Very intense polarized emission in self-assembled room temperature metallomesogens based on Zn(II) coordination complexes: an experimental and computational study†
Massimo
La Deda
 a,
Giuseppe
Di Maio
a,
Angela
Candreva
a,
Giuseppe
Di Maio
a,
Angela
Candreva
 a,
Benoît
Heinrich
a,
Benoît
Heinrich
 b,
Adelina-Antonia
Andelescu
c,
Evelyn
Popa
c,
Emilie
Voirin
b,
Valentin
Badea
d,
Mario
Amati
b,
Adelina-Antonia
Andelescu
c,
Evelyn
Popa
c,
Emilie
Voirin
b,
Valentin
Badea
d,
Mario
Amati
 e,
Otilia
Costişor
e,
Otilia
Costişor
 c,
Bertrand
Donnio
c,
Bertrand
Donnio
 *b and
Elisabeta I.
Szerb
*b and
Elisabeta I.
Szerb
 *c
*c
aDepartment of Chemistry and Chemical Technologies, University of Calabria, Rende 87036, CS, Italy
bInstitut de Physique et Chimie des Matériaux de Strasbourg (IPCMS), UMR7504, CNRS-Université de Strasbourg, Strasbourg 67034, France. E-mail: bertrand.donnio@ipcms.unistra.fr
c“Coriolan Drăgulescu” Institute of Chemistry, Romanian Academy, 24 Mihai Viteazu Bvd, Timisoara 300223, Romania. E-mail: eszerb@acad-icht.tm.edu.ro
dDepartment of Applied Chemistry and Organic and Natural Compounds Engineering, Politehnica University Timisoara, Carol Telbisz 6, Timisoara RO-300001, Romania
eDipartimento di Scienze, Università degli Studi della Basilicata, Campus di Macchia Romana, Potenza 85100, Italy
First published on 11th November 2021
Abstract
Three room-temperature metallomesogens based on Zn(II) cations pentacoordinated by different chelating N^N^N terpyridines and two monodentate gallate co-ligands were designed and synthesized. Depending on the terpyridine ligand's tip functionality, the complexes self-assemble into either a 3D hexagonal mesophase or into a columnar hexagonal mesophase. A systematic study between the structure (molecular architecture, arrangement of the molecules and columns, and chains in both types of mesophases) and photophysical properties (emission maxima and yields, lifetimes of the excited states in the mesophase), correlated with investigations of single molecules in solution, was conducted. Remarkably, one complex exhibited a record quantum yield of 95.0% in solution, while in the mesophase, despite quenching due to the radiative deactivation from the ILCT state, still retained a considerable emission yield of ϕ = 20.2%. The polarized emission of an oriented film, determined for the first time in columnar metallomesogens, shows a dichroic emission ratio (I||/I⊥) of 1.30. DFT and TDDFT computational studies confirmed the origin of the observed fluorescence anisotropy from the transition dipole moment oriented along the C2 symmetry axis of the molecule combined with a high level of order in the 3D mesophase.
Introduction
Liquid crystals with intrinsic light-emitting properties are of great interest because such light-emitting materials may find a large variety of technological applications in various fields, such as OLEDs,1 chemosensors,2 optical information storage,3 and liquid-crystal displays.4 Despite these thrilling prospects, developing high-performance light-emitting liquid crystals remains a challenging task. Aggregation or self-organization is a spontaneous process in the formation of a mesophase, and incorporating luminophores into mesogens with preservation of both mesomorphism and luminescence properties is highly challenging.Up to now, several synthetic strategies have been pursued with success to combine liquid crystal properties (e.g. anisotropy of the physical properties, fluidity, self-organization, dynamics, self-healing, and stimuli responsiveness) with high emission quantum yield materials. Of special interest here, the use of the unique optical properties of metal ions integrated in metallomesogens (MMs)5 and lanthanidomesogens,6i.e. liquid crystalline d- and f-block metal coordination complexes, respectively, is one of such promising emerging approaches. Initially luminescence in the mesophase for MMs was somehow disregarded, mostly due to their high transition temperatures and poor thermal stabilities. In the last decade, the fine tuning of the molecular structure (nature of the metallic center, nature and functionalities of ligands, the presence of non-innocent counterions for ionic derivatives, etc.) to offer the right intermolecular interactions permitted a substantial decrease of the transition temperatures, obtaining low-temperature or even room-temperature MMs based on linearly coordinated Cu(I)7 or Au(I)7b,8 and Ag(I),9 planar Au(III),10 Pt(II) and Pd(II)11 or Ni(II), Zn(II), and Cu(II)12 and even for bulkier coordination geometries like tetrahedral or trigonal bipyramidal Zn(II),13 Ag(I),14 Cu(I),15 octahedral Ir(III),16 Ru(II)17 or Rh(II)18 metal centres.
Mesophase emission is due to either the intrinsic luminescence of the coordination compound, or is induced or enhanced in condensed states as a consequence of aggregation-induced emission (AIE).19 The latter depends on the supramolecular arrangement or to the restriction of intramolecular motion (RIM),20 due to high mesophase viscosity. In some cases, the luminescence of the coordination compounds is specifically due to a large pattern of conjugated aromatic rings of the ligands; therefore, the role of the metal is purely structural, as it confers stability to the compound, without modifying the electronic structure of the emissive state. Closed-shell d-metal ions, such as Zn(II), are ideal candidates to preserve the intense luminescence of polypyridinate molecules acting as ligands; therefore the challenge is to obtain liquid crystals of such complexes without turning the luminescence off.
Terpyridine (tpy) ligands are suitable for the construction of 2D and 3D programmable ordered “soft” and condensed supramolecular structures when coordinated to transition metals, yielding functional materials that gain interest in several application fields: stimuli responsive and/or self-healing supramolecular gels,21 photoactive supramolecular architectures for optical devices,22 MOFs, and catalysts.23
Previously we showed that lipophilic gallate could be a versatile unit for inducing liquid crystallinity in d-block metal coordination complexes13b eliminating several time consuming and money spending complex organic synthesis steps needed to functionalize ligands. Moreover, the presence of the electron donor benzoate group coordinated to a Zn(II) ion was found to considerably increase the emission quantum yield of the complex (24.5%) with respect to its dichloride precursor (6.4%) in solution. However, in condensed states, despite the reduction of non-radiative de-excitation pathways that occurred in the solid phase, a substantial reduction of the quantum yield (7.1%) due to different kinetics of the excited-state was observed. This prompted us to consider differently functionalized tpy ligands to fine-tune both the hexagonal arrangement in the mesophase and the excited state type, correlating changes in photophysical properties as a function of the supramolecular architecture.
