Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of mixtures of ethanol–calcium chloride–carboxymethylcellulose on the bovine milk whey freeze concentration process†
Camilla Soares
Duarte
a,
Adrise Aparecida
Rodrigues
a,
Ana Cristina Freitas de Oliveira
Meira
a,
Luiz Ronaldo
de Abreu
a,
Fabiano Freire
Costa
b and
Jaime Vilela
de Resende
 *a
*a
aFederal University of Lavras, Department of Food Science, Laboratory of Food Refrigeration, P.O. Box 3037, 37200-900 Lavras, Minas Gerais, Brazil. E-mail: jvresende@ufla.br; Tel: +55 35 3829 1659
bFederal University of Juiz de Fora, College of Pharmacy, Departamento of Pharmaceutical Science, Juiz de Fora, Minas Gerais, Brazil
First published on 20th December 2022
Abstract
For a long time bovine milk whey (BMW) was considered a residue. However, considering the protein contents, lactose contents and other constituents, BMW has always presented good potential for the manufacture of various by-products of nutritional value. Due to the low temperature, the BMW freeze concentration influences the lactose crystallization, the protein structures and the bioactive compound preservation resulting from water separation as ice crystals. The separation results in increased content and improved functionality of these constituents. The effects of ethanol, calcium chloride (CaCl2) and carboxymethylcellulose (CMC) concentrations on physicochemical properties, rheological parameters and temperature profiles in the freeze concentration (FC) of BMW from cheese enzymatic coagulation were evaluated. The results showed that the effects of ethanol, CaCl2 and CMC addition were significant (p < 0.05) on percentage variations in density, lactose content, total soluble solids and protein content. The number of stages influenced the freeze-concentration process. In the “concentrate” fraction, the largest variations in density, lactose content, SST content and protein content were found in the treatment consisting of 10% ethanol, 0.5% CaCl2 and 0.05% CMC. The results were supported by the analysis of the levels of subcooling and initial freezing temperatures obtained through temperature profiles and in the increase of viscosities of the “concentrated” and “ice crystal” fractions. The use of substances that interfere in the ice crystal formation in the freeze concentration process that precedes the drying process showed remarkable results in the constitution of the final products, used as ingredients in various food formulations.
Introduction
For decades, the dairy industry has been one of the most important sectors for the economy of industrialized and developing countries. However, of the total volume of milk used to make cheese, 85% of it was considered a residue (bovine milk whey, BMW) which, when discarded directly into nature, could cause great environmental impacts. Currently, whey is considered a by-product and proposed to be used for other purposes, as BMW contains constituents of milk, such as lactose, soluble proteins, lipids and high added-value mineral salts, with high application in the food industry as a complement that is nutritional and functional.1,2Methods based on the crystallization of water for obtaining BMW by freeze concentration (FC) have been studied which do not interfere with the nutritional and functional properties of milk whey.1–3 Crystallization is the key operation in FC which is a process that consists of crystallizing part of the water contained in the medium to separate the soluble components found in the system. This unit operation can be applied in several types of processes, specifically, in the concentration of aqueous foods in the food industry.4 The purpose of FC is to remove water at low temperatures. Therefore, important components such as proteins, flavorings, vitamins and polyphenols remain in the food and do not degrade or undergo changes in their properties during crystallization and separation of the media. Another advantage because it occurs at low temperature is that the microbial growth rate and enzyme activity decrease. The main disadvantages of the process are the high cost and the limitation of crystallization due to the increase in viscosity.5,6 The most applied FC techniques in the bio-food industry are suspension, progressive, eutectic partial block and complete block.5 These techniques have been studied by several authors for application in foods such as fruit juices and dairy products and water desalination.7–13 Progressive freeze concentration forms a single ice crystal in the system; therefore, the system is much simpler as compared with the conventional method of equilibrium suspension crystallization for freeze concentration, in which many small ice crystals are formed.14
Aider et al.1,2 used FC technology for the recovery and enhancement of milk whey as a promising ingredient in the food industry. The objective of the work was to optimize the milk whey concentration process, minimizing the amount of dry matter retained in the ice fraction. This was made possible by recycling the ice fraction. The authors report the concentration of acid milk whey from 5.71 ± 0.01% (w/w) to 24.68 ± 0.03% (w/w) of total dry matter through three FC cycles and recycling. The study also aimed to evaluate the emulsifying and foaming properties of concentrated milk whey as a function of concentration cycles. The results showed that the stability index of the concentrated whey emulsion increased, as the number of FC cycles increased, while the emulsifying activity index decreased.5 After four levels of concentration it was possible to concentrate the whey to 35% of the dry matter and the total proteins were concentrated to 20% of the total dry matter. Lactose was more concentrated in the ice fraction and proteins were more concentrated in the non-frozen fraction. The efficiency of the concentration process decreased when the number of FC stages was increased. The optimization of the process by mathematical models showed that the number of optimal stages is three.3
In the freezing process, water crystallization begins when favourable conditions are reached for aggregation in an ordered arrangement of a group of molecules forming particles called crystallization nuclei. These conditions are determined by the correlation between temperature, cooling rate, concentration of solutes and magnitude of the forces guiding the molecules in the liquid. Ice crystal growth is possible once nucleation occurs. The control of the ice crystal sizes in the presence of additives was studied by Carneiro and Cal-Vidal.15 Based on the factors responsible for the nucleation and ice crystal growth, strategies, depending on the final objectives and frozen biological products or systems, were proposed by Blanshard and Franks16 for the control of water crystallization in food systems. Among these strategies include: (1) the inhibition of nucleation. The freezing point of the product is lowered by the introduction of additives. (2) The control of the nucleation. Relative rates of ice nucleation can be manipulated by appropriately exploiting the heat transfer rates and the conditions of the physicochemical parameters of systems. (3) Controlling the growth of the ice crystal. The presence and accumulation of micro and macromolecular additives can modify the diffusion/colligative properties at the water–ice crystal interface and thereby limit the extensive growth of the ice crystal or recrystallization.
Based on these fundamentals, the hypothesis in this work for the FC process is to act in the opposite direction trying to favour the formation of ice crystals by the use of substances to induce water crystallization. Solutes present in water can also directly influence the morphology of ice crystals.15 In food systems, macromolecules (proteins and/or polysaccharides) form a gel network at low temperatures that, as has been shown, influence and modify the ice crystal growth habits. Another aspect considered in the freezing process is the addition of organic solvents, such as alcohols before the freezing process, which can reduce the solubility of solutes and promote their crystallization through supersaturation of the system and also change the ice crystal growth habits.17
Considering the dairy systems, Costa et al.18 investigated the influence of calcium fortification by the addition of calcium chloride and κ-carrageenan on the parameters of ice cream quality. The results showed that the addition of calcium chloride led to a substantial increase in the size of the ice crystals and partial fat coalescence, which were exacerbated by the addition of κ-carrageenan. The effects of adding calcium to dairy systems are well known, resulting in improved casein interactions.19
The ability of various hydrocolloids to influence water crystallization as a function of temperature and viscosity was studied by Budiaman and Fennema.20,21 Carboxymethylcellulose (CMC), gelatin, microcrystalline cellulose, sodium alginate and some gums were tested for the ability to reduce the water crystallization rate as a function of the initial sub-cooling temperature. All samples showed an increase in the crystallization rate with a reduction in the sub-cooling temperature and with a decrease in the concentration of hydrocolloids, but the effects varied with the nature of the hydrocolloid. The influence on the growth rate of ice crystals by the presence of a gel network was discussed by several authors16,22,23 in terms of the interaction of the front of the ice crystal with the network and others components of the gel system. These systems affect the dynamics of the freeze-concentration process. Dairy protein recovery has gained increasing interest due to the high aggregate value of the product and few studies are found specifically on additives that can influence the polarization mechanism of solutes in BMW. Vuist et al.24 evaluated the effect of the addition of sodium chloride and sucrose on the inclusion behaviour in progressive freeze concentration of whey protein solutions. The authors showed that solutions of whey protein isolate can be successfully concentrated using progressive freeze concentration. Higher concentrations of whey protein lead to a higher concentration near the interface, which may create a gel layer that impedes the mass transfer from the boundary layer and hence promotes solute inclusion. Therefore, this work aimed to evaluate the effects of the addition of CaCl2, CMC and ethanol in progressive freeze-concentration of bovine milk whey (BMW) on the composition and thermal and rheological properties of the concentrate and crystal fractions after the ice separation.
