Open Access Article
Open Access ArticlePhotoinduced large magnetic change at room temperature and radical-quenched spin glass in a cyanide-bridged MnII–FeIII compound†
Li-Zhen
Cai
,
Xiao-Qing
Yu
,
Ming-Sheng
Wang
 * and
Guo-Cong
Guo
* and
Guo-Cong
Guo
 *
*
State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: gcguo@fjirsm.ac.cn; mswang@fjirsm.ac.cn
First published on 24th October 2023
Abstract
By the coordination assembly of a redox photoactive functional motif and a cyanide-bridged moiety, a cyanide-bridged MnII–FeIII compound with large photoinduced magnetic change at room-temperature due to photoinduced electron transfer was obtanied. This compound also shows unprecedented radical-quenched spin glass in molecule based magnets.
Photoresponsive magnetic materials, which change magnetism after the absorption of light,1 have attracted intense interest in the past decades for their potential applications in memory and switches. Since the discovery of the first photomagnetic Prussian blue analog,2 cyanide-bridged coordination compounds have become some of the most promising and most widely studied photomagnets.3 They are usually obtained by photoswitching of spin numbers and/or exchange couplings based on various mechanisms,4–6 such as metal-to-metal charge transfer (MMCT),1,7–9 metal-to-ligand charge transfer (MLCT),10–12 ligand-to-metal charge transfer (LMCT),13–15 spin crossover of octahedrally coordinated d4–d7 transition metal,16–20 isomerization of photochromic molecules,21 and so on. Two critical problems have to be solved for the further development of such photomagnets. One is that the number of optional paramagnetic metals is very limited, although the selection of appropriate cyanide-bridged paramagnetic metals can produce the desired photomagnetism in the light of the well-known orbital-overlap principle.22 The other is that most known examples change magnetism under illumination at cryogenic temperatures. Taking advantage of the light-excitation-in-the-thermal-hysteresis-loop technique, some cyanide-bridged coordination compounds display photomagnetism around room temperature (RT).23,24 Even so, cyanide-bridged coordination compounds with thermal hysteresis loops around RT are rather rare. Therefore, obtaining room-temperature cyanide-bridged photomagnets remains a challenge.
Photochromic organic compounds usually change their molecular structures under illumination.25 Incorporating them into spin systems as ligands or guest molecules/ions has been demonstrated to be an effective approach to modulating spin numbers and/or exchange couplings using light.21,26–34 Their flexibilities of structural modification can enrich the cyanide-bridged photomagnetic systems, avoiding the limitation of optional paramagnetic metals. Their other important characteristic is that the photon mode commonly operates around RT. The presence of photochromic organic units in cyanide-bridged compounds very likely increases the photoswitching temperature for magnetism.
The most commonly used photochromic organic units in known photomagnetic compounds are those undergoing photo-induced cis–trans isomerization21,26,27 or pericyclic reactions.28–30,33,34 They require an adequate “breathing” space to allow photochromic reactions to occur due to their large structural change, otherwise the reactions would be prohibited or corruption of the crystal structure would occur.6,32 In contrast, only minor structural distortion occurs after photocoloration for most electron-transfer (redox) photochromic organic species.35–37 They generally generate radicals upon irradiation. It has been reported that radicals can antiferromagnetically or ferromagnetically couple with paramagnetic metals.38,39 If redox photochromic organic units were incorporated into paramagnetic metal complexes as ligands, the resulting compounds could display photomagnetism at RT in the solid state.40 To our knowledge, the photomagnetic effects of cyanide-bridged coordination compounds with redox photochromic organic units as ligands or guest ions have not been reported yet.