Herein we report the synthesis and characterization of three new Zn(II) coordination complexes, Zn_1-3 represented in Scheme 1, whose liquid crystallinity was induced through the use of two gallate monocoordinating units. The three complexes were mesomorphous over broad temperature ranges, showing a two-dimensional hexagonal columnar mesophase (Colhex) or three-dimensional mesophase made of segmented columns (Mhex). Initially, we investigated the influence of the substituents on the liquid crystalline arrangements and on the luminescence, and then the molecular structure – supramolecular structure – photophysical property relationship. Finally, we selected the complex showing the highest emission quantum yield in the mesophase to evaluate the polarized emission. Polarized photoluminescence from a micropatterned film of columnar liquid crystals is rather difficult to observe, as it requires a high degree of orientational control, and furthermore the emission transition dipole moments give rise to a vector composition whose directions are much more difficult to correlate to the director than in classical nematic liquid crystals.24 The obtained results show that the particular three-dimensional structure of Zn_2 made it possible to obtain a remarkable emission anisotropy value.
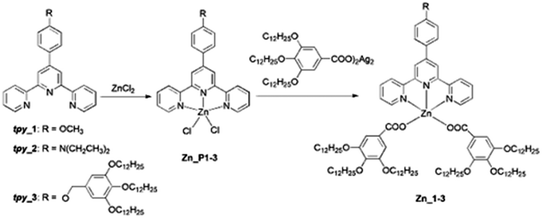 | ||
| Scheme 1 Synthesis and chemical structure of Zn(II) precursor complexes Zn_P1-3 and final complexes Zn_1-3. | ||
Results and discussion
Synthesis and structural characterization
Complexes Zn_1-3 were synthesised following a route reported previously13b that required prior synthesis of Zn(II) dichloride precursors (Zn_P1-3 in Scheme 1). All Zn(II) complexes were obtained as white or yellow air stable solids in good yields (70–90%, see –the ESI†). The coordination of the carboxylate in an exclusive unidentate fashion was revealed by the separation of the stretching vibrations of the COO− group (Δ) of 240–320 cm−1 (see Fig. S3–S5, ESI†).13b The structures and purity of the final Zn(II) complexes were confirmed by elemental analysis, 1H and 13C NMR and MS (see the ESI†).Mesomorphic properties
The thermal behavior was investigated by polarized optical microscopy (POM), differential scanning calorimetry (DSC) and thermal gravimetric analysis (TGA). The thermal properties are presented in Table 1 and in the ESI† (Fig. S15–S20). Complexes Zn_1-3 are room temperature liquid crystals with a wide range of mesophase existence.| Compound | T dec5% [°C] | Mesophases,b transition temperatures (°C) and enthalpies (ΔH [kJ mol−1])c |
|---|---|---|
| a Decomposition temperature at 5% weight-loss (from TGA). b M hex: three-dimensional mesophase with a two-dimensional hexagonal lattice symmetry (see text), Colhex: two-dimensional columnar hexagonal mesophase. c DSC data on the second heating and cooling cycles. | ||
| Zn_1 | 297 | M hex 229 [24.5] Iso/Iso 228 [28.0] Mhex |
| Zn_2 | 301 | M hex 188 [19.7] Iso/Iso 187 [25.1] Mhex |
| Zn_3 | 314 | Colhex 164 [19.7] Iso/Iso 167 [19.7] Colhex |
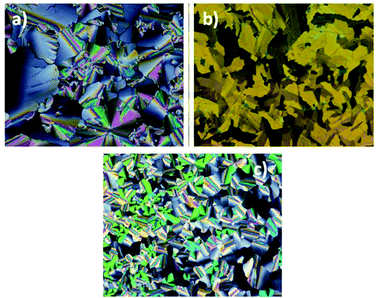
By POM, complexes Zn_1-3 show textures where hexagonal arrangement of columns can be envisaged from the occurrence of focal conic or mix mosaic textures (Fig. 1) and to the presence of homeotropic zones. The complete authentication of the mesophases’ type was nevertheless unambiguously achieved by S/WAXS (Small- and Wide-Angle X-ray Scattering) and Grazing Incidence Wide-Angle X-Ray Scattering (GIWAXS).
In agreement with the POM observations and S/WAXS analysis (see below), Zn_1 and Zn_2 exhibit a three-dimensional mesophase (Mhex), and Zn_3, a two-dimensional hexagonal columnar mesophase (Colhex). The Mhex/Colhex transitions to isotropic liquid phase (Iso) are associated with significant enthalpy change and temperature delay, due to molecular self-assembly into cohesive mesomorphic structures. All complexes show transitions below room temperature to undefined mesomorphic and/or crystalline states (see Fig. S18–S20, ESI†).
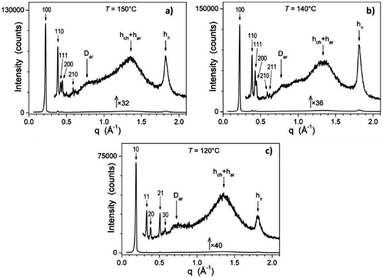
S/WAXS patterns are typical of liquid-crystalline phases, with scattering signals arising from liquid-like lateral distances between molecular segments in the wide-angle region (undifferentiated hch and har signals for aliphatic and aromatic parts, respectively, and stacking periodicity of the molecules along the columns, hπ) and sharp small-angle reflections from the nanosegregated structure formed by the antagonistic aromatic and aliphatic segments (Fig. 2 and Fig. S21–S23, ESI†).
Complex Zn_3 self-assembles into columns surrounded by chains and forms a Colhex structure, as evidenced by the semi-broad wide-angle signal hπ and the sharp small-angle reflections series (hk) of the hexagonal 2D-lattice. For the Mhex phase of Zn_1 and Zn_2, (hk) series become (hk0) and coexist with crossed reflections (hk1) of a hexagonal 3D-structure resulting from the periodic segmentation of the columns. The geometrical parameters of the mesophases (lattice parameters a and c, cross-sectional areas, …) are gathered in Table 2, and do not vary much with temperature (see Table 2).