Materials and methods
Bovine milk whey (BMW)
Bovine milk whey (BMW) was obtained from a dairy located in the municipality of Perdões/MG. It was collected from the cheese-making process by an enzymatic coagulation process. The analytical composition of the original BMW used in the tests was density (1022.96 kg m−3), lactose (2.21%), TSS (3.98%) and protein (1.29%). The BMW was sent to the laboratory where it was filtered using cotton fabric as a filter element and stored between 0 and 4 °C in a refrigerator until its application in the process.Sample preparation and experimental planning
The following reagents were used as additives: carboxymethylcellulose salt sodium USP (CMC) (Exodus Cientifica, São Paulo, Brazil); calcium chloride P.A-ACS (Dinâmica, São Paulo, Brazil) and ethyl alcohol 99.9% (Dinâmica, São Paulo, Brazil). For treatments with different concentrations of CMC, the solubilisation of the additive in the BMW was done using a magnetic stirrer for 30 minutes. After dissolving, the mixture (BMW with CMC) was stored in a refrigerator for 12 hours at temperatures between 0 and 4 °C to complete the hydration of the CMC.After the hydration period, CaCl2 and ethanol were added to the BMW in concentrations defined according to the experimental plan presented in Table 1. The rotational central composite design (RCCD) with 3 dependent variables with 14 axial points and 3 central points was applied and the variables were the concentrations of CMC, CaCl2 and ethanol. After preparation, the treatments were divided into 3 portions to perform the FC process.
| Treatments | Coded variables | Real variables | ||||
|---|---|---|---|---|---|---|
| Ethanol (%) | CaCl2 (%) | CMC (%) | Ethanol (%) | CaCl2 (%) | CMC (%) | |
| 1 | −1 | −1 | −1 | 2.02 | 0.20 | 0.02 |
| 2 | −1 | −1 | 1 | 2.02 | 0.20 | 0.08 |
| 3 | −1 | 1 | −1 | 2.02 | 0.80 | 0.02 |
| 4 | −1 | 1 | 1 | 2.02 | 0.80 | 0.08 |
| 5 | 1 | −1 | −1 | 7.98 | 0.20 | 0.02 |
| 6 | 1 | −1 | 1 | 7.98 | 0.20 | 0.08 |
| 7 | 1 | 1 | −1 | 7.98 | 0.80 | 0.02 |
| 8 | 1 | 1 | 1 | 7.98 | 0.80 | 0.08 |
| 9 | −1.68 | 0 | 0 | 0 | 0.5 | 0.05 |
| 10 | 1.68 | 0 | 0 | 10 | 0.5 | 0.05 |
| 11 | 0 | −1.68 | 0 | 5 | 0 | 0.05 |
| 12 | 0 | 1.68 | 0 | 5 | 1 | 0.05 |
| 13 | 0 | 0 | −1.68 | 5 | 0.5 | 0 |
| 14 | 0 | 0 | 1.68 | 5 | 0.5 | 0.1 |
| 15 | 0 | 0 | 0 | 5 | 0.5 | 0.05 |
| 16 | 0 | 0 | 0 | 5 | 0.5 | 0.05 |
| 17 | 0 | 0 | 0 | 5 | 0.5 | 0.05 |
| Control | — | — | — | 0 | 0 | 0 |
Progressive freeze-concentration process (FC)
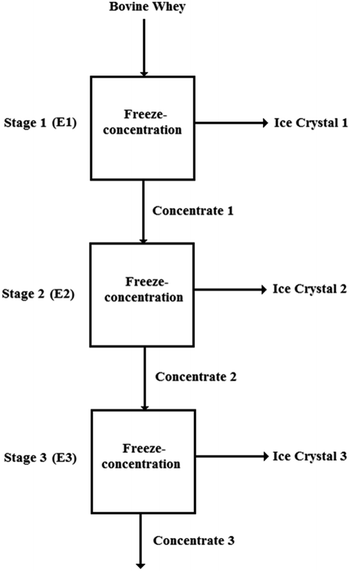
The FC process for each of the treatments including the control was done in batches and in three stages and 4 L of BMW were processed in each stage. A diagram of the process is shown in Fig. 1.Concentrator
The progressive FC of the samples was performed in a concentrator with a 5.0 L volume container built in the laboratory. The formation of ice occurs on the external surface of a 3/8 ” (9.53 mm) diameter stainless steel coil through which secondary refrigerants circulate. The secondary refrigerant used for cooling/freezing was an alcoholic solution (0.8 L of ethanol/liter of solution) at −12 °C recirculated from an ultra-thermostatic bath (Nova Ética, Model: 521/3DF, Vargem Grande Paulista, Brazil). The residence time of the samples in the concentrator was fixed at 40 minutes with mechanical agitation (72 rpm) (IKA Labortechnik, model RW.20).These operating conditions were determined from pre-tests to determine the ideal time and temperature for the process. After removing the concentrate, to accelerate the process of removing the formed ice, water at 30 °C from a thermostatic bath (Brookfield, Model: EX200, Stoughton, USA) was recirculated through the circuit.
The standard BMW (raw material) was submitted to batches under the same operating conditions of time and temperature. After the processes, the fractions in each batch were separated and conditioned in containers labeled as ice crystal fraction and concentrated fraction. The trials were carried out until sufficient concentrated fraction volume (4 L) was obtained to start the second stage. The same procedures were carried out to start the third stage.
To obtain the temperature histories, 4 copper-constantan T-type thermocouples (Omega Engineering Inc., USA – AWG 30) were installed in the radial direction 0.5 cm apart and close to the coil, as shown in Fig. 2. Obtaining the temperature data as a function of time was made by a data signal conditioning system (National Instruments Mod. SCXI – Hungry) and the Lab View 8.5 software was used for data acquisition. The interval between measurements was 10 seconds. Fig. 2 shows a representation of the data acquisition system and the radial distribution of the thermocouples inside the concentrator.
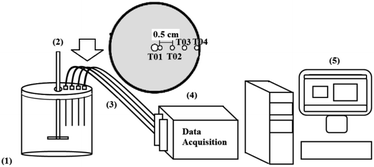 | ||
| Fig. 2 Data acquisition system (1) concentrator; (2) agitator; (3) thermocouples (position of the thermocouples installed on the concentrator); (4) data acquisition; (5) computer. | ||
Physicochemical analysis
Total soluble solid content, density, lactose and protein
The total soluble solid (TSS) content, density, lactose and protein were determined using Lactoscan © equipment (Ultrasonic milk analyzer; Milk Otronic, Bulgaria). The measuring ranges are: TSS from 0 to 50% ± 0.17%; density from 1000 to 1150 kg m−3 ± 0.3 kg m−3; lactose from 0.01 to 20.00% ± 0.20% and proteins from 2 to 7% ± 0.15% in accordance with the manufacturer's instructions. The equipment was previously calibrated using a standard BMW sample and the samples were read in quadruplicate. The analyses of these parameters were made in terms of the results obtained for the concentrated samples compared to those obtained for the standard sample (BMW pure without additives) (eqn (1)): | (1) |
Viscosity
The rheological measurements were performed at 4 °C using a Brookfield DVIII Ultra concentric cylinder rotational viscometer (Brookfield Engineering Laboratories, Stoughton, USA), with an adapter for small samples 13R/RP (19.05 mm in diameter and 64.77 mm in depth) and shear sensor coaxial SC4. The samples were submitted to an increasing shear rate ramp that varied linearly from 0 s−1 to 240 s−1. The measurements were made in triplicate and 13 points were taken in each trial.With the shear stress (τ) and shear rate (γ) values, the rheological parameters, consistency index (k) and fluid behaviour index (n) were calculated from the power law model (eqn (2)). All rheological parameters were obtained using the Rheocalc software (Version V.3.1, Brookfield Engineering Laboratories, Stoughton, USA).
τ = k·![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) n n | (2) |
![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif) = shear rate (s−1); n fluid behaviour index.
= shear rate (s−1); n fluid behaviour index.
