Stable and wide-wavelength tunable luminescence of CsPbX3 nanocrystals encapsulated in metal–organic frameworks†
Hailong
Wu
,
Lijia
Yao
,
Wenqian
Cao
,
Yu
Yang
 ,
Yuanjing
Cui
,
Yuanjing
Cui
 *,
Deren
Yang
*,
Deren
Yang
 and
Guodong
Qian
and
Guodong
Qian
 *
*
State Key Laboratory of Silicon Materials, Cyrus Tang Center for Sensor Materials and Applications, School of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: cuiyj@zju.edu.cn; gdqian@zju.edu.cn
First published on 2nd March 2022
Abstract
Lead-halide perovskite nanocrystals (PeNCs) possess attractive linear and nonlinear optical properties. However, further adjusting their linear and nonlinear optical properties in a facile way remains a great challenge due to the inevitable instabilities. Herein, an effective host–guest system was successfully constructed by encapsulating PeNCs (CsPbX3 NCs) into a metal–organic framework (MOF), ZJU-28, via a sequential deposition method at ambient conditions. The accessible and charged frameworks of ZJU-28 facilitate the confinement growth of CsPbX3 NCs in the channels under a mild environment, and therefore facile halide-composition adjustment can be achieved by simply tuning the halide stoichiometry of CsX in a solution process, enabling wide-wavelength tunable one- and two-photon excited (1PE and 2PE) luminescence from 450 nm to 660 nm. Moreover, besides the confinement effect, effective separation, passivation and protection of CsPbX3 NCs are still achieved in ZJU-28⊃CsPbX3 crystals, resulting in a giant two-photon action cross-section which is comparable to that of colloidal CsPbX3 NCs densely capped by surface ligands, and significantly enhanced 1PE and 2PE photostability especially for the spectral stability of mixed-halide composites. These results will pave the way for the exploitation of highly stable and emission-tunable PeNCs composites in optoelectronic applications and even future integrated photonics.
Introduction
Lead-halide perovskite nanocrystals (PeNCs) have been widely recognized as emerging luminescent materials and promising candidates in the fields of display backlights, light emitting diodes (LEDs), lasers and photodetectors due to the combination of a high photoluminescence quantum yield (PLQY), large absorption coefficient, high color purity and tunable bandgap.1–6 Besides the exceptional linear optical characteristics, PeNCs still exhibit superior nonlinear optical properties especially for two-photon absorption (2PA) compared with traditional semiconductor nanocrystals and organic molecules, which further broaden their applications benefited from longer wavelength absorption.7–9 Many efforts have been devoted to exploring the relationship between the nonlinear absorption capacity and the sizes, shape and also the composition of PeNCs, aiming at promoting and tunning their nonlinear optical properties.10–12 Compositional engineering such as halogen substitution is an effective way to adjust the electronic structure and bandgap of PeNCs, thereby further optimizing their nonlinear absorption capacity to enable full visible spectrum modulation of up-conversion luminescence.13,14 However, PeNCs are vulnerable to light, oxygen, heat, moisture and polar solvent and are severely degraded under ambient conditions due to their inherent ionic nature and low formation energy.15 In particular, iodine-containing PeNCs are more volatile because large-radius iodide ions in the perovskite lattice lead to a lower Goldschmidt tolerance factor (below 0.9), which indicates their intrinsic structural instability under ambient conditions.16 These iodine-containing PeNCs also exhibit poor phase stability and tend to spontaneously undergo phase transition into the undesired non-luminous phase once they are exposed to prolonged illumination, humidity or high temperature.17,18 Furthermore, some mixed-halide PeNCs show compositional instability in the form of the migration of halide ions and the segregation of halides into separate iodide- and bromide-rich domains under illumination or applied bias, which leads to localized variations in the material band gap and adversely impacts the emission spectrum and color.19–21 Although the isolation and passivation effect enabled by anchoring PeNCs on the surface of silica spheres, or embedding PeNCs into stable hosts such as polymers, inorganic salts, SiO2, glass and zeolites, can alleviate the stability issues, the research subjects mainly focus on CsPbBr3 NCs, one of the most stable species of PeNCs.22–27 More efforts are needed to stabilize mix-halide PeNCs and those containing iodine. Therefore, it remains a great challenge to stabilize PeNCs and further optimize and tune their nonlinear optical properties via compositional engineering.Host–guest systems constructed by encapsulating PeNCs into host materials with specific methods are usually utilized to address the above issues. CsPbX3 NCs have been successfully stabilized in glass via a conventional melt-quenching technique, or in zeolites via a modified hot-injection method, both of which enable full spectrum adjustment of up-conversion luminescence.28,29 However, the ultrahigh temperature is indispensable in the melt-quenching technique and tedious conditions such as heating and a nitrogen atmosphere are highly required in the hot-injection method. Recent intriguing porous materials, metal–organic frameworks (MOFs), have been demonstrated to be multifunctional platforms for the successful incorporation of dye molecules, carbon dots and even nanoparticles, owing to their high porosity, tunable pores/channels and specific sites.30–33 The accessible and charged frameworks (e.g. anionic MOF, ZJU-28) enable enrichment of the constituent elements of perovskites (lead and caesium) via ion exchange processes in solution and trigger nucleation and growth of PeNCs inside hydrophobic pores/channels of MOFs under an ambient environment.34,35 In addition, this ion exchange process in synthetic procedures offers great convenience for compositional engineering of the in situ formation of PeNCs under mild conditions. Therefore, the combination of PeNCs and MOFs seems to be a facile and effective strategy to simultaneously stabilize PeNCs and tune their nonlinear optical properties.
