Open Access Article
Open Access ArticleTowards a sustainable and green extraction of curcuminoids using the essential oil of Cinnamomum cassia†
Verena
Huber‡
 *,
Michael
Schmidt‡
,
Didier
Touraud
and
Werner
Kunz
*,
Michael
Schmidt‡
,
Didier
Touraud
and
Werner
Kunz
 *
*
Institute of Physical and Theoretical Chemistry, University of Regensburg, D-93040 Regensburg, Germany. E-mail: werner.kunz@chemie.uni-regensburg.de
First published on 8th March 2023
Abstract
A natural and sustainable alternative to conventional solvents in terms of solubilization and extraction of curcuminoids from Curcuma longa L. was investigated. By mixing ethanol with natural aromas, a drastic increase (up to 30-fold) of curcumin solubility with regard to pure ethanol could be achieved. Through a solubility screening of ten naturally abundant aromas with different functionalities, conducted via UV/vis analysis, cinnamaldehyde was determined to be the most promising one. COSMO-RS calculations combined with 1H and NOESY NMR were conducted to determine the solving mechanism. As a natural source, rich in cinnamaldehyde, essential cinnamon oils were examined concerning their curcumin solving ability with regard to their chemical composition. The best oil, coming from Cinnamomum cassia with a cinnamaldehyde content of 79%, was used successively as a natural solvent for cycle extraction experiments. By encountering a premature saturation, a theoretical model for cycle extractions was proposed.
Introduction
Curcuminoids are bright yellow pigments found in the rhizomes of turmeric, Curcuma longa L. The plant can be harvested throughout Southeast Asia,1,2 where it has been known for ages for its beneficial health effects, like anti-inflammatory and antioxidant effects, in ayurvedic medicine, its colour, and its flavour characteristics as a spice.3–6 Another genus, also native to the tropical climate of Southeast Asia, is Cinnamomum, cinnamon. Just like turmeric, it is a very popular spice, which is known for its medicinal applications, including antioxidant, anti-inflammatory, antidiabetic, anticancer, and cardio protective activities.7,8 Two species, Cinnamomum verum and cassia, have been of interest throughout this study. They can be characterized as tropical, evergreen trees containing cinnamaldehyde (up to 80%)9–11 in the oil of their bark. The two species can be distinguished from one another by looking at their quills, the rolled bark pipes, which are the exportable product. While Cinnamomum verum has a soft, light bark, which rolls in several layers, Cinnamomum cassia has a dark, hard bark, which only rolls to one layer.8The oils of these two species combined with ethanol were investigated in terms of their solvent abilities, solving curcumin, and extracting the curcuminoids successively from their plant matrix. Using the essential oil of cinnamon is an elegant step towards a green and sustainable extraction as nature already provides oils with suitable solvent characteristics.12,13 The close geographical proximity1,7,8,14 of the two investigated plants – turmeric and cinnamon – enables the cut of transport costs along with the emission of CO2. By having all raw materials, the essential oil15 and ethanol16 produced sustainably as well, a completely green extraction could be introduced.
Experimental
Chemicals
The synthetic phytochemicals curcumin (Cur, purity > 97%), cinnamyl acetate (purity > 97.0%), benzyl benzoate (purity > 99.0%), 3-phenylpropylacetate (purity > 98.0%) and alpha-hexylcinnamaldehyde (purity > 90.0%) were purchased from TCI (Eschborn, Germany). Benzaldehyde (purity 99%) and trans-cinnamaldehyde (purity > 98%) were bought from Merck (Darmstadt, Germany). 3-Phenylpropionaldehyde (purity 95%) was purchased from Fisher Scientific GmbH (Schwerte, Germany) and citral (purity N/A) was obtained from All Organic Treasures GmbH (Wiggensbach, Germany). The cinnamon bark oil Cinnamomum zeylanicum was obtained from Jean Pütz (Gelsenkirchen, Germany), while Cinnamomum cassia and Cinnamomum verum were bought from PCW (Grasse, France). The ground turmeric rhizomes were provided by Kwizda (Linz, Austria). Ethanol (EtOH, purity ≥ 99.8%), (R)-(+)-limonene (purity ≥ 93%), trans-anethole (purity ≥ 99%), and deuterated methanol-d4 (purity >99.8%) were bought from Sigma Aldrich (Darmstadt, Germany).All chemicals were used without further purification.
