Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluation of blanching technology in green color preservation in yerba-mate as a substitute for conventional process
Ana Paula
Picolo
a,
Itamar Luís
Gonçalves
 b,
Everson Adelmo
Pasquali
b,
Albanin
Aparecida Mielniczki-Pereira
b and
Alice Teresa
Valduga
b,
Everson Adelmo
Pasquali
b,
Albanin
Aparecida Mielniczki-Pereira
b and
Alice Teresa
Valduga
 *b
*b
aBarão Erva Mate e Chás, Rua Ilma Picolo 368, Barão de Cotegipe, RS, Brazil
bUniversidade Regional Integrada do Alto Uruguai e das Missões – Erechim, Avenida Sete de Setembro, 1621, Erechim, RS, Brazil. E-mail: valice@uricer.edu.br
First published on 29th November 2022
Abstract
To produce enzymatic inactivation, yerba-mate leaves are exposed to heat produced by wood combustion, a step identified as zapeco. This study aimed to optimize enzymatic inactivation in yerba-mate, in a process able to replace the conventional zapeco. The effects of the variables water temperature and exposure time on the chlorophyll and colorimetric coordinates were evaluated through a factorial design 22. The adjustment of the colorimetric coordinate a* and chlorophyll as a function of temperature and exposure time allowed validation of the empirical models. The experimental condition defined by 95 °C yielded the highest chlorophyll levels and the lowest a* coordinate. Considering organoleptic aspects simultaneously with these optimized responses, the independent variable time affected the response to a lesser extent. The exposure of yerba-mate leaves to boiling water allows a product to be obtained with enzymatic inactivation and color similar to the conventional process.
Introduction
Ilex paraguariensis St. Hil (Aquifoliaceae), popularly known as yerba-mate, is a native plant from South America, of social, economic and ecological importance. In this region yerba-mate leaves and small branches are processed for the production of different beverages, among which the most commonly consumed is maté. Yerba-mate contains in its composition secondary metabolites, such as flavonoids, phenolic acids, methylxanthines and saponins, which can promote numerous benefits to health.1,2The processing of yerba-mate leaves to produce maté involves the steps of zapeco, drying and grinding. In industrial zapeco yerba-mate processing, the plant material is put in direct contact with flames originating from burning wood.3 In this step contact of the leaves with polycyclic aromatic hydrocarbons may occur, that can be adsorbed by the yerba-mate biomass.4–6 Human exposure to polycyclic aromatic hydrocarbons may lead to the development of cancer. When these contaminants are metabolized by CYP enzymes, epoxides are produced that can react with the nucleophilic nitrogen from nucleic acids, affecting replication of the genetic material.7 In addition, conventional zapeco has a limitation in that it requires the absence of prior washing of the leaves. The following step involves drying of the leaves at a temperature close 100 °C, yielding the final product with moisture content lower than 5%. After drying the material is subjected to grinding in a granulometer according to consumer market preference.8
Zapeco is a key step for maintaining the green color in yerba-mate. In Brazil consumption priority is given to the freshly processed product with an intense green color, while in Uruguay, Argentine and Paraguay priority is given to the matured product, which is yellow due to degradation of chlorophyll.9–11 In terms of chemical aspects, zapeco has the function of inactivating enzymes present in vegetable tissues, such as polyphenoloxidases (PFO) and peroxidase (POD), which produce enzymatic darkening.12 In this context, to keep the green color in yerba-mate used in some beverage preparations, enzymatic inactivation is necessary. This condition is reached through fast exposure to high temperature.13
The problems associated with conventional zapeco, described above, have a great impact on the safety and quality of processed yerba-mate. Therefore, attempts have been made to find alternatives to conventional zapeco in an effort to improve these aspects in the final product. In addition to the use of liquefied petroleum gas as an energy source in the zapeco step,8 the use of high-frequency radiation11 has been reported as a promising alternative.
