A high refractive index resist for UV-nanoimprint soft lithography based on titanium-containing elemental polymer oligomers†
Junlun
Qiu
 ab,
Getian
Hu
c,
Yuqiao
Wang
ab,
Getian
Hu
c,
Yuqiao
Wang
 a,
Yurui
Wang
a,
Ming
Luo
*a and
Xin
Hu
a,
Yurui
Wang
a,
Ming
Luo
*a and
Xin
Hu
 *a
*a
aSchool of Materials Engineering, Changshu Institute of Techonology, 99 South Sanhuan Road, Changshu 215500, China. E-mail: mingluo@cslg.edu.cn; huxin@cslg.edu.cn
bDepartment of Materials and Chemistry, Soochow University, 199 Ren'ai Road, Suzhou 215123, China
cDepartment of Biological Sciences, Xi'an Jiaotong-Liverpool University, 111 Ren'ai Road, Suzhou 215123, China
First published on 22nd November 2021
Abstract
This paper demonstrates a novel resist with a high refractive index for UV-curing nanoimprint lithography based on an o-phenoxyphenyl acrylated polytitanoxane oligomer. The oligomer is synthesized by hydrolysis and condensation of Ti-OEt and the o-phenoxyphenyl groups are grafted onto the backbone via the reaction between o-phenylphenoxyethyl acrylate (OPPEA) and titanium(IV) ethoxide. Acrylate groups are introduced by the chelate reaction between titanium(IV) ethoxide and 2-(methacryloyloxy)ethyl acetoacetate (MAEAA). The chelate rings and benzyl rings greatly improve the resin stability: the resin retains solubility in organic solvents even when it is stored in an air atmosphere for at least 48 hours at room temperature. The resist has an excellent UV transmittance (80% at 365 nm), a high modulus (1.97 GPa) and a high refractive index (1.7063 at 589.3 nm), and the refractive index can be further improved to 1.7327 after baking. A UV-nanoimprint protocol is also optimized and high-resolution patterns with 50 nm line width are faithfully imprinted into the resist film. Furthermore, the resin patterns can be used as a mask for duplicating more PDMS molds, which greatly reduces the risk of master damage.
1. Introduction
Patterned high refractive index polymers have been increasingly attractive to researchers in both academic and industrial fields due to their potential applications in various advanced optical devices, i.e., photonic crystals,1–3 metamaterials,4 information storage media,5–8 organic optoelectronic devices9 and sensors.10–12 Refractive indices of most polymers are unable to meet the requirement of high-performance applications such as anti-refractive coatings, microlenses for CMOS image sensors, encapsulants for LEDs, and high-n thermoplastic lenses.13 Nanoimprint lithography is widely known as a low-cost, high-throughput method to fabricate sub-wavelength patterns for optical devices. Polymer patterns are obtained by pressing a mold into a polymer film spin-coated on a substrate. Researchers have developed quite mature techniques including mold fabrication and surface treatment, resists with different RIE selectivities and imprint machines. Unfortunately, a high refractive index resist for UV-curing nanoimprint lithography is still a great challenge and quite undeveloped.Thermal nanoimprint using thermoplastic materials requires high temperature, high pressure and special equipment, as well as a very time-consuming heating–cooling cycle. Consequently, UV-curing nanoimprint lithography (UV-NIL) using a soft mold has been one of the most attractive nanoimprint technologies. In this process, the resist is spin-coated onto a wafer, covered with a mold and UV-cured at room temperature. So, it is vital to develop a high refractive index UV-NIL resist for advanced optical device fabrication.
The design of high refractive index polymers is usually based on the Lorentz–Lorenz equation, which means that the refractive index of a polymer increases when substituents with high molar refractions and low molar volumes are introduced.14 Hence, aromatic rings,15,16 halogen atoms except for fluorine,17 and sulfur atoms18,19 are the most common fragments introduced into molecular structures to synthesize high-n polymers. Recently, acrylate polymers containing sulfur atoms and rigid aromatic rings have been used to prepare high refractive index resists for UV-curing nanoimprint (n = 1.6363 and 1.6444)20,21 and thermal nanoimprint (n = 1.707).22 These resists are synthesized by expensive reactants, complicated reaction steps (i.e. further purified by column chromatography), and a lot of organic solvent is consumed, which make the process high cost, time consuming and environmentally unfriendly. Although sulfur-rich materials19 and sulfur-containing aromatic polyimides23 are synthesized and achieve a refractive index of more than 1.72, the solid material is unsuitable for UV-curing nanoimprint. Some special polymeric materials, such as poly(arylene sulfide sulfone) containing pyrimidine unit, polythiophene and conjugated polymers, have a refractive index higher than 1.7,24 but they are impossible to be formulated to prepare UV-curing nanoimprint resists due to their high glass transition temperature and lack of photo-curing functional groups.
Thanks to the rapid development of nanotechnology, high refractive index nanocomposites have been developed by dispersing inorganic nanoparticles (such as TiO2, ZrO2, PbS or ZnS) in organic polymer matrixes. TiO2 nanoparticles are widely used to improve the nanocomposite refractive index due to their low cost, non-toxicity and easy preparation in both the laboratory and industry. For example, highly crystalline TiO2 nanoparticles were synthesized by a solvothermal method and surface modified to be well dispersed into a commercial epoxy resin to improve the refractive index from 1.5 to 1.8.25 TiO2 nanoparticles were also synthesized using a sol–gel technique and dispersed in an acrylic monomer to prepare UV-curable hybrid films.26 So far, the sol–gel approach, hydrothermal reaction and solvothermal reaction have been the most common methods to synthesize oxide nanoparticle dispersions. However, the procedures are usually complicated, time consuming and environmentally unfriendly. All the particles should be separated, washed, surface modified and re-dispersed in a suitable solvent, which is very time consuming and gives extremely low throughput. When particle dispersion is used to improve the refractive index with high particle content, the resist may suffer from poor processability, storage stability and higher optical loss. In UV-nanoimprint lithography, two shortcomings of polymer/TiO2 hybrid materials are difficult to overcome: (1) high TiO2 content not only improves the refractive index but also greatly reduces UV transparency which is vital to UV-curing; (2) particle aggregation is unavoidable at high nanoparticle concentrations, and high-resolution patterns are very difficult to imprint.
