Open Access Article
Open Access ArticleEmerging technologies for value-added use of oil palm biomass
Mohd Nor
Faiz Norrrahim
 a,
Mohammed Abdillah
Ahmad Farid
*b,
Abubakar Abdullahi
Lawal
c,
Tengku Arisyah
Tengku Yasim-Anuar
bd,
Mohd Hafif
Samsudin
b and
Ahmad Aiman
Zulkifli
b
a,
Mohammed Abdillah
Ahmad Farid
*b,
Abubakar Abdullahi
Lawal
c,
Tengku Arisyah
Tengku Yasim-Anuar
bd,
Mohd Hafif
Samsudin
b and
Ahmad Aiman
Zulkifli
b
aResearch Center for Chemical Defence, Universiti Pertahanan Nasional Malaysia, Kem Sungai Besi, 57000 Kuala Lumpur, Malaysia
bDepartment of Bioprocess Technology, Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. E-mail: abdillah.upm@gmail.com
cDepartment of Agricultural and Environmental Resources Engineering, Faculty of Engineering, University of Maiduguri, Maiduguri, Borno State, Nigeria
dNextgreen Pulp & Paper Sdn. Bhd., Menara LGB, Taman Tun Dr Ismail, 60000 Kuala Lumpur, Malaysia
First published on 3rd May 2022
Abstract
The palm oil industry has been continuing to help in mitigating poverty and drive socio-economic growth through job opportunities and infrastructure development in the suburbs. However, as the industry expands rapidly, production goes hand in hand with waste generation. With current utilization by mills, a large quantity of oil palm biomass is left underutilized. Existing practices allow only a proportion of biomass to be used as mulching agents in plantations and fuel boilers, while trunks and fronds are left to decompose for carbon cycling. A lot of work has been done on bio-product development using oil palm biomass, including biochar, activated carbon, bio-oil, compost, nanocellulose, biosugar, bioelectricity, biohythane, bioplastic, and bioenergy. This review puts together the latest pieces of evidence of technological progress in the valorization of oil palm biomass for value-added use. Overall, it was demonstrated that oil palm biomass can be converted into highly valuable feedstock via several pretreatment routes. Moreover, several challenges were identified and urgently need to be improved. This review will give a glimpse of how effective oil palm biomass is as the main feedstock for these high value-added bioproducts.
Environmental significanceOil palm is now one of the major economic crops in a large number of countries, which triggered the expansion of the plantation area around the world. In Malaysia, this industry annually generates about 80 million tons of oil palm biomass. This creates a serious problem of biomass waste overload to the country. The biomass generated has attracted great interest from researchers. This was due to the abundance of this valuable material which can be converted into value-added materials such as bioelectricity, biofuels, biohydrogen, bioplastics, biosugars, and nanocellulose. This review will give a glimpse of how effective this biomass is as the main feedstock for these high value-added bioproducts. This article discusses the feasibility of biomass conversion technologies, including the detailed process involved in each bioproduct development, which wasn't comprehensively highlighted in recent years. |
1 Introduction
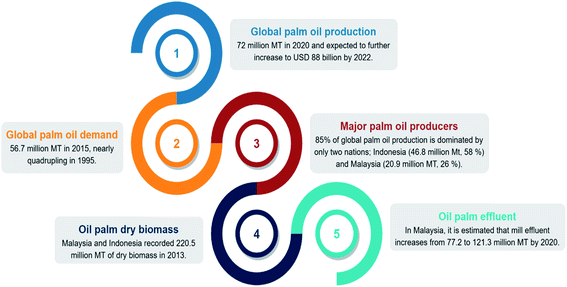
The production of palm oil has increased dramatically over the last five decades, with a projected USD 88 billion worth by 2022.1 Palm oil has advantages over other vegetable oils: (1) superior shelf life of the final products of up to 12 months, (2) the presence of natural antioxidants, tocopherol, and tocotrienols, which contribute to high oxidative stability, and (3) low price.2 In 2015, the global palm oil demand hit 56.4 million metric tons (MT), nearly quadrupling compared to that in 1995. The production continued to rise by 22% in 2020, exceeding 72 million MT.3,4 Almost 85% of palm oil output is generated in only two nations, with Indonesia producing 46.8 million MT or 58% of world supply, while Malaysia produced 20.9 million MT or 26%.5 As the industry expands rapidly, palm oil production goes hand in hand with its biomass generation. Approximately 70% of fresh fruit bunches become waste in the form of empty fruit bunches, shells, and liquid effluent.6 Malaysia and Indonesia recorded 220.5 million MT of dry oil palm biomass production in 2013.7 In Malaysia alone, the government forecasted that solid biomass generation would increase to 93.7 to 122.4 million MT, while mill effluent would increase to 77.2 to 121.3 million MT by 2020.8 The statistics above are summarized in Fig. 1.By looking at the current landscape of the industry, sustainability is a big concern. Despite the outstanding economic and social development that the industry continued to witness, it has drawn global attention attributable to environmental conflicts over the excessive production of biomass. The scenario is further exacerbated after the European Union (EU) and United States (US) boycott of palm oil over the issues of deforestation and global warming.9–11 A bio-based economy is considered one of the feasible alternatives to achieve sustainability by making use of oil palm biomass.
To date, biomass has largely been used as a fuel source for cooking and heating in many developing countries.12 Meanwhile, in developed countries, the use of biomass derived fuels for transportation and for electricity generation is increasing due to the impacts and consequences of CO2 emissions on the environment.12 In most of the palm oil producing countries, oil palm biomass is now underutilised due to its restricted application, which is mostly due to the low or medium technology readiness level of specific bio-product processing, making it difficult for the industry to adapt. This is linked to the technological know-how and financial implications that vary from lab to industrial scale manufacturing. According to Kaniapan et al.,13 oil palm biomass is currently being utilized in various industries such as brooms from oil palm fronds (OPF) and compressed medium density fibreboard from oil palm trunks (OPT), whereas oil palm empty fruit bunches (OPEFB) are being utilized widely as a mulch for fertilizers to promote soil fertility. OPEFB can also be a good source of polymer reinforcement materials. Besides that, processed palm kernel cakes have been commonly used for animal feed given to cattle and chickens.
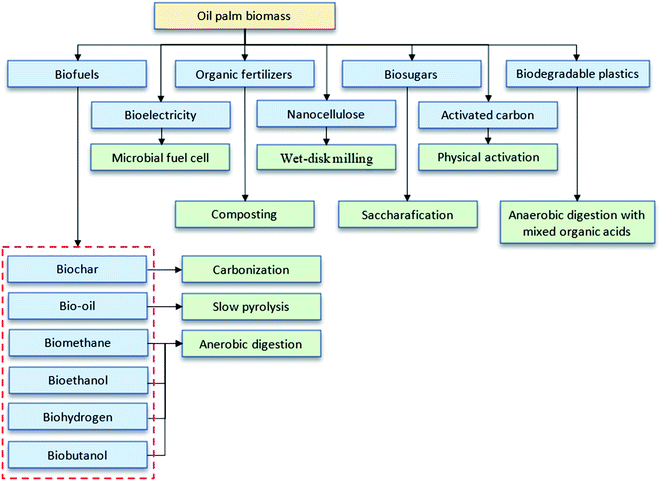
Oil palm biomass is divided into solid and liquid biomass with possible uses for each part. It consists of a complex lignocellulosic structure as represented in Fig. 2. The direct utilization of lignocellulosic biomass for bioproducts is challenging due to the tight bonding within their components. To overcome this problem, several pretreatment methods are usually applied to fractionate this biomass prior to further processing. The scientific community's continuing commitments in research and development (R&D) have expedited technological innovations in potential bio-products, such as biochar, activated carbon, bio-oil, compost, nanocellulose, biosugar, bioelectricity, bioplastics, biogas and bioenergy, as summarized in Fig. 3.14–18 This review highlights the latest findings in that regard.
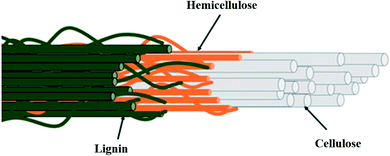 | ||
| Fig. 2 Overview of the complex structure of lignocellulosic fibers. Reproduced from Norrrahim et al.17 | ||
2 Emerging technologies for value-added use of oil palm biomass
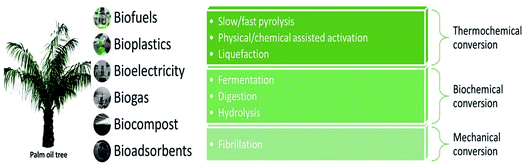
Today's paradigm shift in handling biomass ensures that it is leveraged in an environmentally sound manner so that the benefits are grasped with both hands for profit, people, and the planet. This implies that no spare biomass shall be discarded by mills by any means possible either by converting it into useful derivatives or reclaiming it for in-house use. Much research that has been conducted on numerous processes using oil palm biomass opens up the possibility of creating a valuable product proposal and bringing it to the commercial marketplace.As shown in Fig. 2, the structural arrangement of oil palm biomass and its composition have a significant effect on its conversion efficiency.16 By referring to Fig. 4, the pretreatment of oil palm biomass can be done via thermal, physical, thermomechanical, chemical, and biochemical conversion routes. Physical and thermomechanical pretreatments are usually been applied towards the lignocellulosic fibers. Physical pretreatment, also known as mechanical pretreatment, is a process that uses mechanical methods, such as milling, chipping, grinding, and shredding, to reduce the particle size and to increase the surface area of biomass.17 This pretreatment is also able to partially modify the structure of biomass, reduce cellulose crystallinity, and disrupt the chemical bonding. The chemical composition of natural fibers is usually not affected by physical pretreatment. It is often an essential step prior to or following chemical or biological processing. However, there are some drawbacks to physical pretreatment. Physical pretreatment lacks the ability to remove lignin and hemicellulose, which limits the conversion processes such as enzymatic saccharification and nanocellulose production. Besides that, this pretreatment also requires high energy consumption, which limits its large-scale implementation and causes environmental safety concerns.17
Besides that, thermomechanical pretreatment involves both thermal and physical interactions in the pretreatment process.17 Methods such as steam explosion, superheated steam, hydrothermal, ammonia fibre explosion and liquid hot water are among the most widely used methods. It has the capability of changing the structure of biomass, increasing the surface area, and reducing the degree of biomass polymerization. Thermomechanical pretreatment is considered as the most effective and environmentally friendly method. Interestingly, it has been optimized with a variety of feedstocks on a pilot scale for industrial applications. It is also usually applied to remove hemicellulose and lignin from lignocellulosic biomass.
