Open Access Article
Open Access ArticleNovel naturally derived encapsulation agents in the ionic liquid form for sustainable emulsion-based products†
Ariel A. C.
Toledo Hijo
 *,
Eric Keven
Silva
,
Aureliano A. D.
Meirelles
,
Rosiane L.
Cunha
*,
Eric Keven
Silva
,
Aureliano A. D.
Meirelles
,
Rosiane L.
Cunha
 and
Antonio J. A.
Meirelles
and
Antonio J. A.
Meirelles
School of Food Engineering, University of Campinas, R. Monteiro Lobato 80, Campinas, 13083-862, São Paulo, Brazil. E-mail: arieltoledohijo@gmail.com; hijo@unicamp.br
First published on 9th February 2023
Abstract
We present a strategy to encapsulate bioactive compounds using novel encapsulation agents in the ionic liquid form combining naturally derived functional compounds, such as choline and fatty acids, as exemplified by the resultant emulsion with high encapsulation efficiency (78%) of eucalyptol from rosemary essential oil and high emulsion stability (>1 month).
Bioactive compounds are widely employed in the food industry in the production of functional products and can be obtained from natural sources, such as natural flavors obtained from natural essential oils (NEOs).1 In fact, flavors obtained from NEOs are formed from mixtures of volatile bioactive compounds that could be rapidly lost by diffusion and are susceptible to oxidation when exposed to light, moisture, oxygen, and heat, which could lead to degradation and changes in their sensorial and bioactive properties.1
Encapsulation, classically defined as a coating process (e.g. emulsification) in which an active material is covered by an encapsulating agent,2 is one of the most important techniques used in the food industry to protect, retain, stabilize, deliver, and release bioactive compounds. However, the problems associated with the production of bioactive-based functional products are not completely solved considering the clean label trend in the field and the constant search for more sustainable alternatives. In this context, the search for multifunctional additives (e.g. one having two or more nutritional and/or technological functions to replace various additives) derived from natural sources could be a way to overcome these issues. However, it is a challenge, especially when bioactive compounds from natural sources need to be used to obtain healthier food products with a smaller number of ingredients and lower cost. Such multifunctional naturally based additives seem to be interesting alternatives to replace not-so-clean artificial additives or those obtained from natural sources but associated with allergenic issues or with higher calories, such as soy and some milk derivatives and carbohydrates. This might be more relevant, considering that the food industry has a crucial role in combating the COVID-19 pandemic offering healthier foods and food supplements containing essential nutrients, such as omega fatty acids (FAs) and choline (Ch),3,4 which are required to reach a good nutritional balance necessary to improve the immune system.
Ionic liquids (ILs), defined as salts that melt below 100 °C, are versatile compounds with potential use in the food industry.5–9 Few examples of traditional ILs, such as imidazolium-based, have been used in emulsion encapsulation as the core material and as disperse or continuous phases,10 and they may not be recommended for direct food applications.6 In the fragrance field, ILs have been employed by Procter and Gamble (P&G),11 while FA-based ILs have been used in mixtures with EOs comprising a non-emulsion system to improve the storage and release of fragrance components.12 However, IL application as encapsulation agents is still unexplored. In fact, such a potential application is quite promising for the food industry considering the possibility of combining suitable food ingredients or natural bioactive compounds as precursors to obtain naturally based encapsulating agents in the IL form. In our own ongoing search for new food ingredients in the IL form, we have recently found that combining FAs with a suitable cationic source can lead to naturally based emulsifiers.13
We hypothesize that ILs derived from bioactive compounds that can be obtained from natural sources can be used as encapsulating agents of bioactive compounds from natural EOs for the formulation of functional emulsion-based products with improved stability, functionality, and release. In addition to an IL-based formulation, we propose the high-intensity ultrasound (HIU) technology as an encapsulation process to validate this strategy. This IL approach is based on the use and combination of nutraceuticals already used in the food industry, as exemplified by the essential nutrients vitamin Ch and omega FAs. This approach also expands the application of essential nutraceuticals to be used not only to add nutritional value, but also to have technological application as the encapsulating agent, to replace non-sustainable additives. Considering the clean label trend in the food industry field, the search for a multifunctional and naturally derived encapsulation agent in the IL form has been encouraged.
