 Open Access Article
Open Access ArticleProbing the local structure of FLiBe melts and solidified salts by in situ high-temperature NMR†
Xiaobin
Fu‡
 a,
Yiyang
Liu‡
a,
Hailong
Huang
a,
Huiyan
Wu
ab,
Jianchao
Sun
ab,
Ling
Han
ab,
Min
Ge
a,
Yuan
Qian
*a and
Hongtao
Liu
a,
Yiyang
Liu‡
a,
Hailong
Huang
a,
Huiyan
Wu
ab,
Jianchao
Sun
ab,
Ling
Han
ab,
Min
Ge
a,
Yuan
Qian
*a and
Hongtao
Liu
 *a
*a
aDepartment of Molten Salt Chemistry and Engineering, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, China. E-mail: qianyuan@sinap.ac.cn; liuhongtao@sinap.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing, 100049, China
First published on 20th June 2023
Abstract
The 2LiF-BeF2 (FLiBe) salt melt is considered the primary choice for a coolant and fuel carrier for the generation IV molten salt reactor (MSR). However, the basics of ionic coordination and short-range ordered structures have been rarely reported due to the toxicity and volatility of beryllium fluorides, as well as the lack of suitable high-temperature in situ probe methods. In this work, the local structure of FLiBe melts was investigated in detail using the newly designed HT-NMR method. It was found that the local structure was comprised of a series of tetrahedral coordinated ionic clusters (e.g., BeF42−, Be2F73−, Be3F104−, and polymeric intermediate-range units). Li+ ions were coordinated by BeF42− ions and the polymeric Be–F network through the analysis of the NMR chemical shifts. Using solid-state NMR, the structure of solid FLiBe solidified mixed salts was confirmed to form a 3D network structure, significantly similar to those of silicates. The above results provide new insights into the local structure of FLiBe salts, which verifies the strong covalent interactions of Be–F coordination and the specific structural transformation to the polymeric ions above 25% BeF2 concentration.
Introduction
There has been renewed interest in the research on high-temperature molten salts over the past decades due to the significant advantage of molten salts in areas related to generation IV nuclear energy e.g., molten salt reactors (MSR),1–5 concentrating solar power plants (CSP),6,7 and other clean energy storage industries.8,9 It is known that many multiply charged metal ions can form coordinated local structures in molten salts, which have important effects on the physical and chemical properties of the melt.10,11 The local structure was also important for alkali halide liquid salt, and different ionic structures can significantly adjust the ionic diffusion and dynamics, which is strongly coupled to the viscosity, density, and other salt thermophysical properties.12,13 In the 1960s, FLiBe (2LiF-BeF2) was employed as the coolant and nuclear fuel carrier for the molten salt reactor experiment (MSRE) project at Oak Ridge National Laboratory (ORNL).4 Since then, the LiF/BeF2 binary salt has aroused continuous attention, and the local structure of the FLiBe salt has been widely investigated through theoretical calculation.14–17 However, relevant experimental studies especially in situ studies on the molecular structure of FLiBe have been rarely reported due to the toxicity,17–19 volatilization of beryllium fluorides,20 and the lack of suitable high-temperature high-resolution analytical methods.High-temperature optical absorption spectroscopy, X-ray absorption fine structure (XAFS) spectroscopy, Raman spectroscopy, and Nuclear Magnetic Resonance (NMR) spectroscopy have been widely used in the investigation of the structure of molten salts, which are always combined with theoretical calculations such as molecular dynamics (MD) or ab initio molecular dynamics (AIMD).21 Recently, these high-temperature methods have been reported in research on the structural studies about the alkaline-earth elements, especially magnesium. In 2020, Fei Wu et al.22 used an X-ray scattering perspective and MD simulations to explain the non-Debye–Waller temperature behavior in the intermediate range order for molten MgCl2 and its mixtures with KCl. The result reveals that molten MgCl2 conforms to a network structure rather than an isolated tetrahedral structure. In 2021, Santanu Roy et al.23 employed X-ray scattering and Raman spectroscopy to study the local structure of molten MgCl2. Combined with the AIMD simulations, the five-coordinate MgCl53− complex ions were