Open Access Article
Open Access ArticleSubstituted benzophenone imines for COF synthesis via formal transimination†‡
Josefine
Sprachmann
 a,
Niklas
Grabicki
a,
Niklas
Grabicki
 a,
Anna
Möckel
a,
Jeremy
Maltitz
a,
José Refugio
Monroy
a,
Anna
Möckel
a,
Jeremy
Maltitz
a,
José Refugio
Monroy
 a,
Glen J.
Smales
a,
Glen J.
Smales
 b and
Oliver
Dumele
b and
Oliver
Dumele
 *a
*a
aDepartment of Chemistry & IRIS Adlershof, Humboldt University of Berlin, Berlin 12489, Germany. E-mail: oliver.dumele@hu-berlin.de
bBundesanstalt für Materialforschung und -prüfung (BAM), Berlin 12205, Germany
First published on 18th October 2023
Abstract
Covalent organic frameworks (COFs) are a prominent class of organic materials constructed from versatile building blocks via reversible reactions. The quality of imine-linked COFs can be improved by using amine monomers protected with benzophenone forming benzophenone imines. Here, we present a study on substituted benzophenones in COF synthesis via formal transimination. 12 para-substituted N-aryl benzophenone imines, with a range of electron-rich to electron-poor substituents, were prepared and their hydrolysis kinetics were studied spectroscopically. All substituted benzophenone imines can be employed in COF synthesis and lead to COFs with high crystallinity and high porosity. The substituents act innocent to COF formation as the substituted benzophenones are cleaved off. Imines can be tailored to their synthetic demands and utilized in COF formation. This concept can make access to previously unattainable, synthetically complex COF monomers feasible.
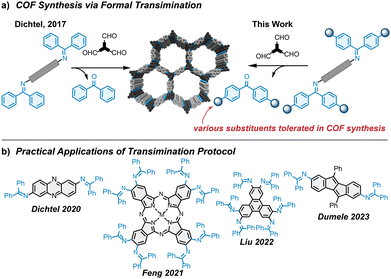
Covalent organic frameworks (COFs) are an emerging class of organic materials.1,2 Linked by dynamic covalent bonds, they are constructed from organic building blocks yielding permanently porous, crystalline, and stable frameworks. Their modular design allows for precise tailoring of the framework structure and properties.3 Owing to these properties, COFs are promising organic materials for various applications including energy storage,4,5 catalysis,6 gas storage and separation,7 and sensing.8 In imine-linked COFs, multivalent amines and aldehydes reversibly form imine bonds, ultimately leading to a crystalline, stable COF. However, the mechanisms of COF formation are complex9–11 and not well understood. Tedious optimizations of reaction conditions are often required to obtain materials of high crystallinity and porosity. Employing specific rigid or geometrically constrained monomers,10 metal triflate catalysis,12 using modulators,13 or reconstruction of COFs14 are strategies that have been developed to improve the quality of COFs.9,10 Dichtel and coworkers proposed a strategy generally applicable to imine COFs, where multivalent free amines are replaced by N-aryl benzophenone imines, which react with aldehydes in a formal transimination reaction yielding high-quality COFs (Fig. 1a).15 This strategy of using benzophenone-protected amines has two key advantages: (1) higher porosity is achieved in comparison to a conventional reaction between a free amine and aldehyde; (2) benzophenone imines are more easily obtained and purified,16 with higher stability than their free amine counterparts.
So far, this strategy has only been utilized on a few examples while most COFs published until now, were constructed from free amines (Fig. 1b). Dichtel and coworkers have used a benzophenone imine substituted phenazine to construct a redox-active COF.17 Feng and coworkers have utilized a benzophenone imine-decorated phthalocyanine in the formation of a conjugated COF to achieve suitable crystallinity.18 For Liu's tribenzimidazole-linked COF, the use of benzophenone imines was hypothesized to ensure a slow release of the COF node before an irreversible reaction locks the COF-linkage.19 In our lab, for the synthesis of antiaromatic dibenzopentalene (DBP) COFs, the use of benzophenone imine protection was essential as a diamino-DBP is not stable and degrades immediately at ambient conditions.20 Hence, the use of benzophenone imines makes the synthesis of a robust, stable COF from an unstable building block feasible.