Methods
Solubility screening
Samples were prepared by mixing the solvent ethanol with either the natural flavour compounds or the cinnamon oils at different weight ratios (0–100 wt%) until the solutions were homogeneous. The preparation of the mixtures used for the solubility map, ternary mixtures of ethanol, water and the cinnamon oil was executed analogously. Then, the samples were saturated with synthetic curcumin and stirred for 1 h at room temperature before filtration with 0.45 μm PTFE filters to remove the excess. All samples were prepared in triplicate.Optical density measurement
A qualitative assessment of the curcumin solubility in the above-mentioned aroma mixtures was done by UV/vis analysis. Samples were adequately diluted in ethanol before the measurement in a spectral range of 350–700 nm, using a Lambda 18 UV/vis spectrometer by PerkinElmer (Waltham, USA). The maximum absorbance at a wavelength of 425 nm was used to compare all samples.COSMO-RS calculations
Based on the predictions by P. Degot et al.,17 the chemical potential of curcumin in solutions of ethanol mixed with natural aromas was calculated based on the solutions' molar ratios using the 19.0.4 version by COSMOlogic.18–22 The COSMObase TZVPD-FINE 19.0 provided some of the precalculated molecules, while the COSMO files of the missing ones were calculated on a TZVPD-FINE level using the templates of COSMOconfX software (version 4.3). For curcumin only the COSMO files of the four sensible conformations of the keto–enol tautomer were calculated.NMR analysis
The 1H and NOESY NMR spectra of trans-cinnamaldehyde and hydroxycinnamaldehyde with curcumin were recorded in deuterated methanol using a solvent ratio of 30/70 (n/n) of aroma/methanol-d4 in which 0.05 mmol curcumin was dissolved. Deuterated methanol was used as a solvent, as it is an alcohol, similar to ethanol. Therefore, the results should be comparable. The NMR spectra were recorded with a Bruker Avance III HD 400 MHz spectrometer equipped with a 5 mm BBO 400SB BB-H-D sample head with Z-gradient. The preinstalled measurement programmes were used to record the spectra, while the scan rate was increased for the NOESY spectra to achieve a better signal/noise ratio. The spectra were processed and evaluated with MestraNova (version 14.1.2).GC-FID analysis
The ratio of cinnamaldehyde in the oil samples was determined via GC-FID analysis. The oils and the synthetic cinnamaldehyde were solved in methanol (1 mg mL−1) and filtered before measurement. 1 μL of sample was injected into a GC-FID 7890A system from Agilent (Santa Clara, USA) with a VF-5 column (30 m × 250 μm × 0.25 μm) with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 split ratio and eluted with a helium flow of 1 mL min−1. Flame ionization detection was performed at 275 °C. A gradient program based on the results presented by A. Chakraborty et al.23 was used: a temperature of 60 °C was held for 5 min at the beginning, which was ramped up to 250 °C with a heating ratio of 20 °C. Successively, the temperature was ramped up to 300 °C with a heating rate of 50 °C min−1. The peak areas were used to determine the ratio of cinnamaldehyde in the cinnamon oils. Cinnamaldehyde in the oils was characterized by the comparison of the retention times with the retention time of the pure cinnamaldehyde sample.
20 split ratio and eluted with a helium flow of 1 mL min−1. Flame ionization detection was performed at 275 °C. A gradient program based on the results presented by A. Chakraborty et al.23 was used: a temperature of 60 °C was held for 5 min at the beginning, which was ramped up to 250 °C with a heating ratio of 20 °C. Successively, the temperature was ramped up to 300 °C with a heating rate of 50 °C min−1. The peak areas were used to determine the ratio of cinnamaldehyde in the cinnamon oils. Cinnamaldehyde in the oils was characterized by the comparison of the retention times with the retention time of the pure cinnamaldehyde sample.
GC-MS analysis
To analyse the volatile contents of the three cinnamon oils qualitatively, GC-MS analysis was performed. Elution was achieved using a 7890B GC system from Agilent (Santa Clara, USA) furnished with a ZB-5MSplus column (30 m × 250 μm × 0.25 μm) from Phenomenex Inc. (Aschaffenburg, Germany). Samples of a volume of 1 μL were injected with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 split ratio and eluted with helium with a flow rate of 1.2 mL min−1. A temperature gradient starting at 60 °C ramping up to 300 °C with 20 °C min−1 was applied. The end temperature was held for 0.5 min. MS detection was carried out using an AccuTOF GCX apparatus from Jeol GmbH (Freising, Germany) with a positive electron impact ionization at a voltage of 70 V. The spectra were assigned to their compounds via the NIST database.
200 split ratio and eluted with helium with a flow rate of 1.2 mL min−1. A temperature gradient starting at 60 °C ramping up to 300 °C with 20 °C min−1 was applied. The end temperature was held for 0.5 min. MS detection was carried out using an AccuTOF GCX apparatus from Jeol GmbH (Freising, Germany) with a positive electron impact ionization at a voltage of 70 V. The spectra were assigned to their compounds via the NIST database.
Determination of a ternary phase diagram
A phase diagram was recorded consisting of water, ethanol, and Cinnamomum cassia oil from PCW. By adding the third compound, either oil or water, dropwise to binary samples of water/ethanol and ethanol/oil respectively, until a visible change of the phase behaviour, the miscibility gap was determined. The phase separation was determined by the naked eye and the exact amounts of all compounds were recorded.Determination of the critical point
The critical point (CP) of the ternary mixture of water/ethanol/cassia oil was determined via dynamic light scattering experiments combined with optical detection. First, correlations in the ternary mixture were examined analogously to V. Huber et al.24 using a temperature-controlled CGS-3 goniometer from ALV (Langen, Germany), with an ALV-7004/FAST Multiple Tau digital correlator and a vertically polarized 22 mW HeNe laser of a wavelength of 632.8 nm. Samples were filtered into dust-free cylindrical cells of 10 mm outer diameter using 0.2 μm PTFE filters. The samples were thermostated in a toluene vat of 25 ± 0.1 °C and measured for 180 s. Conferring to T. Buchecker and S. Krickl et al.,25 the correlation curves were examined qualitatively assuming that higher y-intercepts correspond to more time-stable structuring, indicating the critical points. To verify the location of the CP, an optical determination in the points of the highest correlation was conducted. Three samples in the monophasic domain were prepared and diluted stepwise with 20 μL of water until phase separation. The sample with identical volumes of both samples was determined as the critical point.Cyclic extraction of Curcuma longa L.