2.1 Thermal treatments for biofuels & adsorbents
The biochemical components of oil palm biomass can be decomposed into biochar, bio-oil, and non-condensable gasses when treated under high temperatures in an oxygen-limited environment.19 The classification is based on operating conditions and has been successfully employed in either lab-scale or pilot-scale reactors. Table 1 summarizes previous literature identified using oil palm biomass for potential solid-fuel, carbon-based adsorbent, and bio-oil production.| Biochar production | |||||
|---|---|---|---|---|---|
| Biomass | Process mode | Operating conditions | Yield (wt%) | Higher heating value (HHV) (MJ kg−1) | Reference |
| OPKS | Slow carbonization | 500 °C for 60 min under a 2000 cm3 min−1 N2 flow | 35.3 | 28.9 | 20 |
| OPEFB | 29.1 | 21.3 | |||
| OPMF | 29.8 | 29.1 | |||
| OPEFB | Self-sustained carbonization | 417–590 °C for 15–31.7 h under air-tight conditions | 16.3 | 25.0 | 21 |
| Activated carbon production | |||||
|---|---|---|---|---|---|
| Biomass | Process mode | Operating conditions | Surface area (m2 g−1) | Removal | Reference |
| OPEFB | Physical activation | 900 °C for 30 min under a 2500 cm3 min−1 N2 flow | 635 | 97% cadmium | 22 |
| OPKS | 500–1000 °C under a 12.8–18.2 L min−1 steam flow | 935 | 68% COD and 83% BOD | 23 | |
| OPMF | 600 °C for 1 h | 494 | 23% COD and 88% SS | 24 | |
| Bio-oil production | |||||
|---|---|---|---|---|---|
| Biomass | Process mode | Operating conditions | Oil yield (wt%) | HHV (MJ kg−1) | Reference |
| OPEFB | Slow pyrolysis | 500 °C for 1 h under a continuous N2 flow of 2 L min−1 | 45.8 | 32.6 | 20 |
| OPMF | 43.9 | 28.0 | |||
| OPKS | 47.4 | 29.6 | |||
Abnisa et al.20 reported on biochar production from OPEFB, oil palm mesocarp fiber (OPMF), and oil palm kernel shells (OPKS) via slow pyrolysis. The study found that oil palm biomass contains high fixed carbon, which indicates high energy potential.25 The results found that OPKS-biochar has the highest fixed carbon content of 72.5% compared to OPEFB- and OPMF-biochar at 41.7% and 30.6% respectively. As for the energy content, the samples are characterized at a high heating value of 21.3–29.1 MJ kg−1. To put this into perspective, the high heating value of coal and coke is 30.7 and 33.5 MJ kg−1, respectively, showing that thermochemical conversion of oil palm biomass residues into biochar provides an opportunity as an alternative source for renewable energy.26
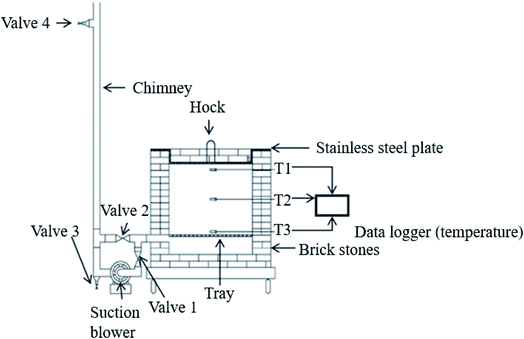
A pragmatic approach to produce oil palm biomass biochar on a large scale was developed by Idris et al.21 using a briquet reactor fitted with an air suction blower. Biomass is thermally pyrolyzed for hot air to flow evenly within the combustion chamber, as shown in Fig. 5. An optimal combustion process is achieved by controlling the temperature and amount of flue gas released so that the pressure inside consistently persists to create partial activation. The combustion is stopped once the temperature falls to <500 °C and the resultant biochar is sprayed using rainwater to halt the combustion from continuing which could burn down the resultant product into ash. The characterization of the biochar produced shows that it has a heating value of 25 MJ kg−1 and productivity reaches 26 wt%.
Another promising bioproduct is activated carbon.27 It typically has a higher surface area and pore volume than biochar between 500 and 1900 m2 g−1 and 0.3 and 1.27 cm3 g−1, respectively.28 Due to its large surface area and specific functional groups that act collectively for adsorption, it has broader applications, e.g., as adsorbents in wastewater treatment, catalyst-support materials for chemical processes, and pseudo-capacitor materials for energy storage.29–31 The process usually includes a two-step process: carbonization and activation; carbonisation is a process to increase the carbon content of organic materials to form char, which is normally non-porous, at 600–900 °C under inert conditions, while activation is a process to increase the surface area of char by increasing the porosity through thermal or chemical modification at 700–900 °C under inert conditions.32,33
Alkhatib et al.22 carbonized OPEFB at 900 °C with an N2 flow rate of 2500 cm3 min−1 for 30 min, followed by physical activation using steam for 15 min. The activated carbon produced is then applied for cadmium removal from an aqueous solution, whereby the adsorption indicates 97% of cadmium removal owing to its high surface area of 635 m2 g−1. Zainal et al.23 investigated OPKS activation in the temperature range 500–1000 °C with a 12.8–18.2 L min−1 steam flow rate. The resulting activated carbon has shown an increase in surface area from 935 m2 g−1, which contributed to its 68% of chemical oxygen demand (COD) and 83% of biochemical oxygen demand (BOD) removal from palm oil mill effluent (POME). In another similar study, Ibrahim et al.24 performed physical activation using steam on OPMF at 600 °C for 1 h, which resulted in an increased surface area of 494 m2 g−1. In treating POME, the activated carbon effectively removed ∼23% COD and 88% suspended solids.
Bio-oil or wood tar is recovered through destructive distillation, as a liquid fraction of biomass that is made up of a complex mixture of 25–30% water, phenolic compounds, and hydrocarbons with a calorific value of 15–16 MJ kg−1.34 During the thermal process, a series of depolymerization, fragmentation, and cracking occurred concurrently to disrupt and restructure chemical bonds within the lignocellulosic components, leading to the production of a dark-brown organic liquid.35 The biomass liquefaction incorporated a slightly different approach, preferring fast pyrolysis to advantageously obtain a high yield of bio-oil, which opposed the usual low heating and long retention in carbonization.36 In a study by Abnisa et al.,20 slow pyrolysis of OPEFB, OPMF, and OPKS is optimized. The best conditions are recorded as 500 °C and 2 L min−1 N2 for 1 h using biomass that is sized at 1–2 mm, where the oil yields obtained from OPKS, OPEFB, and OPMF are 47.4 wt%, 45.8 wt%, and 43.9 wt%, respectively. The energy content of biochar (20–30 MJ kg−1) is still higher than those of the bio-oils obtained, nevertheless.
Despite the great progress achieved in oil palm biomass use for bioenergy production, there are some drawbacks that need to be considered. According to Mohammadi et al.,37 it is important to evaluate several factors such as climate change and human health implications regarding the adoption of biochar technologies as discussed above. The production of biochar may have inconsistent agronomic and soil effects. It depends on their properties, crop species, soil types, and management practices. Moreover, the current method to produce biochar by using kilns is inefficient. It can have a bad impact on human health. Therefore, the development of appropriate technologies based on efficiently engineered pyrolysis facilities as discussed above is believed to mitigate these adverse impacts.
Meanwhile, there are several other challenges that need to be considered for developing future research. Reza et al.38 listed several challenges faced in the application and commercialization of activated carbon as listed below:
(a) Activated carbon does not perform well in the removal of pollutants that are not attracted by carbon like nitrates, sodium, fluoride, and pathogens. Therefore, the process for removing all types of pollutants with activated carbon should be improved.
(b) The service life of activated carbon is short. The adsorption and desorption capability of activated carbon need to be evaluated. Hence, a more thorough evaluation is required to determine its long-term viability.
(c) The development of the second generation of waste during the production process of activated carbon needs to be avoided.
(d) The cost of preparing activated carbon from raw material sources should be taken into account.
2.2 Co-composting for nutrient recycling
Co-composting is the biological degradation of organic materials with microorganisms over a prolonged period under controlled conditions, e.g. temperature, moisture, O2 level, and C/N ratio, to produce nutrient-rich humus or soil-like materials.39–42 A mesophilic stage begins with bacteria proliferation, which simultaneously increases the compost temperature to 50 °C. Thermophilic microbes begin to proliferate as the environment becomes hotter. However, as the temperature continues to rise to 70 °C, pathogenic microbes are exterminated and phytotoxic compounds are broken down. As the thermophilic activity subsides, the pile temperature starts to cool off gradually as it enters the curing phase. The mesophilic microorganisms recolonize and continue to ingest the remaining coarse organic materials at a much slower rate, helping to further degrade potentially toxic organic acids and resistant compounds. O2 is replenished throughout the process by turning the compost pile to provide adequate aeration.42,43 There is a little amount of nitrate-N formed in the primary phase of composting. However, as it enters the curing stage, the mesophilic microbes' proliferation begins to flourish, resulting in high conversion of organic material to ammonium-N and nitrate-N. Therefore, measuring CO2 and NH3 using the Solvita test or C/N ratio is gaining recognition as a useful way of evaluating the degree of compost maturity. Usually, the compost is considered mature once the C/N ratio is <20 and the Solvita Index reaches 7.44,45 The different phases in composting are summarized in Fig. 6.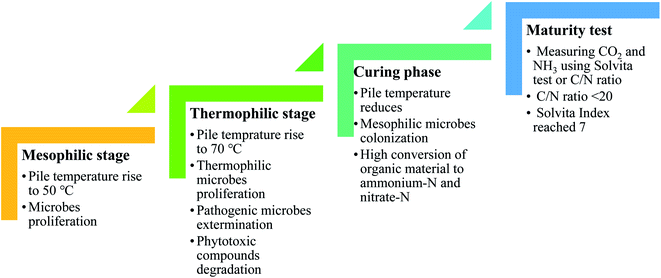 | ||
| Fig. 6 Different phases during composting.42–45 | ||
Composting has gained interest owing to its cheap starting material, simplicity, environmentally friendly nature, and high rate of carbon cycling.46–48 A pile of OPEFB with a block dimension of 4.73 m3 is periodically supplemented with thickened POME sludge at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w).49 The deterioration of OPEFB self-degradation requires a long time to complete, and hence the introduction of POME sludge is to expedite the process by providing nitrogen and inoculum sources. Every 3 days within the first 2 weeks of composting, the sludge is added until the volume reaches a 1
1 (w/w).49 The deterioration of OPEFB self-degradation requires a long time to complete, and hence the introduction of POME sludge is to expedite the process by providing nitrogen and inoculum sources. Every 3 days within the first 2 weeks of composting, the sludge is added until the volume reaches a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) OPEFB to POME sludge ratio. Sufficient aeration is replenished by periodically turning the compost pile 1–3 times a week. After 40 days, the compost achieved maturity as confirmed by the measured C/N ratio of 18.3.
1 (w/w) OPEFB to POME sludge ratio. Sufficient aeration is replenished by periodically turning the compost pile 1–3 times a week. After 40 days, the compost achieved maturity as confirmed by the measured C/N ratio of 18.3.
Another study by Baharuddin et al.50 investigated the physicochemical changes during OPEFB composting with POME. In the first 2 weeks, the pile moisture is kept at 65–75% with aeration regularity once in 3 days. The compost pile is purposefully covered to avoid heat loss and aid raise the temperature to 60–70 °C. After 60 days, the maturity is reached with the C/N ratio reaching 12, an acceptable quality of compost with a N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K ratio of 2.2
K ratio of 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 and a heavy metal content of <10 mg kg−1. Ahmad et al.51 have carried out one-tonne composting of oil palm fronds and POME (1
2.8 and a heavy metal content of <10 mg kg−1. Ahmad et al.51 have carried out one-tonne composting of oil palm fronds and POME (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w oil palm fronds to POME ratio) for 30 days. The POME addition is completed within the first 2 weeks of composting and the turning process is carried out once in 3 days to ensure an even distribution of moisture and provide sufficient aeration. The compost maturity is achieved as the C/N ratio is reduced from 64 to 18 after 60 days. Similar conditions are applied by Hock et al.52 using a different raw material, OPMF. The reported N
1 w/w oil palm fronds to POME ratio) for 30 days. The POME addition is completed within the first 2 weeks of composting and the turning process is carried out once in 3 days to ensure an even distribution of moisture and provide sufficient aeration. The compost maturity is achieved as the C/N ratio is reduced from 64 to 18 after 60 days. Similar conditions are applied by Hock et al.52 using a different raw material, OPMF. The reported N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K content is 2.1
K content is 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.3
0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, which met the USEPA standard.