proven to exist in the molten pure MgCl2 and ZnCl2-MgCl2. In addition, the structure of Ni (II) ions in molten ZnCl2-MgCl2 was also investigated by a multimodal approach combining high-temperature ultraviolet-visible absorption spectra,10 extended XAFS, AIMD simulations, and rate theory of ion exchange. The research reveals that the coordination states of Ni (II) rely on the temperature and content of molten salts. Despite all the above studies on molten salts, the experimental studies on the local structure of beryllium fluoride melts have rarely been reported. It is known that the X-ray method is quite difficult to apply on the ionic structure studies of beryllium salt melts because of the high X-ray transmittance of Be atoms.24 Only some theoretical calculation studies on the ionic structure of beryllium melts were reported.14–17 It has been found that the BeF2-based beryllium fluoride mixed salts adopt an analogue to SiO2 and silicates, which shows a 3D network structure with corner-sharing tetrahedrally coordinated Be2+ cations.25 A series of the tetrahedral coordinated ionic clusters (e.g., BeF42−, Be2F73−, and Be3F104−) and the polymeric intermediate-range units may exist in FLiBe melts.15 Furthermore, the distribution of the different species and the thermo-physical properties as a function of composition were discussed through MD simulation.14–17 Accordingly, systematic experimental local structure studies on LiF/BeF2 binary salts have been particularly desirable in supporting the rapid development of MSR over the past few years.
Nuclear magnetic resonance (NMR) spectroscopy is a high-resolution and powerful method to examine the microstructure and dynamics of complicated multiple salt systems.26 With the development of the high-temperature NMR (HT-NMR) method, the research on the ionic structure of molten salts has deepened. In this work, the local structure of FLiBe melts was investigated in detail using a newly designed HT-NMR method. A laser beam coupled with an optical fiber was used to construct the salt heating system for HT-NMR, which was systematically presented in our previous work.27 The structure and the evolution of different coordinated cluster anions were discussed through the analysis of 19F, 7Li, and 9Be NMR chemical shifts. The strong covalent interactions of Be2+ ions were also discussed based on the above results. Furthermore, the structure of solid FLiBe solidified mixed salts at ambient temperature was also investigated by 1D and 2D solid-state NMR (ss-NMR). The network structure of the FLiBe glass state was certified, which should have close relations with the formation and the amount of the polymeric Be–F network during the solidification of FLiBe melts. Our work deepens the understanding of the local structure of FLiBe salts and demonstrates the significant potential of the NMR method on the structural studies of fluoride salts, which is closely related to their applications in the field of the development of new energy production technology.
Experimental section
Sample preparation
The LiF and BeF2 were purchased from Aladdin. LiF-BeF2 salts were synthesized as follows: 10 g of salts were weighed in proportion and mixed thoroughly in a nickel crucible sealed with a screw cap; then it was heated to 850 °C by 5 °C min−1 and kept for 3 hours in an electrothermal furnace. After that, it was cooled down to room temperature at 5 °C min−1 in the furnace. All the operations were carried out in an argon-atmosphere glovebox.X-ray diffraction
X-ray diffraction measurements were performed on a Bruker D8 ADVANCE using Cu-Kα (1.5406 Å) radiation (40 kV, 20 mA). All samples were mounted on the same sample holder and scanned from 2θ = 10° to 70° at a speed of 10° min−1. To avoid the deliquescence of the salt samples, a Kapton membrane was covered on the sample holder and the background signals are shown in Fig. S3 (ESI†). The experiments were performed at room temperature.DFT calculation
The quantum chemical calculations of Be(1+n)F(4+3n)(2+n)− (n = 0–3) were carried out using the Gaussian 09 program. The geometries of the anions were fully optimized at the B3LYP/AVTZ level, in which the B3LYP hybrid density functional with the aug-cc-pVTZ (AVTZ) basis set was employed. Frequency analyses were performed at the same level of theory to confirm that the optimized geometries were true minimum points. The 19F, 9Be NMR chemical shifts of F and Be atoms in each anionic species were also calculated at the B3LYP/AVTZ level using the optimized structures.NMR experiments