As a proof of the concept of cyclic extraction for solvent economisation introduced by P. Degot et al.26 and continued by V. Huber et al.,24,27 continuously fresh turmeric rhizomes were extracted using the solvent composition of the highest curcumin solubility. The powdered rhizomes with a grain size of 50–100 μm were obtained from Kwizda (Linz, Austria), and specified to contain 81 mg kg−1 water and 35 mL kg−1 essential oils. 2 g of rhizome powder were immersed in 32 g solvent (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 ethanol/cinnamon oil (w/w)) at room temperature. After stirring for 1 h, the samples were centrifuged for 10 min at 4700 G and the supernatant was removed and used for another cycle of extraction. This method was repeated until 12 extraction cycles were achieved. A qualitative analysis of the increasing concentration was done by diluting the extracts adequately in ethanol and analysing the optical density like described above.
70 ethanol/cinnamon oil (w/w)) at room temperature. After stirring for 1 h, the samples were centrifuged for 10 min at 4700 G and the supernatant was removed and used for another cycle of extraction. This method was repeated until 12 extraction cycles were achieved. A qualitative analysis of the increasing concentration was done by diluting the extracts adequately in ethanol and analysing the optical density like described above.
Results and discussion
Screening of natural aromas
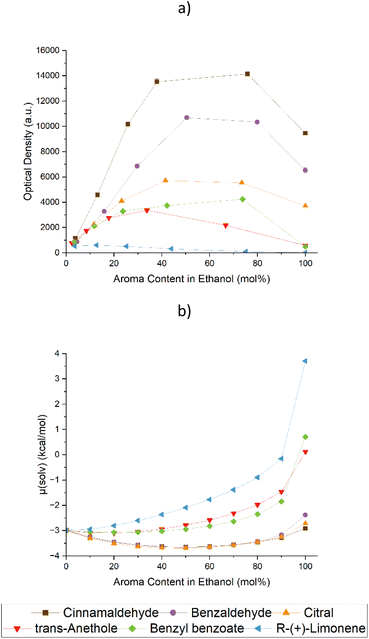
By introducing additives into ethanolic solutions, the solubility of curcumin can be increased depending on the nature of the additives.28Fig. 1 shows the UV/vis screening of six natural aromas in solutions of ethanol.The aromas in question were cinnamaldehyde (Cin), benzaldehyde (Bza), citral, anethole, benzyl benzoate, and limonene. They were chosen based on their different structural elements. While Cin and Bza are aromatic aldehydes with conjugated systems of different sizes, benzyl benzoate is an aromatic ester. Anethole is an aromatic ether, limonene is a non-functionalised terpene and citral is a terpenoid with an aldehyde function. Their structures can be viewed in Table S1 of the ESI.† Along with the experimental results obtained by UV/Vis analysis, the predictions of the chemical potential of curcumin via COSMO-RS are presented in Fig. 1 at the bottom as well. The UV/vis results in wt% are presented in Fig. S1 of the ESI.† Additionally, the UV/vis spectra of curcumin in binary mixtures of EtOH and Cin are given in Fig. S2.†
Looking at the lower part of Fig. 1, the COSMO-RS prediction of the chemical potential of curcumin, three trends of its solubility in the EtOH/aroma mixtures were forecast: strongly decreasing, hardly affecting, and improving the solubility of curcumin in the mixtures.
Regarding the first case, limonene was the compound leading to the low solubility. Comparing it to the other molecules, limonene showed no positive solubility synergy with EtOH. In all other cases, a solving synergy with a maximum solubility of curcumin at an aroma content between 70 and 90 wt% could be achieved. Limonene, on the other hand, exhibited a quasi–linear curve progression leading to lower curcumin contents with higher limonene contents. The experiments were confirmed by COSMO-RS calculations, yielding a continuously worse solubility. Just like stated by V. Huber et al. who looked at hexanoic acid,28 a long carbon backbone without functionalization affected the solubility of curcumin in a negative way.
The second case, hardly any effect on the solubility, is made up by anethole and benzyl benzoate. In comparison to pure EtOH, both molecules managed to enhance the solubility of curcumin in mixtures with EtOH 5-fold. Their curve progressions of the COSMO-RS predictions were very similar with a positive trend of the chemical potential, indicating a bad solubility. Yet, the results of the experiments, especially in the pure aromas, were not as bad as predicted, which was an overall sign that both the ester and ether groups had a seemingly decent effect when solving curcumin.
All aldehydes, Cin, Bza, and citral, make up the third group, showing an almost identical curve progression of the chemical potential. Lower predicted chemical potentials in the EtOH/aldehyde mixtures indicate positive solubility synergies.
Even though the synergies could indeed be observed in the solubility experiments, the overall curves do not look as similar as predicted. A 10-fold solubility augmentation compared to the pure EtOH was achieved in citral mixtures, while mixtures of Bza and Cin with EtOH almost doubled and tripled these results respectively. This led to the hypothesis that COSMO-RS did not consider some interactions Bza and Cin must incur with curcumin. Possible neglected interactions may include π–π interactions or stacking. Bza and Cin both are planar, aromatic molecules. The conjugated system of Cin is larger than the one of Bza and nearly looks like half the structure of curcumin. It can be imagined that curcumin may stack with the two aromas and will form a complex with a preferential solubility in EtOH.
Concerning these results, the augmentation of the curcumin solubility in mixtures of EtOH mixed with natural flavours was driven by two effects: The solubility of curcumin in the aromas was the first, less effect. Depending on the functionalisation of the molecules in question, a trend could be established as follows: aldehyde in conjugation with the aromatic system > aldehyde in conjugation > ester > ether > non-functionalised molecules. The second, more important effect was the preferential intermolecular interactions between the solute curcumin and the natural flavours. These interactions permitted such high solubilities of curcumin.
