Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective adsorption of dihydrogen isotopes on DUT-8 (Ni,Co) monitored by in situ electron paramagnetic resonance†
Muhammad Fernadi
Lukman
 a,
Matthias
Mendt
b,
Volodymyr
Bon
a,
Matthias
Mendt
b,
Volodymyr
Bon
 c,
Stefan
Kaskel
c and
Andreas
Pöppl
c,
Stefan
Kaskel
c and
Andreas
Pöppl
 *a
*a
aFelix Bloch Institute for Solid State Physics, Leipzig University, 04103 Leipzig, Germany. E-mail: poeppl@physik.uni-leipzig.de
bSaxonQ GmbH, Emilienstr. 15, 04107 Leipzig, Germany
cChair of Inorganic Chemistry I, Technische Universität Dresden, 01069 Dresden, Germany
First published on 20th July 2023
Abstract
In situ continuous wave electron paramagnetic resonance investigation has been proven as a powerful method by employing paramagnetic Ni2+–Co2+ pairs as spin probes to follow the isotope-selective gate opening phenomenon on the DUT-8(Ni0.98 Co0.02) framework. This method is very sensitive to detect the phase transition from the closed pore to the open pore phase in response to D2 adsorption in the framework, while no phase transformation has been observed during H2 gas adsorption. More interestingly, it is also able to sense local structural changes around the spin probe during the desorption of D2 gas. Based on these evidences, the in situ continuous wave electron paramagnetic resonance method can be implemented as an efficient and non-invasive technique for the detection of dihydrogen isotopes.
Flexible metal organic frameworks such as MIL-53(Al) and DUT-8(Ni) are considered as very attractive MOFs due to their switchability behaviour towards external stimuli such as the adsorption of specific guest molecules, pressure or temperature.1–5 Recently, Kim and co-workers6 reported a D2-selective breathing behaviour in MIL-53(Al), which is potentially useful for the purification of isotopic mixtures. In addition, some of us also revealed that a flexible DUT-8(Ni) selectively responds to D2 gas, while in a contrasting way, is irresponsive towards HD and H2 gas.7 The flexible DUT-8(Ni) MOF is a primitive cubic (pcu) network that consists of Ni2 paddle wheels as nodes (see Fig. 1), 2,6-naphthalenedicarboxylate (ndc) as a linker and 1,4-diazabicyclo[2,2,2]octane (dabco) as pillars. A pronounced nuclear quantum effect8–11 is suggested to be responsible for the higher adsorption enthalpy of deuterium-containing dihydrogen isotopes to be adsorbed inside the DUT-8(Ni) framework at cryogenic temperatures. Previously, in situ neutron powder diffraction (NPD) and thermal desorption spectroscopy (TDS) investigations were conducted to study this intriguing effect demonstrating a high adsorption selectivity for D2vs. H2 after exposure to 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 gas mixtures.7
1 gas mixtures.7
On the other hand, from the point of view of electron paramagnetic resonance (EPR) spectroscopy, the isomorphous substitution of Co2+ towards DUT-8 (Ni) has been investigated and comprises a noticeable EPR pattern for an open pore (op) phase species that can be interpreted as an effective spin S = 1/2 ground state of the antiferromagnetically coupled mixed Ni2+–Co2+ paddle wheel unit interacting with its 59Co nuclear spin (I = 7/2).5,12 A lengthy discussion on other possible assignments of EPR spectra for the op phase state and its rejection arguments has been discussed by Ehrling and co-workers.5 Previously, an in situ CW-EPR approach enabled us to follow the gate opening mechanism for a series of DUT-8(Ni1−xCox) samples in response to their exposure to N2 gas close to its standard boiling point (71 K).12 Even preceding that, the exotic in situ CW-EPR investigation at X-band frequency has been implemented as a versatile method to follow the gate opening phenomenon of several flexible MOFs triggered by the response toward guest molecule stimuli.12–16 In the present work, we implement this in situ CW-EPR technique for the first time to monitor an isotope-selective phase transition of DUT-8(Ni0.98 Co0.02) towards dihydrogen isotopes.
