 Open Access Article
Open Access ArticleEco-friendly biodegradable nanocomposite materials and their recent use in food packaging applications: a review
Samah M.
El-Sayed
*a and
Ahmed M.
Youssef
 *b
*b
aDairy Science Department, National Research Centre, 33 El Bohouth St. (Former El Tahrir St.), P.O. 12622, Dokki, Giza, Egypt. E-mail: samah_mosbah80@yahoo.com
bPackaging Materials Department, National Research Centre, 33 El Bohouth St. (Former El Tahrir St.), P.O. 12622, Dokki, Giza, Egypt. E-mail: amyoussef27@yahoo.com; Fax: (+20) 33370931; Tel: (+20) 33322418
First published on 8th February 2023
Abstract
There is significant interest in creating biobased polymers and innovative industrial techniques that can minimize fossil fuel use and migration to an eco-friendly and sustainable way of life. The utilization of novel, high-performing, inexpensive green polymeric materials enabled by bionanocomposites makes it possible for them to replace conventional, non-biodegradable petroleum-based plastic packaging materials that generate serious environmental issues. Thus, using polysaccharides (such as starch, chitosan, cellulose derivatives, and carboxymethyl cellulose), biodegradable polymers (e.g., polylactic acid (PLA), polyhydroxybutyrate (PHB), and polycaprolactone (PCL)), and edible films are new approaches that could be investigated to resolve this crisis. Biobased films enhanced the shelf life, food safety, and ease of handling for food packaging, based on international guidelines. The current review provides a comprehensive overview of the development and potential for use of new biobased materials from various sources in antimicrobial food packaging, including carbohydrate (polysaccharide)-based materials, antibacterial agents, and biobased composites. These materials can address the problems of environmental impact as well as the prevention of food-borne pathogens and spoilage microorganisms. Additionally, the use of biobased polymers can be increased as a result of the usage of nanotechnology in food packaging, reducing waste from food-related packaging materials, and promoting food preservation by prolonging the shelf life of foods.
1. Introduction
Sustainable bioplastics, biodegradable packaging, environmentally friendly packaging, coatings, edible films, and sustainable packaging are some of the innovative packaging developments that have been discovered since more than a decade now. In general, the goals of the aforementioned methods were to substitute non-renewable hydrocarbons-based plastic materials used in foodstuff packaging.1Food is a complicated material made up of both large and small molecules that supply important nutrients for energy, metabolism, and smooth operation of the body's basic functions. Around 1.3 billion tons of food are wasted or lost annually, or one-third of all produced food for human use.2 Globally, 2 billion individuals experienced mild to severe food poverty in 2018.3 Food waste happens all along the supply chain, from the agricultural farm to the residential customer, in both developed and developing nations. To reduce rising food waste, infrastructure, transportation, processing, and packaging technologies must advance.2
Packaging waste, particularly that comprising non-biodegradable polymers, has become an important component of community solid waste, raising environmental anxieties. Abandoned packaging presents a substantial waste management concern because it is an obvious source of litter. A petroleum-based polymer most frequently utilized in packaging applications is polyethylene (PE).4 When petroleum-based polymers are disposed of on land, they particularly struggle to biodegrade, which causes variable degrees of contamination. Recently, a lot of focus has been placed on creating biodegradable polymers using renewable resources to address this issue,5,6 which is additionally motivated by global environmental consciousness; by using enzymatic catalysis processes, microorganisms (e.g., bacteria and fungus) typically cause the destruction of biodegradable polymers dumped in bioactive settings (such as landfills). Polymer chains can also be broken down via non-enzymatic processes such as chemical hydration. The end products of biodegraded polymers often consist of biomass, CO2, water, CH4, and other naturally occurring materials with possible advantages for greenhouse gas balances and other environmental effects.7 Materials for biodegradable packaging are crucial for maintaining the ecosystem health. As opposed to conventional food packaging materials, biobased materials have drawbacks, such as poor barrier and mechanical qualities, which often lead to a lower shelf life.8
Instead, in 2015, 79% of all plastic manufactured was deposited in landfills, 12% of it was burned, and 9% of it was recycled.9 By 2050, around 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of plastic trash will enter the environment through landfills if the current rates of plastic manufacturing and garbage management are maintained.10 The packaging sector, which includes food packaging, uses a majority of synthetic polymers. Alternative packaging regulations are, therefore, necessary to manage food waste while lowering environmental impact.
000 metric tons of plastic trash will enter the environment through landfills if the current rates of plastic manufacturing and garbage management are maintained.10 The packaging sector, which includes food packaging, uses a majority of synthetic polymers. Alternative packaging regulations are, therefore, necessary to manage food waste while lowering environmental impact.
Biodegradable polymers are substances that can break down into water and carbon dioxide when exposed to certain environmental microbes including bacteria and fungi.11 Due to the activity of extracellular enzymes produced by microorganisms, the biodegradation mechanisms or decomposition starts on the polymer surface and produces oligomers. Once inside the microorganism cell, these matching oligomers operate as carbon sources and are broken down into carbon dioxide and water.12 Due to their degradability characteristics and minimal environmental burden upon disposal, biopolymers have attracted a lot of interest as “green” or “environmentally friendly” polymeric materials.13 Biopolymers are frequently modified to increase their physical and thermochemical qualities in order to make them more suitable for use in finished products. The improvement is accomplished by adding fillers, binders, or copolymers. As shown in Fig. 1, there are numerous approaches for researching biodegradable polymers, including physical observation; chromatographic, spectroscopic, and respirometric techniques; and meta-analyses.
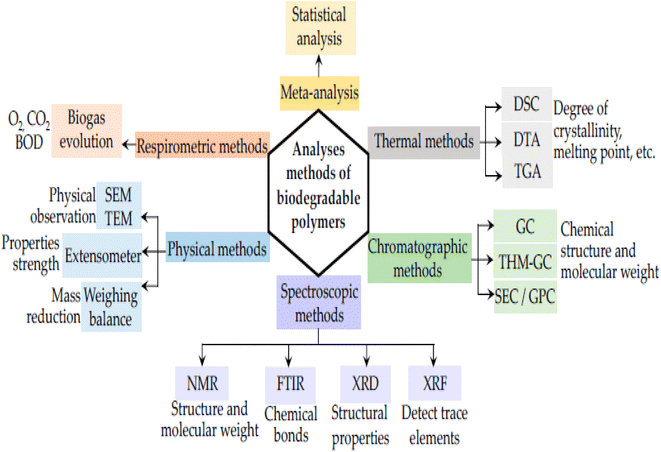 | ||
| Fig. 1 Analytical techniques for evaluation of biodegradable polymers.107 | ||
With an emphasis on packaging applications, the objective of the current review is to discuss the most recent works on the design, development, and characterization of various bionanocomposites based on biodegradable polymers used as packaging materials. The review also investigated biodegradable food packaging made of biomaterials. Biobased packaging has the potential to be a genuine replacement for conventional packaging, which is made of non-biodegradable plastic polymers that could be hazardous to the environment. Biobased packaging, however, has the potential to improve food quality, extend shelf life, and reduce material waste.
2. Biobased materials used in the food packaging
Food safety and palatable flavor are the two most important considerations when constructing biomaterial-based food packaging. It provides a longer shelf life, as well as food safety and easy manipulation, based on global regulations. Biodegradable packaging materials are developed from macromolecules, for example, polysaccharides and proteins. Furthermore, lipids may be added to improve hydrophobicity or plasticization effect on the film matrix, as well as using biodegradable polymer from renewable resources as packaging materials for enhancing the shelf life of food products. While biological–chemical chitosan demonstrated both antioxidant and antibacterial properties in a manner comparable to commercial chitosan, a Ramon starch film did not. Chitosan, a biological–chemical substance, offers a potential method for creating materials with antioxidant and antibacterial properties. Therefore, these materials might be useful for extending the shelf life of food products.14Products that are biodegradable or appropriate for human consumption must have a number of features, including non-toxicity, lipid or water solubility, physicochemical properties, pH dependency, and a moisture/gas barrier. These characteristics are affected by the kind of biomaterial, its conversion, and the processing method. Exclusive plasticizers, texturing reagents, property boosters, and crosslinking agents are introduced during biocomposite synthesis to optimize the qualities for specific applications15 (Fig. 2).