1.2, which met the USEPA standard.
In the development of co-composting of oil palm biomass, some challenges related to health issues are the main concerns and require attention for future research. Usually, heavy metals are present in POME. Based on the data reported by Krishnan et al.,53 POME has metal concentrations (e.g. B 7.6 mg L−1, Fe 46.5 mg L−1, Mn 2.0 mg L−1, Cu 0.89 mg L−1 and Zn 2.3 mg L−1). The presence of these heavy metals is due to the mechanisms such as metal binding, microbial immobilization and oxidation, and humification. Krishnan et al.53 also suggested by profiling that the microbial community in the POME and developed compost can help the future research to increase the effectiveness of the co-composting process. By doing this, species with higher oxidation and immobilization of toxic heavy metals can be identified. Besides that, prolonging the co-composting period is ineffective and not economically practical. The co-composting period is reported to be around 60 days. Therefore, the identification of microbes involved in the co-composting would also provide novel and valuable insights into the mechanism and help to improve the efficiency of the process.
2.3 Nanocellulose as a reinforcement material
Fibrillated cellulose is used as a reinforcement material for composite materials owing to its advantages, e.g. improved deformation, great thermal conductivity and insulation, non-poisonous nature, high durability, and electrical conductivity.54–60 Nanocellulose isolation stages and the types of treatments are summarized in Fig. 7. The latest finding reveals that nanocellulose gives better performance to the composite compared to macro- and micro-sized cellulose, e.g., higher surface area, higher crystallinity, and higher thermal stability as well as mechanical strength.61–64Table 2 summarizes a few studies that focused on utilizing oil palm-derived nanocellulose for biocomposite production.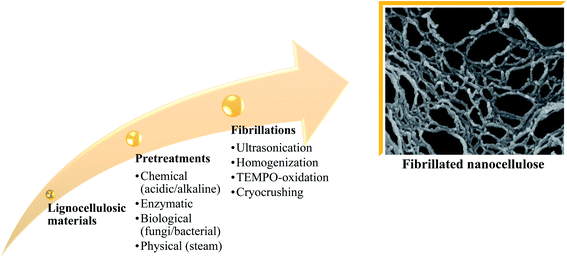 | ||
| Fig. 7 Nanocellulose isolation stages and types of treatments.65–69 | ||
| Biomass | Fabrication | Filler loading (wt%) | Results | Reference |
|---|---|---|---|---|
| OPEFB | Nano-filler epoxy nanocomposites | 3 | (1) Density increased from 1.13–1.25 g cm−3 | 70 |
| (2) Improved thermomechanical properties | ||||
| OPMF | Nanowhisker nanocomposites | 3–6 | (1) Elongation at break increased by 240% at 3 wt% loading | 71 |
| (2) Tensile strength and elastic modulus increased at 6 wt% loading | ||||
| OPMF | PE reinforced nanocellulose nanocomposite | 3 | (1) 139% increase in flexural strength | 61 |
| (2) 195% increase in flexural modulus | ||||
| (3) Increased thermal properties | ||||
| OPMF | PLA reinforced nanocellulose nanocomposite | 3 | (1) 11.6% increase in tensile strength | 72 |
| (2) 27.1% increase in Young's modulus | ||||
| OPMF | PP reinforced nanocellulose nanocomposites | 3 | (1) 34.2% increase in tensile strength | 73 |
| (2) 63.7% increase in Young's modulus | ||||
| OPEFB | PLA reinforced nanocellulose nanocomposites | 3–5 phr | (1) 84% increase in tensile strength at 3 phr | 74 |
| (2) 12.7% increase in degradation temperature at 5 phr | ||||
| OPEFB | PVA/starch film reinforced nanocellulose nanocomposites | 5–10 | (1) Highest tensile strength at 10% (v/v) | 75 |
| (2) Highest elongation at break at 5% (v/v) | ||||
| (3) Water adsorption increased with filler loading |
During the fabrication of composite materials, nanocellulose provides rigidity and porosity so that a stronger interlocking with resins can be achieved, and hence boosts the mechanical strength.76–78 Saba et al.70 found that the use of OPEFB-nanocellulose in epoxy increased the density and overall thermomechanical properties of the resultant composites due to enhanced interfacial bonding. Campos et al.71 studied the feasibility of OPMF-nanowhiskers, which are another form of nanocellulose, for cassava starch nanocomposites via acid hydrolysis and microfluidization, followed by solvent casting into the starch film. This method had improved the morphological and mechanical properties of the nanocomposites. This is because the interaction between nanowhiskers and the starch matrix resulted in better stability and more interfacial bonds, thus providing the obtained composites with higher tensile strength and thermal-mechanical adhesion.
Yasim-Anuar et al.61 discovered the potential of nanocellulose in enhancing the tensile and flexural strengths of a polyethylene (PE) composite by 139% and 195% respectively with 3 wt% OPMF-nanocellulose inclusion into the polymer matrix. A similar trend of increment is also evidenced for polylactic acid (PLA) and polypropylene (PP) nanocomposites.79–81 Ariffin et al.72 revealed that the addition of 3 wt% OPMF-nanocellulose has increased both the tensile strength and Young's modulus of a PLA nanocomposite by 13% and 38% respectively, attributed to the high crystallinity of nanocellulose. Haafiz et al.74 noticed a great improvement in the mechanical and thermal properties of PLA composites with 3 wt% OPEFB-nanowhisker reinforcement. The tensile strength has increased substantially by 84% upon prolonging the degradation temperature (Tmax) from 363 °C to 389 °C. Norrrahim et al.73 reported that the interaction between OPMF-nanocellulose and PP improved the mechanical properties and crystallinity by 33.4% and 9%, respectively. A similar trend of increment is also recorded with 5 wt% OPEFB-nanocellulose inclusion into a polyvinyl alcohol (PVA)/starch film.75 The addition of OPEFB-nanocellulose also improved the water resistance and biodegradability of the PVA/starch film. The water absorption capacity increased to approximately 60% and 59% more of the film's weight was lost after 90 days than the PVA/starch film without nanocellulose reinforcement.
The usefulness of nanocellulose production from oil palm biomass is a promising and exciting area of current and future R&D. Although the effectiveness of nanocellulose as a new green biobased material has been demonstrated through several different studies, further improvements are still needed. The cost-effectiveness and availability of nanocellulose on an industrial scale are the main concerns in the production of nanocellulose from oil palm biomass.82 Indeed, the energy consumption related to the production of nanocellulose is still an issue hampering the scale-up production of nanocellulose. However, to the best of our knowledge, several achievements have been accomplished by many scientists, who were focused on this area. Thus, increase the potential application of nanocellulose in several fields.
2.4 Gasification of biomass
The rapid depletion of gas and oil reserves to sustain global energy demand has driven the exploration of biogas.83 Oil palm biomass is a promising alternative resource to fossil fuels given its significantly lower carbon emission relative to fossil fuels. The gasification of oil palm biomass is a main driver for the interest in the production of versatility gases such as carbon monoxide (CO), hydrogen (H2), methane (CH4) and several other hydrocarbons. These biogases can be used in conventional equipment such as boilers, engines, turbines, and fuel cells for the generation of heat and electricity. It is a mature technology pathway that uses a controlled process involving heat, steam, and oxygen to convert oil palm biomass to gaseous products, without combustion.H2 and CH4 are the most common biogases that have been actively produced from oil palm biomass. This is due to several factors such as the abundance of locally available energy sources, the ability to reduce the greenhouse gas emission and make the energy market less dependent on the supply and fluctuation price of oil and gas. Hydrogen is seen as a promising future clean energy that has the potential to replace fossil fuels thanks to its ability that can provide energy for a wide range of applications, from domestic to industrial, while emitting no hazardous emissions.84 Meanwhile, CH4 is able to produce more heat and light energy than other hydrocarbons or fossil fuels.85 CH4 also produces significantly less carbon dioxide (CO2) and other pollutants that contribute to smog and unhealthy air.
Biogas derived from organic content-rich wastewater through anaerobic digestion could be used for electricity generation. POME is a non-toxic, thick, viscous liquid waste with a high organic content and abundance in quantity.86Table 3 summarizes the previous findings on CH4 and hydrogen H2 production from POME.
| Types of gas | Methods | Production | References |
|---|---|---|---|
| CH4 | Anaerobic digestion | 2.62 thousand MT CH4 per year | 87 |
| 484 MT CH4 per year | 88 | ||
| H2 | Two-stage sequential dark and photo-fermentation | 3.064 ml H2 per mL POME | 89 |
| Anaerobic sequencing batch reactor (ASBR) and upflow anaerobic sludge blanket (UASB) | 73 L H2 per kg-VS at 11% purity | 90 | |
| Two-stage fermentation | 4.1 L H2 per L POME | 91 | |
| POME pre-treatment and UASB | 11.75 L H2 per day at 52% purity | 92 |
Biogas production can be achieved from POME through anaerobic digestion as it contains high methane which is around 65 to 75%.93 For every MT of CPO production, about 2.8 MT of POME is produced, and for every MT of POME being anaerobically digested, nearly 31 m3 of biogas is generated.93 Harsono et al.88 assessed the potential contribution of the anaerobic treatment of POME using an 80 m3 anaerobic digestion plant at a ∼422.4 thousand MT fresh fruit bunch (FFB) per year capacity palm oil mill. The CH4 production reached 484 MT CH4 per year with a calculated energy output of 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 GJ per year, or 7.3 GW h per year, which is approximately valued at around USD 1 million at the current Malaysian tariff. From another similar study, a palm oil mill of 396 thousand MT per year FFB capacity was reported to generate 2.62 thousand MT CH4 per year, which is equivalent to 13.26 GW h per year.87
275 GJ per year, or 7.3 GW h per year, which is approximately valued at around USD 1 million at the current Malaysian tariff. From another similar study, a palm oil mill of 396 thousand MT per year FFB capacity was reported to generate 2.62 thousand MT CH4 per year, which is equivalent to 13.26 GW h per year.87
Besides CH4, the microbial degradation of organic matter under anaerobic conditions also releases H2 gas, which is another valuable source of energy for electricity.94 The production of H2 from biomass is deemed as a viable option to reduce the carbon footprint released by fossil fuels. It is expected that if POME is used as the main ingredient in H2 production, half of the H2 market can be projected. The potential production of H2 could hit 21.6 million MT per year with the current POME generation at 184.6 million MT per year, or 2.59 EJ per year for power output.95
The most widely utilized method involves two-stage microbial fermentation: (1) acidogenesis (formation of H2 and CO2) and (2) acetogenesis (H2 and acetic acid formation). Mishra et al.89 studied H2 production via two-stage sequential dark and photo-fermentation, where the H2 yield reached 3.06 mL H2 per mL POME with 93% COD reduction. Experimentation by Seengenyoung et al.90 conducted a pilot-scale experiment on a two-stage thermophilic for POME-H2 synthesis, using a sequential ASBR and UASB, and the authors managed to create 73 mL H2 per g COD, accounting for 11% of the biogas composition. This is in agreement with the findings reported by O-Thong et al.,91 whereby biogas production from POME was investigated via a two-stage thermophilic-mesophilic reactor with methanogenic effluent containing Thermoanaerobacterium sp., achieved a maximum H2 yield of 4.1 L H2 per L POME. Mahmod et al.92 used a UASB reactor at a hydraulic retention time (HRT) of 6 h, achieving 11.75 L H2 per L POME per day with 52% biogas composition.
