups.27 Similarly, amino acid scores much higher than the one recommended by FAO were observed for leucine and aromatic amino acids (AAAs). In the case of isoleucine, its content determined in the produced protein was adequate for children, adolescents and adults. In contrast, the scores found for lysine and sulphuric amino acids (SAAs) were not in the recommended amino acid scoring range.
Similar studies concerning amino acid analysis of the produced protein in submerged cultivation of different fungi have been reported. Phenylalanine, aspartate, glutamate, and proline were found to have the highest content among other amino acids in the case of Pleurotus pulmonarius, whereas threonine, glycine, and glutamic acid, were the most abundant amino acids in proteins produced by cultivation of Cordyceps militaris.23,49
Protein production in lab scale bioreactors
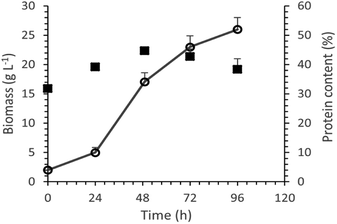 | ||
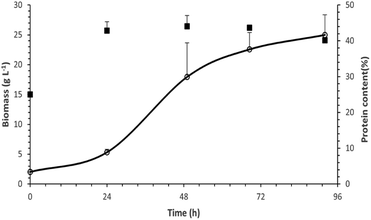
| Fig. 7 Biomass produced (line) and its protein content (black squares) as a function of time for the cultivation of P. ostreatus LGAM 1123 in a 3.5 L stirred tank bioreactor under optimal conditions. | ||
The maximum biomass production value achieved in our work was higher compared to those found in most studies concerning submerged cultivation of different strains of Pleurotus.22,23,50,51 More specifically, the biomass production reached a maximum value of 8.2 g L−1 in a submerged cultivation of Pleurotus sajor caju in a 5 L stirred tank bioreactor with 10 g L−1 glucose. In the case of P. ostreatus, an even lower biomass production of 5.2 g L−1 was achieved in a 3.5 L stirred tank bioreactor with 5 g L−1 sucrose.50,51 Similarly, in a study on P. ostreatus growth in a 15 L stirred tank bioreactor with a 20 g L−1 glucose containing medium, the biomass production reached a maximum at a value of 6 g L−1 after 168 h of cultivation.22
Finally, according to a study on submerged cultivation of P. ostreatus in a 20 L stirred-tank bioreactor in a medium of 57 g L−1 xylose and 37 g L−1 corn steep liquor, the biomass production reached a maximum of 39.2 ± 0.6 g L−1, a value higher than the one found in our study, after 68 h of cultivation. However, the respective protein production achieved was 6.5 ± 0.1 g L−1, a lower value compared to our results.23
Moreover, an analysis of the basic biochemical components of biomass produced in the bioreactor was conducted. As shown in Table 5, the most abundant component of P. ostreatus LGAM 1123 biomass was total carbohydrates, followed by total proteins as determined by the Dumas method, while lipids were found to be the least abundant biochemical component. Concerning the protein content of biomass, our results show a higher maximum value compared to the respective one found in a study on submerged cultivation of P. ostreatus in a 20 L stirred-tank bioreactor (16.7 ± 0.1% protein content, 4.2 ± 0.2% lipids and 62.5 ± 0.9% alimentary fibers).23
| Bioactive compound | Method | g/100 g biomass |
|---|---|---|
| Proteins | Total proteins by the Dumas method | 38.0 ± 2.1 |
| Soluble intracellular proteins | 19.7 ± 0.7 | |
| Lipids | Total lipids | 2.0 ± 0.1 |
| Carbohydrates | Total carbohydrates | 50.9 ± 2.8 |
| Intracellular polysaccharides | 34.7 ± 0.98 |
In other studies, paper and pulp industry wastes have been used as lignin media for P. ostreatus and other white-rot fungi for the production of hydrolytic and oxidative enzymes.52,53 In addition, cultivation of the P. ostreatus mushroom on a solid substrate made of cellulose fibre rejects has been conducted.54 To our knowledge, there is no scientific literature on fungal protein production using pulp and paper wastes. Different waste waters have been used in other studies conducted for SCP production by cultivation of a variety of fungi or yeasts. A protein production of 12.2 ± 0.4 g L−1 and protein content of 36.7 ± 0.5% were achieved after 72 h of fermentation in submerged cultivation of Candida utilis in potato wastewater supplemented with 5% glycerol.8 A protein content range of 46–54% was accomplished by cultivation of different fungi in the pea-processing by-product. Cultivation of Fusarium venenatum in a 2% pea-processing byproduct (PpB) substrate led to the highest protein production of 59.75%.55 In addition, the cultivation of Saccharomyces cerevisiae in variable food wastes including fish, pineapple, bananas, apples, and citrus peels in a 5 L batch fermenter led to a protein content of 40.19 ± 2.13% after 120 h.6 The use of an industrial side stream as a feedstock, instead of pure glucose to produce SCP provides an interesting route for the valorization of this side stream. This conversion route can be regarded as a paradigm, revealing the wide spectrum of such industrial implementation possibilities for P. ostreatus associated not only with lower production costs, but also with the application and promotion of circular economy principles.