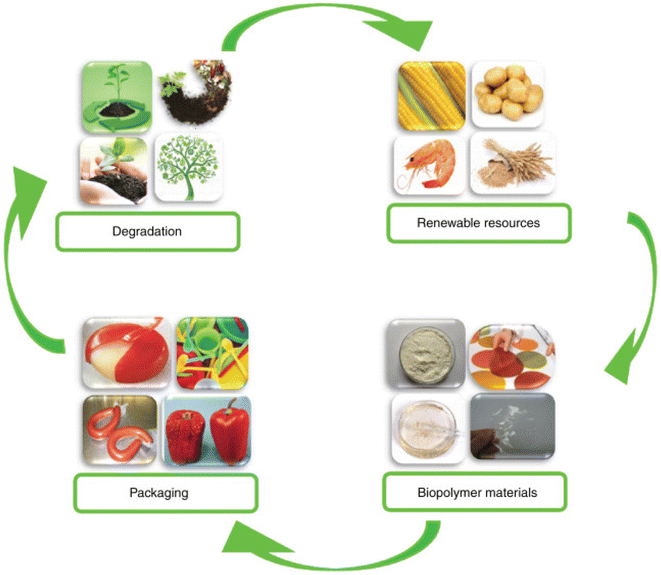 | ||
| Fig. 2 Biopolymer packaging materials for food shelf-life prolongation.15 | ||
3. Major polysaccharide materials used in food packaging applications (Table 1)
In food packaging applications, various polysaccharide types, including alginate, have been investigated.16 Starch films17 could be used in the food industry to make packaging, compostable bags, carry bags, and other molded items.18 Cellulose, pectin, hemicellulose, lignin, and waxy components make up naturally occurring lignocellulosic fibers.19,20 Enzymatic activity allows lignocellulose to be broken down into hemicellulose, lignin, and cellulose.21,22 A complex anionic polysaccharide called pectin is employed in the food industry for a variety of purposes, including gels, thickeners, emulsifiers, and stabilizers.23 Carboxymethyl cellulose (CMC) has a variety of uses in the food industry, including acting as a barrier against a variety of pollutants.24| Source | Extract | Composites | Properties | Application | Reference |
|---|---|---|---|---|---|
| Arabic gum | Gum | Carboxymethyl cellulose, arabic gum and gelatin–garlic extract | Shelf life, antibacterial | Packaging | Youssef et al.,73 |
| Cumin | Cumin essential oil | Butylene adipate-co-terephthalate/clay platelets-cumin essential oil | Shelf life, antibacterial, biodegradable | Active food packaging | Moustafa et al.,82 |
| Chitin | Chitosan | Blended films of BCh/RS | Physicochemical, antimicrobial, and mechanical properties | Packaging | Martín-López et al.,28 |
| Chitosan | Gallic acid (GA) | Chitosan-starch-gallic acid | Rheological behavior, physical, mechanical, microstructural, WVP, optical properties and antioxidant activity | Packaging, edible film | Pacheco et al.,83 |
| Chitin | Chitosan | Chitosan (CCh) and corn starch (CS) | Antioxidant and antimicrobial activity | Bioactive films, coating | Pech-Cohuo et al.,14 |
| Crab shell | Chitin | Polyurethane/chitin/rosin-ZnO-doped-SiO2 nanoparticles | Antimicrobial, biodegradable | Packaging | Moustafa et al.,84 |
| Crab shell | Chitosan | Chitosan and beeswax–pollen grains | Shelf life postharvest preservation | Edible coating | Sultan et al.,65 |
| Crab shell | Chitosan | CS/Alg/CMC | Shelf life, antibacterial | Packaging | El-Sayed et al.,56 |
| Crab shell | Chitosan | PVA/CS–ZnO–SiO2 | Shelf life, antibacterial | Active food packaging | Al-Tayyar et al.,1 |
| Crab shell | Chitin | Chitin-CNF | Bio-compatibility eco-friendly | Packaging | Hai et al.85 |
| Crab shell | Chitosan | Chitosan/guar gum/zinc oxide | Shelf life, antibacterial | Edible coating | El-Sayed et al.,51 |
| Crab shell | Chitin | PVA/chitin | Good barrier | Active packaging | Peng & Chen86 |
| Citrus | Pectin | Pectin/marjoram oil | Antimicrobial | Active packaging | Almasi et al.87 |
| Citrus | Pectin | Clove oil/pectin | Antibacterial, shelf life | Active packaging | Kumar et al.,88 |
| Citrus | Pectin | CMC/pectin/glycerol | Heat stability | Packaging | Seslija et al.23 |
| Citrus | Pectin | Alginate/pectin | Antibacterial | Active packaging | Makaremi et al.89 |
| Grape fruit seed | TPS | PLA/PE/TPS | Antibacterial | Active packaging | Wang and Rhim90 |
| Sugarcane bagasse | Carboxymethyl cellulose | CMC/PVA–zeolite | Antimicrobial | Active packaging | Youssef et al.,91 |
| Sugarcane bagasse | Carboxymethyl cellulose | CMC/PVA/CuO | Shelf life, antibacterial | Packaging | Youssef et al.,92 |
| Jackfruit | Starch | PVA/starch/ZnO | pH sensing | Packaging | Jayakumar et al.93 |
| Corn | Starch | PVA/starch/citric acid | Antibacterial | Packaging | Wu et al.94 |
| Potato | Cellulose | Na-alginate/cellulose/CuO | Antioxidant antimicrobial | Smart packaging | Saravanakumar et al.95 |
Gums are another type of polysaccharide that—depending on pH, rate density, and counter ion—can form gels in solutions.25 Gums, also known as hydrocolloids or polysaccharides, are very adaptable biopolymers that are widely used as ingredients or additives in the food industry. They serve a variety of technological and, occasionally, nutritional functions. These polysaccharides' molecular makeup, which gives them qualities like gelling, thickening, moisture retention, emulsification, and stabilization, is directly related to their versatility. They are widely used in the food industry as beverage stabilizers, clarifiers, food emulsions, ice cream stabilizers, flavor and color microencapsulators, and confectionery stabilizers. Furthermore, chitosan is a food ingredient that has been approved by the FDA for human consumption. The rate of chitosan breakdown is mostly determined by the crystallin shape and degree of acetylation. It is capable of being chemically altered to produce a variety of biomaterials with enhanced physiochemical properties.26,27 Martín-López et al.28 prepared and evaluated chitosan from biologically derived chitin in order to determine its capability to produce biofilms using Melipona honey (MH) and to measure the antimicrobial, physicochemical, and mechanical properties of the resultant biofilm. Interaction with the fabricated biofilms inhibited microbial growth, creating materials that were appropriate for food packaging applications.