Until now, the formal transimination strategy has been limited to pristine benzophenone imines bearing no further substituents, though expanding this concept beyond benzophenone opens up new opportunities. One example has been shown recently where a COF building block equipped with benzophenone imines with –OiPr groups for solubility was successfully used.20 This is the first demonstration of how difficulties in the synthesis of complex COF building blocks can be tackled by functionalization of benzophenone imines. Another example could be conceived where electron-withdrawing groups could help further stabilize oxidatively unstable electron-rich COF building blocks. At the same time, quality optimization of the resulting COF is also in the scope of functionalized imines in COF synthesis. The above reflections have encouraged us to systematically explore the use of substituted imines in the synthesis of COFs via formal transimination. We synthesized a series of 12 N-aryl benzophenone imines with electron donating or -withdrawing groups in para-position. COFs with excellent crystallinity and porosity were constructed from these imines, showing that benzophenone substituents are compatible and innocent in COF formation. While electron donating and -withdrawing groups on the benzophenone moiety influence the rate constant of imine hydrolysis, the quality of COF is not clearly affected. The work shows that a variety of imines is compatible with COF synthesis, offering potential solutions for synthetically challenging COFs.
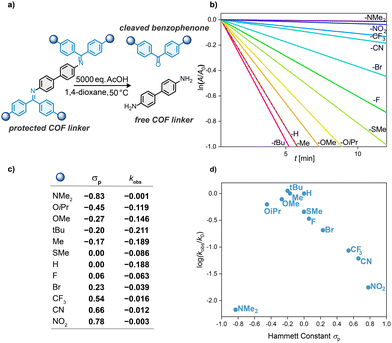
Motivated by the well-studied nonlinear Hammett plot of imine hydrolysis,21–23 we aimed at a substituent analysis with benzophenone imines to investigate the hypothesis of a slow-release mechanism.15 For a systematic substituent analysis we selected the N-benzophenone imines of benzidine (BND) and synthesized a series of benzophenones 1a–l containing electron-withdrawing and electron-donating functional groups in para-position (Scheme 1). The benzophenones 1a–l were coupled to BND, using DABCO and TiCl4.15,24 The reactions gave the imines in moderate to good yields, and the crude products were conveniently purified by recrystallization, avoiding the use of column chromatography. We obtained 12 previously unreported BND-benzophenone imines spanning the Hammett scale from the electron-rich, –NMe2 substituted 2a to the electron-poor, –NO2 substituted 2l. All imines show excellent stability in ambient conditions and exhibit an improved solubility compared to the free-amine BND. Especially OiPr-containing 2b and OMe-containing 2c show significantly enhanced solubility. Single-crystals suitable for single-crystal X-ray crystallography of CF3-substituted imine 2j and unsubstituted imine 2g were grown giving unambiguous proof for the structure of the imines.