However, the production of biogas from oil palm biomass also has some challenges. The implementation of this technology in developing countries still requires advancements at all levels for energy and electricity production. According to Patinvoh et al.,96 some developing countries are also facing problems associated with funding, policy, sustainability, awareness, technical services, and education. These are the important key factors to achieve the full potential of biogas production. Moreover, the production of biogas can also lead to potential explosions, corrosion hazards and greenhouse gas emission problems. Safety and operational risk mitigation in operating biogas trapping facilities is important to overcome these issues. Workers must ensure a stable operation and close monitoring of biogas during the operation and maintenance. Patinvoh et al.96 suggested several approaches to enhance biogas implementation by applying technical training, enforcement of policy, public–private partnership funding, record keeping and advertisement of biogas programmes.
2.5 Bioelectricity from microbial fuel cells
The fundamental concept of a microbial fuel cell (MFC) is converting chemical energy stored in organic materials to electricity through the metabolic activity of microbes.97,98 The MFC is set up by conjoining aerobic (cathode) and anaerobic (anode) chambers, which are separated by a proton-exchange membrane. The electron transfer activity from the anode to the cathode creates an electron current and hence electricity. The MFC was previously used to harness chemical energy from simple organic materials, including acetate, butyrate, and simple sugars.99 As of today, the method is leveraged for complex substrate degradation in wastewater treatment. POME is known to be rich in total organic carbon, and therefore it is a promising carbon source alternative at a lower cost to be utilized in MFCs. As organic compounds in POME are being anaerobically degraded by microbes for growth, the catabolism at the anode (oxidation) releases electrons (H+), only to be received by an oxidant (O2) at the anode chamber. Hence, it creates an electric current that can be harvested by an external circuit. Table 4 summarizes the findings on MFCs using POME for electricity generation. The synergic integration of POME treatment and the MFC system has been demonstrated by Nor et al.100 with the highest energy harvested at 85.11 mW m−2. Mahmood et al.101,102 demonstrated bioelectricity generation from different concentrations of alkaline pretreated OPEFB with a mixed culture. As a result, higher power densities (0.33–0.68 W m−2) are achieved at a lower concentration of substrate loading.According to Bazmi et al.,103 many studies were performed in recent decades to estimate the future demand and supply of bioelectricity. Recently, the world's market for bioenergy has been expected to increase to meet the global demand by year 2050. Shifting the electricity mix from fossil fuels to renewables can now be done using the best existing technologies as discussed here. However, the shift process requires much investment in infrastructure, equipment and R&D related to palm oil biomass.
2.6 Enzymatic saccharification for biosugar conversion
Lignocellulosic materials are composed of complex molecular and recalcitrant polymer structures. To enzymatically convert lignocellulosic materials into biosugar, pretreatment is required to disrupt the lignin polymer and reduce the crystallinity of the cellulosic components.104–106 Alkaline pretreatment using NaOH is recognized as an effective approach, causing the swelling effect on the lignocellulosic structure, thus significantly disrupting the linkages between cellulosic and lignin components.107 Apart from that, biological pretreatment involves the degradation of lignocellulosic materials with lignolytic enzymes produced by a fungus. However, this typical pretreatment has the major drawback of a slow degradation process, suggesting that a combination with another pretreatment process is required. Table 5 lists several pretreatment applications on different oil palm biomass for biosugar production.| Substrates | Pretreatments | Yields | References |
|---|---|---|---|
| OPEFB | Physical SHS-enzymatic laccase | 71.5% g g−1 glucose | 108 |
| OPMF | 63% g g−1 glucose | 108 | |
| OPT | Deep eutectic solvent | 74% glucose conversion | 109 |
| OPEFB | 5% (v/v) acetic acid-steam pretreatment | 696.9 mg g−1 carbohydrate | 110 |
| OPEFB | Sequential mechanical-green solvent | 105.3 g L−1 total reducing sugars | 111 |
| OPMF | Subcritical H2O–CO2 | 29.9% xylose and 84.6% glucose | 112 |
Indeed, the selection of optimum pretreatment before saccharification is crucial to increase the accessibility of enzymes in the degradation process. According to Rizal et al.,108 the combination of physical and biological pretreatment using superheated steam (SHS) and laccase enzyme has greatly improved the glucose yield by 4.6-fold and 4.8-fold that of saccharification of OPEFB and OPMF, respectively. As an interesting note, in this study, they also reported that a proportional interaction between substrate size and glucose yield has been shown, with a concurrent increase in total surface area for enzymatic degradation.113,114 On the other hand, the application of a deep eutectic solvent, which is the high melting point emerged fluid from an ionic class solvent composed of two or three components, has been demonstrated by Zulkefli et al.109 As a result, the increment of 26.4% of the cellulose component in pretreated OPT as compared to untreated OPT resulted in mixed ethyl ammonium chloride and ethylene glycol. Subsequently, the highest glucose conversion achieved was 74% from the saccharification of pretreated OPT at 50 °C for 24 h (celluclast 1.5 L, 50 FPU per g; Novozyme 188, 100 CBU per mL; substrate concentration, 15 mg mL−1).
The mechanical-green solvent concept for the bioconversion of biomass into biosugar is regarded as one of the innovative approaches in the pretreatment process. In brief, green chemistry is aimed at utilizing non-hazardous solvents as an alternative to the synthesis of value-added products. Julio-Altamiranda et al.111 have demonstrated the combination of mechanical and chemical pretreatment using urea on OPEFB. The results showed that a substrate size of 0.5 mm with 4% urea concentration has successfully produced the highest total reducing sugars of 105.3 g L−1 from OPEFB. The denouement of urea causes the polymeric structure of lignocellulosic materials to degrade, thereby preventing the resemblance of cellulose molecules after linkage disintegration.115
Although some pretreatments could achieve a high amount of sugar recovery, the efficiency and the suitability of the pretreatment should be considered in several aspects as listed below:116
(a) Energy and time consumption
(b) Cost (initial capital for setting up the plant)
(c) The inhibitors released after the pre-treatment process
(d) The waste generated from the pre-treatment process
(e) The environmental impact
According to Rizal et al.,116 even though a single pretreatment could save energy and time, a combination of more than two pre-treatments could enhance the sugar recovery. However, the compatibility of combining pretreatments is still limited. Nevertheless, many previous studies have been conducted on a small scale, yet there is a significant disparity between laboratory preliminary findings and industrial-scale results. Therefore, further research is required to address these issues and provide a feasible pretreatment approach for large-scale biosugar production systems.
2.7 Biodegradable plastics from sugar derivatives
Rising concern over the troubling environmental impacts of plastic use encouraged the production of biodegradable plastics.117 However, the most significant challenges to commercialising green plastics, such as polyhydroxyalkanoate (PHA) and polylactic acid (PLA), are consistency and cost of production, thus researchers must discover solution to tackle these issues. The use of biomass as feedstock for the biological processing of plastics is viewed as a feasible solution in light of the zero-discharge policy for the palm oil industry relative to traditional petroleum plastics such as PE, PP, and PVA.118 Naturally, organic plastics are biopolymers generated as a form of intracellular energy storage by microbes via fermentation under unbalanced growth conditions, e.g. nutrient deprivation or excess carbon source. These biopolymers are close to chemically derived polymers characteristically with pronounced biodegradability. However, the thermal and mechanical properties of PHA and PLA differ, which reflects the kinds of monomers included.119 A variety of studies are listed in Table 6 concerning the production of biobased plastics with palm oil biomass.| Microbes | Substrates | Dry cell mass conc. | Yields | References |
|---|---|---|---|---|
| Polyhydroxyalkanoate (PHA) | ||||
| Comamonas sp. EB 172 | Mixed organic acids from POME | 3 g L−1 | 85.8% | 120 |
| 9.8 g L−1 | 59% | 121 | ||
| Rhodobacter sphaeroides | 4 g L−1 | 67% | 122 | |
| Ralstonia eutropha ATCC 17699 | — | 11.4% | 123 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Polylactic acid (PLA) | ||||
| Bacillus coagulans JI12 | OPEFB | — | 80.6 g L−1 | 124 |
| — | 97 g L−1 | 125 | ||
| Lactobacillus lactis ATCC19435 | OPT | — | 89.9% | 126 |
Mumtaz et al.120 produced PHA anaerobically using organic acids from POME. A maximum recovery rate of 97% (83.23 g L−1 total acid concentration) was achieved via a two-step dewatering and acid-distillation process at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 of H2SO4
4 of H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) POME. Fed-batch cultivation of Comamonas sp. EB 172 was continued with the addition of 1 g L−1 POME acids and 20 C/N ratio (carbon source: organic acids; nitrogen source: (NH4)2SO4) for 63 h at pH 7.5, 30 °C, 30% dissolved O2 concentration, and continuous agitation from 200–800 rpm. The maximum dry cell weight reached 3 g L−1 with a PHA yield of 0.31 g g−1 at 85.8% content. Zakaria et al.121 investigated polyhydroxy butyrate (PHB) production from POME using Comamonas sp. EB 172, achieving a maximum cell dry weight of 9.8 g L−1 with 59% PHB composition. The cultures were incubated at 30–37 °C for 4–5 days using 10 g L−1 of POME acids containing a 5
POME. Fed-batch cultivation of Comamonas sp. EB 172 was continued with the addition of 1 g L−1 POME acids and 20 C/N ratio (carbon source: organic acids; nitrogen source: (NH4)2SO4) for 63 h at pH 7.5, 30 °C, 30% dissolved O2 concentration, and continuous agitation from 200–800 rpm. The maximum dry cell weight reached 3 g L−1 with a PHA yield of 0.31 g g−1 at 85.8% content. Zakaria et al.121 investigated polyhydroxy butyrate (PHB) production from POME using Comamonas sp. EB 172, achieving a maximum cell dry weight of 9.8 g L−1 with 59% PHB composition. The cultures were incubated at 30–37 °C for 4–5 days using 10 g L−1 of POME acids containing a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (acetic
2 (acetic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) propionic
propionic![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) butyric) acid ratio.
butyric) acid ratio.