| Compounds phase | T | V mol ρ | a A[Z] | h mol h π | χ χh A core | M hex | |
|---|---|---|---|---|---|---|---|
| c V | Z mol | ||||||
| Colhex | |||||||
| D core,cyl | s ch,cyl | ||||||
| S ZnAr,cyl | q ch,cyl | ||||||
| a T, temperature of the measurement (°C); Vmol, molecular volume (Å3); ρ, density (g cm−3); a, lattice parameter; A = a2√3/2, lattice area (Å2); Z, number of columns per lattice; hmol = Vmol/A/Z, molecular slice thickness (Å); hπ, π-stacking distance from the peak position (Å); χch, calculated aliphatic volume fraction; Acore = (1 − χch) × (A/Z), cross-sectional area of columnar cores (Å2). For Zn_1 and Zn_2: c, cell parameter (Å); V = A × c, cell volume (Å3); Zmol = V/Vmol, number of molecules per cell. For Zn_3: Dcore,cyl = √(4 × Acore/π), core diameter (Å) of equivalent cylinder of cross-sectional area Acore; SZnAr,cyl = πDcore,cyl × hmol, cylinder surface area per Zn(II) complex (Å2); sch,cyl = SZnAr,cyl/nch, cross-section area per chain (Å2), nch being the number of peripheral substituents per molecule (nch = 9 for Zn_3); qch,cyl = sch,cyl/σch, chain packing ratio for the average cylindrical interface. | |||||||
| Zn_1 | 20 | 2758 | 31.44 | 3.22 | 0.714 | 36.40 | 11.3 |
| M hex | 1.06 | 856[1] | 3.36 | 245 | 31160 | ||
| 50 | 2817 | 31.66 | 3.24 | 0.715 | 36.50 | 11.2 | |
| 1.03 | 868[1] | 3.37 | 248 | 31640 | |||
| 150 | 3012 | 32.42 | 3.31 | 0.717 | 35.49 | 10.7 | |
| 0.97 | 910[1] | 3.45 | 257 | 32340 | |||
| Zn_2 | 20 | 2838 | 31.72 | 3.26 | 0.722 | 33.70 | 10.4 |
| M hex | 1.05 | 871[1] | 3.39 | 242 | 29360 | ||
| 60 | 2918 | 31.95 | 3.30 | 0.723 | 34.35 | 10.4 | |
| 1.02 | 884[1] | 3.41 | 244 | 30290 | |||
| 140 | 3080 | 32.56 | 3.35 | 0.725 | 34.08 | 10.2 | |
| 0.97 | 918[1] | 3.46 | 252 | 31290 | |||
| Zn_3 | 20 | 3862 | 37.23 | 3.22 | 0.754 | 19.4 | 21.8 |
| Colhex | 1.02 | 1200[1] | 3.38 | 295 | 196 | 1.02 | |
| 60 | 3973 | 37.58 | 3.25 | 0.755 | 19.5 | 22.1 | |
| 1.00 | 1223[1] | 3.42 | 297 | 199 | 1.01 | ||
| 120 | 4141 | 37.75 | 3.36 | 0.756 | 19.6 | 22.9 | |
| 0.96 | 1234[1] | 3.47 | 301 | 206 | 1.00 | ||
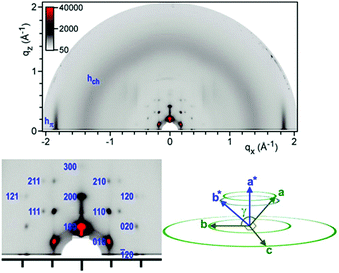
The GIWAXS pattern of Zn_2 thin film recorded at 20 °C (Fig. 3) complies with a three-dimensional mesophase made of segmented columns of π-stacked complexes, a × b being the columnar plane and c the direction of stacking. Domains are oriented with the direction of columns parallel to the substrate and random in-plane orientation. Reflections were indexed in a three-dimensional hexagonal lattice. The space group of highest symmetry compatible with the presence of (hk1)/(hh1) reflections and absence of (h01)/(0k1) is P63/mcm. The space groups of lower symmetry with the same reflection conditions are P63cm and P6c2.
The geometry of the complexes can be deduced from single-crystal structures of molecules including the same complex moiety. Thus, structures of several Zn(II) bis-(benzoato)-phenylterpyridine derivatives are available25 and show irregular trigonal bipyramidal geometries, in which both benzoate ligands lie nearly orthogonal to the tpy ring plane.
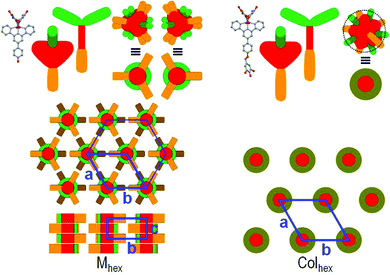
In Zn_1 and Zn_2, the pattern composition and geometrical parameters indicate that both compounds self-organize through π-stacking of tpy rings into columnar structures (Fig. 4, left). The crowding of the long chains connected only on one side of the cores forces the complexes to stack on top of each other with rotation angles in the order of 120° that equally distribute chains and R-benzyloxyphenyl groups around the columnar cores. Consequently, the protruding benzyloxyphenyl moieties confer the shape of a cross with three branches to the cross-section of columns. The columns, disposed at the nodes of the hexagonal lattice, adopt the most compact in-plane arrangement for this shape that consists in directing “crossed branches” toward “cross centers” of neighboring columns. This arrangement by nature follows the hexagonal symmetry but combines the segmentation of columns into smaller subunits. Only three over six neighboring columns are indeed pointed by crossed branches (due to the chains’ deficit on one side of the complex) and optimal space-filling presumably requires that pointed and non-pointed columns regularly swap along columnar axes, resulting in segmentation in groups of stacked molecules with inverted orientations. This segmentation defines the P63/mcm symmetry of the structure and the periodicity of two groups along the c-axis. It is found that the elementary group involves 5.6 Zn_1 molecules and 5.2 Zn_2 molecules.
In Zn_3, beside the aliphatic chains introduced by the gallate units, three additional peripheral alkyl chains are introduced through the benzyloxyphenyl of the tpy ligand. This implies an expansion of the interface between antagonistic aromatic and alkyl chains segments and the aggregation of aromatic segments into columns surrounded by an aliphatic shell. The characteristic π-stacking signature hπ and the matching of the stacking distance with the molecular slice thickness hmol further reveal that columnar cores consist of a single molecular strand of randomly orientated π-stacked tpy rings, each strand being located at the nodes of the hexagonal lattice. This strand is decorated with the gallate segments and R-benzyloxyphenyl groups of the individual complexes, whose average distances explain the additional broad scattering signals Dar and har. This decoration preserves the hexagonal lattice symmetry and the average interface with the aliphatic periphery is therefore a cylinder. Chain packing ratio qch,cyl26 is close to unity, i.e. nearly ideal for an efficient nanosegregation, which improves the cohesion of the structure and obviously contributes to the extended Colhex range.