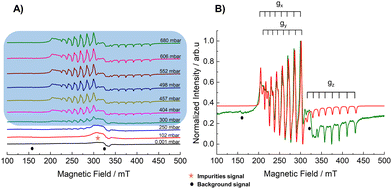
Initially, there was no signal observed for DUT-8(Ni0.98 Co0.02) in the evacuated state (ca. 10−4 mbar), which indicates the presence of closed pore (cp) phase at 21.5 K, in agreement with previous results.5,12 The absence of an EPR signal is probably due to short relaxation times of low symmetric Ni2+–Co2+ paddle wheels. The spectral pattern distinctly evolved after increasing the D2 pressure to 250 mbar, producing a nicely resolved species A (see Fig. 2A and Table 1 for the spin Hamiltonian parameters), which is assigned to the Ni2+–Co2+ paddle wheel signal in the op phase state of DUT-8(Ni0.98 Co0.02). The spin Hamiltonian parameters were determined by spectral simulation of the experimental EPR spectra. The intensity of species was obtained by double integration of the corresponding EPR spectra in Fig. 2A and served as a measure for the volume fraction of the op phase in the sample (Fig. 4). The emergence of the EPR spectrum of the Ni2+–Co2+ paddle wheels at 250 mbar as an indication of the op phase is very well in line with the volumetric D2 adsorption data obtained by Bondorf and co-workers,7 where they also found a similar gate opening pressure and shape of the adsorption hysteresis for D2 but with much longer equilibration times per pressure point.
| Species | g x | g y | g z | A x | A y | A z | Symmetry |
|---|---|---|---|---|---|---|---|
| A | 2.65(4) | 2.59(4) | 1.79(4) | 510(10) | 465(10) | 417(10) | Rhombic |
| B | 2.62(4) | 2.62(4) | 1.80(4) | 468(10) | 468(10) | 427(10) | Axial |
| 12 | 2.62(4) | 2.62(4) | 1.39(4) | 538(100) | 538(100) | 410(100) | Axial |
Interestingly, after desorption to pressures less than 90 mbar, there is a gradual transformation of species A into a new species B with axial symmetry as presented in Fig. 3A (the percentage of its respective species is tabulated in Table SI1, ESI†). Its spectral pattern at 0.001 mbar is shown in Fig. 3B. The transformation of the symmetry of the paddle wheel units from lower symmetry into higher symmetry (C4 as principal rotational axis) at low partial pressure of D2 (p < 35 mbar) indicates less distortion for the paddle wheel units during the evacuation of D2 gas. This phenomenon might originate from the adaptive response of the DUT-8(Ni0.98 Co0.02) framework towards a D2 release via a switching of the linker orientation and can subsequently change the symmetry into a more ordered state.5,17
The sensitivity of the EPR experiment allows one to observe such phase transformation in rather shorter timescales as compared to the volumetric D2 and NPD measurements, which were previously published.7 Note that the intensity of the EPR signal (considering double integration of species A which then transformed into species B) was observed to be constant during the desorption stage and did not revert back to the cp phase at least within the observation times of our EPR measurements (Fig. 4).
 | ||
| Fig. 4 Plot of EPR signal intensity of Ni2+–Co2+ species for DUT-8(Ni0.98 Co0.02) normalized with the highest EPR intensity during adsorption and desorption stages (blue shaded arrows). | ||
Desorption studies of D2 with variability of time shown in Fig. SI3 (ESI†) indicated that even up to 33 minutes during pumping off at 0.001 mbar and 21.5 K, the op phase (e.g. Species B) is still observed with relatively constant EPR intensities. This supports the in situ NPD finding that the transition from op phase to cp phase would only be triggered after 24 h evacuation of D2 at similar temperature (ca. 23.3 K).7 One possible explanation might be that D2 molecules are kinetically trapped in the pores since the dimension of the cp phase (2.366 nm × 0.695 nm) structure may accommodate the residual D2 gas that was trapped during transition from the op to cp phase state. Pollock and co-workers18 previously observed this trapping mechanism during the desorption of D2 gas on another variant of flexible MOF material, MIL-53(Al), even at higher temperature (77 K). Moreover, this molecular clamp appeared to be functional until 120 °C when probed using neutron scattering measurements. We also attempted to increase the temperature while desorbing the D2 gas under vacuum at 0.001 mbar, which resulted in a pore closing mechanism being triggered at 30 K (Fig. SI3, ESI†). The EPR spectra at that temperature suffered from intensity reduction and line broadening before vanishing at T > 35 K, leaving only the background signal. This result is in agreement with previous TDS results that observe the largest D2 desorption peak between 25–30 K, which implies that the desorption temperature plays an important role in the gate closing mechanism in the DUT-8 (Ni) materials.