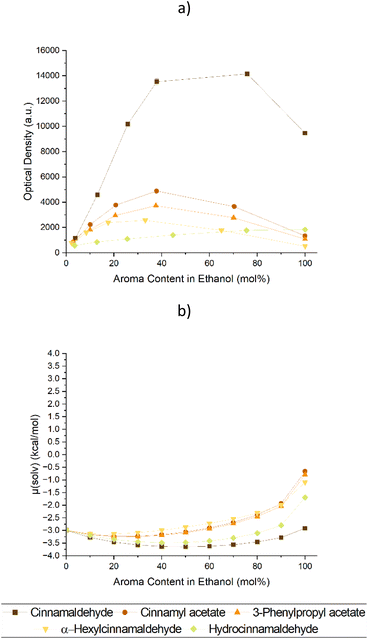
Both prerequisites were fulfilled by Cin, which was also the additive yielding the highest curcumin solubility. Looking at nature, cinnamon bark oil is a natural source of Cin with high contents of up to 80%.10,11 Nevertheless, natural products are rarely pure, and cinnamaldehyde derivatives were inspected to test their effect on the solubility of curcumin. The derivatives which could most likely be encountered in the oils9–11 included cinnamyl acetate, 3-phenylpropyl acetate, α-hexyl cinnamaldehyde, and hydrocinnamaldehyde. Their corresponding experimental and theoretical data regarding the solubility and chemical potential of curcumin are presented in Fig. 2. A depiction depending on the weight ratio of the Cin derivatives is given in Fig. S3 of the ESI† and their structures can be viewed in Table S1.†
Cinnamaldehyde like in Fig. 1 achieved the highest curcumin solubilities, while the rest showed mediocre ability to enhance the curcumin solubility. This find was also supported by the COSMO-RS predictions. However, the curcumin solubility enhancing effect of hydrocinnamaldehyde strayed off course. Instead of causing a solubility synergy upon mixing with EtOH like all other examined molecules, a linear increase of curcumin solubility was achieved. This stood in contrast with the chemical potential calculated via COSMO-RS. According to the prediction, hydrocinnamaldehyde should behave similarly to Cin with a similar effect on the solubility of curcumin. To explain this, 1H NMR measurements in deuterated methanol were made (cf. Fig. S4–8†).
It was suspected that the aldehydes react with the alcohol and form a hemiacetal. This is indeed the case as the hemiacetals can be observed in the 1H-NMR spectra of trans-cinnamaldehyde or hydrocinnamaldehyde in methanol-d4. The hemiacetal amounts to 16% and 89%, respectively (cf. Fig. S4 and 5†). This also explains why COSMO-RS was not able to predict the solubility of curcumin in hydrocinnamaldehyde/ethanol correctly, as only little hydrocinnamaldehyde is present in the solution. The double bond in trans-cinnamaldehyde seems to prevent hemiacetal formation to some extent.29
First, the change of the chemical shift of the curcumin signals in the binary mixture with cinnamaldehyde/hydrocinnamaldehyde in methanol-d4 is investigated, cf.Table 1. No changes of the chemical shifts of the aroma compounds were observed. An upfield shift of the chemical shift corresponds to an increase in electron density at the nucleus, while a downfield shift corresponds to a decrease in electron density at the nucleus. Changes in the chemical shift are in correlation with the mesomeric effects of functional groups.30,31 The allyl protons of curcumin (21,24 & 19,20) experience a slight downfield shift, as well as protons (10,13) of the aromatic ring in the case of hydrocinnamaldehyde. In contrast, the signal of (11,12) is shifted upfield for cinnamaldehyde. In both cases, the methoxy groups of curcumin (1,4) experience an upfield shift.
| #H | 1,4 | 27 | 21,24 | 10,13 | 11,12 | 7,16 | 19,20 | 3,6 | 22 |
| Methanol-d4 | 3.91 | — | 6.63 | 6.82 | 7.11 | 7.22 | 7.57 | — | — |
| Cin | 3.76 | — | 6.88 | — | 7.02 | — | 7.60 | — | — |
| HCi | 3.73 | 5.97 | 6.66 | 6.96 | — | — | 7.74 | — | — |
Combining changes in chemical shift in the 1H spectra (cf. Fig. S6–8†) and the cross peaks found in the respective 1H–1H-NOESY spectra (cf. Fig. S9 and 10†) the different curcumin solubilities can be explained. For cinnamaldehyde in methanol-d4, cross peaks between all hydrogen nuclei of cinnamaldehyde and the methoxy groups of curcumin are visible in the NOESY spectrum. The methoxy groups of curcumin increase the electron density in the aromatic ring (+M-effect), whereas the electron density at the methoxy group itself is reduced. The electron-deprived hydrogen nuclei of the methoxy group can interact with the electron-rich cinnamaldehyde with its aliphatic, allylic and aldehyde protons. This also corresponds to the changes in the chemical shift. With the increased electron density at the methoxy groups (1,4) their +M-effect intensified and thus a higher electron density in the aromatic ring is observed leading to an upfield shift of the protons in the aromatic ring (11,12). Unfortunately, the other signals of the aromatic ring (10,13) and (7,16) are not visible in the NMR spectrum as these signals overlap with the signals of cinnamaldehyde. The allylic hydrogen nuclei in conjugation to carbonyl (−M effect) are shifted downfield. This shift is less pounced for (19,20) while the signal of (21,24) is shifted by +0.25 ppm. The residual cross peaks in the NOESY spectrum show interactions between trans-cinnamaldehyde and curcumin themselves respectively.