Contact with hot water is widely used in vegetable processing to keep their green color and it involves the fast exposure of vegetables to boiling water or steam.14 Another advantage of this process is the possibility of washing the leaves before the next processing steps. Thus, blanching is a viable alternative process that can be utilized to avoid enzymatic darkening in yerba-mate leaves without contamination by polycyclic aromatic hydrocarbons. In this context, the effects of water temperature and exposure time are reported here on the color and enzymatic activity of yerba-mate-leaves subjected to a blanching process. The use of hot water in this process may be an opportunity to use greener energy sources in yerba-mate processing.
Results and discussion
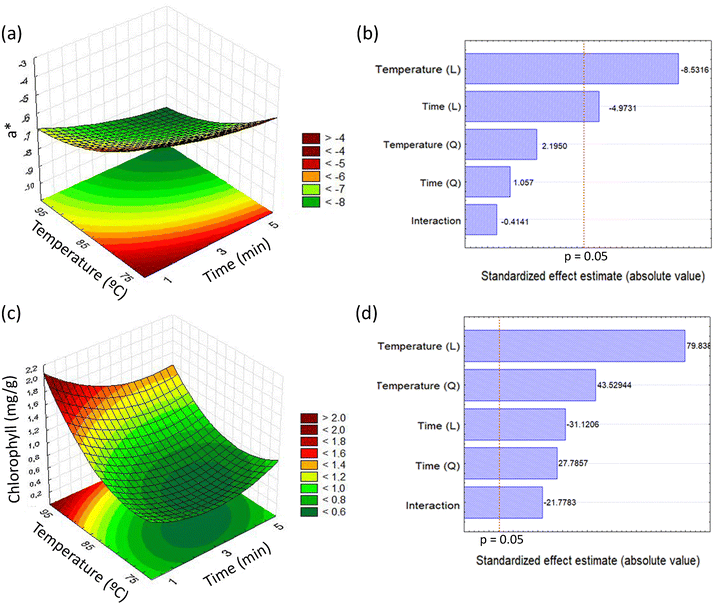
In order to avoid the exposure of yerba-mate leaves to smoke, we performed a set of assays aimed at producing green yerba-mate, replacing the conventional zapeco step by exposure to hot water. The exposure time and water temperature were the variables investigated and the ratio between the leaf mass and water mass was fixed on the basis of previous investigations (50 g of yerba-mate leaves exposed to 1 L of water). The responses measured were the colorimetric coordinates L*, a*, b* (CIELAB scale) and the total chlorophyll level.For colorimetric coordinate L*, all values obtained from the experimental conditions were higher than for the sample subjected to conventional zapeco. In relation to the coordinate a*, the experimental conditions in which the temperature was the lowest were less efficient in preserving the green color, producing high values for this parameter. Preservation of the green color is achieved when small values of a* are obtained. All experimental conditions increased the colorimetric coordinate b* in relation to the sample subjected to conventional processing, and this finding is associated with sample yellowing. On the other hand, the highest chlorophyll value was produced by using high-temperature water for a short time. The values of coordinates b* and L* did not allow a surface response to be produced with good adjustment, while for coordinate a* and chlorophyll the analysis yielded good adjustment.
Adjustment of colorimetric coordinate a* as a function of temperature and exposure time produced a calculated F value corresponding to 6.54, higher than the tabulated F(5,5) = 5.05 adopting 95% as the confidence interval, and r2 = 0.8673, validating the empirical model (p < 0.05) utilized to generate the surface response and the contour curves represented in Fig. 1a. The ANOVA for this response is represented in Table 1. The lower green color loss (a* = −8.85 ± 0.05) was related to the use of a higher temperature (95 °C) and 5 minutes. The linear terms of the variables temperature and time had significant negative effects on the response (Fig. 1b).