Herein, host–guest ZJU-28⊃CsPbX3 was constructed by encapsulating CsPbX3 NCs into an anionic MOF, ZJU-28 (ZJU-28 = [In3(BTB)4] (Me2NH2)3), via a novel sequential deposition approach, during which in situ growth of CsPbX3 NCs was realized by sequentially depositing Pb2+ and CsX into the channels of ZJU-28. The whole growth process of CsPbX3 NCs was carried out under mild conditions and their halide composition can be simply tuned from Cl2Br to BrI2 by adjusting the halide stoichiometry of CsX during the second deposition process, resulting in successfully tuning the 1PE and 2PE luminescence of ZJU-28⊃CsPbX3 crystals covering almost the full-vision region. Thanks to the strong confinement and surface passivation provided by ZJU-28 frameworks, ZJU-28⊃CsPbX3 crystals exhibit a giant two-photo action cross-section (8.66 × 104 GM for ZJU-28⊃CsPbClBr2 at 780 nm, 1.58 × 106 GM for ZJU-28⊃CsPbBr3 at 780 nm and 1.96 × 105 GM for ZJU-28⊃CsPbBrI2 at 980 nm, respectively), which is comparable to that of colloidal CsPbX3 NCs dispersed in toluene. Moreover, ZJU-28 frameworks also separate and passivate the internal CsPbX3 NCs, resulting in significantly enhanced 1PE and 2PE photostability especially for the spectral stability of mixed-halide composites.
Results and discussion
Anionic MOF ZJU-28 was chosen as the host to encapsulate CsPbX3 NCs and ZJU-28⊃CsPbX3 crystals were synthesized via a sequential deposition method at ambient conditions, as shown in Scheme 1. Firstly, lead ions were introduced into the channels of ZJU-28 by replacing the dimethylamine cations (Me2NH2+) to form ZJU-28⊃Pb2+ crystals.36 The obtained ZJU-28⊃Pb2+ crystals were then impregnated into a methanol solution of CsX to trigger the in situ growth of CsPbX3 NCs within ZJU-28. It is worth noting that the CsX solution provides all the halogens in this reaction, therefore, a series of ZJU-28⊃CsPbX3 crystals was successfully synthesized by simply adjusting the halide stoichiometry of CsX from Br to Cl/Br or Br/I, including ZJU-28⊃CsPbCl2Br, ZJU-28⊃CsPbCl1.5Br1.5, ZJU-28⊃CsPbClBr2, ZJU-28⊃CsPbBr3, ZJU-28⊃CsPbBr2I, ZJU-28⊃CsPbBr1.5I1.5, and ZJU-28⊃CsPbBrI2. All experiments were carried out under an ambient environment without any atmosphere protection, which makes the in-situ formation and composition regulation of CsPbX3 NCs facile. The various colors of the acquired samples and their blue to red emission under UV light are indicative of the successful formation of CsPbX3 NCs with different halide compositions in ZJU-28 (Fig. S1, ESI†). As a comparison, a series of colloidal CsPbX3 NCs (X3 = Cl2Br, Cl1.5Br1.5, ClBr2, Br3, Br2I, Br1.5I1.5, BrI2) was also prepared via a typical hot-injection method (Fig. S2, ESI†).13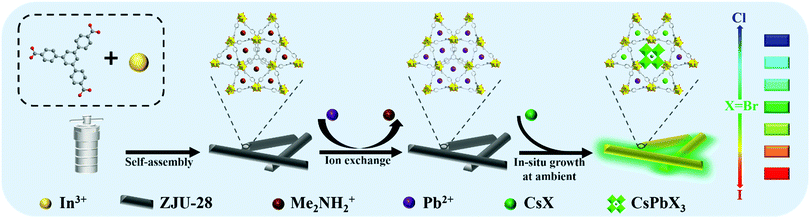 | ||
| Scheme 1 Schematic diagram of the in situ synthesis of ZJU-28⊃CsPbX3 crystals with a controllable halide composition. | ||
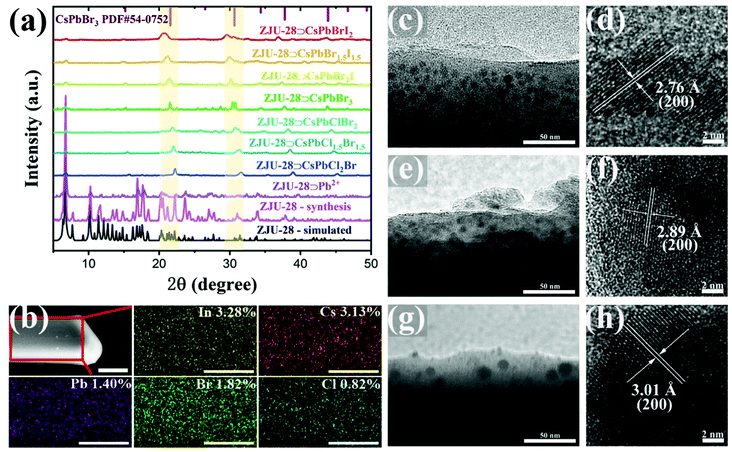
Various methods were carried out to confirm the successful in situ formation of CsPbX3 NCs in ZJU-28. Powder X-ray diffraction (PXRD) was measured to investigate the crystal structure and phase of the ZJU-28⊃CsPbX3 crystals. The XRD patterns of ZJU-28 at different synthesis stages are shown in Fig. 1a, where the XRD patterns of ZJU-28 (magenta line) and ZJU-28⊃Pb2+ (purple line) match well with the simulated ones, indicating the phase purity of ZJU-28 crystals and the structure retention after the ion exchange process. After encapsulation of CsPbBr3 NCs, several new diffraction peaks at around 15.2°, 21.5°, 30.4°, 37.7°, and 43.7° appear, which could be assigned to the (100), (110), (200), (211) and (220) planes of the cubic CsPbBr3 (PDF#54-0752).13 Moreover, these characteristic peaks shift to higher angles with the increase in chlorine content and to lower angles with the increase in iodine content, verifying the diverse halide composition of the CsPbX3 NCs encapsulated in the ZJU-28⊃CsPbX3 crystals.37 The severely weakened and broadened diffraction peaks of ZJU-28 could be attributed to the encapsulation of CsPbX3 with a larger particle size than the channel size of ZJU-28 and the flexible structure of ZJU-28.38 PXRD patterns of the obtained colloidal CsPbX3 NCs present similar results (Fig. S3, ESI†). X-Ray photoelectron spectroscopy (XPS) tests were implemented to determine the chemical composition of ZJU-28⊃CsPbX3 crystals. XPS patterns of ZJU-28⊃CsPbClBr2, ZJU-28⊃CsPbBr3, and ZJU-28⊃CsPbBrI2 all possess characteristic peaks of In 3d, C 1s and O 1s, which are attributed to ZJU-28 (Fig. S4, ESI†). XPS patterns of ZJU-28⊃CsPbClBr2, ZJU-28⊃CsPbBr3, and ZJU-28⊃CsPbBrI2 all possess characteristic peaks of In 3d, C 1s and O 1s, which are attributed to ZJU-28 (Fig. S4, ESI†). Characteristic peaks of Cs 3d (723.7 eV and 737.7 eV), Pb 4f (137.8 eV and 142.6 eV), and Br 3d (67.7 eV and 74.8 