Colorimetry
The color of the BMW samples was measured using a Konica Minolta Spectrophotometer CM-5 colorimeter in the color coordinate system L*, a* and b*. In this color representation system, the values L*, a* and b* describe the uniformity of color in three-dimensional space, where the value L* corresponds to how light and how dark the analyzed product is (0 = black; 100 = white). The values of a* correspond to the scale from green to red (a* negative, green; a* positive, red) and the values of b* correspond to the blue to yellow scale (b* negative, blue; b* positive, yellow).From the values of a* and of b*, Hue angles (h°) were calculated for each test that indicate the chromatic hue (attribute in which the color is perceived), using eqn (3):
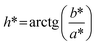 | (3) |
pH
The determination of pH was carried out using a bench pH meter (MPA-210).Statistics
All the readings in the samples were made in quadruplicate and the mean values were used as response variables of the treatments in the RCCD. A RCCD was performed with central points (level 0) and axial points (levels ±α), totalling 17 tests, associated with the response surface methodology (RSM).25 The minimum (α(−1.68) = 0%), central (α(0) = 5%) and maximum (α(+1.68) = 10%) of ethanol concentrations were established according to the work of Bezerra et al.17 Considering that the concentrated product will be freeze-dried, the effects on large ice crystal are minimized with higher ethanol concentrations and the freezing stage becomes more difficult by the reduction in the freezing temperature of the systems. The minimum (α(−1.68) = 0%), central (α(0) = 0.5%) and maximum (α(+1.68) = 1.0%) of CaCl2 concentrations were defined according to daily adequate calcium intake that establishes 1000–1300 mg.18 The minimum (α(−1.68) = 0%); central (α(0) = 0.05%) and maximum (α(+1.68) = 0.10%) of CMC concentrations were defined in pre-tests based on increased viscosities of the systems.21 The complete experimental configuration is shown in Table 1. The second-order polynomial model was adjusted to the experimental data (eqn (4)).| Yi = β0 + β1x1 + β11x12 + β2x2 + β22x22 + β3x3 + β33x32 + β12x1x2 + β13x1x3 + β23x2x3 + e | (4) |
Results and discussion
Physicochemical (step 1) – concentrated 1
Table 2 shows the regression analysis coefficients (RCCD) of the physicochemical parameters of the samples obtained in concentrate 1 from stage 1 added with ethanol, CaCl2 and CMC after freeze concentration. Table 2 also shows the correlation coefficients R2, the calculated F value and the regression coefficients for each order with their respective values of p. These parameters make it possible to evaluate the significant variables involved in the different stages of the process applied in the complete coded model shown in eqn (4). It is observed that practically all the values of F calculated for the curve adjustments presented in Table 2 are above the value F tabled (Ftab = 3.73), indicating that the parameters are significant.25| Concentrate 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Var. density (%) | Var. lactose (%) | Var. TSS (Brix) (%) | Var. protein (%) | |||||
| Regression coefficient | p | Regression coefficient | P | Regression coefficient | p | Regression coefficient | p | |
| a Significant at the level of 5% probability (p < 0.05). Legend: β0 = a constant term; β1 = ethanol (%); β11= (ethanol (%))2; β2 = CaCl2 (%); β22 = (CaCl2 (%))2; β3 = CMC (%); β33 = (CMC (%))2; β12 = ethanol (%) × CaCl2 (%); β13 =ethanol (%) × CMC (%); β23 CaCl2 (%) × CMC (%). | ||||||||
| β 0 | 206.227 | 0.000a | 302.441 | 0.000a | 238.976 | 0.000a | 201.374 | 0.000a |
| β 1 | 77.250 | 0.000a | 163.231 | 0.000a | 107.829 | 0.000a | 61.097 | 0.000a |
| β 11 | 2.955 | 0.562 | 19.693 | 0.112 | 8.160 | 0.332 | 1.003 | 0.808 |
| β 2 | 15.598 | 0.010a | 17.587 | 0.117 | 16.043 | 0.059 | 15.954 | 0.003a |
| β 22 | 10.074 | 0.077 | 16.760 | 0.165 | 8.219 | 0.329 | 3.972 | 0.350 |
| β3 | −13.394 | 0.019a | −17.290 | 0.122 | −25.197 | 0.009a | −12.722 | 0.010a |
| β 33 | 5.637 | 0.284 | 12.228 | 0.296 | 18.569 | 0.050a | −0.687 | 0.867 |
| β 12 | −8.248 | 0.195 | −15.102 | 0.278 | −10.124 | 0.313 | −6.977 | 0.182 |
| β 13 | 10.614 | 0.108 | 21.738 | 0.134 | 14.646 | 0.159 | 7.429 | 0.159 |
| β 23 | −17.830 | 0.017a | −29.657 | 0.054 | −21.346 | 0.055 | −15.633 | 0.013a |
| R 2 | 0.980 | 0.977 | 0.974 | 0.980 | ||||
| F | 38.694 | 32.889 | 29.071 | 37.359 | ||||
| Ice crystal 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Var. density (%) | Var. lactose (%) | Var. TSS (Brix) (%) | Var. protein (%) | |||||
| Regression coefficient | p | Regression coefficient | P | Regression coefficient | p | Regression coefficient | p | |
| β 0 | 158.870 | 0.000a | 239.958 | 0.000a | 185.444 | 0.000a | 147.025 | 0.000a |
| β 1 | 84.998 | 0.000a | 155.126 | 0.000a | 108.485 | 0.000a | 68.591 | 0.000a |
| β 11 | 6.638 | 0.258 | 18.609 | 0.1106 | 10.726 | 0.173 | 3.483 | 0.458 |
| β 2 | 18.716 | 0.007a | 21.415 | 0.0540 | 19.172 | 0.020 | 18.650 | 0.002a |
| β 22 | 5.119 | 0.374 | 11.010 | 0.316 | 7.172 | 0.345 | 3.712 | 0.430 |
| β 3 | −12.762 | 0.035a | −19.107 | 0.078 | −15.309 | 0.049a | −11.639 | 0.024a |
| β 33 | 5.394 | 0.351 | 10.077 | 0.356 | 7.083 | 0.350a | 4.351 | 0.359 |
| β 12 | −5.486 | 0.420 | −9.314 | 0.467 | −7.464 | 0.404 | −4.910 | 0.382 |
| β 13 | 11.133 | 0.126 | 20.136 | 0.140 | 13.369 | 0.156 | 8.786 | 0.139 |
| β 23 | −16.857 | 0.034a | −27.187 | 0.060 | −19.567 | 0.053 | −14.406 | 0.029a |
| R 2 | 0.980 | 0.977 | 0.978 | 0.979 | ||||
| F | 37.211 | 33.605 | 34.526 | 36.886 | ||||
Another parameter presented in Table 2 is the determination coefficient (R2). The value R2 is a measure of the variation percentage of the observed values around the mean explained by the adjusted model. In the analysis of variance shown in Table 2, the percentage of variation explained by the regression is greater than 97%.27 It can be seen in Table 2 that the additives (ethanol, CaCl2 and CMC) linearly influenced the density of the BMW concentrate after stage 1 of the concentration process. These results allowed the construction of the response surfaces shown in Fig. 1S – ESI.† These three additives also significantly influenced the variation in the concentration of proteins in concentrate 1. There was a positive linear increase in the protein content in concentrate 1 with increasing concentrations of ethanol and CaCl2 and a negative linear decrease with increasing CMC concentration. In the statistical analyses of the BMW freeze concentration process, it is observed that the higher the ethanol and CaCl2 concentrations, the more effective and linear is the response and the treatments with the highest concentrations of these additives presented the best results. This characteristic is explained by the interactions between the additives and the BMW. Ethanol is a polar organic compound, soluble in water, and yields hydrogen bonds, assisting in the water separation from BMW components. With the addition of CaCl2 to the system, part of the water molecules bind strongly to salt, contributing to the increase of hydrophobic interactions, resulting in protein–protein interactions. CMC had little interference in the freeze concentration process and in the pre-tests; with agitation, there was a better concentration of pure BMW and lower solute concentrations in the formed ice crystal. However, when CMC is added to BMW, it interacts with the BMW protein and precipitates. With agitation, the system becomes homogeneous and protein molecules tend to incorporate into the crystal.
Only the addition of ethanol had a linear and positive influence on the variation of the lactose concentration in concentrate 1 and the other additives, CaCl2 and CMC did not produce a significant effect on this parameter. The addition of ethanol also increased the variation in the TSS contents and the addition of CMC had a negative influence on the variation of this parameter. The analysis of the coefficients in Table 2 indicates that the higher the concentration of ethanol (%), the greater the density variation values, lactose content, TSS and proteins. Table 2 also shows that the higher concentration of CaCl2 (%) improves the effectiveness of the process by linearly increasing the variation in density and protein content. The amount of CMC has a linear and negative influence on the variation of density, TSS and protein content, that is, the greater the amount of CMC, the smaller the variation of all of these evaluated components.
Table 2 shows that CaCl2 does not significantly influence the lactose content or TSS. CMC does not significantly influence the lactose content but influences TSS in concentrate 1. The negative coefficients show that CMC reduces both the lactose content and TSS and this effect is also noticeable in the system consisting of CMC and CaCl2 (interactive effect β23). The structure of the gel is composed of water strongly bonded by hydrogen bonds to the hydrocolloid being immobilized by the formation of the net which gives texture to the system. The addition of Ca2+ ions competes with structural water causing the breakdown of hydrogen bonds and water is released in the system with a consequent reduction of viscosity. This behaviour is in accordance with Ganz28 and justifies the availability of water necessary for the reduction of lactose and TSS contents in concentrate 1.