To test our hypothesis, we chose Ch and diethanolamine as cation precursors, and FAs as anion precursors, to form ILs (Scheme S1†). Ch (pKa = 13.97) belongs to B-complex vitamins, is a food additive, can be naturally present in several foods and food byproducts, and is considered as an essential and nontoxic nutrient.3 Extra Ch supplements may help pregnant women mitigate the negative effects of respiratory viral infection, including COVID-19, on their newborns.4 FAs are bioactive compounds that can be naturally obtained from edible vegetable oils and formed by the hydrolysis of triacylglycerol molecules. Thus, they need to be removed in the refining of vegetable oils, representing an important byproduct. Also, FAs are food additives and considered as GRAS (Generally Recognized as Safe) substances. Stearic (pKa = 4.75) and oleic (pKa = 5.02) acids are the most significant FAs in the FA profile of the most used food-grade vegetable oils. Oleic acid (Omega-9) was also chosen to evaluate the effect of unsaturation present in its molecular structure, which could be interesting for a better protection of encapsulated bioactive compounds. Alkanolamines, such as diethanolamine (H2EA, pKa = 8.96), are naturally found in phospholipid biological membranes. In this first study, envisioning potential naturally derived encapsulation agents, traditional ionic sources, such as Ch hydroxide and FAs, and a validated straightforward synthesis reaction14–16 were used to evaluate a direct IL application. However, to make this technology feasible for the food industry, food-grade sources, such as Ch- and FA-based salts, could be considered as alternative ionic sources to prepare such ILs, which could be verified by further investigation. Thus, this potential IL application in the food industry field could be more related to Ch- and FA-based compounds. H2EA was used to investigate the potential effect of cation molecular structures. Note that H2EA-based ILs and Ch-based ILs are protic and aprotic ILs, respectively. 1,8 Cineole, known as eucalyptol (Scheme S1†), was chosen because it is one of the most common monoterpenes found in widely used EOs (e.g. rosemary, origanum, eucalyptus, mint and salvia), is volatile and a GRAS substance, used as a food flavoring agent with a camphor-like aroma and for the treatment of respiratory diseases (e.g. hypersecretion, asthma and rhinosinusitis), and has antitussive, decongestant, antimicrobial, antimalarial, anti-inflammatory, antioxidant and analgesic properties.17 Rosemary EO is a natural flavoring and antioxidant food ingredient and naturally composed of bioactive compounds, such as eucalyptol. In this context, Rosemary EO was chosen as the core material and eucalyptol as the marker bioactive compound for encapsulation efficiency.
The ILs were obtained through an acid–base reaction14,18 (NMR and IR spectra, SM) and named diethanolammonium stearate ([H2EA][C18OO]), diethanolammonium oleate ([H2EA][C18:1OO]) and cholinium stearate ([Ch][C18OO]). H2EA-based ILs were synthesized by a neat reaction, affording a white solid and gel-like compound for stearate- and oleate-based ILs, respectively. The Ch-based IL was synthesized by reaction in ethanolic-aqueous solution media followed by drying under high vacuum, affording a white solid.