| QS | DF | QM | F | |
|---|---|---|---|---|
| Coordinate a* | ||||
| Regression | 20.254 | 5 | 4.050 | 6.54 |
| Residue | 3.099 | 5 | 0.619 | |
| Lack of fit | 2.715 | 3 | ||
| Pure error | 0.384 | 2 | ||
| Total | 23.354 | 10 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Chlorophyll total | ||||
| Regression | 1.305 | 5 | 0.261 | 21.75 |
| Residue | 0.061 | 5 | 0.012 | |
| Lack of fit | 0.060 | 3 | ||
| Pure error | 0.001 | 2 | ||
| Total | 1.367 | 10 | ||
Changes in colorimetric parameters and vegetable pigment levels are not exclusively related to yerba-mate processing, but also relate to its shelf-life. After 12 weeks, the parameters L*, a*, b* and chlorophyll showed changes between consecutive measurements. In this same analysis we found a positive correlation between chlorophyll degradation and parameter a* approximating a zero value.15
Another parameter investigated as a response in the factorial design was the total chlorophyll level. The model generated showed that the total chlorophyll level was maximized (1.78 ± 0.03 mg g−1) when the sample was subjected to the conditions defined by a temperature of 95 °C for 1 minute (Fig. 1c). The variance analysis allowed us to obtain an r2 of 0.9546 and a calculated F corresponding to 21.75, resulting in validation of the model shown in Fig. 1c (F(5,5) = 5.05 adopting 95% as the confidence interval). The ANOVA for this response is reported in Table 1. The response was significantly affected by all independent variables, according to the Pareto analysis represented in Fig. 1d.
About 80% of the chlorophyll content present in yerba-mate leaves suffers degradation during the zapeco step.16 Research evaluating the kinetics of chlorophyll degradation in peas showed that its half-life is shorter at low pH values and high temperatures.14 The total chlorophyll values for yerba-mate described in the literature at the beginning of storage range from 2.16 to 6.04 mg g−1,15,17,18 so that the values obtained in this work conform to reported data.
Among the factors that can exercise effects on chlorophyll quantification can be mentioned the material source, the processing, the methodology of quantification and the time between the processing and the analysis.
It may be demonstrated that the polynomial regression produced using the data of a* and chlorophyll allowed us to obtain successful adjustment of the correlation between the predicted and observed values, according to its higher r2 value, as shown in Fig. 2. In other words, for coordinate a* nearly 14% of total variance could not be explained by the model, and for chlorophyll nearly 5% of variance could not be explained by the model. A mathematical model which could predict behavior of the a* and chlorophyll values in the investigated experimental domain is presented in eqn (1) and (2). Eqn (1) and (2) include neglected insignificant coefficients for investigated response variables.
| a* = −7.195 − 0.890x + 0.279x2 − 1527y + 0.604y2 − 0.0901xy | (1) |
| Chlorophyll = 0.635 − 0.136x + 0.187x2 + 0.349y + 0.293y2 − 0.116xy | (2) |
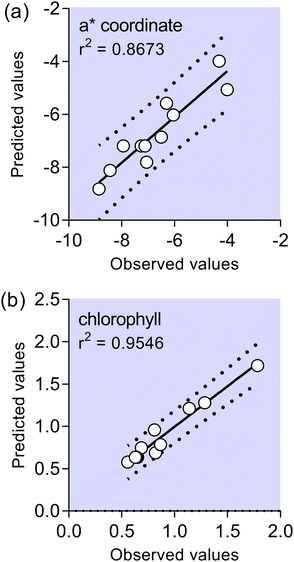 | ||
| Fig. 2 Comparison of experimental and predicted data given by the response surface methodology model for: coordinate a* (a) and total chlorophyll (b). | ||
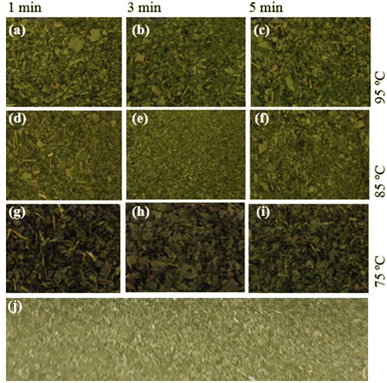
Fig. 3 shows the color of the final product obtained under different experimental conditions. It can be observed that samples located in the same line (same temperature and different exposure times) showed a similar color, while samples located in the same columns (same exposure time and different temperatures) showed different aspects. This result is in accordance with the most pronounced effect of the variable temperature described in the Pareto analysis in Fig. 1. Fig. 3 also shows that high temperature is an important factor for obtaining a color with a high similarity level to traditional zapeco, as shown in Fig. 3j.