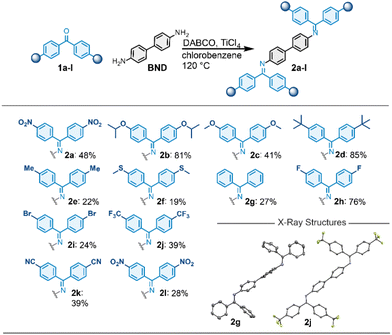 | ||
| Scheme 1 Synthesis of N-benzidine benzophenone imines 2a–l including crystal structures of imines 2g and 2j. | ||
The hydrolysis rates of imine bonds under reaction conditions similar to COF reaction conditions were investigated by following the intensity change at one wavelength using UV-vis spectroscopy (50 °C, 1,4-dioxane, 5000 equiv. AcOH, Fig. 2a and b). These conditions were chosen as the exact COF reaction conditions are not compatible with a spectroscopic setup (biphasic system, 120 °C, above boiling point in a pressurized tube). The hydrolysis of imines 2a–l occurred with different rate constants kobs dependent on the electronic nature of their substituent (Fig. 2c). Neutral to slightly electron-withdrawing substituents, with Hammett constants from −0.2 to 0, resulted in the highest imine hydrolysis rates whereas the moderately electron-donating substituents, –Me and –tBu, did not impact the reaction rate. Higher Hammett parameters led to slower hydrolysis in an approximately linear trend. With increasingly electron-rich substituents –OMe, –OiPr, and –NMe2, the rate of hydrolysis was decreased (Fig. 2d). The results indicate that the conclusions drawn from earlier Hammett studies on imine formation and hydrolysis21–23 are also viable in COF reaction conditions. This inspired us to study the electronic modulation of the benzophenone moieties for improving COF quality. To investigate the implications on COF formation, we reacted the 12 substituted N-benzidine benzophenone imines 2a–l with 1,3,5-triformylbenzene (TFB). The reaction affords BND-TFB COF, while the substituted benzophenone is being cleaved (Fig. 3). COF synthesis was carried out under solvothermal conditions (mesitylene/1,4-dioxane 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
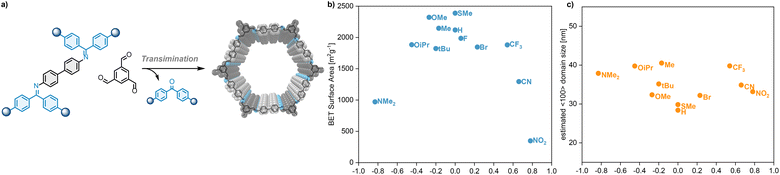
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 120 °C, 3 d). All substituents are tolerated in COF formation, leading to BND-TFB COF (Fig. 3a). Characterization of the material matches with previous reports (ESI,‡ Sections S5 and S6).15,25–27 BND-imines with –OMe, –Me, or –SMe substituent lead to COFs with moderately increased Brunauer–Emmett–Teller surface area (SABET) compared to the COF from unsubstituted benzophenone imine (–H), while other substituents lead to COFs with slightly or markedly decreased SABET. Plotting the Hammett constant of the benzophenone substituents against the SABET of the resulting COF reveals a widely scattered plot (Fig. 3b). The most electron donating substituent –NMe2 and the most electron withdrawing substituents –CN and –NO2 give a comparatively lower SABET. All other entries gave high SABET ranging from 1823 m2 g−1 to 2388 m2 g−1. While surface areas vary considerably, all COFs show high-crystallinity in their powder X-ray diffractograms (Fig. 4). To quantitatively compare crystallinities we used estimated domain sizes as a measure of crystallinity. Scherrer analysis using the full-width half maximum of the (100) reflex, revealed estimated domain sizes between 28 nm and 40 nm, typical values for COFs. The domain sizes are uniformly scattered showing that all substituents across the Hammett scale act innocent in COF formation, leading to high-quality materials (Fig. 3c). Contrary to our initial consideration, the quality of a COF is not modulated by the electronic nature of the protecting imine but gives reliable high-quality materials in the substituent range from –OiPr to –CF3 (−0.45 < σp < 0.54) throughout. This suggests that the mechanism by which formal transimination improves the COF quality is more complex than the previously proposed slow-release mechanism.15 The practical implication of this result lies in the variety of substituents that are tolerated in COF formation. Imine groups can be tailored to any synthetic demands, enabling the incorporation of building blocks that are not attainable with conventional methods. For instance, the –OiPr group can solubilize otherwise poorly soluble monomers. Substitution with electron-withdrawing functional groups (–CN, –NO2) can improve stability of particularly electron-rich monomers.
