Open Access Article
Open Access ArticleCurrent production strategies and sustainable approaches towards the resurgence of non-centrifugal cane sugar production – a review
Venkatesh
T
 *ab,
Nandhu Lal
A. M.
a,
Silpa
V.
a,
Balakrishnan
Dharmalingam
c,
Padma Ishwarya
S.
d,
Reshma
M. V.
ab,
Sajeev
M. S.
e,
Ravi
Pandiselvam
*ab,
Nandhu Lal
A. M.
a,
Silpa
V.
a,
Balakrishnan
Dharmalingam
c,
Padma Ishwarya
S.
d,
Reshma
M. V.
ab,
Sajeev
M. S.
e,
Ravi
Pandiselvam
 f and
Anjineyulu
Kothakota
*ab
f and
Anjineyulu
Kothakota
*ab
aAgro Processing and Technology Division, CSIR – National Institute for Interdisciplinary Science and Technology, Thiruvananthapuram, 695 019, India. E-mail: venkateshsowdhill@gmail.com; kothakotaanjanikumar23@gmail.com
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201 002, India
cDepartment of Chemical and Petroleum Engineering, University of Calgary, Alberta T2N 1N4, Canada
dDepartment of Chemical Engineering, Indian Institute of Technology, Madras, 600 036, India
eDivision of Crop Utilization, ICAR – Central Tuber Crops Research Institute, Thiruvananthapuram, 695 017, India
fPhysiology, Biochemistry and Post-Harvest Technology Division, ICAR-Central Plantation Crops Research Institute (CPCRI), Kasaragod-671124, Kerala, India
First published on 5th January 2023
Abstract
Non-centrifugal cane sugar (NCS) is a traditional unrefined sweetener widely consumed across the globe, in different forms and under different names. Unlike refined sugar which possesses only carbohydrates and negligible health impacts, NCS is healthier and more nutritious as it contains minerals, vitamins and phenolic antioxidants. A shift in consuming patterns focussing on health aspects has opened an opportunity for natural sweeteners such as NCS in the global market, but they are hampered by bottlenecks such as improper final product quality, inefficient production techniques and unauthorized use of chemicals. Considering the status quo of NCS production, this review initially focuses on conventional NCS production methods and their drawbacks. Later, the review session moves into a discussion related to documented scientific interventions and technological advancements in improving the efficiency of NCS production. Finally, recommendations and strategic hygienic policies for the modernization of the NCS industry are discussed. Revival of NCS production requires energy-efficient machinery and technologies. Modern packaging techniques such as modified atmospheric packaging, vacuum packaging, and edible coating can enhance the shelf-life of NCS. Strict implementation of food standards for NCS will aid in preventing adulteration. The revival of NCS manufacturing units can provide employment opportunities, mainly for the rural population. Besides, an increase in NCS production can establish its potential as an alternative to refined sugar.
1. Introduction
The widespread and naturally occurring sweet-tasting mono- and di-saccharides, such as glucose, fructose, and sucrose, found in fruit and vegetables have been an integral part of the human diet, although the quantity and source of sugars ingested have escalated as a result of the accessibility and affordability of sweeteners.1 In addition to sedentary lifestyles, added sugars, i.e., refined sugar included in processed and prepared foods, or added to sweets or foods at the table as delineated by the US Department of Agriculture have been associated with numerous health issues, leading to obesity and other chronic diseases including diabetes, cardiovascular diseases, metabolic syndrome, and cancer, in adults as well as in children.2–4 Reports from studies directed by the World Health Organization5 suggested limiting the quantity of sugar added to foods and reducing consumption of sugar-sweetened beverages (a major source of added sugars) would be beneficial in promoting public health, particularly in terms of lowering the risk of dental caries, type 2 diabetes, and cardiovascular ailments. The Scientific Advisory Committee on Nutrition of the United Kingdom went even further, proposing that “free sugars should not exceed 5% of overall energy consumption”.6 Such reports indicate the compelling necessity for fostering potential sweetening agents as sugar substitutes in diets globally.Natural sweeteners (honey, dates, molasses, and agave nectar), artificial sweeteners (aspartame, sucralose, acesulfame K, and saccharin), sugar alcohols (xylitol, sorbitol, and mannitol), sugars of plant origin (stevia) and traditional sweeteners (non-centrifugal sugars such as cane jaggery, palm jaggery) are the major alternatives presently available for refined sugar. Among these, traditionally produced non-centrifugal sugar obtained by evaporating the juice from various plantation crops is of utmost significance, owing to the presence of bioactive and antioxidant compounds along with higher nutritional value.7 Non-centrifugal sugar can be produced from a wide range of sources such as sugarcane,8 coconut,9–11 palm,12 maple,13 and dates.14 Among all these sources, natural sugar obtained from sugarcane is the most predominantly used. The Food and Agriculture Organization of the United Nations15 adopted the technical term non-centrifugal cane sugar (NCS) to refer to a traditional minimally processed sugarcane sweetener (Saccharum officinarum L.), a solid unrefined product obtained by evaporating sugarcane juice. NCS is widely consumed as an alternative sweetener worldwide in different forms.16 It is also known by other names such as gur/jaggery (Indian subcontinent), kokuto (Japan), panela (Mexico), muscovado (Philippines), vollrohrzucker (Germany), and rapadura (Brazil). NCS is a sucrose-rich product composed of irregularly shaped amorphous particles or crystals surrounded by a variety of elements such as reducing sugars, minerals, vitamins, amino acids, and other traces.17 The nutritional composition of non-centrifugal sugar in solid, liquid, and powder forms and its evaluation with normal white sugar are presented in Table 1.
| Composition per 100 g | Types of non-centrifugal sugar | Sugar | ||
|---|---|---|---|---|
| Solid | Liquid | Granular | ||
| Sucrose (g) | 65–85 | 40–60 | 80–90 | 99.5 |
| Reducing sugar (g) | 9–15 | 15–25 | 5–9 | — |
| Water (g) | 3–10 | 30–35 | 1–2 | 0.2–0.5 |
| Protein (g) | 0.4 | 0.5 | 0.4 | — |
| Fat (g) | 0.1 | 0.1 | 0.1 | — |
| Total minerals (g) | 0.6–1.0 | 0.75 | 0.6–1.0 | 0.05 |
| Calcium (mg) | 8.0 | 3.0 | 9.0 | — |
| Phosphorous (mg) | 4.0 | 3.0 | 4.0 | — |
| Iron (mg) | 11.4 | 8.5–11 | 12 | — |
| Calorific value (kcal) | 383 | 300 | 383 | 398 |
Regardless of nutritional benefits, global NCS consumption has gradually declined over the years.18,19 A significant reason for the decline in NCS consumption is the rapid urbanization of developing nations. The rapid decline in NCS consumption resulted in the closure of many NCS manufacturing units, affecting the subsistence of rural locals. Despite the conception of various initiatives and movements towards promoting organic healthier natural sweeteners, cottage industries producing non-centrifugal sugar/NCS have not been considered much in terms of technical interventions and policy upgrades. The traditional sector still works by using archaic methods that could be riskier for both labourers and consumers—technological interventions from small-scale production levels to integrated large-scale production are necessary. Incompetent cane crushing efficiency, imprudent clarification methods, unscientific and unhygienic operational methods, improper storage and packing methods and most importantly the small-size scale of the industry are some of the significant constraints in delivering a fine quality final product. Comprehensive investigations on technological development, machinery development, automation, optimization of additives, including clarifying agents, storage and shelf-life studies, and development of suitable plantation crop varieties for NCS production are limited. Despite limited studies conducted on improving the quality of NCS and production strategies, there has been a significant surge over the past decade towards the consumption of NCS which is evident from the increased import of such products from India.20
Despite the conception of various initiatives and movements towards promoting organic healthier natural sweeteners, cottage industries producing non-centrifugal sugar have not been considered much in terms of technical interventions and policy upgrades. The traditional sector still works by using archaic methods that could be riskier for both labourers and consumers—technological interventions from farm to fork level needed to be considered. Incompetent cane crushing efficiency, imprudent clarification methods, unscientific and unhygienic operational methods, and improper storage and packing methods are some of the industry's significant constraints. In particular, comprehensive investigations on technological development, machinery development, automation, optimization of additives, including clarifying agents, storage and shelf-life studies, and development of suitable plantation crop varieties for NCS production are limited. Thus, the need-of-the-hour focuses on this unorganized cottage industry, which is indispensable to safeguarding the economic and social prosperity of the rural workers working in this sector. Upgradation of the NCS sector from small-scale to integrated large-scale processing in terms of production using energy-efficient, cost-effective technical interventions, alongside technological improvements in terms of the quality of NCS, is quintessential. This led the authors to analyse the methodologies adopted in traditional NCS production, the issues related to technical and quality aspects, and studies affecting the parameters of NCS for various components at various procedural steps. However, according to literature reports, though recent R & D studies have been carried out on clarification of cane juices and establishing the nutritional aspects of NCS, research in the arenas of pan design, packaging, storage studies, etc., has been seldom explored. For example, there was no reported literature or systematic study on NCS packaging techniques and drying methods in late 2015s.