Previously, we reported that several photochromic halozinc coordination compounds of N-methyl-4,4′-bipyridinium cation (MQ+) exhibit photoinduced halo atom to MQ+ electron transfer with the formation of MQ˙ radicals at RT.41 Inspired by this, by introducing the redox photoactive MQ+ cation into a cyanide-bridged MnII–FeIII inorganic framework as a photochromic organic functional motif,42 we successfully obtained a photoactive semiconductor, [{MnII–HS(MQ)2}{FeIII–LS(CN)6}]Cl (1 HS = high spin, LS = low spin), which showed a clear change of color and electrical properties with the generation of stable radicals after irradiation at RT.43 Given the potential for photogenerated radicals to magnetically couple with paramagnetic centers and alter magnetic behavior,44 we investigated the magnetic properties of compounds 1. Our findings indicate that compound 1 exhibits a high degree of spin frustration, displaying antiferromagnetic coupling at RT and a spin canted antiferromagnetic glassy state at low temperatures before irradiation. After irradiation, compound 1 demonstrated photoinduced large magnetic change at room temperature and an unprecedented radical-quenched spin glass.
Single crystals of 1 were obtained according to our reported procedure and its crystal structure has been thoroughly defined.43 So only the magnetic related structural information is discussed in detail here. The crystal structure of 1 features a 3-D supramolecular network, where 2-D inorganic layers are linked by π⋯π interactions between two adjacent MQ+ ligands (Fig. 1). In the layer, each FeIII atom coordinates to six cyano groups, while each MnII atom is ligated by two MQ+ ligands and four cyano groups from four [Fe(CN)6]3− units. Thus, two cyano groups of each [Fe(CN)6]3− unit are mono-coordinated. This result agrees with the conclusion drawn from the IR spectra. The 2-D inorganic layer is similar to that in RbMnII[FeIII(CN)6]·H2O with a 3-D inorganic framework (Fig. 1).45,46 However, the layers in 1 are stacked in parallel along the c axis without the displacement observed in the structure of RbMnII[FeIII(CN)6]·H2O. The nearest metal atom from adjacent layers for each FeIII atom is also the FeIII atom, giving a Fe⋯Fe separation of ∼13.50 Å. The shortest Fe⋯Mn, Fe⋯Fe and Mn⋯Mn distances in each layer are ∼5.29 Å, 7.40 Å and 7.56 Å, respectively, which are comparable with those found in RbMnII[FeIII(CN)6]·H2O.
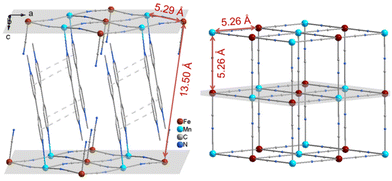 | ||
| Fig. 1 Comparison of crystal structures between 1 (left, H atoms being omitted) and RbMnII[FeIII(CN)6]·H2O (right, only Mn[Fe(CN)6]− is shown). The dashed lines depict π⋯π stacking interactions. | ||
As reported in our previous work,43 compound 1 changed its color from brown (1A) to black (1B) upon irradiation with a Xe lamp (∼50 mw cm−2) at RT. Electron transfer from Cl/CN to MQ+ concomitant with the generation of MQ˙ radicals was proposed to interpret the coloration behavior. Other mechanisms including the photo-induced release of cyanide ligands similar to octacyanomolybdate47 or electron transfer from Fe(III) to Mn(II) in RbMnII[FeIII(CN)6]·H2O were excluded because no obvious changes were observed in the PXRD pattern (Fig. S1†) or IR spectrum (Fig. S2†) after irradiation.
To explore the effect of photogenerated radicals on magnetic properties, the variable-temperature magnetic susceptibilities before and after irradiation were measured under a field of 1000 Oe in the temperature range of 2–300 K. As shown in Fig. 2a, the χMT value of 1A at 300 K is 4.85 cm3 K mol−1 per MnFe unit, which is slightly larger than the spin-only value of 4.75 cm3 K mol−1 for one low-spin FeIII (SFe = 1/2, assuming gFe = 2.00) and one high-spin MnII atom (SMn = 5/2, assuming gMn = 2.00). As the temperature is lowered, the χMT value decreases smoothly to a minimum of 4.36 cm3 K mol−1 at 28.3 K, and then abruptly increases to reach a maximum of 11.31 cm3 K mol−1 at about 4.95 K. Finally, the χMT value decreases again to 7.63 cm3 K mol−1 at 2 K. This temperature-dependent behavior is typical for ferrimagnets as a result of an antiferromagnetic interaction.45,46 The χMvs. T data between 50 and 300 K can be fitted to the Curie–Weiss law (χM = C/(T–θ)) with C = 4.89 cm3 K mol−1 and θ = −5.25 K (Fig. S3†). The small negative Weiss constant indicates a weak antiferromagnetic interaction between FeIII (t52geg0) and MnII (t32geg2) through the cyanide bridge, which is in accord with the theoretical inference based on the orbital-overlap principle.22 The sharp peak of χMT at low temperature implies the long-range magnetic ordering below 5.0 K and the sharp decrease in χMT could possibly be attributed to the field saturation of the magnetic moment.