Photophysics
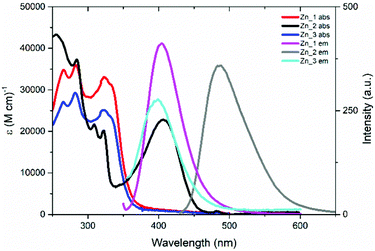
The photophysical properties of Zn_1-3 were collected from solutions and mesophase samples. The electronic states pattern is inferred from the spectra collected in diluted dichloromethane solutions (Fig. 5 and Table 3). The absorption band below 280 nm is present in all the complexes and it is due to ligand-centred (LC) transitions on the gallate ligands13b (see for example in Fig. S24 (ESI†) a comparison between the Zn_2 and its dichloride precursor Zn_P2 absorption spectra). The group of bands between 280 and 325 nm are attributed to charge-transfer (CT) transitions from the gallate to the tpy ligands (see the Computational results section).13b,27 The band centered at 406 nm, present only in the Zn_2 spectrum and responsible for the pale-green color of this sample, is due to a strong intraligand charge-transfer (ILCT) from the diethylaniline fragment to the aromatic rings of the tpy (see the Computational results section).28| Sample | Abs, λmax/nm (ε/M−1 cm−1) | Em, λmax/nm | ϕ/% | τ/ns |
|---|---|---|---|---|
| Zn_1 | 266 (34820), 284 (35910), 323 (33160), 333 (31300) | 405 | 6.5 | 2.0 |
| Zn_2 | 255 (43320), 284 (37310), 309 (21770), 322 (20180), 406 (22830) | 488 | 95.0 | 4.2 |
| Zn_3 | 265 (27100), 282 (29320), 322 (25250), 333 (23410) | 400 | 0.5 | 3.0 |
Fluorescence emission was registered for all samples. The emission from the CT excited state, observed for Zn_1 and Zn_3 at 405 and 400 nm, respectively, is weak, providing an emission quantum yield (ϕ) value of 6.5 and 0.5%, respectively. Interestingly, the fluorescence originated from the ILCT excited state in Zn_2 (centered at 488 nm) reaches the outstanding ϕ-value of 95%. The decay from the excited state follows a first order kinetic for all samples, with lifetime values between 2.0 and 4.2 ns (see Table 3).
The effect of temperature variation on the photophysical properties of Zn_1-3 in the mesophase is reported in Tables S1–S3 (ESI†). The reported data clearly show that after the first heating cycle, the values of the emission maximum and of the quantum yield have a little dependence on the temperature. In particular, the emission maximum wavelength remains fixed, throughout a wide temperature range (30–180 °C), while the emission quantum yield value, as expected, decreases with increasing temperature, due to the rise of non-radiative deactivation. This behaviour suggests that the temperature does not have a direct effect on the emission, but it depends on the supramolecular organization. For this reason, it is worth comparing the photophysical data of the mesophase and solutions at room temperature. Emission spectra recorded from mesophase samples at room temperature (Fig. 6 and Table 4) have the same structure as those collected in solution. All suffer a red-shift with respect to the solutions, showing peaks at 435 nm for Zn_1 (+30 nm) and Zn_3 (+35 nm), and at 542 nm for Zn_2 (+54 nm).
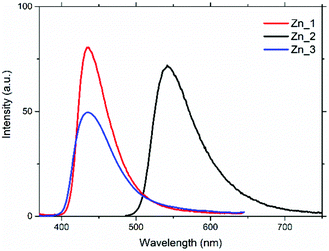 | ||
| Fig. 6 Emission spectra of the samples in the mesophase at room temperature. Excitation wavelength: 340 nm for Zn_1 and Zn_3; 450 nm for Zn_2. | ||
| Sample | Em, λmax/nm | ϕ/% |
|---|---|---|
| Zn_1 | 435 | 4.0 |
| Zn_2 | 542 | 20.2 |
| Zn_3 | 435 | 1.5 |
Compared to that measured in solution, the ϕ-value of Zn_3 increases in the room-temperature mesophase (from 0.5 to 1.5%), while for Zn_1 it decreases from 6.5 to 4.0%; for Zn_2 it undergoes a drastic reduction from 95.0 to 20.2%. Generally, on going from solution to a condensed phase, the molecular packing reduces the vibrational motions and the consequent decrease of the non-radiative rate constant (knr) causes an increase in the luminescence intensity. While this statement is true for Zn_3, it is not for Zn_1 and Zn_2.
Mesophase type of Zn_3 (Colhex) and of Zn_1 and Zn_2 (Mhex) exerts an effect on the quantum yield which adds to the reduction of vibrational motions. In fact, the columnar core of Zn_1 and Zn_2 consist of a three-molecule strand of π-stacked tpy rings, and this aggregation partially quenches luminescence; this is not effectively compensated by the reduction of knr and ultimately causes a reduction in the emission quantum yield for these compounds in the mesophase compared to the solution. In the columnar core of Zn_3, the poor π–π stacking does not effectively counterbalance the decrease in knr, and the quantum yield increases in the mesophase with respect to the solution. The behaviour of Zn_2 (which, as already highlighted, undergoes a drastic reduction of the ϕ-value) deserves a particular explanation, also considering that the mesophase is the same as Zn_1. This depends on the nature of the emissive state of this complex, which (unlike Zn_1 and Zn_3) is an ILCT from the diethylaniline fragment to the aromatic rings of the tpy. This transition became partially symmetry-forbidden in the mesophase, where the packing distorts the orientation between the diethylaniline fragment and the tpy core, causing a noticeable reduction of the emission quantum yield.
Nevertheless, the luminescence quantum yield value of Zn_2 remains considerable even in the mesophase, and for this reason the polarized emission of an oriented film of this complex was measured. To correlate the orientational order of the liquid crystal with its optical anisotropy properties, it is necessary to consider the orientation of the molecular emission transition dipole moment (TM) with respect to the columnar director. Theoretical calculations (see below) demonstrate that in the Zn_2 molecule the TM vector lies on the plane of the aromatic rings of the tpy, oriented by the amino group towards the centre of gravity of the molecule.29 Molecules form π-stacked units according to a symmetry that identifies a ternary axis parallel to the director, as shown in Fig. 4, and the TMs point towards three directions placed at 120° from each other and perpendicular to the director. Considering that the π-stacked groups are offset by 60°, six TM vectors for each column (Fig. 7a) can be identified. The Mhex phase hexagonal arrangement allows an ordered organization of the TMs, and therefore an emission anisotropy can be detected.