In the case of H2 adsorption and desorption on DUT-8(Ni0.98 Co0.02) at 19.5 K, which are provided in Fig. SI1 and SI2 (ESI†), the EPR intensities are constant and show only a low-spin Co2+ impurity signal together with some background signals from the cryostat (Fe3+ and Cu2+ species around 150 and 325 mT, respectively). This result signifies that the op phase with its characteristic EPR signal of the Ni2+–Co2+ paddle wheel signal is not present within the pressure range of 0 ≤ p ≤ 929.2 mbar during the H2 adsorption experiment. However, it cannot be ruled out that a trace amount of H2 gas is adsorbed in the cp phase without triggering a pore transition, simply because the energy barrier for the pore transformation is too high for H2 at 19.5 K and pressures up to 1000 mbar.7 The selective opening of DUT-8(Ni0.98 Co0.02) towards D2 owing to the so-called “sizable” chemical affinity quantum sieving (CAQS) effect originates from a slightly lower ZPE (zero point energy) that allows D2 to bind preferentially to various adsorption sites, such as accessible paddle wheel units or linker sites.7,10,19 We should note that the definition of the CAQS effect is not merely constrained to the adsorption of gases in the framework that poses open binding sites. Savchenko and co-workers20 reported that the selectivity of D2 over H2 is mainly caused by different adsorption enthalpies, which in principle, is rooted fundamentally to the small difference in ZPE of adsorbed dihydrogen isotopes on the funnel-like, metal and linker sites in the MOF MFU-4l (at 50 K) where none of them can be considered as open sites in a straightforward definition.
In conclusion, we have demonstrated that the in situ CW-EPR technique at X-band frequencies can be implemented as an efficient technique to monitor the selective-adsorption of dihydrogen isotopes on DUT-8(Ni0.98 Co0.02) MOF, which further confirms the isotope-selective phase transition in response to D2 adsorption. Moreover, as an outlook, the utilisation of in situ pulsed-EPR techniques is in progress to locate the D2 position with respect to the spin probe within the framework of DUT-8(Ni0.98 Co0.02) and estimate how this gas interacts around the accessible paddle wheel units where our EPR active probe resides. This detailed information would provide more clarity in terms of understanding the nature of adsorption sites of the flexible MOF materials.
Muhammad Fernadi Lukman: conceptualization, methodology, investigation, data curation, writing – original draft, and writing – review & editing. Matthias Mendt: investigation, writing – review & editing. Volodymyr Bon: writing – review & editing. Stefan Kaskel: review and supervision. Andreas Pöppl: conceptualization, review, project administration, funding acquisition and supervision.
The authors would like to acknowledge the DFG funding of GRK 2721: Hydrogen Isotopes 1, 2, 3 H with project number 443871192 and FOR 2433 with project number 279409724.
Conflicts of interest
There are no conflicts to declare.References
- N. Klein, H. C. Hoffmann, A. Cadiau, J. Getzschmann, M. R. Lohe, S. Paasch, T. Heydenreich, K. Adil, I. Senkovska, E. Brunner and S. Kaskel, J. Mater. Chem., 2012, 22(20), 10303–10312 RSC.
- Y. Liu, J. H. Her, A. Dailly, A. J. Ramirez-Cuesta, D. A. Neumann and C. M. Brown, J. Am. Chem. Soc., 2008, 130(35), 11813–11818 CrossRef CAS PubMed.
- M. I. Breeze, G. Clet, B. C. Campo, A. Vimont, M. Daturi, J. M. Grenèche, A. J. Dent, F. Millange and R. I. Walton, Inorg. Chem., 2013, 52(14), 8171–8182 CrossRef CAS PubMed.
