Open Access Article
Open Access ArticleImparting structural robustness of metal–organic cages based on oxo-dimolybdenum clusters†
Solène
Delaporte
 ab,
Isabel
Abánades Lázaro
ab,
Isabel
Abánades Lázaro
 a,
Javier
López-Cabrelles‡
a,
Javier
López-Cabrelles‡
 a,
Eleni C.
Mazarakioti
a,
Eleni C.
Mazarakioti
 a,
Sarah
Chebourou
a,
Iñigo J.
Vitórica-Yrezábal
c,
Mónica
Giménez-Marqués
a,
Sarah
Chebourou
a,
Iñigo J.
Vitórica-Yrezábal
c,
Mónica
Giménez-Marqués
 a and
Guillermo
Mínguez Espallargas
a and
Guillermo
Mínguez Espallargas
 *a
*a
aInstituto de Ciencia Molecular (ICMol), Universitat de València, Catedrático José Beltrán 2, 46980 Paterna, Spain. E-mail: guillermo.minguez@uv.es
bENS Paris-Saclay, Département de Chimie, 4 Av. des Sciences, 91190 Gif-sur-Yvette, France
cSchool of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK
First published on 22nd August 2023
Abstract
A family of robust and stable molybdenum-based metal–organic cages have been obtained based on the [Mo2O2(μ2-O)2]2+ secondary building unit. The resulting cages are decorated with different pyrdine derivatives that impart structural stability, resulting in the structural elucidation of the activated cage with single-crystal diffraction. The chemical robustness of the cage is also demonstrated by the post-synthetic modification of the cage, which allows the exchange of the pyridine derivatives without rupture of the cage.
Introduction
Metal–Organic Cages (MOCs) are a subclass of hybrid compounds composed of metal ions or clusters linked by organic ligands forming discrete porous molecular structures. Although MOCs have been known for over two decades,1 they have attracted recent interest due to their solubility, surpassing the processability of Metal–Organic Frameworks (MOFs), their higher dimensional equivalents. In fact, MOCs have shown promising applicability for gas storage,2,3 catalysis,4 molecular separation5 or biomedicine,6 among others. Efforts have been devoted to designing crystalline MOCs with permanent porosity,7 which can arise both from their inherent molecular pore space or from the packing of MOC molecules. Nevertheless, the study of MOCs remains challenging due to their poor thermal and hydrolytic stability that arises from the lack of extended connectivity.8 Thus, crystals of MOCs often degrade upon activation preventing the structural determination by single crystal X-ray diffraction. This lack of structural data of activated structures hinders precise analysis and understanding of their porosity. In this context, two different scenarios are generally found upon MOCs’ activation: (i) sample amorphization;8 and (ii) changes on crystallinity envisioned in PXRD patterns9 but unable to provide structural information. The retention of the crystal packing upon desolvation is extremely rare.10In most cases, the absence of structural data is substituted by Mass Spectrometry measurements, although this technique does not ensure the structural integrity of the material or the maintained packing. In some cases, this has been resolved with adsorption measurements, proving the inherent molecular pore space. Nevertheless, new approaches to obtain direct structural visualisation of the activated MOCs is needed for the development of the field.
Aiming to circumvent the lack of structural evidence of the activated samples, two alternative approaches have been developed. One of these is the assembly of MOCs via bidentate ligands,11–15 such as dabco ligands that coordinate to the exterior of the MOCs, connecting them in a 3D manner,16 analogous to dabco-pillared MOFs. Another approach is the post-synthetic assembly of MOCs into extended networks17 using secondary building units (clusters) to form analogous MOFs through rigid bridges.18–20 However, although these methods provide meaningful information, direct structural visualisation of the activated MOCs would be the ultimate tool to understand their porosity and gas adsorption properties.