In a similar vein, proteins have also been employed to create biodegradable packaging, including whey proteins,25 soy proteins,28 and gelatin.29 Soybeans play an important part in the manufacture of comestible oils and other foods.30 Soy protein helps in foaming, emulsification, shippability, solubility, adhesiveness, cohesion, and dough formation, among other physiological features. Compared with other protein-based films, these films have exceptional flexibility, clarity, and homogeneity.31 According to Sarode et al.,32 whey proteins are a form of protein that can be extracted from milk serum by adjusting the pH when processing casein and are also present in cheese whey. Whey proteins could help to improve the quality of edible film packaging. Since they can regulate moisture, carbon dioxide, oxygen, lipids, fragrance transfer, tastes, and biodegradability, many whey proteins offer environmental benefits.33,34 Casein is another protein that is found in milk and is generally used in food packaging (Table 2).
| Biopolymers | Antimicrobial agents | Applications | References |
|---|---|---|---|
| Poly(butylene adipate-co-terephthalate) (PBAT) | Copper oxide nanoparticles (CuO-NPs) | Food packaging | Hasanin & Youssef.96 |
| Polyurethane/chitin/rosin composites | ZnO-doped-SiO2 nanoparticles | Green packaging | Moustafa et al.,84 |
| Polyvinyl alcohol (PVA) and chitosan (Cs) | Cinnamon essential oil, TiO2 nanoparticles | Fresh chicken breast fillets | Youssef et al.,97 |
| Polyvinyl alcohol (PVA) | Dapsone-capped TiO2 nanoparticles (DAP-TiO2-NPs) | Food-safe packaging | Moustafa et al.,98 |
| Carboxymethyl cellulose (CMC), arabic gum (AG) & gelatin (GL) | Garlic extracts (GE) and TiO2 nanoparticles (TiO2-NPs) | Fresh Nile tilapia fish fillets | Youssef et al.,73 |
| Chitosan–beeswax | Pollen grains | Le conte pear | Sultan et al.,65 |
| Chitosan | Sodium benzoate/potassium sorbate | Culture media | Chen et al.,99 |
| Acetic/propionic acid | Meat | Ouattara et al.,100 | |
| Corn zein | Lysozyme/nisin | Culture media | Padgett et al.,101 |
| Carrageenan | Chlortetracycline/oxytetracycline | Poultry | Meyer et al.,102 |
| Alginate | Nisin | Beef | Cutter and Siragusa.103 |
| Corn zein | Lysozyme/nisin | Culture media | Padgett et al.,101 |
| Soy protein isolate | Lysozyme/nisin | Culture media | Padgett et al.,101 |
| Wheat gluten | Sorbic acid | Ethanol–water | Redl et al.,104 |
| Cellulose | Pediocin | Meat | Ming et al.,105 |
| Starch and derivatives | Potassium sorbate | Strawberry | Garcia et al.,106 |
Despite being effective in film formation, such biopolymers showed several limits in terms of material characteristics as well as oxygen barrier, poor water vapor formation, inadequate mechanical strength, and higher manufacturing cost.35 Biomaterial advancements make pathogen identification faster and easier; they also offer an excellent barrier, although food packaging is being developed. Biomaterials can be used to increase the physiochemical properties, barrier stability, and biodegradability of standard packaging. Fig. 3 shows the general characteristics of biomaterials for use in food packaging.36
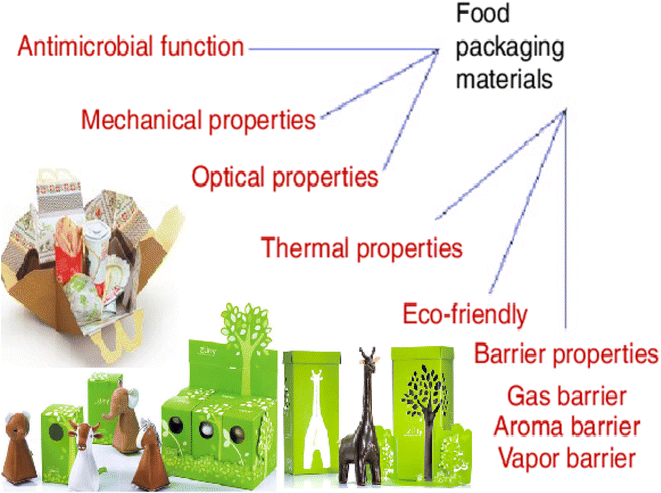 | ||
| Fig. 3 Characteristics of biomaterials for food packaging.36 | ||
4. Utilization of nanotechnology in food packaging
Nanotechnology appears to be a novel source of significant enhancements for today's food security and sustainability challenges. Richard Feynman proposed the concept of nanotechnology in 1959. Nanotechnology is the ability to operate on a scale of around 1–100 nm in order to comprehend, develop, characterize, and use material structures, technologies, and systems with novel features resulting from their nanostructures.37 Although there is potential to increase packaging functionality through the use of nanomaterials, little is known about particle migration and toxicity. Therefore, it is essential that food and food products be handled with the utmost care and properly packaged using non-toxic, secure, and environmentally friendly materials.38 The material's physical and chemical characteristics are markedly different from those of macroscale materials made of the same component when particle size is lowered below this cutoff. Over the last decade, research in the field of nanotechnology has rapidly increased; now, there are numerous companies specializing in the fabrication of new forms of nanosized matter, with anticipated applications including medical therapeutics, molecular computing, diagnostics, and energy production.39 Nearly all the areas of food industry, including agriculture, food processing, food packaging (such as stronger, more impermeable polymer films), dietary supplements, and anti-counterfeiting devices, have already been identified by scientists and industry stakeholders as potential applications for nanotechnology (Fig. 4). The market for nanotechnology-enabled food and beverage packaging was 4.13 billion US dollars in 2008, and it was anticipated to reach 7.3 billion US dollars by 2014, growing at an estimated 11.65 percent annual rate.39 In addition to strength and biodegradability, a material's desirable properties include gas and moisture permeability.40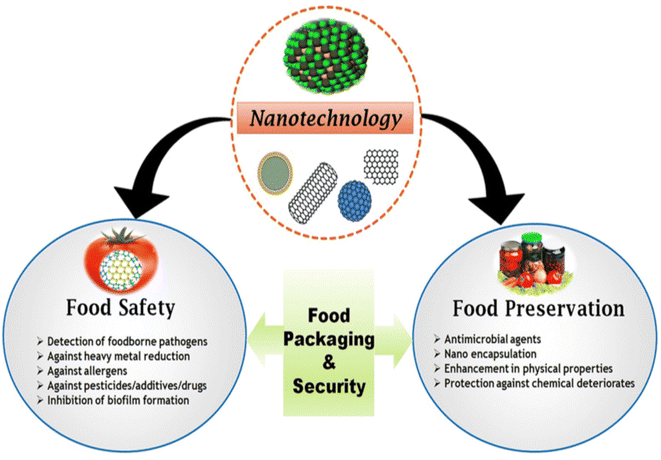 | ||
| Fig. 4 Visions of using nanotechnology for food protection, security, and safety.108 | ||
Despite the fact that foodborne diseases have been technologically controlled over the most recent revolution in “modern food packaging”, intermittently microbial, viral, and bacterial diseases can play a multidisciplinary role in a typical food packaging system. Nanoparticles can improve the antimicrobial properties of packaging materials by mechanically and thermally reinforcing the polymeric texture of the packaging film. Nanosized metal oxides (NMOs) have recently sparked a lot of interest in modern food packaging. The natural antibacterial properties in some NMOs can shield food from pollutants in the environment. They also stop microbial growth on surfaces that come in contact with food. Modern packaging is appropriate for NMOs due to their increased surface-area-to-volume ratios and antibacterial characteristics. Microbial activities are effectively inhibited by antimicrobial bionanocomposite films.41
Nano-based “smart” and “active” food packaging has several advantages over traditional packaging methods, including better mechanical strength, antimicrobial films, and barrier properties, as well as nano-sensing for pathogen discovery and warning consumers to the safety grade of food.42 The packaging of food can be improved by using nanocomposites as active packaging materials and material coatings.43
Many researchers are interested in learning more about the antimicrobial capabilities of organic substances such as bacteriocins, organic acids, and essential oils, as well as how they could be utilized in polymeric forms as antimicrobial food packaging.44,45 These compounds, however, are too sensitive to high temperatures and pressures to be used in the many food processing steps that require them.