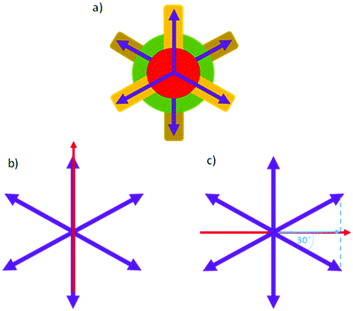 | ||
| Fig. 7 (a) Assumed orientation of the TMs (purple arrows) of Zn_2 seen along the column axis (see Fig. 4); (b) red arrow represents the direction of polarized emission light parallel to the TM; (c) red arrow represents the direction of polarized emission light perpendicular to the TM, while the blue arrow represents the projection of the vector TM along this direction. | ||
Fig. 8 reports the absorption and polarized emission spectra of the thin film of Zn_2, prepared as described in the ESI.† The absorption spectrum of the film shows the same electronic transitions as those reported in Fig. 5. Under un-polarised 440 nm excitation, the film shows a well-defined polarized emission band peaked at 546 nm, superimposable with that reported in Fig. 6. When the emission polarizer (Fig. 7b) is parallel (||) to the direction of one of the TM vectors (see the ESI†), the emission intensity is higher than when the polarizer (Fig. 7c) is perpendicular (⊥), since in this last direction the TM vector projection is lower.
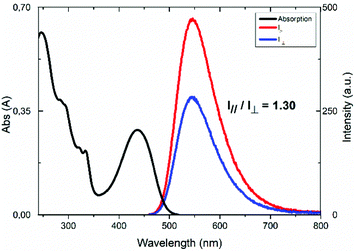 | ||
| Fig. 8 Absorption and polarized emission spectra of Zn_2 on an aligned thin film at room temperature (||: parallel emission light; ⊥: perpendicular emission light; λex = 440 nm). | ||
The value of the I||/I⊥ ratio is 1.30, indicative of the average orientation of the luminescent liquid crystals, considering that the vector composition of the TMs always provide non-zero values of the emission intensity at each angular value; although this ratio is not high, this value is the first obtained for a columnar metallomesogen.
Computational results
Zn_1 and Zn_2 were the object of computational investigations based on the DFT and TD-DFT methods (see “Computational methods” in the ESI†). In particular, the high fluorescence quantum yield of Zn_2 and the fluorescence anisotropy in its mesophase were investigated.In this respect, the main reference point for our theoretical study was the photophysical characterization performed in CH2Cl2 solutions. As evident in the following, inclusion of solvent effects is essential for the correct prediction of Zn_2 spectroscopy. However, the particular nature of the studied complexes induces uncertainness about the way to model the environment. In fact, the presence of six long hydrocarbon chains surrounding the core of the complex can modify the solvation environment of the core itself. Our choice was to perform the computational studies on model systems in which all the hydrocarbon chains were replaced with methyl groups and to carry out separate evaluations of the solvation effects by selecting n-hexane and CH2Cl2 as surrounding solvents. n-Hexane was used to simulate the hydrocarbon chains and the two solvents were chosen as extreme solvation environments, being the real environment in-between in terms of solute perturbation.
Fig. 9 provides a visual description of the computed Zn_2 structure. Its general features can be directly extended to Zn_1 due to the great structural similarity between the two complexes. Table 5 lists some relevant structural parameters computed for Zn_1 and Zn_2 both in the gas phase and in CH2Cl2 solution. Zn_2 belongs to the C2 point symmetry. Accordingly, the three nitrogen aromatic donor atoms are labelled as C2-axial (Nax) and non-C2-axial (Nnax). The coordination geometry can be considered trigonal bipyramidal (TBPY-5), with Nax and both the O atoms of the gallate ligands in the equatorial plane of the bipyramid. An evident deviation from the ideal TBPY-5 coordination geometry is the Nnax–Zn–Nnax angle, which is significantly smaller than 180°. The computed structure is in line with the experimental structural analysis performed on similar complexes.25 Analogue considerations can be applied to Zn_1 (Table 5) which show a high level of local C2 symmetry in most of its structure, in particular in the metal–ligands core of the complex. In both complexes, the phenyl group linked to the tpy core is rotated with respect to that of the tpy core itself. The dihedral angle between the average planes of the two aromatic systems (Ph and tpy in Table 5) are 36.2 and 38.6 degrees (Zn_2 and Zn_1 in vacuum, respectively) and it is reduced by 6–8 degrees in CH2Cl2.
| Zn_1 | Zn_2 | |||
|---|---|---|---|---|
| Vacuum | CH2Cl2 | Vacuum | CH2Cl2 | |
| Zn–Nax | 2.133 | 2.104 | 2.127 | 2.097 |
| Zn–N | 2.238 | 2.224 | 2.240 | 2.226 |
| 2.239 | 2.225 | |||
| Zn–O | 1.969 | 1.992 | 1.971 | 1.991 |
| 1.970 | 1.992 | |||
| N–Zn–N | 148.3 | 149.8 | 148.4 | 150.0 |
| O–Zn–O | 105.6 | 98.8 | 105.4 | 98.1 |
| O–Zn–Nax | 126.4 | 131.1 | 127.3 | 130.9 |
| 128.0 | 130.2 | |||
| Ph-tpy dihedral angle | 38.6 | 32.6 | 36.2 | 28.1 |
No relevant changes are attributed to solvation effects in comparison to computations in vacuum: the Zn–O bond distance increases by about 0.02 Å in CH2Cl2 by virtue of the increased solvation-induced stabilization of the “negatively charged” gallate ligands. As a consequence, the Zn–N bonds shorten by a similar amount. As expected, computed structural features in n-hexane are in-between the gas-phase and CH2Cl2 ones (Table S4 (ESI†) collects all the computed Cartesian coordinates).
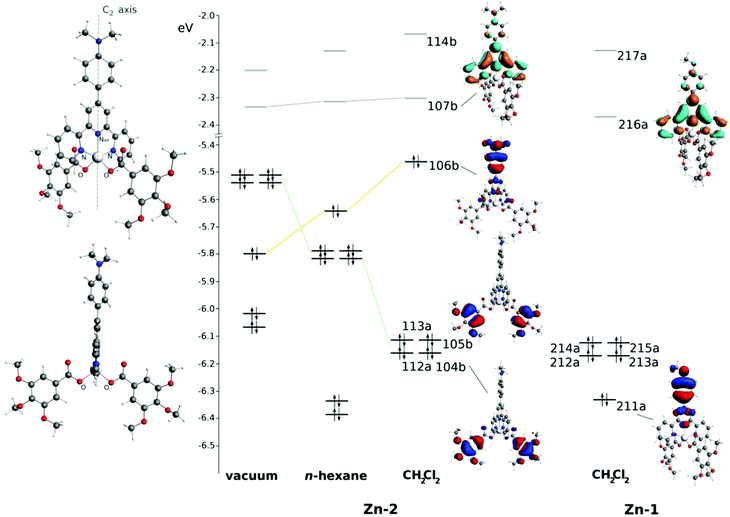
Fig. 9 sketches the molecular orbitals (MOs) energies of Zn_2 and Zn_1 computed in different environments which are important for the description of the low energy part of the absorption spectrum and also for the emission properties. In CH2Cl2, the Zn_2 HOMO (Highest Occupied Molecular Orbital, 106b in Fig. 9) is computed at −5.46 eV and is mainly localized on the diethylaniline group (86.5%). Lower in energy, in the range from −6.11 to −6.16 eV, gallate-localized MOs are present in the form of almost-degenerate symmetric (A symmetry) and corresponding asymmetric (B symmetry) combinations of the highest π bonding orbitals of gallate (for instance, 113a and 105b at −6.11 and −6.12 eV, respectively, only the asymmetric combination is shown in Fig. 9). The Zn_2 LUMO (Lowest Unoccupied Molecular Orbital, 107b in Fig. 9) shows a larger localization on the tpy core (85.9%) with only small contributions from the diethylaniline group (8.0%). The contribution of the central metal atomic orbitals on all these MOs is negligible, being always lower than 1.2%. Passing from CH2Cl2 to n-hexane, the shape of the shown MOs does not significantly change but their energies shift in different ways. The gallate-localized MOs are destabilised as a consequence of the negative charge on them and the lower ability of n-hexane to polarize itself in the proximity. On the contrary, the HOMO (106b) is stabilized, being the polarization of the solvent less destabilizing on this part of the molecule in n-hexane (see Fig. S25, ESI,† for details about the COSMO surface around the complex). Computations in vacuum show that the lack of solvation effects further destabilizes the gallate-localized MOs, which become the highest in energy occupied orbitals.