Herein, we report the synthesis of a novel family of pyridine capped Mo2-benzene tricarboxylate MOCs, MUV-27 (MUV = Material of the University of Valencia), which can be obtained through a simple self-assembly synthetic route. Contrary to the previous Mo-MOCs,21–25 the MUV-27 family exhibit an original geometry that is not based on a paddlewheel unit, unstable in the case of Mo(II), but on a [Mo2O2(μ2-O)2]2+ core. In addition, this family of MOCs can be functionalized through the use of different auxiliary ligands (based on pyridine) which results, in one specific case, in an enhancement of the MOC structural stability, allowing the structural elucidation of the activated samples using single crystal X-ray diffraction. Thus, we unequivocally determine the retention of the cage structure upon removal of all solvents.
Results and discussion
Synthesis and structural study
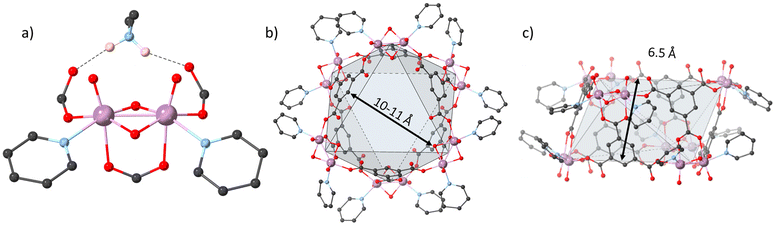
The solvothermal reaction in dimethylformamide (DMF) of molybdenum acetate [Mo2(OAc)4] and trimesic acid (H3BTC) in the presence of pyridine (py) results in a mixture of orange crystals and red gel from which single crystals could be isolated and purified. The hexagonal crystals crystallized in the monoclinic P21/c space group (see details in section S2 in the ESI†). Structure solution from single crystal diffraction data revealed a MOC, namely MUV-27-py. The secondary-building unit of this cage is formed by a dimolybdenum-oxo cluster [Mo2O2(μ2-O)2]2+, denoted [Mo2O4]2+, in which two bonded molybdenum atoms bearing oxo-ligands are connected by a non-planar double oxygen bridge (Fig. 1a). Six bimetallic Mo2-units are located at the vertices of an irregular octahedron of approximately 10–11 Å diameter (see Fig. 1b), and form two antiparallel equilateral triangles, which are approximately 6.5 Å apart (see Fig. 1c). The six remaining isosceles triangles faces are occupied by the BTC3− linkers that connect the Mo2-units together, with the overall structure displaying a triangular antiprismatic geometry.The bimetallic [Mo2O4]2+ fragment is a structural unit that has been commonly observed in Mo(V) chemistry. Indeed, numerous complexes containing [Mo2O4]2+ centres have been investigated,26 with bond-distances that are comparable with those obtained from SXRD data of MUV-27-py.27 The Mo–Mo distances (Table S3†) are in the range of 2.547(1)–2.555(1) Å, suggesting a single-bond between the two Mo(V) atoms. The Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O linkages exhibit distances in the range of 1.679(8)–1.690(8) Å and the oxo-bridges have the Mo–O distance between 1.917(6) and 1.941(7) Å, which are also in the typical range for other reported [Mo2O4]2+ clusters.27 To complete the distorted octahedral environment of the Mo centres, one pyridine is coordinated to each metal, with Mo–N bond lengths in the range 2.230(1)–2.248(9) Å. Three BTC3− are coordinated to the [Mo2O4]2+ unit, two of them having an equatorial monodentate coordination mode to each metal, and the third linker is bridging both Mo atoms, in an axial syn,syn bidentate manner.
O linkages exhibit distances in the range of 1.679(8)–1.690(8) Å and the oxo-bridges have the Mo–O distance between 1.917(6) and 1.941(7) Å, which are also in the typical range for other reported [Mo2O4]2+ clusters.27 To complete the distorted octahedral environment of the Mo centres, one pyridine is coordinated to each metal, with Mo–N bond lengths in the range 2.230(1)–2.248(9) Å. Three BTC3− are coordinated to the [Mo2O4]2+ unit, two of them having an equatorial monodentate coordination mode to each metal, and the third linker is bridging both Mo atoms, in an axial syn,syn bidentate manner.