5. Biodegradable nanocomposite films in food preservation
Edible films and coatings are used to preserve cheese, meat, fish, fruit, and pastries, as revealed in Fig. 5. As a preventive layer, a thin layer of films and coatings is applied to or wrapped around the surface of a food product. There are numerous successful examples of biodegradable-nanocomposite-based packaging films used to extend the shelf life of food products.46,47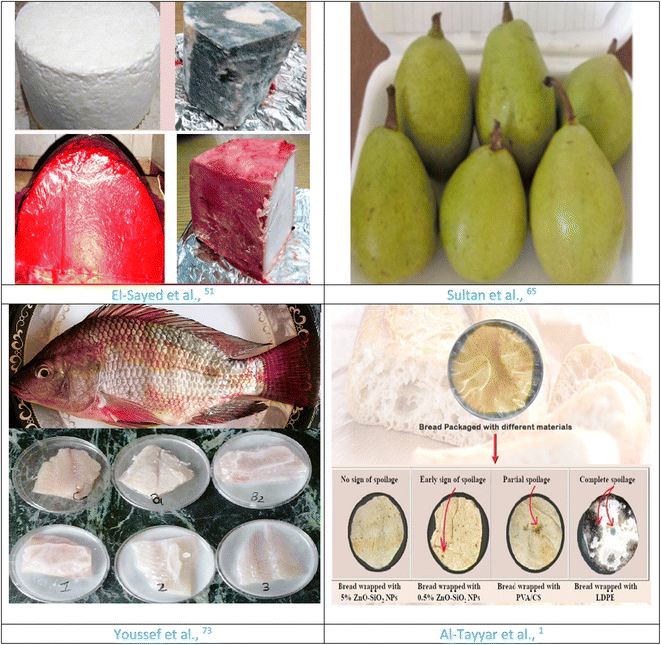 | ||
| Fig. 5 Utilization of biodegradable nanocomposites films in many types of food preservation techniques. | ||
5.1. Different types of cheese
Cheese packaging has traditionally been limited to the production of hard cheeses that need extended ripening times. This stage is performed to reduce weight losses as well as to inhibit bacterial contamination and spoilage without interfering with usual cheese ripening.Youssef et al.50 fabricated packaging materials for Ras cheese; bionanocomposite materials based on chitosan and polyvinyl alcohol (PVA) were created with a 0.5–2% loading of titanium dioxide nanoparticles (TiO2-NPs). They discovered that a cheese coating reduced weight and moisture losses while having no effect on the normal ripening changes in the chemical, microbiological, and textural properties of Ras cheese. Coating cheese with a film containing 2% TiO2-NPs removed mold growth on the cheese surface. Moreover, El-Sayed et al.51 prepared chitosan/guar gum/zinc oxide bionanocomposites-containing Roselle calyx extract (RE-ZnO) as a green technique. They discovered that adding RE-ZnO nanocomposites enhanced the permeability, tensile, antioxidant, and antibacterial properties of bionanocomposite films. For about three months, the bionanocomposite film, which contains 3% RE-ZnO nanocomposites, shielded the surface of Ras cheese from the growth of mold, yeast, and bacteria.
Li et al.54 incorporated TiO2 nanoparticles and/or Ag nanoparticles in a poly(lactic acid) (PLA) matrix to develop a novel antimicrobial packaging system for cottage cheese preserved at 5 ± 1 °C for 25 days. A low-density polyethylene (LDPE) film was applied as a control. In comparison to cheese packed with PLA and LDPE film, those with PLA/TiO2 and PLA/TiO2–Ag film exhibited good pH value, LAB, sensory quality, and antibacterial activity. According to the results, adding TiO2 or Ag nanoparticles to the PLA matrix could allow the cheese to maintain its quality and have a shelf life of up to 25 days.
Furthermore, Youssef et al.55 produced materials for packaging Karish cheese that are inexpensive and environmentally friendly by combining chitosan, PVA, glycerol, and TiO2-NPs. A bionanocomposite containing 1%, 2%, and 3% TiO2-NPs was manufactured and applied to Karish cheese. They realized that various pathogenic bacteria and fungus were inhibited by the fabricated bionanocomposite in different ways. Similarly, the coated Karish cheese maintained its better quality for a total of 25 days of storage, whereas uncoated Karish cheese began to develop surface fungus growth and decreased quality after 15 days. The Karish cheese coated with the bionanocomposite containing 3% TiO2-NPs exhibited the highest acceptability at the end of the storage period. El-Sayed et al.56 also investigated the effectiveness of new antibacterial edible coatings using chitosan, CMC, and sodium alginate, as well as environmentally friendly antibacterial microcrystalline cellulose and probiotic strains (Lactobacillus acidophilus, Lactobacillus casei, and Bifidobacterium lactis). In addition, for 45 days, these edible coating materials were used as preservers for UF soft cheese. They established that the fashioned probiotic edible films with chitosan and sodium alginate had a high antimicrobial effect against the pathogenic microbes. Additionally, all the films exhibited probiotic counts of more than 8.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU g−1 after 45 days of storage, and sodium alginate, CMC, and chitosan were preferred over the control for the cheese's general acceptability.
log CFU g−1 after 45 days of storage, and sodium alginate, CMC, and chitosan were preferred over the control for the cheese's general acceptability.
5.2. Films and coatings for fruit
Fresh or cut fruits are perishable items. Packaging is a critical tool to enhance the shelf life of packed fresh-cut agricultural produce. Fresh fruits are traditionally wrapped in petroleum-based films. However, because they are non-biodegradable and derived from non-renewable resources, these films cause serious environmental issues. Various efforts have been made to resolve this concern, with a focus on biodegradable and renewable films derived from natural polymers.58El-Magied et al.59 prepared edible coatings and films from wheat gluten with different concentrations of glycerol at pH 10 for packed strawberry fruits. They establish that the best prepared film properties were for 25% glycerol content. Strawberries packed in a wheat gluten film showed improvement for all the tested parameters, namely, visible decay, weight loss, firmness loss, surface color development, and sensory characteristics compared with fruits packed in perforated polypropylene. Moreover, they prepared different coating layers based on wheat gluten and they found that gluten coatings were more capable of controlling decay than gluten films. Consistently, strawberries coated with gluten were more acceptable compared with the control at the end of storage. Furthermore, soy or wheat gluten protein is used to coat strawberries as a carrier of thymol and calcium chloride.60 The weight-loss percentage was reduced by coating strawberries with thymol carried by soy protein or white gluten and CaCl2. Strawberries' appearance did not change after nine days of storage with a coated material containing thymol carried by soy protein or white gluten. The most effective treatments observed in fruit coated with thymol loaded with soy protein or white gluten were lower values of anthocyanin.
The surfaces of strawberries were coated using four types of edible coatings: pectin, gluten, starch, and soy protein.61 Strawberries coated with pectin had a large influence on the preservation of firmness; displayed better results in physicochemical analyses, and exhibited reduced weight loss compared with the control fruit and other coatings. Strawberries coated with gluten were the second choice while starch- and soy-protein-based coatings had the third level of positive effect (strawberries covered with starch and soy protein shrank during 16 days of storage). Correspondingly, strawberries coated with pectin and gluten layers preserved the visual quality of the fruit and the taste was acceptable to consumers during storage time.
Elabd and Gomma62 used gelatin and aloe vera as the coating material on fresh-cut kiwi fruits and stored it for 12 days. The quality of the stored kiwi fruit slices improved with an aloe vera coating and they observed that the aloe vera gel and mixture of gelatin plus aloe vera gel (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) had the lowest variations and achieved the best results in the preference panel test. The weight loss increased but the coating with gelatin and aloe vera gel (5
100) had the lowest variations and achieved the best results in the preference panel test. The weight loss increased but the coating with gelatin and aloe vera gel (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) had a major impact on reducing weight loss with storage time and had the best microbiological quality. Indumathi et al.63 prepared chitosan–cellulose acetate phthalate (CS-CAP) films. The shelf life of black grape fruits was increased by up to nine days by the CS-CAP film, which contained 5% (w/w) nano-ZnO. The CS-CAP-ZnO films demonstrated barrier and food protection properties, confirming their suitability as a principal food packaging material that could be utilized for extending the shelf life of black grape fruits.