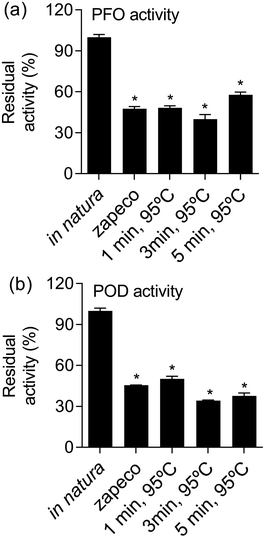
From the results of the response surface methodology, we performed an analysis of PFO and POD activity in yerba-mate samples treated with the most promising conditions able to make a* values low and chlorophyll levels high. This effect was obtained by yerba-mate leaves exposed to 95 °C for 1, 3 and 5 minutes. Treating yerba-mate leaves under these conditions yielded residual levels of POD and PFO very similar to yerba-mate subjected to conventional zapeco, which was nearly half of that in in natura leaves. The levels of enzymatic activity are shown in Fig. 4.
Several technologies are available to avoid enzymatic darkening in vegetables,19 despite thermal treatment being the classical approach, and recent investigations have explored the use of microwave radiation20 and ultrasound.21 Decreasing the yerba-mate zapeco time, without color changes, was a target of the research, and the best results were shown for enzyme inactivation after heat treatment at 450 °C for 15 seconds and at 500 °C for 10 seconds.13 Peroxidase inactivation is challenging, considering that it is a thermostable enzyme, and its activity may be regenerated after thermal treatment.22 The absence of enzymatic inactivation in yerba-mate leaves produces darkening and oxidation. The oxidative process in yerba-mate leaves was previously utilized for the development of a functional beverage, similar to black tea obtained from Camellia sinensis leaves.23,24
The process reported here, in addition to promoting the washing of leaves, and possibly avoiding exposure to aromatic polycyclic hydrocarbons, can minimize the degradation of phenolic compounds presents in yerba-mate leaves. A previous study reported the high oxidation rate of 5-O-caffeoylquinic acid in the temperatures utilized in the conventional enzymatic inactivation and drying of yerba-mate leaves.25 To find alternative processing methods that can be applied to yerba-mate leaves is an emerging necessity, aiming at an improvement in food security while maintaining its phytochemical composition and sensory acceptance.26,27 Future studies will be undertaken to assess the changes in phytochemical composition of yerba-mate produced by this form of enzymatic inactivation.
Conclusion
The condition characterized by sample exposure at 95 °C produced lower chlorophyll degradation and lower coordinate a* values. The exposure time appears to affect organoleptic aspects to a smaller extent. The experimental conditions also produced enzymatic inactivation similar to that produced by the conventional process. Exposure of yerba-mate leaves to boiling water for a short period of time allowed us to obtain processed yerba-mate, with a color similar to that produced in conventional processing.Experimental
Obtaining samples
Yerba-mate leaves obtained from the URI-Erechim gardens were subjected to two processes: (i) conventional zapeco: in a laboratory-scale prototype powered by liquefied petroleum gas energy (operational conditions in batches, corresponding to: 100 g of leaves each cycle, 180 °C, 5 minutes and 60 Hertz),8 and (ii) blanching: 50 g of leaves by exposure to 1 L of water at a temperature and time defined by the factorial design. In both experimental conditions the samples were dried in fixed bed with hot air at 70 °C until the moisture content was less than 5%.Experimental design
The experimental conditions were defined by a factorial design 22. The independent variables which were considered were the temperature and the time, ranging respectively from 75 °C to 95 °C, and from 1 to 5 minutes, with the central point corresponding to 85 °C and 3 minutes. These ranges were adopted from results obtained in previous assays. 95 °C was considered as being the water boiling temperature, as a function of the altitude of the region in which the studies were performed.Obtaining the enzymatic extract