Conclusions
Overall, P. ostreatus LGAM 1123 should be cultivated under optimal conditions to produce large amounts of proteins. Carbon and nitrogen sources are the main factors that affect protein production. Biomass derived from submerged cultivation could stand as an alternative vegan protein source due to its high protein content in contrast to fruiting bodies. The dietary value of the produced protein in submerged cultivation of P. ostreatus LGAM 1123, after an amino acid analysis, was found to be in accordance with the recommended amino acid scoring patterns in human nutrition (FAO Expert Consultation for Dietary Protein Quality Evaluation, 2013). Single-cell protein which contains the essential amino acids and has a protein content of over 40% is suitable to be used for food applications.3 It is noteworthy, however, that an important factor that should be taken into consideration regarding SCP consumption is its nucleic acid level (ranging from 2 to 18% of dry matter). Nucleic acid content in the diet should not exceed 2 g per day since it could cause an increased deposition of uric acid crystals in the kidneys or joints, therefore being a potential threat to the human body.1 In addition, protein production could be achieved using industrial side streams decreasing the process cost. Waste valorisation is very important for industrial use since it provides the opportunity to exploit a non-value waste to produce a valuable food product. Of course, safety and organoleptic tests conducted on biomass produced in pilot-scale bioreactors are crucial to produce valuable food products such as meat analogs.Author contributions
Georgios Bakratsas: data curation, formal analysis, investigation, methodology, validation, visualization, writing – original draft, Angeliki Polydera: data curation, formal analysis, writing – original draft, Oskar Nilson: investigation, data curation, Charilaos Xiros: investigation, data curation, formal analysis, resources, writing – review & editing, Lalie Kossatz: investigation, Petros Katapodis: conceptualization, supervision, writing – review & editing, and Haralambos Stamatis: investigation, resources, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
“Co-financed by the European Regional Development Fund of the European Union and Greek National Funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH – CREATE – INNOVATE (project code: T2EDK-02830)”. We gratefully acknowledge Prof. Georgios I. Zervakis from the Laboratory of General and Agricultural Microbiology, Agricultural University of Athens, Iera Odos 75, 11855 Athens, Greece; for providing the fungal strain.References
- C. E. Boyd, A. A. McNevin and R. P. Davis, Food Secur., 2022, 14, 805–827 CrossRef PubMed.
- J. Ahlborn, A. Stephan, T. Meckel, G. Maheshwari, M. Rühl and H. Zorn, International Journal of Recycling Organic Waste in Agriculture, 2019, 8, 447–455 CrossRef.
- A. Ritala, S. T. Häkkinen, M. Toivari and M. G. Wiebe, Front. Microbiol., 2017, 8, 2009 CrossRef PubMed.
- E. J. Derbyshire and J. Delange, Frontiers in Sustainable Food Systems, 2021, 5, 581682 CrossRef.
- M. I. Ahmad, S. Farooq, Y. Alhamoud, C. Li and H. Zhang, Trends Food Sci. Technol., 2022, 121, 14–29 CrossRef CAS.
- A. Tropea, A. Ferracane, A. Albergamo, A. G. Potortì, V. Lo Turco and G. Di Bella, Fermentation, 2022, 8, 1–11 Search PubMed.
- P. Thiviya, A. Gamage, R. Kapilan, O. Merah and T. Madhujith, Separations, 2022, 9, 178 CrossRef CAS.
- A. Kurcz, S. Błażejak, A. M. Kot, A. Bzducha-Wróbel and M. Kieliszek, Waste Biomass Valorization, 2018, 9, 57–64 CrossRef CAS.
- M. Kieliszek, A. M. Kot, A. Bzducha-Wróbel, S. Błażejak, I. Gientka and A. Kurcz, Fungal Biol. Rev., 2017, 31, 185–198 CrossRef.