- F. M. Mulder, B. Assfour, J. Huot, T. J. Dingemans, M. Wagemaker and A. J. Ramirez-Cuesta, J. Phys. Chem. C, 2010, 114(23), 10648–10655 CrossRef CAS.
- S. Ehrling, E. M. Reynolds, V. Bon, I. Senkovska, T. E. Gorelik, J. D. Evans, M. Rauche, M. Mendt, M. S. Weiss, A. Pöppl, E. Brunner, U. Kaiser, A. L. Goodwin and S. Kaskel, Nat. Chem., 2021, 13(6), 568–574 CrossRef CAS PubMed.
- J. Y. Kim, J. Park, J. Ha, M. Jung, D. Wallacher, A. Franz, R. Balderas-Xicohténcatl, M. Hirscher, S. G. Kang, J. T. Park, I. H. Oh, H. R. Moon and H. Oh, J. Am. Chem. Soc., 2020, 142(31), 13278–13282 CrossRef CAS PubMed.
- L. Bondorf, J. L. Fiorio, V. Bon, L. Zhang, M. Maliuta, S. Ehrling, I. Senkovska, J. D. Evans, J.-O. Joswig, S. Kaskel, T. Heine and M. Hirscher, Sci. Adv., 2022, 8(15), 7035 CrossRef PubMed.
- H. Oh and M. Hirscher, Eur. J. Inorg. Chem., 2016, 4278–4289 CrossRef CAS.
- J. Y. Kim, H. Oh and H. R. Moon, Adv. Mater., 2019, 31, 1805293 CrossRef PubMed.
- H. Oh, I. Savchenko, A. Mavrandonakis, T. Heine and M. Hirscher, ACS Nano, 2014, 8(1), 761–770 CrossRef CAS PubMed.
- J. Y. Kim, J. Park, J. Ha, M. Jung, D. Wallacher, A. Franz, R. Balderas-Xicohténcatl, M. Hirscher, S. G. Kang, J. T. Park, I. H. Oh, H. R. Moon and H. Oh, J. Am. Chem. Soc., 2020, 142(31), 13278–13282 CrossRef CAS PubMed.
- S. Ehrling, M. Mendt, I. Senkovska, J. D. Evans, V. Bon, P. Petkov, C. Ehrling, F. Walenszus, A. Pöppl and S. Kaskel, Chem. Mater., 2020, 32(13), 5670–5681 CrossRef CAS.
- D. M. Polyukhov, S. Krause, V. Bon, A. S. Poryvaev, S. Kaskel and M. V. Fedin, J. Phys. Chem. Lett., 2020, 11(15), 5856–5862 CrossRef CAS PubMed.
- V. Bon, E. Brunner, A. Pöppl and S. Kaskel, Adv. Funct. Mater., 2020, 30(41), 1907847 CrossRef CAS.
- M. Mendt, P. Vervoorts, A. Schneemann, R. A. Fischer and A. Pöppl, J. Phys. Chem. C, 2019, 123(5), 2940–2952 CrossRef CAS.
- K. Thangavel, F. Walenszus, M. Mendt, V. Bon, S. Kaskel and A. Pöppl, J. Phys. Chem. C, 2023, 127(17), 8217–8234 CrossRef CAS.
- H. Miura, V. Bon, I. Senkovska, S. Ehrling, S. Watanabe, M. Ohba and S. Kaskel, Dalton Trans., 2017, 46(40), 14002–14011 RSC.
- R. A. Pollock, J. H. Her, C. M. Brown, Y. Liu and A. Dailly, J. Phys. Chem. C, 2014, 118(31), 18197–18206 CrossRef CAS.
- J. Teufel, H. Oh, M. Hirscher, M. Wahiduzzaman, L. Zhechkov, A. Kuc, T. Heine, D. Denysenko and D. Volkmer, Adv. Mater., 2013, 25(4), 635–639 CrossRef CAS PubMed.
- I. Savchenko, A. Mavrandonakis, T. Heine, H. Oh, J. Teufel and M. Hirscher, Microporous Mesoporous Mater., 2015, 216, 133–137 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc02938g |
| This journal is © The Royal Society of Chemistry 2023 |