100) had a major impact on reducing weight loss with storage time and had the best microbiological quality. Indumathi et al.63 prepared chitosan–cellulose acetate phthalate (CS-CAP) films. The shelf life of black grape fruits was increased by up to nine days by the CS-CAP film, which contained 5% (w/w) nano-ZnO. The CS-CAP-ZnO films demonstrated barrier and food protection properties, confirming their suitability as a principal food packaging material that could be utilized for extending the shelf life of black grape fruits.
El-Eryan and Tarabih64 coated Egyptian Banzahir lime fruits with 10% Arabic gum for three days for marketing followed by two months of cold storage after treating them with aqueous ozone for five or ten minutes. They discovered that this treatment provided preferred physiological vision characteristics such as decay, chilling injury, juice percentage, fruit weight loss, respiratory rate, fruit firmness, skin hue color, and technological index. Furthermore, it reflects an improvement in fruit chemical compositions over time. Furthermore, Sultan et al.65 fabricated chitosan–beeswax-based film for preserving Le Conte pear post-harvest. They demonstrated that all the prepared films displayed respectable self-healing abilities ranging from 86.7 to 96.3. Moreover, the prepared chitosan–beeswax/pollen grains composites film improved the water contact angle and revealed a two-fold lower WVTR value contrary to the control film based on chitosan, as well as having a tendency to increase the stiffness of the chitosan–beeswax/pollen grains composites film. Mechanical properties such as elongation percentage at break declined from 35.81 to 14.09. Likewise, after 7 days, the qualitative characteristics of Le Conte pears stored in cold storage for 105 days or more were evaluated as “simulating marketing time.” Chitosan–beeswax/pollen grain composites extensively coated on Le Conte pears reduced weight loss, degradation, and softening rate.
5.3. Films and coatings for meats and bread
Fresh meats (chicken, fish, and meat) are an extremely important consumable product owing to its biological composition.66 Microbial growth and metabolism are the primary causes of food spoilage.67 Several guidelines have been developed to prevent pathogenic microorganisms and spoilage.68 One of these is adding antimicrobial substances to edible films and coatings to stop surface growth in food, which is a major contributor to deterioration and contamination.69 Meat packaging implies extending the shelf life by slowing the activity of spoilage bacteria, oxidative processes, and changes in sensory properties like color and flavor.Ejaz et al.70 produced active packaging for peeled shrimp using bovine skin gelatin (BSG) composite films with 2% zinc oxide nanorods (ZnO NRs) and clove essential oil (CEO) (25 and 50% w/w of protein). They demonstrated that adding ZnO NRs to BSG/CEO films reduced porosity. Composite films containing 50% CEO demonstrated the highest antibacterial activity against Listeria monocytogenes and Salmonella typhimurium inoculated in shrimp during refrigerated storage. They suggested that the developed BSG/CEO/ZnO NR film could be used as active packaging for peeled shrimp.
Echeverría et al.71 investigated the potential use of active nanocomposite films based on soy protein isolate (SPI)–montmorillonite (MMT)–CEO for bluefin tuna (Thunnus thynnus) muscle fillet preservation during 17 days of refrigerated storage. Protein films nano-reinforced with 10 g MMT/100 g SPI and activated with CEO reduced microbial growth and lipid auto-oxidation in tuna fillets during storage. The occurrence of clay materials appeared to support the release of clove oil's active principals by spreading out its antimicrobial activity (particularly active against Pseudomonas spp.) as well as without seeing the migration of the clay's own metals (Si and Al) from the nanocomposite materials to fish muscle over time; scientists additionally evaluated its antioxidant properties.
To prevent Salmonella typhimurium from growing in chicken meat, Lin et al.72 developed cold-plasma-treated thyme essential oil (TO)/silk fibroin (SF) nanofibers. They demonstrated that cold plasma treatment significantly increased the TO release quantity of plasma-TO/SF nanofibers, producing enhanced antibacterial activity in plasma-TO/SF nanofibers than TO/SF nanofibers. At 25 °C, Salmonella typhimurium levels in duck and chicken meat declined by 6.1 and 6.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU g−1 respectively, after treatment with plasma-TO/SF nanofibers. They declared that the plasma-TO/SF nanofibers membrane was a suitable antimicrobial packaging to extend the shelf life of food, with a variety of applications in the field of food preservation.
log CFU g−1 respectively, after treatment with plasma-TO/SF nanofibers. They declared that the plasma-TO/SF nanofibers membrane was a suitable antimicrobial packaging to extend the shelf life of food, with a variety of applications in the field of food preservation.
After 21 days of storage period, Youssef et al.73 developed a new bionanocomposite based on CMC, Arabic gum (AG), and gelatin (GL), incorporating garlic extract (GE) and nano-TiO2, as a coating for Nile tilapia fish fillets. The addition of GE and nano-TiO2 improved the oxygen transmission rate (OTR), water vapor transmission rate (WVTR), thermal, antimicrobial, and mechanical properties of the fabricated films from the new bionanocomposite materials. They discovered that GE in combination with nano-TiO2 raises the protection properties of CMC/AG/GL/GE-TiO2 bionanocomposites for the preservation of tilapia fish fillets, which reduce weight loss and control the microbial growth during tilapia fish fillet storage.
Al-Tayyar et al.1 fabricated the antimicrobial bionanocomposite films as a packaging film for bread, comprising PVA, chitosan (CS), and silicon dioxide nanoparticles doped with zinc oxide nanoparticles (ZnO–SiO2) nanocomposites with different ratios (0.50%, 1.0%, 3.0%, and 5.0%). They displayed that the fabricated bionanocomposite films exhibited superior antibacterial activity against Staphylococcus aureus, S33R, and Escherichia coli, and IRAQ 3, as well as improved visual appearance of the bread and increase in shelf life and decreased food-borne pathogens in packaged bread.
6. Potential risks of nanotechnology in food and food packaging
Food safety has become a major public health concern on a global scale, and consumers are concerned about the safety of the food they eat. According to reports, foodborne microbial diseases account for more than 20 million recorded deaths worldwide each year. The major goal of food safety worldwide is to ensure that customers do not suffer any harm as a result of the preparation and consumption of food.74 The careful use of nanotechnology could have a significant impact on food packaging.75 Numerous studies have shown that customers are more receptive to nanomaterials in food packaging than in actual food.76 However, there is concern that the nanomaterials employed in food packaging could migrate to the food and cause health concerns to those who consume such items. Titania, silver, and CNT nanoparticles have been the subject of studies by a number of researchers, and it has been found that these nanomaterials enter the bloodstream and—attributable to their insolubility—may accumulate in bodily organs with negative health effects77Nanocomposites released trace amounts of particles into food through food packaging, according to Avella et al.78 The amount of migration was minimal and within the restrictions set for nanocomposites by the European Commission (EC). Particle migration from nanoparticles to food was found to be lower than the EC's limitations, as determined by Panea et al.79 using Ag and ZnO nanoparticles. However, according to a study by Sharma et al.,80 ZnO nanoparticles—even at small doses—may cause genotoxicity in epidermal cells. According to Huang et al.,81 both duration and temperature at which packaging is stored have an impact on the migration of nanoparticles from packaging to foods. Only when foods and food products are processed and packaged with the proper materials can quality be retained. Although the use of nanomaterials in the packaging of food and food products offers a great opportunity to enhance packaging functionality, little is known about particle migration and toxicity. Therefore, it is essential that food and food products be handled with utmost care and properly packaged using non-toxic, secure, and environmentally friendly materials.38
7. Conclusions
The utilization of innovative biomaterials and their implementation at the industrial level could confirm the quality and safety of foodstuffs, as well as lowering prices and increasing efficacy. Additionally, nanotechnology appears to be a new source of considerable improvements to the existing problems associated with sustainability and security of food. It is obvious that advances in nano-biotechnology greatly contribute to the health requirements of the commodity in bioactive food components and packaging. The food industry is currently developing a variety of biomaterials to be utilized as a part or packaging; such components could be used as pathogen-control agents, stabilizers, or indeed, edible films. To ensure quality, opportunities concerning the interfaces among ambient circumstances, packaging materials, and food must be deliberated during the improvement of food packaging. Because of environmental challenges, biomaterials have gained popularity among customers, financial institutions, and global markets in recent years. The majority of these bionanocomposites have exceptional properties such as mechanical strength, gas or water barrier, biodegradability, bioassimilation, compostability, and efficient molding.Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Samah El-Sayed and Ahmed Youssef: project administration; conceptualization; formal analysis; investigation; resources; writing – review & editing.Conflicts of interest
The authors declare that there is no conflict of interest.References
- N. A. Al-Tayyar, A. M. Youssef and R. R. Al-Hindi, Food Packag. Shelf Life, 2020, 25, 100523, DOI:10.1016/j.fpsl.2020.100523.