eV) indicate the existence of all constituent elements of CsPbBr3 in ZJU-28⊃CsPbBr3 (Fig. S6, ESI†). Furthermore, the additional peaks of Cl 2p (197.3 eV and 198.9 eV) for ZJU-28⊃CsPbClBr2 and these of I 3d (618.3 eV and 629.7 eV) for ZJU-28⊃CsPbBrI2 reveal a mixed-halide composition (Fig. S5 and S7, ESI†). Scanning electron microscopy (SEM) tests show that pristine ZJU-28 and ZJU-28⊃Pb2+ crystals exhibit a topical morphology of a triangular prism with a smooth and clean surface, and the ZJU-28⊃CsPbX3 crystals maintain a triangular prism shape without any perovskite crystallites on the surface of the ZJU-28 crystals, possibly resulting from the formation of CsPbX3 NCs inside the ZJU-28 crystals (Fig. S8, ESI†). The elemental mapping of the ZJU-28⊃Pb2+ and ZJU-28⊃CsPbX3 crystals was further performed to investigate the element distribution. Fig. S9 (ESI†) shows that lead ions are uniformly distributed in ZJU-28 after the ion exchange process. After in situ formation of CsPbX3 NCs, taking ZJU-28⊃CsPbClBr2 as an example, characteristic elements such as caesium, lead, chlorine and bromine are evenly distributed in the whole selected area of the ZJU-28 single crystal (Fig. 1b). Similar results are also obtained for other samples (Fig. S10–S15, ESI†). Moreover, although the atomic ratios of caesium, lead and halogen are deviated from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 due to the anionic frameworks of ZJU-28, the atomic ratio of different halogens matches well with the halide stoichiometry of the CsPbX3. As shown in Fig. 1b, the atomic ratio of chlorine to bromine for ZJU-28⊃CsPbClBr2 is 0.82
3 due to the anionic frameworks of ZJU-28, the atomic ratio of different halogens matches well with the halide stoichiometry of the CsPbX3. As shown in Fig. 1b, the atomic ratio of chlorine to bromine for ZJU-28⊃CsPbClBr2 is 0.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.82 and very close to the corresponding stoichiometric ratio of 1
1.82 and very close to the corresponding stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, validating the successful regulation of the halide composition of the inner CsPbX3 NCs. Transmission electron microscopy (TEM) measurements were carried out, in which uniformly distributed, monodispersed and nearly spherical particles can be directly and clearly observed in the thin edge of ZJU-28⊃CsPbClBr2, ZJU-28⊃CsPbBr3, and ZJU-28⊃CsPbBrI2 crystals (Fig. 1c, e and g). High-resolution TEM tests were used to determine the observed particles. Fig. 1d, f and h clearly present the lattice fringes of these particles with interplanar distances of 2.76 Å, 2.89 Å and 3.01 Å, which can be identified as the (200) planes of cubic CsPbClBr2, CsPbBr3 and CsPbBrI2 respectively. The shape of the observed CsPbX3 NCs in ZJU-28⊃CsPbX3 is different to that of colloidal CsPbX3 NCs, which could be attributed to the confinement of ZJU-28 frameworks (Fig. S17, ESI†). Furthermore, the average particle sizes of the CsPbClBr2 NCs, CsPbBr3 NCs and CsPbBrI2 NCs encapsulated in ZJU-28 are 8.33 nm, 9.09 nm and 12.2 nm respectively, which are very close to those of the corresponding colloidal perovskite nanocrystals (9.20 nm for CsPbClBr2 NCs, 9.27 nm for CsPbBr3 NCs and 11.5 nm for CsPbBrI2 NCs) (Fig. S16 and S17, ESI†). All the above results strongly confirm that CsPbX3 NCs are successfully and uniformly in situ encapsulated in ZJU-28 crystals and their halide composition can be tuned easily.
2, validating the successful regulation of the halide composition of the inner CsPbX3 NCs. Transmission electron microscopy (TEM) measurements were carried out, in which uniformly distributed, monodispersed and nearly spherical particles can be directly and clearly observed in the thin edge of ZJU-28⊃CsPbClBr2, ZJU-28⊃CsPbBr3, and ZJU-28⊃CsPbBrI2 crystals (Fig. 1c, e and g). High-resolution TEM tests were used to determine the observed particles. Fig. 1d, f and h clearly present the lattice fringes of these particles with interplanar distances of 2.76 Å, 2.89 Å and 3.01 Å, which can be identified as the (200) planes of cubic CsPbClBr2, CsPbBr3 and CsPbBrI2 respectively. The shape of the observed CsPbX3 NCs in ZJU-28⊃CsPbX3 is different to that of colloidal CsPbX3 NCs, which could be attributed to the confinement of ZJU-28 frameworks (Fig. S17, ESI†). Furthermore, the average particle sizes of the CsPbClBr2 NCs, CsPbBr3 NCs and CsPbBrI2 NCs encapsulated in ZJU-28 are 8.33 nm, 9.09 nm and 12.2 nm respectively, which are very close to those of the corresponding colloidal perovskite nanocrystals (9.20 nm for CsPbClBr2 NCs, 9.27 nm for CsPbBr3 NCs and 11.5 nm for CsPbBrI2 NCs) (Fig. S16 and S17, ESI†). All the above results strongly confirm that CsPbX3 NCs are successfully and uniformly in situ encapsulated in ZJU-28 crystals and their halide composition can be tuned easily.