1, 120 °C, 3 d). All substituents are tolerated in COF formation, leading to BND-TFB COF (Fig. 3a). Characterization of the material matches with previous reports (ESI,‡ Sections S5 and S6).15,25–27 BND-imines with –OMe, –Me, or –SMe substituent lead to COFs with moderately increased Brunauer–Emmett–Teller surface area (SABET) compared to the COF from unsubstituted benzophenone imine (–H), while other substituents lead to COFs with slightly or markedly decreased SABET. Plotting the Hammett constant of the benzophenone substituents against the SABET of the resulting COF reveals a widely scattered plot (Fig. 3b). The most electron donating substituent –NMe2 and the most electron withdrawing substituents –CN and –NO2 give a comparatively lower SABET. All other entries gave high SABET ranging from 1823 m2 g−1 to 2388 m2 g−1. While surface areas vary considerably, all COFs show high-crystallinity in their powder X-ray diffractograms (Fig. 4). To quantitatively compare crystallinities we used estimated domain sizes as a measure of crystallinity. Scherrer analysis using the full-width half maximum of the (100) reflex, revealed estimated domain sizes between 28 nm and 40 nm, typical values for COFs. The domain sizes are uniformly scattered showing that all substituents across the Hammett scale act innocent in COF formation, leading to high-quality materials (Fig. 3c). Contrary to our initial consideration, the quality of a COF is not modulated by the electronic nature of the protecting imine but gives reliable high-quality materials in the substituent range from –OiPr to –CF3 (−0.45 < σp < 0.54) throughout. This suggests that the mechanism by which formal transimination improves the COF quality is more complex than the previously proposed slow-release mechanism.15 The practical implication of this result lies in the variety of substituents that are tolerated in COF formation. Imine groups can be tailored to any synthetic demands, enabling the incorporation of building blocks that are not attainable with conventional methods. For instance, the –OiPr group can solubilize otherwise poorly soluble monomers. Substitution with electron-withdrawing functional groups (–CN, –NO2) can improve stability of particularly electron-rich monomers.
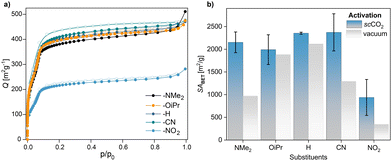 | ||
| Fig. 4 (a) N2 adsorption–desorption isotherms at 77 K and (b) improved SABET of COFs after scCO2 activation compare to SABET after vacuum activation. | ||
In addition to the COF synthesis conditions, the so-called activation process (removal of solvents from the microporous material) plays a crucial role in obtaining high-quality COFs.28,29 Among several methods, activation by supercritical CO2 (scCO2) has proven to be especially superior.30 To shed further light to our study and to further investigate a possible correlation between Hammett parameter and COF quality, we replicated the COF syntheses, this time followed by scCO2 activation. We picked five benzophenone imines across the Hammett scale and repeated the COF synthesis in duplicates. For all examples we achieved significantly higher SABET with this method compared to using vacuum activation (Fig. 4). scCO2 might be more efficient than solvent washes in removing residual benzophenone from the COF pores. With scCO2 activation, using benzophenone imines substituted with –NMe2, –OiPr, –H or –CN all gave very high, similar surface areas, independent of the imine-substituent. Only para-nitro benzophenone imine consistently gave lower porosity COFs of SABET = 939 m2 g−1. The highest surface area for BND-TFB COF was achieved using –CN substituted benzophenone imine 2d. With 2783 m2 g−1, the BET surface area is only 60 m2 g−1 below the theoretical surface area and the highest value for BND-TFB COF reported so far in the literature. It is interesting to note that the reaction of unsubstituted BND-benzophenone imine (R = H) with TFB very consistently yields COFs with a high surface area. In comparison, the functionalized benzophenone imines (R = NMe2, OiPr, CN, NO2) also lead to high surface area COFs though with BET surface areas varying in a wider range for repeated experiments.