Differently from the occupied MOs, the LUMO is less affected by environmental effects, being mainly localized on the inner part of the tpy ligand and less exposed to the solvent in comparison to the diethylaniline and gallate ligands.
In Zn_1, MOs close to the HOMO and LUMO are very similar to the Zn_2 ones. MO 211a corresponds to MO 106b in Zn_2 (Fig. 9). However, in Zn_1, the 211a MO is not the HOMO, neither in CH2Cl2, being lower in energy in comparison to the gallate-localized MOs (already described in Zn_2 and found very similar also in Zn_1). This fact can be referred back to the lower π-conjugation of the OMe group (in comparison to the NEt2) with the phenyl ring and also to the high electronegativity of the oxygen atom and consequently to its electron withdrawing ability. The Zn_1 LUMO strongly resembles the Zn_2 one, both in shape and in energy, because it is barely affected by the phenyl group and localized “internally” to the complex.
TD-DFT computations confirm the nature of the first singlet excited state (S1) of Zn_2 and Zn_1 as already reported in the discussion of photophysical data in CH2Cl2 solution. Zn_2 S1 corresponds to an ILCT transition from the diethylaniline fragment to the tpy aromatic rings. In Zn_1, S1 is predicted as a CT transition from the gallate to the tpy ligands. Table 6 lists some relevant features of S1 of Zn_2 and Zn_1. In Zn_2, S1 can be assigned to a substantially pure HOMO–LUMO monoelectronic excitation (99.3% of contribution in CH2Cl2, 99.1% in n-hexane). The intensity of the related vertical electronic transition from the ground state (oscillator strength in Table 6) is computed to be rather large and it decreases passing from CH2Cl2 to the less polarizable n-hexane (in vacuum it is further lowered to 0.26, not reported in Table 6). Finally, being S1 an A-symmetry state like the ground state, the transition dipole moment is fully directed along the C2 axis of the molecule. All the higher singlet states (from S2 to S6) are characterised by a very low oscillator strength, hence no significant absorptions are predicted till 3.50 eV (354 nm, in CH2Cl2) or 3.13 eV (396 nm, in n-hexane). Thus, the low energy part of the Zn_2 absorption spectrum is dominated by a single intense absorption toward S1. In the experimental absorption spectrum (Fig. 5 and Table 3), Zn_2 differs from Zn_1 and Zn_3 for the presence of a well-defined band at 406 nm, which now can be safely assigned to the HOMO–LUMO transition. Interestingly, this absorption is computed at 452 nm in CH2Cl2, in good agreement with the experiment. However, the agreement improves when n-hexane is used as the solvent (418 nm). This could be an indication of the importance of the long hydrocarbon chains in determining the S1 energy with respect to the ground state. As reported in Table 6, S2 consists of an almost pure monoelectronic excitation from 106b (HOMO) to 114a, whereas the four following transitions consist of charge transfers from the gallate-localized MOs (112a, 113a, 104b and 105b) to the tpy-localized LUMO. The involved excited states consist of almost-degenerate couples of A and B symmetry states (for instance S3 and S4). For symmetry reasons, the transition dipole moment of the B states is perpendicular to the C2 rotation axis.
| State | Energy | Oscillator strength | Composition |
|---|---|---|---|
| Zn_2 (CH2Cl2 solution) | |||
| S1 (2A) | 2.74 eV; 452 nm | 0.59 | 106b → 107b 99.3% |
| S2 (1B) | 2.90 eV; 428 nm | 2.6 × 10−4 | 106b → 114a 99.1% |
| S3 (2B) | 3.44 eV; 360 nm | 4.5 × 10−5 | 113a → 107b 99.8% |
| S4 (3A) | 3.44 eV; 360 nm | 9.6 × 105 | 105b → 107b 99.8% |
| S5 (3B) | 3.50 eV; 354 nm | 3.7 × 10−5 | 112a → 107b 99.8% |
| S6 (4A) | 3.50 eV; 350 nm | 3.6 × 10−4 | 104b → 107b 99.8% |
| Zn_2 (n-hexane solution) | |||
| S1 (2A) | 2.96 eV; 419 nm | 0.47 | 106b → 107b 99.1% |
| S2 (1B) | 3.02 eV; 411 nm | 4.1 × 10−5 | 106b → 114a 98.9% |
| S3 (2B) | 3.09 eV; 401 nm | 1.7 × 10−5 | 113a → 107b 99.7% |
| S4 (3A) | 3.09 eV; 401 nm | 6.5 × 10−5 | 105b → 107b 99.6% |
| S5 (3B) | 3.13 eV; 396 nm | 3.7 × 10−5 | 112a → 107b 99.7% |
| S6 (4A) | 3.13 eV; 396 nm | 4.8 × 10−9 | 104b → 107b 99.7% |
| Zn_1 (CH2Cl2 solution) | |||
| S1 | 3.38 eV; 367 nm | 3.1 × 10−5 | 215a → 216a 68.8% |
| 214a → 216a 31.1% | |||
| S2 | 3.38 eV; 367 nm | 1.2 × 10−4 | 215a → 216a 31.1% |
| 214a → 216a 68.8% | |||
| S3 | 3.44 eV; 360 nm | 1.3 × 10−4 | 213a → 216a 94.3% |
| S4 | 3.44 eV; 360 nm | 9.1 × 10−6 | 212a → 216a 94.3% |
| S5 | 3.47 eV; 357 nm | 0.56 | 211a → 216a 98.7% |
| S6 | 3.63 eV; 342 nm | 8.6 × 10−5 | 215a → 217a 66.5% |
| 214a → 217a 31.4% | |||
Passing to Zn_1, low-intensity gallate-to-tpy CTs become the lowest electronic transitions (Table 6). The intense tpy-centred absorption is now associated to S5 rather than S1, and it is predicted at 357 nm, in good agreement with the first intense features measured at 333 nm (Table 3). Interestingly, the weak absorption around 400 nm (appearing like a tail at lower energies respect to the first absorption band in Fig. 5) confirms the presence of lower in energy singlet states (from S1 to S4) with low absorption intensities. This tail is not visible in the Zn_2 spectrum, a fact that further confirms the accuracy of the performed computations.