With hydrocinnamaldehyde, the methoxy groups of curcumin (1,4) are also shifted upfield. However, looking into NOESY, no cross peaks between curcumin and hydrocinnamaldehyde can be observed.
This is surprising as at least the aromatic ring of hydrocinnamaldehyde should be able to interact with the electron-deprived methoxy groups. Looking closer into the NOESY spectrum a weak cross-peak, close to the background, between the aromatic protons of hydrocinnamaldehyde and the methoxy groups of curcumin can be seen. This observation correlates with the observed solubilities, whereas hydrocinnamaldehyde only slightly increased the solubility of curcumin in ethanol. In addition, no interaction between the methoxy groups and the aldehyde proton can be seen, as the hemiacetal is predominately present. However, no cross peak between the hemiacetal and the methoxy group is observed either. Its methoxy group (+M-effect) and additional hydroxy group (+M-effect) increase the electron density at the hemiacetal which should be favourable for interactions with the methoxy group of curcumin. As already observed by Huber V., hydroxy groups have a strong negative effect on the solubility of curcumin and probably prevent further interactions.28 This will need to be further evaluated in the future.
After the solubility assessment of pure compounds, three natural cinnamon bark oils were examined:
Cinnamomum cassia oil from PCW (cassia), and two Cinnamomum verum oils, one from PCW (verum) and one from Jean Pütz (zeylanicum). To characterize the oils, the total Cin content was determined via GC-FID and by-products were identified using GC-MS. All chromatograms and mass spectra are presented in Fig. S11–45 of the ESI.†
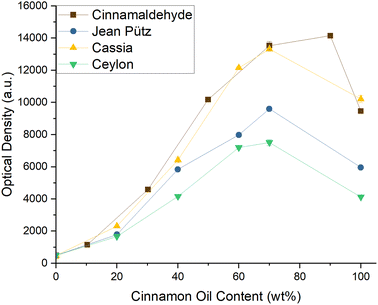
The oil with the highest Cin content was the cassia oil from PCW with 79%, followed by the zeylanicum oil with 74%, and the verum oil with 62%. The reference, pure, and synthetic cinnamaldehyde had a total content of 97%. As the oils contained considerably less of the aroma in question, it was not expected that any of them could meet the high curcumin solubility achieved in Cin/EtOH mixtures. Fig. 3, however, shows that the curve progression of oil cassia matched the curve of Cin almost perfectly, enhancing the curcumin solubility in solutions of EtOH almost identically.
The zeylanicum oil contained only 5% less Cin than the cassia oil. Yet, its solubilisation power was far weaker, only dissolving 65% of the amount of curcumin that was solved in solutions of ethanol and cassia. While the Cin content in the zeylanicum and verum oils differed by 12%, their power of solubilizing curcumin was more comparable. An explanation was found in the GC-MS data which are presented in Fig. S15–45. The Cinnamomum cassia oil predominantly contained derivatives of Cin, while the other two oils were also rich in terpenoids. By reviewing Fig. 1, limonene, a non-functionalised terpene, corrupted the solubility of curcumin in EtOH significantly. Thus, indeed, the presence of terpenes and terpenoids in both Cinnamomum verum oils had a negative impact on the solubility of curcumin, which could not be compensated by Cin.
The cassia oil, on the other hand, did not contain any terpenoids or terpenes but only aromatic esters and aldehydes. As stated above, the curcumin solubility could be enhanced by functionalised, aromatic additives. This is the reason why the cassia oil managed to yield such high curcumin solubilities. Remembering that the oil contained 79% Cin but achieved almost equally high solubilities as the reference, the high abundance of Cin could not be the sole reason for the high solubility. This led to the assumption that the mixture of all ingredients of the oil could create their own solubility synergy, which preferentially solved curcumin. Subsequently, all further experiments of this study were performed using the Cinnamomum cassia oil from PCW.
It still has to be noticed, regarding the mass spectrum of Fig. S21,† that oil from the species Cinnamomum cassia contains coumarin. Coumarins are phytochemicals found in many plants like, in this case, cinnamon or tonka beans, which can cause liver disease in rats and dogs.32 Though, the metabolomic pathway in the human body is different, coumarins and their corresponding metabolites can still be harmful. Hence, coumarins as flavouring agents in food were barred by the United States Food and Drug Administration (FDA), whereas Europe's Food Safety Authority (EFSA), on the other hand, approved a tolerable daily intake of 0.1 mg kg−1 body weight for adults. Regulations about foodstuffs, which are already carriers of coumarin, like cinnamon, however, are ambiguous and these foodstuffs can still be used without problems. Thus, utilizing Cinnamomum cassia oil for applications is still approved.15,32,33 Processes, in which the presence of coumarins is undesired, can bypass this problem by processing the oils. Oils with a high curcumin solving power should be obtainable by removing coumarin from oil cassia, or by rectifying Cinnamomum verum oils, depleting them of terpenoids. Both methods can yield oils of low coumarin content and high solving capacity of curcumin.