The encapsulation of eucalyptol was performed through an emulsification process in two steps. 1% of IL (concentration based on the Brazilian food legislation19) was dissolved in the aqueous phase, and then 1% of rosemary EO containing eucalyptol was gradually added as the first step by using Ultra Turrax (U). The system was submitted to a HIU-assisted encapsulation process as the second step. We observed high encapsulation efficiency (EE) of eucalyptol. Interestingly, [H2EA][C18OO], [H2EA][C18:1OO] and [Ch][C18OO] presented EE close to 75.11%, 81.57% and 77.55% (Fig. 1), respectively. Among the evaluated ILs, the EE was statistically similar. This suggests that the encapsulation might be mainly ruled by intermolecular electrostatic interactions and less ruled by steric interactions under the used formulation conditions, and the combination of such interactions resulted in a high EE. Unlike expected, aprotic ILs as in the case of [Ch][C18OO] that commonly present lower ionic interactions than protic ILs (e.g. [H2EA][C18OO]) afforded enough electrostatic interactions to form an encapsulating layer and reach high EE, which is promising due to the nutritional importance of Ch and FAs for the food industry field. Among the proposed ILs, Omega FA based-ILs, as in the case of [H2EA][C18:1OO], are expected to have potential to protect bioactive compounds susceptible to oxidation, due to the antioxidant properties of Omega FAs, an effect that should be further investigated. In addition, preliminary results of Zeta Potential (ZP) of H2EA-based ILs in aqueous solution may indicate that an isoelectric point may be observed at pH values between 2 and 4 (ZP varying from values closed to 13 mV to −19 mV, respectively), suggesting that the proposed IL-based encapsulating system might potentially allow the delivery or controlled release of an encapsulated compound in the body, possibly through a micellar rupture in the gastric system at pH ∼1–2, or may be related to the pKa values of the involved compounds.
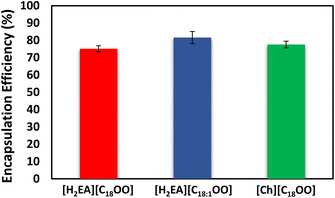 | ||
| Fig. 1 Encapsulation efficiency of eucalyptol in emulsions prepared with [H2EA][C18OO], [H2EA][C18:1OO] and [Ch][C18OO]. | ||
The HIU-assisted encapsulation by emulsification is based on the bubble cavitation phenomenon that produces intense microshear. This means that the physicochemical properties of the ILs were probably preserved, and their arrangements well formed to afford a suitable interfacial layer able to encapsulate eucalyptol, after submitting to acoustic cavitation by HIU, leading to high EE. Otherwise, without the addition of an efficient encapsulating agent, volatile compounds present in EOs, such as eucalyptol, are expected to be rapidly lost by diffusion and degraded when submitted to U + HIU due to compound's high volatility, heat, oxygen, moisture, and light. Therefore, ILs acted as promising encapsulating agents at low concentration affording high EE values, which are quite competitive when compared to those observed using traditional commercial additives, such as gum acacia and chemically modified starches (e.g., snow-flake and Hi-cap), among others.20 Previously, rosemary EO emulsions obtained by HIU using gum Arabic as the encapsulation agent at 10% (w/w) presented EE of eucalyptol from 83% to 97%, depending on the ultrasound energy strategy.21 Moreover, soy lecithin and hydrolyzed whey protein have been shown to be as alternatives to polysaccharides.22 In fact, affording NEO-based emulsions with high kinetic stability with some traditional commercial encapsulating agents could be a challenge, and bioactive compounds are better retained in highly stable emulsions. In these cases, a higher concentration of some commercial encapsulating agents can be used to achieve the desire performance,20,21 unlike the proposed ILs used at 1% (w/w) with the possibility of being used at much lower concentrations.