The concentration of components in concentrate 1 was more effective for treatments consisting of higher concentrations of ethanol and CaCl2 and this characteristic can be explained by competition of these additives through covalent and ionic bonds with water in BMW releasing more free water in the systems and favouring the formation of ice. The effects of molecular interactions between these additives and BMW proteins inducing the formation of large ice crystals have also been discussed in the literature.18 Various studies29,30 show that in heat-set and cold-set whey protein gels, the addition of calcium salts promotes aggregation and gelation through a reduction in electrostatic repulsion. These aggregated whey proteins were investigated by analyses of the rheological, textural and microstructural properties of the gel. Information on the interactions of added calcium with whey proteins at lower temperature is limited.
Physicochemical analysis – step 1 – ice crystal 1
The ice crystal 1 fraction was formed and adhered to the concentrator coil and was removed after stage 1. The results also are shown in Table 2. The response surfaces for the variables in ice crystal 1 showed behaviours in the graph that follow the same trend that was observed for concentrate 1 (Fig. 1S – ESI†).Physicochemical analysis – effects of other stages in the concentration process
Tables 3 and 4 show the results of the regression analyses of the physicochemical parameters of the concentrate and ice crystal fractions obtained in stages 2 and 3, respectively, after adding ethanol, CaCl2 and CMC and freeze concentration.| Concentrate 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Var. density (%) | Var. lactose (%) | Var. TSS (Brix) (%) | Var. protein (%) | |||||
| Regression coefficient | p | Regression coefficient | P | Regression coefficient | p | Regression coefficient | p | |
| a Significant at the level of 5% probability (p < 0.05). Legend: β0 = a constant term; β1 = ethanol (%); β11 = (ethanol (%))2; β2 = CaCl2 (%); β22 = (CaCl2 (%))2; β3 = CMC (%); β33 = (CMC (%))2; β12 = ethanol (%) × CaCl2 (%); β13 = ethanol (%) × CMC (%); β23 CaCl2 (%) × CMC (%). | ||||||||
| β 0 | 228.151 | 0.000a | 329.522 | 0.000a | 261.677 | 0.000a | 214.328 | 0.000a |
| β 1 | 76.004 | 0.000a | 164.975 | 0.000a | 109.687 | 0.0000a | 61.184 | 0.000a |
| β 11 | −3.598 | 0.397 | 15.220 | 0.155 | 6.908 | 0.263 | −1.063 | 0.677 |
| β 2 | 16.146 | 0.003a | 17.607 | 0.082 | 16.283 | 0.016a | 17.009 | 0.000a |
| β 22 | 6.639 | 0.140 | 13.940 | 0.188 | 8.211 | 0.192 | 2.728 | 0.302 |
| β 3 | −15.297 | 0.004a | −20.930 | 0.047a | −18.033 | 0.010a | −14.903 | 0.000a |
| β 33 | 3.454 | 0.416 | 8.927 | 0.381 | 4.436 | 0.461 | −0.0124 | 0.996 |
| β 12 | −9.698 | 0.080 | −16.384 | 0.192 | −12.458 | 0.107 | −7.429 | 0.038a |
| β 13 | 15.480 | 0.014a | 29.242 | 0.037a | 18.467 | 0.029a | 11.240 | 0.006a |
| β 23 | −13.694 | 0.023a | −26.301 | 0.054 | −17.253 | 0.038a | −11.628 | 0.005a |
| R 2 | 0.980 | 0.982 | 0.982 | 0.992 | ||||
| F | 56.143 | 43.200 | 54.863 | 99.811 | ||||
| Ice crystal 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Var. density (%) | Var. lactose (%) | Var. TSS (Brix) (%) | Var. protein (%) | |||||
| Regression coefficient | p | Regression coefficient | P | Regression coefficient | p | Regression coefficient | p | |
| β 0 | 161.665 | 0.000a | 238.339 | 0.000a | 189.063 | 0.000a | 145.693 | 0.000a |
| β 1 | 86.746 | 0.000a | 165.508 | 0.000a | 114.191 | 0.000a | 70.683 | 0.000a |
| β 11 | 5.055 | 0.407 | 18.191 | 0.107 | 8.487 | 0.2980 | 2.381 | 0.556 |
| β 2 | 19.470 | 0.007a | 29.605 | 0.013a | 21.217 | 0.018a | 20.150 | 0.001a |
| β 22 | 11.350 | 0.088 | 18.004 | 0.110 | 9.331 | 0.256 | 10.877 | 0.026a |
| β 3 | −9.905 | 0.099 | −22.108 | 0.043a | −15.133 | 0.063 | −9.632 | 0.028a |
| β 33 | 6.777 | 0.275 | 12.085 | 0.259 | 8.250 | 0.310 | 6.492 | 0.136 |
| β 12 | −7.618 | 0.300 | −23.538 | 0.084 | −14.816 | 0.142 | −7.979 | 0.124 |
| β 13 | 10.587 | 0.163 | 33.228 | 0.025a | 20.071 | 0.060 | 9.271 | 0.082 |
| β 23 | −16.453 | 0.046a | −12.888 | 0.306 | −13.304 | 0.181 | −13.662 | 0.020a |
| R 2 | 0.978 | 0.982 | 0.977 | 0.985 | ||||
| F | 34.357 | 42.142 | 33.766 | 52.645 | ||||
| Concentrate 3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Var. density (%) | Var. lactose (%) | Var. TSS (Brix) (%) | Var. protein (%) | |||||
| Regression coefficient | p | Regression coefficient | P | Regression coefficient | p | Regression coefficient | p | |
| a Significant at the level of 5% probability (p < 0.05). Legend: β0 = a constant term; β1 = ethanol (%); β11= (ethanol (%))2; β2 = CaCl2 (%); β22 = (CaCl2 (%))2; β3 = CMC (%); β33 = (CMC (%))2; β12 = ethanol (%) × CaCl2 (%); β13 = ethanol (%) × CMC (%); β23 CaCl2 (%) × CMC (%). | ||||||||
| β 0 | 233.972 | 0.000a | 352.844 | 0.000a | 271.034 | 0.000a | 215.389 | 0.000a |
| β 1 | 73.366 | 0.000a | 163.789 | 0.000a | 88.357 | 0.002a | 60.077 | 0.000a |
| β 11 | −5.867 | 0.082 | 9.321 | 0.267 | 12.262 | 0.572 | 0.745 | 0.848 |
| β 2 | 7.710 | 0.022a | 5.910 | 0.428 | 27.850 | 0.182 | 8.976 | 0.034a |
| β 22 | 6.796 | 0.051 | 6.254 | 0.445 | 13.017 | 0.549 | 5.222 | 0.206 |
| β 3 | −14.945 | 0.001a | −24.463 | 0.010a | −38.817 | 0.078 | −15.218 | 0.003a |
| β 33 | 4.024 | 0.207 | 4.415 | 0.586 | 10.574 | 0.625 | 1.933 | 0.622 |
| β 12 | −6.430 | 0.103 | −13.820 | 0.176 | −41.928 | 0.132 | −0.614 | 0.894 |
| β 13 | 11.496 | 0.012a | 17.817 | 0.094 | 46.660 | 0.099 | 9.528 | 0.070 |
| β 23 | −15.663 | 0.003a | −33.692 | 0.008a | −56.983 | 0.053a | −11.789 | 0.033a |
| R 2 | 0.992 | 0.988 | 0.854 | 0.980 | ||||
| F | 96.785 | 64.093 | 4.566 | 39.072 | ||||
| Ice crystal 3 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Var. density (%) | Var. lactose (%) | Var. TSS (Brix) (%) | Var. protein (%) | |||||
| Regression coefficient | p | Regression coefficient | P | Regression coefficient | p | Regression coefficient | p | |
| β 0 | 178.203 | 0.000a | 264.713 | 0.000a | 206.713 | 0.000a | 166.032 | 0.000a |
| β 1 | 82.348 | 0.000a | 157.689 | 0.000a | 107.969 | 0.000a | 65.970 | 0.000a |
| β 11 | 7.997 | 0.233 | 25.569 | 0.064 | 14.669 | 0.104 | 5.020 | 0.327 |
| β 2 | 15.625 | 0.026a | 15.623 | 0.183 | 15.652 | 0.065 | 16.318 | 0.007a |
| β 22 | 7.402 | 0.266 | 13.251 | 0.293 | 8.925 | 0.294 | 5.1567 | 0.315 |
| β 3 | −11.664 | 0.074 | −17.010 | 0.152 | −13.034 | 0.111 | −9.826 | 0.058 |