Therefore, MUV-27-py contains one BTC3− linker per [Mo2O4]2+ unit and, as indicated by the structural measurements, the presence of dimethylammonium (DMA+) cation balances the charge. This countercation is located in the periphery of the cavity, with the NH2+ group forming N–H⋯O hydrogen bonds with the two non-coordinated oxygen atoms of the BTC3− linker (Fig. 1a). 1H NMR studies confirm the presence of 6 DMA+ per cage, suggesting that MUV-27-py is a −6 negatively charged MOC of general formula (DMA)6[MoV12O12(μ2-O)12(BTC)6(py)12], which is further confirmed with additional characterization including PXRD, FT-IR, acid-digested 1H NMR, TGA and UV-vis studies (see sections S3–S8 in the ESI†).
Functionalization of MUV-27
As commonly found in MOCs, activation of MUV-27-py causes a change in the crystal packing, as evidenced by X-ray powder diffraction (Fig. S16†). In fact, structural changes are also observed simply upon drying the materials (Fig. S8†). In order to circumvent these issues, the supramolecular packing structure was reinforced by introducing functionalised capping ligands during the synthesis. Thus, following the same synthetic procedure as for MUV-27-py, three functionalized MOCs have been synthesized using 4-picoline (py-CH3), 1,2-bis(4-pyridyl)ethane (bpy-C2) and 1,3-bis(4-pyridyl)propane (bpy-C3), resulting in three new MOCs denoted MUV-27-py-CH3, MUV-27-bpy-C2 and MUV-27-bpy-C3, respectively. These MOCs were also obtained as single-crystals, thus allowing their structural elucidation revealing, in the three cases, “Mo12” MOCs analogous to MUV-27-py (Fig. 2), with molecular formulas (DMA)6[MoV12O12(μ2-O)12(BTC)6(py-CH3)12], (DMA)6[MoV12O12(μ2-O)12(BTC)6(bpy-C2)6] and (DMA)6[MoV12O12(μ2-O)12(BTC)6(bpy-C3)6]. Remarkably, the cages with bipyridine ligands (bpy-C2 and bpy-C3) were not assembled together forming MOFs or amorphous supramolecular soft polymers as previously reported with other MOCs and similar bidentate ligands,12,15,20 but instead each ditopic ligand bridged two molybdenum atoms from neighbouring [Mo2O4]2+ fragments of the same cage (Fig. 2c and d). Importantly, activation of these three MOCs is accompanied by retention of crystallinity (Fig. S16†), but the crystal packing is not maintained likely caused from a re-organization of the MOCs, which unfortunately could not be followed by single crystal diffraction.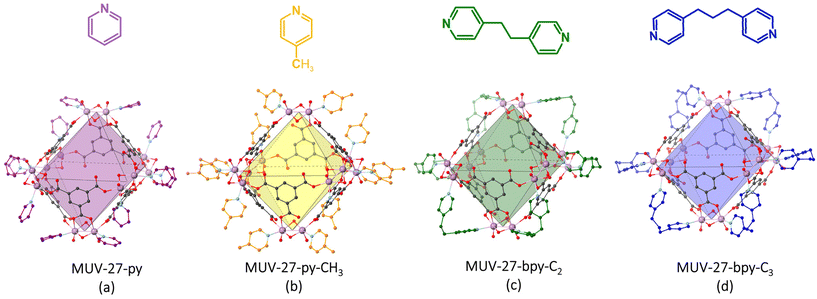 | ||
| Fig. 2 Structures of MUV-27 with different capping ligands (shown in different colours): (a) MUV-27-py; (b) MUV-27-py-CH3; (c) MUV-27-bpy-C2; (d) MUV-27-bpy-C3. | ||
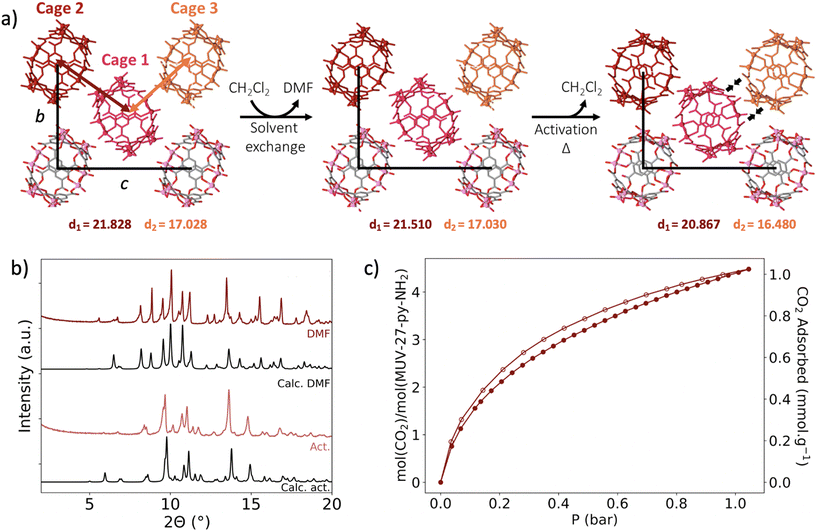
