Open Access Article
Open Access ArticleKinetics or thermodynamics? Extolling their role to modulate the crystal phases and luminescence of KY3F10:Eu3+ powders†
Pablo
Serna-Gallén
 *,
Héctor
Beltrán-Mir
*,
Héctor
Beltrán-Mir
 and
Eloísa
Cordoncillo
and
Eloísa
Cordoncillo

Departamento de Química Inorgánica y Orgánica, Universitat Jaume I, Av. Sos Baynat s/n 12071, Castelló de la Plana, Spain. E-mail: pserna@uji.es
First published on 28th September 2023
Abstract
KY3F10, a fluoride compound with two polymorphs (α and δ), has been a subject of study due to its unique properties. Obtaining the metastable δ-phase has been challenging, but this study presents an enhanced synthetic methodology using coprecipitation to isolate specific crystal phases. Varying the reaction temperature and time allows for the modulation of polymorph formation. The structural analysis of the synthesized powders reveals the influence of kinetic and thermodynamic control. The morphology of the particles is also affected by these factors, with different reaction conditions leading to distinct particle shapes. The luminescent response of Eu3+-doped powders helps understand the structural stability. It is demonstrated that time-resolved fluorescence spectroscopy is a sensitive and direct measurement to follow the observed changes. Overall, this study demonstrates the interplay between thermodynamics and kinetics in materials synthesis and the impact on crystal phase formation and properties.
1. Introduction
When it comes to materials synthesis, the corresponding thermodynamic phase diagram should be used as a starting point to yield the most stable product. However, it is not difficult to find some crystal phases that, although predicted as thermodynamically stable, cannot be synthesized experimentally and, instead of them, metastable phases are formed.1–3 This fact evinces the competitive nature between the thermodynamic and the kinetic factors.Thermodynamics is the cornerstone of materials and solids engineering because it helps us to study, predict, and explain the existence of different crystal phases, their range of stabilities, and their physicochemical properties.4 On the other hand, the kinetics of a given chemical system involves the study of the concentration of reagents, reaction medium, pressure, temperature, catalysts, etc. that affect the reaction time or the formation of a specific product to the detriment of others. Given that, designing new synthetic approaches becomes a powerful strategy to tune the reaction pathways and thus the formation of the final product. Commonly, kinetic/metastable products (which are less stable thermodynamically) are favored under reaction conditions of low temperatures.5 In contrast, high temperatures or pressures facilitate stabilizing thermodynamic products.6,7
In materials science, the controlled synthesis of polymorphs (i.e., compounds with the same chemical formula but different crystal phases) has attracted significant interest because it opens up a plethora of possibilities regarding the functionalization and applications of solid materials.8,9 However, understanding the involved crystal phase transformations is still a challenge in some compounds.10,11
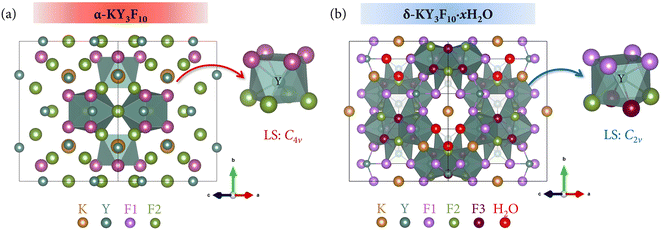
In that sense, the KY3F10 host lattice emerges as a fluoride that deserves special attention. This compound presents two polymorphs with cubic structure12,13 but completely different properties: the thermodynamic α-phase and the metastable δ-phase. Indeed, a δ → α phase transformation is observed around 440 °C,14 thus corroborating the higher thermodynamic stability of α-KY3F10. α-KY3F10 crystallizes in a fluorite-type cubic structure with the Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (no. 225) space group (SG). The lattice parameter is a = 11.536 Å, and the cell volume is V = 1535.20 Å3 with 8 formula units per unit cell (Z = 8).12 The structure can be described by means of square antiprisms formed by YF8 units, having the Y3+ central cation a C4v local symmetry, Fig. 1(a). On the other hand, the δ-KY3F10·xH2O compound has also a cubic structure with a similar SG = Fd
m (no. 225) space group (SG). The lattice parameter is a = 11.536 Å, and the cell volume is V = 1535.20 Å3 with 8 formula units per unit cell (Z = 8).12 The structure can be described by means of square antiprisms formed by YF8 units, having the Y3+ central cation a C4v local symmetry, Fig. 1(a). On the other hand, the δ-KY3F10·xH2O compound has also a cubic structure with a similar SG = Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (no. 227), Z = 16, and a = 15.492 Å (cell volume V = 3717.90 Å3).14 The crystal structure is also composed of YF8 square antiprisms, having the Y3+ central cation a C2v local symmetry (e.g., a symmetry lower than in α-KY3F10) Fig. 1(b). Additionally, the δ-phase incorporates crystalline water molecules which gives rise to a zeolitic behavior in this unique phase.
m (no. 227), Z = 16, and a = 15.492 Å (cell volume V = 3717.90 Å3).14 The crystal structure is also composed of YF8 square antiprisms, having the Y3+ central cation a C2v local symmetry (e.g., a symmetry lower than in α-KY3F10) Fig. 1(b). Additionally, the δ-phase incorporates crystalline water molecules which gives rise to a zeolitic behavior in this unique phase.
While the α-phase has been widely studied in the literature and different synthetic procedures have been developed,15–19 the obtention of single-phase δ-KY3F10 has been more challenging.20,21 In a recent article, we employed a sonochemical synthesis to obtain both the α and δ compounds by accurately adjusting the pH of the reaction medium.22 The complex formation mechanism of the δ-phase was discussed in detail employing equilibrium reactions. Inspired by this peculiar and delicate system, we published another study in which a novel and easy coprecipitation method was developed to obtain nanospheres of the δ-phase at room temperature.21 The results highlighted the delicacy of this fluoride-based system because by only changing the speed of addition of the fluoride source (KF/HF solution) into the Y3+ solution it was possible to obtain the single δ-phase or a mixture of α + δ. Additionally, the site-selective emission of the Eu3+-doped δ-phase was found to be 20 times more intense than that of the α-phase, a fact that illustrated the importance of exploring this metastable phase.
Therefore, this last synthesis process was a good candidate to explore the influence of kinetic and thermodynamic factors on the reaction system and the final product. However, as outlined in the ESI† of the present study, it is not possible to properly achieve only the thermodynamic phase (α) by performing several experiments in which the influence of temperature is implemented (thermodynamic conditions). Moreover, another secondary phase starts to appear.
Herein we introduce an enhanced synthetic methodology with some modifications of the coprecipitation strategy previously mentioned that allows to properly follow the transition-phase pathway. To consider both the kinetics and thermodynamics and see how they affect the formation of one or another polymorph, the reaction temperature was varied from 25 to 80 °C, and the reaction time was varied from 1 hour up to 7 days. The results revealed the possibility of isolating both crystal phases depending on the experimental conditions. In addition, due to the different optical responses of both phases, the samples were doped with Eu3+, and all the structural changes were followed by the luminescence of the lanthanide ion.