In this context, this article is intended to present an inclusive discussion on the shortfalls of the conventional methods of NCS production, emphasizing their energy efficiency and use of clarificants. Based on the above information, the latter sections of this work discuss modern processing techniques and recent R & D advances in NCS production. Furthermore, this paper will also highlight the policy recommendations for the modernization of NCS units and the revival of NCS consumption.
2. Conventional processing methods – technology adopted, issues and challenges
The technologies adopted for non-centrifugal sugar processing slightly vary from place to place according to the indigenous knowledge and machinery available in that particular region or nation. However, all processes involve the clarification and concentration steps. In most places, processing is accomplished at the sugarcane farm in situ. Even though the procedure may vary accordingly, the procedures, viz.-a-viz. juice extraction, evaporation, clarification, concentration, and solidification remain common for NCS production.The harvested sugarcane is crushed in a horizontal crusher having around 55–60% crushing efficiency. The crushed cane (juice) is filtered generally via a clean cotton cloth or wired mesh (with a pore size of a few microns) and the filtered juice is then transferred to a wide-open boiling pan (usually made of iron) and is filled to 1/3rd of its total capacity for unit operations, viz., evaporation and concentration. In the NCS production process, dried sugarcane bagasse obtained after juice extraction is burnt as fuel for the evaporation and concentration processes. Sugarcane contains about 23–37% bagasse.21 Rao et al.22 reported that around 650 kg of sugarcane juice and 350 kg of bagasse could be obtained after crushing 1000 kg of sugarcane. Sahu23 reported that fresh bagasse primarily consists of an equal amount of water and fibres (around 47–49%) and remaining dissolved sugars (2–5%). The traditional NCS industry mostly uses dried bagasse as fuel, thereby making the process highly energy inefficient and laborious in nature.24
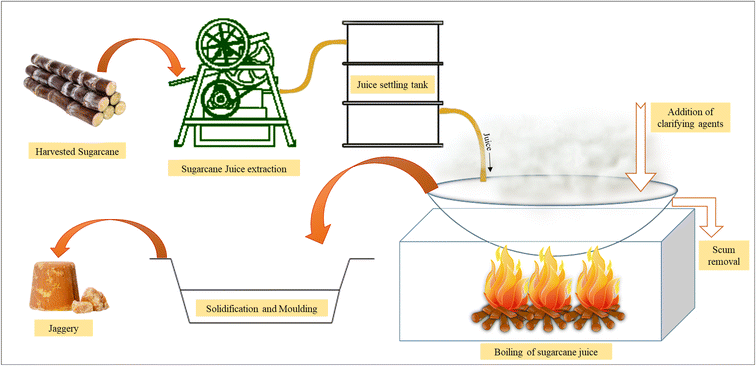
Sugarcane juice undergoes three major processes25 during open pan heating. Raising the temperature of cane juices to their boiling point constitutes the first stage, where around 6% of the total energy produced is utilized, wherein in the second stage, water present in juices gets converted into steam with the simultaneous concentration of cane juices. The conversion of water into steam with the requirement of latent heat is an energy-intensive process with around 85% of the total energy produced being utilized in the concentration process. In the final stage, the heat supplied raises the temperature to the striking point, marking the culmination of the evaporation process and forming a semi-solid product. However, the furnace temperature and open flame temperature reach around 500–1000 °C, thereby posing serious safety concerns also. During the first stage, a measured quantity of clarificants is added. During this process, floating residue called molasses (around 0.75–1.3 kg/100 kg of sugarcane juice) is formed at the top of the boiling juices in the form of foams, which is removed by skimming the top layer. To avoid excess frothing, a small quantity of mustard/groundnut oil may be sprinkled during the late boiling stages to facilitate more effortless movement of hot syrup between containers.26 The endpoint is usually determined by taking a small quantity of syrup poured into cool water, and cooling and shaping it using a finger (Fig. 2). Formation of shape by the NCS ball test is the confirmation test for determining the product's endpoint, marking the time to pour it for cooling and moulding purposes. Endpoint determination is an essential factor since overheating can burn and alter the product taste. In contrast, under-heating adversely affects the proper solidification of the product. The operation ends when the concentrated product is transferred for the cooling process. During the cooling process, concentrated juice is transferred to a wooden tray and allowed to cool for about 15–20 minutes. The cooled product is then moulded into desired shapes and sizes.27 The obtained product then undergoes storage and packaging. Traditional packaging methods followed across the globe include various methods such as a blanket of wheat straw, plastic canisters, clothes lined with polyethene sheets, earthen pots, aluminium foil, and jute bags.28 The NCS is then sold through local markets, reaching out to consumers. The traditional scheme of NCS production is represented in Fig. 1 and 2.
2.1 Constraints in existing machinery for NCS production
The majority of NCS producers still adopt traditional methods. Usually, local artisans manage NCS units with limited knowledge of energy losses. Hence, the design may usually be below thermal efficiency.24 Rao et al.29 conducted studies on local production units and found that the efficiency of units is as low as 13–15%, with a fuel consumption of 385 kg/100 kg of NCS produced. Sharon et al.30 reported very low crushing efficiency of cane (around 60%) and concentration efficiency (14.75%) in traditional NCS plants. The majority of the fuel gets wasted in the atmosphere as flue gases. The lengthy evaporation process demands many skilled labourers. The production capacity is restricted by the capacity of the pan used, thus rendering it a significant bottleneck for mass production. Therefore, only batch operation is possible with the existing process.2.2 Constraints in sugarcane juice quality used for NCS production
The initial quality of the sugarcane juices usually gets unchecked, which affects the final product quality. The juice may get contaminated by microbe load and debris passed through unhygienic initial clarification, which may alter the constituents of the juice, affecting the attributes of the final product. Maintaining juice quality is vital for obtaining a good product with better taste and longer shelf life. The use of unsafe and unwanted chemicals without proper limits is the primary constraint in traditional NCS production.31 There is no proper monitoring authority or safety limits on chemical usage in traditional NCS production methods.32 There are inadequate safety systems and monitoring authorities and no proper waste disposal facilities in traditional NCS production units. In addition to the environmentally harmful and unhealthy combustion, the working conditions are reasonably arduous, increasing the hesitation of labourers to work in the sector.The hygroscopic nature of NCS favours moisture absorption, which initiates the growth of a wide range of microorganisms including fungi and bacteria. The simultaneous growth of fungus with an increase in moisture absorption leads to increased water activity, thereby leading to degradation. Moreover, the presence of fungus in NCS leads to the production of organic acids with simultaneous degradation of the product quality. The widespread presence of Bacillus subtilis, B. mesentericus, B. vulgatus, Aerobacter aerogens, Actinomyces, Saccharomyces, Penicillium, Mucor, and Aspergillus in raw sugar-cane juice is also reported.33,34 Oliveira et al.35 analysed the inhabitancy of harmful micro-organisms such as salmonella and other parasites in sugarcane juice. They reported poor sanitary conditions in 25% of the samples, with thermotolerant coliform levels exceeding Brazilian norms, alarmingly reporting the presence of harmful thermotolerant coliforms on the hands of 37% of vendors. Singh et al.36 isolated nine microbes from NCS. Few were reported to be pathogenic, thus demanding precautionary measures as the rural population consumes the product in large quantities.