Hassan et al.122 experimented with Rhodobacter sphaeroides to produce PHA using POME acids as a substrate. The organic acids were obtained through dark-fermentation of treated POME and mesophilic sludge in a photobioreactor at 30 °C and pH 6 for 24 h, producing 8.7 g L−1 total acid concentration. Initially, the R. sphaeroides were cultured in a basic medium containing glucose and ammonium to increase the cell concentration before transferring into the medium containing the POME acids. According to the organic acid profile, acetic and propionic acids were consumed at a much higher rate compared to formic acid. At pH 7, the highest PHA yield of 0.5 g g−1 was obtained with 65.1% PHA content. Hong et al.123 employed a 2 L reactor to synthesize PHA by using Ralstonia eutropha ATCC 17699 using POME-extracted organic acids as a carbon source. The acids were first extracted using a 50 L continuously stirred tank reactor (CSTR) under controlled conditions of pH 6.5, and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sludge to POME ratio for 4 days, achieving the maximum yield at 15.3 g L−1 of total organic acids. Following a two step evaporation process, the organic acid-containing distillate was concentrated with a recovery of 76% to a total concentration of 87.2 g L−1, comprising 44.6 g L−1 acetic acid, 20.2 g L−1 propionic acid, and 22.5 g L−1 butyric acid. These organic acids were then supplemented into a 2 L fed-batch fermenter for PHA accumulation by using Ralstonia eutropha ATCC 17699 under a nitrogen deficient environment and C/N ratio of 40, achieving the highest PHA yield at 11.4 g L−1.
1 sludge to POME ratio for 4 days, achieving the maximum yield at 15.3 g L−1 of total organic acids. Following a two step evaporation process, the organic acid-containing distillate was concentrated with a recovery of 76% to a total concentration of 87.2 g L−1, comprising 44.6 g L−1 acetic acid, 20.2 g L−1 propionic acid, and 22.5 g L−1 butyric acid. These organic acids were then supplemented into a 2 L fed-batch fermenter for PHA accumulation by using Ralstonia eutropha ATCC 17699 under a nitrogen deficient environment and C/N ratio of 40, achieving the highest PHA yield at 11.4 g L−1.
Generally, the synthesis of lactic acid from lignocellulosic materials is done mainly using Lactobacillus sp. or Lactococcus sp., due to their fast-growing characteristics and capability of producing a high yield of acid. The process of lactic acid synthesis includes a few stages in succession: (1) pre-treatment of biomass, (2) saccharification, (3) separate hydrolysis, and (4) fermentation. However, there are some disputes over its unattractive and uneconomical approach. With the current state-of-the-art implementation of simultaneous saccharification and fermentation (SSF), all the fermentation processes can be merged into a single step. Ye et al.124 investigated the production of L-lactic acid from Bacillus coagulans JI12 within a simultaneous detoxification, saccharification, and co-fermentation (SDSCF) system. By consuming the recovered cellulosic and hemicellulosic fractions from OPEFB as substrates, which were recovered via acid hydrolysis, a lactic acid yield of 80.6 g L−1 was obtained with an efficiency of 3.4 g L−1 h−1. Another study by Ye et al.125 was reported on lactic acid production from Bacillus coagulans JI12 using hydrolysate from OPEFB via the batch fermentation process. The acid hydrolysis of OPEFB was conducted at 130 °C for 1 h containing 2% (w/v) H2SO4 and 0.8% (w/v) H3PO4 in a high-pressure reactor. The lactic acid production was then carried out in a 2 L fermenter containing 600 mL hydrolysate, 1% (v/v) yeast extract, and 0.2% (v/v) (NH4)2SO4. Within 9.5 h, a maximum lactic acid yield of 59.2 g L−1 (97%) with an efficiency of 6.2 g L−1 h−1 was achieved. Kosugi et al.126 studied lactic acid production from OPT from the homolactic acid bacterium Lactobacillus lactis ATCC19435. The fermentation resulted in a lactic acid yield of 89.9%, which was comparable to the efficiency of the reference fermentation using glucose as a substrate.
However, the main problem in the development of bioplastics is price competition. Based on the data reported by Hassan et al.,118 the price of biodegradable plastics in the US is about five times higher than that of common thermoplastics. This will limit the demand on bioplastics especially in packaging industries. Besides that, the cost involved in the production process also needs to be reduced. In designing future research, the overall production costs of bioplastics should not only focus on the raw material, bacterial strain and the fermentation system but also on the downstream process which affects the economics of the overall process.
2.8 Acetone–butanol–ethanol fermentation for bioenergy harvesting
Anaerobic fermentation is a series of processes that use anaerobic microorganisms, to break down biodegradable materials such as simple sugars to produce required products in the absence of oxygen. Since the process is in the absence of oxygen, the metabolic reaction could use other substituent components to replace the oxygen as aerobic microorganisms do. Fossil fuels as a major energy source take a toll on the environment. Thus, renewed interest in exploring alternative routes for industrial biofuel production has been evident since the oil crisis of the 1970s, of which acetone–butanol–ethanol (ABE) fermentation is considered one of the early approaches. It employs anaerobic bacteria such as Clostridia sp. to convert fermentable sugars into acetone, butanol, and ethanol. ABE fermentation is divided into two metabolic pathways, which are solventogenesis and acidogenesis. Biofuels have been produced during solventogenesis with a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of acetone, butanol, and ethanol, respectively. Table 7 shows a summary of biobutanol and bioethanol production from oil palm biomass.
1 of acetone, butanol, and ethanol, respectively. Table 7 shows a summary of biobutanol and bioethanol production from oil palm biomass.
Microbial butanol production has been afflicted by grievous fermentation and a high recovery cost resulting in low productivity of butanol. Over the years, several studies have been conducted to overcome these hurdles. The integration of biobutanol separation might be one of the innovative solutions including cell immobilization, extractive fermentation, and pervaporation.141 Hastuti et al.127 have demonstrated the utilization of nanocellulose described as TEMPO-oxidized cellulose nanofibers (TOCN) to improve the stability of C. saccharoperbutylacetonicum N1-4 ATCC 13564. As a result, the total biobutanol concentration obtained was 36.6 g L−1 from OPEFB-derived TOCN, which was 54.3% higher as compared to that of TOCN-free. The study also found that the cell immobilization technique has significantly improved biobutanol production by 27%. The positive effect of TOCN on biobutanol production is due to the safeguard action of TOCN that prevents the loss of calcium ions caused by the tightly packed nanofibrous structure, which subsequently retains a high level of cell viability.142 In another study, by considering the time consumption and extra vessel required, the SSF approach has been introduced to overcome these obstacles. Several studies have been conducted to evaluate the performance of SSF in biobutanol production using OPEFB as a substrate. Ibrahim et al.128 have produced 2.75 g L−1 biobutanol concentration at a yield coefficient of 0.11 g g−1 using 50 g L−1 OPEFB as a substrate. Razali et al.129 studied biobutanol optimization via SSF from pretreated OPEFB by using Clostridium acetobutylicum ATCC 824. The highest biobutanol yield obtained was 3.97 g L−1 concentration at a 0.16 g g−1 yield coefficient, under controlled conditions of pH 5.5, 35 °C, and 15 FPU per g cellulose loading. It is worth mentioning that the optimum biobutanol was achieved at a fermentation period of 120 h with a productivity of 0.03 g L−1 h−1.
Based on Table 7, several studies have been conducted on the feasibility of oil palm biomass as a substrate for the production of bioethanol. Sukhang et al.130 implemented acid-alkali (H2SO4 and NaOH) delignification of OPEFB as a pretreatment before enzymatic hydrolysis using cellulase and β-glucosidase to produce fermentable sugars. The fermentation through SSF and separate hydrolysis and fermentation (SHF) using Klyveromyces marxinus were compared. In SHF, 0.584 g g−1 of sugars obtained has given the maximum bioethanol concentration of 28.1 g L−1 at a yield coefficient of 0.258 g g−1. Meanwhile, a higher bioethanol yield coefficient was observed in the SSF process at 0.281 g g−1 with a concentration of 25.8 g L−1, under optimum conditions of 48 h, 37.5 °C, 10% (w/v) substrate loading, 1% (v/v) culture, and pH 5. Therefore, these findings suggested that better performance of SSF was achieved over the SHF process. Another study by Farah Amani et al.131 utilized OPF hydrolysate (63.7% cellulose, 21.9% hemicellulose, and 14.4% lignin) for bioethanol production. The enzymatic hydrolysis of pretreated OPF was carried out using 40 U per g of cellulase and 10 U per g of hemicellulase at 50 °C for 150 min, resulting in the maximum yield of 2421 μg g−1 and 2418 μg g−1 of glucose and xylose, respectively. The obtained hydrolysate was then used to incubate Saccharomyces cerevisiae HC 10 under controlled conditions of 30 °C, and agitation at 150 rpm for 60 h. The highest bioethanol concentration obtained was 13.8 g L−1 at a 0.12 g g−1 ethanol yield coefficient.
The production of bioethanol from oil palm biomass can lead to the production of vinasse, which is dangerous to the environment. Vinasse is a dark-brown, acidic aqueous with a high COD value. According to Yusof et al.,146 the amount of vinasse production was about 10–20 times that of ethanol produced, with a COD range of 27.5–299.3 kg m−3 (depending on the types of raw materials and the operating conditions). To overcome this problem, the initial separation of suspended solids in the stillage that contained yeast and other materials needs to be done prior to proceeding with other pretreatment methods.
3 Biomass technology by-products
Despite the great incentives, the emerging technologies are not without challenges emanating from the secondary wastes generated during production. In other words, the by-products generated are sources of environmental concern posing a question on the sustainability of the emerging technologies. For instance, technologies employing high temperature treatment are associated with the production of various pollutants as exhaust gas containing condensable gases and non-condensable gases such as NH3, hydrogen sulfide (H2S), sulfur oxide (SO2), CH4, methene (C2H4), CO2 and CO.147,148 Pyrolytic gas capture has been the best applicable solution to pollution caused by biomass pyrolysis. The captured gas, commonly referred to as bio-oil, has potential uses as a bioplastic, asphalt amendment, wood preservative, biocide and crop protection and plant growth enhancer.149The challenging secondary wastes associated with co-composting are odors, bioaerosols and heavy metals, which can lead to health risks such as respiratory disorders and eye membrane irritation. Control measures such as aeration optimization, bulk agent addition and biofiltration for end-of-pipe systems have been shown to be effective technologies to minimize odor emission. Source-separation co-composting is effective in minimizing aerosol generation and reducing the content of heavy metals in compost materials.150 However, anaerobic digestion produces a substantial volume of undesirable byproducts that must be treated to avoid odour and aquatic contamination. Digestate was demonstrated to be an effective organic fertilizer suitable for varieties of crops due to the diverse contents of macroelements and heavy metals.151,152 In general, most biomass conversion technologies aim at zero emission, where value is added to the by-products generated for utilization.
4 Conclusion
There is an issue with oil palm biomass disposal across the world. Therefore, it is vital to continuously explore for alternatives to manage the problem effectively. This review also demonstrates that oil palm biomass is a highly valuable feedstock for value-added use via thermal, physical, chemical, and biochemical conversion routes. It can be inferred that the downstream application has a profound effect on the selection and optimization of a feasible pretreatment technique. Among all, physical and thermomechanical pretreatments are gaining popularity since they are more advantageous due to their chemical-free processability, cost-effectiveness, and sustainability. This is because an ideal oil palm biomass pretreatment should have minimum or no solvent costs and also the capacity to process at high solid loadings with shorter treatment times and minimal secondary waste formation.Overall, the progress conversion technologies for potential bioproducts are summarized as follows:
(1) Solid biomass contains high combustible residues that could turn into biochar through pyrolysis at 400–600 °C under an oxygen-limited environment, reaching a high energy potential of 29 MJ kg−1.
(2) At a higher pyrolysis temperature of 500–1000 °C, solid biomass is thermally activated to form 500–900 m2 g−1 of highly porous activated carbon by further removing the remaining volatile matter.
(3) A liquid fraction of biomass, known as bio-oil, is recoverable at a slower pyrolysis rate that held an energy content of about 30 MJ kg−1.