2. Experimental section
2.1. Materials and methods
The reagents used for the synthesis of the powders were yttrium(III) nitrate hexahydrate [Y(NO3)3·6H2O 99.8%], europium(III) nitrate pentahydrate [Eu(NO3)3·5H2O 99.9%], potassium fluoride [KF 99.5%], and hydrofluoric acid aqueous solution [HF 40% wt]. All of them were purchased from Sigma-Aldrich and used without further purification.The compounds were prepared by a coprecipitation method. Different temperatures were fixed (25, 40, 60, and 80 °C) combined with different reaction times (1 h, 1 day, and 7 days) yielding a total number of 12 samples. The amounts of reagents were adjusted to obtain approximately 0.5 g of the final product. First, 3.0 mmol of the hydrated Ln(NO3)3 were dissolved in 30 mL of water (0.1 M Ln3+ solution; Ln = Y, Eu; 3 mol% Eu3+) in a double-necked round-bottom flask. One of the necks was fitted with a refluxing condenser and the other one was sealed with a rubber septum. The flask was put in a silicon oil bath with the desired temperature fixed. The system was maintained under stirring for 10 min to ensure that the Ln3+ solution had achieved the fixed temperature.
After that, the fluoride-source solution was prepared by dissolving 6 mmol of KF in 10 mL of water and, subsequently, adding 270 μL of HF aqueous solution 40% wt. (6 mmol HF). The resulting solution (0.6 M KF/HF) was added dropwise to the previous Ln3+ solution in the flask. For that purpose, the rubber septum was removed and it was placed again after finalizing the addition process. Immediately, a white precipitate appeared and it was allowed to mature at the desired time under continuous stirring. Finally, the products were collected by centrifugation, washed twice with water, and dried under an infrared lamp. A summary of the abbreviations used for the samples according to the fixed temperature and reaction time is indicated in Table 1 and a scheme of the experimental procedure is depicted in Fig. 2.
| T (°C) | 1 hour | 1 day | 7 days |
|---|---|---|---|
| 25 | R25-1 h | R25-1 d | R25-7 d |
| 40 | R40-1 h | R40-1 d | R40-7 d |
| 60 | R60-1 h | R60-1 d | R60-7 d |
| 80 | R80-1 h | R80-1 d | R80-7 d |
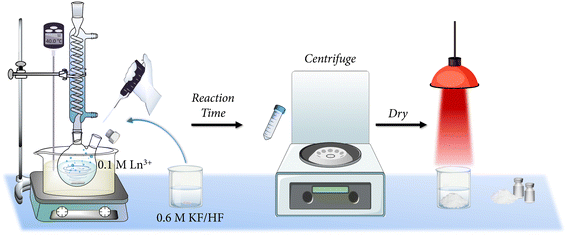 | ||
| Fig. 2 Illustrative representation of the experimental procedure followed for the synthesis of the powders. | ||
2.2. Characterization techniques
Powder X-ray diffraction (XRD) of the samples was performed at room temperature using a Bruker-AX D8-Advance X-ray diffractometer with CuKα1 radiation from 2θ = 20° to 90° at a scan speed of 2.25° min−1. To characterize the morphology of the particles, a JEOL 7001F scanning electron microscope (SEM) was used (acceleration voltage = 30 kV, measuring time = 20 s, and working distance = 8 mm). Previously, the powders had been deposited on double-sided carbon stickers (adhered to the surface of aluminum stubs) and had been sputtered with platinum (10 s).The photoluminescence response of the materials was recorded with an Eclipse Fluorescence Spectrophotometer (Varian). All the photoluminescence experiments were carried out at room temperature and recorded with a detector delay time (DT) of 0.2 ms to observe the contributions from the higher excited level 5D1 in the emission spectra and discriminate between the optical response of α, δ, and (α + δ) phases. Excitation spectra were recorded in the range 250–500 nm fixing the emission wavelength at 593 nm, while the emission spectra were collected with an excitation at 395 nm in the 500–750 nm range. Time-resolved luminescence measurements were performed at different emission wavelengths (593 nm to collect the 5D0 → 7F1 emission and 554 nm to record the response arising from the 5D1 → 7F2 transition). The excitation wavelength was monitored at 395 nm (7F0 → 5L6 transition) and lifetime values were extracted from the corresponding decay profiles.
3. Results and discussion
3.1. Structural characterization
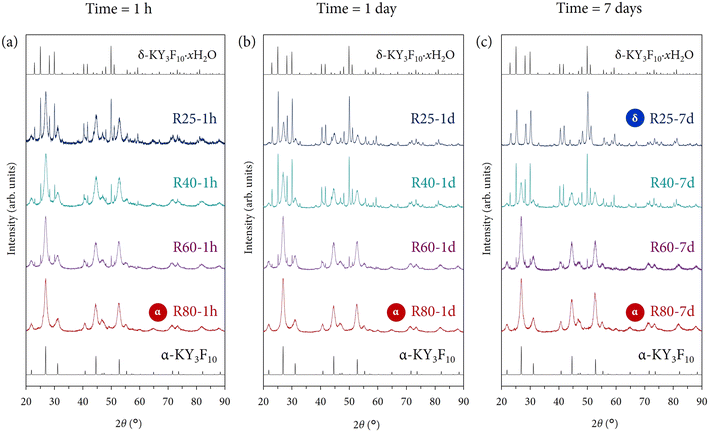
Fig. 3 presents the XRD patterns of the resulting powders under reaction times of (a) 1 h, (b) 1 day, and (c) 7 days. To facilitate the interpretation of the XRD peaks, especially when there is a coexistence of α + δ phases, the theoretical main reflection peaks for each crystal phase have also been incorporated. The pattern standards used were ICSD card 00-040-9643 (α-KY3F10) and ICDD card 04-016-7073 (δ-KY3F10·xH2O). Additionally, Fig. S2 of the ESI† depicts the most useful diffraction peaks that can be used to assign and/or corroborate the presence of the above crystal phases(s).Regarding Fig. 3(a), with a reaction time of 1 h, it is important to note that at 25 °C a clear mixture of both crystal phases is obtained. This result is optimum to be used as a starting point to study the influence of kinetics and thermodynamics on this particular system. Then, the kinetic factor will be ascribed to the reaction time while the thermodynamic factor, to the reaction temperature. The subsequent increase in the temperature produces a profound effect on the resulting material. The presence of the polymorph δ decreases and only α single-phase is obtained at the maximum temperature of work (80 °C). This tendency evidences the clear importance of the thermodynamic effect to yield the “thermodynamic phase”, i.e., α.
Very similar conclusions can be extracted from Fig. 3(b) and (c) if one compares the evolution of the resulting crystal phase(s) with the increase in temperature. However, to analyze the kinetic factor (time) and its influence, one can compare the XRD patterns of samples obtained at the same reaction temperature, but at different maturation times. Perhaps, the most evident and fascinating results are found evaluating the powders obtained at 25 °C. Since the temperature is mild enough to avoid thermodynamic control, it is expected that the kinetic effect becomes more prominent. Indeed, comparing samples R25-1 h, R25-1 d, and R25-7 d, it is clearly shown that the metastable δ-phase is stabilized and R25-7 d contains only this crystal phase without any traces or impurities from α-KY3F10. However, it is quite curious that the metastable phase can be stabilized to detriment of the thermodynamic α-phase, as one may expect a priori. Further explanations are given below in a subsection.
At 40 °C, the thermodynamic control starts to become slightly more important since the temperature is higher, although not enough to allow the thermodynamics to govern the whole reaction process and neglect the kinetics. The most substantial change occurs between samples R40-1 h and R40-1 d. The XRD pattern corresponding to the latter one presents a higher presence of the metastable δ-phase. This major contribution is even slightly higher at a long reaction time (sample R40-7 d). These results underscore that we are under experimental conditions where the competitiveness between kinetics and thermodynamics plays an important role and it is not possible to obtain either single α or δ-phase.