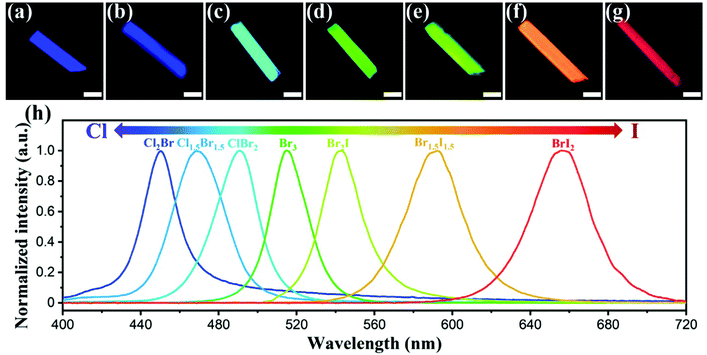
Successfully encapsulating CsPbX3 NCs into ZJU-28 endows ZJU-28⊃CsPbX3 crystals with unique optical properties. Fig. S18 (ESI†) shows that an isolated ZJU-28 microcrystal emits violet-blue light centered at 415 nm under 365 nm excitation and maintains a similar emission (centered at 418 nm) after ion exchange with Pb2+. After encapsulation of CsPbX3 NCs, ZJU-28⊃CsPbX3 crystals exhibit obviously changed color and luminescence. Fig. 2d and h show that the ZJU-28⊃CsPbBr3 microcrystal emits bright green light centered at 515 nm with the full width at half maximum (FWHM) of 22.3 nm under 365 nm excitation. In the case of gradually replacing Br with Cl, microcrystals of ZJU-28⊃CsPbClBr2, ZJU-28⊃CsPbCl1.5Br1.5 and ZJU-28⊃CsPbCl2Br exhibit gradually blue-shifted emission from cyan luminescence (centered at 491 nm) to blue luminescence (centered at 470 nm) and then to dark blue luminescence (centered at 450 nm), of which the corresponding FWHM is 25.7 nm, 31.3 nm and 21.3 nm, respectively (Fig. 2a–c and h). Conversely, in the case of gradually replacing Br with I, microcrystals of ZJU-28⊃CsPbBr2I, ZJU-28⊃CsPbBr1.5I1.5 and ZJU-28⊃CsPbBrI2 display gradually bathochromic-shifted emission from yellow-green luminescence (centered at 542 nm) to orange luminescence (centered at 593 nm) and then to red luminescence (centered at 661 nm), of which the corresponding FWHM is 25 nm, 34.5 nm and 35.4 nm, respectively (Fig. 2e–g and h). Therefore, ZJU-28⊃CsPbX3 crystals exhibit halide composition-dependent emission and their emission can be tuned to cover almost the full visible spectrum.13,37 Moreover, almost the entire ZJU-28⊃CsPbX3 microcrystals emit bright light, confirming the uniform formation of CsPbX3 NCs within ZJU-28 crystals. Fig. S19 (ESI†) shows the absorption spectra of ZJU-28⊃CsPbX3 crystals, in which the lowest absorption of ZJU-28⊃CsPbX3 crystals feature a similar evolution trend to their emission with the change in halide composition. It is worth mentioning that both the emission and absorption characteristics of ZJU-28⊃CsPbX3 crystals are close to these of the synthesized colloidal CsPbX3 NCs, indicating that the main luminescent centre of ZJU-28⊃CsPbX3 is the internal CsPbX3 NCs (Fig. S20 and Table S1, ESI†). The overlaps of the absorbance of CsPbX3 NCs and the emission of ZJU-28 give rise to the possibility of energy transfer between each other and cause the disappearance of the characteristic emission of the later.39 The shortened life time of the characteristic emission of the ZJU-28 (centered at around 415 nm) is further evidence for the possible energy transfer (Fig. S21, ESI†). The quantum yields of ZJU-28⊃CsPbX3 crystals range from 2.45% to 27.0%, which are lower than these of colloidal CsPbX3 NCs. The lower quantum yields could be attributed to different sample states during tests and no organic ligands capping on the surface of the CsPbX3 NCs encapsulated in ZJU-28. More details about the optical properties of ZJU-28⊃CsPbX3 crystals and colloidal CsPbX3 NCs are listed in Table S1 (ESI†). It is found that encapsulation of CsPbX3 NCs endows ZJU-28⊃CsPbX3 crystals with the optical properties of CsPbX3 NCs, which are similar to these of the colloidal counterparts, and tunable 1PE emission covering almost the whole visible region is achieved by simply adjusting the halide stoichiometry of CsX.