(4) Biological-assisted degradation of OPEFB using POME over 30–60 days under controlled conditions helps to produce an organic fertilizer with the highest reported NPK ratio of 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 with all heavy metals measured to be <10 mg kg−1.
2.8 with all heavy metals measured to be <10 mg kg−1.
(5) The extraction and fibrillation of cellulose from oil palm fibers produce natural fillers that reinforce composites by improving the tensile and flexural strengths by 13–84% and 139%, respectively.
(6) The anaerobic digestion of POME potentially generates 0.4–2.4 thousand tonnes of CH4 per year at mill capacity production, and 4–12 L H2 per L POME in a lab-scale biohydrogen production set-up.
(7) Microbial fuel cells have made electricity harvesting from oil palm biomass possible, with the highest energy reported to be 85.11 mW m−2 using POME.
(8) Pretreatment and saccharification convert lignocellulosic biomass into glucose at a conversion rate of 63–85%.
(9) Organic plastics from oil palm biomass are synthesized through fermentation under stress growth conditions, where the highest PHA and PLA yields were obtained as 85.8% and 97%, respectively.
(10) Biobutanol and bioethanol are synthesized from Clostridia sp. by ABE fermentation, with the highest yields reported to be 36.6 g L−1 and 0.28 g g−1, respectively.
It can be concluded that there are no preferable technologies for each individual oil palm biomass-based bioproduct, as each has its own set of advantages and disadvantages. In comparison to using a single process, integrating numerous processes can improve the efficiency, economic feasibility, and environmental feasibility of bioproduct production. Despite the never-ending growth of technologies, the manufacturer or producer must examine the goal, technical efficiency, and economic feasibility before making a decision. This is not just to ensure that the company makes a profit, but it also has to be able to meet the company's and targeted customers' needs.
Conflicts of interest
The authors declare no conflict of interest in the preparation of this review.Acknowledgements
The authors would like to highly acknowledge the support of Universiti Pertahanan Nasional Malaysia (National Defence University of Malaysia), Universiti Putra Malaysia and University of Maiduguri in the preparation of this review.References
-
B. Grace, What is palm oil?, https://www.livescience.com/palm-oil.html, accessed 22 December 2021 Search PubMed
.
-
Malaysian Palm Oil Council, Stability and desired attributes of palm olein in deep frying applications, https://mpoc.org.my/stability-and-desired-attributes-of-palm-olein-in-deep-frying-applications/, accessed 22 December 2021 Search PubMed
.
- G. K. Sathishkumar, M. Ibrahim, M. M. Akheel, G. Rajkumar, B. Gopinath, R. Karpagam, P. Karthik, M. M. Charles, G. Gautham and G. G. Shankar, J. Nat. Fibers, 2014, 1–24 Search PubMed
.
- S. N. Arisht, P. M. Abdul, J. Jasni, N. H. Mohd Yasin, S. K. Lin, S. Y. Wu, M. S. Takriff and J. M. Jahim, Ecotoxicol. Environ. Saf., 2020, 203, 110991 CrossRef CAS PubMed
.
-
M. Niall, Which countries produce the most palm oil?, https://www.forbes.com/sites/niallmccarthy/2020/10/02/which-countries-produce-the-most-palm-oil-infographic/?sh=64b4de041e42, accessed 22 December 2021 Search PubMed
.
-
Z. Salman, Biomass wastes from palm oil mills, https://www.bioenergyconsult.com/palm-biomass/, accessed 22 December 2021 Search PubMed
.
-
G. Anli, in Biomass and Bioenergy, Springer, Cham, Switzerland, 2015, pp. 187–209 Search PubMed
.
-
EU-Malaysia Chamber of Commerce and Industry, Oil Palm Biomass & Biogas in Malaysia, 2017 Search PubMed
.
-
S. Claire, Malaysia relies on sustainable palm oil to keep entry into European market, https://www.euractiv.com/section/agriculture-food/news/trashed-61/, accessed 22 December 2021 Search PubMed
.
-
P. Yiswaree, Malay Mail Newsletter, 2020 Search PubMed
.
-
T. Huileng, CNBC Newsletter, 2019 Search PubMed
.
-
Biomass explained – U.S. Energy Information Administration (EIA), https://www.eia.gov/energyexplained/biomass/, accessed 3 April 2022 Search PubMed
.
- S. Kaniapan, S. Hassan, H. Ya, K. Patma Nesan and M. Azeem, Sustainability, 2021, 13, 3110 CrossRef CAS
.
- R. Ilyas, S. Sapuan, M. Atikah, M. Asyraf, S. A. Rafiqah, H. Aisyah, N. M. Nurazzi and M. Norrrahim, Text. Res. J., 2021, 91, 152–167 CrossRef CAS
.
- M. N. Norizan, M. H. Moklis, A. H. Alias, A. I. Rushdan, M. N. F. Norrrahim, K. Abdan and N. Abdullah, Funct. Compos. Struct., 2021, 3, 024002 CrossRef
.
- M. N. F. Norrrahim, R. A. Ilyas, N. M. Nurazzi, M. S. A. Rani, M. S. N. Atikah and S. S. Shazleen, Appl. Sci. Eng. Prog., 2021, 14, 588–605 Search PubMed
.
- M. N. F. Norrrahim, M. R. M. Huzaifah, M. A. A. Farid, S. S. Shazleen, M. S. M. Misenan, T. A. T. Yasim-Anuar, J. Naveen, N. M. Nurazzi, M. S. A. Rani, M. I. Hakimi, R. A. Ilyas and A. Jenol, Polymers, 2021, 13(17), 1–23 CrossRef PubMed
.
-
C. H. Lee, S. H. Lee, F. N. M. Padzil, Z. M. A. Ainun, M. N. F. Norrrahim and K. L. Chin, in Composite Materials, CRC Press, 2021, pp. 29–60 Search PubMed
.
- S. Wang, G. Dai, H. Yang and Z. Luo, Prog. Energy Combust. Sci., 2017, 62, 33–86 CrossRef
.
- F. Abnisa, A. Arami-Niya, W. M. A. W. Daud and J. N. Sahu, BioEnergy Res., 2013, 6, 830–840 CrossRef CAS
.
- J. Idris, Y. Shirai, Y. Andou, A. A. Mohd Ali, M. R. Othman, I. Ibrahim and M. A. Hassan, J. Cleaner Prod., 2015, 89, 257–261 CrossRef CAS
.
- M. F. Alkhatib, S. A. Muyibi and J. O. Amode, Environmentalist, 2011, 31, 349–357 CrossRef
.
- N. H. Zainal, A. A. Aziz, J. Idris, N. F. Jalani, R. Mamat, M. F. Ibrahim, M. A. Hassan and S. Abd-Aziz, J. Cleaner Prod., 2018, 182, 830–837 CrossRef CAS
.
- I. Ibrahim, M. A. Hassan, S. Abd-Aziz, Y. Shirai, Y. Andou, M. R. Othman, A. A. M. Ali and M. R. Zakaria, J. Cleaner Prod., 2017, 141, 122–127 CrossRef CAS
.
- Anshariah, A. M. Imran, S. Widodo and U. R. Irvan, IOP Conf. Ser. Earth Environ. Sci., 2020, 473, 1–7 Search PubMed
.
-
Mechanical Engineer's Data Handbook, ed. J. Carvill, Butterworth-Heinemann, 1993, pp. 102–145 Search PubMed
.
- A. A. Lawal, M. A. Hassan, M. R. Zakaria, M. Z. M. Yusoff, M. N. F. Norrrahim, M. N. Mokhtar and Y. Shirai, Bioresour. Technol., 2021, 332, 1–7 CrossRef PubMed
.
- W. Li, K. Yang, J. Peng, L. Zhang, S. Guo and H. Xia, Ind. Crops Prod., 2008, 28, 190–198 CrossRef CAS
.
- L. Wang, H. Zhang, G. Cao, W. Zhang, H. Zhao and Y. Yang, Electrochim. Acta, 2015, 186, 654–663 CrossRef CAS
.
- F. Wang, S. Tan, Y. Cao, D. Wang, J. Wu, F. Luo, Q. Liu, X. Xie, S. Li and M. Zhou, Energy Fuels, 2020, 35(5), 6168–6177 CrossRef
.
- V. J. Watson, C. Nieto Delgado and B. E. Logan, Environ. Sci. Technol., 2013, 47, 6704–6710 CrossRef CAS PubMed
.
- H. Marsh and F. Rodríguez-Reinoso, Carbon, 2006, 454–508 Search PubMed
.
- M. J. Prauchner and F. Rodríguez-Reinoso, Microporous Mesoporous Mater., 2012, 152, 163–171 CrossRef CAS
.
-
H. Chen, Lignocellulose biorefinery product engineering, Woodhead Publishing Series, 2015 Search PubMed
.
- R. K. Liew, W. L. Nam, M. Y. Chong, X. Y. Phang, M. H. Su, P. N. Y. Yek, N. L. Ma, C. K. Cheng, C. T. Chong and S. S. Lam, Process Saf. Environ. Prot., 2018, 115, 57–69 CrossRef CAS
.
- F. Abnisa, W. M. A. W. Daud, W. N. W. Husin and J. N. Sahu, Biomass Bioenergy, 2011, 35, 1863–1872 CrossRef CAS
.
- A. Mohammadi, B. Khoshnevisan, G. Venkatesh and S. Eskandari, Processes, 2020, 8, 1–21 Search PubMed
.
- M. S. Reza, C. S. Yun, S. Afroze, N. Radenahmad, M. S. A. Bakar, R. Saidur, J. Taweekun and A. K. Azad, J. Assoc. Arab Univ. Basic Appl. Sci., 2020, 27, 208–238 Search PubMed
.
- A. N. Kabbashi, Z. A. MD and A. Othan, Nat. Res. Eng. Technol., 2006, 176–181 Search PubMed
.
- N. Mohammad, M. Z. Alam and N. A. Kabashi, J. Mater. Cycles Waste Manage., 2013, 15, 348–356 CrossRef CAS
.
- W. Shi, J. M. Norton, B. E. Miller and M. G. Pace, Appl. Soil Ecol., 1999, 11, 17–28 CrossRef
.
- J. J. Thambirajah, M. D. Zulkali and M. A. Hashim, Bioresour. Technol., 1995, 52, 133–144 CrossRef CAS
.
- R. P. Singh, M. H. Ibrahim, N. Esa and M. S. Iliyana, Rev. Environ. Sci. Biotechnol., 2010, 9, 331–344 CrossRef CAS
.
- Z. Dzulkurnain, M. A. Hassan, M. R. Zakaria, P. E. M. Wahab, M. Y. Hasan and Y. Shirai, Waste Biomass Valorization, 2017, 8, 695–705 CrossRef CAS
.
- V. Sim, J. Wei, C. H. Bing, A. Saptoro and J. Nandong, Iran. J. Energy Environ., 2016, 7(2), 156–162 Search PubMed
.
- D. Meyer-Kohlstock, G. Hädrich, W. Bidlingmaier and E. Kraft, Journal of Waste Management, 2013, 33, 536–539 CrossRef PubMed
.
- A. Yahya, C. P. Sye, T. A. Ishola and H. Suryanto, Bioresour. Technol., 2010, 101, 8736–8741 CrossRef CAS PubMed
.