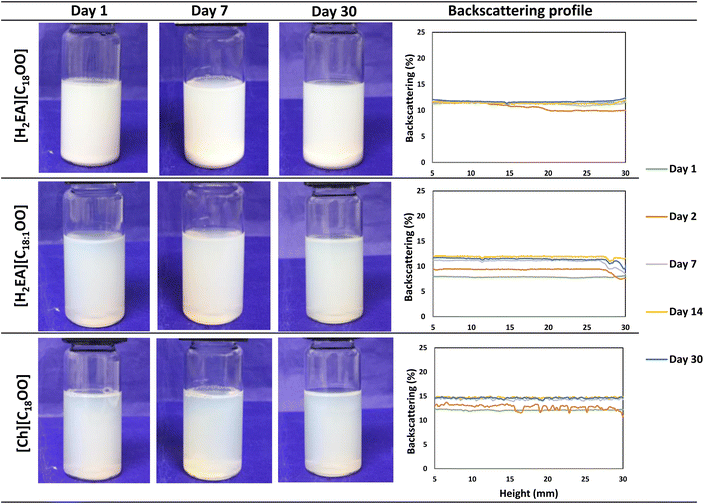
To understand the similarity in EE of ILs, we investigated the emulsifying properties of ILs by emulsion characterization. Due to the importance of emulsion stability on encapsulation, the kinetic stability of the IL-based emulsions was characterized by visual aspects and using an optical scanning device (Fig. 2). Emulsions presented high kinetic stability without phase separation of at least one month. This is interesting because emulsions with high kinetic stability are highly required for a better retention of bioactive compounds and can increase the shelf life of an encapsulated-based product. IL-based emulsions presented similar visual stability, although [H2EA][CnOO]-based ILs present lower critical micellar concentration (CMC) values (CMC = 0.092 and 0.184 mM for n = 18 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)23 than [Ch][CnOO]-based ILs (CMC = 6.4, 1.8 for n = 14, 16),15 and although they might present higher intermolecular interactions since they are protic ILs.
1)23 than [Ch][CnOO]-based ILs (CMC = 6.4, 1.8 for n = 14, 16),15 and although they might present higher intermolecular interactions since they are protic ILs.
Emulsions presented low backscattering values and a nanoemulsion like appearance trend (Fig. 2) and were not suitable for the master sizer after characterization attempts. Nanoemulsions obtained by HIU using orange EO and Tween 80 and gums have been previously reported.24 This is interesting, since possible smaller droplets are directly related to higher emulsion stability according to Stokes' law and to higher EE,25 which might support the fact that the proposed naturally based ILs presented similar EE. According to micrographs (Fig. 3), after storage, emulsions still presented very small droplets. The high electrostatic repulsion of IL-formed micelles might lead to an equilibrated system and may avoid increase in the droplet size over storage time. This might be related to high kinetic stability observed for emulsions after storage (Fig. 2). Considering that emulsions stabilized by these ILs presented less or no apparent changes over time under the used conditions, the evaluation of these emulsifiers at lower concentration (<1%, w/w) should be further investigated.
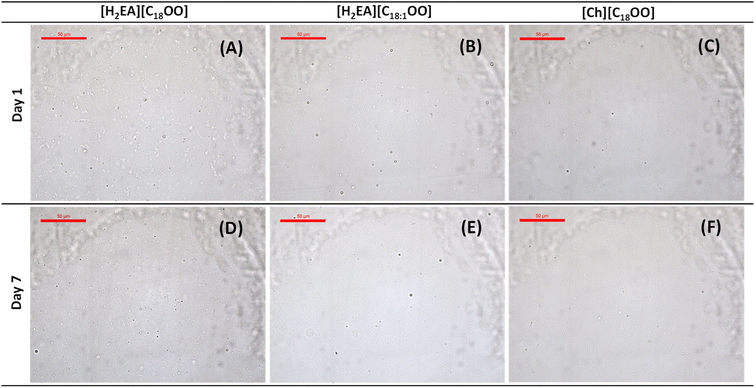 | ||
| Fig. 3 Optical micrographs of [H2EA][C18OO], [H2EA][C18:1OO] and [Ch][C18OO] emulsions after 1 and 7 days of preparation. Scale bar: 50 μm. | ||
We further characterized the IL-based emulsions by rheology (Table S1†). An effect of the type of ILs and storage time on the rheological properties and apparent viscosity of emulsions was observed. Emulsions with C18OO-based ILs presented non-Newtonian rheological behavior with a shear-thickening profile, whereas that with C18:1OO-based ILs presented a Newtonian profile with lower apparent viscosity. Emulsions with Ch-based ILs presented viscosity decrease after 7 days of storage. Therefore, IL-based emulsions presented low viscosity and high mechanical stability that is desired to design emulsion-based encapsulation systems with a long shelf-life.