- J. Gustafsson, C. Cederberg, U. Sonesson, and A. Emanuelsson, The Methodology of the FAO Study: Global Food Losses and Food Waste-Extent, Causes and Prevention”-FAO, 2011, 2013 Search PubMed.
- FAO, Prevention and Disposal of Obsolete Pesticides, Rome, Food and Agriculture Organization of the United Nations, 2019, http://www.fao.org/agriculture/crops/obsoletepesticides/prevention-and-disposal-of-obsolete-pesticides/en/ Search PubMed.
- S. M. Emadian, T. T. Onay and B. Demirel, J. Waste Manag., 2017, 59, 526–536, DOI:10.1016/j.wasman.2016.10.006.
- M. P. Arrieta, M. D. Samper, M. Aldas and J. López, Materials, 2017, 10(9), 1008, DOI:10.3390/ma10091008.
- J. Wróblewska-Krepsztul, T. Rydzkowski, G. Borowski, M. Szczypiński, T. Klepkaand and V. K. Thakur, Int. J. Polym. Anal. Charact., 2018, 23(4), 383–395, DOI:10.1080/1023666X.2018.1455382.
- Y. Zhong, P. Godwin, Y. Jin and H. Xiao, Adv. Ind. Eng. Polym. Res., 2020, 3(1), 27–35 Search PubMed , http://creativecommons.org/licenses/by-nc-nd/4.0.
- N. A. Al-Tayyar, A. M. Youssef and R. R. Al-Hindi, Food Chem., 2020, 310, 125915, DOI:10.1016/j.foodchem.2019.125915.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3(7), e1700782, DOI:10.1126/sciadv.1700782.
- O. O. Ayeleru, S. Dlova, O. J. Akinribide, F. Ntuli, W. K. Kupolati, P. F. Marina and P. A. Olubambi, J. Waste Manag., 2020, 110, 24–42, DOI:10.1016/j.wasman.2020.04.017.
- Y. Ichikawa and T. Mizukoshi, Synthetic Biodegradable Polymers, 2011, 285–313, DOI:10.1007/12_2011_135.
- Y. Ichikawa and T. Mizukoshi, Chem. & Mater. Sci., 2012, 24, 285–313 Search PubMed.
- O. Alam, M. Billah and D. Yajie, Resour. Conserv. Recycl., 2018, 132, 121–129, DOI:10.1016/j.resconrec.2018.01.037.
- S. C. Pech-Cohuo, H. Martín-López, J. Uribe-Calderón, N. G. González-Canché, I. Salgado-Tránsito, A. May-Pat and N. Pacheco, Polymers, 2022, 14(7), 1346, DOI:10.3390/polym14071346.
- S. Z. Popović, V. L. Lazić, N. M. Hromiš, D. Z. Šuput and S. N. Bulut, Biopolymer packaging materials for food shelf-life prolongation, in Biopolymers for food design, Academic Press, 2018, pp. 223–277 Search PubMed.
- Z. Mahcene, A. Khelil, S. Hasni, P. K. Akman, F. Bozkurt, K. Birech and F. Tornuk, Int. J. Biol. Macromol., 2020, 145, 124–132, DOI:10.1016/j.ijbiomac.2019.12.093.
- C. V. Dhumal, J. Ahmed, N. Bandara and P. Sarkar, Food Packag. Shelf Life, 2019, 21, 100380, DOI:10.1016/j.fpsl.2019.100380.
- H. Xiong, S. Tang, H. Tang and P. Zou, Carbohydr. Polym., 2008, 71(2), 263–268, DOI:10.1016/j.carbpol.2007.05.035.
- M. M. Kabir, H. Wang, K. T. Lau and F. Cardona, Compos. B. Eng., 2012, 43(7), 2883–2892, DOI:10.1016/j.compositesb.2012.04.053.
- R. Reshmy, E. Philip, S. A. Paul, A. Madhavan, R. Sindhu, P. Binod and A. Pandey, Biomass Convers. Biorefin., 2021, 11(3), 861–870, DOI:10.1007/s13399-020-00961-1.
- P. Phanthong, P. Reubroycharoen, X. Hao, G. Xu, A. Abudula and G. Guan, Carbon Resour. Convers., 2018, 1(1), 32–43, DOI:10.1016/j.crcon.2018.05.004.
- H. Charreau, E. Cavallo and M. L. Foresti, Carbohydr. Polym., 2020, 237, 116039, DOI:10.1016/j.carbpol.2020.116039.
- S. Šešlija, A. Nešić, M. L. Škorić, M. K. Krušić, G. Santagata and M. Malinconico, Macromol. Symp., 2018, 378, 1600163, DOI:10.1002/masy.201600163.
- B. Ghanbarzadeh, H. Almasi and A. A. Entezami, Innov. Food Sci. Emerg. Technol., 2020, 11(4), 697–702, DOI:10.1016/j.ifset.2010.06.001.
- C. V. Dhumal, K. Pal and P. Sarkar, Polym.-Plast. Technol. Mater., 2019, 58(3), 255–269, DOI:10.1080/03602559.2018.1466179.
- M. B. Vásconez, S. K. Flores, C. A. Campos, J. Alvarado and L. N. Gerschenson, Int. Food Res. J., 2009, 42(7), 762–769, DOI:10.1016/j.foodres.2009.02.026.
- R. Reshmy, E. Philip, A. Madhavan, R. Sindhu, A. Pugazhendhi, P. Binod and A. Pandey, Environ. Pollut., 2021, 283, 117071, DOI:10.1016/j.envpol.2021.117071.
- H. Martín-López, S. C. Pech-Cohuo, T. Ayora-Talavera, J. C. Cuevas-Bernardino, A. Ramos-Díaz, H. Espinosa-Andrews and N. Pacheco, MRS Adv., 2021, 6(38), 885–892, DOI:10.1557/s43580-021-00168-0.
- L. Scartazzini, J. V. Tosati, D. H. C. Cortez, M. J. Rossi, S. H. Flôres, M. D. Hubinger and A. R. Monteiro, J. Food Sci. Technol., 2019, 56(9), 4045–4056, DOI:10.1007/s13197-019-03873-9.
- J. Liu, Q. Ru and Y. Ding, Int. Food Res. J., 2012, 49(1), 170–183, DOI:10.1016/j.foodres.2012.07.034.
- S. N. Swain, S. M. Biswal, P. K. Nanda and P. l. Nayak, J. Polym. Environ., 2004, 12(1), 35–42 CrossRef CAS.
- A. R. Sarode, P. D. Sawale, C. D. Khedkar, S. D. Kalyankar and R. D. Pawshe, Casein and caseinate: methods of manufacture, 2016, pp. 676–682, DOI:10.1016/B978-0-12-384947-2.00122-7.