All of the 19F, 7Li, and 9Be NMR experiments were performed on a Bruker AVANCE NEO 400 WB spectrometer operated at 376.61 MHz, 155.55 MHz, and 56.24 MHz for 19F, 7Li, and 9Be, respectively. For all the high-temp NMR experiments, a 7.0 mm double-resonance laser-heating HT probe was used. Homemade sample containers were used during the experiments, which were reported in our previous work.27 The experiment temperature was set to 50 °C above the melting point of each LiF/BeF2 binary molten salt with different BeF2 concentrations. The melting points of the samples were obtained from the calculated LiF-BeF2 phase diagram in Fig. S5 in the ESI.†25 The experiment temperatures were set to 810 °C (15%), 795 °C (17%), 750 °C (22%), 680 °C (25%), 654 °C (27%), 554 °C (32%), 506 °C (35%), and 499 °C (37%) for LiF/BeF2 binary salt samples with different BeF2 concentrations respectively. The experiment temperatures were calculated by using the KBr external standard method. The recycle delay was set to 2 s for all of the 19F, 7Li and 9Be HT-NMR experiments. For the solid-state NMR experiments at room temperature, a 3.2 mm double-resonance magic angle spinning (MAS) probe was used. For single pulse excitation (SP) NMR experiments, the recycle delay was set to 60 s, 5 s, and 30s for 19F, 7Li, and 9Be, respectively. The spinning rate was set to 20 kHz. For 19F-7Li and 19F-9Be HETCOR NMR experiments, the cross polarization time was set to 200 us. The MAS rate was set to 10 kHz. To suppress the spin diffusion, FSLG decoupling was applied during t1 (the 19F dimension) and the 19F RF field was set to 100 kHz. The 19F, 7Li and 9Be chemical shifts were calibrated using C2H4O2F3N (δ = −74.5 ppm), the LiCl aqueous solution (1 mol L−1, δ = 0 ppm) and BeF2 (solid, δ = 0 ppm), respectively.Results and discussion
To investigate the ionic structure of the FLiBe melts, 19F HT-NMR (Fig. 1a) was firstly performed on LiF/BeF2 binary salts with different BeF2 concentrations. The experiment temperature was set to 50 °C above the melting point of each LiF/BeF2 binary molten salt. Only one signal was observed in each 19F spectrum because of the rapid and dynamic exchange between the different species involving the observed nucleus. The peak position was the average between the chemical shifts of the different units present in the melt weighted by their relative proportions. It was observed that the 19F signal shifted to the low field with the increase of the BeF2 concentration. To exhibit the changes of the signals clearly, the chemical shift evolution of LiF-BeF2 molten salts with different BeF2 concentrations is illustrated in Fig. 1b. To obtain the averaged chemical shifts accurately, signal simulation was carried out and the detailed expatiation is given in the ESI† (Fig. S7 and Tables S1, S2). It is clearly seen that 19F chemical shifts follow an almost linear change when the BeF2 concentration is below 25%. As reported and described above, the linear change always implies a dynamic exchange of two species due to the average between the two specific chemical shifts. However, when the BeF2 concentration was above 25%, a non-linear monotonous evolution of the 19F chemical shifts was observed. The deviation thus demonstrated the existence of, at least, a third type of fluorine anion complexes in FLiBe melts. | ||
| Fig. 1 19F HT-NMR spectra and chemical shift evolution of LiF-BeF2 molten salts with different BeF2 concentrations. | ||
9Be HT-NMR was also performed on the above salt samples to investigate the coordinated structure of Be2+ ions and the chemical shift evolution plot is illustrated in Fig. 2a. Unlike the monotonous evolution of 19F NMR, the plot of 9Be NMR showed quite a different trend. First, with the increase of BeF2 concentration, the 9Be NMR signal stayed almost unchanged when the BeF2 concentration was below 25%. This indicates that the chemical structure of Be2+ ions also remained unchanged. When the BeF2 concentration reached 25%, the 9Be NMR signal started to go into high field, suggesting the formation of the new structure species of Be–F complexes. Such a change is also consistent with the 19F NMR results.
