Te4+-doped zero-dimensional Cs2ZnCl4 single crystals for broadband yellow light emission†
Xiaoxia
Liu
,
Chengdong
Peng
*,
Lijie
Zhang
,
Daying
Guo
and
Yuexiao
Pan
 *
*
Key Laboratory of Carbon Materials of Zhejiang Province, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, P. R. China. E-mail: cdpeng@wzu.edu.cn; yxpan@wzu.edu.cn; Fax: +86-577-8837-3017; Tel: +86-577-8837-3017
First published on 26th November 2021
Abstract
As an all-inorganic zero-dimensional (0D) lead-free metal halide, Cs2ZnCl4 is a potentially excellent matrix for preparing chemically stable and environmentally friendly photoluminescent materials through an ion-doping strategy. Therefore, it is necessary to study the emission properties of every kind of doping ion in the tetrahedral coordination of Cs2ZnCl4. Here, for the first time, we successfully synthesized Te4+-doped Cs2ZnCl4 single crystals via a facile hydrothermal method. X-ray crystallography clearly reveals the strong distortion of the tetrahedral units in Cs2ZnCl4 caused by doping with Te4+. Broadband yellow-light emission covering the range from 450 nm to 700 nm with a large Stokes shift is observed in Cs2ZnCl4:Te4+ single crystals, and this is attributed to self-trapped excitons (STEs) caused by the lattice vibration of the distorted structure. It is worth mentioning that this broadband yellow phosphor can be excited by a wide range of blue light, implicitly giving it an excellent ability to adapt to multiple models of blue LED chips, thus serving as a suitable yellow phosphor that can be applied to fabricate WLEDs without the need to worry about the lack of red light.
Introduction
Luminescent all-inorganic metal halides with flexible crystallography/electronic structures have been widely studied as optoelectronic materials in recent years.1,2 Among them, lead halide perovskites have drawn most attention due to their high photoluminescence quantum yields (PLQYs) and color tunability, which are beneficial for their application in light-emitting diodes (LEDs).3 However, the toxicity of lead and the poor stability of lead halide perovskites have limited their practical applications in solid-state lighting devices.4 To avoid these two nonnegligible shortcomings of lead-based perovskites, a series of lead-free metal halides have been developed at present.5 Because of their similar electronic configurations to Pb2+, the ions Ge2+, Sn2+, Sb3+ and Bi3+ are considered to be several of the most promising candidates to take the place of Pb2+ in the B-site of ABX3.6–9 Nevertheless, most lead-free halides belong to the perovskite-type structure that consists of BX6 octahedral units, and only a few examples adopt non-perovskite structures. It is generally believed that low-dimensional metal halides are more conducive to lattice deformation; therefore it is easier to produce high-efficiency light emission.10 At present, 1D and 0D metal halide materials with various color luminescence and high PLQYs have been realized in Sn-, Cu-, In- and Sb-based single crystals.11–14Among many lead-free metal halides, Cs2ZnCl4 did not attract too much attention in the field of optoelectronics in the early years. Under X-ray irradiation, Cs2ZnCl4 exhibits an ultraviolet light emission with a maximum at 310 nm and two well-defined shoulders at 260 and 410 nm.15 Benefiting from rapid Auger-free luminescence in the ultraviolet spectral region, this compound is usually used as a scintillator in high-energy X-ray detection and has the advantages of high timing resolution and detection efficiency.16 As an all-inorganic 0D metal halide featuring easy synthesis, good chemical stability and a wide band gap, Cs2ZnCl4 is a potentially excellent host to study the emission properties of every kind of doping ion in tetrahedral coordination.
A large number of reports have confirmed that doping is an effective mean to induce new emission centers and boost the photoluminescent properties of metal halides.17 Among the positive examples, several doping studies with Cs2ZnCl4 as the host show inspiring results as efficient emitters in recent studies. Among them, Cu+-doped Cs2ZnCl4 was synthesized and found to generate an intense sky-blue emission with a high photoluminescence quantum yield.18 Meanwhile, Mn2+- and Sn2+-doped Cs2ZnCl4 produce green and red light-emission, respectively.19,20 Remarkably, it was found that the incorporation of Sb3+ into the Cs2ZnCl4 matrix led to a high-efficiency broadband near-infrared emission.21 However, an intense broadband yellow light emission based on all-inorganic 0D Cs2ZnCl4 through a doping strategy in tetrahedral coordination, which is more desirable for display and solid-state lighting,22 has remained unexplored up to the present. Usually, one of the implementation methods for a white LED is to utilize blue InGaN LEDs coated with a yellow-emitting YAG:Ce3+ phosphor, but this kind of white light source often looks too “cold” to the naked eye due to the narrow range emission of yellow phosphor.23,24 Therefore, it is significant to develop chemically stable and environmentally friendly 0D metal halides with high-efficiency broadband yellow PL emission.
In this work, we realized broadband yellow light emission by incorporating Te4+ ions which have a relatively high cationic valence state into a zero-dimensional all-inorganic and stable metal halide Cs2ZnCl4 single crystal via a facile hydrothermal method. Under blue light excitation, the resultant material Cs2ZnCl4:Te4+ shows bright broadband yellow emission peaking at 570 nm and spanning 450 nm to 700 nm, which should be attributed to the self-trapped exciton (STE) emission of the distorted [TeCl4] and [ZnCl4]2− units being formed by doping Te4+ into the Cs2ZnCl4 host. Moreover, by combining the Cs2ZnCl4:Te4+ powder with a blue InGaN LED (450 nm) chip, a tentative white LED has been fabricated showing the CIE chromaticity coordinates (0.3422, 0.3543) with a correlated color temperature (CCT) of 5116 K. These corresponding parameters indicate that the described yellow phosphor of Cs2ZnCl4:Te4+ entirely satisfies the color properties required by WLEDs.
Experimental
Chemicals
The raw materials tellurium dioxide (TeO2, 99.9%), cesium chloride (CsCl, 99.9%), zinc chloride (ZnCl2, 99.9%), and ethanol (C2H5OH, 99.8%) were purchased from the Aladdin Chemical Reagent Factory. Hydrochloric acid (HCl, 37 wt% in water) was purchased from the Zhongxing Chemical Reagent Factory. All the chemical reagents used for synthesis were commercially purchased and used without further purification.Synthesis of Cs2ZnCl4 single crystals
The Cs2ZnCl4 single crystals were synthesized through a hydrothermal method. Here is an example to illustrate the experimental details. First, 2 mmol CsCl (99.9%), 1 mmol ZnCl2 (99.9%), and 4 mL of hydrochloric acid (37 wt%) were successively loaded into a 15 mL Teflon autoclave container. Then, the autoclave was put into a drying oven, followed by heating up to the target temperatures (160 °C). After keeping the reaction at the preset temperature for 24 h, the solution was slowly cooled down to room temperature (RT) overnight. Finally, the products in the form of lamellar crystals were obtained after immediate filtration and washing with ethanol three times.Growth of Cs2ZnCl4:Te4+ single crystals
The procedure was identical to that described above for Cs2ZnCl4, except for the addition of TeO2.Characterization
The crystal structure and phase composition were checked using powder X-ray diffraction and single-crystal X-ray diffraction. Powder X-ray diffraction (PXRD) data were collected on a Bruker D8 Advance X-ray diffractometer with Cu Kα radiation (λ = 1.5406 Å). The data were collected in a step-scanning mode with a step size of 0.02° and 5 s counting time per step. The single-crystal X-ray diffraction data were collected using a SMART APE II DUO X-ray four-circle single-crystal diffractometer (Bruker) equipped with a CCD-detector, a graphite monochromator, and a Cu Kα radiation source.UV-vis diffuse reflectance spectra (DRS) were measured on a UV-visible diffuse reflectance spectrometer (SolidSpec-3600) consisting of an integration sphere using BaSO4 as a reference. The photoluminescence excitation and emission spectra were collected using a FluoroMAX-4-TCSPC fluorescence spectrophotometer, equipped with a 450 W xenon arc lamp and a photomultiplier tube (measurement range 200–900 nm).
Results and discussion
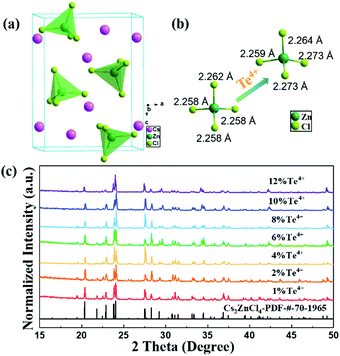
The hydrothermal reactions of cesium chloride, zinc chloride and hydrochloric acid at 160 °C afforded colorless single crystals of Cs2ZnCl4. X-ray crystallography reveals that Cs2ZnCl4 has a 0D structure and adopts an orthogonal Pnma space group. Crystallographically, every ZnII center is surrounded by four chlorine atoms in a highly symmetrical elongated tetrahedral geometry, thus forming [ZnCl4]2− tetrahedra, as shown in Fig. 1a. Each unit cell contains two complete [ZnCl4]2− tetrahedra and two angular tetrahedra and each [ZnCl4]2− tetrahedron consists of a longer Zn–Cl bond (2.262 Å) and three identical shorter Zn–Cl bonds (2.258 Å). Overall, the crystal structure of Cs2ZnCl4 is composed of ordered but disconnected [ZnCl4]2− tetrahedra with charge-balancing Cs+ cations filling the vacant positions and further enhancing the structural stability. The crystal structure of Cs2ZnCl4:Te4+ obtained through incorporating Te4+ into Cs2ZnCl4 has also been characterized in detail by single-crystal X-ray crystallography and shows a similar structure to that of the undoped crystal. The small increase in cell volume (Table S1, ESI†) conforms with the fact that Te4+ (r = 0.97 Å) is bigger than Zn2+ (r = 0.60 Å). The obvious results shown in Fig. 1b demonstrate that the [ZnCl4]2− tetrahedron becomes more distorted compared with its predecessor after doping with Te4+ ions, because the distance between ZnII and the surrounding chlorine atoms ranges from 2.259 Å to 2.273 Å, which is distinctly different from the Zn–Cl bonds in the matrix lattice. In addition, the adjacent bond angles of Cl–Zn–Cl of the [ZnCl4]2− tetrahedra also clearly changed after doping with Te4+. As can be seen in Fig. S1 (ESI†), the four Cl–Zn–Cl bond angles parallel to the page in a [ZnCl4]2− tetrahedron include two 106.488° and two 109.884° in pristine Cs2ZnCl4. After doping with Te4+, the angles become different to each other with a range from 105.925° to 115.684°. These significant structural differences between the host and Cs2ZnCl4:Te4+ also provide evidence that Te4+ ions have substituted for a small part of the Zn2+ sites. As a common phenomenon in metal halide materials, the distorted coordination units and the resultant close contact of Cl⋯Cl (3.62–3.83 Å) between nearest neighbours in Cs2ZnCl4:Te4+ will probably contribute to the formation of Cl2− species upon excitation, affording self-trapped holes and self-trapped electrons that originate from short-range electron–phonon coupling in the strongly deformable lattice.25–27 The powder X-ray diffraction (PXRD) patterns of x% Te-doped Cs2ZnCl4 (x = 0–12) samples, which correspond well with the standard PXRD pattern of Cs2ZnCl4 (JCPDS card 70-1965), are shown in Fig. 1c and no impurity phase has been detected. This uniformity of the patterns also confirms that the Cs2ZnCl4:Te4+ single crystals retain the isomorphic structure of pristine Cs2ZnCl4.The elemental composition of Te4+-doped Cs2ZnCl4 has also been strictly qualitatively and quantitatively characterized. The energy dispersive spectroscopy (EDS) elemental mapping of the Cs2ZnCl4:Te4+ crystal shows even distributions of Cs, Zn, Cl, and Te elements (Fig. S2 and S3, ESI†), indicating that Te4+ ions have been uniformly doped in the lattice of Cs2ZnCl4 with high phase purity. On the other hand, characterization with an inductively coupled plasma optical emission spectrometer (ICP-OES) reveals that the actual Te4+ concentrations in Cs2ZnCl4:Te4+ single crystals are lower than the experimentally expected values (Fig. S4 and Table S2, ESI†). When the molar ratio of the feeding amount based on Te atoms in the experiment is 1–12%, the actual percentage of atoms entering the lattice of Cs2ZnCl4 is only 0.21–4.02%. This may be due to the larger radius and higher valence state of the Te4+ ion which means it does not easily replace the lattice site of Zn2+. Additionally, it should be noted that the experimentally expected values will be used to express the doping contents for convenience of description in the full text. In order to further determine the valence state of Te4+ doping in Cs2ZnCl4, X-ray photoelectron spectroscopy (XPS) measurement is employed and gives high-resolution spectra of the 2p orbital of Zn2+, the 3d orbital of Cs+, the 2p orbital of Cl− and the 3d orbital of Te4+, which are shown in Fig. S5 (ESI†). The two peaks observed at 586.6 and 576.3 eV can be assigned to Te 3d3/2 and Te 3d5/2,28 proving the presence of Te in the as-synthesized samples. This result also demonstrates the oxidative stability of tellurium because of its unchanged valence state compared with the precursor. Evidence for the high yield and phase purity of Cs2ZnCl4:Te4+ single crystals was also provided by Fourier-transform infrared spectroscopy (FTIR) and Raman spectra (Fig. S6, ESI†).
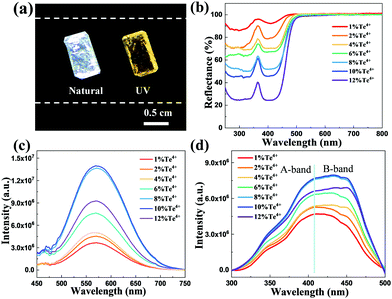
The Te4+-doped Cs2ZnCl4 single crystal exhibits transparency and colourlessness under natural light and emits bright yellow light when it is exposed to the irradiation of a 4 W and 365 nm ultraviolet lamp (Fig. 2a), whereas the undoped crystals are transparent and show no luminescence under UV light excitation (Fig. S7, ESI†). The excellent transmittance of the crystals verifies their high crystallinity. To investigate the optical properties of undoped and Te4+-doped Cs2ZnCl4 single crystals, steady-state UV-Vis diffuse reflectance spectra with different doping contents of Te4+ were first carried out and are presented in Fig. 2b. The Cs2ZnCl4 host shows a plateau of high reflection in the wavelength range of 500–800 nm and then starts to decrease gradually from 300 to 500 nm. However, according to relevant reports of metal halide luminescent materials, it is natural to consider that the differences between the Cs2ZnCl4:Te4+ single crystals and the pristine host in the region of 360–500 nm originate from the characteristic of free excitons in the low-dimensional and deformable structure of metal halides with Te4+ ions introduced into the lattice.29,30 Along with the increase in the concentration of Te4+ in the Cs2ZnCl4 host, the corresponding band edge absorption intensities are also significantly enhanced and the absorption edges gradually shift to a low energy band. Referring to the relevant literature, the band gap (Eg) of a Cs2ZnCl4 matrix is defined to be 4.60 eV.21 According to the Kubelka–Munk equation, as the doping content of Te4+ increases, the band gaps of 1, 2, 4, 6, 8, 10 and 12 mol% Te4+-doped Cs2ZnCl4 are obtained as 4.01, 3.35, 2.79, 2.70, 2.67, 2.63 and 2.32 eV, respectively. There is no doubt that the reduction in these Eg values should be attributed to the doping by Te.
Upon 417 nm excitation, Te4+-doped Cs2ZnCl4 single crystals exhibit a broadband yellow-light emission spanning the visible light region from 450 nm to 700 nm with a Stokes shift of about 140 nm. The PL intensities of different feeding ratios of Te4+-doped Cs2ZnCl4 are significantly transformed with the trend rising first and then falling because of the concentration quenching effect, and reaching a maximum with a half-maximum full width (FWHM) of up to 118 nm when the concentration of Te4+ is 8 mol% (Fig. 2c). These results indicate the successful conquering of the problem of self-absorption in the near-UV region. It is very clear that the emission spectrum of Te4+-doped Cs2ZnCl4 single crystals consists of a shoulder band at 470 nm and a main broad band at 570 nm. So that it is reasonable to attribute the high-energy shoulder to the free excitons and the low-energy broadband emission to self-trapped excitons because of the similar two-band emission profiles to those metal halides.31,32 The excitation spectra of different Te4+ feed ratios are shown in Fig. 2d, which show a certain correlation to the absorption spectra. Because of the similar electronic configurations between Te4+ and Sb3+, its energy band is composed of ground state 1S0, singlet excited state 1P1 and triplet excited state 3Pn (n = 0, 1, 2).33,34 We can see in Fig. 2d that when the doping concentration increases, the optimal excitation wavelength changes from 417 nm (A-band) at 1 mol% to 451 nm (B-band) at 12 mol% Te4+-doped Cs2ZnCl4. According to the transition selection law and considering the contribution of lattice vibration to the transition process, the A-band is due to the 1S0 → 3P1 transition of Te4+ through spin–orbit coupling which is a double peak caused by the dynamic Jahn–Teller effect, and the B band is due to the 1S0 → 3P2 transition of Te4+.28 For the above-mentioned reasons, this broad excitation band also indicates that the broadband yellow phosphor can be excited by a wide range of blue light, making it promising for applications in the field of solid-state lighting.
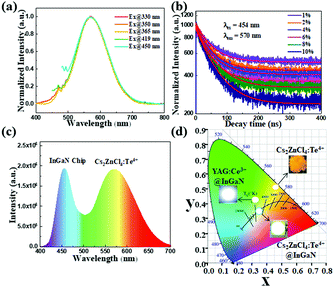
Importantly, no apparent change to the color properties of the broadband yellow emission is observed in Cs2ZnCl4:Te4+ single crystals when adjusting the excitation from 330 nm to 450 nm, confirming its nature as a broadband monochromatic emitter. Time-resolved photoluminescence decay experiments indicate the lifetime of the emission measured at 570 nm is 53.2 ns for 8 mol% Te4+-doped Cs2ZnCl4 single crystals, which is close to the decay time of some low-dimensional lead halide perovskites that originated from self-trapped excitons.10,35 It can easily be found from Fig. 3b and Table S3 (ESI†) that, upon 454 nm excitation, the decay times of the intrinsic radiation for various concentration of Te4+ monitored at 570 nm decrease slightly from 62.94 to 50.87 ns with the increase in the concentration of Te4+, which is related to the increased non-radiative transition. Given the intense distortion of the tetrahedra caused by the introduction of Te4+ into the 0D all-inorganic cesium zinc chloride, we sought to investigate the origin of the broadband yellow-light photoluminescence of Te4+-doped Cs2ZnCl4 single crystals. In the cases of Sb3+- and Ce3+-doped Cs2ZnCl4, the possibility of intrinsic defect-induced emission has been excluded.21,36 So here we also ignore the impact of defects on luminescence. On the other hand, the light emission caused by the energy level transition of Te4+ itself always features a long lifetime of tens of microseconds or milliseconds, which is inconsistent with those lifetimes we obtained.37–39 Thus, the lifetime of Te4+ ions is strongly dependent on the crystal field in the host lattices. Considering that the emission spectra of Cs2ZnCl4:Te4+ single crystals possess the characteristics of broadband emission and a large Stokes shift, it is reasonable to attribute the emission centered at 570 nm to the recombination of STEs. Actually, the pristine Cs2ZnCl4 single crystals give a weak narrowband emission at 491 nm upon 267 nm excitation (Fig. S8, ESI†) that has been defined as originating from their STEs.21,36 Additionally, the luminescence is too weak to be observed by the naked eye. Based on the above mentioned factors, we ascribe the low-energy broadband yellow-light emission peak at 570 nm and the small high-energy shoulder peak at 470 nm of Cs2ZnCl4:Te4+ single crystals to the recombination of STEs, which are formed by the strong lattice vibration originating from the distorted [TeCl4] and [ZnCl4]2− units in the Te4+-doped Cs2ZnCl4 single crystals. For Cs2ZnCl4, the STEs produced by the doping of Te4+ ions cause a greater Stokes shift, broaden the emission spectra and greatly enhance the intensity. And as for the characteristics of fluorescence emission, the average slightly longer lifetimes of Cs2ZnCl4:Te4+ with different Te4+ concentrations compared to previously reported lead halide perovskites can presumably be attributed to the populated self-trapped excitons.
Photographs and color coordinates of WLEDs made from YAG:Ce3+, the as-fabricated WLEDs driven at 30 mA current and Cs2ZnCl4:Te4+ single crystals are shown together in Fig. 3d. The broadband light emission was determined to have Commission International de l’Eclairage (CIE) chromaticity coordinates of (0.4578, 0.5126) for Cs2ZnCl4:Te4+ single crystals, indicating that it could be a potential yellow phosphor. To demonstrate the commercial value of Cs2ZnCl4:Te4+, a white LED was fabricated by combining Cs2ZnCl4:Te4+ powder with a blue InGaN LED (450 nm) chip. The emission spectrum of the WLED is shown in Fig. 3c. The white light generated shows CIE chromaticity coordinates (x = 0.3422, y = 0.3543) and a correlated color temperature (CCT) of 5116 K, which is wamer than a WLED fabricated with YAG:Ce3+ with CIE chromaticity coordinates (x = 0.3101, y = 0.4087) and a CCT of 8312 K. The obtained results indicate that the as-synthesized Cs2ZnCl4:Te4+ is a potential candidate yellow phosphor that can be applied to white WLEDs.
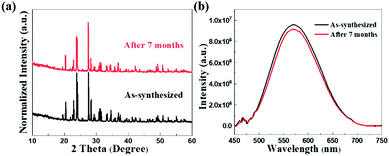
In addition to excellent PL properties as a broadband yellow phosphor, Cs2ZnCl4:Te4+ single crystals also have good humidity stability, as certified by the unchanged PXRD patterns after exposure to the ambient atmosphere for 7 months, overcoming the moisture sensitivity of lead halide perovskites (Fig. 4a). Likewise, the PL intensity of the samples also remained almost unchanged after being stored in air for 7 months. The above results confirm that the Te4+-doped Cs2ZnCl4 single crystals have excellent air stability. Additionally, we combined thermogravimetric and differential thermal analysis (TG-DTA) of Cs2ZnCl4 and Cs2ZnCl4:Te4+ single crystals to investigate their thermal stability. The corresponding test results show that they both have a water loss platform up to about 600 °C and then undergo a phase transition up to 800 °C (Fig. S9, ESI†). Thus, although the introduction of Te4+ ions into Cs2ZnCl4 single crystals brings about a change in the photoluminescence property, it still retains the inherent stability of the matrix. The temperature-dependent spectra indicate that no spectral shift occurred and the spectral shapes were perfectly preserved at various temperatures (Fig. S10, ESI†). The PL intensity retained 73% of the initial value at 343 K. Therefore, Te4+-doped 0D Cs2ZnCl4 metal halide single crystals display impressive stability, which lays a solid foundation for their application in actual light-emitting devices.
Conclusions
In conclusion, via incorporating Te4+ into the tetrahedral coordination environment in zero-dimensional Cs2ZnCl4 through simple and convenient hydrothermal synthesis, structurally stable and broadband yellow light-emitting single crystals covering the range from 450 nm to 700 nm (Cs2ZnCl4:Te4+) have been obtained successfully. After carefully analyzing the crystallographic structures of both the pristine and Te4+-doped Cs2ZnCl4 single crystals, it is easy to see that the [ZnCl4]2− tetrahedra in the lattice of Cs2ZnCl4:Te4+ become more distorted, whether from changes in bond lengths or angles; this is beneficial for exciton trapping and STE emission. In particular, this broadband yellow phosphor can be excited by a wide range of blue light, so it is able to perfectly adapt to blue light-emitting LED chips which have been mass produced industrially, allowing the assembly of natural-colored WLEDs and thus serving as a potential alternative for commercial applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation (NSF) of China (52172152, 51572200), Key Projects of NSF of Zhejiang Province (LZ20E020003), and Wenzhou Major Scientific and Technological Innovation Project (G20170005).Notes and references
- V. Morad, Y. Shynkarenko, S. Yakunin, A. Brumberg, R. D. Schaller and M. V. Kovalenko, J. Am. Chem. Soc., 2019, 141, 9764–9768 CrossRef CAS PubMed.
- Y. Jiang, X. Wang and A. Pan, Adv. Mater., 2019, 31, 1806671 CrossRef CAS.
- M. I. Saidaminov, J. Almutlaq, S. Sarmah, I. Dursun, A. A. Zhumekenov, R. Begum, J. Pan, N. Cho, O. F. Mohammed and O. M. Bakr, ACS Energy Lett., 2016, 1, 840–845 CrossRef CAS.
- B. Philippe, B. W. Park, R. Lindblad, J. Oscarsson, S. Ahmadi, E. M. J. Johansson and H. Rensmo, Chem. Mater., 2015, 27, 1720–1731 CrossRef CAS.
- Z. Xiao, Z. Song and Y. Yan, Adv. Mater., 2019, 31, 1803792 CrossRef CAS PubMed.
- S. Attique, N. Ali, S. Ali, R. Khatoon, N. Li, A. Khesro, S. Rauf, S. Yang and H. Wu, Adv. Sci., 2020, 7, 1903143 CrossRef CAS.
- P. P. Sun, Q. S. Li, L. N. Yang and Z. S. Li, Nanoscale, 2016, 8, 1503–1512 RSC.
- L. Liang and P. Gao, Adv. Sci., 2018, 5, 1700331 CrossRef.
- Z. Wu, Q. Zhang, B. Li, Z. Shi, K. Xu, Y. Chen, Z. Ning and Q. Mi, Chem. Mater., 2019, 31, 4999–5004 CrossRef CAS.
- Z. Yuan, C. Zhou, Y. Tian, Y. Shu, J. Messier, J. C. Wang, L. J. van de Burgt, K. Kountouriotis, Y. Xin, E. Holt, K. Schanze, R. Clark, T. Siegrist and B. Ma, Nat. Commun., 2017, 8, 14051 CrossRef CAS.
- H. Peng, S. Yao, Y. Guo, R. Zhi, X. Wang, F. Ge, Y. Tian, J. Wang and B. Zou, J. Phys. Chem. Lett., 2020, 11, 4703–4710 CrossRef CAS PubMed.
- L. Zhou, J. F. Liao, Z. G. Huang, J. H. Wei, X. D. Wang, W. G. Li, H. Y. Chen, D. B. Kuang and C. Y. Su, Angew. Chem., Int. Ed., 2019, 58, 5277–5281 CrossRef CAS PubMed.
- C. Zhou, M. Worku, J. Neu, H. Lin, Y. Tian, S. Lee, Y. Zhou, D. Han, S. Chen, A. Hao, P. I. Djurovich, T. Siegrist, M. H. Du and B. Ma, Chem. Mater., 2018, 30, 2374–2378 CrossRef CAS.
- B. M. Benin, D. N. Dirin, V. Morad, M. Wörle, S. Yakunin, G. Rainò, O. Nazarenko, M. Fischer, I. Infante and M. V. Kovalenko, Angew. Chem., Int. Ed., 2018, 57, 11329–11333 CrossRef CAS PubMed.
- K. Takahashi, M. Arai, M. Koshimizu, Y. Fujimoto, T. Yanagida and K. Asai, Jpn. J. Appl. Phys., 2020, 59, 072002 CrossRef CAS.
- N. Yahaba, M. Koshimizu, Y. Sun, T. Yanagida, Y. Fujimoto, R. Haruki, F. Nishikido, S. Kishimoto and K. Asai, Appl. Phys. Express, 2014, 7, 062602 CrossRef CAS.
- X. Zhang, L. Li, Z. Sun and J. Luo, Chem. Soc. Rev., 2019, 48, 517–539 RSC.
- D. Zhu, M. L. Zaffalon, V. Pinchetti, R. Brescia, F. Moro, M. Fasoli, M. Fanciulli, A. Tang, I. Infante, L. D. Trizio, S. Brovelli and L. Manna, Chem. Mater., 2020, 32, 5897–5903 CrossRef CAS.
- V. Morad, I. Cherniukh, L. Pöttschacher, Y. Shynkarenko, S. Yakunin and M. V. Kovalenko, Chem. Mater., 2019, 31, 10161–10169 CrossRef CAS PubMed.
- X. Wang, Q. Shen, Y. Chen, N. Ali, Z. Ren, G. Bi and H. Wu, Nanoscale, 2021, 13, 15285–15291 RSC.
- B. Su, M. Li, E. Song and Z. Xia, Adv. Funct. Mater., 2021, 31, 2105316 CrossRef.
- J. Liang, L. Ying, F. Huang and Y. Cao, J. Mater. Chem. C, 2016, 4, 10993–11006 RSC.
- L. C. Chen, Z. L. Tseng, W. W. Chang and Y. W. Lin, Ceram. Int., 2018, 44, 3868–3872 CrossRef CAS.
- B. Janjua, T. K. Ng, C. Zhao, A. Prabaswara, G. B. Consiglio, D. Priante, C. Shen, R. T. Elafandy, D. H. Anjum, A. A. Alhamoud, A. A. Alatawi, Y. Yang, A. Y. Alyamani, M. M. El-Desouki and B. S. Ooi, ACS Photonics, 2016, 3, 2089–2095 CrossRef CAS.
- E. R. Dohner, E. T. Hoke and H. I. Karunadasa, J. Am. Chem. Soc., 2014, 136, 1718–1721 CrossRef CAS.
- L. Mao, Y. Wu, C. C. Stoumpos, M. R. Wasielewsk and M. G. Kanatzidis, J. Am. Chem. Soc., 2017, 139, 5210–5215 CrossRef CAS PubMed.
- C. Peng, Z. Zhuang, H. Yang, G. Zhang and H. Fei, Chem. Sci., 2018, 9, 1627–1633 RSC.
- T. Chang, Q. Wei, R. Zeng, S. Cao, J. Zhao and B. Zou, J. Phys. Chem. Lett., 2021, 12, 1829–1837 CrossRef CAS PubMed.
- E. R. Dohner, A. Jaffe, L. R. Bradshaw and H. I. Karunadasa, J. Am. Chem. Soc., 2014, 136, 13154–13157 CrossRef CAS.
- D. Cortecchia, J. Yin, A. Bruno, S. Z. A. Lo, G. G. Gurzadyan, S. Mhaisalkar, J. L. Brédas and C. Soci, J. Mater. Chem. C, 2017, 5, 2771–2780 RSC.
- R. Zhang, X. Mao, Y. Yang, S. Yang, W. Zhao, T. Wumaier, D. Wei, W. Deng and K. Han, Angew. Chem., 2019, 131, 2751–2755 CrossRef.
- M. D. Smith and H. I. Karunadasa, Acc. Chem. Res., 2018, 51, 619–627 CrossRef CAS.
- T. V. Sedakova and A. G. Mirochnik, Opt. Spectrosc., 2016, 120, 268–273 CrossRef CAS.
- Y. Jing, Y. Liu, J. Zhao and Z. Xia, J. Phys. Chem. Lett., 2019, 10, 7439–7444 CrossRef CAS PubMed.
- M. D. Smith, B. L. Watson, R. H. Dauskardt and H. I. Karunadasa, Chem. Mater., 2017, 29, 7083–7087 CrossRef CAS.
- K. Sugawara, M. Koshimizu, T. Yanagida, Y. Fujimoto, R. Haruki, F. Nishikido, S. Kishimoto and K. Asai, Opt. Mater., 2015, 41, 53–57 CrossRef CAS.
- Y. Fujimoto, K. Saeki, D. Nakauchi, H. Fukada, T. Yanagida, H. Kawamoto, M. Koshimizu and K. Asai, Mater. Res. Bull., 2018, 105, 291–295 CrossRef CAS.
- A. Yao, X. Zhou, W. Wu, H. Song, Y. Hong, S. Hu, B. Wang, S. Lu and Y. Wang, Chem. Phys., 2021, 546, 111170 CrossRef CAS.
- H. Song, W. Wu, Z. Xing, X. Zhou, A. Yao, S. Hu, Y. Hong, B. Wang, S. Lu and Y. Wang, Ceram. Int., 2021, 47, 17286–17292 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Crystal data and structure refinement data, change of bond angles, ICP, EDS, and XPS analysis, FT-IR and Raman spectra, elemental mapping, TG-DTA data for Te4+-doped Cs2ZnCl4, and PL and PLE data for Cs2ZnCl4. See DOI: 10.1039/d1tc04990a |
| This journal is © The Royal Society of Chemistry 2022 |