In order to overcome the structural arrangement observed in the previous MUV-27 materials upon activation, a ligand with potentially increased supramolecular interactions was used, namely 4-aminopyridine (py-NH2). Following a similar synthetic protocol results in homogeneous red rhombohedral crystals of MUV-27-py-NH2-DMF, which also forms Mo12 MOCs analogous to the previous compounds. However, in this case a DMF molecule is coordinated to a Mo atom substituting a py-NH2 ligand (Fig. S6†), leading to the molecular formula (DMA)6[MoV12O12(μ2-O)12(BTC)6(py-NH2)11(DMF)]. Close inspection of the crystal packing reveals the presence of N–H⋯O hydrogen bonds (2.073–2.694 Å range) between the amino-groups and oxygen atoms from surrounding cages (Fig. 3). Activation of MUV-27-py-NH2-DMF crystals was successfully achieved in a two-step process: (1) DMF solvent molecules were replaced by DCM via washing, direct soaking for 2 days and further washing, leading to single crystals that were analysed by X-ray single crystal diffraction. We could observe a significant decrease in the a and b unit cell parameters, although minor changes in the crystal packing had occurred (see Table 1 and Fig. 4). (2) Then, these crystals containing DCM molecules were dried at air for 24 hours, obtaining the activated compound, denoted MUV-27-py-NH2-act. Despite of the two-step transformation, suitable single crystals for X-ray diffraction of the activated product were isolated, allowing to obtain unequivocal evidence of the structural changes suffered in the activation process of MUV-27-py-NH2-DMF. Thus, while the cage's molecular structure is maintained with minor deformations, the crystal packing is affected significantly (see section S2.7 in the ESI†), with a shortening in the intermolecular distances as shown in Fig. 4a, which is translated in a reduction of the unit cell parameters (a, b and c). Nevertheless, the typical crystal collapse that is normally observed upon activation has been avoided, likely due to the presence of N–H⋯O hydrogen bonds between MOCs, that have reinforced the overall crystal stability.
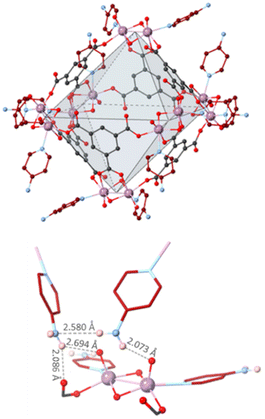 | ||
| Fig. 3 (top) Structure of MUV-27-py-NH2; (bottom) H-bonds between the [Mo2O4]2+ subunit of one MOC and 4-aminopyridine ligands of a different MOC. | ||
| Parameter | DMF | DCM | act |
|---|---|---|---|
| a/Å | 19.7115(3) | 19.3279(4) | 19.0877(7) |
| b/Å | 20.0909(2) | 19.6862(3) | 18.2843(6) |
| c/Å | 27.2180(4) | 27.2810(10) | 26.7480(7) |
| β/° | 110.345(2) | 109.113(3) | 108.715(3) |
| Space group | P21/n | P21/n | P21/n |
Subsequently, the porosity of the activated MUV-27-py-NH2-act (activation procedure as for the SCXRD measurements) was investigated through N2 and CO2 isotherms, at 77 K and 273 K respectively. While N2 adsorption was almost negligible (Fig. S28†), moderate CO2 sorption was observed (Fig. 4c), with ca. 4 molecules of CO2 per cage, demonstrating the retention of porosity upon activation. Interestingly, the other MOCs also show some CO2 sorption capacity (Fig. S29†), indicating that the intrinsic porosity of the cages is accessible upon activation.