 | ||
| Fig. 2 (a) 9Be HT-NMR chemical shift evolution of LiF-BeF2 molten salts with different BeF2 concentrations. (b) Ionic structure of the BeF42− ion and the Be–F polymeric ions. | ||
As revealed by the 19F and 9Be NMR results, when the BeF2 concentration is lower than or higher than 25%, the ionic coordinated structure of Be–F ions should be significantly different. The theoretical studies have suggested that BeF42− ions will form first and gradually become the main species with the addition of BeF2 into the LiF salt.15 At a low BeF2 concentration, the melt is essentially well-dissociated, and it comprises Li+, BeF42−, and F− species. With the increase of BeF2 concentration, BeF42− ions continuously increase, and free F− anions decrease. As a result, the fast exchange between BeF42− anions and F− anions makes the 19F NMR chemical shifts follow a linear variation. Meanwhile, all the Be2+ ions form BeF42− complexed ions and thus 9Be signal keep almost unchanged. When the BeF2 concentration reaches 25%, the evolution of 19F and 9Be NMR chemical shifts tend to be particularly different from that lower than 25%, suggesting the formation of the new coordinated ionic structure.
According to the theoretical calculation results by Smith et al.,15 polymeric species (e.g., Be2F73−, Be3F104−, and Be4F135−) were formed progressively when the BeF2 concentration increased, until a fully connected network was constructed for pure BeF2. When BeF2 concentration was higher than 25%, the polymeric Be–F network tended to be the main species in the FLiBe melt. The formation of the polymeric network should account for the changes in 19F and 9Be evolution. The ionic structure of the BeF42− ions and the Be–F polymeric ions are presented in Fig. 2b. The polymeric Be–F network is made of tetrahedral corner-sharing Be2+ cations linked by F− anions. The formation of the network will lead to the increase of corner-sharing Be2+ ions and the linked F−, thus resulting in the different 19F and 9Be NMR chemical shifts.
DFT calculations were carried out to further investigate the relations between ionic structure and the chemical shift variation trend. The calculated average chemical shifts of the NMR signals are listed in Table 1. The detailed calculation results and the data processing method are provided in the ESI† in Fig. S9 and S10. It could be observed that 19F chemical shifts would go into low field when the polymeric ions (Be3F104−, Be4F135−) formed while for 9Be HT-NMR signals, it would go into high field with the formation of the polymeric ions. The variation tendency is consistent with the experimental 19F and 9Be HT-NMR results, although the values of the calculated chemical shifts are a little different. Based on the above results, we conclude that at low BeF2 concentration, the melt comprises the isolated species (F−, BeF42− and Be2F73−). With the increase of the concentration, especially after 25%, polymeric Be–F network (Be3F104−, Be4F135− and even polymer chains) is formed and gradually becomes the main species in the FLiBe melt.
| 19F c.s./ppm | 9Be c.s./ppm | |
|---|---|---|
| BeF42− | −197.44 | −1.92 |
| Be2F73− | −192.09 | −2.28 |
| Be3F104− | −189.75 | −2.37 |
| Be4F135− | −187.69 | −2.38 |
Formation of the polymeric Be–F network should be probably derived from the strong covalent interactions between Be and F atoms. Similar to silica SiO2 and some silicates,28,29 the strong interactions can facilitate the formation of tetrahedral network. The similarity between FLiBe and silicates thus illustrates that Be–F bonds should have some covalent properties. As reported in the literature,13–15 the Be–F distances in FLiBe melts with high BeF2 concentration is about 1.57 Å, which is calculated via MD and AIMD simulation. The relatively short ionic distance thus indicates that the Be–F bonds exhibit the particular covalency. Moreover, compared to Mg, the Be–F interactions are much higher than Mg–F interactions although they are the same alkali earth group atoms. According to the literature,30 the calculated bond dissociation energy of BeF− ions is 88.7 kcal/mol, which is also much higher than that of MgF− ions (61.9 kcal/mol). This could also indicate the strong covalent interactions of Be–F bonds. The strong covalent interactions of Be2+ ions thus can facilitate the coordination between Be2+ ions and F− ions, leading to the formation of the network structure in FLiBe melts with high BeF2 concentration.