2.3 Constraints in packaging and storage practices adopted for NCS production
The traditional storage method is less effective at storing the NCS produced. Generally, sound long-term storage methods are unavailable for the NCS produced during the winter/spring season. The primary constraint is that NCS absorbs moisture, which alters its properties and causes deterioration as a consequence of fermentation and microbial spoilage, thus reducing the product's shelf-life. Also, NCS is sold under unhygienic open conditions without any proper packing at the retail level, causing faster product deterioration. Other key factors influencing NCS production and sales include the lack of proper infrastructure facilities and poor pricing and distribution.37NCS may be adulterated with many substances. Reports state that white refined sugar is added as an adulterant to quicken the process and increase colour. Besides, the addition of molasses and stone powder is also reported.38 Toxic adulterants such as metanil yellow, sodium hydrosulphite, calcium carbonate as well as sugar, maida, superphosphate, artificial colouring agents and several chemicals are added without any control. Some common adulterants in NCS and their detection methods are cited in Table 2. Adulteration in NCS can be determined by a mildly salty taste which indicates the presence of mineral salts and a lighter colour which is an indication of the addition of bleaching agents.
| Adulterant | Purpose and effect | Detection method |
|---|---|---|
| Washing soda | Added for improving colour. Washing soda can cause intestinal disorders, such as diarrhoea and vomiting | Adulteration using washing soda could be detected by addition of HCL; effervescence confirms the presence of washing soda as an adulterant |
| Metanil yellow colour | Metanil yellow is an unpermitted dye, added to improve the colour to bright yellow. It is extremely dangerous since it harms the stomach lining, kidneys, and liver | Addition of a ¼ teaspoon of jaggery followed by 3 ml of alcohol in a test tube, followed by vigorous shaking to blend the contents. The existence of metanil yellow as an adulterant is confirmed by the formation of pink colour after the addition of 10 drops of HCl |
| Zinc formaldehyde sulphoxylate (ZFS) | ZFS is a dangerous chemical, widely used in textile manufacturing, is reported allegedly to be used in the jaggery industry to improve colour | GC-MS, GC, IR-MS, NMR, HPLC and DNA based techniques38 and by analysing the ratio of reducing sugar to non-reducing sugars |
| Calcium carbonate | CaCO3 addition is to improve colour as well as the weight of jaggery | The presence of CaCO3 could be identified by visual appearance, light colour, hardness and the taste |
| Sodium hydrosulphide | This chemical is also added as an adulterant to improve colour | GC-MS, GC, IR-MS, NMR, HPLC and DNA based techniques38 and by analysing the ratio of reducing sugar to non-reducing sugars |
| Chalk powder | Added to increase quantity | The presence of chalk powder also could be determined by addition of HCl, with effervescence as confirmation for the presence, or just by mixing small quantity of sample, the chalk powder settles down, confirming the presence of adulterants |
3. Modern production techniques in NCS production units
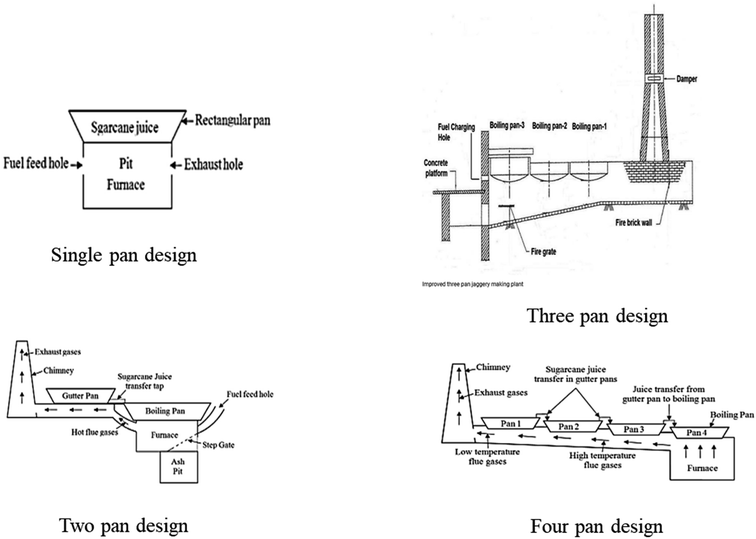
Instead of open pan evaporation, modern NCS production units employ a steam-jacketed kettle powered by a bagasse-operated boiler, thereby enhancing the efficiency of NCS production by 2.5–3 times.39 Recent developments in NCS production techniques include the use of solar energy and heat pumps. However, these techniques are in the early stage of development, and the literature indicates that these techniques should be used in conjunction with traditional ones to improve the performance of jaggery-making plants. The major energy requirement in NCS processing includes both mechanical energy (for sugarcane juice extraction) and thermal energy (for the evaporation process). Most of the energy loss occurs during thermal processes. Replacement of a portion of this thermal energy consumption with solar energy could save energy, thereby making the process efficient. Solar collectors can be employed for heating sugarcane juice to boiling temperature before transferring it to a boiling pan. Solar driers can be a viable option to reduce the moisture content of the fuel (bagasse) and pre-heat the pans, thereby reducing the processing time and energy demand. Jakkamputi & Mandapati25 conducted energy calculations of the combustion process and reported a loss of 55% of energy, and only 45% of energy is efficiently used for the evaporation process, out of which 39.22% of energy is used as latent heat and the remaining as sensible heat to raise the temperature of sugarcane juice from its initial temperature to boiling temperature. They suggested that the combustion efficiency of a furnace can be increased to a great extent by using pre-heated air instead of atmospheric air. They also estimated energy savings of 2360 kJ kg−1 NCS and fuel savings of around 23% by suitable adoption of solar energy. Modern production units generally adopt double/four pan designs which could effectively use the flue heat from the previous pan as a pre-heat source for the second pan and so on, which will eventually reduce the energy requirement of the process.40,414. Research advancements in cane juice processing and NCS production
Under this section, a detailed review of pre-processing (sugarcane juice handling), processing (development of economic furnaces and design of pans for evaporation) and post-processing aspects of NCS (enhancement of shelf life by novel packaging and drying operations) have been discussed in detail.4.1. Research developments in sugarcane juice processing techniques
A wide range of studies ranging from filtration to food-grade radiation has been studied to prolong the shelf life of cane juices. For example, Alcarde et al.42 reported that a reduction in microbial contamination upon application of gamma radiation in treating sugarcane must be obtained after the clarification process. Sankhla et al.43 conducted an analysis of the effect of hurdle technology using a combination of pasteurization, chemical treatment, irradiation and storage for sugarcane juice. They reported the method's efficiency as the hurdles were efficient enough to prevent microbial contamination and marked storage of sugarcane juice at the average temperature for 60 days and at lower temperatures for 90 days. Activated carbon produced from bagasse is utilized to remove impurities from the liquid and gaseous phases and finds applications in removing coloured impurities, pigments, and inorganic constituents from sugarcane juices.44 Laksameethanasana et al.45 conducted comparative studies on bentonite and activated carbon as clarificants against lime that is conventionally used to clarify sugarcane juice. Using 3% bentonite and 0.3% activated carbon as clarificants was found to be effective, as colour retention to a light colour was achieved in these, compared to the dark colour formed during lime addition. The sucrose content, as well as sensory quality, were also superior compared to those obtained by lime addition. However, the availability of activated carbon from other sources and bentonite needs to be studied in detail for large-scale feasibility. Disposal of activated carbon and bentonite after clarification is again an environmental concern. Solís-Fuentes et al.44 experimented with bagasse-activated carbon and ultrafiltration techniques to purify sugarcane juice and use the purified juice for NCS production. The results indicated an improvement in the final colour without impacting the physicochemical properties of NCS. Ultrafiltration also provided similar results. However, using bagasse-activated carbon was preferred over ultrafiltration as a promising alternative for the clarification of sugarcane juice since the cost intensiveness of the latter was found unaffordable for small-scale industries. Since light coloured NCS is preferred, farmers tend to mix hazardous chemicals such as sodium hydrosulphite/sodium dithionite (hydros), which are dangerous for consumption.46Conservation of sucrose content is vital for improving the colour and shelf life of NCS. Hussain et al.47 conducted experiments to prepare light golden-coloured NCS by controlling polyphenol oxidase activity, thereby conserving sucrose by pre-treating the juice with inert gases in distinct experiments. They concluded that treatment with nitric oxide and CO2 was optimal among the tested gases. Sulphur-related gases were not recommended owing to their adverse health effects. They studied sugarcane juice pre-heating and heating inlet air using solar energy. Jakkamputi and Mandapati25 confirmed the improvement in the efficiency of NCS production units. Nikam et al.48 utilized thermal energy from the solidification process of freshly prepared NCS for pre-heating of juice and found increased NCS production efficiency. Besides sugars, sugarcane juice contains a minute amount of bagasse, salts, proteins, organics and polysaccharides. Sugarcane juice clarification plays an essential part in the NCS manufacturing process. Clarification is the technique of removing unwanted materials, viz. mud or soil particles, proteins, and colloidal materials, from fresh cane juices. Such compounds present in the juice will alter the solidification process, and hence the removal of these particles is of utmost importance to NCS processing. Also, clarification performed at a temperature of around 80 °C is efficient as natural flocs created at this temperature will enhance the clarification step. Thus, an efficient clarification process is quintessential in defining the quality and yield of the product. Clarification can be performed by either chemical or physical means.49 Different studies on the effect of different clarification methods on sugarcane juice are reported in Table 4.