| β 33 | 3.352 | 0.601 | 7.358 | 0.548 | 4.765 | 0.564 | 2.553 | 0.609 |
| β 12 | −10.767 | 0.182 | −16.629 | 0.2684 | −12.720 | 0.215 | −9.464 | 0.138 |
| β 13 | 11.086 | 0.171 | 22.738 | 0.144 | 14.939 | 0.154 | 8.043 | 0.198 |
| β 23 | −16.476 | 0.058 | −26.621 | 0.096 | −19.797 | 0.072 | −14.244 | 0.040a |
| R 2 | 0.985 | 0.972 | 0.973 | 0.974 | ||||
| F | 27.051 | 26.647 | 27.705 | 29.382 | ||||
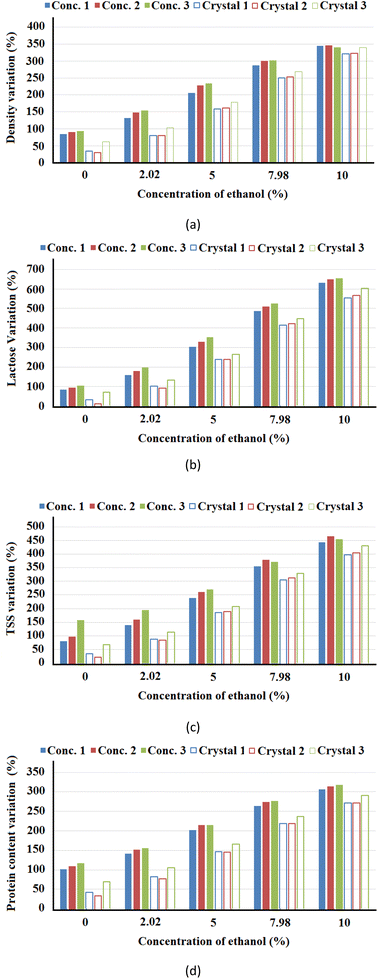
The coefficients in Tables 3 and 4 when applied to the model described by eqn (4) allow the comparison of the response variables at different stages. Fig. 3 shows the variations in density, lactose content, TSS and protein content in relation to a standard sample as a function of the variation in the ethanol concentration and fixing the concentration of CaCl2 at 0.5% and CMC at 0.05%. Fig. 3 shows that these response variables increase notably with the increase in the concentration of ethanol. A fact that must be highlighted is the reduction of the percentage variation in the concentrate currents in the sequence of stages 1, 2 and 3. On the other hand, these variations increase in the ice crystal fraction, with the largest variations observed in stage 3 of the freeze concentration process. This is explained because the amount of water is reduced in the systems at the end of each stage and density, lactose content, TSS and protein content in the ice fraction increased compared to the concentrate fraction. There was an inclusion of lactose, TSS and protein in the ice layer.24
The variations in density, lactose content, TSS and protein content in relation to a standard sample as a function of the variation in CaCl2 concentration and fixing the ethanol concentration at 5% and 0.05% CMC are shown in Fig. 2S of the ESI.† In these systems, the magnitudes of the variations were lower.
Fig. 3S of the ESI† shows the variations in density, lactose content, TSS and protein content in relation to a standard sample as a function of the variation in CMC concentration and fixing the ethanol concentration at 5% and 0.5% CaCl2. In general and compared with added CaCl2 and ethanol, it can be seen in Fig. 3S,†Tables 3 and 4 that the concentration of CMC had little significant effect on the response variables for both concentrates and ice crystals.
Fig. 3, 2S, and 3S† allow observing those treatments with ethanol, CaCl2 and CMC where the differences between concentrates, between concentrates and ice crystals and between stages were statistically significant. In stage 1, both the crystal and the concentrate had close values of F and p (Table 2). In stage 2, the behaviour was different in the variations (%) of density and TSS; the value of Fconc. > Fcristal (Table 3) and this characteristic was more evident in the protein content response variable, where, Fconc. ≈ 99.811 and Fcristal ≈ 52.645. In this case, the concentrate had a lesser dispersion of data, and it can be said that the addition of ethanol and CaCl2 favoured the concentrate.
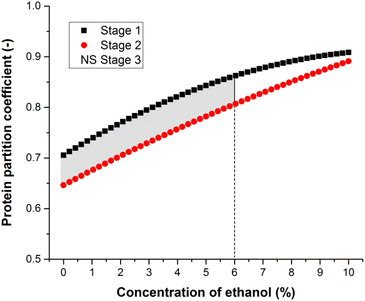
The results indicated that the number of stages and the concentration of ethanol influenced the effectiveness of the processes. Although Fig. 3 shows that the protein increases in the concentrated fraction and in the ice fraction as the ethanol concentration increases, the partition coefficient (protein concentration in the ice/protein concentration in the concentrate) increases as shown in Fig. 4S and Table 1S (ESI†). Table 1S (ESI†) shows that ethanol and CaCl2 concentrations and stages 1 and 2 influence significantly the partition coefficient. The effect is notable in increasing the ethanol concentration and stage 3 has no significant effect (Table 2S – ESI†). In freeze concentration systems, the partition coefficient is an important parameter that varies between 0 and 1 and is desired to be as low as possible. When the partition coefficient is equal to or very close to 1, it means that the ice and concentrate have the same concentration, and the process is not effective.
The optimum ethanol concentrations in stages 1 and 2 were determined to obtain minimum protein partition coefficients. The optimum range for the partition coefficient was determined by superimposing the contour surfaces of all the stage results. Fig. 4 shows the overlaid contour plots for the significant responses, which were evaluated as a function of ethanol concentration at constant 0.5% CaCl2 and 0.05% CMC concentrations. Fig. 4 reveals that an ethanol concentration range of 0–6.0% has the best combination of factors yielding maximum values of protein partition coefficient of 0.86 (stage 1) and 0.80 (stage 2).
The process with two stages and intermediate concentrations of ethanol resulted in greater effectiveness of the freeze concentration. To be effective in BMW freeze concentration, CaCl2 must have a concentration of 0.5% in stage 2 as shown in Fig. 4Sb. (ESI†).
Statistical analysis of the results of the color parameters was made in the BMW samples at all stages. The results showed that in the first stage the data obtained in the ANOVA table were not significant at the 95% level, and it was not possible to adjust to the model in eqn (4). In the second stage, the results obtained for the Hue angle of concentrate 2 were significant and it was possible to perform the data regression and for ice crystal 2 this parameter was not significant. In the third stage, the variation of the Hue angle was significant in ice crystal 3 fractions. The results of the regression analyses are shown in Table 5 and the response surfaces for concentrate 2 and ice crystal 3 as a function of ethanol concentrations and CaCl2 are shown in Fig. 5Sa and b (ESI†), respectively. This parameter indicates how much closer to neutral colors the analysed concentrate or ice crystal samples are. The increase in the hue angle parameter is analyzed considering that values close to zero are related to colors close to red that have an angular value equal to 0°. For yellow, the angular value is equal to 90° and for green the angular value is equal to 180°. Fig. 5Sa and b† show that intermediate ethanol and CaCl2 concentrations presented values indicating a color closer to yellow. The pH variation did not significantly influence the freeze concentration process and does not interfere with the interactions of the additives with BMW.