Following this line of reasoning, when the temperature is increased and set to 60 °C, the thermodynamic control is more than evident because all the XRD patterns of samples R60-1 h, R60-1 d, and R60-7 d exhibit virtually the same profile regardless of the total reaction time. The powders are mainly composed of α-KY3F10 with some minor impurities of δ-KY3F10, thus highlighting that the kinetics do not play any significant contribution at this point.
This fact is even accentuated considering samples synthesized at 80 °C because independently of the reaction time (1 h, 1 day, or even 7 days), the final product only exhibits the main XRD reflections ascribed to α-KY3F10. Thereby, thermodynamics governs the whole reaction pathway.
In summary, the structural analysis of the synthesized powders has revealed the possibility of modulating the desired polymorph(s) of KY3F10 thanks to thermodynamic and kinetic control.
Additionally, all the experimental diffraction patterns of the powders were refined using the Rietveld method. The weight fractions (%) of α-KY3F10 and δ-KY3F10·xH2O crystal phases are indicated in Table 2. The values are in very good agreement with the previous discussion of the XRD results and the kinetic/thermodynamic effects. Fig. 4 and 5 depict the Rietveld refinements of the XRD patterns for samples obtained at 25/40 °C, and at 60/80 °C, respectively. Further information about the Rietveld refinement can be found in Section S2 of the ESI.† Table S1 of the ESI† complements the results with the refined unit cell parameter (a = b = c) for the different experimental XRD patterns, although no substantial changes seem to take place comparing all the samples.
| T (°C) | % α | % δ | ||||
|---|---|---|---|---|---|---|
| 1 hour | 1 day | 7 days | 1 hour | 1 day | 7 days | |
| 25 | 68.3 | 41.9 | 0 | 31.7 | 58.1 | 100 |
| 40 | 92.8 | 63.7 | 61.3 | 7.2 | 36.3 | 38.7 |
| 60 | 95.1 | 93.9 | 93.6 | 4.9 | 6.1 | 6.4 |
| 80 | 100 | 100 | 100 | 0 | 0 | 0 |
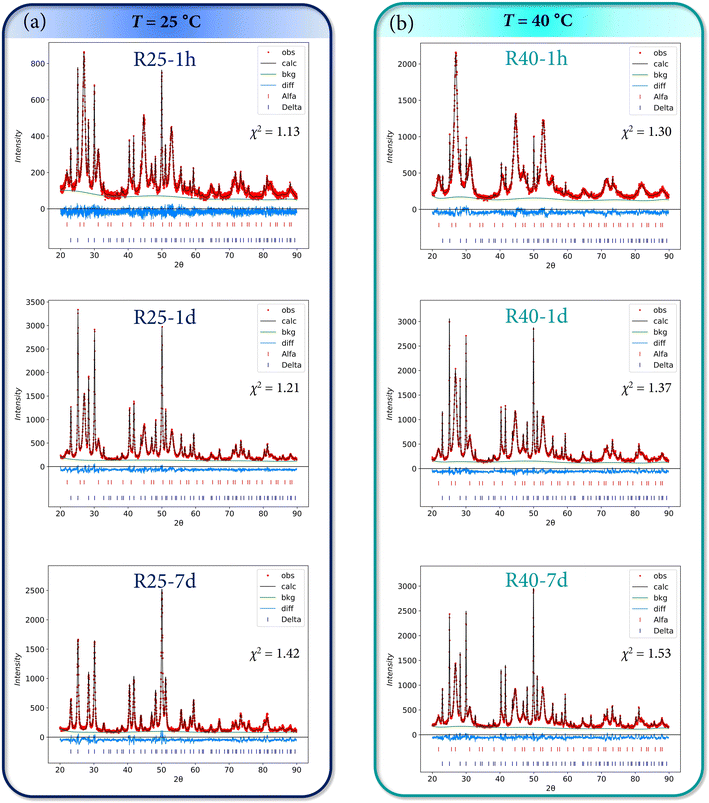 | ||
| Fig. 4 Rietveld refinements of the XRD patterns for samples obtained at (a) 25 °C and (b) 40 °C. The goodness of fit χ2 parameter is also indicated in each plot. | ||
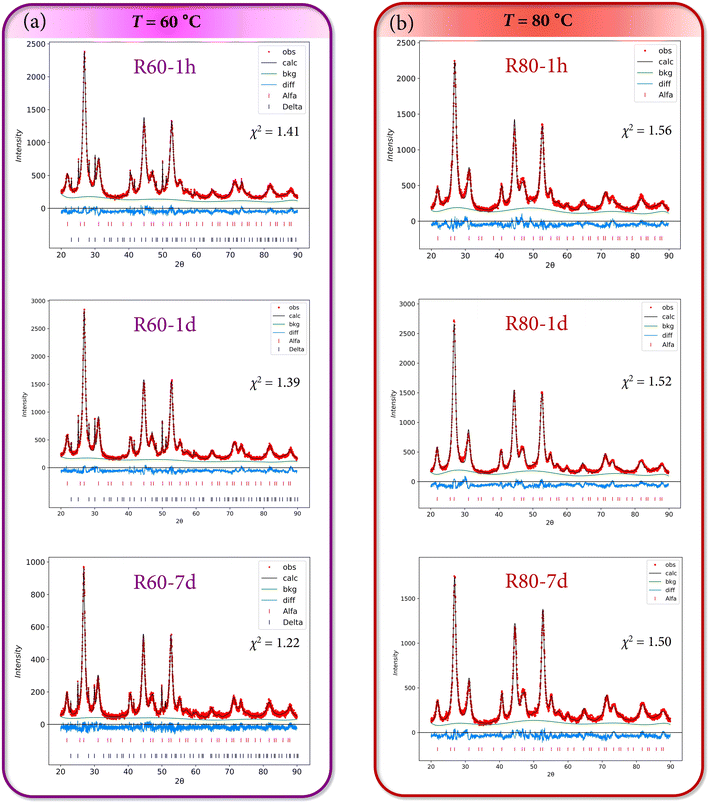 | ||
| Fig. 5 Rietveld refinements of the XRD patterns for samples obtained at (a) 60 °C and (b) 80 °C. The goodness of fit χ2 parameter is also indicated in each plot. | ||
To shed some light on the mechanism underlying such crystal phase transformation, further experiments were conducted to prove whether it would be possible, or not, for α to dissociate into the corresponding ions in the reaction medium and then form the less stable δ-phase. For that purpose, the pure α-KY3F10 was synthesized following the same experimental conditions as those described for sample R80-1 h (T = 80 °C, reaction time = 1 hour). Then, the resulting precipitate in the reaction medium was allowed to cool down until room temperature under continuous stirring. To have a better understanding of the process of transformation, several aliquots of the mixture were taken during one week. The dried powders were characterized by XRD diffraction and the results showed that the sample did not experience any partial α to δ phase transformation (see Fig. S3 of the ESI†).
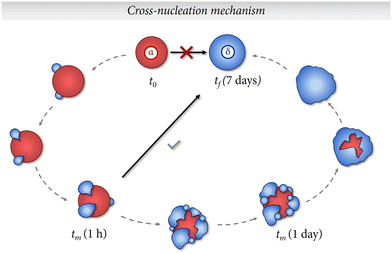
Although this fact seems to be somewhat counterintuitive, there are several studies in the literature that address the crystallization of polymorphs that do not follow Ostwald's rule of stages.24–26 Quite often, a process can result in the simultaneous crystallization of multiple polymorphic structures, further complicating the control of polymorphism, as is our case (e.g. sample R25-1 h). Various mechanisms have been suggested to explain this occurrence, which is referred to as concomitant polymorphism. This phenomenon has been attributed to competing processes of nucleation that can lead to the cross-nucleation of a metastable polymorph on the stable polymorph and finally obtain the less stable polymorph.27 Therefore, the observed results could indicate that the α to δ phase transformation might undergone a cross-nucleation mechanism, as depicted schematically in Fig. 6.