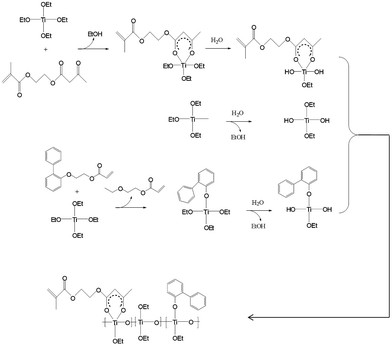
In this paper, we demonstrate a low-cost and high-throughput method to fabricate a high refractive index resist for UV-NIL combining the advantage of a pure organic polymer resist and an organic/inorganic hybrid composite. A very simple and environmentally friendly strategy is suggested to prepare a UV-curable high refractive index Ti-containing resin (Ti-resin) via stirring at room temperature and evaporation. The product can be directly used to formulate a UV-nanoimprint resist without further purification. The resin is highly UV transparent and can be UV-cured in the presence of a UV initiator. The synthesis reaction mechanism is suggested in Scheme 1. The protocol and formulations were optimized to improve the moisture stability, refractive index and UV transparency. Additionally, a PDMS mold can be directly used without further anti-stick treatment and the imprinted resist can be directly used as the mask to duplicate a new PDMS mold, which greatly reduces the risk of original master damage.
2. Experimental
2.1 Chemicals
Titanium(IV) ethoxide and 2-(methacryloyloxy)ethyl acetoacetate (MAEAA) were purchased from Macklin, China. O-Phenylphenoxyethyl acrylate (OPPEA) was a commercial product of Miwon Commercial Co., Ltd, Korea. The UV initiator Irgacure 184 was purchased from Ciba. All other chemicals were obtained commercially from Sinopharm Chemical Co, Ltd (China) and were utilized without further refinement.2.2 Synthesis of Ti-resist
Titanium(IV) ethoxide (9.03 g, 39.58 mmol), MAEAA (1.01 g, 4.71 mmol) and OPPEA (6.00 g, 22.35 mmol) were added to a conical flask equipped with magnetic stirring. Afterward, deionized water (0.71 g, 39.24 mmol) was slowly added to the system at room temperature. After water was completely added, the liquid was continuously stirred at room temperature for 1 hour. A yellow viscous liquid (9.76 g) was obtained called Ti-resin after rotary evaporation. The Ti-resist was prepared by dissolving Ti-resin with Irgacure 184 (3 wt%, vs. Ti-resin) in xylene.2.3 Characterization
Fourier transform infrared spectroscopy (FTIR) spectra and solution state 1H NMR spectra were recorded using a ThermoFisher Nicolet IS10 spectrometer and a Bruker DRX-300 spectrometer, respectively. FTIR spectra were measured at 4 cm−1 resolution at 10 scans per point using an IR microscope. In 1H NMR characterization, all chemical shifts were reported in ppm downfield from TMS using the residual protonated solvent as an internal standard (CDCl3, 1H 7.29 ppm). The morphology observation was conducted with a scanning electron microscope (SEM, ULTRA55, Zeiss and Helios Nanolab 600i, FEI) and an atomic force microscope (AFM, XE7, Park). The modulus of the UV-cured Ti-resist was measured using the contact mode of AFM. The refractive indices of resist films were measured using an ellipsometer (RC2-XI, J.A. Woollam Co. Ltd). The UV-vis spectra were monitored with a fiber optic spectrometer (PG2000-Pro-EX, Ideaoptics, China). The contact angles were measured using a DropMaster 300 contact angle goniometer and water droplets of deionized water.2.4 High-resolution imprint and mold duplicate
PDMS (Sylgard 184, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 curing agent to prepolymer weight ratio) was cast onto an anti-stick treated silicon mask. Then, it was degassed in a vacuum desiccator at room temperature for 30 min and heated in an oven at 60 °C for 240 minutes, respectively. After it was cooled to room temperature, PDMS was peeled off the silicon mask and the patterns were duplicated to the PDMS membrane.
10 curing agent to prepolymer weight ratio) was cast onto an anti-stick treated silicon mask. Then, it was degassed in a vacuum desiccator at room temperature for 30 min and heated in an oven at 60 °C for 240 minutes, respectively. After it was cooled to room temperature, PDMS was peeled off the silicon mask and the patterns were duplicated to the PDMS membrane.
Silicon wafers were cleaned using piranha solution (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 by volume of 30% H2O2 and H2SO4) to remove surface organic contaminants. This was followed by washing with deionized water and blow-drying using a nitrogen gas gun. After cleaning, the Ti-resist was spin-coated on the cleaned silicon wafer. Then, the resist film was covered with a PDMS mold, heated in a hot stage, and irradiated with a 100 W 365 nm UV LED lamp in a nitrogen atmosphere for 5 minutes, respectively. In the end, the PDMS mold was peeled off and a resist pattern was obtained.
7 by volume of 30% H2O2 and H2SO4) to remove surface organic contaminants. This was followed by washing with deionized water and blow-drying using a nitrogen gas gun. After cleaning, the Ti-resist was spin-coated on the cleaned silicon wafer. Then, the resist film was covered with a PDMS mold, heated in a hot stage, and irradiated with a 100 W 365 nm UV LED lamp in a nitrogen atmosphere for 5 minutes, respectively. In the end, the PDMS mold was peeled off and a resist pattern was obtained.