Conclusions
In summary, we have presented new encapsulating agents of bioactive compounds obtained in the IL form. By this approach, multifunctional naturally derived encapsulating agents can be obtained by combining two bioactive compounds, such as vitamin Ch and omega FAs, with the ability to provide high EE and stability, and possibly a controlled release of bioactive compounds. Considering that emulsions were highly stable, future studies should include in-depth investigation of long-term stability of emulsions through accelerated phase separation methods, characterization by Dynamic Light Scattering (DLS) to verify droplet sizes and interfacial tension evaluation. Considering the clean label trend in the food industry field, a naturally derived multifunctional ingredient that provides the nutritional value of at least two nutrients and presents technological use as an encapsulating agent is quite promising for the formulation of sustainable emulsion-based products and may be considered as an interesting alternative over traditional additives.Conflicts of interest
Drs Ariel A. C. Toledo Hijo, Antonio J. A. Meirelles, Rosiane L. Cunha, and Aureliano A. D. Meirelles are named inventors on related patent applications.Acknowledgements
Ariel A. C. Toledo Hijo thanks the São Paulo Research Foundation (FAPESP) (Grant # 2019/18608-1, 2022/04614-2, and 2016/24461-5) for scholarships and financial support. The authors thank the funding agencies FAPESP (2014/21252-0, 2016/24461-5, 2019/18608-1, and 2022/04614-2) and National Council for Scientific and Technological Development (CNPq) (307398/2019-6 and 406963/2016-9) for financial support and scholarships. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance code 001.Notes and references
- A. E. Asbahani, K. Miladi, W. Badri, M. Sala, E. H. A. Addi, H. Casabianca, A. E. Mousadik, D. Hartmann, A. Jilale, F. N. R. Renaud and A. Elaissari, Int. J. Pharm., 2015, 483, 220–243 CrossRef CAS PubMed.
- N. Mehta, J. S, P. Kumar, A. K. Verma, P. Umaraw, S. K. Khatkar, A. B. Khatkar, D. Pathak, U. Kaka and A. Q. Sazili, Foods, 2022, 11, 2973 CrossRef CAS PubMed.
- S. H. Zeisel and K.-A. da Costa, Nutr. Rev., 2009, 67, 615–623 CrossRef PubMed.
- R. Freedman, S. K. Hunter, A. J. Law, A. D'Alessandro, K. Noonan, A. Wyrwa and M. Camille Hoffman, J. Psychiatr. Res., 2020, 128, 1–4 CrossRef PubMed.
- A. A. C. Toledo Hijo, H. D. F. Q. Barros, G. J. Maximo, C. B. B. Cazarin, L. B. E. da Costa, J. F. B. Pereira, M. R. Maróstica Junior and A. J. A. Meirelles, Food Res. Int., 2020, 134, 109125 CrossRef CAS PubMed.
- A. A. C. Toledo Hijo, G. J. Maximo, M. C. Costa, E. A. C. Batista and A. J. A. Meirelles, ACS Sustainable Chem. Eng., 2016, 4, 5347–5369 CrossRef CAS.
- A. A. C. Toledo Hijo, C. Alves, F. O. Farias, V. S. Peixoto, A. J. A. Meirelles, G. H. F. Santos and G. J. Maximo, Food Res. Int., 2022, 157, 111194 CrossRef CAS PubMed.
- M. C. Ferreira, A. A. C. Toledo Hijo, F. O. Farias, E. A. C. Batista, G. J. Maximo and A. J. A. Meirelles, Fluid Phase Equilib., 2022, 555, 113350 CrossRef CAS.