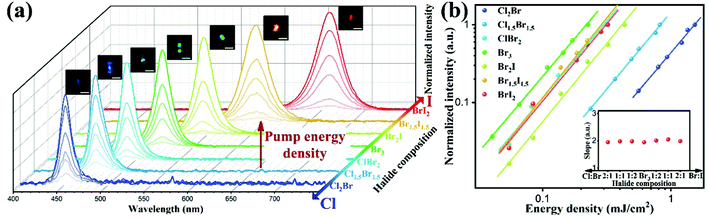
The two-photon excited (2PE) luminescence properties of the ZJU-28⊃CsPbX3 crystals were investigated under femtosecond (fs) pulsed laser excitation with a home-built system (Fig. S22, ESI†). ZJU-28⊃CsPbClxBr3−x (x = 0, 1, 1.5, 2) crystals were excited at 780 nm and ZJU-28⊃CsPbBr3−yIy (y = 1, 1.5, 2) crystals were excited at 980 nm under conditions of 25 °C and 55% humidity. Fig. 3a shows that the intensity of the 2PE luminescence for all ZJU-28⊃CsPbX3 crystals gradually increases without accompanying the obvious emission peak shift and split as the pump energy density increases. Fig. 3b shows that the plots of the logarithm of the emission intensity and the logarithm of the pump energy density display a linear relationship for the ZJU-28⊃CsPbX3 crystals. The slopes of the fitting lines for X3 = Cl2Br, Cl1.5Br1.5, ClBr2, Br3, Br2I, Br1.5I1.5 and BrI2 are 1.96, 1.98, 1.96, 1.95, 2.01, 2.01 and 1.99 respectively, which are all close to 2 and verify the 2PE luminescence process.38 The emission color of the ZJU-28⊃CsPbX3 crystals under infrared fs pulsed laser excitation is the same as these under excitation of 365 nm or 480 nm and the peak position of the 2PE luminescence is very close to that of the 1PE luminescence (Fig. 2a–h and 3a). Therefore, the 2PE luminescence of the ZJU-28⊃CsPbX3 crystals is also halide composition-dependent and can be tuned to cover almost the full visible spectrum. Moreover, the required minimum pump energy density to generate 2PE luminescence for ZJU-28⊃CsPbX3 is different, which could be attributed to their composition-dependent two-photon absorption (2PA) (Fig. S23–S29, ESI†).40,41 The two-photon action cross-section (ησ2, that is, the product of photoluminescence quantum yield (η) and 2PA cross-section (σ2)) and the 2PE luminescence reference method were chosen to evaluate the 2PE luminescence and quantitatively determine the ησ2, respectively (see the ESI†).38,42 According to the previous reports,43 the ησ2 of the ZJU-28⊃CsPbX3 crystals can be calculated from the ratio of 2PE luminescence intensity (eqn (1), ESI†):
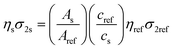 | (1) |
In order to meet the urgent requirements of the stability improvement of perovskite NCs in practical applications, the photostability of ZJU-28⊃CsPbX3 crystals and colloidal CsPbX3 NCs (for the comparation) was mainly studied under 25 °C and 55% humidity. For the 1PE photostability tests, ZJU-28⊃CsPbX3 crystals were continuously illuminated at 365 nm or 480 nm, of which the corresponding power densities are 598 mW cm−2 and 336 mW cm−2, respectively. Fig. S33 (ESI†) shows the plots of the relative PL intensity of the ZJU-28⊃CsPbX3 crystals and the corresponding colloidal CsPbX3 NCs as a function of irradiation time, in which about 79.9%, 90.7%, 75.1%, 80.7%, 78.2%, 66.5% and 28.5% of the initial luminescence intensity were preserved after 90 min of irradiation for ZJU-28⊃CsPbCl2Br, ZJU-28⊃CsPbCl1.5Br1.5, ZJU-28⊃CsPbClBr2, ZJU-28⊃CsPbBr3, ZJU-28⊃CsPbBr2I, ZJU-28⊃CsPbBr1.5I1.5 and ZJU-28⊃CsPbBrI2, respectively. However, the luminescence intensity of colloidal CsPbX3 NCs dropped much faster especially when the solvent volatilized completely and there was no obvious luminescence after no more than 30 min irradiation. These results indicate that encapsulating CsPbX3 NCs into ZJU-28 crystals provides significant enhancement of 1PE photostability. As for colloidal CsPbX3 NCs, the nanocrystals are densely in contact with each other after the solvent evaporated completely and light-induced regrowth and deterioration might occur due to the dynamic bounding of the surface ligands to CsPbX3 NCs.47 In contrast, the ZJU-28 frameworks effectively separate the CsPbX3 NCs and make them monodispersed in ZJU-28⊃CsPbX3 crystals (Fig. 1c, e and g). We speculate that this isolation effect is one reason for the enhancement of the photostability.22 In addition, time-resolved PL decays of ZJU-28⊃CsPbX3 crystals and colloidal CsPbX3 NCs were measured (Fig. S34, ESI†) and fitted by a biexponential decay function. The slower component τ2, which is attributed to surface trap assisted recombination, is longer in the ZJU-28⊃CsPbX3 crystals than in colloidal CsPbX3 NCs, indicating effective surface passivation derived from ZJU-28 frameworks.48 This effective surface passivation could be another reason for the improvement of photostability. Furthermore, after storing in the air without additional protection for 6 months, ZJU-28⊃CsPbBr3 crystals still exhibit bright green emission upon excitation, and maintain around 78% of the initial emission intensity after 90 min irradiation at 365 nm with the power density of 598 mW cm−2 (Fig. S35, ESI†), indicating the excellent long-term stability of the ZJU-28⊃CsPbX3 crystals.