Previous work has suggested that Li+ ions do not form any complex with Be2+ or F− ions.26 To examine the coordinated structure of Li+ ions, 7Li HT-NMR was also performed on these FLiBe salt samples and the spectra are presented in Fig. 3a. The evolution of 7Li chemical shifts is also primarily similar to 19F and 9Be results, indicating the formation of the polymeric Be–F networks. Unexpectedly, two broad signals could be distinguished in the spectra of FLiBe melts with 22% and 25% BeF2 concentration. In these two samples, the melt consists of both BeF42− ions and the polymeric Be–F network. The two 7Li signals should be assigned to the Li+ ions coordinated with the dissociated BeF42− ions and the polymeric Be–F network. The separation of the two 7Li signals may be due to the reduced exchange rate of these two species, which is caused by the formation of the polymeric Be–F network. This indicates that Li+ ions don’t simply follow the dissociative state which doesn’t form any complex with F− ions. The two separable 7Li signals thus verified that Li+ ions should be also coordinated by the dissociated F−, BeF42− ions, and the polymeric Be–F network, respectively. Furthermore, considering the reduced exchange behavior between the two species, we should also observe two signals in the 19F HT-NMR spectra. However, such individual signals were hardly observed due to the relatively low resolution of 19F signals.
 | ||
| Fig. 3 7Li HT-NMR spectra and chemical shift evolution of LiF-BeF2 molten salts with different BeF2 concentrations. | ||
Fluoride crystals have wide applications in the optical industry such as luminescence materials and laser crystals. In general, BeF2 is believed to adopt a structure analogue to SiO2, with a 3D network of corner-sharing tetrahedrally coordinated structure.28 The crystal behavior and the glass state of the beryllium fluorides can significantly influence their applications. As discussed above, the addition of alkali-metal elements, LiF, can adjust the ionic structure of FLiBe melts. Formation of the polymeric Be–F network would reduce the crystallization and thus lead to the glass state during the solidification of FLiBe melts.
To investigate the local structure of solidified FLiBe mixed salts, high-resolution solid-state19F MAS NMR spectroscopy was performed on LiF/BeF2 salts with different BeF2 concentrations and the spectra are presented in Fig. 4. Four signals could be observed in the above spectra, which are named F-1 (−205.6 ppm), F-2 (−202.0 ppm), F-3 (−195.8 ppm), and F-4 (−187.3 ppm) respectively. To exhibit the differences of the signals, the software “Dmfit” was employed for signal decomposition and the calculated intensities of the signals are presented in Fig. S2 (ESI†). With the increase of BeF2 concentration, the intensity of F-1 and F-2 signals tended to decrease gradually and the intensity of the F-3 signal increased. When BeF2 concentration reached 60%, F-1 and F-2 signals disappeared, and a new F-4 signal appeared in the spectrum. Signal assignment should be conducted to further analyze the local structure of FLiBe mixed salts.
 | ||
| Fig. 4 Solid-state 19F MAS NMR spectra of FLiBe mixed salts with different BeF2 concentrations. The software “Dmfit” was used for signal decomposition. | ||
To assign the 19F signals, 2D 19F-7Li and 19F-9Be Heteronuclear Correlation (HETCOR) NMR spectroscopy was performed on the LiF/BeF2 salts (40% BeF2) and the spectra are presented in Fig. 5. It could be observed that F-1 (19F) signal has cross peaks with 7Li signal only. No cross peaks could be observed between F-1 and 9Be signals, suggesting the above F− ions are only bonded to Li+ ions. Thus, we assign F-1 signal to the F− ions of LiF crystals. For F-2 and F-3 signals, they have cross peaks with both 7Li signal and 9Be signal, indicating that these F− ions are bonded to Li+ ions and Be2+ ions. It is known that Li2BeF4 crystals can be formed when the molar ratio of LiF:BeF2 is about 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. To verify if Li2BeF4 crystals exist in the mixed salts, XRD was performed on the samples and the XRD patterns are presented in Fig. S3 (ESI†). As shown, the characteristic diffraction peaks of Li2BeF4 crystals could be clearly observed, illustrating the existence of Li2BeF4 crystal lattices. Thus F-2 signal is assigned to the F− ions of Li2BeF4 crystals.