Following this line of reasoning, the formation of crystal nuclei consisting of α particles would take place first and fast. Then, nuclei of δ particles would start to crystallize on the surfaces of the most stable α-nuclei. Subsequently, the amount of δ-phase would start to become more prominent until finally this polymorph is isolated despite being less stable thermodynamically. We believe that these interesting results should be subjected to further study and confirmed with future lines of research.
3.2. Morphological characterization
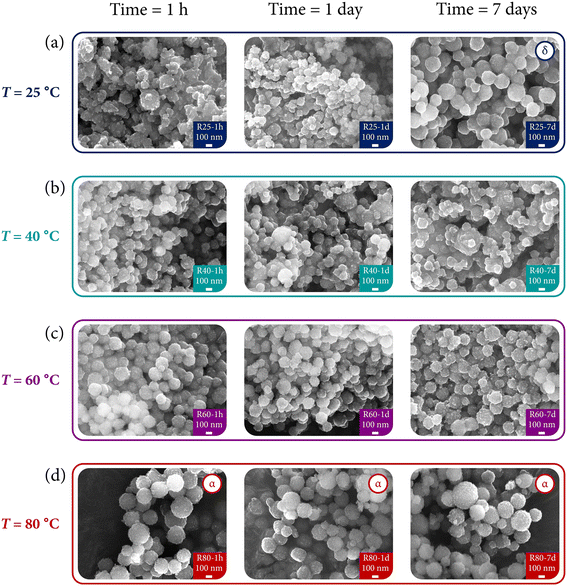
Given the structural results, it is of interest to analyze the morphology of the particles in order to illustrate their evolution and corroborate the kinetic/thermodynamic effects. Fig. 7 presents the SEM images of the Eu3+-doped powders prepared under different reaction conditions of time and temperature.Fig. 7(a) shows the micrographs of samples synthesized at 25 °C with different maturing times. Sample R25-1 h is mainly composed of particle aggregates without a morphology clearly defined. Notwithstanding, with the evolution of time, the aggregates tend to form spherical particles, with the most notable effect appreciated in sample R25-7 d (single δ-phase), in which the coalescence of spheres is demonstrated. Thus, not only does the kinetics serve as a director of the crystal structure but also as a morphological modulator.
Regarding Fig. 7(b), i.e., samples synthesized at 40 °C, no substantial changes can be appreciated depending on the reaction time. However, a major degree of order (particles with more regular shapes instead of large aggregates) is formed at this temperature. Compare for instance sample R40-1 h with R25-1 h.
In a similar way to the results issued by the XRD analysis, where no difference was appreciated among the samples at different reaction times, the SEM images of Fig. 7(c) corresponding to powders obtained at 60 °C reflect the same behavior. As previously commented with samples of Fig. 7(b), the temperature (and, thus the thermodynamic factor) also contributes to the formation of more regular sphere-shaped particles.
Finally, Fig. 7(d) underscores two main points: (1) the good correlation between the structural and morphological characterization, and (2) the prevalence of the thermodynamic control over the particle shapes. Interestingly, all the powders (which present the α-KY3F10 polymorph) exhibit the same morphology regardless of the reaction time: spherical particles resulted from the self-assembly of subunits around 20 nm. More intriguing is the fact that this morphology is the same that has been possible to obtain under the aforementioned process of synthesis mediated by sonochemistry.22
Hence, the evolution of the polymorphs is governed by kinetic and/or thermodynamic control, also reflected in the evolution of the morphology of particles. As a schematic representation, Fig. 8 summarizes the above effects. At the beginning (sample R25-1 h), a mixture of both α and δ phases is obtained, but with the incorporation of the thermodynamic (temperature) or kinetic (time) factors it is possible to modulate the formation of a single crystal phase.
 | ||
| Fig. 8 Schematic representation of the influence of the thermodynamic (temperature) and kinetic (time) factors that allow to obtain both crystal phases. | ||
3.3. Photoluminescence properties
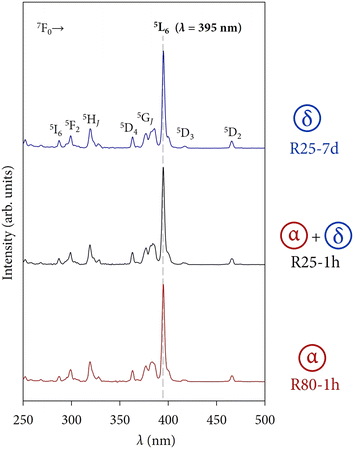
Fig. 9 shows the room temperature excitation spectra for different samples as an example of the δ-phase (R25-7 d), α-phase (R80-1 h), and α + δ (R25-1 h). Regardless of the sample composition, there are no substantial changes in the excitation spectra of the different powders. All the bands can be attributed to the transitions of Eu3+ from the ground 7F0 level to higher excited levels. As outlined with the dashed line, the maximum intensity corresponds to the band associated with the 7F0 → 5L6 transition (395 nm).
In addition, there are no bands associated with charge transfers (CTs) from the anions of the host lattice (F−) to the Eu3+ ion. For Eu3+-doped fluorides, the F−–Eu3+ CT appears at high-energy positions in comparison with oxidic matrices because of the band gap of compounds and the optical electronegativity of the ions implied.28–30 These CTs usually have low intensity and are expected to appear in the 150–170 nm spectral region so they might be difficult to detect sometimes due to instrumental limitations.31 Therefore, the emission spectra of the powders have been recorded under an excitation wavelength of 395 nm.
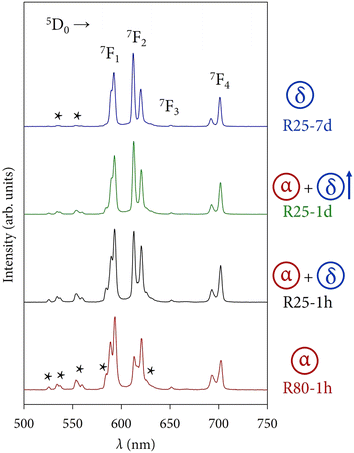
Regarding the emission profiles of the powders, Fig. 10 depicts a selection of four spectra as an example to appreciate at first glance the modulation and changes in the photoluminescence response of the compounds depending on the polymorph(s). The observed bands correspond to the common 5D0 → 7FJ transitions of Eu3+ but, due to the different electronic nature of the α and δ phases, the presence of bands associated with transitions arising from the higher excited state 5D1 can also be observed (marked with black stars in Fig. 10).32 The presence of transitions from higher excited states is attributed to the typical low phonon energies of fluorides (300–550 cm−1).33,34 The internal vibrations of the host lattice are low enough to avoid fast and non-radiative multiphonon relaxation processes from the 5L6 state to the lowest-lying excited state 5D0, allowing the observation of such 5D1 → 7FJ emissions.35,36
Sample R80-1 h (α) is dominated by the 5D0 → 7F1 magnetic dipole transition, whose main peak appears at 395 nm. Samples R25-1 h (α + δ) and R25-1 d (α + δ, with a major contribution of the δ-phase) exhibit an intermediate optical response between the two crystal phases. It can be well appreciated how the peaks corresponding to the 5D1 → 7FJ transitions start to almost disappear and the 5D0 → 7F2 electric dipole transition (with the most intense peak at 621 nm) becomes more prominent. Finally, the photoluminescence of sample R25-7 d (δ) is governed by the 5D0 → 7F2 transition and the 5D1 → 7FJ emissions are still present, albeit with very low intensity, a fact that will be directly related to the lifetimes of these states in the next section.