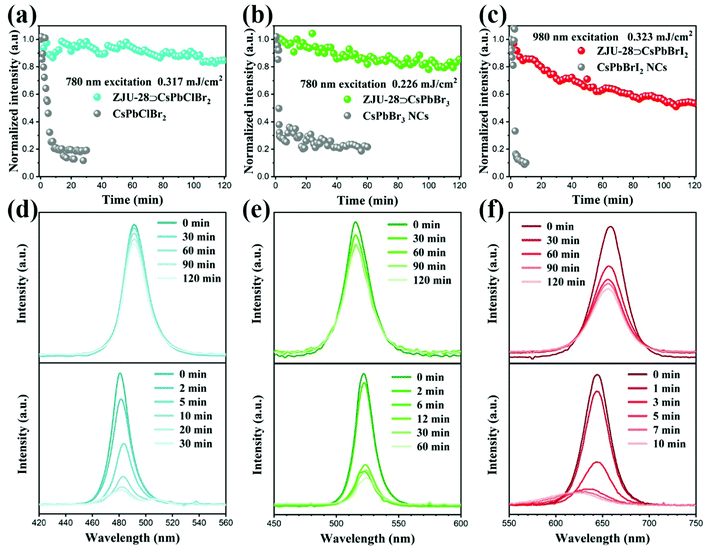
The 2PE photostability of ZJU-28⊃CsPbX3 crystals was investigated upon fs laser excitation at 780 nm (for X3 = Cl2Br, Cl1.5Br1.5, ClBr2 and Br3) and 980 nm (for X3 = Br2I, Br1.5I1.5 and BrI2) under 25 °C and 55% humidity. Fig. 4 shows the illumination-time-dependent 2PE luminescence intensity of ZJU-28⊃CsPbClBr2, ZJU-28⊃CsPbBr3 and ZJU-28⊃CsPbBrI2 crystals upon infrared fs laser excitation, which clearly displays a much slower drop than the corresponding colloidal CsPbX3 NCs. Therein, about 83.3% and 20% of the initial luminescence intensity was maintained for ZJU-28⊃CsPbBr3 and CsPbBr3 NCs respectively after 120 min irradiation of 780 nm (0.226 mJ cm−2) (Fig. 4b) and there was no emission peak shift and split under continuous irradiation for both (Fig. 4e). The ZJU-28⊃CsPbClBr2 crystals kept about 85.1% of the initial luminescence intensity after 120 min irradiation of 780 nm (0.317 mJ cm−2), which was much higher than that of CsPbClBr2 NCs (about 18.7%) (Fig. 4a). During continuous irradiation, ZJU-28⊃CsPbClBr2 crystals still presented no obvious peak shift and split but there was a new emission peak centered at around 506 nm for the CsPbClBr2 NCs (Fig. 4d). Similarly, new emission peaks centered at 415 nm and 484 nm for CsPbCl2Br NCs and CsPbCl1.5Br1.5 NCs appeared respectively but no emission peak split was observed for ZJU-28⊃CsPbCl2Br and ZJU-28⊃CsPbCl1.5Br1.5 crystals under continuous irradiation (Fig. S36 and S37, ESI†). ZJU-28⊃CsPbBrI2 crystals held 53.5% of the initial luminescence intensity after 120 min irradiation of the 980 nm fs laser with a single pulse energy density of 0.323 mJ cm−2 but CsPbBrI2 NCs only preserved around 10% after 10 min irradiation under the same conditions (Fig. 4c). In addition, the emission peak of the CsPbBrI2 NCs shifted significantly from 644 nm to 619 nm but no obvious emission peak shift was observed for ZJU-28⊃CsPbBrI2 crystals upon continuous excitation of the 980 nm fs laser (Fig. 4f). Fig. S38 and S39 (ESI†) also show that CsPbBr2I NCs and CsPbBr1.5I1.5 NCs have experienced an obvious emission peak shift while ZJU-28⊃CsPbBr2I and ZJU-28⊃CsPbBr1.5I1.5 crystals have no emission peak shift under continuous irradiation. Besides greatly slowed attenuation of the 2PE luminescence intensity, ZJU-28⊃CsPbX3 crystals also exhibit stable emission characteristics without any emission peak split and shift under continuous irradiation of an infrared fs pulse laser. We speculate that this emission peak split and shift of the mixed-halide perovskite NCs can be imputed to the light-induced ion segregation and phase separation.19,20 ZJU-28 frameworks can isolate the internal CsPbX3 NCs and passivate their surface defects, thus allowing them to retain their original size and a highly stable homogenous phase.49,50 Therefore, confinement of CsPbX3 NCs in the ZJU-28 crystals leads to enhancement of 2PE spectral stability and photostability.
Apart from the photostability, the thermal and water stability of the ZJU-28⊃CsPbX3 crystals were also tested. Fig. S40 (ESI†) shows the thermal stability test of ZJU-28⊃CsPbClBr2, ZJU-28⊃CsPbBr3 and ZJU-28⊃CsPbBrI2 crystals and their PL intensity monotonically decreases as the temperature rises. ZJU-28⊃CsPbBr3 crystals present the best thermal stability and still maintain half of the initial luminous intensity up to 80 °C. Despite the inferior thermal stability of the ZJU-28⊃CsPbClBr2 and ZJU-28⊃CsPbBrI2 crystals, their emission peak remains constant with temperature changes, which indicates the composition stability at different temperatures. It is known that lead halide perovskites are very sensitive to water and degrade fast in water. Surprisingly, after immersion in water for 2 hour, ZJU-28⊃CsPbBr3 crystals still emit bright green light and about half of the initial PL intensity was maintained, which confirms their excellent water stability (Fig. S41, ESI†).