| Concentrate 2 | Ice crystal 3 | |||
|---|---|---|---|---|
| Regression coefficient | p | Regression coefficient | p | |
| a Significant at the level of 5% probability (p < 0.05). Legend: β0 = a constant term; β1 = ethanol (%); β11 = (ethanol (%))2; β2 = CaCl2 (%); β22 = (CaCl2 (%))2; β3 = CMC (%); β33 = (CMC (%))2; β12 = ethanol (%) × CaCl2 (%); β13 = ethanol (%) × CMC (%); β23 CaCl2 (%) × CMC (%). Temperature profiles during freeze concentration. | ||||
| β 0 | 87.384 | 0.000a | 90.932 | 0.000a |
| β 1 | −0.904 | 0.415 | 1.864 | 0.062 |
| β 11 | 1.150 | 0.350 | 1.103 | 0.272 |
| β 2 | −1.986 | 0.098 | −0.505 | 0.567 |
| β 22 | 2.689 | 0.051 | 1.525 | 0.144 |
| β 3 | 0.556 | 0.610 | −1.411 | 0.137 |
| β 33 | 6.370 | 0.001a | 0.351 | 0.716 |
| β 12 | −0.628 | 0.658 | −1.477 | 0.221 |
| β 13 | 4.517 | 0.013a | 4.512 | 0.005a |
| β 23 | −5.191 | 0.007a | −2.906 | 0.033a |
| R 2 | 0.899 | 0.841 | ||
| F | 6.950 | 4.119 | ||
Temperature profiles during freeze concentration
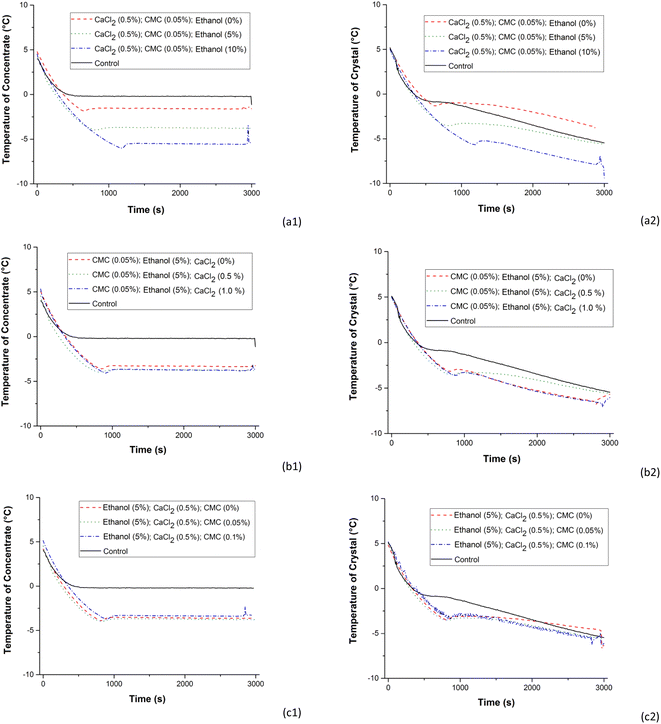
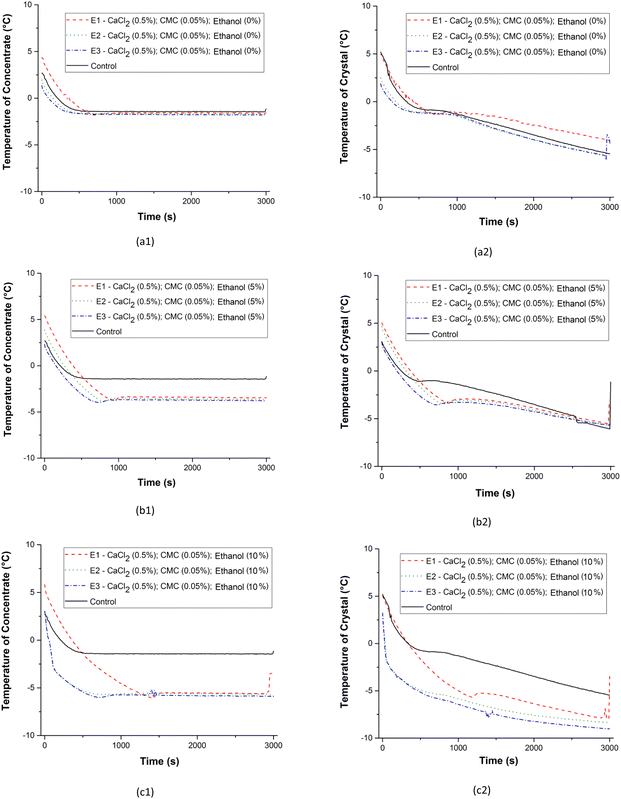
The graphs in Fig. 5 were obtained from readings of thermocouples installed near the central region of the concentrator (T02) to obtain the temperature as a function of time for the concentrates and near the cooled surface (T04) for the ice crystals. In Fig. 5, the treatments consisted of different concentrations of ethanol, 0.5% CaCl2 and 0.05% CMC (Fig. 5a); different concentrations of CaCl2, 5% ethanol and 0.05% CMC (Fig. 5b) e; different concentrations of CMC, 5% ethanol and 0.5% CaCl2 (Fig. 5c) which were selected to evaluate the individual effects of each additive on the temperature histories of the samples.Fig. 5a1, b1 and c1 (T02, concentrate) show that during the entire processing time the temperatures of the concentrates remained at constant levels after reaching the initial freezing temperature, characterizing an equilibrium state solution-ice crystal. In these cases, the temperature readings were obtained in the concentrate samples located in the centre of the concentrator container and with mechanical agitation. Fig. 5a2, b2 and c2 (T04, ice crystal, close to the concentrator coil) show the fast formation of ice crystals adhered to the coil. Compared with the cooling history of the control sample, Fig. 5 shows the individual effects of the additives in reducing the initial freezing temperature and in the ice crystal formation rates adhered to the serpentine. The freezing front movement occurs from the cooled surface of the concentrator towards the centre and an increase in the solute concentrations in the non-frozen phase occurs near to ice formed in the serpentine. The accumulation of solutes near the ice that is forming causes concentration polarization in progressive freeze concentration systems and is responsible for reducing the freezing temperatures observed in Fig. 5a2, b2 and c2. In this context, the effect is notable and proportional to the increase in the ethanol concentration and also, with less intensity, in the increase in the CaCl2 concentration. The increase in the ethanol concentration provided a delay in the formation of ice crystals that occurred at extremely low temperatures. For the addition of CaCl2 and CMC, no major changes are observed in temperature profiles, initial freezing points or ice crystal formation rates.
The effects of the stages are shown in Fig. 6 for treatments with 0.5% CaCl2, 0.05% of CMC and different ethanol concentrations for the concentrates and ice crystals separated in stages 1, 2 and 3. Fig. 6 shows that for the fraction of BMW concentrate without the addition of ethanol, the differences among initial freezing temperatures were small considering the number of stages. The lower initial freezing temperatures were in stages E2 and E3. This is an indication that the changes in the compositions of solutes in the concentrates increase with the increase in the number of stages. In systems consisting of 5% ethanol, there were notable changes in the sub-cooling temperatures of BMW samples in the three stages. In stage 1 (E1), the cooling rate was slower and becomes faster in stages 2 (E2) and 3 (E3), respectively. Sub-cooling and initial freezing temperatures are dependent on the levels of TSS. Sub-cooling also depends on the type of solute. At the same mass concentration (% w/w), the lower molecular weight (MW) solutes have higher molality and therefore lower freezing point. This could help explain the marked effect of ethanol (MW ethanol < MW CaCl2 < MW CMC). The higher the content of TSS, the lower the sub-cooling levels and the presence of ethanol decreases the initial freezing temperature and the ice crystal formation onset.
In the BMW samples with ethanol and additives, the increase in the number of stages improved the efficiency of the freeze concentration process. These observations are also valid when the added ethanol concentration to the BMW was increased to 10%. However, in this case the effect is observed only by comparing stages E1 and E2. Stages E2 and E3 showed small differences in terms of temperature profiles. What stands out is that these effects were most noticeable in the fractions of ice crystals and can be proven and associated with increased density, lactose content, soluble solid content and protein content in the ice crystal fractions shown in Fig. 3. This behaviour in the BMW freeze concentration process is partially in line with the work of Aider et al.,3 where lactose was found more concentrated in the ice fraction, while proteins were more concentrated in the non-frozen fraction (thawed, freeze-concentrated).
Vuist et al.24 evaluated the progressive freeze concentration of whey protein–sucrose–salt mixtures if the inclusion of protein in the ice was influenced by the change in freezing point due to the low-molecular weight components that accumulate in the concentration polarization layer. The authors concluded that at higher protein concentrations the inclusions are caused by the increase in viscosity in the boundary layer, impeding mass transfer and sucrose caused a similar effect. In this work, a higher protein fraction was also found in the non-frozen fraction and is attributed to the presence of ethanol. Aider et al.3 observed that the efficiency of the concentration process decreases when the number of freeze concentration stages increases and that the number of optimal stages is 3. In this work, the presence of additives mainly CaCl2 and ethanol influenced the efficiencies of stages 1 and 2 and showed that the increase in the number of stages increased the lactose concentration, TSS and protein content in the ice crystal fraction.