A proof of concept – preparation for extraction experiments
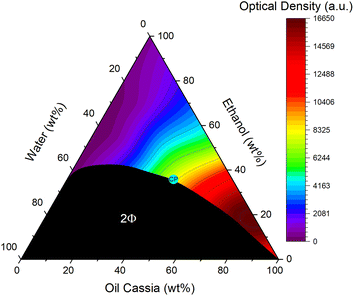
A ternary phase diagram of water, EtOH, and oil cassia, the most powerful oil in terms of solving curcumin was recorded. It is depicted in Fig. 4. Despite the oil being a multiple component mixture, it will be treated as a unit, which allows the classification of the diagram as a ternary one. The mixture of water and cinnamon bark oil was enabled by using EtOH as a co-solvent and solubilizer, reminiscent of the formulation of spear mint oil by C. Benkert et al.34 Between the hydrophilic and hydrophobic compounds, a large miscibility gap was found. Neither water nor oil cassia was soluble at any proportion in one another. Water and EtOH on the other hand were fully miscible. This is not visible in the diagram, as only one small drop of Cinnamomum cassia oil turned the binary water/EtOH mixtures with a content of less than 40 wt% two-phasic. Ultimately, to achieve a full miscibility of all compounds at any proportion a minimum content of 40 wt% EtOH was necessary. The critical point of the system was determined visually and via DLS measurements (cf. Fig. S46†). It was located on the oil side of the system.The solubility of curcumin in various ternary mixtures is characterized by the contour map, covering the monophasic area. Red signified a high solubility, while purple was an indication for a bad one. The area close to the oil cassia apex exhibited the highest curcumin solubility, while the lowest one was found in the binary mixtures of water and EtOH. A correlation of the best solubility of curcumin with the critical point was not found. The solubility seemed rather to depend on the content of the oil. A decrease of solubility was found in a diagonal direction from high cinnamon oil contents to low ones.
Hence, it was assumed that the solubility of curcumin was principally governed by the amount of oil in the mixtures. The theory about the preferential solubility of curcumin being a result of special interactions that are shared by the target and cinnamaldehyde and potentially its derivatives as suggested via NMR was supported by the trend of solubility in the ternary systems.
70–80 wt% of cinnamaldehyde and accordingly the essential oil was found to be the optimum additive content to reach the maximum of curcumin solubility in the binary mixtures with EtOH. The same was true for the ternary ones. An oil content of 80 wt% achieved the highest solubility of curcumin. Despite water limiting its solubility throughout previous studies,17,26 in this case, it had a positive influence on the solubility. A small addition of water, here 5 wt%, had an enhancing effect on the solubility of curcumin. This has already been described by P. Degot et al. in the mixture of water/sodium salicylate/ethyl acetate, who also found that water could change the solvent environment to an extent allowing even higher solubilities of curcumin.35 From these results, an optimum composition of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 (w/w/w) was found in the water/EtOH/oil cassia ternary system. This composition was also used to perform the successive extraction experiments.
73 (w/w/w) was found in the water/EtOH/oil cassia ternary system. This composition was also used to perform the successive extraction experiments.
To prove whether the enrichment of curcuminoids in the ternary water/EtOH/cinnamon oil system was possible, the solvent was used to perform several cycles of extraction. In previous studies by P. Degot and V. Huber et al.24,26 the issues of the cycle extractions included fast saturation due to a low solubility of the solvent and the change of solvent, limiting the cycles and, thus, the enrichment procedure. The trialled system of water/EtOH/oil cassia was expected not to encounter these problems. The extracts were analysed via UV/vis in conformity with P. Degot et al.26 after every extraction cycle. The single curcuminoids could not be distinguished from one another in that way, of course, due to their similar wavelength of highest absorption. However, this was enough to see the trend. Fig. 5 presents the results. The absorbance and with it the concentration of curcumins increased fairly linearly with each extraction cycle in the ternary cinnamon oil system. Environmental conditions, like temperature, were the reason for the deviations from the linear behaviour. In contrast to the reference system of water/EtOH//TriA, achieving twelve cycles of extraction took a duration of two days instead of only a few hours. Hence, the system achieving fewer cycles, of course, did not suffer under the environmental conditions. Nevertheless, the curcuminoid content could be enriched similarly in all three systems, proving the concept of cycle extraction.
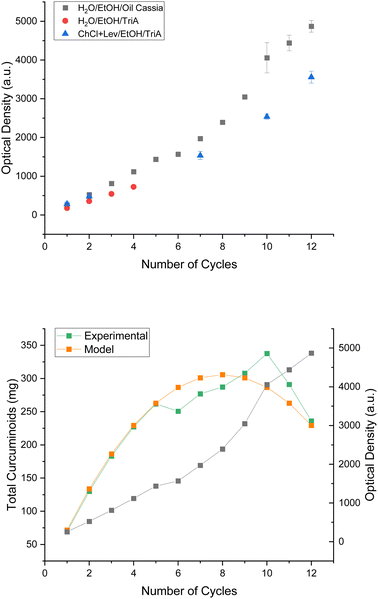 | ||
| Fig. 5 Top: absorbance of curcuminoid extracts in water/EtOH/oil cassia (black squares), water/EtOH/TriA (red circles), and ChCl + Lev/EtOH/TriA (blue triangles) after several cycles of extraction. The references were taken from P. Degot26 and V. Huber et al.24 and were calculated according to the calibration curve presented in Fig. S47.† Bottom: total mass of extracted curcuminoids in the water/EtOH/oil cassia system through experimental analysis (green squares) and modelling (orange squares). | ||
Regarding the mass of curcuminoids (cf.Fig. 5 green curve on the bottom), similarly to the experiments using the natural deep eutectic solvent,24 indeed a saturation seemed to occur after ten cycles, although the solubility limit should not have been reached yet. Beforehand, the blame of premature saturation was placed on the change of solvent, due to the loss of choline chloride to the plant matrix. However, hardly such interactions should take place in this system. An explanation was found when regarding the loss of solvent after every cycle of extraction. Approximately 2 g of solvent was lost after each extraction of 2 g rhizome powder. This altered the powder to solvent ratio drastically. As this ratio has a strong influence on the quality of extraction,36 this may play a key role during these experiments. In the following equation, a theoretical model was proposed to determine the maximum number of cycles before saturation, where m is the mass of curcuminoids, n the number of cycles, A the absorbance (in this case, the mean increase of absorbance after each extraction cycle), ε the molar extinction coefficient, l the illuminated layer thickness, V the total volume of the solvent and V2 the loss of solvent after one cycle: m(n) = nA/εl(V − nV2).