In summary, we have prepared a series of 12 para-substituted N-benzidine benzophenone imines and studied their hydrolysis under COF formation conditions. The imines were employed in COF reactions with TFB, all yielding high-quality BND-TFB COF. Unsubstituted benzophenone-imines give the most reliable and reproducible results while substitution with electron-donating or electron-withdrawing groups increases the variance in surface area. Although the Hammet parameter of the substituents clearly influences the hydrolysis rate of the imines, no clear trend can be observed for COF surface areas. The results indicate that electronic effects of the imines are not the decisive factor for COF quality (based on SABET). It is likely that imine hydrolysis occurs too fast for the imines to efficiently act as slow-release agent but they rather act as initial solubilizing group and modulators in the dynamic COF formation reaction. Therefore the hydrolysis rate of the imine monomer does not influence the quality of the resulting COF (i.e. SABET), although the imine hydrolysis rates are in a similar time range as the initial COF formation.31 Further research is necessary to elucidate the complex mechanism of COF formation involving benzophenone imines. We show that para-substitution of benzophenone imines is well tolerated in the synthesis of COFs, emphasizing the generality of the formal transimination approach. This can facilitate the synthesis of previously unattainable, synthetically complex COF monomers as benzophenone imines can be tailored to the synthetic demands, e.g. with solubilizing or electronically stabilizing substituents. Utilizing such substituted benzophenone imines or even imines beyond benzophenone will be a vital tool in the preparation of synthetically demanding high-performance imine COFs in the future.
We thank Edon Vitaku for helpful discussions.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O’Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, 923 CrossRef CAS PubMed.
- N. Huang, P. Wang and D. Jiang, Nat. Rev. Mater., 2016, 1, 16068 CrossRef CAS.
- S. Kandambeth, V. S. Kale, O. Shekhah, H. N. Alshareef and M. Eddaoudi, Adv. Energy Mater., 2022, 12, 2100177 CrossRef CAS.
- H. Gao, A. R. Neale, Q. Zhu, M. Bahri, X. Wang, H. Yang, Y. Xu, R. Clowes, N. D. Browning, M. A. Little, L. J. Hardwick and A. I. Cooper, J. Am. Chem. Soc., 2022, 144, 9434–9442 CrossRef CAS PubMed.
- M. Traxler, S. Gisbertz, P. Pachfule, J. Schmidt, J. Roeser, S. Reischauer, J. Rabeah, B. Pieber and A. Thomas, Angew. Chem., Int. Ed., 2022, 61, e202117738 CrossRef CAS PubMed.
- H. Lyu, H. Li, N. Hanikel, K. Wang and O. M. Yaghi, J. Am. Chem. Soc., 2022, 144, 12989–12995 CrossRef CAS PubMed.
- L. Wei, T. Sun, Z. Shi, Z. Xu, W. Wen, S. Jiang, Y. Zhao, Y. Ma and Y. B. Zhang, Nat. Commun., 2022, 13, 7936 CrossRef CAS PubMed.
- F. Haase and B. V. Lotsch, Chem. Soc. Rev., 2020, 49, 8469–8500 RSC.
- L. Bourda, C. Krishnaraj, P. Van Der Voort and K. Van Hecke, Mater. Adv., 2021, 2, 2811–2845 RSC.
- H. Li, A. M. Evans, W. R. Dichtel and J. L. Bredas, ACS Mater. Lett., 2021, 3, 398–405 CrossRef CAS.
- M. Matsumoto, R. R. Dasari, W. Ji, C. H. Feriante, T. C. Parker, S. R. Marder and W. R. Dichtel, J. Am. Chem. Soc., 2017, 139, 4999–5002 CrossRef CAS PubMed.
- T. Ma, E. A. Kapustin, S. X. Yin, L. Liang, Z. Zhou, J. Niu, L.-H. Li, Y. Wang, J. Su, J. Li, X. Wang, W. D. Wang, W. Wang, J. Sun and O. M. Yaghi, Science, 2018, 361, 48–52 CrossRef CAS PubMed.
- W. Zhang, L. Chen, S. Dai, C. Zhao, C. Ma, L. Wei, M. Zhu, S. Y. Chong, H. Yang, L. Liu, Y. Bai, M. Yu, Y. Xu, X. W. Zhu, Q. Zhu, S. An, R. S. Sprick, M. A. Little, X. Wu, S. Jiang, Y. Wu, Y. B. Zhang, H. Tian, W. H. Zhu and A. I. Cooper, Nature, 2022, 604, 72–79 CrossRef CAS PubMed.