Viscosity
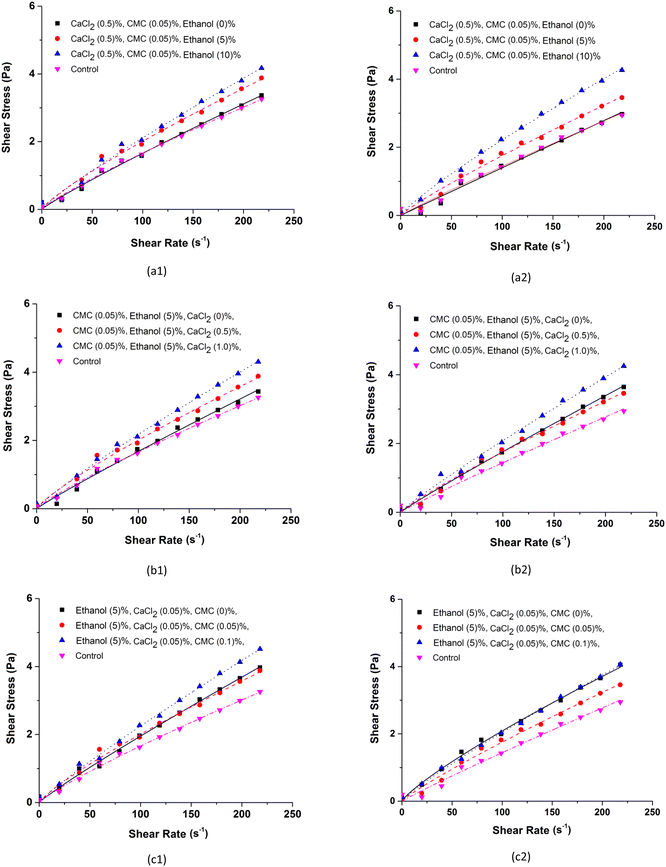
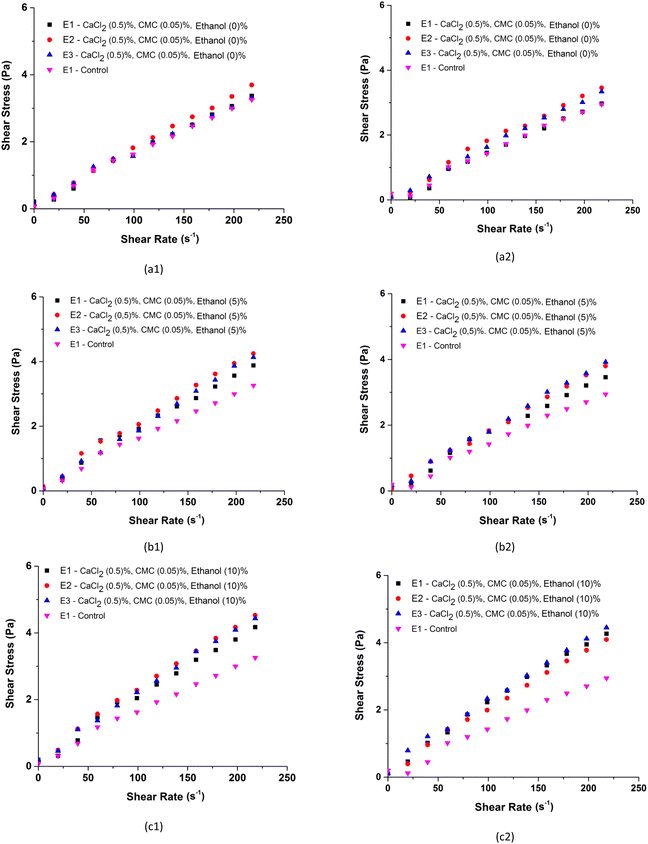
Fig. 7 shows the shear stress curves as a function of the shear rate for BMW samples with 0.5% CaCl2 and 0.05% CMC and added with different concentrations of ethanol (Fig. 7a); with 5% ethanol and 0.05% CMC added with different CaCl2 concentrations (Fig. 7b); and with 5% ethanol and 0.5% CaCl2 added with different concentrations of CMC (Fig. 7c), in the concentrates and ice crystals separated in stage 1. The effects of the number of stages of the freeze concentration process on the shear stress as a function of the shear rate are shown in Fig. 8. The results of the rheological parameters, consistency index (k) and flow behaviour index (n) obtained by adjusting the experimental data to the power law model are shown in Table 6.| Rheological parameters (power law) | ||||||
|---|---|---|---|---|---|---|
| Concentrate | Ice crystal | |||||
| k | n | R 2 | k | n | R 2 | |
| Ethanol 0% | 0.025 | 0.910 | 0.992 | 0.015 | 0.992 | 0.987 |
| Ethanol 5% | 0.044 | 0.831 | 0.992 | 0.030 | 0.882 | 0.991 |
| Ethanol 10% | 0.047 | 0.876 | 0.991 | 0.042 | 0.857 | 0.998 |
| CaCl2 0% | 0.022 | 0.936 | 0.992 | 0.023 | 0.944 | 0.995 |
| CaCl2 0.5% | 0.044 | 0.831 | 0.992 | 0.030 | 0.882 | 0.991 |
| CaCl2 1.0% | 0.038 | 0.880 | 0.996 | 0.031 | 0.915 | 0.995 |
| CMC 0% | 0.029 | 0.917 | 0.994 | 0.046 | 0.830 | 0.996 |
| CMC 0.05% | 0.044 | 0.831 | 0.992 | 0.030 | 0.882 | 0.991 |
| CMC 0.1% | 0.036 | 0.894 | 0.996 | 0.036 | 0.875 | 0.998 |
| Control | 0.032 | 0.860 | 0.997 | 0.018 | 0.943 | 0.986 |
Compared with the control sample and the individual effect of each additive, Fig. 7a shows that the shear stress as a function of the shear rate increases with the addition of ethanol, which indicates an increase in the apparent viscosity of the BMW samples after the stage (E1). This increase is most evident in the samples of the ice crystal fractions as shown in Fig. 7a2. This behaviour of increased viscosity after the concentration process was also observed in the BMW samples containing CaCl2 and CMC and is caused by the increase in the amount of solutes after the stage (E1). However, in these systems, the differences between the shear stresses of concentrates and ice crystals are more difficult to identify and the behaviours seem similar. The effects of stages E1, E2 and E3 on the increase in shear stresses are shown in Fig. 8. The effects are notable with the increase in the concentration of ethanol compared to the control system and less evident when comparing the effects between stages E1, E2 and E3. The level of ethanol addition, CaCl2 and CMC in the BMW samples influenced the consistency index (k). Higher values were observed with the increase in the level of incorporation of additives in the formulations (Table 6). The consistency index (k) is an informative parameter of the BMW sample viscosities after Stage (E1); higher values of k indicate that the concentrated BMW is more viscous. The presence of additives led to an increase in the viscosity of the concentrate and ice crystal fractions.
The rheological properties of BMW dispersions with additives are associated with greater or lesser water retention capacity, which are favourable or unfavourable to the formation of ice as indicated in the curves that describe the freezing histories.
The flow behaviour index (n) in all treatments was less than 1 (for the concentrate fraction and for the ice crystal fraction), indicating a pseudoplastic behaviour (Table 6). Increasing concentration of ethanol, CaCl2 and CMC in BMW causes it to deviate from Newtonian behaviour and therefore has a greater pseudoplasticity after the freeze concentration process.
The consistency index (k) and the flow behaviour index (n) showed opposite variations in relation to the level of concentration of additives. The k values increased and the values of n decreased as these concentrations increased (Table 6). High values of k and low values of n indicate that BMW is more viscous.
In this work, the presence of additives influenced the rheological behaviour of BMW systems and consequently the freeze concentration process. The additives influence the loss of secondary and tertiary structures of proteins during denaturation which leads to an increase in the volume occupied by the protein. The result is increased viscosity if more denatured proteins are present. The presence of a large number of high molecular weight aggregates increases the resistance to flow, increasing apparent viscosity.31 Huan et al.32 evaluated the influence of molecular weight and CMC concentration on the stability and properties of whey protein isolate (WPI) stabilized in emulsions. Emulsions stabilized by mixed WPI-CMC had improved surface properties as well as reduced droplet flocculation. Increased viscosity due to no adsorbed CMC also contributed to increased stability at high CMC concentrations. The binding of calcium ions to whey proteins reduces electrostatic repulsions and promotes interactions of the hydrophobic domain that favours the aggregate formation and influences the rheological behaviour of BMW systems.28
Ethanol can effectively denature BMW proteins, as well as change secondary and tertiary structures and induce protein aggregation. Various studies33–35 have evaluated the ethanol effects on the microstructure and rheological properties of whey proteins. Ethanol is less polar than water and has less permissiveness, which could easily disrupt non-covalent interactions and therefore facilitate protein denaturation. According to Feng et al.,33 ethanol induces molecular unfolding of native whey proteins and subsequent aggregation that is caused by the formation of disulphide bonds and intramolecular hydrogen bonds, as well as hydrophobic interactions have significant effects on the structural, morphological and functional properties. In these studies, the results showed that with the increase in ethanol concentration, structural changes occurred and were detected.