Finally, an important aspect for the use of MOCs is their robustness upon application of chemical stimuli. In this sense, despite the ample number of cages that has been reported, there is only one example of a Rh-based cage has been shown to be robust enough to make composites.28 Thus, we examined the robustness of these Mo-based cages in order to enhance the chemical variety of stable MOCs. For this, we evaluated the robustness and effects of post-synthetic modification (PSM)28–33 in the MUV-27 family of cages, by preparing a suspension of the insoluble cages in DMF with the solubilized pyridine capping ligands and heating at 50 °C upon stirring overnight. In particular, the transformation of MUV-27-py and MUV-27-py-CH3 to all the aforementioned materials was successful, leading to crystalline materials with a complete exchange of the pyridine capping ligands confirmed by 1H NMR, PXRD and FT-IR (section S11 in the ESI†). However, when using other MUV-27 cages as synthetic platforms (i.e.MUV-27-bpy-C2, MUV-27-bpy-C3 and MUV-27-NH2), only a partial exchange of the ligands was observed, possibly due to the bis-coordination mode of the capping ligands in MUV-27-bpy-C2, MUV-27-bpy-C3 and to the H-bonding of the NH2 group in MUV-27-py-NH2. This successful PSMs prove the chemical robustness of the cages and could also be used as a platform multi-functionalised MOCs.
Conclusions
A novel family of metal–organic cages composed of 12 Mo(V) centres, denoted MUV-27, has been prepared bearing a novel secondary building unit in MOC chemistry, [Mo2O2(μ2-O)2]2+. The general synthetic approach is demonstrated with the preparation of five different compounds possessing different functional groups. Pure and crystalline cages can be easily obtained through a self-assembly synthesis and functionalized with pyridine derivatives. Importantly, the introduction of –NH2 groups enhances the structural stability of the resultant MOC through hydrogen bonding between cages, resulting in crystals suitable for structural determination after activation. This important aspect has been elusive despite the large interest in this type of compounds. Specifically, we unequivocally demonstrate that the Mo12MUV-27-py-NH2 MOC is structurally robust upon activation, despite the effective shrinkage of the crystal packing. We have also confirmed the possibility to conduct post-synthetic modifications on MUV-27 cages, which is encouraging in the prospect of future experiments to tune the functionalities of MUV-27 and highlights the robustness of the cages.These results evidence the plausible retainment of structural integrity in MOCs which enables direct determination of the crystal structure. Therefore, we believe that the methods here reported –enhancing stability through inter-cages interactions between functionalised ligands– could have applicability in other MOC systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work has been supported by the European Union (ERC-2016-CoG 724681-S-CAGE), grants PID2020-117177GB-I00, PID2020-118564GA-I00 and CEX2019-000919-M, funded by MCIN/AEI/10.13039/501100011033, and the Generalitat Valenciana (CIPROM/2022/48). This study forms part of the Advanced Materials program and was supported by MCIN with funding from European Union NextGenerationEU (PRTR-C17.I1) and by Generalitat Valenciana (project MAF/2022/31). M. G.-M, and I. A.-L. thank MICINN for a Ramón y Cajal fellowship (RYC2019-027902-I), a Juan de la Cierva Incorporación fellowship (IJC2020-044374-I), respectively. J. L.-C. acknowledges the Universitat de València for an “Atracció de Talent” grant.References
- D. Zhang, T. K. Ronson, Y.-Q. Zou and J. R. Nitschke, Nat. Rev. Chem., 2021, 5, 168–182 CrossRef CAS PubMed.