- M. E. Gounga, S. Y. Xu and Z. Wang, J. Food Eng., 2007, 83(4), 521–530, DOI:10.1016/j.jfoodeng.2007.04.008.
- M. Ozdemir and J. D. Floros, J. Food Eng., 2008, 84(1), 116–123, DOI:10.1016/j.jfoodeng.2007.04.029.
- C. Maraveas, Polymers, 2020, 12(5), 1127, DOI:10.3390/polym12051127.
- G. Keskin, G. Kızıl, M. Bechelany, C. Pochat-Bohatier and M. Öner, Pure Appl. Chem., 2017, 89(12), 1841–1848, DOI:10.1515/pac-2017-0401.
- M. C. Roco, Curr. Opin. Biotechnol., 2003, 14(3), 337–346, DOI:10.1016/S0958-1669(03)00068-5.
- S. A. O. Adeyeye and T. J. Ashaolu, J. Food Process Eng., 2021, 44(7), e13708, DOI:10.1111/jfpe.13708.
- T. V. Duncan, J. Colloid Interface Sci., 2011, 363(1), 1–24, DOI:10.1016/j.jcis.2011.07.017.
- Y. D. Livney, Food nanotechnology: proposed uses, safety concerns and regulations, Curr. Opin. Food Sci., 2014, 3, 125–135, DOI:10.1016/j.cofs.2015.06.010.
- S. Jafarzadeh, A. Salehabadia and S. M. Jafari, Handbook of Food Nanotechnology, 2020, pp. 379–414, DOI:10.1016/b978-0-12-815866-1.00010-8.
- S. D. F. Mihindukulasuriya and L. T. Lim, Nanotechnology development in food packaging: a review, Trends Food Sci. Technol., 2014, 40, 149–167, DOI:10.1016/j.tifs.2014.09.009.
- M. Z. Khajavi, A. Ebrahimi, M. Yousefi, S. Ahmadi, M. Farhoodi, A. M. Alizadeh and M. Taslikh, Food Eng. Rev., 2020, 12(3), 346–363, DOI:10.1007/s12393-020-09235-y.
- A. Gálvez, H. Abriouel, R. L. López and N. B. Omar, Int. J. Food Microbiol., 2007, 120, 51–70, DOI:10.1016/j.ijfoodmicro.2007.06.001.
- B. C. Schirmer, R. Heiberg, R. Eie, T. Møretrø, T. Maugesten and M. Carlehøg, Int. J. Food Microbiol., 2009, 133, 154–160, DOI:10.1016/j.ijfoodmicro.2009.05.015.
- C. Pastor, L. Sánchez-González, A. Marcilla, A. Chiralt, M. Cháfer and C. González-Martínez, Postharvest Biol. Technol., 2011, 60(1), 64–70, DOI:10.1016/j.postharvbio.2010.11.003.
- P. Talens, R. Pérez-Masía, M. Fabra, M. Vargas and A. Chiralt, J. Food Eng., 2012, 112(1–2), 86–93, DOI:10.1016/j.jfoodeng.2012.03.022.
- M. F. Pocas and M. Pintado, Packaging and the shelf life of cheese, in Food Packaging and Shelf Life: A Practical Guide, ed. G. L. Robertson, 2010, pp. 103–126 Search PubMed.
- A. S. El-Sisi, A. M. Gapr and K. M. Kamaly, Biolife, 2015, 3(2), 564–570, DOI:10.17812/blj2015.32.32.
- A. M. Youssef, F. M. Assem, M. E. Abdel-Aziz, M. Elaaser, O. A. Ibrahim, M. Mahmoud and M. H. Abd El-Salam, Food Chem., 2019, 270, 467–475, DOI:10.1016/j.foodchem.2018.07.114.
- S. M. El-Sayed, H. S. El-Sayed, O. A. Ibrahim and A. M. Youssef, Carbohydr. Polym., 2020, 239, 116234, DOI:10.1016/j.carbpol.2020.116234.
- A. M. Youssef, S. M. El-Sayed, H. H. Salama, H. S. El-Sayed and A. Dufresne, Carbohydr. Polym., 2015, 132, 274–285, DOI:10.1016/j.carbpol.2015.06.075.
- A. M. Youssef, S. M. El-Sayed, H. S. El-Sayed, H. H. Salama and A. Dufresne, Carbohydr. Polym., 2016, 151, 9–19, DOI:10.1016/j.carbpol.2016.05.023.
- W. Li, L. Li, H. Zhang, M. Yuan and Y. Qin, J. Food Process. Preserv., 2018, 42(1), e13362, DOI:10.1111/jfpp.13362.
- A. M. Youssef, S. M. El-Sayed, H. S. El-Sayed, H. H. Salama, F. M. Assem and M. H. Abd El-Salam, Int. J. Biol. Macromol., 2018, 115, 1002–1011, DOI:10.1016/j.ijbiomac.2018.04.165.
- H. S. El-Sayed, S. M. El-Sayed, A. M. Mabrouk, G. A. Nawwar and A. M. Youssef, J. Polym. Environ., 2021, 29(6), 1941–1953, DOI:10.1007/s10924-020-02003-3.
- A. M. Youssef, F. M. Assem, H. S. El-Sayed, S. M. El-Sayed, M. Elaaser and M. H. Abd El-Salam, RSC Adv., 2020, 10, 37857, 10.1039/D0RA07898K.
- H. P. S. Abdul Khalil, A. Banerjee, C. K. Saurabh, Y. Y. Tye, A. B. Suriani, A. Mohamed and M. T. Paridah, Food Eng. Rev., 2018, 10(3), 139–153, DOI:10.1007/s12393-018-9180-3.
- M. M. El-Magied, N. A. Salama, K. S. Nagy and M. R. Ali, Univ. Cairo Bull. Fac. Agric., 2009, 60(2), 168–177 Search PubMed.
- S. H. A. Amal, M. M. El-Mogy, H. E. Aboul-Anean and B. W. Alsanius, J. hortic. sci. ornam. plants, 2010, 2(3), 88–97 Search PubMed.
- A. M. Yossef, Middle East J. Appl. Sci., 2014, 4, 416–424 Search PubMed.
- M. A. Elabd and M. M. Gomma, Egypt. J. Food Sci., 2018, 46, 113–123 Search PubMed.
- M. P. Indumathi, K. S. Sarojini and G. R. Rajarajeswari, Int. J. Biol. Macromol., 2019, 132, 1112–1120, DOI:10.1016/j.ijbiomac.2019.03.171.
- E. E. El-Eryan and M. E. Tarabih, Int. J. Food Sci. Technol., 2020, 13, 9–21, DOI:10.47277/JETT/9(3)620.
- M. Sultan, O. M. Hafez, M. A. Saleh and A. M. Youssef, RSC Adv., 2021, 11(16), 9572–9585, 10.1039/D0RA10671B.
- A. L. De Lacey, M. E. López-Caballero and P. Montero, LWT–Food Sci. Technol., 2014, 55(2), 559–564, DOI:10.1016/j.lwt.2013.09.028.
- L. Gram and P. Dalgaard, Curr. Opin. Food Sci., 2002, 13(3), 262–266, DOI:10.1016/S0958-1669(02)00309-9.
- J. Gómez-Estaca, A. L. De Lacey, M. E. López-Caballero, M. C. Gómez-Guillén and P. Montero, Food Microbiol., 2010, 27(7), 889–896, DOI:10.1016/j.fm.2010.05.012.
- R. Gyawali and S. A. Ibrahim, Food Control, 2014, 46, 412–429, DOI:10.1016/j.foodcont.2014.05.047.
- M. Ejaz, Y. A. Arfat, M. Mulla and J. Ahmed, Food Packag. Shelf Life, 2018, 15, 113–121, DOI:10.1016/j.fpsl.2017.12.004.
- I. Echeverría, M. E. López-Caballero, M. C. Gómez-Guillén, A. N. Mauri and M. P. Montero, Int. J. Food Microbiol., 2018, 266, 142–149, DOI:10.1016/j.ijfoodmicro.2017.10.003.