At this point we are able to explain the differences in fluorescence properties between Zn_2 and Zn_1. The first difference is the fluorescence quantum yield. Zn_2 is strongly emissive due to the large oscillator strength (transition dipole moment) of S1 in this complex, which leads to fast fluorescence kinetics. In Zn_1, on the contrary, the lowest singlet states are different and associated to very low oscillator strengths. Accordingly, they are barely emissive due to unfavourable electronic properties. Also, the longer emission wavelength in Zn_2 can be easily explained as a consequence of the lower HOMO–LUMO gap in comparison to Zn_1. In this case, a key role is played by the NEt2 substituent of the diethylaniline of Zn_2, which is able to destabilize the phenyl-localized π MOs much more than the OMe group in Zn_1.
Zn_3 is expected to be similar to Zn_1 in terms of electronic structure and (consequently) spectroscopy. The coupling between the OR substituent and the phenyl group in the tpy-based fragment is likely to be comparable to the OMe coupling in Zn_1. This prediction is corroborated by the similarity in absorption and emission spectroscopy between Zn_3 and Zn_1 (see Fig. 5).
Now it is possible to explain the observed fluorescence anisotropy from the Zn_2 mesophase. In the previous paragraph, this observation was supposed to be originated by a transition dipole moment oriented along the metal-tpy direction, obviously combined to a high level of order in the columnar arrangement of the liquid crystal. This is in perfect agreement with the computational results. As discussed previously, in Zn_2 the emission transition dipole must be directed along the C2 symmetry axis of the molecule (Fig. 9), like the absorption transition dipole associated to S1. This is imposed by symmetry, since the first excited state is of A symmetry.
Conclusions
With the increased importance in obtaining functional optical materials for practical applications, luminescent metallomesogens are promising for the next generation of emitters. The research is highly challenging due to the necessity of matching the structural requirements for inducing mesomorphic properties, high luminescence properties and fine tuning of mesophase architecture for preserving or improving the molecular properties. Among liquid crystal architectures, a columnar one is pivotal for electrical conduction; however, it may be detrimental for the metallomesogen optical properties, favouring quenching of luminescence due to the formation of new excited states by the close packing of emitters. On the other hand, its high viscosity that increases its rheological stability, favour aggregation induced emission as a result of RIM.Therefore we designed and synthesized new room temperature metallomesogens based on bio-available low-cost Zn(II) ions through the monodentate coordination of two lipophilic gallate units. The complexes show different 3D (Zn_1 and Zn_2: Mhex) or 2D (Zn_3: Colhex) liquid crystalline columnar organizations as a function of the functional group inserted on the chelating N^N^N terpyridine ligand.
Systematic structural, computational and photophysical studies allowed the correlation between liquid crystalline architecture and emitting properties in the mesophase. While complexes Zn_1 and Zn_3 emit modestly, the fluorescence originated from the ILCT excited state in Zn_2 from the electron-donating –NEt2 group to the electron-withdrawing aromatic rings of the tpy ligand reach a record luminescence quantum yield in solution (95%). Although the efficiency is reduced in the mesophase due to distortion of the diethylaniline with respect to the aromatic rings of tpy, it still remains as high as 20%.
Taking advantage of this high emission quantum yield of the mesophase, the polarized fluorescence of an oriented film of a columnar metallomesogen was measured for the first time, giving a I||/I⊥ ratio of 1.30, while theoretical calculations on the orientation of the transition dipole moment support the discussion.
Author contributions
Conceptualization, M. L. D., B. D., M. A. and E. I. S.; methodology, E. I. S.; investigation, G. D. M., A. C., B. H., A. A. A., E. P., E. V., V. B. and M. A.; resources, E. I. S.; data curation, M. L. D., B. H., M. A., B. D. and E. I. S.; writing – original draft M. L. D., M. A., O. C., B. D. and E. I. S.; writing – review and editing, M. L. D., M. A., B. D. and E. I. S.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a grant of the Romanian Ministry of Education and Research, CNCS – UEFISCDI, project number PN-III-P4-ID-PCE-2020-1958, within PNCDI III. Italian Ministry of University and Research supported this research through DEMETRA project (PON ARS01_00401). We thank Pohang Accelerator Laboratory (PAL) for giving us the opportunity to perform the GIWAXS experiment, MEST and POSTECH for supporting this experiment, Dr Hyungju Ahn for adjustment of beamline and for doing the measurement. A. A. A., E. P., E. I. Sz. and O. C. acknowledge the Romanian Academy, Program 4. A. A. A. is grateful for the “Ion Heliade Radulescu” mobility scholarship. We acknowledge the Laboratoire de Spectrométrie de Masse BioOrganique (LSMBO) IPHC UMR 7178 for mass spectrometry analysis. BD and BH thank CNRS and the University of Strasbourg for support.Notes and references
- (a) C. Keum, D. Becker, E. Archer, H. Bock, H. Kitzerow, M. C. Gather and C. Murawski, Adv. Opt. Mater., 2020, 2000414 CrossRef CAS; (b) G. Qian, X. Yang, X. Wang, J. D. Herod, D. W. Bruce, S. Wang, W. Zhu, P. Duan and Y. Wang, Adv. Opt. Mater., 2020, 8(20), 2000775 CrossRef CAS; (c) J. De, W.-Y. Yang, I. Bala, S. P. Gupta, R. A. K. Yadav, D. K. Dubey, A. Chowdhury, J.-H. Jou and S. K. Pal, ACS Appl. Mater. Interfaces, 2019, 11, 8291 CrossRef CAS PubMed; (d) J. Han, S. Guo, H. Lu, S. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 1800538 CrossRef; (e) Y. Wang, J. Shi, J. Chen, W. Zhu and E. Baranoff, J. Mater. Chem. C, 2015, 3, 7993 RSC; (f) Y. Wang, J. Fan, J. Shi, H. Qi, E. Baranoff, G. Xie, Q. Li, H. Tan, Y. Liu and W. Zhu, Dyes Pigm., 2016, 133, 238 CrossRef CAS.