Obtaining an optimum pH for clarification is a challenge. However, it is already known that the utilization of freshly made lime (pH 7.5–8.5) can bring satisfactory clarification. The main function of clarification aids is to make the floccules denser and assist in decanting. Examples of clarification aids include magnesium oxide, polyelectrolytes, bentonite, activated silica, and phosphoric acid.52
Using bentonite, lime, and activated carbon,45 colour, turbidity, pH, and sensory properties were analysed to understand the effect of these clarifying agents on the clarification process (Table 3). pH, the concentrations of bentonite and activated carbon and their combinations were considered the process variables. The authors suggested that clarification using 0.3% activated carbon and 3% bentonite would give the best results in terms of colour and sensory attributes.
| Clarification method | pH of syrup | Absorbance 540 nm (colour) | Glucose (%) | Fructose (%) | Sucrose (%) | Colour likeness |
|---|---|---|---|---|---|---|
| Lime – pH 7 | 5.80a ± 0.01 | 2.8542a ± 0.0002 | 2.50c ± 0.10 | 1.75c ± 0.98 | 52.24c ± 2.31 | 5.84a ± 0.35 |
| Lime – pH 7 and activated carbon – 0.3% | 5.85a ± 0.07 | 1.9544c ± 0.0011 | 1.95d ± 0.17 | 1.54d ± 0.17 | 55.28a ± 1.80 | 5.42ab ± 0.19 |
| Bentonite – 3% | 5.15b ± 0.05 | 2.4241b ± 0.0023 | 3.73a ± 0.21 | 2.88b ± 0.33 | 51.91b ± 0.50 | 4.78b ± 0.84 |
| Bentonite – 3% and activated carbon 0.3% | 5.14b ± 0.08 | 1.3038d ± 0.0012 | 3.66b ± 0.14 | 3.13a ± 0.31 | 52.40d ± 0.79 | 5.27ab ± 0.72 |
| Clarification method | Colour removal (%) | Turbidity (%) | Starch removal (%) | Dextran removal | Total polysaccharide | Calcium hardness | Ref. |
|---|---|---|---|---|---|---|---|
| Tannin extract (300–500 ppm), pH 7.3, anionic flocculent 3 ppm | 32–34 | 89–93 | 90 | 98% | — | 18% reduction compared to that in the conventional method | 51 |
| Conventional method | — | — | 69 | 96% | — | — | 51 |
| Aluminium PC = 60 ppm, pH = 8, polyelectrolyte = 0 ppm | — | 92.39 | — | 15, 75 (μg ml−1) | 29.22 (μg ml−1) | — | 52 |
| Natural sugarcane juice | — | 88.21 | — | 21, 15 (μg ml−1) | 54.4 (μg ml−1) | — | 52 |
| Carbon dioxide at a flow rate of 200 nL h−1 and pH values of 8 and 9 | 92.93 & 91.66 (ICUMSA colour) | — | 89.19 & 85.75 | — | — | — | 53 |
A recent study by Leite & Barbosa51 investigated the efficacy of natural black Acacia tannin extract to clarify sugarcane juice. Their results proved that along with lime, the addition of 3 ppm of anionic flocculent and 300–500 ppm of tannin resulted in the best output concerning colour removal (32–34%) and turbidity reduction (89–93%). While conventional methods removed 69% starch and 96% dextran, respectively, this alternative removed 90% starch and 98% dextran. Compared to conventional methods, tannin extract also helped reduce the hardness of calcium by up to 18%, which helps prevent fouling reactions in the subsequent steps.
Prati & Moretti52 reported the usage of aluminium polychloride and negatively charged polyelectrolyte, i.e., Magnafloc LT-27, for the clarification of sugar syrups at various pH levels.
With the aim of developing a technique for sugarcane juice clarification and obtaining a cloudy, greenish-yellow beverage, Prati & Moretti52 combined heat treatment (the temperature at 65 °C for about 50 minutes) with various concentrations of flocculent (aluminium polychloride) and clarifying aid (positively charged electrolyte, Magnafloc) maintained at different pH levels. The researchers analysed the turbidity, colour, appearance, dextran content, and total polysaccharide content. Results proved that adding aluminium polychloride at an optimum concentration of 60 ppm maintained at a pH of 8 yielded a maximum turbidity removal of 90%.
Favero et al.53 analysed sugarcane juice clarification by a laboratory carbonation technique. The basic principle behind carbonation is that CO2 and calcium hydroxide form a calcium carbonate complex, which subsequently helps form the precipitate. They have analysed the starch removal rate, calcium left in the juice and ICUMSA (International Commission for Uniform Methods of Sugar Analysis) colour. Their results showed 89.19% and 85.75% starch removal and 92.93 and 91.6% ICUMSA colour which were obtained in trials using carbon dioxide at a flow rate of 200 nL h−1 and pH of 8 and 9. However, CO2 aided clarification is not economically feasible in a large scale production scenario.
Ultrafiltration is being explored by researchers across the globe as an effective alternative for fruit juice clarification to retain the quality and natural taste of juices with a negligible effect on the product.54 The use of ultrafiltration in sugarcane juice clarification has been found to be an effective and promising alternative. The use of ultrafiltration significantly reduced the occlusion index for sugarcane juice. Due to the better removal of polysaccharides, the formation of colourants was also inhibited, which subsequently resulted in the reduced colour of sucrose crystals. Through soda ash addition and secondary clarification, heavy molecular weight components were formed with cations of calcium, and hence they were removed by ultrafiltration. The ultrafiltration treatment produced a juice with lower turbidity, larger crystal size, lower viscosity, higher crystal growth, and better colour reduction.55 Akhtar et al.56 evaluated the clarification efficiency of cane juice using an ultrafiltration membrane coated with lanthanum phosphate. The researchers observed that the enzyme activity (polyphenol oxidase) was 70% lower in permeate with a low bacterial count. Also, they analysed the loss of sucrose with respect to time in raw, clarified and limed sugarcane juice and found no significant loss in sucrose throughout the process. Hence, they concluded that using a lanthanum phosphate coated ultrafiltration membrane to clarify raw sugarcane juice was viable together with the use of physical cleaning. However, all these reports mentioned above show the issue of serious fouling, which limits the use of ultrafiltration for commercial large-scale operation.
Ozonation is an advanced oxidation process in which ozone reacts in its molecular form in neutral and acidic media, whereas in a basic medium, ozone gets decomposed, forming hydroxyl radicals. Ozone cleaves the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds giving out carboxylic acids, ketones, and aldehydes, and also opens aromatic rings.57 Thus, ozonation removes compounds, mainly responsible for the colour, as well as colour precursors, viz. amines and phenolic compounds. According to Sartori et al.,57 ozonation is an effective technique for reducing low molecular weight pigments that are present in sugarcane juice. Fonseca et al.58 observed that on ozonation at 15 mg min−1 for 2 hours, 80% colour was removed from sugarcane juice as well as around 50% removal in an hour. Rodrigues et al.59 elucidated the mechanism by which the ozonation process would affect the turbidity and colour (ICUMSA) in sugarcane juice. In their experiment, they divided their samples into two lots, and the process of ozonation was performed with 13, 17, and 21 mg of ozone per min at temperatures of 30, 60, and 90 °C for a duration of 0, 30 & 60 min. Among the above factors, temperature, ozone generation and ozonation time contributed to a significant reduction of colour. Furthermore, ozonation time showed a more significant influence. Their result showed that ozone is capable of reducing the colour of sugarcane juice, which in turn means that ozone has a positive effect on the clarification process. Thus, the product after ozonation was proved to be equivalent to that obtained from traditional methods.