- L. Lin, X. Liao and H. Cui, Food Packag. Shelf Life, 2019, 21, 100337, DOI:10.1016/j.fpsl.2019.100337.
- A. M. Youssef, H. S. El-Sayed, E. N. Islam and S. M. El-Sayed, RSC Adv., 2021, 11(37), 22571–22584, 10.1039/D1RA03819B.
- M. Pal and R. Mahendra, Sanitation in Food Establishments, LAMBERT Academic Publishers, Saarbruchen, Germany, 2015 Search PubMed.
- X. He, H. Deng and H. H. wang, J. Food Drug Anal., 2019, 27, 1–21, DOI:10.1016/j.jfda.2018.12.002.
- M. Siegrist, M. E. Cousin, H. Kastenholz and A. Wiek, Appetite, 2007, 49, 459–466, DOI:10.1016/j.appet.2007.03.002.
- J. W. Rhim, H. M. Park and C. S. Ha, Prog. Polym. Sci., 2013, 38, 1629–1652, DOI:10.1016/j.progpolymsci.2013.05.008.
- M. Avella, J. J. de Vlieger, M. E. Errico, S. Fischer, P. Vacca and M. G. Volpe, Food Chem., 2005, 93, 467–474, DOI:10.1016/j.foodchem.2004.10.024.
- B. Panea, G. Ripoll, J. González, A. Fernández-Cuello and P. Albertí, J. Food Eng., 2013, 123, 104–112, DOI:10.1016/j.jfoodeng.2013.09.029.
- V. Sharma, R. K. Shukla, N. Saxena, D. Parmar, M. Das and A. Dhawan, Toxicol. Lett., 2009, 185, 211–218, DOI:10.1016/j.toxlet.2009.01.008.
- Y. Huang, S. Chen, X. Bing, C. Gao, T. Wang and B. Yuan, Packag. Technol. Sci., 2011, 24, 291–297, DOI:10.1002/pts.938.
- H. Moustafa, S. M. El-Sayed and A. M. Youssef, J. Thermoplast. Compos. Mater., 2023, 36(1), 96–117, DOI:10.1177/0892705721989771.
- N. Pacheco, M. G. Naal-Ek, T. Ayora-Talavera, K. Shirai, A. Román-Guerrero, M. F. Fabela-Morón and J. C. Cuevas-Bernardino, Int. J. Biol. Macromol., 2019, 125, 149–158, DOI:10.1016/j.ijbiomac.2018.12.060.
- H. Moustafa, N. A. Darwish and A. M. Youssef, Food Chem., 2022, 371, 131193, DOI:10.1016/j.foodchem.2021.131193.
- L. Hai, E. S. Choi, L. Zhai, P. S. Panicker and J. Kim, Green nanocomposite made with chitin and bamboo nanofibers and its mechanical, thermal and biodegradable properties for food packaging, Int. J. Biol. Macromol., 2020, 1, 491–499, DOI:10.1016/j.ijbiomac.2019.12.124.
- C. Peng and G. Chen, Materials, 2018, 11(10), 1883, DOI:10.3390/ma11101883.
- H. Almasi, S. Azizi and S. Amjadi, Food Hydrocoll., 2020, 99, 105338, DOI:10.1016/j.foodhyd.2019.105338.
- M. Kumar, M. Tomar, V. Saurabh, T. Mahajan, S. Punia, M. del Mar Contreras and J. F. Kennedy, Trends Food Sci. Technol., 2020, 105, 223–237, DOI:10.1016/j.tifs.2020.09.009.
- M. Makaremi, H. Yousefi, G. Cavallaro, G. Lazzara, C. B. Goh, S. M. Lee and P. Pasbakhsh, Polymers, 2019, 11(10), 1594, DOI:10.3390/polym11101594.
- L. F. Wang and J. W. Rhim, Grape fruit seed extract incorporated antimicrobial LDPE and PLA films: effect of type of polymer matrix, LWT, 2016, 74, 338–345, DOI:10.1016/j.lwt.2016.07.066.
- H. F. Youssef, M. E. El-Naggar, F. K. Fouda and A. M. Youssef, Food Packag. Shelf Life, 2019, 22, 100378, DOI:10.1016/j.fpsl.2019.100378.
- A. M. Youssef, F. M. Assem, H. S. El-Sayed, S. M. El-Sayed, M. Elaaser and M. Abd El-Salam, RSC Adv., 2020, 10(62), 37857–37870, 10.1039/D0RA07898K.
- A. Jayakumar, K. V. Heera, T. S. Sumi, M. Joseph, S. Mathew, G. Praveen and E. K. Radhakrishnan, Int. J. Biol. Macromol., 2019, 136, 395–403, DOI:10.1016/j.ijbiomac.2019.06.018.
- Z. Wu, J. Wu, T. Peng, Y. Li, D. Lin, B. Xing and G. Han, Polymers, 2017, 9(3), 102, DOI:10.3390/polym9030102.
- K. Saravanakumar, A. Sathiyaseelan, A. V. A. Mariadoss, H. Xiaowen and M. H. Wang, Int. J. Biol. Macromol., 2020, 153, 207–214, DOI:10.1016/j.ijbiomac.2020.02.250.
- M. S. Hasanin and A. M. Youssef, Food Packag. Shelf Life, 2022, 34, 100979, DOI:10.1016/j.fpsl.2022.100979.
- A. M. Youssef, H. S. El-Sayed, S. M. El-Sayed, M. Fouly and M. E. Abd El-Aziz, Food Bioprocess Technol., 2023, 16, 356–367, DOI:10.1007/s11947-022-02934-w.
- H. Moustafa, A. M. Karmalawi and A. M. Youssef, Environ. Nanotechnol. Monit. Manag., 2021, 16, 100482, DOI:10.1016/j.enmm.2021.100482.
- M. C. Chen, G. H. C. Yeh and B. H. Chiang, Antimicrobial and physicochemical properties of methylcellulose and chitosan films containing a preservative, J. Food Process. Preserv., 1966, 20, 379–390, DOI:10.1111/j.1745-4549.1996.tb00754.x.
- B. Ouattara, R. E. Simard, G. Piette, A. Begin and R. A. Holley, Int. J. Food Microbiol., 2000, 62, 139–148, DOI:10.1016/S0168-1605(00)00407-4.
- T. Padgett, I. Y. Han and P. L. Dawson, J. Food Prot., 1998, 61, 1330–1335, DOI:10.4315/0362-028X-61.10.1330.
- R. C. Meyer, A. R. Winter and H. H. Weiser, Edible protective coatings for extending the shelf life of poultry, Food Technol., 1959, 13, 146–148 Search PubMed.
- C. N. Cutter and G. R. Siragusa, Lett. Appl. Microbiol., 1996, 23, 9–12, DOI:10.1111/j.1472-765X.1996.tb00018.x.
- A. Redl, N. Gontard and S. Guilbert, J. Food Sci., 1996, 61(1), 116–120, DOI:10.1111/j.1365-2621.1996.tb14739.x.
- X. Ming, G. H. Weber, J. W. Ayres and W. E. Sandine, J. Food Sci., 1997, 62(2), 413–415, DOI:10.1111/j.1365-2621.1997.tb04015.x.
- M. A. Garcia, M. N. Martino and N. E. Zaritzky, J. Agric. Food Chem., 1998, 46(9), 3758–3767, DOI:10.1021/jf980014c.
- S. Baidurah, Polymers, 2022, 14(22), 4928, DOI:10.3390/polym14224928.
- V. K. Bajpai, M. Kamle, S. Shukla, D. K. Mahato, P. Chandra, S. K. Hwang and Y. Han, J. Food Drug Anal., 2018, 26(4), 1201–1214, DOI:10.1016/j.jfda.2018.06.011.
| This journal is © The Royal Society of Chemistry 2023 |