Combined with a highly tunable, narrow emission and significantly enhanced photostability, ZJU-28⊃CsPbX3 crystals exhibit great potential in backlight displays. A facile LED device was constructed by combining a UV chip, ZJU-28⊃CsPbBr3 crystals and ZJU-28⊃CsPbBrI2 crystals. As shown in Fig. S42 (ESI†), the fabricated LED device presents a mixed output of red, green and blue light with corresponding color coordinates of (0.330, 0.347), which is very close to (0.33, 0.33). In addition, due to the pure color emission of ZJU-28⊃CsPbX3 crystals, the color gamut of the fabricated device covers almost 112% of the NTSC standard.51
Conclusions
In summary, the host–guest ZJU-28⊃CsPbX3 was successfully constructed by encapsulating CsPbX3 NCs in ZJU-28 crystals via the sequential deposition method, during which the composition of the in situ encapsulated CsPbX3 NCs can be precisely tuned by simply adjusting the halide stoichiometry of CsX. The encapsulation of CsPbX3 NCs and adjustment of their halide composition can be realized in ambient conditions owing to the unique features of MOFs. The 1PE and 2PE emission of the acquired ZJU-28⊃CsPbX3 crystals can be modulated from 450 nm to 661 nm, which covers almost the whole visible region. Importantly, the confinement and surface passivation of CsPbX3 NCs derived from ZJU-28 frameworks endow ZJU-28⊃CsPbX3 crystals with giant ησ2. The values of ησ2 for ZJU-28⊃CsPbClBr2 (at 780 nm), ZJU-28⊃CsPbBr3 (at 780 nm) and ZJU-28⊃CsPbBrI2 (at 980 nm) were calculated to be 8.66 × 104 GM, 1.58 × 106 GM and 1.96 × 105 GM respectively, which is comparable to those of colloidal CsPbX3 NCs dispersed in toluene. Moreover, the ZJU-28 frameworks still separate, passivate and protect the internal CsPbX3 NCs, resulting in a great enhancement of 1PE and 2PE photostability for the ZJU-28⊃CsPbX3 crystals. These unique, tunable and photostable ZJU-28⊃CsPbX3 crystals show great potential in wide color-gamut backlight display, bioimaging, sensing and even future integrated photonics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (No. 52025131 and 61721005).Notes and references
- J. Shamsi, A. S. Urban, M. Imran, L. D. Trizio and L. Manna, Chem. Rev., 2019, 119, 3296–3348 CrossRef CAS PubMed.
- F. Yan, S. T. Tan, X. Li and H. V. Demir, Small, 2019, 15, 1902079 CrossRef CAS PubMed.
- H. Wang and D. H. Kim, Chem. Soc. Rev., 2017, 46, 5204–5236 RSC.
- X. Wang, Z. Bao, Y.-C. Chang and R. S. Liu, ACS Energy Lett., 2020, 5, 3374–3396 CrossRef CAS.
- J. Song, J. Li, X. Li, L. Xu, Y. Dong and H. Zeng, Adv. Mater., 2015, 27, 7162–7167 CrossRef CAS PubMed.
- Y. Wang, X. Li, J. Song, L. Xiao, H. Zeng and H. Sun, Adv. Mater., 2015, 27, 7101–7108 CrossRef CAS PubMed.
- Y. Wang, X. Li, X. Zhao, L. Xiao, H. Zeng and H. Sun, Nano Lett., 2016, 16, 448–453 CrossRef CAS PubMed.
- Y. Xu, Q. Chen, C. Zhang, R. Wang, H. Wu, X. Zhang, G. Xing, W. W. Yu, X. Wang, Y. Zhang and M. Xiao, J. Am. Chem. Soc., 2016, 138, 3761–3768 CrossRef CAS PubMed.
- J. Yu, Y. Cui, C. D. Wu, Y. Yang, B. Chen and G. Qian, J. Am. Chem. Soc., 2015, 137, 4026–4029 CrossRef CAS PubMed.
- J. Chen, K. Žídek, P. Chábera, D. Liu, P. Cheng, L. Nuuttila, M. J. Al-Marri, H. Lehtivuori, M. E. Messing, K. Han, K. Zheng and T. Pullerits, J. Phys. Chem. Lett., 2017, 8, 2316–2321 CrossRef CAS PubMed.
- T. He, J. Li, X. Qiu, S. Xiao, C. Yin and X. Lin, Adv. Opt. Mater., 2018, 6, 1800843 CrossRef.
- A. Pramanik, K. Gates, Y. Gao, S. Begum and P. Chandra Ray, J. Phys. Chem. C, 2019, 123, 5150–5156 CrossRef CAS.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- F. Zhao, J. Li, X. Gao, X. Qiu, X. Lin, T. He and R. Chen, J. Phys. Chem. C, 2019, 123, 9538–9543 CrossRef CAS.
- Y. Wei, Z. Cheng and J. Lin, Chem. Soc. Rev., 2019, 48, 310–350 RSC.
- Y. Lin, X. Zheng, Z. Shangguan, G. Chen, W. Huang, W. Guo, X. Fan, X. Yang, Z. Zhao, T. Wu and Z. Chen, J. Mater. Chem. C, 2021, 9, 12303–12313 RSC.
- Q. Zhang, Y. Zhou, Y. Wei, M. Tai, H. Nan, Y. Gu, J. Han, X. Yin, J. Li and H. Lin, J. Mater. Chem. C, 2020, 8, 2569–2578 RSC.
- Q. Zhang, H. Nan, Y. Zhou, Y. Gu, M. Tai, Y. Wei, F. Hao, J. Li, D. Oron and H. Lin, J. Mater. Chem. C, 2019, 7, 6795–6804 RSC.
- H. Zhang, X. Fu, Y. Tang, H. Wang, C. Zhang, W. W. Yu, X. Wang, Y. Zhang and M. Xiao, Nat. Commun., 2019, 10, 1088 CrossRef CAS PubMed.
- P. Vashishtha and J. E. Halpert, Chem. Mater., 2017, 29, 5965–5973 CrossRef CAS.
- Y. Hassan, J. H. Park, M. L. Crawford, A. Sadhanala, J. Lee, J. C. Sadighian, E. Mosconi, R. Shivanna, E. Radicchi, M. Jeong, C. Yang, H. Choi, S. H. Park, M. H. Song, F. De Angelis, C. Y. Wong, R. H. Friend, B. R. Lee and H. J. Snaith, Nature, 2021, 591, 72–77 CrossRef CAS PubMed.
- X. Li, Y. Wang, H. Sun and H. Zeng, Adv. Mater., 2017, 29, 1701185 CrossRef PubMed.
- Y. J. Yoon, Y. Chang, S. Zhang, M. Zhang, S. Pan, Y. He, C. H. Lin, S. Yu, Y. Chen, Z. Wang, Y. Ding, J. Jung, N. Thadhani, V. V. Tsukruk, Z. Kang and Z. Lin, Adv. Mater., 2019, 31, 1901602 CrossRef PubMed.