The enzymatic crude extract was obtained according to procedures previously described,28–30 with adaptations. A sample composed of 20 g of in natura yerba-mate leaves, subjected to heat treatments defined by factorial design was homogenized in 0.05 mol L−1 phosphate buffer (pH 7.5) and 3.0% (p/p) polyvinylpyrrolidone. The mixture was filtered and centrifuged at 8.400 Hertz, 4 °C for 30 minutes. The supernatant was used as a crude enzymatic extract, and kept on an ice bath until the assessment of enzymatic activity.PFO and POD activity determination
The PFO and POD activities were measured at 420 nm and 470 nm, respectively, according to the methodology defined in previous studies.28–30 For quantification of PFO activity, to a glass tube containing 2.8 mL of 0.05 M phosphate buffer (pH 8.5) and 100 μL of 0.1 M pyrocatechol in 0.1% polysorbate (Tween 80), was added 100 μL of crude extract. In relation to the POD activity measurement, to a glass tube containing 2.725 mL of 0.05 M phosphate buffer (pH 4.7), 100 μL of 0.2 M hydrogen peroxide solution, and 100 μL of guaiacol in 0.1% polysorbate (Tween 80), was added 100 μL of crude extract. In both cases, the absorbance values were read, and enzymatic activity was expressed as residual activity in relation to the in natura leaves. All reagents were purchased from Sigma-Aldrich.Instrumental color measurement
A Minolta CR400 colorimeter was used for color evaluation with the CIELAB system scale, where L* represents luminosity ranging from black (0) to white (100) and the colorimetric coordinates “a*” and “b*” represent different colors (−a = green, +a = red; −b = blue and +b = yellow).Determination of chlorophyll concentrations
The pigments were extracted by trituration of 0.125 g of sample in the presence of N,N-dimethylformamide for 1 min. The suspension was filtered, and the volume made up with solvent in a 25 mL volumetric flask. Pigment quantification was performed with absorbance readings at 664 and 647 nm using eqn (3), as previously developed.31 The results are expressed as milligrams of pigment per gram of material.| [Total chlorophyll, μg mL−1] = 7.04A664 + 20.27A647 | (3) |
Data analysis
The colorimetric coordinates and the enzymatic activity levels were expressed as mean ± standard deviation of three independent experiments, and compared by variance analysis followed by a Tukey test, adopting 5% as the significance level in Statistic 7.0 software. To assess the effect of the variables, different responses were studied with Pareto charts, surface responses and contour lines in Statistic 7.0 software.Conflicts of interest
There are no conflicts to declare.Notes and references
- A. T. Valduga, I. L. Gonçalves, E. Magri and J. R. Delalibera Finzer, Food Res. Int., 2019, 120, 478–503 CrossRef CAS PubMed.
- W. Masson, L. Barbagelata, M. Lobo, J. P. Nogueira, P. Corral and A. Lavalle-Cobo, Plant Foods Hum. Nutr., 2022, 77, 353–366 CrossRef CAS PubMed.
- A. P. Butiuk, M. A. Martos, O. Adachi and R. A. Hours, J. Appl. Res. Med. Aromat. Plants, 2016, 3, 27–33 Search PubMed.
- E. Oranuba, H. Deng, J. Peng, S. M. Dawsey and F. Kamangar, J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev., 2019, 37, 26–41 CrossRef CAS PubMed.
- V. A. García Londoño, P. S. Pok and S. Resnik, Polycyclic Aromat. Compd., 2018, 1381–1389 Search PubMed.
- M. V. Panzl, J. M. S. Almeida, M. Pedrozo-Peñafiel, D. Menchaca, R. Q. Aucélio and A. Rodríguez-Haralambides, Polycyclic Aromat. Compd., 2022, 1–15 Search PubMed.
- B. Moorthy, C. Chu and D. J. Carlin, Toxicol. Sci., 2015, 145, 5–15 CrossRef CAS.
- A. T. Valduga, J. R. D. Finzer and S. H. Mosele, Processamento de erva-mate, EdiFAPES, 2003 Search PubMed.
- I. Zaions, A. P. Picolo, I. L. Gonçalves, A. C. P. Borges and A. T. Valduga, Braz. Arch. Biol. Technol., 2014, 57, 663–667 CrossRef.
- C. S. Lewinski, I. L. Gonçalves, A. C. P. Borges, N. Dartora, L. M. d. Souza and A. T. Valduga, Nutr. Food Sci., 2015, 45, 221–228 CrossRef.