1. To verify if Li2BeF4 crystals exist in the mixed salts, XRD was performed on the samples and the XRD patterns are presented in Fig. S3 (ESI†). As shown, the characteristic diffraction peaks of Li2BeF4 crystals could be clearly observed, illustrating the existence of Li2BeF4 crystal lattices. Thus F-2 signal is assigned to the F− ions of Li2BeF4 crystals.
It was observed that the intensity of F-1 and F-2 signals continuously decreased with increasing the concentration of BeF2. After the concentration of BeF2 reached 60%, F-1 and F-2 signals almost disappeared, demonstrating the absence of LiF and Li2BeF4 crystals in the salt samples with high BeF2 concentration. Moreover, the intensity of F-3 signal increased gradually and a new signal F-4 appeared at high BeF2 concentration. As expected, FLiBe should be also in a glassy state similar to silicate glasses with a 3D network structure (such as Na2SiO3). The above results reveal that F-3 and F-4 signals should be assigned to the F− ions of FLiBe glasses.
In comparison with silicate glasses, Be–F glasses could be also distinguished by these different structure models, which were named Q1, Q2, Q3, and Q4 respectively. The structure models are shown in Fig. 6b. F-4 signal existed only in the 19F ss-NMR spectrum of FLiBe salts with a significantly high BeF2 concentration and we assign F-4 signal to the F− ions of Q3 or Q4 model with Be–F 3D networks. For F-3 signal, the signal intensity increased with the increase of BeF2 concentration. Thus F-3 signal was assigned to the F− ions of Q1 and Q2 models with Be–F dimers or chains. Changes of the 9Be NMR spectra of LiF-BeF2 solidified mixed salts with different BeF2 concentrations also follow the above signal assignment. 9Be ss-NMR spectra and signal simulation of LiF-BeF2 mixed salts are presented in Fig. 6a. Two signals were observed in the spectra, named Be-1 and Be-2. Be-1 should be the Be2+ ions of Li2BeF4 crystals and Q1 model with Be–F dimers. Be-2 should be the Be2+ ions of Q2, Q3, or Q4 models. The abundant corner-sharing tetrahedrally coordinated Be2+ cations of Q2 and Q3 should account for the distinguished chemical shifts of Be-1 and Be-2.
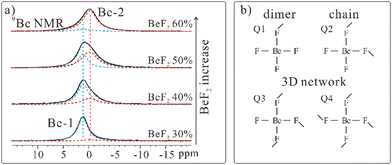 | ||
| Fig. 6 (a) 9Be NMR spectra and signal simulation of LiF-BeF2 mixed salts with different BeF2 concentrations. (b) Illustration of the structure models of Be–F glasses. | ||
The structure transition of FLiBe solidified mixed salts can be investigated through the above signal assignment of 19F and 9Be ss-NMR. At a low BeF2 concentration, the LiF-BeF2 mixed salts consisted of LiF crystals and Li2BeF4 crystals. Moreover, there was also a small amount of the oligomer (e.g., Q1 Be–F tetrahedral ions). With the increase of BeF2 concentration, LiF crystals and Li2BeF4 crystals became gradually disordered. Instead, numerous corner-shared Be–F tetrahedra formed and the number of Q1/Q2 structures increased, thus becoming the main species. This should be caused by the strong Be–F covalent interactions, which is quite similar to some silicates. When the BeF2 concentration was extremely high (>60%), the Be–F network (Q3 and Q4) was also formed, and most the LiF crystals and Li2BeF4 crystals disappeared. Obvious signal broadening could be observed of the XRD patterns, indicating a decreased degree of crystallinity. This also illustrates that the main part of the samples transforms into the glass state. Upon further increasing the BeF2 concentration, the mixed salt would transform completely into the glass state (similar to BeF2). In addition, the formation of the FLiBe glass state should also have close relations with a large amount of the polymeric Be–F structures. The strong Be–F covalent interactions of the Be–F network of FLiBe melts would reduce the crystallization and thus lead to the formation of FLiBe glass. Upon adding LiF into the system, the alkali metal ions will break the long chains or networks, which is beneficial to the crystallization during the solidification of FLiBe melts. This should be the transition process of LiF-BeF2 mixed salts from crystals into glasses.