- P. L. G. Martins, A. R. Braga and V. V. de Rosso, Trends Food Sci. Technol., 2017, 66, 117–124 CrossRef CAS.
- Q. Luo, Y. Wang, Z. Chen, P. Wei, E. Yoo and E. Pentzer, ACS Appl. Mater. Interfaces, 2019, 11, 9612–9620 CrossRef CAS PubMed.
- L. A. M. Holland, O. Todini, D. M. Eike, J. M. Velazquez Mendoza, S. A. Tozer, P. M. Mcnamee, J. R. Stonehouse, W. E. Staite, H. C. R. Fovargue, J. A. Gregory, K. R. Seddon, H. Q. N. Gunaratne, A. V. Puga, J. Estager, F.-L. Wu, S. D. Devine, M. Blesic and F. M. Ferrero Vallana, PCT Int. Appl., WO2017075299A120170504, 2017, 1–89 Search PubMed.
- P. Berton, K. Bica and R. D. Rogers, Fluid Phase Equilib., 2017, 450, 51–56 CrossRef CAS.
- A. A. C. Toledo Hijo, A. A. D. Meirelles, G. J. Maximo, R. L. Cunha, M. Cristianini, T. S. Leite, J. F. B. Pereira and A. J. A. Meirelles, ACS Sustainable Chem. Eng., 2022, 10, 15017–15024 CrossRef CAS.
- A. A. C. T. Hijo, E. Pereira, A. M. S. Magalhães, G. J. Maximo, M. C. Costa, J. F. B. Pereira and A. J. A. Meirelles, Fluid Phase Equilib., 2021, 548, 113168 CrossRef CAS.
- R. Klein, D. Touraud and W. Kunz, Green Chem., 2008, 10, 433–435 RSC.
- R. Klein, H. Dutton, O. Diat, G. J. T. Tiddy and W. Kunz, J. Phys. Chem. B, 2011, 115, 3838–3847 CrossRef CAS PubMed.
- G. Jalilzadeh-Amin and M. Maham, Pharm. Biol., 2015, 53, 594–599 CrossRef CAS PubMed.
- A. A. C. Toledo Hijo, G. J. Maximo, R. L. Cunha, F. H. S. Fonseca, L. P. Cardoso, J. F. B. Pereira, M. C. Costa, E. A. C. Batista and A. J. A. Meirelles, Phys. Chem. Chem. Phys., 2018, 20, 6469–6479 RSC.
- ANVISA, RESOLUÇÃO-RDC N° 23, http://portal.anvisa.gov.br/legislacao#/visualizar/27496, (accessed 12/08/2019).
- G. A. Pereira, E. K. Silva, N. M. Peixoto Araujo, H. S. Arruda, M. A. A. Meireles and G. M. Pastore, Food Hydrocolloids, 2019, 97, 105190 CrossRef CAS.
- A. A. C. Toledo Hijo, R. E. Guinosa and E. K. Silva, J. Mol. Liq., 2022, 358, 119179 CrossRef CAS.
- E. Hebishy, L. Collette, P. Iheozor-Ejiofor and B. A. Onarinde, J. Food Process. Preserv., 2022, 46, e16840 CAS.
- G. J. Maximo, R. J. B. N. Santos, J. A. Lopes-Da-Silva, M. C. Costa, A. J. A. Meirelles and J. A. P. Coutinho, ACS Sustainable Chem. Eng., 2014, 2, 672–682 CrossRef CAS.
- A. M. Hashtjin and S. Abbasi, Food Hydrocolloids, 2015, 44, 40–48 CrossRef CAS.
- C. R. L. Francisco, F. D. de Oliveira Júnior, G. Marin, I. D. Alvim and M. D. Hubinger, Colloids Surf., Ac, 2020, 607, 125470 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fb00054g |
| This journal is © The Royal Society of Chemistry 2023 |