C double bonds giving out carboxylic acids, ketones, and aldehydes, and also opens aromatic rings.57 Thus, ozonation removes compounds, mainly responsible for the colour, as well as colour precursors, viz. amines and phenolic compounds. According to Sartori et al.,57 ozonation is an effective technique for reducing low molecular weight pigments that are present in sugarcane juice. Fonseca et al.58 observed that on ozonation at 15 mg min−1 for 2 hours, 80% colour was removed from sugarcane juice as well as around 50% removal in an hour. Rodrigues et al.59 elucidated the mechanism by which the ozonation process would affect the turbidity and colour (ICUMSA) in sugarcane juice. In their experiment, they divided their samples into two lots, and the process of ozonation was performed with 13, 17, and 21 mg of ozone per min at temperatures of 30, 60, and 90 °C for a duration of 0, 30 & 60 min. Among the above factors, temperature, ozone generation and ozonation time contributed to a significant reduction of colour. Furthermore, ozonation time showed a more significant influence. Their result showed that ozone is capable of reducing the colour of sugarcane juice, which in turn means that ozone has a positive effect on the clarification process. Thus, the product after ozonation was proved to be equivalent to that obtained from traditional methods.
4.2 Research on improving the energy efficiency of evaporators/open pans
4.3 Research developments in post-production aspects
Tiwari et al.27 designed a green-house drying model and concluded the efficiency of green-house dryers to be higher than that of open sun drying in terms of mass transfer coefficient. The effective mass transfer coefficient in a forced convection green-house drying model was around 20 W m−2 K−1 compared to 6–8 W m−2 K−1 for open drying, making the process faster and more efficient. Kumar and Tiwari83 designed an effective thermal model for greenhouse drying of NCS in natural and forced convection modes, predicting drying characteristics and practical importance of the size and shape characteristics of NCS as an important factor in greenhouse gas drying of NCS. Prakash and Kumar84 developed models based on an ANN and fuzzy networks based on MATLAB to predict the drying behaviour of NCS under various conditions of temperature, humidity and air velocity. All the reports reported proposed the effective usage of solar energy for the final drying of NCS to enhance the shelf life of the final product.
Uppal et al.87 conducted storage studies at low temperatures and affirmed the possibility of storing NCS for about eight months. He observed that the optimal way to preserve NCS was based on visual observations and the results showed that drying NCS at room temperature for 75 days until its moisture content reached 3.5 percent, and then transferring it to a tightly sealed glass container was found to be the best method for preserving NCS's good quality, colour, and appearance, and ensuring complete control of microbial growth. In their studies, Chand et al.86 investigated the storage of commercial NCS in an open pan, storage bins and polyethene bags for a period of six months and observed the change in various parameters such as moisture content, reducing and non-reducing sugar content and colour values. Compared to that stored in an open pan and polythene bags, the NCS stored in bins showed minimal change in quality parameters, thereby ensuring good quality.
The Indian Institute of Sugarcane Research, Lucknow (IISR, Lucknow) designed custom-made drying cum storage bins made of galvanized iron for the storage of NCS. A comparative study on storage of NCS in IISR bins against various traditional methods was conducted, and it was found that IISR bins were effective in maintaining a final moisture level of 6–7%, whereas, in traditional methods, the values of moisture content increased over a period of time.88 Anwar et al.89 compared the shelf life of normal NCS with that of vitamin C enriched NCS and found a negligible difference in the shelf lives of the stored products. The application and effect of various novel processing methods on the storage and preservation of NCS are delineated in Table 5.
| Technology | Inference | Ref. |
|---|---|---|
| Irradiation | Studied the effect of packaging material and different irradiation doses (3, 5, & 7 kGy) on the storage stability of jaggery and reported LDPE & paper bags subjected to medium dose irradiation of 7 kGy as the best for jaggery storage | 43 |
| Modified atmospheric packaging | Observed the aftereffects of modified atmospheric packaging (MAP) and reported that usage of PET films with 100% nitrogen could extend shelf life of jaggery for a long time.94 Compared and confirmed that PET films were better than LDPE, PP and laminated aluminium films, under modified atmospheric conditions, and were able to store up to 210 days | 95 |
| Vacuum packaging | Found the triple layer vacuum packaging material to be a promising packaging material for preserving the quality characteristics of jaggery | 94 |
| Pre-sterilization | Studied application of a pre-sterilized PET bottle on the shelf life of jaggery and reported positive benefits with efficient storage at 7 °C | 96 |
| Edible coating | Characterized the outcomes of edible coating on microbial quality in jaggery and reported the efficacy of whey as a coating material in jaggery preservation | 97 |
| Investigated the impact of quality constraints on quality aspects of jaggery and reported that drying cum storage bins with an edible coating of whey protein could extend the shelf life of jaggery | 98 | |
| Reported the efficient usage of carboxy methylcellulose (CMC) and whey protein in extension of the shelf life of jaggery and aiding in moisture retention to a desired level | 99 | |
| Reported the usage of edible coating materials viz. carboxy methylcellulose (CMC) and hydroxyl propyl methyl cellulose (HPMC) which could extend the shelf life of jaggery to about 225 days | 100 |
4.4 Freeze concentration of sugarcane juices for NCS production
Rane and Jabade90 reported a decrease in equivalent bagasse consumption and an increase in productivity using a dehumidifier-based freeze concentration system for NCS production. In this study, cane juices were concentrated in a freeze dryer set-up up to 40° brix, after which concentration was performed by conventional methods. Despite producing sugarcane juice of superior quality, the freeze concentration procedure was not effective at producing highly concentrated juice.91 Sahasrabudhe et al.92 established a mathematical model of freeze concentration systems and analysed its pros and cons using the designed mathematical model. Rane and Uphade93 analysed the response of freeze pre-concentration on the production of NCS and reported higher thermal efficiency, with drawbacks incurring additional production costs and an increase in sugar loss. However, despite higher thermal efficiencies employing freeze concentration for NCS production is economically not feasible and needs technological interventions.5. Strategic policies for modernization of NCS industries
The NCS production sector required interventions from the technical and infrastructural sides, besides policy adaptations from the government side. Policies should be adopted to ensure support for the rejuvenation of the industry along with farmers and labourers while ensuring assured quality products for consumers. NCS processing industries could be modernized with various changes. The plant layout could be designed following standard norms such as Hazard Analysis Critical Control Point (HACCP) standards. Hygiene could be prioritized as an important factor in NCS production from farm to fork. Research studies and technological implementations should ensure protocols such as FSSAI, Agricultural Mark (AGMARK), and ISO 22000:2005 by the International Organization for Standardization so as to explore good export marketing potential and ensure quality NCS products in local markets. Proper awareness of the benefits of using NCS as a substitute for sweeteners and its beneficial effects on health should be emphasized through possible methods of communication.Technical interventions in all unit operations of NCS production could be modernized to boost productivity. Cane crushing efficiency could be increased to increase juice extraction efficiency by at least 70–75%. The juice handling process and related processes could be automated to achieve hygienic juice processing. The industry could benefit from developing better pan systems for efficient heat utilization and energy conservation.
Setting up safety limits for chemical usage and constant monitoring could ensure safe products for the consumer. Promoting the usage of natural clarificants could ensure better quality products. Technological adaptations on juice concentration, for instance, the implementation of technologies such as steam boiling, vacuum boiling, mechanical vapour recompression technology, as well as the inclusion of multiple effect evaporators, could positively impact NCS production with respect to fuel efficiency, energy consumption, and time consumption.
The moulding and cooling processes could be semi-automated, and the absence of microbial activity should be ensured during the cooling process as it may adversely affect the shelf life of NCS. Packaging and storage methods should be chosen in such a way that they do not interfere with product quality and ensure that the product has a long shelf life. The packing system should be consumer-friendly, ensuring traceability and providing relevant information to consumers.
Along with waste reduction, possibilities for proper waste disposal and waste reutilization could also be investigated to improve the efficiency of NCS units. Utilization of by-products such as bagasse and molasses can be used in the paper and pulp industry for producing papers and cardboard.
From the government side, mandatory regulations should be made for NCS units to stick to norms to ensure hygiene and quality. State governments should afford incentives to modernize NCS production units in terms of enhanced crushing efficiency, juice concentration units, moulding facilities and adequate packaging facilities. The involvement of self-help groups and cooperative societies in NCS production could also be advantageous for the industry. Proper policy adaptations and technological interventions considering farmers, labourers and quality end products for consumers could revive the NCS industry.