- D. N. Dirin, B. M. Benin, S. Yakunin, F. Krumeich, G. Raino, R. Frison and M. V. Kovalenko, ACS Nano, 2019, 13, 11642–11652 CrossRef CAS PubMed.
- X. Liang, M. Chen, Q. Wang, S. Guo and H. Yang, Angew. Chem., Int. Ed., 2019, 58, 2799–2803 CrossRef CAS PubMed.
- S. Yuan, D. Chen, X. Li, J. Zhong and X. Xu, ACS Appl. Mater. Interfaces, 2018, 10, 18918–18926 CrossRef CAS PubMed.
- R. Li, Z. Wei, H. Zhao, H. Yu, X. Fang, D. Fang, J. Li, T. He, R. Chen and X. Wang, Nanoscale, 2018, 10, 22766–22774 RSC.
- H. Zhang, M. Jin, X. Liu, Y. Zhang, Y. Yu, X. Liang, W. Xiang and T. Wang, Nanoscale, 2019, 11, 18009–18014 RSC.
- Y. Tong, Q. Wang, E. Mei, X. Liang, W. Gao and W. Xiang, Adv. Opt. Mater., 2021, 9, 2100012 CrossRef CAS.
- Y. Cui, J. Zhang, H. He and G. Qian, Chem. Soc. Rev., 2018, 47, 5740–5785 RSC.
- H. He, E. Ma, Y. Cui, J. Yu, Y. Yang, T. Song, C. D. Wu, X. Chen, B. Chen and G. Qian, Nat. Commun., 2016, 7, 11087 CrossRef CAS PubMed.
- Z. G. Gu, D. J. Li, C. Zheng, Y. Kang, C. Woll and J. Zhang, Angew. Chem., Int. Ed., 2017, 56, 6853–6858 CrossRef CAS PubMed.
- Z. Yuan, L. Zhang, S. Li, W. Zhang, M. Lu, Y. Pan, X. Xie, L. Huang and W. Huang, J. Am. Chem. Soc., 2018, 140, 15507–15515 CrossRef CAS PubMed.
- S. K. Yadav, G. K. Grandhi, D. P. Dubal, J. C. de Mello, M. Otyepka, R. Zboril, R. A. Fischer and K. Jayaramulu, Small, 2020, 16, 2004891 CrossRef CAS PubMed.
- C. Zhang, W. Li and L. Li, Angew. Chem., Int. Ed., 2021, 60, 7488–7501 CrossRef CAS PubMed.
- J. Yu, Y. Cui, C. Wu, Y. Yang, Z. Wang, M. O'Keeffe, B. Chen and G. Qian, Angew. Chem., Int. Ed., 2012, 51, 10542–10545 CrossRef CAS PubMed.
- Q. A. Akkerman, V. D'Innocenzo, S. Accornero, A. Scarpellini, A. Petrozza, M. Prato and L. Manna, J. Am. Chem. Soc., 2015, 137, 10276–10281 CrossRef CAS PubMed.
- H. He, Y. Cui, B. Li, B. Wang, C. Jin, J. Yu, L. Yao, Y. Yang, B. Chen and G. Qian, Adv. Mater., 2019, 31, 1806897 CrossRef PubMed.
- S. Bhattacharyya, D. Rambabu and T. K. Maji, J. Mater. Chem. A, 2019, 7, 21106–21111 RSC.
- W. Chen, F. Zhang, C. Wang, M. Jia, X. Zhao, Z. Liu, Y. Ge, Y. Zhang and H. Zhang, Adv. Mater., 2021, 33, 2004446 CrossRef CAS PubMed.
- J. Xu, X. Li, J. Xiong, C. Yuan, S. Semin, T. Rasing and X. H. Bu, Adv. Mater., 2020, 32, 1806736 CrossRef CAS PubMed.
- W. Chen, S. Bhaumik, S. A. Veldhuis, G. Xing, Q. Xu, M. Grätzel, S. Mhaisalkar, N. Mathews and T. C. Sum, Nat. Commun., 2017, 8, 15198 CrossRef CAS PubMed.
- R. Medishetty, J. K. Zareba, D. Mayer, M. Samoc and R. A. Fischer, Chem. Soc. Rev., 2017, 46, 4976–5004 RSC.
- N. S. Makarov, M. Drobizhev and A. Rebane, Opt. Express, 2008, 16, 4029 CrossRef CAS PubMed.
- H. He and T. C. Sum, Dalton Trans., 2020, 49, 15149–15160 RSC.
- W. Liu, J. Xing, J. Zhao, X. Wen, K. Wang, P. Lu and Q. Xiong, Adv. Opt. Mater., 2017, 5, 1601045 CrossRef.
- J. Chen, D. Liu, M. J. Al-Marri, L. Nuuttila, H. Lehtivuori and K. Zheng, Sci. China Mater., 2016, 59, 719–727 CrossRef CAS.
- S. Li, D. Lei, W. Ren, X. Guo, S. Wu, Y. Zhu, A. L. Rogach, M. Chhowalla and A. K. Y. Jen, Nat. Commun., 2020, 11, 11 CrossRef PubMed.
- X. Wang, Y. Ling, X. Lian, Y. Xin, K. B. Dhungana, F. Perez-Orive, J. Knox, Z. Chen, Y. Zhou, D. Beery, K. Hanson, J. Shi, S. Lin and H. Gao, Nat. Commun., 2020, 11(10), 695 Search PubMed.
- A. J. Knight and L. M. Herz, Energy Environ. Sci., 2020, 13, 2024–2046 RSC.
- H. C. Wang, S. Y. Lin, A. C. Tang, B. P. Singh, H. C. Tong, C. Y. Chen, Y. C. Lee, T. L. Tsai and R. S. Liu, Angew. Chem., Int. Ed., 2016, 55, 7924–7929 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The synthesis and supplementary figures and table of the characterization of the as-prepared materials. See DOI: 10.1039/d2tc00075j |
| This journal is © The Royal Society of Chemistry 2022 |