According to this model, the number of cycles depends largely on V2, as this is the only variable which can be affected actively during the process. Increasing the number of cycles should be facilitated by decreasing the lost volume. This, in turn, should make it possible to take advantage of the effectivity of the solvent. On the laboratory scale, the reduction of solvent loss can only be achieved by replacing the centrifugation with a more effective method or by changing the extraction process from a batch extraction to a continuous one. The topic of a follow-up study should be the proof of this theory and an amelioration of the process in order to achieve more cycles and to fully use the potential of the solvent.
Conclusions
The prospect of finding alternative, natural solvents for the solubilization and extraction of curcuminoids from turmeric was the motivation of this study. Additionally, it is one of the few studies, entertaining the idea of using essential oils as solvents. Natural aromas were investigated using UV/vis examinations regarding the solubility of curcumin along with predictions of its chemical potential via COSMO-RS, combined with NMR measurements, and GC analysis to determine suitable natural products able to dissolve curcuminoids.Natural aromas, abundant in essential oils, like cinnamaldehyde, benzaldehyde, citral, anethole, benzyl benzoate, and limonene, were investigated in terms of their ability to dissolve curcumin. A passable increase of the curcumin solubility of a factor of 10–30 in comparison to pure EtOH was achieved by the former three phytochemicals, which were all aldehydes. The remaining three hardly affected the solubility in a positive way. According to this screening, structural features like an aromatic system or an aldehyde function had positive effects on the curcumin solubility in ethanolic solutions. Both of these features were found in cinnamaldehyde, which yielded the best solubility results. Interactions between the conjugated systems of cinnamaldehyde and curcumin were determined via NOESY NMR to be the basis for the strong augmentation of the solubility of curcumin in EtOH. Successively, cinnamon bark oils were examined as solvents while being a natural source of cinnamaldehyde.
Three oils in total were examined, one Cinnamomum cassia oil and two Cinnamomum verum oils. While all three oils achieved decent solubility results, only the Cinnamomum cassia oil reached almost identical results comparable to the pure cinnamaldehyde. This oil had the highest cinnamaldehyde content of 79%, which was enough to imitate the pure reference. Even though the two Cinnamomum verum oils were similarly rich in cinnamaldehyde, they exhibited higher terpene and terpenoid contents, which limited the curcumin solubility. Cinnamomum cassia, on the other hand, mostly contained structural analogues of cinnamaldehyde while no terpenoids were present. Concludingly, side compounds with functionalities similar to the ones of cinnamaldehyde were found to be beneficial for the curcumin solubility in ethanolic solutions.
A ternary phase diagram of water/EtOH/oil cassia with a corresponding solubility map was recorded. The content of cinnamon oil essentially dictated the solubility of curcumin in the ternary systems and reached a maximum in the ternary at high oil contents and low water contents. At the point of the highest solubility, curcuminoid enrichment experiments were performed via cycle extraction. By reusing the solvent several times, it was certainly possible to increase the concentration of curcuminoids. However, after twelve cycles, also a premature saturation occurred in the cinnamon oil-based solvent. This preterm saturation prompted a theoretical model linking it to the loss of solvent after every cycle of extraction. This model should be verified and refined in the future while also ideas to amend the process should be proposed.
The extraction of phytochemicals using essential oils of natural sources as alternatives to conventional solvents also has to be examined in more detail, while also the influence of water on the solubility and extraction ability should be focused. This study could pave the way for novel extraction processes of phytochemicals relying on only sustainable sources.
Author contributions
Verena Huber: methodology, data curation, conceptualization, and writing-original draft. Michael Schmidt: methodology, data curation, validation, formal analysis, software, and writing-original draft. Didier Touraud: conceptualization and supervision. Werner Kunz: conceptualization and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thankfully acknowledge Antoine Fourriere, Mira Jahn, and Jennifer Schuster for their support during the lab phase, Tuan Anh Nguyen for recording the NMR spectra, Jamal Chaboun and Valérie Jeannot from the firm Phytotagante (Toulouges, France) for their fruitful ideas, and Gwenn Atheaux from Bioval (La Possession, La Réunion, France). We also thank the reviewers for their critical reading. No external funding was received, and the authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.References
- K. P. Nair, Turmeric (Curcuma Longa L.) and Ginger (Zingiber Officinale Rosc.) - World's Invaluable Medicinal Spices, Springer, 2019 Search PubMed.
- S. Akbar, in Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications, Springer Nature Switzerland AG, 1st edn, 2020, pp. 781–807 Search PubMed.
- S. Prasad and B. B. Aggarwal, in Herbal Medicine: Biomolecular and Clinical Aspects, ed. I. F. F. Benzie and S. Wachtel-Galor, CRC Press/Taylor&Francis, 2nd edn, 2011 Search PubMed.
- K. Priyadarsini, Molecules, 2014, 19, 20091–20112 CrossRef PubMed.
- A. Geethanjali, P. Lalitha and M. J. Firdhouse, Int. J. Coal Prep. Util., 2016, 2, 55–62 Search PubMed.