6. Conclusion
As people's mindset gradually shifts towards organic and healthier foods, non-centrifugal sugar has immense potential as an alternative sweetener to refined white sugar. As understood from the above discussions, current challenges in NCS production include a lack of pilot-scale proven energy-efficient technologies and cost economics, packaging issues and poor marketing strategies. Hence, future strategies pertaining to NCS production should prioritize the development of energy conservative unit operations that involve less capital investment and hygienic practices. In brevity, NCS manufacturing should be optimized with respect to chemical usage, fuel consumption, and moisture content to achieve a sustainable process. Also, government interventions concerning subsidies and better marketing of NCS products should be made to promote rural technologies and decentralized food production.Ethical statement
Not applicable.Consent to participate
On behalf of all authors, the corresponding author declares that all authors are aware of the manuscript submission.Consent for publication
On behalf of all authors, the corresponding author declares that all authors are giving their consent for publication.Data availability
The authors declare that data supporting the findings of this study are available within the article.Author contributions
Venkatesh T. – writing the original draft & editing; Nandhu Lal A. M. & Silpa V. – methodology & data curation; writing – review and editing; Balakrishnan Dharmalingam – writing – review and editing; Padma Ishwarya S., Reshma M. V., Sajeev M. S. & Pandiselvam R. – investigation, proof reading & validation; Anjineyulu Kothakota – investigation, project administration, funding acquisition, supervision & correspondence.Conflicts of interest
The authors have no conflicts with each other while submitting this manuscript.Acknowledgements
The first author, Venkatesh T., would like to thank the Department of Science and Technology, Government of India for their funding vide no. DST/TDT/AGRO-46/2019. The first author, Venkatesh T., would like to thank his senior colleagues Shri Venugopalan V. V. & Dr Reshma M. V. for their constant support and motivation throughout his scientific career.References
- C. H. Edwards, M. Rossi, C. P. Corpe, P. J. Butterworth and P. R. Ellis, The role of sugars and sweeteners in food, diet and health: alternatives for the future, Trends Food Sci. Technol., 2016, 56, 158–166, DOI:10.1016/j.tifs.2016.07.008.
- M. B. Vos, J. L. Kaar, J. A. Welsh, L. v van Horn, D. I. Feig, C. A. M. Anderson, M. J. Patel, J. Cruz Munos, N. F. Krebs and S. A. Xanthakos, Added sugars and cardiovascular disease risk in children: a scientific statement from the American heart association, Circulation, 2017, 135, e1017–e1034, DOI:10.1161/CIR.0000000000000439.
- L. Paglia, The sweet danger of added sugars, Eur. J. Paediatr. Dent., 2019, 20, 89, DOI:10.23804/ejpd.2019.20.02.01.
- J. M. Rippe and T. J. Angelopoulos, Relationship between added sugars consumption and chronic disease risk factors: current understanding, Nutrients, 2016, 8, 697, DOI:10.3390/nu8110697.
- WHO, Guideline: sugars intake for adults and children, World Health Organization, 2015, https://www.who.int/publications/i/item/9789241549028 Search PubMed.
- SACN, SACN Carbohydrates and Health Report, United Kingdom, 2015 Search PubMed.
- C. Barrera, N. Betoret and L. Seguí, Phenolic profile of cane sugar derivatives exhibiting antioxidant and antibacterial properties, Sugar Tech, 2020, 22, 798–811, DOI:10.1007/s12355-020-00817-y.
- S. S. Abhai Kumar, in Dietary Sugar, Salt and Fat in Human Health, 2020, pp. 347–359 Search PubMed.
- R. Pandiselvam, K. B. Hebbar, M. R. Manikantan, B. K. Prashanth, S. Beegum and S. Ramesh, Microwave treatment of coconut inflorescence sap (Kalparasa®): a panacea to preserve quality attributes, Sugar Tech, 2020, 22, 718–726, DOI:10.1007/s12355-020-00828-9.
- A. Nath, D. Dutta, P. Kumar and J. P. Singh, Review on recent advances in value addition of jaggery based products, J. Food Process. Technol., 2015, 6 DOI:10.4172/2157-7110.1000440.
- R. Pandiselvam, S. Subhashini, E. P. Banuu Priya, A. Kothakota, S. v Ramesh and S. Shahir, Ozone based food preservation: a promising green technology for enhanced food safety, Ozone: Sci. Eng., 2019, 41, 17–34, DOI:10.1080/01919512.2018.1490636.
- K. B. Hebbar, R. Pandiselvam, M. R. Manikantan, M. Arivalagan, S. Beegum and P. Chowdappa, Palm sap—quality profiles, fermentation chemistry, and preservation methods, Sugar Tech, 2018, 20, 621–634, DOI:10.1007/s12355-018-0597-z.
- T. D. Perkins and A. K. van den Berg, Maple syrup-production, composition, chemistry, and sensory characteristics, Adv. Food Nutr. Res., 2009, 56, 101–143, DOI:10.1016/S1043-4526(08)00604-9.
- M. I. Hussain, M. Farooq and Q. A. Syed, Nutritional and biological characteristics of the date palm fruit (Phoenix dactylifera L.) – a review, Food Biosci., 2020, 34, 100509 CrossRef CAS.
- FAO, Classification of commodities; 3. sugar crops and sweeteners and derived products, 2014 Search PubMed.
- J. S. Lee, S. Ramalingam, I. G. Jo, Y. S. Kwon, A. Bahuguna, Y. S. Oh, O.-J. Kwon and M. Kim, Comparative study of the physicochemical, nutritional, and antioxidant properties of some commercial refined and non-centrifugal sugars, Food Res. Int., 2018, 109, 614–625, DOI:10.1016/j.foodres.2018.04.047.
- WCO, Sugars and Sugar Confectionery, 2012 Search PubMed.
- W. Jaffé, Non-centrifugal sugar: world production and trade, Panela Monitor, 2012, 4–48 Search PubMed.
- M. Rodríguez-Entrena, M. Salazar-Ordóñez, R. Cordón-Pedregosa and J. L. Cardenas, Br. Food J., 2016, 118(2), 495–512 CrossRef.
- R. Revathy, P. Murali, V. Venkatasubramanian, D. P. Prathap and S. Balamurali, An appraisal of Indian jaggery and confectionery exports in the global market: Markov chain model approach, Sugar Tech, 2021, 23, 118–129, DOI:10.1007/s12355-020-00866-3.
- A. Agarwal and V. Maroo, Bagasse power in India: meeting challenges of energy, environment and sustainable development, Int. J. Recent Sci. Res., 2013, 4(7), 1098–1102 Search PubMed.
- P. V. K. Rao, M. Das and S. K. Das, Jaggery – a traditional Indian sweetener, Indian J. Tradit. Knowl., 2007, 6(1), 95–102 Search PubMed.
- O. Sahu, Assessment of sugarcane industry: suitability for production, consumption, and utilization, Ann. Agrar. Sci., 2018, 16, 389–395, DOI:10.1016/j.aasci.2018.08.001.
- R. Kumar and M. Kumar, Upgradation of jaggery production and preservation technologies, Renewable Sustainable Energy Rev., 2018, 96, 167–180, DOI:10.1016/j.rser.2018.07.053.
- L. P. Jakkamputi and M. J. K. Mandapati, Improving the performance of jaggery making unit using solar energy, Perspect. Sci., 2016, 8, 146–150, DOI:10.1016/j.pisc.2016.04.019.
- P. Verma, S. R. Iyer, N. Shah and S. Mahajani, Insights into the crystallization phenomenon in the production of non-centrifugal sugar, J. Food Eng., 2021 DOI:10.1016/j.jfoodeng.2020.110259.
- G. N. Tiwari, S. Kumar and O. Prakash, Evaluation of convective mass transfer coefficient during drying of jaggery, J. Food Eng., 2004, 63, 219–227, DOI:10.1016/j.jfoodeng.2003.07.003Get.
- Pa. Kumar, R. Nirmala and E. P. Bhavya, Processing packaging and storage of jaggery from sugarcane, International Journal of Processing and Post Harvest Technology, 2013, 4, 7–12 Search PubMed.
- K. S. S. RO, A. Sampathrajan and S. A. Ramjani, Efficiency of traditional jaggery making furnace, Madras Agric. J., 2003, 90, 1 CrossRef.