Conclusions
In summary, the local structures of LiF-BeF2 molten salts and the solidified mixed salts are systematically interpreted in detail using the HT-NMR and solid-state MAS NMR methods. In this study, it was suggested that the strong covalent interactions of Be2+ ions can facilitate the coordination between Be2+ ions and F− ions. As a result, polymeric Be–F chains and networks can be formed, which will be closely related to the ionic dynamics. 19F, 7Li, and 9Be NMR experiments were used to understand the local structure and the structural transition of the investigated materials. As revealed from the NMR results, the BeF42−, Be2F73−, and Be3F104− coordinated species as well as the polymeric Be–F chains/networks exist in FLiBe melts, the amounts of which can be adjusted via the BeF2 concentration. Moreover, the structure of solid FLiBe mixed salts at ambient temperatures was also investigated by 1D and 2D solid-state NMR (ss-NMR). The network structure of FLiBe was verified to be similar to those of silicate glasses. According to the results of the observations, the local structure of FLiBe salts was elucidated using experimental methods, and more insights were provided into the transition of the structure from the isolated ionic systems into the polymeric networks and were found to be significantly related to the thermo-physical properties and their applications in new energy production technology. This work also demonstrates that HT-NMR methods are promising in the investigation of the micro-structure and dynamics of fluoride salts. Our work shows that HT-NMR is also applicable to research on other fluorine-containing materials.Data availability
The authors declare that data relating to the characterization of materials and products, general methods, experimental procedures, mechanistic studies, XRD data, and NMR spectra are available within the article and the ESI† or from the corresponding author upon request.Author contributions
Y. Q. and H. L. conceived and designed the experiments. X. F. and Y. L. performed the experiments and analyzed the data. H. H., H. W. and J. S. helped synthesize the BeF2-LiF salts. L. H. helped draw the structure diagram. X. F. wrote the original manuscript. M. G. helped revise it. All authors proofread the paper, made comments, and approved the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (22103094), the K. C. Wong Education Foundation (Grant No. GJTD-2018-10), and the Young Potential Program of Shanghai Institute of Applied Physics, Chinese Academy of Sciences. The authors also express their gratitude to Professor Ian Farnan from the University of Cambridge for his advice on manuscript modification.Notes and references
- M. Jiang, H. Xu and Z. Dai, Bull. Chin. Acad. Sci., 2012, 27, 366–374 Search PubMed.
- H. G. MacPherson, Nucl. Sci. Eng., 1985, 90, 374–380 CrossRef CAS.
- J. Serp, M. Allibert, O. Beneš, S. Delpech, O. Feynberg, V. Ghetta, D. Heuer, D. Holcomb, V. Ignatiev, J. L. Kloosterman, L. Luzzi, E. Merle-Lucotte, J. Uhlíř, R. Yoshioka and D. Zhimin, Prog. Nucl. Energy, 2014, 77, 308–319 CrossRef CAS.
- D. F. Williams, Assessment of Candidate Molten Salt Coolants for the Advanced High Temperature Reactor (AHTR), Oak Ridge, TN, 2006 Search PubMed.
- Y. Wang, J. Tian, S.-W. Wang, C. Zhou and N.-X. Wang, Nucl. Sci. Tech., 2021, 32, 92 CrossRef CAS.
- S. Kuravi, J. Trahan, D. Y. Goswami, M. M. Rahman and E. K. Stefanakos, Prog. Energy Combust. Sci., 2013, 39, 285–319 CrossRef.
- M. Liu, N. H. Steven Tay, S. Bell, M. Belusko, R. Jacob, G. Will, W. Saman and F. Bruno, Renewable Sustainable Energy Rev., 2016, 53, 1411–1432 CrossRef CAS.
- O. Garbrecht, M. Bieber and R. Kneer, Energy, 2017, 118, 876–883 CrossRef CAS.
- A. Giaconia, M. de Falco, G. Caputo, R. Grena, P. Tarquini and L. Marrelli, AIChE J., 2008, 54, 1932–1944 CrossRef CAS.
- S. Roy, Y. Liu, M. Topsakal, E. Dias, R. Gakhar, W. C. Phillips, J. F. Wishart, D. Leshchev, P. Halstenberg, S. Dai, S. K. Gill, A. I. Frenkel and V. S. Bryantsev, J. Am. Chem. Soc., 2021, 143, 15298–15308 CrossRef CAS PubMed.