- R. F. Tayyem, D. D. Heath, W. K. Al-Delaimy and C. L. Rock, Nutr. Cancer, 2006, 55, 126–131 CrossRef CAS PubMed.
- P. V. Rao and S. H. Gan, Evidence-Based Complementary Altern. Med., 2014, 2014, 642942 CrossRef PubMed.
- P. Kawatra and R. Rajagopalan, Pharmacogn. Res., 2015, 7, S1–S6 CrossRef PubMed.
- R. Bauer, W. Blaschek, W. Buff, B. Classen, E. M. Heise, A. Hensel, L. Krenn, J. J. Lichius, U. Lindequist, D. Loew, M. F. Melzig, E. Stahl-Biskup, E. Teuscher, R.-B. Volk and M. Wichtl, in Wichtl - Teedrogen und Phytopharmaka: Ein Handbuch für die Praxis, ed. M. Wichtl, Wissenschaftliche Verlagsgesellschaft, 2015, vol. 6, pp. 180–182 Search PubMed.
- S. Akbar, in Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications, Springer Nature Switzerland AG, 2020, pp. 629–643 Search PubMed.
- S. Akbar, in Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications, Springer Nature Switzerland AG, 2020, pp. 645–661 Search PubMed.
- V. Marturano, V. Bizzarro, A. De Luise, A. Calarco, V. Ambrogi, M. Giamberini, B. Tylkowski and P. Cerruti, Nano Res., 2018, 11, 2783–2795 CrossRef CAS.
- Y. Li, A.-S. Fabiano-Tixier and F. Chemat, 2014, 55–61.
- P. Scartezzini and E. Speroni, J. Ethnopharmacol., 2000, 71, 23–43 CrossRef CAS PubMed.
- Y. H. Wang, B. Avula, N. P. D. Nanayakkara, J. Zhao and I. A. Khan, J. Agric. Food Chem., 2013, 61, 4470–4476 CrossRef CAS PubMed.
- F. Chemat, M. Abert Vian, A. S. Fabiano-Tixier, M. Nutrizio, A. Režek Jambrak, P. E. S. Munekata, J. M. Lorenzo, F. J. Barba, A. Binello and G. Cravotto, Green Chem., 2020, 22, 2325–2353 RSC.
- P. Degot, V. Huber, E. Hofmann, M. Hahn, D. Touraud and W. Kunz, Food Chem., 2021, 336, 127660 CrossRef CAS PubMed.
- A. Klamt and G. Schüürmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805 RSC.
- A. Klamt, G. J. P. Krooshof and R. Taylor, AIChE J., 2002, 48, 2332–2349 CrossRef CAS.
- A. Klamt, J. Phys. Chem., 1995, 99, 2224–2235 CrossRef CAS.
- COSMOlogic GmbH & Co. KG, A Dassault Systèmes, company, 2019 Search PubMed.
- COSMOlogic GmbH & Co. KG, COSMOthermX User Guide Version 19.0, 2019 Search PubMed.
- A. Chakraborty, V. Sankaran, M. Ramar and D. R. Chellappan, J. Chem. Pharm. Sci., 2015, 8, 476–479 CAS.
- V. Huber, L. Muller, J. Hioe, P. Degot, D. Touraud and W. Kunz, Molecules, 2022, 26(24), 7702–7721 CrossRef PubMed.
- T. Buchecker, S. Krickl, R. Winkler, I. Grillo, P. Bauduin, D. Touraud, A. Pfitzner and W. Kunz, Phys. Chem. Chem. Phys., 2017, 19, 1806–1816 RSC.
- P. Degot, V. Huber, D. Touraud and W. Kunz, Food Chem., 2021, 339, 128140 CrossRef CAS PubMed.
- V. Huber, L. Muller, P. Degot, D. Touraud and W. Kunz, Food Chem., 2021, 355, 129624–129631 CrossRef CAS.
- V. Huber, J. Hioe, D. Touraud and W. Kunz, J. Mol. Liq., 2022, 361, 119661 CrossRef CAS.
- A. P. Philipps and J. G. Murphy, J. Org. Chem., 1951, 16(6), 954–962 CrossRef.
- C. K. Ingold, Chem. Rev., 1934, 15, 225–274 CrossRef CAS.
- H. Shimizu, M. Katayama and S. Fujiwara, Bull. Chem. Soc. Jpn., 1959, 32, 419–420 CrossRef CAS.
- M. Lončar, M. Jakovljević, D. Šubarić, M. Pavlić, V. B. Služek, I. Cindrić and M. Molnar, Foods, 2020, 9(5), 645–679 CrossRef PubMed.
- S. C. Heghes, O. Vostinaru, C. Mogosan, D. Miere, C. A. Iuga and L. Filip, Front. Pharmacol., 2022, 13, 1–9 Search PubMed.
- C. Benkert, A. Freyburger, V. Huber, D. Touraud and W. Kunz, Food Chem., 2022, 372, 131230 CrossRef CAS PubMed.
- P. Degot, V. Huber, A. El Maangar, J. Gramüller, L. Rohr, D. Touraud, T. Zemb, R. M. Gschwind and W. Kunz, J. Mol. Liq., 2021, 329, 115538–115549 CrossRef CAS.
- Q. W. Zhang, L. G. Lin and W. C. Ye, Chin. Med., 2018, 13, 1–26 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fb00026a |
| ‡ Both authors contributed equally to the work and are the primary authors of this manuscript. |
| This journal is © The Royal Society of Chemistry 2023 |