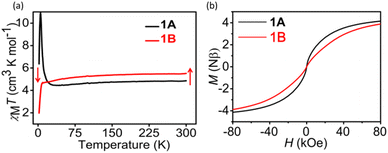 | ||
| Fig. 2 (a) χMT versus T under H = 1000 Oe for 1A and 1B; (b) hysteresis loop of 1A and 1B measured at 2.0 K. | ||
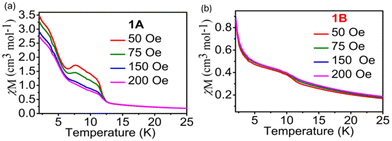
The variable-temperature alternating current (ac) susceptibility was recorded under zero dc to further confirm the magentic phase transition behavior. Apparent frequency-dependent peaks in the in-phase (χ′) and out-of-phase (χ′′) component are observed below 10 K. The peaks of the χ′′ component are located at 3.18 K and 3.59 K for 10 and 1000 Hz, respectively, indicating the zero-field slow relaxation of magnetization. The shift of the peak temperature (Tp) in the plots of χ′′–T was calculated to be 0.05 using the Mydosh parameter ϕ = (ΔTp/Tp)/(Δlog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f), falling in the normal range for spin glass (ϕ < 0.08) (Fig. 3a). In addition, the temperature dependences of FCM (field-cooled magnetization) and ZFCM (zero-field-cooled magnetization) under an applied field of 5 Oe show an obvious bifurcation below 5.0 K, further suggesting the blocking of the magnetization caused by spin glass behavior (Fig. S4†).
f), falling in the normal range for spin glass (ϕ < 0.08) (Fig. 3a). In addition, the temperature dependences of FCM (field-cooled magnetization) and ZFCM (zero-field-cooled magnetization) under an applied field of 5 Oe show an obvious bifurcation below 5.0 K, further suggesting the blocking of the magnetization caused by spin glass behavior (Fig. S4†).
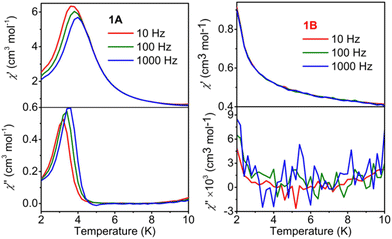 | ||
| Fig. 3 χ′ and χ′′ versus T for 1A (a) and 1B (b) in 0 Oe dc and 2.5 Oe ac fields at various ac frequencies (10, 100, and 1000 Hz). | ||
To check the magnetic hysteretic behavior of 1A suggested by the irreversibility of the ZFC/FC curves below 10 K, the hysteresis loop at 2.0 K was measured. As can be seen, a hysteresis loop is observed with a coercive field (Hc) of 24 Oe and a remnant magnetization (Mr) of 0.014Nβ (Fig. 2b and Fig. S5a†), further indicating the presence of spontaneous magnetization, consistent with the spin-glass antiferromagnetic behavior. Furthermore, the field dependence of the magnetization curve of 1A, measured at 2 K, illustrates that the saturation of magnetization is completed at ∼80 kOe (Fig. S5b†). At this point, the magnetization of ∼4.15 Nβ is very close to the expected value of 4 Nβ for a FeIII–LS–MnII–HS system with antiferromagnetic coupling (Ms = gMnSMnNβ–gFeSFeNβ, assuming gFe = gMn = 2.0). The M(T) data measured at different applied fields in FC mode (Fig. 4a) exhibit a fine transition peak at about 4.5 K and a broad transition peak between 7.5 K and 12.5 K. Furthermore, the magnetization curves become flatter with increasing applied magnetizing fields, and the obvious transition peak almost disappears at 200 Oe (Fig. 4a) due to saturation of magnetization. All these results indicate that compound 1A has a spin glass behavior below 5 K. These observations align with the alternating current (ac) data, which indicate spin glass ordering occurring at approximately 4.5 K (Fig. 3a).
Notably, the black color of the photoproduct is undesirable for the penetration of light to the interior of crystals.48,49 So, we chose a YAG laser (4.1 mJ cm−2) instead of the Xe lamp as a light source to obtain a colored sample for the magnetic study. The wavelength of the output light from the laser was monitored to the optimal photoresponsive wavelength of 1A, i.e. 320 nm. As shown in Fig. 2a, after irradiation, the χMT value increased to 5.49 cm3 K mol−1and was 13.2% larger than that before irradiation, implying that new spins were generated after irradiation.
The photomagnetism of RbMnII[FeIII(CN)6]·H2O was caused by photo-induced MnII-to-FeIII charge transfer.45,46 As mentioned above, the photoinduced coloration of 1 was caused by Cl/CN → MQ+ electron transfer, instead of MnII-to-FeIII charge transfer, CN → FeIII charge-transfer, d–d transition of FeIII, and MnII-to-MQ+ electron transfer. The increase of the χMT value for 1 upon irradiation can further rule out the occurrence of MnII-to-FeIII charge transfer and MnII-to-MQ+ electron transfer. The reason is that the χMT value would be largely reduced with the formation of diamagnetic FeII–LS (t2g6eg0), if the MnII-to-FeIII charge transfer happens. In case of MnII-to-MQ+ charge transfer, the χMT value would be almost retained for MnIII–MQ˙ ferromagnetic coupling or reduced for MnIII–MQ˙ antiferromagnetic coupling. All in all, the new spins are ascribed to the photogenerated radicals.
As shown in Fig. 2a, the χMT value for 1B gradually decreased as the temperature was lowered, and went down sharply below 10 K to reach a minimum of 1.26 cm3 K mol−1 at 2 K. The χMT values for 1B are larger than those for 1A in the range of 25–300 K but smaller than those for 1A below 25 K. The χMvs. T data between 50 and 300 K can be fitted to the Curie–Weiss law with C = 5.56 cm3 K mol−1 and θ = −5.65 K (Fig. S3b†). This θ value is smaller than that of 1A, which means that the photogenerated radicals antiferromagnetically couple with metals. Koga et al. reported that MnII can antiferromagnetically couple with carbene radicals through the pyridyl bridge.50 For 1B, the metal–radical antiferromagnetical coupling should occur between MnII and MQ˙. The M vs. H curve for 1B becomes flat and is moved down compared with that of 1A, but M does not increase linearly to H as occurs for a typical antiferromagnet (Fig. S5b†). So, the photoproduct of 1 is a ferrimagnet in nature.
The hysteresis loop at 2.0 K and variable-temperature alternating current (ac) susceptibility measurements were also performed for 1B under zero dc. No hysteresis loop (Fig. S5a†), in-phase (χ′) and out-of-phase (χ′′) signals (Fig. 3b) as well as field-dependent transition peaks (Fig. 4b) can be found in the measured temperature range. All these results imply that the spin glass behavior was quenched by room temperature photoinduced coloration in 1B. For 1A, the slow magnetic relaxation and hysteresis loop are attributed to the spin glass behavior, which is most likely because of the disorder of the MQ ligands. However, the structural disorder should still exist in 1B because no obvious structural change is observed after irradiation. The quenching of spin glass behavior for 1B may be attributed to the generation of radicals, which have been reported to quench slow magnetic relaxation in molecule-based magnets.51
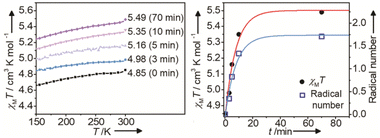
Ideally, one molecule of 1 can generate two radicals after irradiation. The χMT values at 300 K for 1A and 1B have a difference of 0.64 cm3 K mol−1, which theoretically corresponds to ∼1.71 radicals per [{MnII–HS(MQ)2}{FeIII–LS(CN)6}]Cl molecule (Sradical = 1/2, gradical = 2.0). We conducted a time-dependent magnetic susceptibility measurement at a field of 1000 Oe under YAG laser irradiation at 300 K for 1. As shown in Fig. 5, with the increase in irradiation time, the curve of the radical number showed a trend similar to that of the χMT value. This reveals that the photomagnetism of 1 originates from the photogeneration of radicals, and the change in the χMT value is positively related to the number of radicals.
Conclusions
In summary, we successfully found a cyanide-bridged MnII–FeIII compound with room-temperature photomagnetism, by incorporating the redox photoactive ligand MQ+ into a 2-D cyanide-bridged inorganic framework. This photomagnet undergoes photoinduced electron transfer and yields stable radicals after irradiation at RT. The antiferromagnetic coupling between MnII and the photogenerated radical significantly changes the magnetism. Interestingly, this photomagnet shows spin glass behavior before irradiation, which can be turned off by room temperature light irradiation, showing the first example of radical-quenched spin glass in molecule based magnets. The new design strategy described in this work may help to obtain room temperature cyanide-bridged photomagnets with on/off slow magnetic relaxation behavior.Author contributions
G.-C. Guo and M.-S. Wang conceived and supervised the project. L.-Z. Cai and X.-Q. Yu planned and implemented the synthesis and characterization of materials, and analysed data. L.-Z. Cai and M.-S. Wang wrote the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support by the National Key Research and Development Program Ministry of Science and Technology (2021YFB3801604) and the National Natural Science Foundation (22371279, U21A20508, 22073102, 21827813, 21871264) of China.References
- W. Huang, X. Ma, O. Sato and D. Wu, Chem. Soc. Rev., 2021, 50, 6832 RSC.
- O. Sato, T. Iyoda, A. Fujishima and K. Hashimoto, Science, 1996, 272, 704 CrossRef CAS PubMed.
- S. Ohkoshi and H. Tokoro, Acc. Chem. Res., 2012, 45, 1749 CrossRef CAS PubMed.
- Y. Avila, P. Acevedo-Pena, L. Reguera and E. Reguera, Coord. Chem. Rev., 2022, 453, 214274 CrossRef CAS.
- D. Aguila, Y. Prado, E. S. Koumousi, C. Mathoniere and R. Clerac, Chem. Soc. Rev., 2016, 45, 203 RSC.
- J. H. Wang, Z. Y. Li, M. Yamashita and X. H. Bu, Coord. Chem. Rev., 2021, 428, 213617 CrossRef CAS.
- T. Liu, Y.-J. Zhang, S. Kanegawa and O. Sato, J. Am. Chem. Soc., 2010, 132, 8250 CrossRef CAS PubMed.
- E. S. Koumousi, I.-R. Jeon, Q. Gao, P. Dechambenoit, D. N. Woodruff, P. Merzeau, L. Buisson, X. Jia, D. Li, F. Volatron, C. Mathonière and R. Clérac, J. Am. Chem. Soc., 2014, 136, 15461 CrossRef CAS PubMed.
- J.-X. Hu, L. Luo, X.-J. Lv, L. Liu, Q. Liu, Y.-K. Yang, C.-Y. Duan, Y. Luo and T. Liu, Angew. Chem., Int. Ed., 2017, 56, 7663 CrossRef CAS PubMed.
- L. Reguera, Y. Avila and E. Reguera, Coord. Chem. Rev., 2021, 434, 213764 CrossRef CAS.
- J.-D. Cafun, G. Champion, M.-A. Arrio, C. C. Moulin and A. Bleuzen, J. Am. Chem. Soc., 2010, 132, 11552 CrossRef CAS PubMed.
- T. Liu, H. Zheng, S. Kang, Y. Shiota, S. Hayami, M. Mito, O. Sato, K. Yoshizawa, S. Kanegawa and C. Duan, Nat. Commun., 2013, 4, 2826 CrossRef.
- G. Li, T. Akitsu, O. Sato and Y. Einaga, J. Am. Chem. Soc., 2003, 125, 12396 CrossRef CAS PubMed.
- H. Tokoro, T. Matsuda, T. Nuida, Y. Moritomo, K. Ohoyama, E. D. L. Dangui, K. Boukheddaden and S.-i. Ohkoshi, Chem. Mater., 2008, 20, 423 CrossRef CAS.
- H. Svendsen, M. R. V. Jørgensen, J. Overgaard, Y.-S. Chen, G. Chastanet, J.-F. Létard, K. Kato, M. Takata and B. B. Iversen, Inorg. Chem., 2011, 50, 10974 CrossRef CAS PubMed.
- L. Zhao, Y.-S. Meng, Q. Liu, O. Sato, Q. Shi, K. Oshio and T. Liu, Nat. Chem., 2021, 13, 698 CrossRef CAS PubMed.
- S. Ohkoshi, K. Imoto, Y. Tsunobuchi, S. Takano and H. Tokoro, Nat. Chem., 2011, 3, 564 CrossRef CAS PubMed.
- S. Chorazy, T. Charytanowicz, D. Pinkowicz, J. H. Wang, K. Nakabayashi, S. Klimke, F. Renz, S. Ohkoshi and B. Sieklucka, Angew. Chem., Int. Ed., 2020, 59, 15741 CrossRef CAS PubMed.
- M. Reczynski, D. Pinkowicz, K. Nakabayashi, C. Nather, J. Stanek, M. Koziel, J. Kalinowska-Tluscik, B. Sieklucka, S. I. Ohkoshi and B. Nowicka, Angew. Chem., Int. Ed., 2021, 60, 2330 CrossRef CAS PubMed.
- Q. Liu, J.-X. Hu, Y.-S. Meng, W.-J. Jiang, J.-L. Wang, W. Wen, Q. Wu, H.-L. Zhu, L. Zhao and T. Liu, Angew. Chem. Int. Ed., 2021, 60, 10537 CrossRef CAS PubMed.
- K.-P. Xie, Z.-Y. Ruan, B.-H. Lyu, X.-X. Chen, X.-W. Zhang, G.-Z. Huang, Y.-C. Chen, Z.-P. Ni and M.-L. Tong, Angew. Chem., Int. Ed., 2021, 60, 27144 CrossRef CAS PubMed.
- T. Mallah, S. Thiebaut, M. Verdaguer and P. Veillet, Science, 1993, 262, 1554 CrossRef CAS PubMed.
- S. Koshihara, Y. Tokura, K. Takeda and T. Koda, Phys. Rev. Lett., 1992, 68, 1148 CrossRef CAS PubMed.
- S. Bonhommeau, G. Molnár, A. Galet, A. Zwick, J.-A. Real, J. J. McGarvey and A. Bousseksou, Angew. Chem., Int. Ed., 2005, 44, 4069 CrossRef CAS PubMed.
- H. Bouas-Laurent and H. Dürr, Pure Appl. Chem., 2001, 73, 639 CrossRef CAS.
- M. Estrader, J. S. Uber, L. A. Barrios, J. Garcia, P. LloydWilliams, O. Roubeau, S. J. Teat and G. Aromí, Angew. Chem., Int. Ed., 2017, 56, 15622 CrossRef CAS PubMed.
- M. M. Paquette, D. Plaul, A. Kurimoto, B. O. Patrick and N. L. Frank, J. Am. Chem. Soc., 2018, 140, 14990 CrossRef CAS PubMed.
- N. Kojima, M. Okubo, H. Shimizu and M. Enomoto, Coord. Chem. Rev., 2007, 251, 2665 CrossRef CAS.
- K. Matsuda, Pure Appl. Chem., 2008, 80, 555 CrossRef CAS.
- M. Morimoto, H. Miyasaka, M. Yamashita and M. Irie, J. Am. Chem. Soc., 2009, 131, 9823 CrossRef CAS PubMed.
- S. Venkataramani, U. Jana, M. Dommaschk, F. D. Sönnichsen, F. Tuczek and R. Herges, Science, 2011, 331, 445 CrossRef CAS PubMed.
- K. Takahashi, Y. Hasegawa, R. Sakamoto, M. Nishikawa, S. Kume, E. Nishibori and H. Nishihara, Inorg. Chem., 2012, 51, 5188 CrossRef CAS PubMed.
- B. Rösner, M. Milek, A. Witt, B. Gobaut, P. Torelli, R. H. Fink and M. M. Khusniyarov, Angew. Chem., Int. Ed., 2015, 54, 12976 CrossRef PubMed.
- K. S. Kumar and M. Ruben, Coord. Chem. Rev., 2017, 346, 176 CrossRef.
- T. M. Bockman and J. K. Kochi, J. Org. Chem., 1990, 55, 4127 CrossRef CAS.
- G. Xu, G.-C. Guo, M.-S. Wang, Z.-J. Zhang, W.-T. Chen and J.-S. Huang, Angew. Chem., Int. Ed., 2007, 46, 3249 CrossRef CAS PubMed.
- Q.-K. Liu, J.-P. Ma and Y.-B. Dong, J. Am. Chem. Soc., 2010, 132, 7005 CrossRef CAS PubMed.
- J.-X. Hu, Q. Li, H.-L. Zhu, Z.-N. Gao, Q. Zhang, T. Liu and G.-M. Wang, Nat. Commun., 2022, 13, 2646 CrossRef CAS PubMed.
- B. Xia, Q. Gao, Z.-P. Hu, Q.-L. Wang, X.-W. Cao, W. Li, Y. Song and X.-H. Bu, Research, 2021, 549048 Search PubMed.
- Y.-J. Ma, J.-X. Hu, S.-D. Han, J. Pan, J.-H. Li and G.-M. Wang, J. Am. Chem. Soc., 2020, 142, 2682 CrossRef CAS PubMed.
- X.-Y. Lv, M.-S. Wang, C. Yang, G.-E. Wang, S.-H. Wang, R.-G. Lin and G.-C. Guo, Inorg. Chem., 2012, 51, 4015 CrossRef CAS PubMed.
- G.-C. Guo, Y.-G. Yao, K.-C. Wu, L. Wu and J.-S. Huang, Prog. Chem., 2001, 13(2), 151 CAS.
- X.-Q. Yu, C. Sun, M.-S. Wang and G.-C. Guo, Nat. Commun., 2020, 11, 1179 CrossRef CAS PubMed.
- L.-Z. Cai, Q.-S. Chen, C.-J. Zhang, P.-X. Li, M.-S. Wang and G.-C. Guo, J. Am. Chem. Soc., 2015, 137(34), 10882 CrossRef CAS PubMed.
- S. Ohkoshi, H. Tokoro and K. Hashimoto, Coord. Chem. Rev., 2005, 249, 1830 CrossRef CAS.
- E. J. M. Vertelman, T. T. A. Lummen, A. Meetsma, M. W. Bouwkamp, G. Molnar, P. H. M. van Loosdrecht and P. J. van Koningsbruggen, Chem. Mater., 2008, 20, 1236 CrossRef CAS.
- X. Qi, S. Pillet, C. de Graaf, M. Magott, E.-E. Bendeif, P. Guionneau, M. RouziHres, V. Marvaud, O. Stefańczyk, D. Pinkowicz and C. MathoniHre, Angew. Chem., Int. Ed., 2020, 59, 3117 CrossRef CAS PubMed.
- J. Harada, R. Nakajima and K. Ogawa, J. Am. Chem. Soc., 2008, 130, 7085 CrossRef CAS PubMed.
- N. Leblanc, M. Allain, N. Mercier and L. Sanguinet, Cryst. Growth Des., 2011, 11, 2064 CrossRef CAS.
- N. Koga, Y. Ishimaru and H. Iwarnura, Angew. Chem., Int. Ed. Engl., 1996, 35, 755 CrossRef CAS.
- Q. Zhang, S.-D. Han, Q. Li, J.-X. Hu and G.-M. Wang, Sci. China Mater., 2022, 65(3), 788 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. Fig. S1–S5. See DOI: https://doi.org/10.1039/d3dt03080f |
| This journal is © The Royal Society of Chemistry 2023 |