- S. A. Holowaty, A. E. Thea, C. Alegre and M. E. Schmalko, J. Food Process Eng., 2018, 41, e12911 CrossRef.
- J. R. Whitaker, A. G. Voragen and D. W. Wong, Handbook of Food Enzymology, Marcel Dekker, 2003 Search PubMed.
- J. G. Provesi, G. H. Nabechima, M. A. Vieira and E. R. Amante, Int. J. Food Sci. Technol., 2010, 45, 971–977 CrossRef CAS.
- N. Koca, F. Karadeniz and H. S. Burdurlu, Food Chem., 2007, 100, 609–615 CrossRef CAS.
- G. Cabral-Malheiros, L. H. R. Hecktheuer, M. W. d. Canto and G. M. Balsamo, Cienc. Rural, 2010, 40, 654–660 CrossRef.
- M. E. Schmalko and S. M. Alzamora, Drying Technol., 2001, 19, 599–610 CrossRef CAS.
- R. O. Morawicki, M. E. Schmalko and R. G. Känzig, Braz. Arch. Biol. Technol., 1999, 42, 1–5 Search PubMed.
- K. Santos, Dissertação de mestrado, Universidade Federal do Paraná, 2004.
- J. I. R. De Corcuera, R. P. Cavalieri and J. R. Powers, in Encyclopedia of Agricultural, Food, and Biological Engineering, Marcel Dekker, Inc, New York, 2004, pp. 1–5 Search PubMed.
- K. Liburdi, I. Benucci and M. Esti, LWT–Food Sci. Technol., 2019, 99, 497–504 CrossRef CAS.
- F. Ren, C. A. Perussello, Z. Zhang, J. P. Kerry and B. K. Tiwari, LWT–Food Sci. Technol., 2018, 87, 102–111 CrossRef CAS.
- O. Fatibello-Filho and I. d. C. Vieira, Quim. Nova, 2002, 25, 455–464 CrossRef CAS.
- R. F. Molin, N. Dartora, A. C. P. Borges, I. L. Gonçalves, M. Di Luccio and A. T. Valduga, Braz. Arch. Biol. Technol., 2014, 57, 997–1003 CrossRef CAS.
- R. F. Molin, A. T. Valduga, M. Di Luccio, N. Dartora, A. J. Cichoski, M. Pistore and E. Rigo, Braz. Arch. Biol. Technol., 2011, 54, 337–345 CrossRef.
- C. Benincá, G. Kaskantzis and E. F. Zanoelo, Biosyst. Eng., 2009, 104, 503–509 CrossRef.
- J. d. C. Tomasi, G. G. d. Lima, I. Wendling, C. V. Helm, F. A. Hansel, R. C. B. d. Godoy, R. L. Grunennvaldt, T. O. d. Melo, M. M. Tomazzoli and C. Deschamps, Int. J. Food Eng., 2021, 17, 551–560 CrossRef CAS.
- J. de Cássia Tomasi, G. Goetten de Lima, M. Mendes Duarte, R. Catie Bueno de Godoy, I. Wendling, C. Vieira Helm, F. Augusto Hansel, R. Lúcia Grunennvaldt, M. Maciel Tomazzoli and C. Deschamps, J. Food Process. Preserv., 2021, 45, e15944 Search PubMed.
- M. Primo, G. Ceni, N. Marcon, O. Antunes, D. Oliveira, J. V. Oliveira and C. Dariva, J. Supercrit. Fluids, 2007, 43, 283–290 CrossRef CAS.
- G. C. Ceni, E. M. Baldissera, O. A. Antunes, J. V. Oliveira, C. Dariva and D. de Oliveira, Bioprocess Biosyst. Eng., 2008, 31, 541–550 CrossRef CAS PubMed.
- G. H. Nabechima, J. G. Provesi, J. d. O. Frescura, M. B. H. Mantelli, M. A. Vieira, E. S. Prudêncio and E. R. Amante, CyTA–J. Food, 2014, 12, 399–406 CrossRef CAS.
- R. Moran, Plant Physiol., 1982, 69, 1376–1381 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |