N-Heterocyclic carbene-based tetradentate platinum(II) complexes for phosphorescent OLEDs with high brightness†
Guijie
Li
 *,
Shun
Liu
,
Yulu
Sun
,
Weiwei
Lou
,
Yun-Fang
Yang
*,
Shun
Liu
,
Yulu
Sun
,
Weiwei
Lou
,
Yun-Fang
Yang
 and
Yuanbin
She
*
and
Yuanbin
She
*
College of Chemical Engineering, Zhejiang University of Technology, Hangzhou, Zhejiang 310014, P. R. China. E-mail: guijieli@zjut.edu.cn; sheyb@zjut.edu.cn
First published on 20th November 2021
Abstract
Organic light-emitting diode (OLED) is an attractive technology for display applications in high-end electronics. Tetradentate Pt(II) complexes are phosphorescence emitters of great importance for OLED fabrication. A novel series of N-heterocyclic carbene (NHC), benzocarbene (Ph/NHC) and pyridinocarbene (Py/NHC)-based tetradentate Pt(II) complexes with fused 5/6/6 metallocycles was designed and developed. Their electrochemical and photophysical properties were systematically studied through experimental and theoretical investigations, which were greatly affected by the NHC, Ph/NHC and Py/NHC. Pt(NHC-1) shows a dominant emission peak at 509 nm with a quantum efficiency of 40% and an excited lifetime of 1.8 μs in a 5 wt% doped PMMA film at room temperature. A Pt(NHC-1)-doped green OLED using 26mCPy as the host material demonstrated a very high Lmax of 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 cd m−2, which was a record-high value for the reported Pt(II) complex-based phosphorescent OLED. The OLED also achieved a peak EQE of 13.1% with small efficiency roll-off, which still maintained EQEs of 12.8%, 11.6% and 8.6% at a high brightness of 100, 1000 and 10
416 cd m−2, which was a record-high value for the reported Pt(II) complex-based phosphorescent OLED. The OLED also achieved a peak EQE of 13.1% with small efficiency roll-off, which still maintained EQEs of 12.8%, 11.6% and 8.6% at a high brightness of 100, 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2.
000 cd m−2.
Introduction
N-Heterocyclic carbenes (NHCs) are electrically neutral species possessing two nonbonding electrons, which may be spin-paired for the singlet state or spin-non-paired for the triplet state.1 In 1968, Wanzlick2 and Öfele3 almost simultaneously published their research on the isolation of the first NHC-based metal complex. By 1991, the first thermally stable and free NHC was successfully separated by Arduengo.4 After that, NHCs have been comprehensively investigated in mainstream and modern chemistry. They are widely used as organic catalysts5 and ligands in organometallic chemistry,6 main group chemistry7 and materials science.8,9 In particular, luminescent NHC-based metal complexes have attracted great attention in the past decades, because of their potential for application as emitters in organic light-emitting diodes (OLEDs) for lighting and display, such as linear-type Pd(0), Au(I), Ag(I) and Cu(I) complexes,10–18 square planar-type Pt(II)19–36 and Pd(II)37 complexes, and octahedron-type Ir(III)38–50 and Fe(III) complexes.51,52Many NHC complex-based OLEDs demonstrated good device performances, such as high quantum efficiencies,13–18,21,29,33 high colour purities21,29,33,35 and long device operational lifetimes.21,22,35,53–61 NHC-based tris-bidentate Ir(III) complexes were studied first, in particular, for many blue and deep-blue OLEDs.38–40,48 Recently, through judicious molecular design, Chi, Chou and co-workers successfully developed a novel series of bis-tridentate NHC-based Ir(III) complexes for efficient blue and deep-blue OLEDs.42,44,46,47,49,50 Phosphorescent Pt(II) complexes were recognized to be potentially commercial emitters for efficient and stable OLED applications until tetradentate ligands were employed for the molecular design.21,22,53–61 In 2017, Credgington and co-workers demonstrated that cyclic (alkyl)(amino)-carbene (CAAC)-based linear Au(I) complexes could act as efficient emitters for solution-processed OLEDs with near-100% internal quantum efficiency (IQE).13 Two years later, Thompson and co-workers successfully eliminated the nonradiative decay in NHC-based linear Cu(I) and Ag(I) emitters and realized >99% quantum efficiencies and microsecond lifetimes.14,17 Recently, we designed and developed the first series of NHC-based Pd(II) complexes for narrow-band deep-blue emitters with a dominant peak at 437–439 nm and a full-width at half-maximum (FWHM) of 34–48 nm.37
Luminescence intensity is an important parameter of OLEDs, especially for lighting applications. In 2015, Forrest and co-workers found that NHC-based fac-Ir(pmp)3 could simultaneously act as a phosphorescent deep-blue emitter, hole transporter and electron-blocking material, which enabled the OLED to achieve a very high brightness of >7800 cd m−2.40 Typically, Pt(II) complex-based deep-blue OLEDs exhibited maximum brightness (Lmax) less than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2, by comparison, green OLED doped Pt(II) emitters could achieve much higher brightness. Che and co-workers in 2019 showed that tetra-Pt-S1-doped green OLEDs realized a Lmax of 49
000 cd m−2, by comparison, green OLED doped Pt(II) emitters could achieve much higher brightness. Che and co-workers in 2019 showed that tetra-Pt-S1-doped green OLEDs realized a Lmax of 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cd m−2 and a peak EQE of 16.6%.62 In 2020, we fabricated a green OLED using tetradentate Pt(ppy-1) as an emitter which achieved a Lmax of 40
600 cd m−2 and a peak EQE of 16.6%.62 In 2020, we fabricated a green OLED using tetradentate Pt(ppy-1) as an emitter which achieved a Lmax of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 979 cd m−2 and a peak EQE of 18.5%.63 Recently, we developed a new tetradentate Pt(II) complex Pt(pbiz) and enabled green OLEDs to demonstrate a Lmax of 55
979 cd m−2 and a peak EQE of 18.5%.63 Recently, we developed a new tetradentate Pt(II) complex Pt(pbiz) and enabled green OLEDs to demonstrate a Lmax of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 481 cd m−2 and a peak EQE of 21.6% using 2,6-di(9H-carbazol-9-yl)pyridine (26mCPy) as the host material.64 However, issues still remain, such as heavy efficiency roll-off at high brightness. Thus, the design of stable phosphorescence emitters, which can efficiently luminesce at a large current density, is important for the potential commercial applications of display and lighting.
481 cd m−2 and a peak EQE of 21.6% using 2,6-di(9H-carbazol-9-yl)pyridine (26mCPy) as the host material.64 However, issues still remain, such as heavy efficiency roll-off at high brightness. Thus, the design of stable phosphorescence emitters, which can efficiently luminesce at a large current density, is important for the potential commercial applications of display and lighting.
In the transition metal complexes discussed above, the NHC carbon typically is in the singlet state and adopts bent sp2-hybridization, the electron pair occupied sp2 orbital possesses a strong σ-donating ability and bonds with a vacant d orbital of the metal, such as 5dxz for Pt(II). Meanwhile, the vacant pz orbital of the carbon has weak π-accepting ability and can bond with a completely filled d orbital of the metal, such as 5dx2–y2 for Pt(II), both of which enable the NHCs to be ideal ligands to form stable organometallic complexes for phosphorescence emitters (Fig. 1). In this work, we report a novel series of NHC, benzocarbene (Ph/NHC) and pyridinocarbene (Py/NHC)-based tetradentate Pt(II) complexes with fused 5/6/6 metallocycles (Fig. 1). The tert-butyl group was incorporated into the phenyl group to improve the solubilities and also prevent the intermolecular interaction, which might induce triplet–triplet annihilation (TTA). The electrochemical and photophysical properties of the Pt(II) complexes are systematically studied. The Pt(II) complexes show dominant emission peak of 495–505 nm with quantum efficiency of 5–55% in 5 wt% doped poly(methyl methacrylate) (PMMA) film at room temperature. The Pt(NHC-1)-doped green OLED using 26mCPy as the host material demonstrated a very high Lmax of 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 cd m−2 and a peak EQE of 13.1% with small efficiency roll-off.
416 cd m−2 and a peak EQE of 13.1% with small efficiency roll-off.
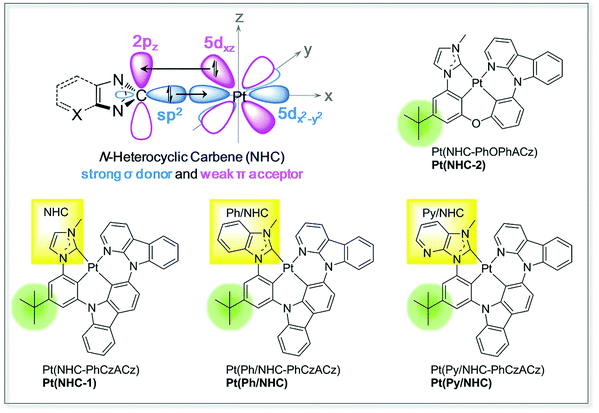 | ||
| Fig. 1 Chemical structures of NHC, Ph/NHC and Py/NHC-based tetradentate Pt(II) complexes studied in this work. ACz, aza carbazolyl group. | ||
Results and discussion
The synthetic route of the NHC, Ph/NHC and Py/NHC-based tetradentate Pt(II) complexes is shown in Fig. 2. Pd(0)-catalyzed C–N bond cross-coupling of 1-(3-bromo-5-(tert-butyl)phenyl)-1H-imidazole or derivatives (1) with 9-(9H-carbazol-2-yl)-9H-pyrido[2,3-b]indole (2) using SPhos as the ligand and t-BuONa as the base in toluene afforded intermediate 3 in 57–76% yields, which was methylated with CH3I and then underwent anion exchange with NH4PF6 to give the desired ligand(NHC-1), ligand(Ph/NHC) and ligand(Py/NHC) in 61–86% yields for the two steps. Ligand(NHC-2) could be similarly synthesized through Cu(I)-catalyzed C–O bond cross-coupling of 1(NHC-1) with 3-(9H-pyrido[2,3-b]indol-9-yl)phenol (4) using 2-picolinic acid as ligand and K3PO4 as the base to afford intermediate 5 in 72% yields, and then methylation and anion exchange in 65% yield. Pt(NHC-1), Pt(Ph/NHC), Pt(Py/NHC) and Pt(NHC-2) were synthesized by metalation of the corresponding ligands with Pt(COD)Cl2 in the presence of NaOAc in 1-methoxy-2-(2-methoxyethoxy)ethane (DME) at 120 °C to form the desired sole Pt(II) complex in 16–73% yields. The metalation yield for the synthesis of Pt(NHC-2) (73%) was much higher than those of the others, because the flexible molecular geometry of the ligand(NHC-2) using oxygen atom as the linking group facilitates the coordination with the Pt(II) ions. The synthetic details are provided in the ESI.† The key intermediates 3 and 5, and all the ligands and the newly developed tetradentate Pt(II) complexes were confirmed by 1H and 13C NMR spectroscopy, and high resolution mass spectroscopy (HRMS).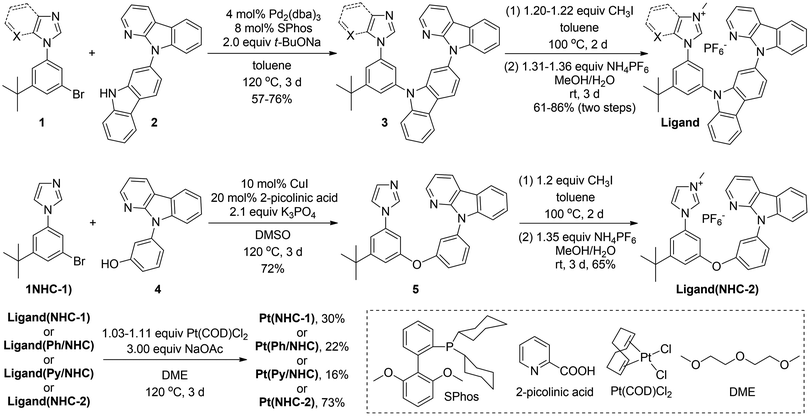 | ||
| Fig. 2 The synthesis of NHC, Ph/NHC and Py/NHC-based tetradentate Pt(II) complexes. X = CH for Ph/NHC or X = N for Py/NHC. | ||
All the Pt(II) complexes exhibit irreversible redox processes in N,N-dimethyl formamide (DMF) under a nitrogen atmosphere (Fig. S1, ESI†), which can be attributed to the square planar molecular geometry and the vacant d orbital of the Pt(II) complexes to be attacked by the nucleophilic solvent molecules.33,64,65 The values of their redox potentials were measured by different pulsed voltammetry (DPV) relative to an internal reference ferrocenium/ferrocene (Cp2Fe/Cp2Fe+) (Fig. S2, ESI†) and listed in Table 1. The Pt(II) complexes show oxidation potentials in 0.29–0.48 V. Pt(NHC-1), Pt(Ph/NHC) and Pt(Py/NHC) have similar oxidation potentials with 0.29, 0.36 and 0.38 V, respectively, suggesting the oxidation processes dominantly took place at the PhCz and the metal moieties. However, Pt(NHC-2) has a much larger oxidation potential of 0.48 V compared to the other Pt(II) complexes, suggesting the oxidation process mainly occurred at the PhOPh and the metal moieties. The Pt(II) complexes exhibit reduction potentials in a small range between −2.33 and −2.39 V, demonstrating the reduction processes dominantly occurred at the ACz moieties. The differences of the redox potentials of the Pt(II) complexes indicate the NHC, Ph/NHC and Py/NHC moieties also have significant influence on their electrochemical properties.
| Metal complex | Dihedral anglea | Calc. HOMOb/LUMOb/ΔEgc [eV] | E ox [V] | E red [V] | HOMOe/LUMOf/ΔEgc [eV] | E T1 [eV] |
|---|---|---|---|---|---|---|
| a Dihedral angle between terminal NHC and ACz planes on the basis of optimized S0. b From DFT calculations at the B3LYP/6-31G(d)/LANL2DZ level based on optimized S0. c ΔEg = LUMO–HOMO. d From different pulsed voltammetry (DPV) measurements (Fig. S2, ESI). e HOMO = −(Eox + 4.8) eV. f LUMO = −(Ered + 4.8) eV. g Triplet energy ET1 estimated from the peak of the phosphorescence emission spectrum at 77 K in 2-MeTHF. | ||||||
| Pt(NHC-1) | 46.72 | −4.59/−1.47/3.12 | 0.29 | −2.39 | −5.09/−2.41/2.68 | 2.58 |
| Pt(Ph/NHC) | 48.47 | −4.64/−1.53/3.11 | 0.36 | −2.33 | −5.16/−2.47/2.69 | 2.58 |
| Pt(Py/NHC) | 48.15 | −4.63/−1.59/3.04 | 0.38 | −2.33 | −5.18/−2.47/2.71 | 2.56 |
| Pt(NHC-2) | 49.19 | −4.69/−1.57/3.12 | 0.46 | −2.37 | −5.28/−2.43/2.85 | 2.63 |
Density functional theory (DFT) calculations63,66–68 reveal that the tetradentate Pt(II) complexes show distorted planar molecular geometries with dihedral angles between the terminal NHC and ACz planes of 46.72°, 48.47°, 48.15° and 49.19° in their ground states respectively (Table S1 and Fig. S1). The bond length of Pt–C1NHC (2.082 Å) in Pt(NHC-1) is significantly shorter than that of the Pt–N1ACz (2.172 Å) in Pt(NHC-1), and the Pt–NPy (2.200 Å) in Pt(ACzCCz-3)67 which employed a similar tetradentate ligand (Table S1, ESI†). Similar results are also found in Pt(Ph/NHC), Pt(Py/NHC) and Pt(NHC-2), indicating the high bond strength of the Pt–C1NHC in the newly developed Pt(II) complexes. All the tetradentate Pt(II) complexes exhibit similar the highest occupied molecular orbital (HOMO) and HOMO−1 distributions, however, their lowest unoccupied molecular orbital (LUMO) and LUMO+1 distributions are significantly different (Fig. 3 and Fig. S3, ESI†). Pt(NHC-1), Pt(Ph/NHC) and Pt(Py/NHC) have the HOMO distributions on πPhCz orbitals and dPt centers with energy levels of −4.59, −4.64 and −4.63 eV, respectively, and Pt(NHC-1) has the HOMO distribution on the πPhOPh orbital and the dPt center with energy levels of −4.69 eV (Fig. 3). All the Pt(II) complexes show LUMO distributions on πACz orbitals with energy levels of −1.47to −1.59 eV besides Pt(Py/NHC), also having some distribution on πPy/NHC (Fig. 3). The LUMO+1 distributions are mainly on the πNHC–Ph orbitals for Pt(NHC-1) and Pt(NHC-2), and on the πPh/NHC and πPy/NHC orbitals for Pt(Ph/NHC) and Pt(Py/NHC) respectively (Fig. 3 and Fig. S3, ESI†). These results indicate that the electron-withdrawing abilities are in the order ACz > Py/NHC > Ph/NHC > NHC. The energy levels for the Pt(II) complexes from the calculation results are well in agreement with the trend of their HOMO/LUMO levels from their redox potentials (Table 1).
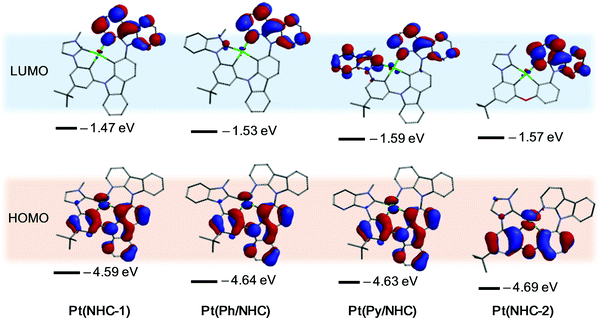 | ||
| Fig. 3 Density functional theory calculations of frontier orbitals for Pt(II) complexes based on optimized S0 geometries. The H atoms were omitted for clarity. | ||
The UV–vis absorption spectra of the tetradentate Pt(II) complexes at room temperature (RT) in dichloromethane (DCM) solution are illustrated in Fig. 4. Pt(NHC-1), Pt(Ph/NHC) and Pt(Py/NHC) show significantly stronger absorption in the region of less than 375 nm compared to Pt(NHC-2) due to the small conjugation in Pt(NHC-2). Between 375 and 475 nm, all the four Pt(II) complexes have similar absorption strength, but Pt(NHC-2) has a significantly large blue-shift compared to those of the other Pt(II) complexes. The above results reveal that, for all the Pt(II) complexes, the very strong absorption bands less than 375 nm are attributed to spin-allowed 1(π → π*) transitions in the cyclometalating ligands. The absorption bands at 375–475 nm are identified as spin-allowed metal-to-ligand charge-transfer (1MLCT) transitions. The absorption for the 3MLCT transitions could be also observed above 480 nm, which were structureless and very different from the previous reported Pt(II) complexes.29,30,33,63,66–68
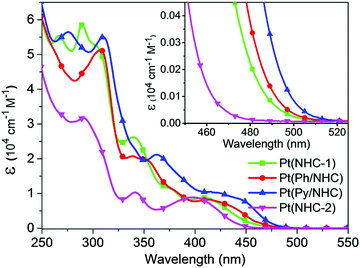 | ||
| Fig. 4 Absorption spectra of the NHC, Ph/NHC and Py/NHC-based Pt(II) complexes in dichloromethane at room temperature. | ||
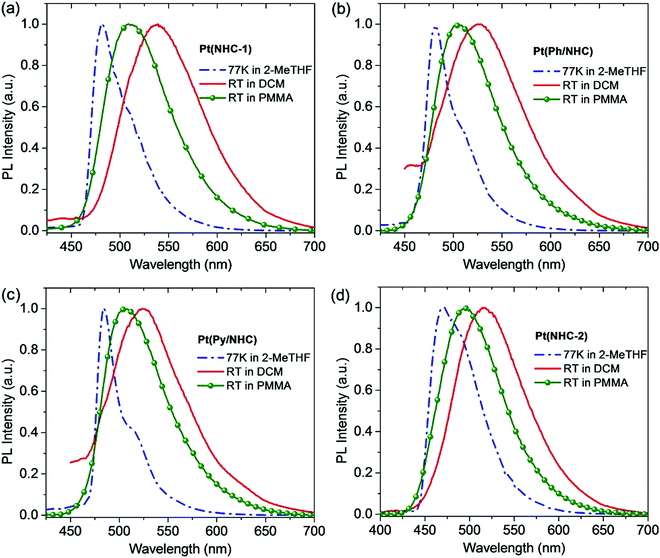
The comparison of the emission spectra and transient decay spectra of the Pt(II) complexes under various conditions is shown in Fig. 5 and Fig. S4 (ESI†), and the data of their photophysical properties are listed in Table 2. At 77 K in 2-methyltetrahydrofuran (2-MeTHF), the Pt(II) complexes show vibronic emission spectral peaks at 472–484 nm indicating strong admixed ligand-centered (3LC) and mixed 3MLCT characters in their T1 states.33 Pt(NHC-2) has a much longer excited-state lifetime (τ) of 11.1 μs compared to those of the other Pt(II) complexes of 4.0–5.6μs, indicating the significant influence of the tetradentate ligands. At room temperature in dichloromethane, the Pt(II) complexes exhibit a significant red-shift of 58, 44, 40 and 55 nm for Pt(NHC-1), Pt(Ph/NHC), Pt(Py/NHC) and Pt(NHC-2), respectively. Importantly, they all show broad and structureless emission spectra indicating significantly enhanced 3MLCT (πPhdPt* → πACz) characters in their T1 states. By comparison, the NHC-based Pd(II) complexes typically show dominantly 3LC characters in their T1 states because of their relatively weak SOC effect.37 The quantum efficiencies (ΦPL) of the Pt(II) complexes are relatively low (2–18%). However, in the doped PMMA films, the ΦPL could be significantly enhanced even measured in air, and Pt(NHC-1) and Pt(NHC-2) demonstrated the ΦPL of up to 40% and 55% respectively. This is attributed to the suppression of molecular vibrations and the oxygen exclusion by the film. The Pt(II) complex-doped films also show broad and structureless emission spectral peaks at 495–509 nm with a τ of 1.0–3.9 μs. The improved ΦPL and short τ enable Pt(NHC-1) and Pt(NHC-2) to achieve radiative rate (kr) of 2.22 × 105 s−1 and 1.41 × 105 s−1, indicating that they can act as ideal phosphorescence emitters for device fabrication.
| Complex | Emission at 77 K in 2-MeTHF | Emission at RT in DCM | Emission at RT in 5 wt% PMMA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λ max [nm] | τ [μs] | λ max [nm] | τ [μs] | Φ PL [%] | k r [104 s−1] | k nr [104 s−1] | λ max [nm] | τ [μs] | Φ PL [%] | k r [104 s−1] | k nr [104 s−1] | |
| a Measured in air using an integrating sphere. DCM, dichloromethane; 2-MeTHF, 2-methyltetrahydrofuran; PMMA, poly (methyl methacrylate). | ||||||||||||
| Pt(NHC-1) | 481 | 5.6 | 539 | 0.7 | 18 | 25.7 | 117.1 | 509 | 1.8 | 40 | 22.2 | 33.3 |
| Pt(Ph/NHC) | 481 | 4.5 | 525 | 1.4 | 2 | 1.4 | 70.0 | 505 | 1.3 | 19 | 14.6 | 62.3 |
| Pt(Py/NHC) | 484 | 4.0 | 524 | 1.8 | 2 | 1.0 | 54.6 | 506 | 1.0 | 5 | 5.0 | 95.0 |
| Pt(NHC-2) | 472 | 11.1 | 517 | 0.7 | 18 | 8.6 | 39.0 | 495 | 3.9 | 55 | 14.1 | 11.5 |
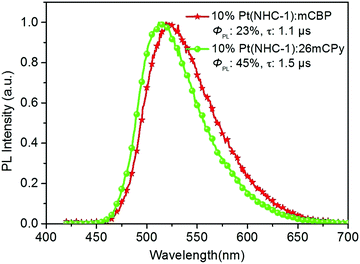
The photoluminescence properties of the Pt(NHC-1) in 10 wt% doped host materials mCBP [3,3-di(9H-carbazol-9-yl)biphenyl] and 26mCPy [2,6-di(9H-carbazol-9-yl)pyridine] films were studied (Fig. 6). In mCBP film, Pt(NHC-1) shows a broad emission spectra at 519 nm with a relatively low ΦPL of 23% and a τ of 1.1 μs. However, the ΦPL can be significantly enhanced to 45% in 26mCPy film, and the emission spectrum shows a blue-shift with a dominant peak at 512 nm. The enhanced ΦPL in 26mCPy enables Pt(NHC-1) to act as an emitter for OLED fabrication.
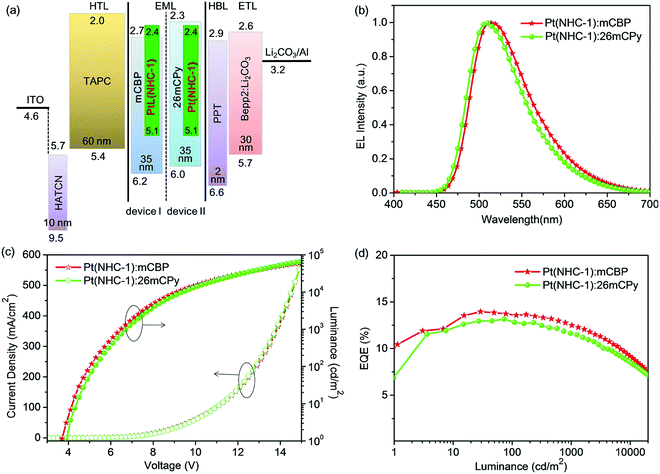
To evaluate the electroluminescence properties of Pt(NHC-1), OLEDs were fabricated using mCBP (device I) and 26mCPy (device II) as host materials respectively. The device architecture was ITO/HATCN (10 nm)/TAPC (60 nm)/Pt(NHC-1):host (10%, 35 nm)/PPT (2 nm)/Bepp2:Li2CO3 (30 nm)/Li2CO3 (1 nm)/Al (100 nm), where HATCN was 2,3,6,7,10,11-hexacyano-1,4,5,8,9,12-hexaazatriphenylene and employed as a hole injection material, TAPC was 1,1′-bis[4-(di-p-tolylamino)phenyl]-cyclohexane and used as a hole-transporting material, and PPT was dibenzo[b,d]thiophene-2,8-diylbis(diphenylphosphine oxide) and acted as a hole-blocking material for its deeper LUMO level (−6.6 eV) than those of the host materials mCBP (−6.2 eV) and 26mCPy (−6.0 eV), Bepp2 was bis [2-2-(hydroxyphenyl) pyridinato]beryllium and Bepp2:Li2CO3 was used as electron-transporting material69–71 (Fig. 7a). The device performances are summarized in Table 3.
| Device | EML | V on (V) | λ EL (nm) | EQEa (%) | L max (cd m−2) | CIE (x, y) |
|---|---|---|---|---|---|---|
a Maximum EQE, and EQE at 100, 1000 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2. Von: turn-on voltage at 1 cd m−2. Lmax: maximum brightness. CIE: Commission Internationale de l’Eclairage. 000 cd m−2. Von: turn-on voltage at 1 cd m−2. Lmax: maximum brightness. CIE: Commission Internationale de l’Eclairage.
|
||||||
| Device I | 10% Pt(NHC-1):mCBP | 3.5 | 512 | 13.9/13.6/12.5/9.2 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 275 |
(0.288, 0.588) |
| Device II | 10% Pt(NHC-1):26mCPy | 3.9 | 509 | 13.1/12.8/11.6/8.6 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 416 |
(0.261, 0.575) |
Both device I and device II showed turn-on voltages (Von) of 3.5 V and 3.9 V, respectively. They also exhibited sole and similar EL spectral peaks at 512 nm with CIE coordinates of (0.288, 0.588) and (0.261, 0.575) respectively (Fig. 8), device I had a slight red-shift of 3 nm compared to device II (Fig. 7b and Table 3). The distorted molecular geometry of the Pt(NHC-1) and the introduction of the tert-butyl group (Table S1, ESI†) prevents the intermolecular interaction between the dopant molecules, and no excimer or aggregation emission was observed, which is helpful for improving the colour purity of the OLEDs. Compared to the photoluminescence spectra of Pt(NHC-1), the electroluminescence of both device I and device II exhibited similar spectra with blue-shift of 7 nm and 3 nm, respectively. The maximum brightness (Lmax) of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 and 64
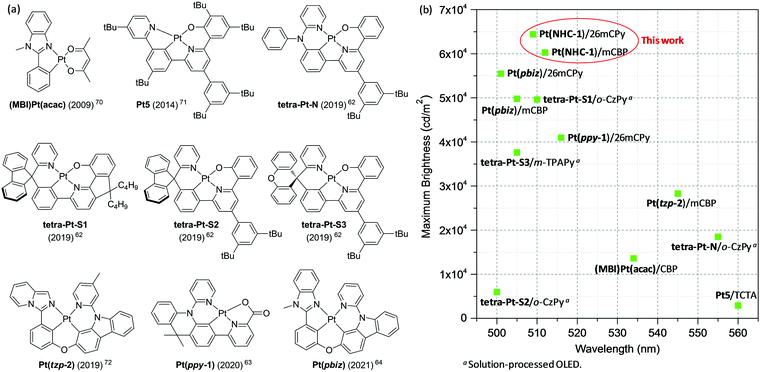
275 and 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 cd m−2 could be achieved for device I and device II, respectively (Fig. 7c and Table 3). As far as we know, the Lmax values of the device I and device II are record-high values for the reported Pt(II) complex-based phosphorescent OLEDs (Fig. 9 and Fig. S5, Table S3, ESI†).62,63,66,72–74 This result suggested the superiority of NHC-based Pt(II) complex as emitters for OLED applications because of the chemical stability. The high brightness facilitates the emitter for potential lighting application. Additionally, both the devices demonstrated small efficiency roll-off, the maximum EQE, and EQE at 100 and 1000 cd m−2 were 13.9%, 13.6%, 12.5% and 13.1%, 12.8%, 11.6% for device I and device II, respectively, yet maintained EQEs of 9.2% and 8.6% at a high brightness of 10
416 cd m−2 could be achieved for device I and device II, respectively (Fig. 7c and Table 3). As far as we know, the Lmax values of the device I and device II are record-high values for the reported Pt(II) complex-based phosphorescent OLEDs (Fig. 9 and Fig. S5, Table S3, ESI†).62,63,66,72–74 This result suggested the superiority of NHC-based Pt(II) complex as emitters for OLED applications because of the chemical stability. The high brightness facilitates the emitter for potential lighting application. Additionally, both the devices demonstrated small efficiency roll-off, the maximum EQE, and EQE at 100 and 1000 cd m−2 were 13.9%, 13.6%, 12.5% and 13.1%, 12.8%, 11.6% for device I and device II, respectively, yet maintained EQEs of 9.2% and 8.6% at a high brightness of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cd m−2 (Fig. 7d and Table 3). The TAPC, which possesses a higher HOMO level (−2.0 eV) than those of the host materials mCBP (−2.7 eV) and 26mCPy (−2.3 eV), and the hole-blocking material PPT can confine the electrogenerated triplet excitons inside of the emitting layer and achieve a high device performance. It is believed that the performance of the OLED can be further improved by using the state-of-the-art device structure.
000 cd m−2 (Fig. 7d and Table 3). The TAPC, which possesses a higher HOMO level (−2.0 eV) than those of the host materials mCBP (−2.7 eV) and 26mCPy (−2.3 eV), and the hole-blocking material PPT can confine the electrogenerated triplet excitons inside of the emitting layer and achieve a high device performance. It is believed that the performance of the OLED can be further improved by using the state-of-the-art device structure.
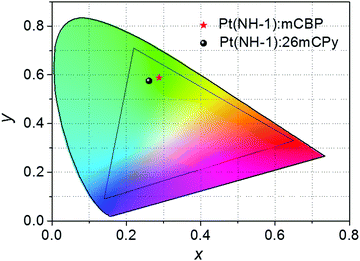 | ||
| Fig. 8 Electroluminescence colour coordinates on the CIE 1931 chromaticity diagram of the Pt(NHC-1)-doped OLEDs. | ||
Conclusions
In summary, a new series of NHC, Ph/NHC and Py/NHC-based tetradentate Pt(II) complexes with fused 5/6/6 metallocycles were designed and developed. Systematic experimental and theoretical studies indicate that the NHC, Ph/NHC and Py/NHC had a significant influence on the electrochemical properties, frontier orbitals and photophysical properties. The Pt(II) complexes show a dominant emission peak at 517–539 nm with a quantum efficiency of 2–18% in dichloromethane solution at room temperature. In a 5 wt% doped PMMA film at room temperature, the Pt(II) complexes exhibit significant blue-shift peaks at 495–505 nm and greatly enhanced quantum efficiencies of 5–55%. Pt(NHC-1)-doped green OLEDs were fabricated using mCPB and 26mCPy as host materials with small efficiency roll-off, which demonstrated very high Lmax values of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 and 64
275 and 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416 cd m−2 and peak EQEs of 13.9% and 13.1% respectively. This work could offer a valuable reference for the development of novel stable phosphorescent Pt(II) emitters, which can efficiently luminesce at large current density, for OLED fabrication for potentially commercial display and lighting applications.
416 cd m−2 and peak EQEs of 13.9% and 13.1% respectively. This work could offer a valuable reference for the development of novel stable phosphorescent Pt(II) emitters, which can efficiently luminesce at large current density, for OLED fabrication for potentially commercial display and lighting applications.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We thank the National Natural Science Foundation of China (Grant no. 21878276, 22178319 and 22138011) and the Fundamental Research Funds for the Provincial Universities of Zhejiang (RF-A2019013) for financial support.References
- G. P. Moss, P. A. S. Smith and D. Tavernier, Pure Appl. Chem., 1995, 67, 1307 Search PubMed.
- H.-W. Wanzlick and H.-J. Schönherr, Angew. Chem., Int. Ed. Engl., 1968, 80, 154 CrossRef.
- K. Öfele, J. Organomet. Chem., 1968, 12, 42 CrossRef.
- A. J. Arduengo, III, R. L. Harlow and M. Kline, J. Am. Chem. Soc., 1991, 113, 361 CrossRef.
- Y. Liu, Q. Chen, C. Mou, L. Pan, X. Duan, X. Chen, H. Chen, Y. Zhao, Y. Lu, Z. Jin and Y. R. Chi, Nat. Commun., 2019, 10, 1675 CrossRef.
- H. V. Huynh, The Organometallic Chemistry of N-heterocyclic Carbenes, John Wiley & Sons Inc, 2017 Search PubMed.
- V. Nesterov, D. Reiter, P. Bag, P. Frisch, R. Holzner, A. Porzelt and S. Inoue, Chem. Rev., 2018, 118, 9678 CrossRef CAS.
- C. A. Smith, M. R. Narouz, P. A. Lummis, I. Singh, A. Nazemi, C.-H. Li and C. M. Crudden, Chem. Rev., 2019, 119, 4986 CrossRef CAS.
- G. Hong, X. Gan, C. Leonhardt, Z. Zhang, J. Seibert, J. M. Busch and S. Bräse, Adv. Mater., 2021, 2005630 CrossRef CAS.
- G. Li, Z.-Q. Zhu, Q. Chen and J. Li, Org. Electron., 2019, 69, 135 CrossRef CAS.
- M. J. Leitl, V. A. Krylova, P. I. Djurovich, M. E. Thompson and H. Yersin, J. Am. Chem. Soc., 2014, 136, 16032 CrossRef PubMed.
- A. F. Henwood, M. Lesieur, A. K. Bansal, V. Lemaur, D. Beljonne, D. G. Thompson, D. Graham, A. M. Z. Slawin, I. D. W. Samuel, C. S. J. Cazin and E. Zysman-Colman, Chem. Sci., 2015, 6, 3248 RSC.
- D. Di, A. S. Romanov, L. Yang, J. M. Richter, J. P. H. Rivett, S. Jones, T. H. Thomas, M. A. Jalebi, R. H. Friend, M. Linnolahti, M. Bochmann and D. Credgington, Science, 2017, 356, 159 CrossRef CAS PubMed.
- R. Hamze, J. L. Peltier, D. Sylvinson, M. Jung, J. Cardenas, R. Haiges, M. Soleilhavoup, R. Jazzar, P. I. Djurovich, G. Bertrand and M. E. Thompson, Science, 2019, 363, 601 CrossRef CAS PubMed.
- T. Li, D. S. M. Ravinson, R. Haiges, P. I. Djurovich and M. E. Thompson, J. Am. Chem. Soc., 2020, 142, 6158 CrossRef.
- S. Shi, M. C. Jung, C. Coburn, A. Tadle, M. R. Daniel Sylvinson, P. I. Djurovich, S. R. Forrest and M. E. Thompson, J. Am. Chem. Soc., 2019, 141, 3576 CrossRef CAS.
- R. Hamze, S. Shi, S. C. Kapper, D. S. M. Ravinson, L. Estergreen, M.-C. Jung, A. C. Tadle, R. Haiges, P. I. Djurovich, J. L. Peltier, R. Jazzar, G. Bertrand, S. E. Bradforth and M. E. Thompson, J. Am. Chem. Soc., 2019, 141, 8616 CrossRef CAS.
- M. Gernert, L. Balles-Wolf, F. Kerner, U. Müller, A. Schmiedel, M. Holzapfel, C. M. Marian, J. Pflaum, C. Lambert and A. Steffen, J. Am. Chem. Soc., 2020, 142, 8897 CrossRef CAS PubMed.
- X. Yang, G. Zhou and W.-Y. Wong, Chem. Soc. Rev., 2015, 44, 8484 RSC.
- S. Huo, J. Carroll and D. A. K. Vezzu, Asian J. Org. Chem., 2015, 4, 1210 CrossRef CAS.
- T. Fleetham, G. Li and J. Li, Adv. Mater., 2017, 29, 1601861 CrossRef.
- G. Li and Y. She, Tetradentate Cyclometalated Platinum Complexes for Efficient and Stable Organic Light-Emitting Diodes, In Light Emitting Diode-An Outlook On the Empirical Features and Its Recent Technological Advancements, IntechOpen, London, 2018, pp 77–101 Search PubMed.
- Y. Unger, D. Meyer, O. Molt, C. Schildknecht, I. Munster, G. Wagenblast and T. Strassner, Angew. Chem., Int. Ed., 2010, 49, 10214 CrossRef CAS.
- K. Li, X. Guan, C.-W. Ma, W. Lu, Y. Chen and C.-M. Che, Chem. Commun., 2011, 47, 9075 RSC.
- Z. M. Hudson, C. Sun, M. G. Helander, Y.-L. Chang, Z.-H. Lu and S. Wang, J. Am. Chem. Soc., 2012, 134, 13930 CrossRef CAS PubMed.
- K. Li, G. Cheng, C. Ma, X. Guan, W.-M. Kwok, Y. Chen, W. Lu and C.-M. Chen, Chem. Sci., 2013, 4, 2630 RSC.
- T. Fleetham, J. Ecton, Z. Wang, N. Bakken and J. Li, Adv. Mater., 2013, 25, 2573 CrossRef CAS PubMed.
- X.-C. Hang, T. Fleetham, E. Turner, J. Brooks and J. Li, Angew. Chem., Int. Ed., 2013, 52, 6753 CrossRef CAS PubMed.
- G. Li, T. Fleetham and J. Li, Adv. Mater., 2014, 26, 2931 CrossRef CAS.
- T. Fleetham, G. Li, L. Wen and J. Li, Adv. Mater., 2014, 26, 7116 CrossRef CAS PubMed.
- A. Tronnier, A. Pöthig, S. Metz, G. Wagenblast, I. Münster and T. Strassner, Inorg. Chem., 2014, 53, 6346 CrossRef CAS PubMed.
- A. Tronnier, U. Heinemeyer, S. Metz, G. Wagenblast, I. Muenster and T. Strassner, J. Mater. Chem. C, 2015, 3, 1680 RSC.
- G. Li, A. Wolfe, J. Brooks, Z.-Q. Zhu and J. Li, Inorg. Chem., 2017, 56, 8244 CrossRef CAS PubMed.
- H. Fukagawa, T. Shimizu, Y. Iwasaki and T. Yamamoto, Sci. Rep., 2017, 7, 1735 CrossRef.
- H. Fukagawa, T. Oono, Y. Iwasaki, T. Hatakeyama and T. Shimizu, Mater. Chem. Front., 2018, 2, 704 RSC.
- J.-S. Huh, M. J. Sung, S.-K. Kwon, Y.-H. Kim and J.-J. Kim, Adv. Funct. Mater., 2021, 31, 2100967 CrossRef CAS.
- G. Li, J. Zheng, X. Fang, K. Xu, Y.-F. Yang, J. Wu, L. Cao, J. Li and Y. She, Organometallics, 2021, 40, 472 CrossRef CAS.
- K. S. Yook and J. Y. Lee, Adv. Mater., 2012, 24, 3169 CrossRef CAS.
- Y. Im, S. Y. Byun, J. H. Kim, D. R. Lee, C. S. Oh, K. S. Yook and J. Y. Lee, Adv. Funct. Mater., 2017, 27, 1603007 CrossRef.
- J. Lee, H.-F. Chen, T. Batagoda, C. Coburn, P. I. Djurovich, M. E. Thompson and S. R. Forrest, Nat. Mater., 2016, 15, 92 CrossRef CAS PubMed.
- C.-Y. Kuei, W.-L. Tsai, B. Tong, M. Jiao, W.-K. Lee, Y. Chi, C.-C. Wu, S.-H. Liu, G.-H. Lee and P.-T. Chou, Adv. Mater., 2016, 28, 2795 CrossRef CAS.
- H.-H. Kuo, Y.-T. Chen, L. R. Devereux, C.-C. Wu, M. A. Fox, C.-Y. Kuei, Y. Chi and G.-H. Lee, Adv. Mater., 2017, 29, 1702464 CrossRef.
- A. K. Pal, S. Krotkus, M. Fontani, C. F. R. Mackenzie, D. B. Cordes, A. M. Z. Slawin, I. D. W. Samuel and E. Zysman-Colman, Adv. Mater., 2018, 30, 1804231 CrossRef.
- Y.-K. Chen, H.-H. Kuo, D. Luo, Y.-N. Lai, W.-C. Li, C.-H. Chang, D. Escudero, A. K.-Y. Jen, L.-Y. Hsu and Y. Chi, Chem. Mater., 2019, 31, 6453 CrossRef CAS.
- T.-L. Lam, K.-C. Tong, C. Yang, W.-L. Kwong, X. Guan, M.-D. Li, V. K.-Y. Lo, S. L.-F. Chan, D. L. Phillips, C.-N. Lok and C.-M. Che, Chem. Sci., 2019, 10, 293 RSC.
- H.-H. Kuo, Z.-l. Zhu, C.-S. Lee, Y.-K. Chen, S.-H. Liu, P.-T. Chou, A. K.-Y. Jen and Y. Chi, Adv. Sci., 2018, 5, 1800846 CrossRef.
- L.-Y. Hsu, D.-G. Chen, S.-H. Liu, T.-Y. Chiu, C.-H. Chang, A. K.-Y. Jen, P.-T. Chou and Y. Chi, ACS Appl. Mater. Interfaces, 2020, 12, 1084 CrossRef CAS.
- H.-Y. Park, A. Maheshwaran, C.-K. Moon, H. Lee, S. S. Reddy, V. G. Sree, J. Yoon, J. W. Kim, J. H. Kwon, J.-J. Kim and S.-H. Jin, Adv. Mater., 2020, 32, 2002120 CrossRef CAS PubMed.
- W.-S. Tai, L.-Y. Hsu, W.-Y. Hung, Y.-Y. Chen, C.-L. Ko, X. Zhou, Y. Yuan, A. K.-Y. Jen and Y. Chi, J. Mater. Chem. C, 2020, 8, 13590 RSC.
- W.-S. Tai, P. Gnanasekaran, Y.-Y. Chen, W.-Y. Hung, X. Zhou, T.-C. Chou, G.-H. Lee, P.-T. Chou, C. You and Y. Chi, ACS Appl. Mater. Interfaces, 2021, 13, 15437 CrossRef CAS PubMed.
- P. Chábera, Y. Liu, O. Prakash, E. Thyrhaug, A. E. Nahhas, A. Honarfar, S. Essén, L. A. Fredin, T. C. B. Harlang, K. S. Kjær, K. Handrup, F. Ericson, H. Tatsuno, K. Morgan, J. Schnadt, L. Häggström, T. Ericsson, A. Sobkowiak, S. Lidin, P. Huang, S. Styring, J. Uhlig, J. Bendix, R. Lomoth, V. Sundström, P. Persson and K. Wärnmark, Nature, 2017, 543, 695 CrossRef.
- K. S. Kjær, N. Kaul, O. Prakash, P. Chábera, N. W. Rosemann, A. Honarfar, O. Gordivska, L. A. Fredin, K.-E. Bergquist, L. Häggström, T. Ericsson, L. Lindh, A. Yartsev, S. Styring, P. Huang, J. Uhlig, J. Bendix, D. Strand, V. Sundström, P. Persson, R. Lomoth and K. Wärnmark, Science, 2019, 363, 249 CrossRef.
- H. Fukagawa, T. Shimizu, H. Hanashima, Y. Osada, M. Suzuki and H. Fujikake, Adv. Mater., 2012, 24, 5099 CrossRef CAS PubMed.
- G. Li, J. Ecton, B. O’Brien and J. Li, Org. Electron., 2014, 15, 1862 CrossRef CAS.
- G. Li, T. Fleetham, E. Turner, X.-C. Hang and J. Li, Adv. Opt. Mater., 2015, 3, 390 CrossRef CAS.
- T. Fleetham, G. Li and J. Li, ACS Appl. Mater. Interfaces, 2015, 7, 16240 CrossRef CAS PubMed.
- T. B. Fleetham, L. Huang, K. Klimes, J. Brooks and J. Li, Chem. Mater., 2016, 28, 3276 CrossRef CAS.
- Z.-Q. Zhu, K. Klimes, S. Holloway and J. Li, Adv. Mater., 2017, 29, 1605002 CrossRef PubMed.
- G. Li, K. Klimes, T. Fleetham, Z.-Q. Zhu and J. Li, Appl. Phys. Lett., 2017, 110, 113301 CrossRef.
- K. Klimes, Z.-Q. Zhu and J. Li, Adv. Funct. Mater., 2019, 29, 1903068 CrossRef.
- G. Li, L. Ameri, T. Fleetham, Z.-Q. Zhu and J. Li, Appl. Phys. Lett., 2020, 117, 253301 CrossRef CAS.
- G. Cheng, Y. Kwak, W.-P. To, T.-L. Lam, G. S. M. Tong, M.-K. Sit, S. Gong, B. Choi, W. Choi, C. Yang and C.-M. Che, ACS Appl. Mater. Interfaces, 2019, 11, 45161 CrossRef CAS.
- G. Li, G. Shen, X. Fang, Y.-F. Yang, F. Zhan, J. Zheng, W. Lou, Q. Zhang and Y. She, Inorg. Chem., 2020, 59, 18109 CrossRef CAS PubMed.
- J. Brooks, Y. Babayan, S. Lamansky, P. I. Djurovich, I. Tsyba, R. Bau and M. E. Thompson, Inorg. Chem., 2002, 41, 3055 CrossRef CAS.
- P. I. Kvam, M. V. Pyzyk, K. P. Balashev and J. Songstad, Acta Chem. Scand., 1995, 49, 335 CrossRef CAS.
- G. Li, H. Guo, X. Fang, Y.-F. Yang, Y. Sun, W. Lou, Q. Zhang and Y. She, Chin. J. Chem., 2022, 40, 223–234 Search PubMed.
- Y. She, K. Xu, X. Fang, Y.-F. Yang, W. Lou, Y. Hu, Q. Zhang and G. Li, Inorg. Chem., 2021, 60, 12972 CrossRef CAS.
- G. Li, F. Zhan, J. Zheng, Y.-F. Yang, Q. Wang, Q. Chen, G. Shen and Y. She, Inorg. Chem., 2020, 59, 3718 CrossRef CAS PubMed.
- G. Li, W. Lou, D. Wang, C. Deng and Q. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 32209 CrossRef CAS.
- G. Li, F. Zhan, W. Lou, D. Wang, C. Deng, L. Cao, Y. Yang, Q. Zhang and Y. She, J. Mater. Chem. C, 2020, 8, 17464 RSC.
- Q. Ai, J. Chai, W. Lou, T. Liu, C. Deng, D. Wang, C. Wang, G. Li, X. Liu, Z. Liu and Q. Zhang, ACS Appl. Mater. Interfaces, 2020, 12, 6127 CrossRef.
- H. Li, J. Ding, Z. Xie, Y. Cheng and L. Wang, J. Organomet. Chem., 2009, 694, 2777 CrossRef.
- G. Cheng, S. C. F. Ang, W.-H. Kui, M.-Y. Ko, P.-K. Chow, C.-L. Kwong, C.-C. Kwok, C. Ma, X. Guan, K.-H. Low, S.-J. Su and C.-M. Che, Chem. Sci., 2014, 5, 4819 RSC.
- G. Li, Q. Chen, J. Zheng, Q. Wang, F. Zhan, W. Lou, Y.-F. Yang and Y. She, Inorg. Chem., 2019, 58, 14349 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1tc05207a |
| This journal is © The Royal Society of Chemistry 2022 |