The great advantage is that these physicochemical parameters (such as the well-known asymmetry ratio R, or the Judd Ofelt parameters) can be directly extracted from the emission spectra, a fact that allows for the avoidance of many complex mathematical and computational calculations, as it is the case in other rare earth ions.
Probably, the most popular parameter of Eu3+ is the asymmetry ratio, which can be calculated from the ratio between the integrated intensities of the 5D0 → 7F2 electric dipole (ED) and 5D0 → 7F1 magnetic dipole (MD) transitions. This ED transition has a hypersensitive character to the ion's environment, while the intensity of the above MD transition is relatively independent of the Eu3+ symmetry for a given material. Thereby, R provides some useful information about the local symmetry of the luminescent ion.
In direct connection with the structural analysis from the emission spectrum of the luminescent centers, the Judd Ofelt (JO) theory and the corresponding parameters emerge as a robust framework for understanding and predicting the optical properties of rare earth ions. The Ω2 parameter is linked to the polarizability and covalent nature of the RE3+ ion, offering valuable insights into the surrounding crystal structure of Eu3+. Consequently, it is regarded as a parameter that primarily reflects the local environment.39 In fact, Ω2 ∝ R for the europium ions of the material under consideration.40 Hence, larger values of R and Ω2 tend to indicate lower symmetry of Eu3+ ions in the host lattice.41,42 On the other hand, Ω4 is known as the long-range JO parameter because it is sensitive to macroscopic properties such as viscosity and rigidity.43
The above parameters can be calculated by making use of some physical equations,44,45 although recent application software packages incorporate them and highly facilitate the calculus.46,47 The JO and R parameters were calculated using the LUMPAC software package and are presented in Table 3. It must be noted that these parameters refer exclusively to the 5D0 → 7FJ transitions. Therefore, to accurately calculate them, they were extracted from the emission spectra of the samples that were recorded again setting a detector delay time (DT) of 10 ms, which ensures the avoidance of contribution from the higher excited state 5D1.34 An example of the emission spectrum at different DTs is presented in Fig. S4 of the ESI.†
| T (°C) | R | Ω 2 (10−20 cm2) | Ω 4 (10−20 cm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 hour | 1 day | 7 days | 1 hour | 1 day | 7 days | 1 hour | 1 day | 7 days | |
| 25 | 1.11 | 1.24 | 1.49 | 2.02 | 2.19 | 2.62 | 1.87 | 1.90 | 1.99 |
| 40 | 1.09 | 1.12 | 1.17 | 1.89 | 1.99 | 2.08 | 1.87 | 1.99 | 1.94 |
| 60 | 1.08 | 1.07 | 1.05 | 1.97 | 1.94 | 1.88 | 2.04 | 2.00 | 1.91 |
| 80 | 0.99 | 0.95 | 0.92 | 1.82 | 1.78 | 1.69 | 1.86 | 1.89 | 1.92 |
As mentioned, Ω2 ∝ R, therefore, the discussion of the obtained results will be addressed for the asymmetry ratio (the same conclusions can be extracted from the analysis of Ω2). Theoretically, the Eu3+ ions occupying the yttrium positions in the α phase would have an ideal C4v local symmetry, while it would be C2v for Eu3+ ions incorporated into the δ phase. As a result, it is expected to obtain, a priori, lower values of R (more symmetric sites) for samples containing major fractions of the α phase.
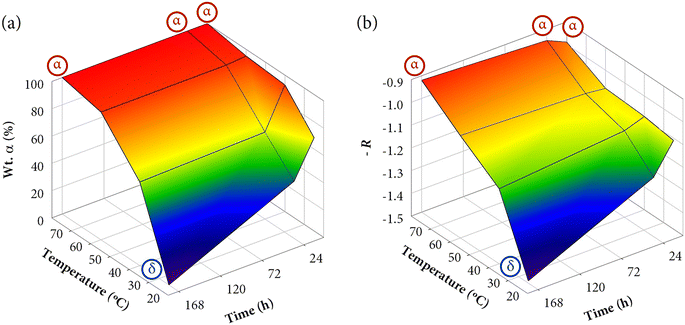
The analysis of the results presented in Table 3 confirms this hypothesis. The evolution of the α/δ content was more remarkable in samples synthesized at 25 and 40 °C. For example, the weight fractions of α phase in samples R25-1 h (68.3%), R25-1 d (41.9%), and R25-7 d (0%) are directly related to the occupation of C2v sites in a major proportion, as reflected in the increase of the asymmetry ratio values: R25-1 h (1.11), R25-1 d (1.24), and R25-7 d (1.49). To appreciate at first glance the correlation between the weight fractions and R, 3D plots (fraction-time–temperature and asymmetry-time–temperature) are depicted in Fig. 11. For visual purposes, instead of plotting the R values, it has been plotted the negation of the asymmetry ratio (i.e., −R). Thus, higher values of −R indicate more symmetry in the crystal environment of Eu3+ ions (more fraction of α phase).
On the other hand, it must be recalled that the Ω4 JO parameter is ascribed to bulk properties. For this reason, due to the wide range of morphologies observed and other macroscopic properties that can present the materials, it is complicated to establish a direct relationship among the different series of samples.
Herein, taking advantage of the presence of 5D1 → 7FJ transitions, it is possible to measure the lifetimes of the materials for different excited states (5D0 and 5D1) since their sensitivity to the structural changes might be different. Thus, it is pretended to extract more information about the photoluminescence properties of the compounds and check to what extent such an inherent relationship can be established.
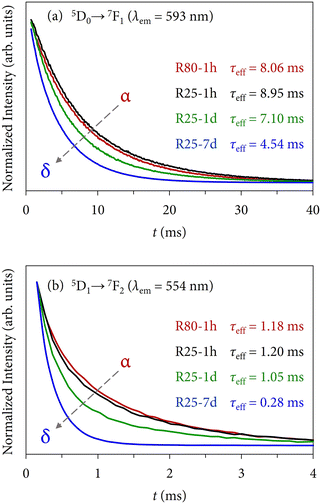
In direct connection with the emission results previously discussed, Fig. 12 displays the decay curves of some selected samples (the same used in Fig. 10). For the 5D0 state, Fig. 12(a), the emission was collected at 593 nm (5D0 → 7F1 transition), while for the 5D1, Fig. 12(b), it was collected at 554 nm (5D1 → 7F2 transition). At first glance, it can be observed that the decay profiles change during the phase transformation. In both cases, the red (R80-1 h, α) and blue curves (R25-7 d, δ) are very different. The decay curves were best fitted to a double exponential model (R2 ≥ 0.999) following the expression:
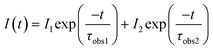 | (1) |
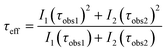 | (2) |
| T (°C) | τ eff 5D0 → 7F1 (ms) | τ eff 5D1 → 7F2 (ms) | ||||
|---|---|---|---|---|---|---|
| 1 hour | 1 day | 7 days | 1 hour | 1 day | 7 days | |
| 25 | 8.95(6) | 7.10(4) | 4.54(1) | 1.20(3) | 1.05(3) | 0.28(4) |
| 40 | 8.72(4) | 7.87(3) | 7.49(3) | 1.15(2) | 1.09(3) | 1.03(2) |
| 60 | 8.63(4) | 9.15(1) | 9.25(5) | 1.17(3) | 1.23(2) | 1.22(2) |
| 80 | 8.06(3) | 8.37(1) | 8.68(4) | 1.18(2) | 1.20(2) | 1.23(2) |
The results indicate that the lifetimes corresponding to the 5D1 state are shorter than those of the 5D0, a phenomenon strictly linked to the cross-relaxation process in which Eu3+ ions populating the higher excited level decay to the metastable 5D0.52 Further information about the fitting procedure can be found in Section S4 of the ESI.†
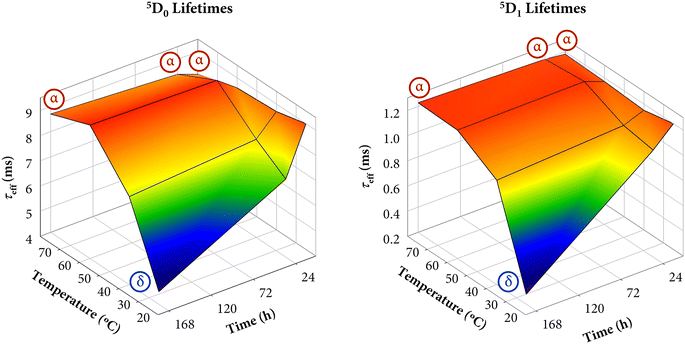
First, the 5D0 lifetimes will be analyzed, which are also in good agreement with the XRD and SEM results. Samples synthesized at 25 °C display a progressive shortening of the lifetimes (from 8.95 ms of the α + δ mixture to 4.54 ms of single δ). Similarly, samples obtained at 40 °C also exhibit shorter lifetimes with the evolution of time, which can be well ascribed to the major presence of the δ-phase. On the other hand, although the XRD results showed that the crystal structure composition of samples synthesized at 60 °C (mainly α + some minor contribution of δ) and at 80 °C (α) was constant regardless of the maturing time because the thermodynamic control governed the reaction pathways, there is a tendency in the lifetimes to become longer as the reaction time increases, which is the opposite trend in comparison with the above series of samples. A priori, one could think that this fact could be associated with morphological aspects, however, the micrographs outlined that there were no substantial changes among each series of samples. Therefore, the main possible explanation could be associated with a local rearrangement or ordering of Eu3+ ions when the precipitate is exposed to high reaction times that allow for a more uniform distribution of the dopant in the crystal lattice, as might be inferred from the asymmetry ratio and the Ω2 JO parameter, see Table 3. Thus, even though the thermodynamics govern the crystallization of the compound in the latter samples, the kinetics (maturation time) has also some particular influence.
Moreover, it is interesting to note that when both polymorphs coexist in the sample, slightly longer lifetimes are obtained. The most evident examples can be found comparing samples synthesized in 1 h at different temperatures: the τeff shortens progressively from 8.95 ms (R25-1 h) to 8.06 ms (R80-1 h). This behavior has also been reported previously21 and might be related to different coupling mechanisms between Eu3+ ions embedded in the different host lattices that, as a result, tend to elongate the lifetime.
On the other hand, regarding the lifetimes associated with the 5D1 state, similar conclusions can be drawn. However, the optical response of the 5D1 → 7FJ transitions is primarily attributed to the presence of Eu3+ ions in α-KY3F10 with an almost negligible contribution of the dopant in δ-KY3F10 (as depicted in Fig. 10 for sample R25-7 d). Therefore, the variation of the 5D1 lifetime values in the different series of samples is less notable (τeff ≈ 1.0–1.2 ms) in comparison to the 5D0 lifetimes. Remarkably, a drastic change is observed for sample R25-7 d (δ-KY3F10) whose lifetime is 0.28 ms.
Additionally, 3D plots (lifetime-time–temperature) are depicted in Fig. 13 to compare the kinetic and thermodynamic effects on the resulting lifetimes. As can be appreciated, both graphics evince the perfect relationship and good agreement with the tendency observed in the results previously discussed. The color gradient is very useful to easily interpret the changes. Moreover, they closely resemble the 3D plots of Fig. 11 (weight fractions of α-KY3F10 and asymmetry ratio R).
4. Conclusions
To control and follow the transition-phase pathway of the two existent polymorphs of KY3F10 (α and δ), an enhanced coprecipitation strategy has been used to synthesize different Eu3+-doped powders, which contain a mixture of both crystal phases in different proportions or single-phases. The kinetics and thermodynamics effects have been able to study varying reaction conditions of temperature (25–80 °C) and time (1 hour to 7 days).The results underscored the competitiveness between kinetics and thermodynamics. At low reaction temperature (25 °C), it is possible to modulate the crystal structure with the maturing time and the metastable δ-phase can be isolated after 7 days. However, when the temperature starts to increase (40 °C) the thermodynamic control becomes more prominent. Although the kinetics still play an important role and an evolution toward the formation of the δ-phase is evident with the reaction time, it is no longer possible to isolate this compound and only a mixture of polymorphs is obtained. Finally, higher working temperatures change dramatically the system and evidence the total thermodynamic control of the reaction pathway. Indeed, the α polymorph is always obtained at 80 °C regardless of the maturing time.
The microstructural analysis of the powders matches very well with the above structural results, being able to observe the influence of thermodynamics and kinetics in the particle morphology.
Finally, bearing in mind the good adequacy of the Eu3+ ion to act as a sensitive structural probe, the optical response of the prepared materials is in direct connection with the presence of one or both polymorphs and thus it serves as a perfect tool to control the kinetics and thermodynamics effects in the reaction pathway.
Author contributions
P. Serna Gallén: conceptualization, methodology, investigation, writing (original draft, review & editing). E. Cordoncillo and H. Beltrán-Mir: conceptualization, funding acquisition, writing (review & editing).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported financially by the Spanish MCIN (Grant PID2020-116149GB-I00 funded by MCIN/AEI/10.13039/501100011033). P. Serna-Gallén also thanks the Spanish MCIN for an FPU predoctoral contract (FPU18/04511 funded by MCIN/AEI/10.13039/501100011033).References
- M. Bianchini, J. Wang, R. J. Clément, B. Ouyang, P. Xiao, D. Kitchaev, T. Shi, Y. Zhang, Y. Wang, H. Kim, M. Zhang, J. Bai, F. Wang, W. Sun and G. Ceder, The interplay between thermodynamics and kinetics in the solid-state synthesis of layered oxides, Nat. Mater., 2020, 19, 1088–1095, DOI:10.1038/s41563-020-0688-6.
- B. Hu, S. Sridar, L. Hao and W. Xiong, A new thermodynamic modeling of the Ti–V system including the metastable ω phase, Intermetallics, 2020, 122, 106791, DOI:10.1016/j.intermet.2020.106791.
- D. Rabadjieva, K. Sezanova, R. Gergulova, R. Titorenkova and S. Tepavitcharova, Precipitation and phase transformation of dicalcium phosphate dihydrate in electrolyte solutions of simulated body fluids: Thermodynamic modeling and kinetic studies, J. Biomed. Mater. Res., Part A, 2020, 108, 1607–1616, DOI:10.1002/jbm.a.36929.