Conclusions
The substances (ethanol, CaCl2 and CMC) significantly and linearly influenced the density of the BMW concentrate after stage 1. Only the addition of ethanol had a linear and positive influence on the variation of the lactose concentration in concentrate 1 and the other additives, CaCl2 and CMC did not have a significant effect on this parameter. The addition of ethanol also increased the variation in the TSS content and the addition of CMC negatively influenced the variation of this parameter. Increases in concentrations of ethanol and CaCl2 are favourable for greater ice formation and more concentrated solutions in concentrate 1. These variations increased in the ice crystal fraction, with the largest variations observed in stage 3 of the freeze concentration process. The process with two stages and intermediate concentrations of ethanol resulted in greater effectiveness of the freeze concentration.With the cooling histories of the BMW samples, it was possible to observe the individual effects of the additives in reducing the initial freezing temperature and the ice crystal formation rate adhered to the serpentine. In this context, the effect is notable and proportional to the increase in the concentration of ethanol and also, with little intensity, in the increase in the concentration of CaCl2.
The rheological parameters increase with the addition of ethanol, which indicates an increase in the apparent viscosity of the BMW samples after the (E1) stage of the freeze concentration process. This increase is most evident in the samples of the ice crystal fractions. The level of ethanol addition, CaCl2 and CMC in BMW samples influenced the consistency index (k).
A justification for the use of these additives in the freeze concentration process is that the effect of adding ethanol, for example, becomes interesting if this process is applied in a pre-drying process where the ethanol is easily eliminated. In addition, ethanol is low-cost and sustainably produced and accelerates the drying process. The result is a product with higher protein content, lactose and TSS compared to the control sample. These products can be used as wall material in microencapsulation processes of bioactive compounds and as ingredients in various food formulations.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Camilla Soares Duarte: project administration; conceptualization; data curation; formal analysis; investigation; writing – original draft. Adrise Aparecida Rodrigues: conceptualization; methodology. Ana Cristina Freitas de Oliveira Meira: conceptualization; methodology. Luiz Ronaldo de Abreu: conceptualization, methodology; Fabiano Freire Costa: supervision, conceptualization, methodology; Jaime Vilela de Resende: funding acquisition; project administration; resources; supervision; methodology, writing – review & editing.Conflicts of interest
The authors declare that there is no conflict of interest.Acknowledgements
The authors wish to thank the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil) for financial support for this research.References
- M. Aider, D. Halleux and I. Melnikova, Innovative Food Sci. Emerging Technol., 2009, 10(3), 334, DOI:10.1016/j.ifset.2009.01.005.
- M. Aider, D. De Halleux and I. Melnikova, Food Bioprocess Technol., 2009, 2, 80, DOI:10.1007/s11947-007-0023-0.
- M. Aider, D. De Halleux and A. Akbache, J. Food Eng., 2007, 82(1), 92, DOI:10.1016/j.jfoodeng.2007.01.025.
- R. P. Chhabra and B. Gurappa, in Coulson and Richardson's Chemical Engineering: Volume 2A: Particulate Systems and Particle Technology, Elsevier Butterworth-Heinemann, 6th edn, 2019 Search PubMed.
- M. Aider and D. Halleux, LWT--Food Sci. Technol., 2009, 42(3), 679, DOI:10.1016/j.lwt.2008.08.013.
- Z. Zhang and R. W. Hartel, J. Food Eng., 1996, 29(1), 23, DOI:10.1016/0260-8774(95)00049-6.
- M. Aider and W. B. Ounis, Int. J. Food Sci. Technol., 2012, 47(1), 195, DOI:10.1111/j.1365-2621.2011.02826.x.
- J. Sánchez, Y. Ruiz, M. Raventós, J. M. Auleda and E. Hernández, Innovative Food Sci. Emerging Technol., 2010, 11(4), 644, DOI:10.1016/j.ifset.2010.06.006.
- E. Hernández, M. Raventós, J. M. Auleda and A. Ibarz, Innovative Food Sci. Emerging Technol., 2010, 11(1), 130, DOI:10.1016/j.ifset.2009.08.014.
- O. Miyawaki, et al. , J. Food Eng., 2016, 171, 153, DOI:10.1016/j.jfoodeng.2015.10.022.
- J. Sánchez, E. Hernández, J. M. Auleda and M. Raventós, J. Food Eng., 2011, 103, 147, DOI:10.1016/j.jfoodeng.2010.10.009.
- V. G. Gude, Water Res., 2016, 89, 87, DOI:10.1016/j.watres.2015.11.012.
- A. Zambrano, Y. Ruiz, E. Hernández, M. Raventós and F. L. Moreno, Desalination, 2018, 436, 56, DOI:10.1016/j.desal.2018.02.015.
- O. Miyawaki and T. Inakuma, Food Bioprocess Technol., 2021, 14, 39, DOI:10.1007/s11947-020-02517-7.
- C. S. Carneiro and J. Cal-Vidal, Pesqui. Agropecu. Bras., 2000, 35(2), 423, DOI:10.1590/S0100-204X2000000200021.
- J. M. V. Blanshard and F. Franks, in Food Structure and Behavior, ed. J. M. V. Blanshard and P. Lilford, Academic Press, London, 1987, p. 51 Search PubMed.
- T. S. Bezerra, C. G. Pereira, M. E. T. Prado, E. V. B. Vilas Boas and J. V. Resende, Drying Technol, 2018, 36(10), 1250, DOI:10.1080/07373937.2017.1399275.
- F. F. Costa, J. V. Resende, L. R. Abreu and H. D. Goff, J. Dairy Sci., 2008, 91(6), 2165, DOI:10.3168/jds.2007-0932.
- P. F. Fox, T. Uniacke-Lowe, P. L. H. McSweeney and J. A. O'Mahony, in Dairy Chemistry and Biochemistry, Springer International Publishing Switzerland, 2nd edn, 2015 Search PubMed.
- E. R. Budiaman and O. Fennema, J. Dairy Sci., 1987, 70(3), 535, DOI:10.3168/jds.S0022-0302(87)80038-3.
- E. R. Budiaman and O. Fennema, J. Dairy Sci., 1987, 70(3), 547, DOI:10.3168/jds.S0022-0302(87)80039-5.
- A. H. Muhr, J. M. V. Blanshard and S. J. Sheard, Int. J. Food Sci. Technol., 1986, 21(5), 587, DOI:10.1111/j.1365-2621.1986.tb00397.x.
- A. H. Muhr and J. M. V. Blanshard, Int. J. Food Sci. Technol., 1986, 21(6), 683, DOI:10.1111/ijfs1986216683.
- J. E. Vuist, R. M. Boom and M. A. I. Schutyser, Innovative Food Sci. Emerging Technol., 2021, 74, 102829, DOI:10.1016/j.ifset.2021.102829.
- M. I. Rodrigues and A. F. Iemma, Planejamento de Experimentos e otimização de Processos, Casa de Pão:Campinas, 2005 Search PubMed.
- A. I. Khuri and J. A. Cornell, Response Surfaces – Designs and Analyses, Marcel Dekker, Inc., 2nd edn, 1996 Search PubMed.
- B. Barros Neto, I. S. Scarminio and R. E. Bruns, Planejamento e otimização de experimentos. 2a Edição, Editora da UNICAMP, 1996 Search PubMed.
- A. J. Ganz, Cereal Sci. Today, 1973, 18(12), 398 CAS.
- Z. Y. Ju and A. Kilara, J. Dairy Sci., 1998, 81, 1236, DOI:10.3168/jds.S0022-0302(98)75684-X.
- L. Lin, H. E. Oh, H. C. Deeth and M. Wong, Int. Dairy J., 2021, 113(2), 104893, DOI:10.1016/j.idairyj.2020.104893.
- G. Krešić, V. Lelas, A. R. Jambrak, Z. Herceg and S. R. Brnčic, J. Food Eng., 2008, 87, 64, DOI:10.1016/j.jfoodeng.2007.10.024.
- Y. Huan, S. Zhang and B. Vardhanabhuti, J. Dairy Sci., 2016, 99, 3305, DOI:10.3168/jds.2015-10278.
- Y. Feng, X. Ma, B. Kong, Q. Chen and Q. Liu, Food Hydrocolloids, 2021, 111, 106379, DOI:10.1016/j.foodhyd.2020.106379.
- A. Nikolaidis and T. Moschakis, Food Hydrocolloids, 2018, 84, 389, DOI:10.1016/j.foodhyd.2018.05.051.
- J. Wagner, M. Andreadis, A. Nikolaidis, C. G. Biliaderis and T. Moschakis, LWT--Food Sci. Technol., 2021, 139, 110518, DOI:10.1016/j.lwt.2020.110518.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fb00024e |
| This journal is © The Royal Society of Chemistry 2023 |