- M. E. M. Sharon, C. V. K. Abirami and K. Alagusundaram, Energy losses in traditional jaggery processing, Indian Food Industry Mag, 2013, vol. 32(3) Search PubMed.
- N. Prabhu, Jaggery, not so healthy after all, Report by Cooperation Department points to extensive use of chemicals and adulterants, Additional Secretary, Cooperation Department, Govt of India, 2017 Search PubMed.
- G. P. Rao and P. Singh, Value addition and fortification in non-centrifugal sugar (jaggery): a potential source of functional and nutraceutical foods, Sugar Tech, 2021, 1–10, DOI:10.1007/s12355-021-01020-3.
- M. F. Abdallah, K. Audenaert, L. Lust, S. Landschoot, B. Bekaert, G. Haesaert, M. de Boevre and S. de Saeger, Risk characterization and quantification of mycotoxins and their producing fungi in sugarcane juice: a neglected problem in a widely-consumed traditional beverage, Food Control, 2020, 108, 106811, DOI:10.1016/j.foodcont.2019.106811.
- M. A. Daum, Controlling bacterial contaminants in sugarcane ethanol fermentations, 2016 Search PubMed.
- A. C. G. Oliveira, A. S. S. Seixas, C. P. Sousa and C. W. O. Souza, Cad Saude Publica, 2006, 22, 1111–1114 CrossRef PubMed.
- S. Singh, A. Dubey, L. Tiwari and A. K. Verma, Microbial profile of stored jaggery: a traditional Indian sweetener, Sugar Tech, 2009, 11, 213–216, DOI:10.1007/s12355-009-0034-4.
- I. V. Y. Ramarao, An economic appraisal of manufacturing and marketing of jaggery in Andhra Pradesh state, India, Sugar Tech, 2011, 13, 236–244, DOI:10.1007/s12355-011-0093-1.
- P. Hirpara, N. Thakare, V. D. Kele and D. Patel, Jaggery: a natural sweetener, J. Pharmacogn. Phytochem., 2020, 9, 3145–3148 CAS.
- S. K. Tyagi, S. Kamboj, N. Tyagi, R. Narayanan and V. V. Tyagi, Technological advancements in jaggery-making processes and emission reduction potential via clean combustion for sustainable jaggery production: an overview, J. Environ. Manage., 2022, 301, 113792, DOI:10.1016/j.jenvman.2021.113792.
- R. K. Madanrao, M. D. Mohankumar and P. S. Vijaykumar, International Journal of Innovations in Engineering Research and Technology, 2017, 4, 22–26 Search PubMed.
- N. Malik, S. Tripathi, R. Aniruddha, V. S. Babu and I. Sreedhar, Strategy to enhance thermal efficiency of evaporators in an industrial jaggery production, Mater. Today: Proc., 2022 DOI:10.1016/j.mtpr.2022.01.006.
- A. R. Alcarde, J. M. M. Walder and J. Horii, Comparison between gamma radiation and Kamoran HJ in the contamination of sugarcane must, J. Food Process. Preserv., 2001, 25, 137–147, DOI:10.1111/j.1745-4549.2001.tb00449.x.
- S. Sankhla, A. Chaturvedi, A. Kuna and K. Dhanlakshmi, Preservation of sugarcane juice using hurdle technology, Sugar Tech, 2012, 14, 26–39, DOI:10.1007/s12355-011-0127-8.
- J. A. Solís-Fuentes, F. Galán-Méndez, M. del Rosario Hernandez-Medel, R. S. García-Gómez, M. Bernal-González, S. Mendoza-Pérez and M. del Carmen Durán-Domínguez-de, Effectiveness of bagasse activated carbon in raw cane juice clarification, Food Biosci., 2019, 32, 100437, DOI:10.1016/j.fbio.2019.100437.
- P. Laksameethanasana, N. Somla, S. Janprem and N. Phochuen, Clarification of sugarcane juice for syrup production, Procedia Eng., 2012, 32, 141–147, DOI:10.1016/j.proeng.2012.01.1248.
- P. Verma, N. Shah and S. Mahajani, Effect of sodium hydrosulphite treatment on the quality of non-centrifugal sugar: jaggery, Food Chem., 2019, 299, 125043, DOI:10.1016/j.foodchem.2019.125043.
- Z. Hussain, M. Islam, Z. Mohammad, K. M. Khan, S. Perveen and M. Afzal, The effect of pre-treatment of juice on the properties and composition of jaggery, Sugar Tech, 2012, 14, 291–294, DOI:10.1007/s12355-012-0154-0.
- K. H. Nikam, B. D. Nelge and V. A. Meshram, Study of jaggery processing plant to improve the thermal performance, Int. J. Sci. Res. Dev., 2015, 3, 575–577 CAS.
- J. P. Patil, U. S. Shinde, G. S. Nevkar and J. Singh, Clarification efficiency of synthetic and herbal clarificants in quality jaggery production, Sugar Tech, 2005, 7, 77–81, DOI:10.1007/BF02942535.
- S. Asokan and T. R. Rupa, Sugarcane chemistry including sugar and gur technology, Directorate of Open and Distance Learning, Tamilnadu Open University, 2008 Search PubMed.
- I. R. Leite and R. D. Barbosa, Tannin extract for sugarcane juice clarification, Sugar Tech, 2021, 23, 682–691, DOI:10.1007/s12355-020-00923-x.
- P. Prati and R. H. Moretti, Study of clarification process of sugar cane juice for consumption, Food Sci. Technol., 2010, 30, 776–783, DOI:10.1590/S0101-20612010000300033.
- D. M. Favero, F. Hamerski and A. D. de Aquino, Starch and ICUMSA color removal in sugarcane juice clarified by carbonatation, Acta Sci., Technol., 2014, 36, 745–751, DOI:10.4025/actascitechnol.v36i4.17660.
- C. Shi, D. W. Rackemann, L. Moghaddam, B. Wei, K. Li, H. Lu, C. Xie, F. Hang and W. O. S. Doherty, Ceramic membrane filtration of factory sugarcane juice: effect of pretreatment on permeate flux, juice quality and fouling, J. Food Eng., 2019, 243, 101–113, DOI:10.1016/j.jfoodeng.2018.09.012.
- T. Vu, J. LeBlanc and C. C. Chou, Clarification of sugarcane juice by ultrafiltration membrane: toward the direct production of refined cane sugar, J. Food Eng., 2020, 264, 109682, DOI:10.1016/j.jfoodeng.2019.07.029.
- A. Akhtar, S. Subbiah, K. Mohanty, R. Sundar, R. Unnikrishnan and U. S. Hareesh, Sugarcane juice clarification by lanthanum phosphate nanofibril coated ceramic ultrafiltration membrane: PPO removal in absence of lime pre-treatment, fouling and cleaning studies, Sep. Purif. Technol., 2020, 249, 117157, DOI:10.1016/j.seppur.2020.117157.
- J. A. S. Sartori, C. F. Figueiredo Angolini, M. N. Eberlin and C. L. Aguiar, Reactions involved in phenolics degradation from sugarcane juice treated by ozone, Ozone: Sci. Eng., 2019, 41, 369–375, DOI:10.1080/01919512.2018.1547183.
- G. C. Fonseca, C. B. B. Costa and A. J. G. Cruz, Superstructural economic optimization of sugarcane bagasse exploitation in an ethanol distillery connected to Rankine cycle, BIGCC system and second generation ethanol process, Computer Aided Chemical Engineering, Elsevier, 2017, vol. 40, pp. 889–894 Search PubMed.
- R. Rodrigues, L. C. C. Sperandio and C. M. G. Andrade, Investigation of color and turbidity in the clarification of sugarcane juice by ozone, J. Food Process Eng., 2018, 41, e12661, DOI:10.1111/jfpe.12661.
- A. Manjare and J. Hole, Exhaust heat recovery of jaggery making furnace, Int. J. Sci. Res., 2013, 5, 1349–1351 Search PubMed.
- S. I. Anwar, Improving thermal efficiency of open pan jaggery furnaces–a novel concept, Indian Journal of Sugarcane Technology, 2014, 29, 32–34 Search PubMed.
- G. B. Agalave, Performance improvement of a single pan traditional jaggery making furnace by using fins and baffle, Int. J. Adv. Res. Sci.Eng., 2015, 4, 85–89 Search PubMed.