- T. Jiang, F. Schuchardt, G. Li, R. Guo and Y. Zhao, J. Environ. Sci., 2011, 23, 1754–1760 CrossRef CAS
.
- M. H. M. Zainudin, N. Ramli, M. A. Hassan, Y. Shirai, K. Tashiro, K. Sakai and Y. Tashiro, J. Ind. Microbiol. Biotechnol., 2017, 44, 869–877 CrossRef CAS PubMed
.
- A. S. Baharuddin, M. Wakisaka, Y. Shirai, S. Abd-Aziz, N. A. Abdul Rahman and M. A. Hassan, Int. J. Agric. Res., 2009, 4, 69–78 CrossRef CAS
.
- M. N. Ahmad, M. N. Mokhtar, A. S. Baharuddin, L. S. Hock, S. R. A. Ali, S. Abd-Aziz, N. A. A. Rahman and M. A. Hassan, BioResources, 2011, 6, 4762–4780 CAS
.
- L. Hock, A. S. Baharuddin, M. N. Ahmad, U. K. M. Shah, N. A. A. Rahman, S. Abd-Aziz, M. A. Hassan and Y. Shirai, Aust. J. Basic Appl. Sci., 2009, 3, 2809–2816 CAS
.
- Y. Krishnan, C. P. C. Bong, N. F. Azman, Z. Zakaria, N. Othman, N. Abdullah, C. S. Ho, C. T. Lee, S. B. Hansen and H. Hara, J. Cleaner Prod., 2017, 146, 94–100 CrossRef CAS
.
- A. B. A. Hariharan and H. P. S. A. Khalil, J. Compos. Mater., 2005, 39, 663–684 CrossRef CAS
.
- C. A. S. Hill and H. P. S. Abdul, J. Appl. Polym. Sci., 2000, 77, 1322–1330 CrossRef CAS
.
- G. Raju, C. T. Ratnam, N. A. Ibrahim, M. Z. Ab. Rahman and W. M. Z. Wan Yunus, J. Appl. Polym. Sci., 2008, 110, 368–375 CrossRef CAS
.
- H. D. Rozman, M. J. Saad and Z. A. Mohd Ishak, Polym. Test., 2002, 22, 335–341 CrossRef
.
- M. S. Sreekala, M. G. Kumaran, M. L. Geethakumariamma and S. Thomas, Adv. Compos. Mater., 2004, 13, 171–197 CrossRef CAS
.
- B. F. Yousif and N. S. M. El-Tayeb, Proc. Inst. Mech. Eng., Part J, 2008, 222, 637–646 CrossRef CAS
.
-
M. N. F. Norrrahim, T. A. T. Yasim-Anuar, S. M. Sapuan, R. A. Ilyas, M. I. Hakimi, S. U. F. S. Najmuddin and M. A. Jenol, in Bio-Based Packaging: Material, Environmental and Economic Aspects, Wiley Online Library, 2021 Search PubMed
.
- T. A. T. Yasim-Anuar, H. Ariffin, M. N. F. Norrrahim, M. A. Hassan, Y. Andou, T. Tsukegi and H. Nishida, Polymers, 2020, 12, 1–17 CrossRef PubMed
.
- M. N. F. Norrrahim, H. Ariffin, T. A. T. Yasim-Anuar, M. A. Hassan, N. A. Ibrahim, W. M. Z. W. Yunus and H. Nishida, Polymers, 2021, 13, 1064 CrossRef CAS PubMed
.
- M. N. F. Norrrahim, N. A. M. Kasim, V. F. Knight, N. A. Halim, N. A. A. Shah, S. A. M. Noor, S. H. Jamal, K. K. Ong, W. M. Z. W. Yunus, M. A. A. Farid, M. A. Jenol and R. A. Ilyas, Functional Composites and Structures, 2021, 3(2), 024001 CrossRef
.
- M. N. F. Norrrahim, H. Ariffin, M. A. Hassan, N. A. Ibrahim, W. M. Z. W. Yunus and H. Nishida, Int. J. Nanotechnol., 2019, 16, 668–679 CrossRef CAS
.
- T. A. T. Yasim-Anuar, H. Ariffin and M. A. Hassan, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 368, 012033 Search PubMed
.
- K. Pacaphol and D. Aht-Ong, J. Cleaner Prod., 2017, 142, 1283–1295 CrossRef CAS
.
- L. N. Megashah, H. Ariffin, M. R. Zakaria and M. A. Hassan, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 368, 012049 Search PubMed
.
- B. Soni, E. B. Hassan, M. W. Schilling and B. Mahmoud, Carbohydr. Polym., 2016, 151, 779–789 CrossRef CAS PubMed
.
- A. Alemdar and M. Sain, Bioresour. Technol., 2008, 99, 1664–1671 CrossRef CAS PubMed
.
- N. Saba, M. Jawaid, M. T. Paridah and O. Alothman, Ind. Crops Prod., 2017, 108, 840–843 CrossRef CAS
.
- A. de Campos, A. R. d. Sena Neto, V. B. Rodrigues, B. R. Luchesi, F. K. V. Moreira, A. C. Correa, L. H. C. Mattoso and J. M. Marconcini, Carbohydr. Polym., 2017, 175, 330–336 CrossRef CAS PubMed
.
-
H. Ariffin, M. N. F. Norrrahim, T. A. T. Yasim-Anuar, H. Nishida, M. A. Hassan, N. A. Ibrahim and W. M. Z. W. Yunus, in Bionanocomposites for Packaging Applications, Springer, 2018, pp. 95–105 Search PubMed
.
- M. N. F. Norrrahim, H. Ariffin, T. A. T. Yasim-Anuar, M. A. Hassan, H. Nishida and T. Tsukegi, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 368, 1–9 Search PubMed
.
- M. K. M. Haafiz, A. Hassan, H. P. S. A. Khalil, I. Khan, I. M. Inuwa, M. S. Islam, M. S. Hossain, M. I. Syakir and M. R. N. Fazita, Polym. Test., 2015, 48, 133–139 CrossRef
.
- N. S. Lani, N. Ngadi, A. Johari and M. Jusoh, J. Nanomater., 2014, 1–10 Search PubMed
.
-
N. S. Sharip, T. A. T. Yasim-Anuar, M. N. F. Norrrahim, S. S. Shazleen, N. M. Nurazzi, S. M. Sapuan and R. A. Ilyas, in Composites in Biomedical Applications, CRC Press, 2020, pp. 161–190 Search PubMed
.
-
R. A. Ilyas, S. M. Sapuan, M. N. F. Norrrahim, T. A. T. Yasim-Anuar, A. Kadier, M. S. Kalil, M. S. N. Atikah, R. Ibrahim, M. Asrofi, H. Abral, A. Nazrin, R. Syafiq, H. A. Aisyah and M. R. M. Asyraf, in Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers, ed. F. M. Al-Oqla and S. M. Sapuan, Elsevier Inc., Amsterdam, Netherland, 1st edn, 2020, pp. 65–88 Search PubMed
.
- R. A. Ilyas, S. M. Sapuan, R. Ibrahim, H. Abral, M. R. Ishak, E. S. Zainudin, M. Asrofi, M. S. N. Atikah, M. R. M. Huzaifah, A. M. Radzi, A. M. N. Azammi, M. A. Shaharuzaman, N. M. Nurazzi, E. Syafri, N. H. Sari, M. N. F. Norrrahim and R. Jumaidin, J. Mater. Res. Technol., 2019, 8(3), 2753–2766 CrossRef CAS
.
-
R. Ilyas, S. Sapuan, A. Kadier, M. S. Kalil, R. Ibrahim, M. Atikah, N. M. Nurazzi, A. Nazrin, C. Lee, M. N. F. Norrrahim, N. H. Sari, E. Syafri, H. Abral, L. Jasmani and M. Ibrahim, in Advanced Processing, Properties, and Applications of Starch and Other Bio-Based Polymers, Elsevier, 2020, pp. 111–138 Search PubMed
.
- R. A. Ilyas, S. M. Sapuan, M. M. Harussani, M. Y. A. Y. Hakimi, M. Z. M. Haziq, M. S. N. Atikah, M. R. M. Asyraf, M. R. Ishak, M. R. Razman, N. M. Nurazzi, M. N. F. Norrrahim, H. Abral and M. Asrofi, Polymers, 2021, 13, 1326 CrossRef CAS PubMed
.
-
M. N. F. Norrrahim, T. A. T. Yasim-Anuar, M. A. Jenol, N. Mohd Nurazzi, R. A. Ilyas and S. Sapuan, in Biocomposite and Synthetic Composites for Automotive Applications, Woodhead Publishing Series, Amsterdam, Netherland, 2020, pp. 119–215 Search PubMed
.
- M. N. F. Norrrahim, N. A. M. Kasim, V. F. Knight, F. A. Ujang, N. Janudin, M. A. I. A. Razak, N. A. A. Shah, S. A. M. Noor, S. H. Jamal, K. K. Ong and W. M. Z. W. Yunus, Mater. Adv., 2021, 2, 1485–1506 RSC
.
- S. S. Shahlan, K. Kidam, T. A. T. Abdullah, M. W. Ali, L. M. Pejic and H. Kamarden, Journal of Energy and Safety Technology, 2019, 2(1), 2637–1030 Search PubMed
.
- M. S. Ahmad, M. S. Ali and N. Abd Rahim, Energy Strategy Rev., 2021, 35, 100632 CrossRef
.
- A. Górniak, K. Midor, J. Kaźmierczak and W. Kaniak, Multidiscip. Asp. Prod. Eng., 2018, 1, 241–247 Search PubMed
.
- S. R. Sharvini, Z. Z. Noor, C. S. Chong, L. C. Stringer and D. Glew, Energy, 2020, 191, 116513 CrossRef CAS
.
- M. J. Chin, P. E. Poh, B. T. Tey, E. S. Chan and K. L. Chin, Renew. Sustain. Energy Rev., 2013, 26, 717–726 CrossRef CAS
.
- S. S. Harsono, P. Grundmann and S. Soebronto, J. Cleaner Prod., 2014, 64, 619–627 CrossRef CAS
.
- P. Mishra, S. Thakur, L. Singh, Z. Ab Wahid and M. Sakinah, Int. J. Hydrogen Energy, 2016, 41, 18431–18440 CrossRef CAS
.
- J. Seengenyoung, C. Mamimin, P. Prasertsan and S. O-Thong, Int. J. Hydrogen Energy, 2019, 44, 3347–3355 CrossRef CAS
.
- S. O-Thong, W. Suksong, K. Promnuan, M. Thipmunee, C. Mamimin and P. Prasertsan, Int. J. Hydrogen Energy, 2016, 41, 21702–21712 CrossRef CAS
.
- S. S. Mahmod, A. M. Azahar, J. P. Tan, J. M. Jahim, P. M. Abdul, M. S. Mastar, N. Anuar, M. F. Mohammed Yunus, A. J. Asis and S. Y. Wu, Renewable Energy, 2019, 134, 1262–1272 CrossRef CAS
.
- M. M. A. Aziz, K. A. Kassim, M. ElSergany, S. Anuar, M. E. Jorat, H. Yaacob, A. Ahsan, M. A. Imteaz and P. Arifuzzaman, Renew. Sustain. Energy Rev., 2020, 119, 109603 CrossRef CAS
.
- Y. S. Wong, T. T. Teng, S. A. Ong, N. Morad and M. Rafatullah, RSC Adv., 2014, 4, 64659–64667 RSC
.