- A. C. Sudik, A. R. Millward, N. W. Ockwig, A. P. Côté, J. Kim and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 7110–7118 CrossRef CAS PubMed.
- S. Furukawa, N. Horike, M. Kondo, Y. Hijikata, A. Carné-Sánchez, P. Larpent, N. Louvain, S. Diring, H. Sato, R. Matsuda, R. Kawano and S. Kitagawa, Inorg. Chem., 2016, 55, 10843–10846 CrossRef CAS PubMed.
- L. Chen, T. Yang, H. Cui, T. Cai, L. Zhang and C.-Y. Su, J. Mater. Chem. A, 2015, 3, 20201–20209 RSC.
- D. Zhang, T. K. Ronson, Y.-Q. Zou and J. R. Nitschke, Nat. Rev. Chem., 2021, 5, 168–182 CrossRef CAS PubMed.
- H. Sepehrpour, W. Fu, Y. Sun and P. J. Stang, J. Am. Chem. Soc., 2019, 141, 14005–14020 CrossRef CAS PubMed.
- A. J. Gosselin, C. A. Rowland and E. D. Bloch, Chem. Rev., 2020, 120, 8987–9014 CrossRef CAS PubMed.
- S. Mollick, S. Fajal, S. Mukherjee and S. K. Ghosh, Chem. – Asian J., 2019, 14, 3096–3108 CrossRef CAS PubMed.
- B. Lerma-Berlanga, J. Castells-Gil, C. R. Ganivet, N. Almora-Barrios, J. González-Platas, O. Fabelo, N. M. Padial and C. Martí-Gastaldo, J. Am. Chem. Soc., 2021, 143, 21195–21199 CrossRef CAS PubMed.
- (a) M. Zhou, G. Liu, Z. Ju, K. Su, S. Du, Y. Tan and D. Yuan, Cryst. Growth Des., 2020, 20, 4127–4134 CrossRef CAS; (b) C. A. Rowland, G. R. Lorzing, A. J. Gosselin, B. A. Trump, G. P. A. Yap, C. M. Brown and E. D. Bloch, J. Am. Chem. Soc., 2018, 140, 11153–11157 CrossRef CAS PubMed.
- H.-J. Jung, D.-H. Moon and H.-P. Chun, Bull. Korean Chem. Soc., 2011, 32, 2489–2492 CrossRef CAS.
- A. Carné-Sánchez, G. A. Craig, P. Larpent, T. Hirose, M. Higuchi, S. Kitagawa, K. Matsuda, K. Urayama and S. Furukawa, Nat. Commun., 2018, 9, 2506 CrossRef PubMed.
- A. Legrand, L.-H. Liu, P. Royla, T. Aoyama, G. A. Craig, A. Carné-Sánchez, K. Urayama, J. J. Weigand, C.-H. Lin and S. Furukawa, J. Am. Chem. Soc., 2021, 143, 3562–3570 CrossRef CAS PubMed.
- A. Carné-Sánchez, G. A. Craig, P. Larpent, V. Guillerm, K. Urayama, D. Maspoch and S. Furukawa, Angew. Chem., Int. Ed., 2019, 58, 6347–6350 CrossRef PubMed.
- Z. Wang, C. V. Santos, A. Legrand, F. Haase, Y. Hara, K. Kanamori, T. Aoyama, K. Urayama, C. M. Doherty, G. J. Smales, B. R. Pauw, Y. J. Colón and S. Furukawa, Chem. Sci., 2021, 12, 12556–12563 RSC.
- G. R. Lorzing, A. J. Gosselin, B. A. Trump, A. H. P. York, A. Sturluson, C. A. Rowland, G. P. A. Yap, C. M. Brown, C. M. Simon and E. D. Bloch, J. Am. Chem. Soc., 2019, 141, 12128–12138 CrossRef CAS PubMed.