- B. Baboo and S. I. Anwar, Recent developments in jaggery (Gur) research, Indian Institute of Sugarcane Research Technical Bulletin No. IISR/JKS/94, 1994 Search PubMed.
- R. D. Singh, B. Baboo, A. K. Singh and S. I. Anwar, Performance evaluation of two pan furnace for jaggery making, IE (I) Journal-AG, 2009, 90, 27–30 Search PubMed.
- S. I. Anwar, Determination of moisture content of bagasse of jaggery unit using microwave oven, J. Eng. Sci. Technol., 2010, 5, 472–478 Search PubMed.
- A. Manjare and J. Hole, Exhaust heat recovery and performance improvement of jaggery making furnace, Int. J. Curr. Eng. Technol., 2016, 5, 165–170 Search PubMed.
- T. R. Shanthy and K. Baburaj, Socio–economic impact of multiple furnace over single furnace in jaggery preparation, J. Sugarcane Res., 2015, 5, 65–73 Search PubMed.
- H. K. Madan, U. K. Jaiswal, J. S. Kumar and S. K. Khanna, Improvement in gur (jaggery) making plant for rural areas, J. Rural Technol., 2004, 1, 194–196 Search PubMed.
- J. Singh, Indian Journal of Sugarcane Technology, 2009, 24, 45–47 Search PubMed.
- P. K. Arya, S. Kumar and U. K. Jaiswal, Int. J. Eng. Res., 2013, 2, 266–270 Search PubMed.
- R. Kumar and M. Kumar, Performance evaluation of improved and traditional two pan jaggery making plants: a comparative study, Sustain. Energy Technol. Assess., 2021, 47, 101462, DOI:10.1016/j.seta.2021.101462.
- V. R. Sardeshpande, D. J. Shendage and I. R. Pillai, Thermal performance evaluation of a four pan jaggery processing furnace for improvement in energy utilization, Energy, 2010, 35, 4740–4747, DOI:10.1016/j.energy.2010.09.018.
- S. Khattak, R. Greenough, V. Sardeshpande and N. Brown, Exergy analysis of a four pan jaggery making process, Energy Rep., 2018, 4, 470–477, DOI:10.1016/j.egyr.2018.06.002.
- M. Shankar, U. Ravindra, B. Kalpana, S. M. Vidyashree, M. C. Gowda and K. Mohith, Evaluation of multipan furnace over traditional pan furnaces used for jaggery production in Mandya district of Karnataka, Environ. Ecol., 2009, 27, 316–319 Search PubMed.
- K. Y. Shiralkar, S. K. Kancharla, N. G. Shah and S. M. Mahajani, Energy improvements in jaggery making process, Energy Sustainable Dev., 2014 DOI:10.1016/j.esd.2013.11.001.
- R. la Madrid, E. M. Orbegoso, R. Saavedra and D. Marcelo, Improving the thermal efficiency of a jaggery production module using a fire-tube heat exchanger, J. Environ. Manage., 2017, 204, 622–636, DOI:10.1016/j.jenvman.2017.09.035.
- Zabala and Bonilla, Development of a heat transfer calculation model for fire-tubular exchangers (paila) of the panela industry, 2010 Search PubMed.
- G. C. P. Espinoza Pariona, Fluid dynamic and structural study of firetube pans, BSc thesis, University of Piura, Peru, 2017 Search PubMed.
- R. la Madrid, D. Marcelo, E. M. Orbegoso and R. Saavedra, Heat transfer study on open heat exchangers used in jaggery production modules – computational fluid dynamics simulation and field data assessment, Energy Conversion and Management, 2016, 125, 107–120, DOI:10.1016/j.enconman.2016.03.005.
- R. Gupta, P. Singh and A. Suman, in Proceedings of National Seminar on Status, Problems and Prospects of Jaggery & Khandsari, ed. J. Singh, 2002, pp. 193–196 Search PubMed.
- D. Mandal, S. Tudu, S. R. Mitra and G. C. De, Effect of common packing materials on keeping quality of sugarcane jaggery during monsoon season, Sugar Tech, 2006, 8, 137–142, DOI:10.1007/BF02943648.
- J. Singh and R. D. Singh, Processing, handling and storage of sugarcane jaggery, Indian Institute of Sugarcane Research, Lucknow, 2011 Search PubMed.
- A. Kumar and G. N. Tiwari, Effect of shape and size on convective mass transfer coefficient during greenhouse drying (GHD) of jaggery, J. Food Eng., 2006, 73, 121–134, DOI:10.1016/j.jfoodeng.2005.01.011.
- O. Prakash and A. Kumar, Application of artificial neural network for prediction of jaggery mass during drying inside natural convection greenhouse dryer, Int. J. Ambient Energy, 2014, 35, 186–192, DOI:10.1080/01430750.2013.793455.
- P. P. Said and R. C. Pradhan, Preservation and value addition of jaggery, Int. J. Agric. Eng., 2013, 6, 569–574 Search PubMed.
- K. Chand, N. C. Shahi, U. C. Lohani and S. K. Garg, Effect of storage conditions on keeping qualities of jaggery, Sugar Tech, 2011, 13, 81–85, DOI:10.1007/s12355-010-0059-8.
- S. K. Uppal, S. Sharma and G. S. Sidhu, Keeping quality of jaggery under cold storage technology, in proceeding international symposium on “Food, Nutrition and Economic Security Through Diversification in Sugarcane Production and Processing Systems”, J. Food Sci. Technol., 2002, 39, 549–551 Search PubMed.
- K. Chand and A. M. Kulshrestha, Jaggery quality effected by hilly areas, Indian J. Tradit. Knowl., 2012, 11(1), 172–176 Search PubMed.
- S. I. Anwar, R. D. Singh, V. K. Pandey and P. R. Pandey, Storage studies of natural vitamin C enriched jaggery, Agr. Eng. Today, 2015, 39, 8–10 Search PubMed.
- M. v Rane and S. K. Jabade, Energy efficient liquid desiccant-based dryer, Appl. Therm. Eng., 2005, 25, 2122–2137, DOI:10.1016/j.applthermaleng.2004.07.015.
- S. Songsermpong and W. Jittanit, Comparison of peeling, squeezing and concentration methods for the sugarcane juice production, Suranaree J. Sci. Technol., 2010, 17(1), 49–55 Search PubMed.
- A. B. Sahasrabudhe, R. R. Desai and S. K. Jabade, Freeze concentration of sugarcane juice in a jaggery making process-modeling, International Journal of Modeling and Optimization, 2011, 1, 118 CrossRef.
- M. v Rane and D. B. Uphade, Energy efficient jaggery making using freeze pre-concentration of sugarcane juice, Energy Procedia, 2016, 90, 370–381, DOI:10.1016/J.EGYPRO.2016.11.204.
- D. Kumar, J. Singh, D. R. Rai, S. Bhatia and A. K. Singh, Colour changes in jaggery cubes under modified atmosphere packaging in plastic film packages, Agrotechnology, 2013, 5, 2, DOI:10.4172/2168-9881.1000S1-005.
- P. A. Kumar, R. Kailappan and R. Nirmala, Effect of different clarifying agents on the quality of jaggery, Mysore J. Agric. Sci., 2012, 46, 784–789 Search PubMed.
- S. D. Patil and S. v Anekar, Effect of different parameters and storage conditions on liquid jaggery without adding preservatives, Int. J. Res. Eng. Technol., 2014, 3, 280–283 Search PubMed.
- P. Shukla, Prime Journal of Microbiology Research, 2012, 2, 121–125 Search PubMed.
- K. Chand, A. K. Verma, A. Kumar and N. C. Shahi, Effect of edible coating on quality parameters of jaggery during storage, Sugar Tech, 2014, 16, 80–85, DOI:10.1007/s12355-013-0244-7.
- M. Ritesh, P. K. Omre, S. K. Khan Chand and A. S. Bist, Efficacy of a coating composed of carboxymethyl cellulose and whey protein concentrate to control the quality of jaggery, Int. J. Eng. Sci. Res. Technol., 2014 Search PubMed.
- A. Kumar, K. Chand, N. C. Shahi, A. Kumar and A. K. Verma, Optimization of coating materials on jaggery for augmentation of storage quality, Indian J. Agric. Sci., 2017, 87, 1391–1397 CAS.
| This journal is © The Royal Society of Chemistry 2023 |