- A. K. Das, P. K. Nanda, P. Dandapat, S. Bandyopadhyay, P. Gullón, G. K. Sivaraman, D. J. McClements, B. Gullón and J. M. Lorenzo, Molecules, 2021, 26, 2463 CrossRef CAS PubMed.
- G. Bakratsas, A. Polydera, P. Katapodis and H. Stamatis, Future Foods, 2021, 4, 100086 CrossRef CAS.
- A. González, M. Cruz, C. Losoya, C. Nobre, A. Loredo, R. Rodríguez, J. Contreras and R. Belmares, Food Funct., 2020, 11, 7400–7414 RSC.
- F. Bach, C. V. Helm, M. B. Bellettini, G. M. Maciel and C. W. I. Haminiuk, Int. J. Food Sci. Technol., 2017, 52, 2382–2392 CrossRef CAS.
- V. Lavelli, C. Proserpio, F. Gallotti, M. Laureati and E. Pagliarini, Food Funct., 2018, 9, 1353–1372 RSC.
- C. Phat, B. Moon and C. Lee, Food Chem., 2016, 192, 1068–1077 CrossRef CAS PubMed.
- M. L. Fazenda, R. Seviour, B. McNeil and L. M. Harvey, Adv. Appl. Microbiol., 2008, 63, 33–103 CAS.
- V. Elisashvili, M. Penninckx, E. Kachlishvili, N. Tsiklauri, E. Metreveli, T. Kharziani and G. Kvesitadze, Bioresour. Technol., 2008, 99, 457–462 CrossRef CAS PubMed.
- J. A. Bentil, A. Thygesen, M. Mensah, L. Lange and A. S. Meyer, Appl. Microbiol. Biotechnol., 2018, 102, 5827–5839 CrossRef CAS PubMed.
- H. Morais, E. Forgács and T. Cserháti, Eng. Life Sci., 2005, 5, 152–157 CrossRef CAS.
- L. M. Papaspyridi, N. Aligiannis, E. Topakas, P. Christakopoulos, A. L. Skaltsounis and N. Fokialakis, Molecules, 2012, 17, 2714–2724 CrossRef CAS PubMed.
- L. Papaspyridi, V. Sinanoglou, I. Strati, P. Katapodis and P. Christakopoulos, Acta Aliment., 2013, 42, 328–337 CrossRef CAS.
- H. El-Enshasy, A. Daba, M. El-Demellawy, A. Ibrahim, S. El Sayed and I. El-Badry, J. Appl. Sci., 2010, 10, 2523–2529 CrossRef CAS.
- L. M. Papaspyridi, P. Katapodis, Z. Gonou-Zagou, E. Kapsanaki-Gotsi and P. Christakopoulos, Biochem. Eng. J., 2010, 50, 131–138 CrossRef CAS.
- A. Mumpuni, N. Ekowati, P. Purnomowati and E. S. Purwati, Biosaintifika: Journal of Biology & Biology Education, 2017, 9, 572 Search PubMed.
- Y. Hadar and E. Cohen-Arazi, Appl. Environ. Microbiol., 1986, 51, 1352–1354 CrossRef CAS PubMed.
- W. Manu-Tawiah and A. M. Martin, Food Microbiol., 1987, 4, 303–310 CrossRef CAS.
- J. Gang, H. Liu and Y. Liu, Int. J. Med. Mushrooms, 2016, 18, 745–752 CrossRef PubMed.
- M. Klimek-Ochab, M. Brzezińska-Rodak, E. Zymańczyk-Duda, B. Lejczak and P. Kafarski, Folia Microbiol., 2011, 56, 469–475 CrossRef CAS PubMed.
- S. Bleakley and M. Hayes, Foods, 2017, 6, 1–34 CrossRef PubMed.
- Y. W. Sari, W. J. Mulder, J. P. M. Sanders and M. E. Bruins, Biotechnol. J., 2015, 10, 1138–1157 CrossRef CAS PubMed.
- Y. Sun, M. Zhang and Z. Fang, Trends Food Sci. Technol., 2020, 105, 468–482 CrossRef CAS.
- Q. Xiao, F. Ma, Y. Li, H. Yu, C. Li and X. Zhang, Front. Microbiol., 2017, 8, 480 Search PubMed.
- P. K. Smith, R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson and D. C. Klenk, Anal. Biochem., 1985, 150, 76–85 CrossRef CAS PubMed.
- J. Folch, M. Lees and G. H. Sloane Stanley, J. Biol. Chem., 1957, 226, 497–509 CrossRef CAS PubMed.