- Q. Luo, Y. Guo, B. Liu, Y. Feng, J. Zhang, Q. Li and K. Chou, Thermodynamics and kinetics of phase transformation in rare earth–magnesium alloys: A critical review, J. Mater. Sci. Technol., 2020, 44, 171–190, DOI:10.1016/j.jmst.2020.01.022.
- T. Wang, Q. Fan and J. Zhu, Steering On-Surface Reactions by Kinetic and Thermodynamic Strategies, J. Phys. Chem. Lett., 2023, 14, 2251–2262, DOI:10.1021/acs.jpclett.3c00001.
- V. V. Gostishchev, I. A. Astapov, A. V. Seredyuk, S. N. Khimukhin and R. Hosen, High-temperature synthesis of composites based on nickel aluminides, Inorg. Mater., 2016, 52, 419–422, DOI:10.1134/S0020168516040051.
- X. Gong, H. Noh, N. C. Gianneschi and O. K. Farha, Interrogating Kinetic versus Thermodynamic Topologies of Metal-Organic Frameworks via Combined Transmission Electron Microscopy and X-ray Diffraction Analysis, J. Am. Chem. Soc., 2019, 141, 6146–6151, DOI:10.1021/jacs.9b01789.
- H. Liu, J. Han, C. McBean, C. S. Lewis, P. Kumar Routh, M. Cotlet and S. S. Wong, Synthesis-driven, structure-dependent optical behavior in phase-tunable NaYF4:Yb,Er-based motifs and associated heterostructures, Phys. Chem. Chem. Phys., 2017, 19, 2153–2167, 10.1039/c6cp07648c.
- B. Shao, Q. Zhao, Y. Jia, W. Lv, M. Jiao, W. Lü and H. You, A novel synthetic route towards monodisperse β-NaYF4:Ln3+ micro/nanocrystals from layered rare-earth hydroxides at ultra low temperature, Chem. Commun., 2014, 50, 12706–12709, 10.1039/c4cc05191b.
- D. Gentili, M. Gazzano, M. Melucci, D. Jones and M. Cavallini, Polymorphism as an additional functionality of materials for technological applications at surfaces and interfaces, Chem. Soc. Rev., 2019, 48, 2502–2517, 10.1039/c8cs00283e.
- R. Alvarez-Roca, A. F. Gouveia, C. C. De Foggi, P. S. Lemos, L. Gracia, L. F. Da Silva, C. E. Vergani, M. San-Miguel, E. Longo and J. Andrés, Selective Synthesis of α-, β-, and γ-Ag2WO4 Polymorphs: Promising Platforms for Photocatalytic and Antibacterial Materials, Inorg. Chem., 2021, 60, 1062–1079, DOI:10.1021/acs.inorgchem.0c03186.
- KY3F10 Crystal Structure: Datasheet from PAULING FILE Multinaries Edition – 2012 in Springer Materials, ed. P. Villars and K. Cenzual, (https://materials.springer.com/isp/crystallographic/docs/sd_0552093).
- δ-KY3F10·xH2O (KY3F10[H2O]) Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition – 2012” in Springer Materials, ed. P. Villars and K. Cenzual, (https://materials.springer.com/isp/crystallographic/docs/sd_1004004).
- F. Le Berre, E. Boucher, M. Allain and G. Courbion, Synthesis, stability and zeolitic behavior of δ-ALn3F10,xH2O and γ-ThLn2F10,H2O phases (Ln=lanthanide), J. Mater. Chem., 2000, 10, 2578–2586, 10.1039/b002520h.
- L. Zhu, J. Meng and X. Cao, Sonochemical synthesis of monodispersed KY3F10:Eu3+ nanospheres with bimodal size distribution, Mater. Lett., 2008, 62, 3007–3009, DOI:10.1016/j.matlet.2008.01.096.
- C. Cao, Hydrothermal synthesis, phase evolution, and optical properties of Eu3+-doped KF-YF3 system materials, J. Mater. Res. Soc., 2012, 27, 2988–2995, DOI:10.1557/jmr.2012.331.
- S. Goderski, M. Runowski and S. Lis, Synthesis of luminescent KY3F10 nanopowder multi-doped with lanthanide ions by a co-precipitation method, J. Rare Earths, 2016, 34, 808–813, DOI:10.1016/S1002-0721(16)60098-4.
- M. Runowski, Color-tunable up-conversion emission of luminescent-plasmonic, core/shell nanomaterials-KY3F10:Yb3+,Tm3+/SiO2-NH2/Au, J. Lumin., 2017, 186, 199–204, DOI:10.1016/j.jlumin.2017.02.032.
- M. Chen, P. Loiko, J. M. Serres, S. Veronesi, M. Tonelli, M. Aguiló, F. Díaz, S. Y. Choi, J. E. Bae, F. Rotermund, S. Dai, Z. Chen, U. Griebner, V. Petrov and X. Mateos, Fluorite-type Tm3+:KY3F10: A promising crystal for watt-level lasers at ∼1.9 μm, J. Alloys Compd., 2020, 813, 152176, DOI:10.1016/j.jallcom.2019.152176.
- P. Serna-Gallén, H. Beltrán-Mir and E. Cordoncillo, The unexplored δ-phase of KY3F10: toward novel Eu3+-doped nanoplates with a ‘super-diamond’ structure for optical applications, J. Mater. Res. Technol., 2021, 15, 6940–6946, DOI:10.1016/j.jmrt.2021.11.060.
- P. Serna-Gallén, H. Beltrán-Mir, E. Cordoncillo, R. Balda and J. Fernández, A site-selective fluorescence spectroscopy study of the crystal phases of KY3F10: Leveraging the optical response of Eu3+ ions, J. Alloys Compd., 2023, 953, 170020, DOI:10.1016/j.jallcom.2023.170020.
- P. Serna-Gallén, H. Beltrán-Mir and E. Cordoncillo, The pH-dependent reactions in the sonochemical synthesis of luminescent fluorides: The quest for the formation of KY3F10 crystal phases, Ultrason. Sonochem., 2022, 87, 106059, DOI:10.1016/j.ultsonch.2022.106059.
- P. T. Cardew, Ostwald Rule of Stages–Myth or Reality?, Cryst. Growth Des., 2023, 23, 3958–3969, DOI:10.1021/acs.cgd.2c00141.
- S. Chen, H. Xi and L. Yu, Cross-nucleation between ROY polymorphs, J. Am. Chem. Soc., 2005, 127, 17439–17444, DOI:10.1021/ja056072d.
- J. Russo and H. Tanaka, Selection mechanism of polymorphs in the crystal nucleation of the Gaussian core model, Soft Matter, 2012, 8, 4206–4215, 10.1039/c2sm07007c.
- S. F. S. P. Looijmans, D. Cavallo, L. Yu and G. W. M. Peters, Cross-Nucleation between Polymorphs: Quantitative Modeling of Kinetics and Morphology, Cryst. Growth Des., 2018, 18, 3921–3926, DOI:10.1021/acs.cgd.8b00254.
- C. Desgranges and J. Delhommelle, Molecular mechanism for the cross-nucleation between polymorphs, J. Am. Chem. Soc., 2006, 128, 10368–10369, DOI:10.1021/ja063218f.
- G. Blasse, The europium(III)-fluorine charge-transfer transition, J. Phys. Chem. Solids, 1989, 50, 99, DOI:10.1016/0022-3697(89)90479-4.