3. Results and discussion
3.1 Synthesis of Ti-resin
In our previous work, we synthesized a photo-curable Ti-containing monomer by the chelate reaction between titanium(IV) ethoxide and 2-(methacryloyloxy)ethyl acetoacetate with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.27 The resist was used to imprint nanopatterns on both planer and curved substrates using a visible light initiator system due to its UV opaque. Unfortunately, the refractive index was as low as about 1.55 at 593 nm.
2.27 The resist was used to imprint nanopatterns on both planer and curved substrates using a visible light initiator system due to its UV opaque. Unfortunately, the refractive index was as low as about 1.55 at 593 nm.
In this work, the refractive index was improved based on a new strategy: benzyl groups were introduced onto the side of the backbone which not only improved the refractive index but also retained high solubility in organic solvents. The alkyl group content was also reduced via controlled hydrolysis and condensation of titanium(IV) ethoxide in the presence of acrylate chelate, which improved the refractive index, and retained the moisture stability in UV-curable films simultaneously. In detail, titanium(IV) ethoxide was used for building the target polytitanoxane backbone. Acrylate groups were introduced into polytitanoxane by the chelate reaction between an acrylate β-diketone MAEAA and titanium(IV) ethoxide. The diketone also greatly reduced hydrolysis and condensation of titanium(IV) ethoxide at room temperature.
Ti(OEt)4 was first stirred with MAEAA and OPPEA in a conical bottle at room temperature. Then, deionized water was slowly added to the mixture and was continuously stirred for 60 minutes. This process should be carefully handled to avoid vigorous hydrolysis and the condensation reaction which forms TiO2-like particles. During the reaction, Ti-OEt was converted to Ti–OH in the presence of water, but the condensation was hindered by the chelate rings. The chelate reaction can also be observed by the color: after Ti(OEt)4 was mixed with MAEAA for a few minutes, the mixture became clear and the color turned yellow.
When we started this project, OPPEA was designed as a high refractive index active diluent, but it was not the case. In the synthesis of Ti-resin, the total amount of Ti(OEt)4, OPPEA and MAEAA was 16.91 g, while the product quality was 9.76 g and the weight loss was 6.28 g. If Ti(OEt)4 (39.58 mmol) only reacted with MEAEA (4.71 mmol) and H2O (39.24 mmol), the weight loss should be 3.12 g (0.22 g + 2.90 g), which is much less than the investigated weight loss. So, the reaction mechanism should be carefully reconsidered which is vital to future quality control. At first, OPPEA was designed to serve as a reactive high refractive index monomer to reduce viscosity. But FTIR and 1H NMR gave a different result and the reaction mechanism (Scheme 1) was suggested based on the spectra in Fig. 1. From the FTIR spectra of OPPEA, MAEAA, mixture before reaction and Ti-resin (Fig. 1a), it can be observed that the absorption of Ti-resin at 1725 cm−1 is quite weak, which means that most C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O disappeared after the reaction. Simultaneously, the absorption of C–O in ether at 1180 cm−1 disappeared in Ti-resin. So, it is reasonable to assume that OPPEA reacted with Ti(OEt)4 and gave titanium phenoxide and 2-ethoxyethyl acrylate (EEA). The latter was slowly removed by rotary evaporation and the absorption of C
O disappeared after the reaction. Simultaneously, the absorption of C–O in ether at 1180 cm−1 disappeared in Ti-resin. So, it is reasonable to assume that OPPEA reacted with Ti(OEt)4 and gave titanium phenoxide and 2-ethoxyethyl acrylate (EEA). The latter was slowly removed by rotary evaporation and the absorption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1640 cm−1 was greatly reduced. The absorption of about 1615 cm−1 is attributed to the vibration of C
C at 1640 cm−1 was greatly reduced. The absorption of about 1615 cm−1 is attributed to the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the six-membered ring. Fig. 1b gives more structural information that the resin is a mixture of Ti-containing an oligomer and a residual by-product EEA. Peaks from 7.56 to 6.98 are attributed to the protons on the benzene rings, and peaks at 4.421–3.389 ppm are attributed to the protons of –CH2– in the reactants, products and by-products. More information can be found from other peaks. Chemical shifts from 5.63 to 5.55 are attributed to a proton of CH2 in CH2
C in the six-membered ring. Fig. 1b gives more structural information that the resin is a mixture of Ti-containing an oligomer and a residual by-product EEA. Peaks from 7.56 to 6.98 are attributed to the protons on the benzene rings, and peaks at 4.421–3.389 ppm are attributed to the protons of –CH2– in the reactants, products and by-products. More information can be found from other peaks. Chemical shifts from 5.63 to 5.55 are attributed to a proton of CH2 in CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH3)COO–, and peaks at 1.25 ppm are attributed to –CH3 in Ti(OEt)4. It can also be calculated that the area ratio of 5.6 ppm to 1.94 ppm (CH3 in acrylate) was 1
CH(CH3)COO–, and peaks at 1.25 ppm are attributed to –CH3 in Ti(OEt)4. It can also be calculated that the area ratio of 5.6 ppm to 1.94 ppm (CH3 in acrylate) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, which means that the chelated MAEAA did not polymerize significantly. Additionally, all the chemical shifts of protons in acrylate groups moved to a lower field by 0.1 ppm, which indicates that OPPEA was completely converted. A little amount of the by-product EEA was also found and the chemical shifts of the peaks are illustrated in Fig. 2b. The residual acrylate groups can be linked to the cross-linking matrix and would not be extracted by solvents.
3, which means that the chelated MAEAA did not polymerize significantly. Additionally, all the chemical shifts of protons in acrylate groups moved to a lower field by 0.1 ppm, which indicates that OPPEA was completely converted. A little amount of the by-product EEA was also found and the chemical shifts of the peaks are illustrated in Fig. 2b. The residual acrylate groups can be linked to the cross-linking matrix and would not be extracted by solvents.
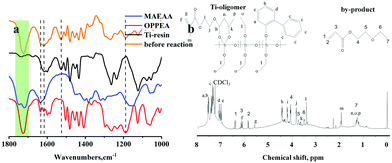 | ||
| Fig. 1 (a) FTIR spectra of MAEAA, OPPEA, mixture before reaction and final Ti-resin; (b) 1H NMR spectra of Ti-resin. | ||
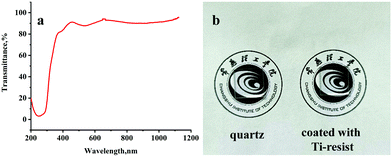 | ||
| Fig. 2 (a) UV-vis spectrum of a quartz wafer coated with 300 nm Ti-resist; (b) optical pictures of a quartz wafer and a quartz wafer coated with Ti-resist. | ||
An Abbe refractometer was used to monitor the condensation reaction during resist synthesis. It was found that the dosage of water greatly influences the reactive index of Ti-resin. When the molar ratio of Ti(OEt)4 to water was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, the refractive index at 589.3 nm (nD) was 1.671. If the molar ratio of Ti(OEt)4 to water increased to 1
0.5, the refractive index at 589.3 nm (nD) was 1.671. If the molar ratio of Ti(OEt)4 to water increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, nD increased to 1.706. A higher ratio was able to further improve the refractive index, but the product turned into a thermoplastic solid due to the higher polymerization degree and possible slight cross-linking. Hence, the molar ratio of Ti(OEt)4 to H2O was optimized to 1
1, nD increased to 1.706. A higher ratio was able to further improve the refractive index, but the product turned into a thermoplastic solid due to the higher polymerization degree and possible slight cross-linking. Hence, the molar ratio of Ti(OEt)4 to H2O was optimized to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
3.2 Preparation and performance of Ti-resist
Ti-resist was prepared by dissolving Ti-resin in xylene (20 wt%) with a UV initiator Irgacure 184 (3 wt%, vs. Ti-resin). After the solvent was spin-coated onto a cleaned quartz wafer with a speed of 3000 rpm for 120 seconds, the UV-vis spectrum was monitored with a fiber-optic spectrometer using a transmission integrating sphere (Fig. 2a). The resist transmittance was greater than 80% over 365 nm, which is able to promise a UV-curing process. In our previous work, we found that the acrylated titanoxane dipolymer is completely UV opaque.28 This shortcoming was overcome by reducing the chelate content. The optical image (Fig. 2b) also shows that the film is colorless and highly transparent.FTIR is a convenient method to investigate UV-curing polymerization kinetics by monitoring the decreased absorption of acrylate double bond C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 1635 cm−1. Unfortunately, the absorption of C
C at 1635 cm−1. Unfortunately, the absorption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C is too weak due to its low content and high absorption of the chelate ring at 1615 cm−1. The FTIR spectra of Ti-resist before and after thermal treatment at 80 °C for 5 minutes are shown in Fig. S1 (ESI†). Two spectra almost coincide, which indicates that Ti-resist is stable to moisture in an air atmosphere even when it is heated. Ti-resist was spin-coated onto silicon wafers and cured with an LED lamp for different times. Afterward, all the wafers were washed with acetone and the optical images are shown in Fig. S2 (ESI†). When the sample was UV-cured for 1 minute, a white powder can be found on the film surface. Ti-resist was not completely cross-linked and the resin rapidly formed TiO2-like particles via hydrolysis and condensation. If the film was UV-cured for more time, the powder amount reduced and completely disappeared and the film color did not change when the resist was cured for 5 minutes. If the resist was completely cross-linked, the Ti-OEt groups would not be able to form Ti–O–Ti bonds even when they are hydrolyzed due to the poor molecular movement ability.
C is too weak due to its low content and high absorption of the chelate ring at 1615 cm−1. The FTIR spectra of Ti-resist before and after thermal treatment at 80 °C for 5 minutes are shown in Fig. S1 (ESI†). Two spectra almost coincide, which indicates that Ti-resist is stable to moisture in an air atmosphere even when it is heated. Ti-resist was spin-coated onto silicon wafers and cured with an LED lamp for different times. Afterward, all the wafers were washed with acetone and the optical images are shown in Fig. S2 (ESI†). When the sample was UV-cured for 1 minute, a white powder can be found on the film surface. Ti-resist was not completely cross-linked and the resin rapidly formed TiO2-like particles via hydrolysis and condensation. If the film was UV-cured for more time, the powder amount reduced and completely disappeared and the film color did not change when the resist was cured for 5 minutes. If the resist was completely cross-linked, the Ti-OEt groups would not be able to form Ti–O–Ti bonds even when they are hydrolyzed due to the poor molecular movement ability.
The double bond concentration is very low in Ti-resist, so the cross-linking mechanism is impossible to be a simple radical approach. Fig. 3a illustrates the FTIR spectrum of Ti-resist films heated at 80 °C and UV irradiated for different times. Absorption at 1720 cm−1 is attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, while the peaks at 1615 cm−1 and 1527 cm−1 are attributed to C
O, while the peaks at 1615 cm−1 and 1527 cm−1 are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, respectively. When Ti-resist was UV irradiated, the open ring of the chelate ring improved the absorption of C
C, respectively. When Ti-resist was UV irradiated, the open ring of the chelate ring improved the absorption of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and meanwhile, the absorption of chelate rings decreased. So, a two-stage cross-linking mechanism is suggested in Fig. 3b: Ti-resist was first partly UV-cured via a radical mechanism in the presence of Irgacure 184. A large proportion of chelate rings opened gradually under UV irradiation, and the resist was fully cross-linked via hydrolysis and condensation of Ti-OEt groups. The modulus of the cured Ti-resist was also high enough (1.97 GPa) for future high-resolution nanoimprint.
O, and meanwhile, the absorption of chelate rings decreased. So, a two-stage cross-linking mechanism is suggested in Fig. 3b: Ti-resist was first partly UV-cured via a radical mechanism in the presence of Irgacure 184. A large proportion of chelate rings opened gradually under UV irradiation, and the resist was fully cross-linked via hydrolysis and condensation of Ti-OEt groups. The modulus of the cured Ti-resist was also high enough (1.97 GPa) for future high-resolution nanoimprint.
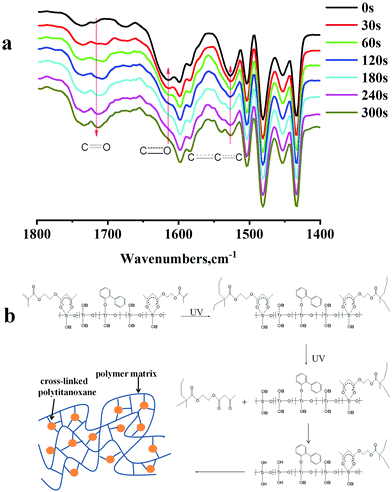 | ||
| Fig. 3 (a) FTIR spectrum of the UV-cured Ti-resist film at 80 °C for different times; (b) suggested cross-linking mechanism of Ti-resist. | ||
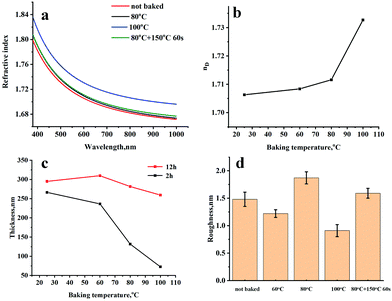
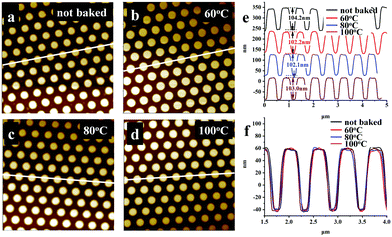
Fig. 4a illustrates the dispersion curves of Ti-resist films baked at different temperatures before UV-curing and an 80 °C baked resist film heated at 150 °C for 60 seconds. The resist viscosity is a little bit high and it should be slightly heated to improve flowability and meet the requirement of nanoimprint using a PDMS soft mold. The refractive index slightly increased from 1.7063 to 1.7116 if the baking temperature was not more than 80 °C, while nD was able to be further improved to 1.7327 if Ti-resist was baked for 5 minutes at 100 °C (Fig. 4b). The resist seems to be not thermally stable, but the stability can be greatly improved by baking before UV-curing. A Ti-resist film was heated at 150 °C for 60 seconds after it was baked at 80 °C and UV-cured. It can be found from Fig. 4a that the two curves almost coincide, which means that baking is able to remove the residual byproduct and thermally unstable compounds. During the synthesis of Ti-resist, rotary evaporation served as a heated vacuum reactor which is more efficient than a three-necked flask. Two resists (evaporated for 2 hours and 12 hours) are coated on a silicon wafer and baked for different times at 80 °C before UV-curing. The two-hour evaporated resist thickness rapidly shrank from 266.4 nm to 72.5 nm when the baking temperature improved from 25 °C to 100 °C. This phenomenon is mainly attributed to the hydrolysis and condensation of Ti-OEt groups and the evaporation of the residual byproduct. Although the condensation ratio was greatly slowed due to the steric hindrance of the six-membered ring chelated structure, the reaction was stimulated at a higher temperature, forms Ti–O–Ti cross-linking bonds and gives H2O during the reaction. When it turns to the resist which was evaporated for 12 hours, OPPEA reacted with it quite completely and most of the byproducts were removed, which greatly improved the resist stability. The film thickness was not remarkably observed when the baking temperature was not higher than 80 °C. Even when the resist film was baked at 100 °C, the film thickness only reduced from 295 nm to 260 nm (Fig. 4c).
In an advanced low film, roughness is vital because light scattering from interface imperfections is one of the main loss mechanisms.29Fig. 4d illustrates the roughness of resist films baked at different temperatures and after thermal treatment which are shown in Fig. 4a. No matter whether they are baked or not, all the films have extremely low roughness ranging from 0.91 to 1.87 nm. The thermal treatment of the UV-cured film reduced the film roughness by 15%.
3.3 High-resolution nanoimprint
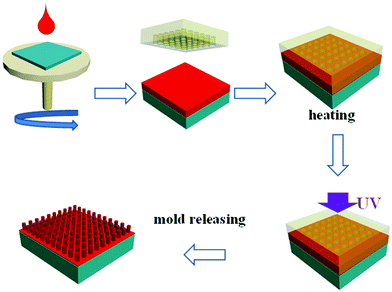
The UV-curing nanoimprint process in this paper is shown in Scheme 2. Ti-resist was spin-coated onto a silicon wafer and covered with a PDMS soft mold. Then, the wafer was heated for 5 minutes and UV irradiated for 5 minutes, respectively. After the wafer was cooled to room temperature, the PDMS mold was peeled off and the Ti-resist pattern was left on the wafer.PDMS molds are widely used in UV-curing nanoimprint lithography because of their low cost and convenient fabrication. Due to the highly improved contact area between the mold structure and the imprinted resist, adhesion force must be reduced to meet the requirement of a smooth de-molding process. When the mold is peeled off the resist, high adhesion may deform the imprinted structures and strip the resist from the substrate. The most successful strategy is grafting a self-assembled fluorinated monolayer onto the mold structure. This process is quite complex and time consuming: the mold surface should be oxidized to introduce Si–OH for the following grafting reaction, and then the fluorosilane coupling agent is grafted onto the surface via condensation between the Si and OH groups. Abrasion of the monolayer during the repeated de-molding process also shortens the mold service life.
The surface energy of Ti-resist is greatly improved and the PDMS mold can be directly used without further anti-stick treatment. The water contact angles of Ti-resist remarkably decreased from 90° to 76° due to the decreased organic content, which improved surface energy contrast between the resist and PDMS mold (108°) and reduced adhesion (Fig. S3, ESI†). A silicon column array was used as the master (Fig. 5a) and the PDMS was cast on the master and cured to fabricate a soft mold (Fig. 5b). The PDMS mold was used as the imprint mold and the pattern was precisely transferred to a Ti-resist film (Fig. 5c). The smooth de-molding process should be attributed to the great surface energy contrast between the PDMS and Ti-resist, the high modulus of the cured Ti-resist, and the high adhesion between the Ti-resist and silicon wafer. Additionally, the mold can be repeatedly used at least 50 times without structural failure.
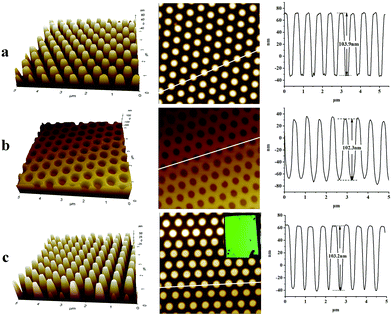
It has become a consensus that high volume shrinkage does harm the imprinted pattern resolution and a number of low curing shrinkage resists have been reported.30–33 In this work, it is not much of a big problem and we managed to develop an optimized protocol to duplicate high-resolution patterns. After Ti-resist was spin-coated onto silicon wafers, the resist films were covered with a PDMS mold with 600 period column structures before baking. Then, the samples were baked at different temperatures for 5 minutes and UV-cured for 5 minutes, respectively. The perspective AFM images and sectional-profile images of the imprinted 600 period column arrays cured under different conditions are shown in Fig. 6a–e. Although the resist film shrank during baking, all the imprinted patterns have a similar shape and baking did not change the pattern morphology: all the column arrays have the same diameter of about 300 nm and the same height of about 100 nm. Whether they are baked or not, all the imprinted pattern profiles almost completely coincided (Fig. 6f). This phenomenon should be attributed to the cross-linking mechanism. The uncured Ti-resist has two reactive groups: C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and Ti-OEt. At room temperature, the hydrolysis of Ti-OEt was hindered by the chelate rings and o-phenoxyphenyl groups, which greatly improved the resist stability in an air atmosphere. When the resist was UV irradiated, the chelate rings opened and Ti-OEt became more active and condensation gradually occurred. With the process of reaction, the molecular weight of Ti-containing molecules increased and began to cross-link. The PDMS mold subsided together with the shrunk film. Consequently, the mold was continuously filled with resists during film shrinkage. The byproduct permeated through the PDMS mold and volatilized in the atmosphere. After UV-curing, Ti-resist was cross-linked via both polymerization of acrylate groups and condensation of Ti–OH, which consequently resulted in a resist matrix.
C and Ti-OEt. At room temperature, the hydrolysis of Ti-OEt was hindered by the chelate rings and o-phenoxyphenyl groups, which greatly improved the resist stability in an air atmosphere. When the resist was UV irradiated, the chelate rings opened and Ti-OEt became more active and condensation gradually occurred. With the process of reaction, the molecular weight of Ti-containing molecules increased and began to cross-link. The PDMS mold subsided together with the shrunk film. Consequently, the mold was continuously filled with resists during film shrinkage. The byproduct permeated through the PDMS mold and volatilized in the atmosphere. After UV-curing, Ti-resist was cross-linked via both polymerization of acrylate groups and condensation of Ti–OH, which consequently resulted in a resist matrix.
High-resolution nanoimprint may be a great challenge when nanoparticles are used to improve the refractive index. Ti-resist was successfully used to fabricate high-resolution patterns which are shown in Fig. 7. Two kinds of soft molds are used to imprint patterns with different resolutions. A grating pattern (550 nm period and 110 nm groove depth) and a column pattern (600 nm period and 100 column height) were imprinted using a PDMS mold. When it turns to higher resolution, the modulus of the PDMS mold cannot meet the requirement of pattern duplication. So, a circle pattern (200 nm period and 50 nm line width) and a dot pattern (200 nm period and 100 nm width) were imprinted using a hybrid soft mold. Fabrication of transparent nanoimprint hybrid soft molds can be found elsewhere.34
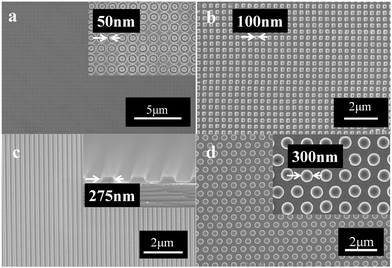 | ||
| Fig. 7 High-resolution imprint patterns: (a) circle pattern, line width 50 nm; (b) dot pattern, 50 nm width; (c) grating pattern, line width 275 nm; (d) dot pattern, diameter 300 nm. | ||
3.4 PDMS mold duplicate
Nanoimprint lithography is a low-cost technique to duplicate nanopatterns, but fabrication of masters is high cost and time consuming because they are usually fabricated by photolithography or e-beam lithography. If a silicon master is stained with PDMS, it is a great challenge to re-new the master. PDMS is a thermoset polymer, so it is impossible to be completely removed using organic solvents. Oxygen plasma or ozone is widely used to clean the surfaces of substrates, but they cannot remove PDMS from the stained master because PDMS would be converted to a SiO2-like material. Although fluorine-based reactive ion etching (RIE) can remove the silicon-containing resist from the mold, it will also permanently damage the structure of the siliceous mold.32Here, the Ti-resist pattern also demonstrates its potential to be a mask to fabricate PDMS molds. The high surface energy of Ti-resist not only reduces the adhesion force between the resist and PDMS but also greatly improves the adhesion between the resist and Si substrate. The former makes the demolding process smoother, and the latter prevents the resist from being peeled off from the substrate. Fig. S4 (ESI†) illustrates an imprinted Ti-resist pattern used as a mask to fabricate the PDMS mold. First, PDMS was cast onto a silicon wafer with 550 nm pitch grating the Ti-resist pattern and cured at 60 °C for 4 hours. When it was cooled to room temperature, the PDMS film was peeled off and a hole pattern on the PDMS was obtained for future application as a soft nanoimprint mold. The 550 nm pitch gratings were faithfully imprinted into the Ti-resist film using the PDMS mold. The pattern can be repeatedly used as a master to fabricate more PDMS molds, which greatly reduced the risk of Si mask damage. Even if the Ti-resist mask is damaged during mold duplication, the Ti-resist mask can be easily fabricated just by repeatedly nanoimprinting.
4. Conclusion
In this paper, we designed and prepared a high refractive index Ti-containing resin (Ti-resin) for UV-curing nanoimprint lithography based on an o-phenoxyphenyl acrylated polytitanoxane oligomer. The refractive index was improved by hydrolysis and condensation of Ti-OEt and grafting the o-phenoxyphenyl groups onto the backbone. The refractive index is improved due to the decrease of organic content and the increase of Ti content. The resist has a high refractive index (1.7063 at 589 nm) which can be further improved to 1.7327 after baking at 80 °C for 5 minutes. The excellent UV transmittance (80% at 365 nm), high modulus (1.97 GPa) and UV-curable films made the resist meet the requirement of UV-nanoimprint, and the high-resolution patterns with a minimum line width of 50 nm were imprinted on a Ti-resist film. Furthermore, Ti-resin patterns can also be used as a mask to fabricate more and more new PDMS molds, which greatly reduces the risk of Si mask damage.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Key Research and Development Plan of Jiangsu Province (Grant No. BE2019002) and the National Natural Science Foundation of China (Grant No. 21704007). We also thank Ideaoptics Co. Ltd, who provided free service for ellipsometer measurement.References
- P. G. Hermannsson, K. T. Sorensen, C. Vannahme, C. L. Smith, J. J. Klein, M. M. Russew, G. Grutzner and A. Kristensen, All-polymer photonic crystal slab sensor, Opt. Express, 2015, 23(13), 16529–16539 CrossRef CAS.
- K. Luo, S. Zhou and L. Wu, High refractive index and good mechanical property UV-cured hybrid films containing zirconia nanoparticles, Thin Solid Films, 2009, 517(21), 5974–5980 CrossRef CAS.
- S. Gazzo, G. Manfredi, R. Pötzsch, Q. Wei, M. Alloisio, B. Voit and D. Comoretto, High refractive index hyperbranched polyvinylsulfides for planar one-dimensional all-polymer photonic crystals, J. Polym. Sci., Part B: Polym. Phys., 2016, 54(1), 73–80 CrossRef CAS.
- M. Choi, S. H. Lee, Y. Kim, S. B. Kang, J. Shin, M. H. Kwak, K. Y. Kang, Y. H. Lee, N. Park and B. Min, A terahertz metamaterial with unnaturally high refractive index, Nature, 2011, 470(7334), 369–373 CrossRef CAS.
- Q. Dong, G. Li, C.-L. Ho, M. Faisal, C.-W. Leung, P. W.-T. Pong, K. Liu, B.-Z. Tang, I. Manners and W.-Y. Wong, A Polyferroplatinyne Precursor for the Rapid Fabrication of L10-FePt-type Bit Patterned Media by Nanoimprint Lithography, Adv. Mater., 2012, 24(8), 1034–1040 CrossRef CAS.
- Z. Meng, G. Li, S. C. Yiu, N. Zhu, Z. Q. Yu, C. W. Leung, I. Manners and W. Y. Wong, Nanoimprint Lithography-Directed Self-Assembly of Bimetallic Iron-M (M = Palladium, Platinum) Complexes for Magnetic Patterning, Angew. Chem., Int. Ed., 2020, 59(28), 11521–11526 CrossRef CAS.
- Q. Dong, Z. Meng, C. L. Ho, H. Guo, W. Yang, I. Manners, L. Xu and W. Y. Wong, A molecular approach to magnetic metallic nanostructures from metallopolymer precursors, Chem. Soc. Rev., 2018, 47(13), 4934–4953 RSC.
- Z. Meng, C.-L. Ho, H.-F. Wong, Z.-Q. Yu, N. Zhu, G. Li, C.-W. Leung and W.-Y. Wong, Lithographic patterning of ferromagnetic FePt nanoparticles from a single-source bimetallic precursor containing hemiphasmidic structure for magnetic data recording media, Sci. China Mater., 2018, 62(4), 566–576 CrossRef.
- Z. Meng, K. Fu, Y. Zhao, Y. Zhang, Z. Wei, Y. Liu, X.-K. Ren and Z.-Q. Yu, Aggregation-induced red-shifted emission and fluorescent patterning of poly(aryleneethynylene) with a lateral AIEgen substituent, J. Mater. Chem. C, 2020, 8(3), 1010–1016 RSC.
- I. Alessandri and J. R. Lombardi, Enhanced Raman Scattering with Dielectrics, Chem. Rev., 2016, 116(24), 14921–14981 CrossRef CAS PubMed.
- F. Fathi, M. R. Rashidi, P. S. Pakchin, S. Ahmadi-Kandjani and A. Nikniazi, Photonic crystal based biosensors: Emerging inverse opals for biomarker detection, Talanta, 2021, 221, 121615 CrossRef CAS PubMed.
- F. M. Hinterholzinger, A. Ranft, J. M. Feckl, B. Rühle, T. Bein and B. V. Lotsch, One-dimensional metal–organic framework photonic crystals used as platforms for vapor sorption, J. Mater. Chem., 2012, 22(20), 10356 RSC.
- J.-g. Liu and M. Ueda, High refractive index polymers: fundamental research and practical applications, J. Mater. Chem., 2009, 19(47), 8907–8919 RSC.
- A. Javadi, A. Shockravi, A. Rafieimanesh, A. Malek and S. Ando, Synthesis and structure-property relationships of novel thiazole-containing poly(amide imide)s with high refractive indices and low birefringences, Polym. Int., 2015, 64(4), 486–495 CrossRef CAS.
- T. Badur, C. Dams and N. Hampp, High Refractive Index Polymers by Design, Macromolecules, 2018, 51(11), 4220–4228 CrossRef CAS.
- H. Jiang, X. Pan, N. Li, Z. Zhang, J. Zhu and X. Zhu, Selenide-containing high refractive index polymer material with adjustable refractive index and Abbe's number, React. Funct. Polym., 2017, 111, 1–6 CrossRef CAS.
- T. Higashihara and M. Ueda, Recent Progress in High Refractive Index Polymers, Macromolecules, 2015, 48(7), 1915–1929 CrossRef CAS.
- N.-H. You, T. Higashihara, S. Ando and M. Ueda, Highly refractive polymer resin derived from sulfur-containing aromatic acrylate, J. Polym. Sci., Part A: Polym. Chem., 2010, 48(12), 2604–2609 CrossRef CAS.
- T. S. Kleine, N. A. Nguyen, L. E. Anderson, S. Namnabat, E. A. LaVilla, S. A. Showghi, P. T. Dirlam, C. B. Arrington, M. S. Manchester, J. Schwiegerling, R. S. Glass, K. Char, R. A. Norwood, M. E. Mackay and J. Pyun, High Refractive Index Copolymers with Improved Thermomechanical Properties via the Inverse Vulcanization of Sulfur and 1,3,5-Triisopropenylbenzene, ACS Macro Lett., 2016, 5(10), 1152–1156 CrossRef CAS.
- Y. Tang, S. Cabrini, J. Nie and C. Pina-Hernandez, High-refractive index acrylate polymers for applications in nanoimprint lithography, Chin. Chem. Lett., 2020, 31(1), 256–260 CrossRef CAS.
- H. Kim, H. Yeo, M. Goh, B.-C. Ku, J. R. Hahn and N.-H. You, Preparation of UV-curable acryl resin for high refractive index based on 1,5-bis(2-acryloylenethyl)-3,4-ethylenedithiothiophene, Eur. Polym. J., 2016, 75, 303–309 CrossRef CAS.
- Y. Tang, C. Pina-Hernandez, Q. Niu, J. Nie and S. Cabrini, A novel high-refractive index episulfide-thiol polymer for nanoimprinting optical elements, J. Mater. Chem. C, 2018, 6(32), 8823–8831 RSC.
- H. Kim, B.-C. Ku, M. Goh, H. Yeo, H. C. Ko and N.-H. You, Synthesis and characterization of phosphorus- and sulfur-containing aromatic polyimides for high refractive index, Polymer, 2018, 136, 143–148 CrossRef CAS.
- G. Zhang, H.-h. Ren, D.-s. Li, S.-r. Long and J. Yang, Synthesis of highly refractive and transparent poly(arylene sulfide sulfone) based on 4,6-dichloropyrimidine and 3,6-dichloropyridazine, Polymer, 2013, 54(2), 601–606 CrossRef CAS.
- P. Tao, Y. Li, A. Rungta, A. Viswanath, J. Gao, B. C. Benicewicz, R. W. Siegel and L. S. Schadler, TiO2 nanocomposites with high refractive index and transparency, J. Mater. Chem., 2011, 21(46), 18623–18629 RSC.
- C.-C. Chang, L.-P. Cheng, F.-H. Huang, C.-Y. Lin, C.-F. Hsieh and W.-H. Wang, Preparation and characterization of TiO2 hybrid sol for UV-curable high-refractive-index organic–inorganic hybrid thin films, J. Sol-Gel Sci. Technol., 2010, 55(2), 199–206 CrossRef CAS.
- M. Luo and X. Hu, Direct imprinting of TiO2 patterns on highly curved substrates, J. Vac. Sci. Technol., B: Nanotechnol. Microelectron.: Mater., Process., Meas., Phenom., 2020, 38(6), 062604 CAS.
- X. Zhang, X. Hu, L. Shen, X. Chen and H. Ge, A Tunable Nanoimprint System to Create New Features, Macromol. Mater. Eng., 2018, 303(9), 1800257 CrossRef.
- M. Trost, S. Schröder, T. Feigl, A. Duparré and A. Tünnermann, Influence of the substrate finish and thin film roughness on the optical performance of Mo/Si multilayers, Appl. Opt., 2011, 50(5), C148–C153 CrossRef CAS.
- M. Zhang, S. Jiang, Y. Gao, J. Nie and F. Sun, Design of a disulfide bond-containing photoresist with extremely low volume shrinkage and excellent degradation ability for UV-nanoimprinting lithography, Chem. Eng. J., 2020, 390, 124625 CrossRef CAS.
- H. Lin, X. Wan, X. Jiang, Q. Wang and J. Yin, A Nanoimprint Lithography Hybrid Photoresist Based on the Thiol-Ene System, Adv. Funct. Mater., 2011, 21(15), 2960–2967 CrossRef CAS.
- X. Hu, T. Yang, R. Gu, Y. Cui, C. Yuan, H. Ge, W. Wu, W. Li and Y. Chen, A degradable polycyclic cross-linker for UV-curing nanoimprint lithography, J. Mater. Chem. C, 2014, 2(10), 1836 RSC.
- L. J. G. Carlos Pina-Hernandez and F. Peng-Fei, High-Resolution Functional Epoxysilsesquioxane-Based Patterning Layers for Large-Area Nanoimprinting, ACS Nano, 2010, 4, 4776–4784 CrossRef.
- Z. Li, Y. Gu, L. Wang, H. Ge, W. Wu, Q. Xia, C. Yuan, Y. Chen, B. Cui and R. Stanley Williams, Hybrid Nanoimprint-Soft Lithography with Sub-15 nm Resolution, Nano Lett., 2009, 9(6), 2306–2310 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1tc04578d |
| This journal is © The Royal Society of Chemistry 2022 |