- A. Khobotov-Bakishev, L. Hernández-López, C. von Baeckmann, J. Albalad, A. Carné-Sánchez and D. Maspoch, Adv. Sci., 2022, 9, 2104753 CrossRef CAS PubMed.
- A. Khobotov-Bakishev, C. von Baeckmann, B. Ortín-Rubio, L. Hernández-López, A. Cortés-Martínez, J. Martínez-Esaín, F. Gándara, J. Juanhuix, A. E. Platero-Prats, J. Faraudo, A. Carné-Sánchez and D. Maspoch, J. Am. Chem. Soc., 2022, 144, 15745–15753 CrossRef CAS PubMed.
- T. Grancha, A. Carné-Sánchez, F. Zarekarizi, L. Hernández-López, J. Albalad, A. Khobotov, V. Guillerm, A. Morsali, J. Juanhuix, F. Gándara, I. Imaz and D. Maspoch, Angew. Chem., Int. Ed., 2021, 60, 5729–5733 CrossRef CAS PubMed.
- J.-R. Li, D. J. Timmons and H.-C. Zhou, J. Am. Chem. Soc., 2009, 131, 6368–6369 CrossRef CAS PubMed.
- Y. Ke, D. J. Collins and H.-C. Zhou, Inorg. Chem., 2005, 44, 4154–4156 CrossRef CAS PubMed.
- J.-R. Li, A. A. Yakovenko, W. Lu, D. J. Timmons, W. Zhuang, D. Yuan and H.-C. Zhou, J. Am. Chem. Soc., 2010, 132, 17599–17610 CrossRef CAS PubMed.
- F. A. Cotton, L. M. Daniels, C. Lin, C. A. Murillo and C. A. Murillo, Chem. Commun., 1999, 841–842 RSC.
- G. R. Lorzing, A. J. Gosselin, B. S. Lindner, R. Bhattacharjee, G. P. A. Yap, S. Caratzoulas and E. D. Bloch, Chem. Commun., 2019, 55, 9527–9530 RSC.
- M. M. Deegan and E. D. Bloch, Dalton Trans., 2021, 50, 3127–3131 RSC.
- H. K. Chae, W. G. Klemperer and T. A. Marquart, Coord. Chem. Rev., 1993, 128, 209–224 CrossRef CAS.
- B. Modec and J. Zubieta, Materials, 2010, 3, 150–157 CrossRef CAS.
- A. Carné-Sánchez, J. Albalad, T. Grancha, I. Imaz, J. Juanhuix, P. Larpent, S. Furukawa and D. Maspoch, J. Am. Chem. Soc., 2019, 141(9), 4094–4102 CrossRef PubMed.
- J. Liu, Z. Wang, P. Cheng, M. J. Zaworotko, Y. Chen and Z. Zhang, Nat. Rev. Chem., 2022, 6, 339–356 CrossRef PubMed.
- E. Sánchez-González, M. Y. Tsang, J. Troyano, G. A. Craig and S. Furukawa, Chem. Soc. Rev., 2022, 51, 4876–4889 RSC.
- M. L. Schneider, O. M. Linder-Patton and W. M. Bloch, Chem. Commun., 2020, 56, 12969–12972 RSC.
- M. T. Yong, O. M. Linder-Patton and W. M. Bloch, Inorg. Chem., 2022, 61, 12863–12869 CrossRef CAS PubMed.
- V. Brega, M. Zeller, Y. He, H. Peter Lu and J. K. Klosterman, Chem. Commun., 2015, 51, 5077–5080 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Containing experimental conditions and detailed characterisation. CCDC 2258028–2258034. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt02482b |
| ‡ Current address: Institute for Integrated Cell–Material Sciences (WPI-iCeMS), Kyoto University Yoshida, Sakyo-ku, Kyoto 606-8501, Japan. |
| This journal is © The Royal Society of Chemistry 2023 |