- T. L. Kelly-Yong, K. T. Lee, A. R. Mohamed and S. Bhatia, Energy Policy, 2007, 35, 5692–5701 CrossRef
.
- R. J. Patinvoh and M. J. Taherzadeh, Curr. Opin. Environ. Sci. Health, 2019, 12, 30–37 CrossRef
.
- G. Drendel, E. R. Mathews, L. Semenec and A. E. Franks, Appl. Sci., 2018, 8, 2384 CrossRef CAS
.
- M. A. Jenol, M. F. Ibrahim, E. K. Bahrin, S. W. Kim and S. Abd-Aziz, Molecules, 2019, 24, 2397 CrossRef CAS PubMed
.
- D. Pant, G. Van Bogaert, L. Diels and K. Vanbroekhoven, Bioresour. Technol., 2010, 101, 1533–1543 CrossRef CAS PubMed
.
- M. H. M. Nor, M. F. M. Mubarak, H. S. A. Elmi, N. Ibrahim, M. F. A. Wahab and Z. Ibrahim, Bioresour. Technol., 2015, 190, 458–465 CrossRef CAS PubMed
.
- N. A. N. Mahmood, N. F. Ghazali, K. A. Ibrahim and M. A. Ali, Chem. Eng. Trans., 2017, 56, 1795–1800 Search PubMed
.
- N. A. N. Mahmood, L. K. Thong, K. A. Ibrahim, J. B. Chyan and N. F. Ghazali, Chem. Eng. Trans., 2015, 45, 79–84 Search PubMed
.
- A. A. Bazmi, G. Zahedi and H. Hashim, Renew. Sustain. Energy Rev., 2011, 15, 574–583 CrossRef
.
- C. Hang Shu, R. Jaiswal and J. Syong Shih, Biotechnol. Bioprocess, 2015, 5, 1–7 CrossRef
.
- X. Chen, Y. Zhang, Y. Gu, Z. Liu, Z. Shen, H. Chu and X. Zhou, Appl. Energy, 2014, 122, 34–41 CrossRef CAS
.
- M. R. Zakaria, M. N. F. Norrrahim, S. Hirata and M. A. Hassan, Bioresour. Technol., 2015, 181, 263–269 CrossRef CAS PubMed
.
- G. Bali, X. Meng, J. I. Deneff, Q. Sun and A. J. Ragauskas, ChemSusChem, 2015, 8, 275–279 CrossRef CAS PubMed
.
- N. F. A. A. Rizal, M. F. Ibrahim, M. R. Zakaria, E. K. Bahrin, S. Abd-Aziz and M. A. Hassan, Molecules, 2018, 23(811), 1–14 Search PubMed
.
- S. Zulkefli, E. Abdulmalek and M. B. Abdul Rahman, Renewable Energy, 2017, 107, 36–41 CrossRef CAS
.
- P. L. Tang, P. M. Abdul, N. S. Engliman and O. Hassan, Cellulose, 2018, 25, 4677–4694 CrossRef CAS
.
- Y. T. Julio-Altamiranda, J. D. Mercado-Pacheco, E. L. Sánchez-Tuirán, Á. D. González-Delgado and K. A. Ojeda, Sustainable Chem. Pharm., 2020, 16, 100256 CrossRef
.
- N. Ahmad, M. R. Zakaria, M. Z. M. Yusoff, S. Fujimoto, H. Inoue, H. Ariffin, M. A. Hassan and Y. Shirai, Molecules, 2018, 23(1310), 1–14 Search PubMed
.
- P. Alvira, A. D. Moreno, D. Ibarra, F. Sáez and M. Ballesteros, Biotechnol. Prog., 2013, 29, 74–82 CrossRef CAS PubMed
.
- N. Nnaemeka and N. MacManus, J. Eng. Technol. Res., 2016, 8, 47–57 CrossRef
.
- Y. Dai, M. Si, Y. Chen, N. Zhang, M. Zhou, Q. Liao, D. Shi and Y. Liu, Bioresour. Technol., 2015, 198, 725–731 CrossRef CAS PubMed
.
- N. F. A. A. Rizal, M. F. Ibrahim, M. R. Zakaria, S. Abd-Aziz, P. L. Yee and M. A. Hassan, Molecules, 2018, 23, 1–14 CrossRef PubMed
.
- A. Z. M. Rus, Sci. Prog., 2010, 93, 285–300 CrossRef CAS PubMed
.
- M. A. Hassan, L. N. Yee, P. L. Yee, H. Ariffin, A. R. Raha, Y. Shirai and K. Sudesh, Biomass Bioenergy, 2013, 50, 1–9 CrossRef CAS
.
- K. Sudesh and T. Iwata, Clean: Soil, Air, Water, 2008, 36, 433–442 CAS
.
- T. Mumtaz, N. A. Yahaya, S. Abd-Aziz, N. Abdul Rahman, P. L. Yee, Y. Shirai and M. A. Hassan, J. Cleaner Prod., 2010, 18, 1393–1402 CrossRef CAS
.
- M. R. Zakaria, M. Tabatabaei, F. M. Ghazali, S. Abd-Aziz, Y. Shirai and M. A. Hassan, World J. Microbiol. Biotechnol., 2010, 26, 767–774 CrossRef CAS
.
- M. A. Hassan, Y. Shirai, N. Kusubayashi, M. I. A. Karim, K. Nakanishi and K. Hashimoto, J. Ferment. Bioeng., 1996, 82, 151–156 CrossRef CAS
.
- S. K. Hong, Y. Shirai, A. R. N. Aini and M. A. Hassan, Res. J. Environ. Sci., 2009, 3, 552–559 CrossRef CAS
.
- L. Ye, M. S. Bin Hudari, Z. Li and J. C. Wu, Biochem. Eng. J., 2014, 83, 16–21 CrossRef CAS
.
- L. Ye, M. S. Bin Hudari, X. Zhou, D. Zhang, Z. Li and J. C. Wu, Appl. Microbiol. Biotechnol., 2013, 97, 4831–4838 CrossRef CAS PubMed
.
- A. Kosugi, R. Tanaka, K. Magara, Y. Murata, T. Arai, O. Sulaiman, R. Hashim, Z. A. A. Hamid, M. K. A. Yahya, M. N. M. Yusof, W. A. Ibrahim and Y. Mori, J. Biosci. Bioeng., 2010, 110, 322–325 CrossRef CAS PubMed
.
- N. Hastuti, R. F. Darmayanti, S. D. Hardiningtyas, K. Kanomata, K. Sonomoto, M. Goto and T. Kitaoka, BioResources, 2019, 14, 6936–6957 CAS
.
- M. F. Ibrahim, S. Abd-Aziz, M. E. M. Yusoff, L. Y. Phang and M. A. Hassan, Renewable Energy, 2015, 77, 447–455 CrossRef CAS
.
- N. A. A. Md Razali, M. F. Ibrahim, E. K. Bahrin and S. Abd-Aziz, Molecules, 2018, 23(1944), 1–17 Search PubMed
.
- S. Sukhang, S. Choojit, T. Reungpeerakul and C. Sangwichien, Cellulose, 2020, 27, 301–314 CrossRef CAS
.
- A. H. Farah Amani, S. M. Toh, J. S. Tan and C. K. Lee, Waste Biomass Valorization, 2018, 9, 539–548 CrossRef CAS
.
- C. Ouephanit, C. Virunanon, V. Burapatana and W. Chulalaksananukul, Water Sci. Technol., 2011, 64, 1774–1780 CrossRef CAS PubMed
.
- C. N. Hipolito, E. Crabbe, C. M. Badillo, O. C. Zarrabal, M. A. Morales Mora, G. P. Flores, M. d. A. Hernández Cortazar and A. Ishizaki, J. Cleaner Prod., 2008, 16, 632–638 CrossRef
.
- N. Qureshi, V. Singh, S. Liu, T. C. Ezeji, B. C. Saha and M. A. Cotta, Bioresour. Technol., 2014, 154, 222–228 CrossRef CAS PubMed
.
- J.-J. Dong, J.-C. Ding, Y. Zhang, L. Ma, G.-C. Xu, R.-Z. Han and Y. Ni, FEMS Microbiol. Lett., 2016, 363(4), 1–6 CrossRef PubMed
.
- H. Husin, M. F. Ibrahim, E. Kamal Bahrin and S. Abd-Aziz, Energy Sci. Eng., 2019, 7, 66–75 CrossRef CAS
.
- M. F. Ibrahim, S. Linggang, M. A. Jenol, P. L. Yee and S. Abd-Aziz, BioResources, 2015, 10, 3890–3907 CrossRef CAS
.
- M. N. A. Razak, M. F. Ibrahim, P. L. Yee, M. A. Hassan and S. Abd-Aziz, BioResources, 2013, 8, 1758–1770 Search PubMed
.
- L. D. Gottumukkala, B. Parameswaran, S. K. Valappil, K. Mathiyazhakan, A. Pandey and R. K. Sukumaran, Bioresour. Technol., 2013, 145, 182–187 CrossRef CAS PubMed
.
- G. Qi, L. Xiong, H. Li, Q. Huang, M. Luo, L. Tian, X. Chen, C. Huang and X. Chen, Biomass Bioenergy, 2019, 122, 76–83 CrossRef CAS
.
- N. Qureshi, S. Hughes, I. S. Maddox and M. A. Cotta, Bioprocess Biosyst. Eng., 2005, 27, 215–222 CrossRef CAS PubMed
.
- M. Park, D. Lee and J. Hyun, Carbohydr. Polym., 2015, 116, 223–228 CrossRef CAS PubMed
.
- V. Chaturvedi and P. Verma, 3 Biotech, 2013, 3, 415–431 CrossRef PubMed
.
- S. Kusch-Brandt, Resources, 2019, 8, 139 CrossRef
.
- K. A. Gray, L. Zhao and M. Emptage, Curr. Opin. Chem. Biol., 2006, 10, 141–146 CrossRef CAS PubMed
.
- S. J. H. Mohd Yusof, A. M. Roslan, K. N. Ibrahim, S. S. S. Abdullah, M. R. Zakaria, M. A. Hassan and Y. Shirai, Sustainability, 2019, 11, 1–14 CrossRef
.
- H. Yang, R. Yan, H. Chen, D. H. Lee, D. T. Liang and C. Zheng, Fuel Process. Technol., 2006, 87, 935–942 CrossRef CAS
.
- H. Yang, R. Yan, T. Chin, D. T. Liang, H. Chen and C. Zheng, Energy Fuels, 2004, 18, 1814–1821 CrossRef CAS
.
- H. P. Schmidt, A. Anca-Couce, N. Hagemann, C. Werner, D. Gerten, W. Lucht and C. Kammann, GCB Bioenergy, 2019, 11, 573–591 CrossRef CAS
.
- Y. Wei, J. Li, D. Shi, G. Liu, Y. Zhao and T. Shimaoka, Resour., Conserv. Recycl., 2017, 122, 51–65 CrossRef
.
- M. Koszel and E. Lorencowicz, Agriculture and Agricultural Science Procedia, 2015, 7, 119–124 CrossRef
.
- T. J. Barzee, A. Edalati, H. El-Mashad, D. Wang, K. Scow and R. Zhang, Front. Sustain. Food Syst., 2019, 3, 58 CrossRef
.
| This journal is © The Royal Society of Chemistry 2022 |