- R. R. Reddy, Y. N. Ahammed, K. R. Gopal and D. V. Raghuram, Optical electronegativity and refractive index of materials, Opt. Mater., 1998, 10, 95–100, DOI:10.1016/S0925-3467(97)00171-7.
- P. Dorenbos, The Eu3+ charge transfer energy and the relation with the band gap of compounds, J. Lumin., 2005, 111, 89–104, DOI:10.1016/j.jlumin.2004.07.003.
- R. T. Wegh, H. Donker, K. D. Oskam and A. Meijerink, Visible quantum cutting in Eu3+-doped gadolinium fluorides via downconversion, J. Lumin., 1999, 82, 93–104, DOI:10.1126/science.283.5402.663.
- K. Binnemans, Interpretation of europium(III) spectra, Coord. Chem. Rev., 2015, 295, 1–45, DOI:10.1016/j.ccr.2015.02.015.
- A. Gulzar, J. Xu, P. Yang, F. He and L. Xu, Upconversion processes: Versatile biological applications and biosafety, Nanoscale, 2017, 9, 12248–12282, 10.1039/c7nr01836c.
- P. Serna-Gallén, H. Beltrán-Mir and E. Cordoncillo, Tuning the optical and photoluminescence properties of high efficient Eu3+-doped KY3F10 phosphors by different synthetic approaches, Opt. Laser Technol., 2021, 136, 106734, DOI:10.1016/j.optlastec.2020.106734.
- T. Yamase, T. Kobayashi, M. Sugeta and H. Naruke, Europium(III) Luminescence and Intramolecular Energy Transfer Studies of Polyoxometalloeuropates, J. Phys. Chem. A, 1997, 101, 5046–5053 CrossRef CAS.
- R. G. Geitenbeek, H. W. De Wijn and A. Meijerink, Non-Boltzmann Luminescence in NaYF4:Eu3+: Implications for Luminescence Thermometry, Phys. Rev. Appl., 2018, 10, 1, DOI:10.1103/PhysRevApplied.10.064006.
- S. K. Gupta, C. Reghukumar and R. M. Kadam, Eu3+ local site analysis and emission characteristics of novel Nd2Zr2O7:Eu phosphor: Insight into the effect of europium concentration on its photoluminescence properties, RSC Adv., 2016, 6, 53614–53624, 10.1039/c6ra11698a.
- J. Cheng, J. Cheng, S. Zhang, C. Ma, X. Bian and Z. Zhai, Photoluminescence, site occupation and optical thermometry of Ba2La6Y2(SiO4)6O2: Eu3+ phosphors with high thermal stability, J. Lumin., 2022, 252, 119265, DOI:10.1016/j.jlumin.2022.119265.
- T. Lan, T. Han, D. Jiang, Y. Wen, X. Y. Sun, Z. Hua, S. Qian, H. Ban, H. Cai, J. Han, H. Liu, S. Liu, L. Ma, L. Qin, J. Ren, G. Tang, Z. Wang, Z. Le Wang and Y. Zhu, Study on the luminescent properties of Eu3+-doped TeO2-GeO2-BaO scintillation glasses, Opt. Mater., 2022, 133, 113000, DOI:10.1016/j.optmat.2022.113000.
- P. Serna-Gallén, H. Beltrán-Mir and E. Cordoncillo, Practical guidance for easily interpreting the emission and physicochemical parameters of Eu3+ in solid-state hosts, Ceram. Int., 2023 DOI:10.1016/j.ceramint.2023.01.141.
- D. K. Patel, B. Vishwanadh, V. Sudarsan and S. K. Kulshreshtha, Difference in the Nature of Eu3+ Environment in Eu3+-Doped BaTiO3 and BaSnO3, J. Am. Ceram. Soc., 2013, 96, 3857–3861, DOI:10.1111/jace.12596.
- C. de Mello Donegá, S. A. Junior and G. F. de Sá, Synthesis, luminescence and quantum yields of Eu(III) mixed complexes with 4,4,4-trifluoro-1-phenyl-1,3-butanedione and 1,10-phenanthroline-N-oxide, J. Alloys Compd., 1997, 250, 422–426, DOI:10.1016/S0925-8388(96)02562-5.
- S. K. Gupta, M. A. Penilla Garcia, J. P. Zuniga and Y. Mao, pH induced size tuning of Gd2Hf2O7:Eu3+ nanoparticles and its effect on their UV and X-ray excited luminescence, J. Lumin., 2020, 228, 117605, DOI:10.1016/j.jlumin.2020.117605.
- Y. Tian, B. Chen, R. Hua, J. Sun, L. Cheng, H. Zhong, X. Li, J. Zhang, Y. Zheng, T. Yu, L. Huang and H. Yu, Optical transition, electron-phonon coupling and fluorescent quenching of La2(MoO4)3:Eu3+ phosphor, J. Appl. Phys., 2011, 109, 053511, DOI:10.1063/1.3551584.
- M. Luo, X. Sha, B. Chen, X. Zhang, H. Yu, X. Li, J. Zhang, S. Xu, Y. Cao, Y. Wang, X. Wang, Y. Zhang, D. Gao and L. Wang, Optical transition properties, internal quantum efficiencies, and temperature sensing of Er3+ doped BaGd2O4 phosphor with low maximum phonon energy, J. Am. Ceram. Soc., 2022, 105, 3353–3363, DOI:10.1111/jace.18299.
- J. D. L. Dutra, T. D. Bispo and R. O. Freire, LUMPAC lanthanide luminescence software: Efficient and user friendly, J. Comput. Chem., 2014, 35, 772–775, DOI:10.1002/jcc.23542.
- A. Ćirić, S. Stojadinović, M. Sekulić and M. D. Dramićanin, JOES: An application software for Judd-Ofelt analysis from Eu3+ emission spectra, J. Lumin., 2019, 205, 351–356, DOI:10.1016/j.jlumin.2018.09.048.
- N. Pathak, S. Mukherjee, B. P. Mandal, A. K. Yadav, S. N. Jha and D. Bhattacharyya, Interplay between local distortion at lattice sites with optical and electrical properties of Eu3+-doped MNbO3 (M = Na and K) compounds, Mater. Adv., 2020, 1, 2380–2394, 10.1039/d0ma00335b.
- M. M. Fernandes, M. Schmidt, T. Stumpf, C. Walther, D. Bosbach, R. Klenze and T. Fanghänel, Site-selective time-resolved laser fluorescence spectroscopy of Eu3+ in calcite, J. Colloid Interface Sci., 2008, 321, 323–331, DOI:10.1016/j.jcis.2008.01.017.
- Y. Hui, Y. Zhao, S. Zhao, L. Gu, X. Fan, L. Zhu, B. Zou, Y. Wang and X. Cao, Fluorescence of Eu3+ as a probe of phase transformation of zirconia, J. Alloys Compd., 2013, 573, 177–181, DOI:10.1016/j.jallcom.2013.03.248.
- P. Li, Y. Zhang, L. Zhang, F. Li, Y. Guo, Y. Li and W. Gao, Phase Control of Eu3+-Doped YPO4 Nano−/Microcrystals, Cryst. Growth Des., 2017, 17, 5935–5944, DOI:10.1021/acs.cgd.7b01038.
- X. Liu, L. Yan and J. Lin, Tunable Photoluminescence and Cathodoluminescence Properties of Eu3+-Doped LaInO3 Nanocrystalline Phosphors, J. Electrochem. Soc., 2009, 156, 1–6, DOI:10.1149/1.3002378.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ce00614j |
| This journal is © The Royal Society of Chemistry 2023 |