- P. Biller and A. B. Ross, Algal Res., 2014, 6, 91–97 CrossRef.
- P. F. Visca Andrea, D. C. Fabrizio, S. Roberta, A. Pietro, C. Agnese, I. Gaetano and T. Luigi, Chem. Eng. Trans., 2017, 57, 127–132 Search PubMed.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- N. Cohen, J. Cohen, M. D. Asatiani, V. K. Varshney, H. T. Yu, Y. C. Yang, Y. H. Li, J. L. Mau and S. P. Wasser, Int. J. Med. Mushrooms, 2014, 16, 273–291 CrossRef CAS PubMed.
- F. R. Smiderle, L. M. Olsen, A. C. Ruthes, P. A. Czelusniak, A. P. Santana-Filho, G. L. Sassaki, P. A. J. Gorin and M. Iacomini, Carbohydr. Polym., 2012, 87, 368–376 CrossRef CAS PubMed.
- B. Ribeiro, P. B. Andrade, B. M. Silva, P. Baptista, R. M. Seabra and P. Valentão, J. Agric. Food Chem., 2008, 56, 10973–10979 CrossRef CAS PubMed.
- M. B. Ruilova Cueva, A. Hernández and Z. Niño-Ruiz, Rev. Fac. Cienc. Agrar., Univ. Nac. Cuyo, 2017, 49, 331–344 Search PubMed.
- R. S. Liu, D. S. Li, H. M. Li and Y. J. Tang, Process Biochem., 2008, 43, 868–876 CrossRef CAS.
- L. de Souza Kirsch, A. C. dos Santos Pinto, T. S. Porto, A. L. F. Porto and M. F. S. Teixeira, Int. J. Med. Mushrooms, 2011, 13, 185–192 CrossRef PubMed.
- D. B. Choi, J. H. Lee, Y. S. Kim, M. S. Na, O. Y. Choi, H. D. Lee, M. K. Lee and W. S. Cha, Korean J. Chem. Eng., 2011, 28, 1427–1432 CrossRef CAS.
- Z. Rahgo, H. R. Samadlouie, S. Mojerlou and K. Jahanbin, BioMed Res. Int., 2019, 2019, 7326590 Search PubMed.
- D. Durán-Sequeda, D. Suspes, E. Maestre, M. Alfaro, G. Perez and A. G. Pisabarro, J. Fungi, 2022, 8, 7 CrossRef PubMed.
- V.-B. Horincar, A. Popa, G. Parfene and T. Balaes, Innovative Rom. Food Biotechnol., 2014, 15, 58–62 Search PubMed.
- L. M. Papaspyridi, P. Katapodis, Z. Gonou-Zagou, E. Kapsanaki-Gotsi and P. Christakopoulos, Eng. Life Sci., 2011, 11, 65–74 CrossRef CAS.
- F. R. Smiderle, L. M. Olsen, A. C. Ruthes, P. A. Czelusniak, A. P. Santana-Filho, G. L. Sassaki, P. A. J. Gorin and M. Iacomini, Carbohydr. Polym., 2012, 87, 368–376 CrossRef CAS PubMed.
- F. G. Confortin, R. Marchetto, F. Bettin, M. Camassola, M. Salvador and A. J. P. Dillon, J. Ind. Microbiol. Biotechnol., 2008, 35, 1149–1155 CrossRef CAS PubMed.
- F. Bettin, F. Cousseau, K. Martins, N. A. Boff, S. Zaccaria, M. Moura da Silveira and A. J. Pinheiro Dillon, J. Environ. Manage., 2019, 236, 581–590 CrossRef CAS PubMed.
- M. Skočaj, A. Gregori, M. Grundner, K. Sepčić and M. Sežun, Holzforschung, 2018, 72, 813–817 CrossRef.
- Y. Hong, M. Dashtban, S. Chen, R. Song and W. Qin, J. Microb. Biochem. Technol., 2015, 07, 177–181 CAS.
- A. Grimm, L. Eilertsen, F. Chen, R. Huang, L. Atterhem and S. Xiong, Waste Biomass Valorization, 2021, 12, 4331–4340 CrossRef.
- P. F. S. Filho, R. B. Nair, D. Andersson, P. R. Lennartsson and M. J. Taherzadeh, Fungal Biol. Biotechnol., 2018, 5, 1–10 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fb00058j |
| ‡ Current address: Metsä Board Husum AB, Bruksvägen 86, 89680 Husum, Sweden. |
| This journal is © The Royal Society of Chemistry 2023 |