- S. Roy, F. Wu, H. Wang, A. S. Ivanov, S. Sharma, P. Halstenberg, S. K. Gill, A. M. Milinda Abeykoon, G. Kwon, M. Topsakal, B. Layne, K. Sasaki, Y. Zhang, S. M. Mahurin, S. Dai, C. J. Margulis, E. J. Maginn and V. S. Bryantsev, Phys. Chem. Chem. Phys., 2020, 22, 22900–22917 RSC.
- J. Wang, J. Wu, Z. Sun, G. Lu and J. Yu, J. Mol. Liq., 2015, 209, 498–507 CrossRef CAS.
- J. Wu, J. Wang, H. Ni, G. Lu and J. Yu, J. Mol. Liq., 2018, 253, 96–112 CrossRef CAS.
- J. Dai, H. Han, Q. Li and P. Huai, J. Mol. Liq., 2016, 213, 17–22 CrossRef CAS.
- A. L. Smith, E. Capelli, R. J. M. Konings and A. E. Gheribi, J. Mol. Liq., 2020, 299, 112165 CrossRef CAS.
- M. Salanne, C. Simon, P. Turq, R. J. Heaton and P. A. Madden, J. Phys. Chem. B, 2006, 110, 11461–11467 CrossRef CAS PubMed.
- R. J. Heaton, R. Brookes, P. A. Madden, M. Salanne, C. Simon and P. Turq, J. Phys. Chem. B, 2006, 110, 11454–11460 CrossRef CAS PubMed.
- D. Naglav, M. R. Buchner, G. Bendt, F. Kraus and S. Schulz, Angew. Chem., Int. Ed., 2016, 55, 10562–10576 CrossRef CAS PubMed.
- M. Müller and M. R. Buchner, Angew. Chem., Int. Ed., 2018, 57, 9180–9184 CrossRef PubMed.
- K. A. Sense, M. J. Snyder and J. W. Clegg, J. Phys. Chem., 1954, 58, 223–224 CrossRef CAS.
- D. G. Lovering, Molten Salt Technology, SpringerNew York, NY, United States, 1982 Search PubMed.
- F. Wu, S. Sharma, S. Roy, P. Halstenberg, L. C. Gallington, S. M. Mahurin, S. Dai, V. S. Bryantsev, A. S. Ivanov and C. J. Margulis, J. Phys. Chem. B, 2020, 124, 2892–2899 CrossRef CAS PubMed.
- S. Roy, M. Brehm, S. Sharma, F. Wu, D. S. Maltsev, P. Halstenberg, L. C. Gallington, S. M. Mahurin, S. Dai, A. S. Ivanov, C. J. Margulis and V. S. Bryantsev, J. Phys. Chem. B, 2021, 125, 5971–5982 CrossRef CAS PubMed.
- A. Snigirev, I. Snigireva, V. G. Kohn and S. M. Kuznetsov, Nucl. Instrum. Methods Phys. Res., Sect. A, 1996, 370, 634–640 CrossRef CAS.
- J. R. Allwardt, B. C. Schmidt and J. F. Stebbins, Chem. Geol., 2004, 213, 137–151 CrossRef CAS.
- J. K. M. Sanders and B. K. Hunter, Modern NMR spectroscopy: a guide for chemists, Oxford University Press, New York, NY, United States, 1988 Search PubMed.
- Y. Liu, R. Lan, C. Dong, K. Wang, X. Fu, H. Liu, Y. Qian and J. Wang, J. Phys. Chem. C, 2021, 125, 4704–4709 CrossRef CAS.
- I. Farnan and J. F. Stebbins, Science, 1994, 265, 1206–1209 CrossRef CAS PubMed.
- H. Krebs, Angew. Chem., Int. Ed. Engl., 1966, 5, 544–554 CrossRef.
- R. Liu, L. Qin, Z. Zhang, L. Zhao, F. Sagan, M. Mitoraj and G. Frenking, Chem. Sci., 2023, 2, 4872–4887 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp01096a |
| ‡ These authors contributed equally. |
| This journal is © the Owner Societies 2023 |