- E. Vitaku and W. R. Dichtel, J. Am. Chem. Soc., 2017, 139, 12911–12914 CrossRef CAS PubMed.
- J. P. Wolfe, J. Åhman, J. P. Sadighi, R. A. Singer and S. L. Buchwald, Tetrahedron Lett., 1997, 38, 6367–6370 CrossRef CAS.
- E. Vitaku, C. N. Gannett, K. L. Carpenter, L. Shen, H. D. Abruña and W. R. Dichtel, J. Am. Chem. Soc., 2020, 142, 16–20 CrossRef CAS PubMed.
- H. Zhong, M. Wang, M. Ghorbani-Asl, J. Zhang, K. H. Ly, Z. Liao, G. Chen, Y. Wei, B. P. Biswal, E. Zschech, I. M. Weidinger, A. V. Krasheninnikov, R. Dong and X. Feng, J. Am. Chem. Soc., 2021, 143, 19992–20000 CrossRef CAS PubMed.
- Q. Zhang, F. Zhang, J. Dong, M. Shao, M. Zhu, D. Wang, Y. Guo, J. Zhang and Y. Liu, Chem. Mater., 2022, 34, 6977–6984 CrossRef CAS.
- J. Sprachmann, T. Wachsmuth, M. Bhosale, D. Burmeister, G. J. Smales, M. Schmidt, Z. Kochovski, N. Grabicki, R. Wessling, E. J. W. List-Kratochvil, B. Esser and O. Dumele, J. Am. Chem. Soc., 2023, 145, 2840–2851 CrossRef CAS PubMed.
- G. M. Santerre, C. J. Hansrote and T. I. Crowell, J. Am. Chem. Soc., 1958, 80, 1254–1257 CrossRef CAS.
- James O. Schreck, J. Chem. Educ., 1971, 48, 103–107 CrossRef CAS.
- E. H. Cordes and W. P. Jencks, J. Am. Chem. Soc., 1962, 84, 832–837 CrossRef CAS.
- M. Higuchi, M. Tsuruta, H. Chiba, S. Shiki and K. Yamamoto, J. Am. Chem. Soc., 2003, 125, 9988–9997 CrossRef CAS PubMed.
- Q. Gao, L. Bai, Y. Zeng, P. Wang, X. Zhang, R. Zou and Y. Zhao, Chem. – Eur. J., 2015, 21, 16818–16822 CrossRef CAS PubMed.
- L. Bai, S. Z. F. Phua, W. Q. Lim, A. Jana, Z. Luo, H. P. Tham, L. Zhao, Q. Gao and Y. Zhao, Chem. Commun., 2016, 52, 4128–4131 RSC.
- L. Bai, Q. Gao and Y. Zhao, J. Mater. Chem. A, 2016, 4, 14106–14110 RSC.
- C. Gropp, S. Canossa, S. Wuttke, F. Gándara, Q. Li, L. Gagliardi and O. M. Yaghi, ACS Cent. Sci., 2020, 6, 1255–1273 CrossRef CAS PubMed.
- D. Zhu and R. Verduzco, ACS Appl. Mater. Interfaces, 2020, 12, 33121–33127 CrossRef CAS PubMed.
- C. H. Feriante, S. Jhulki, A. M. Evans, R. R. Dasari, K. Slicker, W. R. Dichtel and S. R. Marder, Adv. Mater., 2020, 32, 1905776 CrossRef CAS PubMed.
- C. Feriante, A. M. Evans, S. Jhulki, I. Castano, M. J. Strauss, S. Barlow, W. R. Dichtel and S. R. Marder, J. Am. Chem. Soc., 2020, 142, 18637–18644 CrossRef CAS PubMed.
Footnotes |
| † The PXRD data generated or analysed during this study are provided as a Source Data file at https://doi.org/10.5281/zenodo.8337800. |
| ‡ Electronic supplementary information (ESI) available. CCDC 2240743, 1942397 and 2240744. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc03735e. |
| This journal is © The Royal Society of Chemistry 2023 |