- (a) X. Hao, B. Xiong, M. Ni, B. Tang, Y. Ma, H. Peng, X. Zhou, I. I. Smalyukh and X. Xie, ACS Appl. Mater. Interfaces, 2020, 12, 53058 CrossRef CAS PubMed; (b) C. Cuerva, J. A. Campo, P. Ovejero, M. R. Torres, E. Oliveira, S. M. Santos, C. Lodeiro and M. Cano, J. Mater. Chem. C, 2014, 2(43), 9167 RSC.
- Y. L. Zhuang, X. L. Ren, X. T. Che, S. J. Liu, W. Huang and Q. Zhao, Adv. Photonics, 2021, 3(1), 014001 Search PubMed.
- (a) H.-W. Chen, J.-H. Lee, B.-Y. Lin, S. Chen and S.-T. Wu, Light: Sci. Appl., 2018, 7, 17168 CrossRef CAS PubMed; (b) D. Zhao, H. He, X. Gu, L. Guo, K. S. Wong, J. W. Y. Lam and B. Z. Tang, Adv. Opt. Mater., 2016, 4, 534 CrossRef CAS.
- (a) D. Pucci and B. Donnio, Non-Conventional, Supramolecular, Chromonic and Amphiphilic Liq. Cryst., in Handbook of Liquid Crystals: Metal-containing liquid crystals, ed. J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. Gleeson and P. Raynes, Wiley-VCH, Weinheim, 2014, vol. 5 Search PubMed; (b) C. Cuerva, M. Cano and C. Lodeiro, Chem. Rev., 2021, 121(20), 12966 CrossRef CAS PubMed.
- K. Rajendiran, S. T. Yoganandham, S. Arumugam, D. Arumugam and K. Thananjeyan, J. Mol. Liq., 2021, 321, 114793 CrossRef CAS.
- (a) R. Giménez, O. Crespo, B. Diosdado and A. Elduque, J. Mater. Chem. C, 2020, 8, 6552 RSC; (b) A. Kishimura, T. Yamashita, K. Yamaguchi and T. Aida, Nat. Mater., 2005, 4, 546 CrossRef CAS PubMed; (c) R. Chico, C. Dominguez, B. Donnio, B. Heinrich, S. Coco and P. Espinet, Cryst. Growth Des., 2016, 16(12), 6984–6991 CrossRef CAS.
- E. Beltrán, J. Barberá, J. L. Serrano, A. Elduque and R. Giménez, Phase, Eur. J. Inorg. Chem., 2014, 1165–1173 CrossRef.
- (a) J. Jiménez, J. A. Sanz, J. L. Serrano, J. Barberá and L. Oriol, Inorg. Chem., 2020, 59(7), 4842 CrossRef PubMed; (b) P. Dechambenoit, S. Ferlay, B. Donnio, D. Guillon and M. W. Hosseini, Chem. Commun., 2011, 47, 734–736 RSC.
- R. R. Parker, D. H. Liu, X. K. Yu, A. C. Whitwood, W. G. Zhu, J. A. G. Williams, Y. F. Wang, J. M. Lynam and D. W. Bruce, J. Mater. Chem. C, 2021, 9(4), 1287 RSC.
- (a) G. Qian, X. Yang, X. Wang, J. D. Herod, D. W. Bruce, S. Wang, W. Zhu, P. Duan and Y. Wang, Adv. Opt. Mater., 2020, 2000775 CrossRef CAS; (b) B. Yang, H. Ni, H. Wang, Y. Hu, K. Luo and W. Yu, J. Phys. Chem. C, 2020, 124(43), 23879 CrossRef CAS; (c) L. Zhao, B. Yang, L. Zeng, K. Luo, H. Wang, H. Ni, C. Yang and Q. Li, Dyes Pigm., 2019, 164, 398 CrossRef CAS; (d) C. Cuerva, J. A. Campo, M. Cano and C. Lodeiro, Chem. – Eur. J., 2019, 25(52), 12046 CrossRef CAS PubMed; (e) C. Cuerva, J. A. Campo, M. Cano, R. Schmidt and C. Lodeiro, J. Mater. Chem. C, 2018, 2, 9723 RSC; (f) E. Tritto, R. Chico, J. Ortega, C. L. Folcia, J. Etxebarria, S. Coco and P. Espinet, J. Mater. Chem. C, 2015, 3(36), 9385 RSC; (g) V. N. Kozhevnikov, B. Donnio and D. W. Bruce, Angew. Chem., Int. Ed., 2008, 47, 6286 CrossRef CAS PubMed.
- (a) E. de Domingo, C. L. Folcia, J. Ortega, J. Etxebarria, R. Termine, A. Golemme, S. Coco and P. Espinet, Inorg. Chem., 2020, 59(15), 10482 CrossRef CAS PubMed; (b) M. Park, D.-G. Kang, H. Ko, M. Rim, D. T. Tran, S. Park, M. Kang, T.-W. Kim, N. Kim and K.-U. Jeong, Mater. Horiz., 2020, 7(10), 2635 RSC; (c) S. Chakraborty, S. K. Prasad, D. S. S. Rao and C. R. Bhattacharjee, Liq. Cryst., 2019, 46(6), 872 CrossRef CAS; (d) R. Zhang, H. Gao, Y. Ren, Y. Xiao, J. Hu and X. Cheng, Chem. – Asian J., 2018, 13(5), 536 CrossRef CAS PubMed; (e) S. Chakraborty, P. Mondal, S. K. Prasad, D. S. S. Rao and C. R. Bhattacharjee, Eur. J. Inorg. Chem., 2016, 4604 CrossRef CAS; (f) S. Debnath, H. F. Srour, B. Donnio, M. Fourmigué and F. Camerel, RSC Adv., 2012, 2, 4453 RSC; (g) C. Cretu, L. Cseh, B. J. Tang, V. Sasca, V. Badea, E. I. Szerb, G. Mehl, S. Shova and O. Costisor, Liq. Cryst., 2015, 42, 1139 CrossRef CAS.
- (a) N. S. S. Kumar, M. Z. Shafikov, A. C. Whitwood, B. Donnio, P. B. Karadakov, V. N. Kozhevnikov and D. W. Bruce, Chem. – Eur. J., 2016, 22, 8215 CrossRef CAS PubMed; (b) A.-A. Andelescu, B. Heinrich, M. A. Spirache, E. Voirin, M. La Deda, G. Di Maio, E. I. Szerb, B. Donnio and O. Costisor, Chem. – Eur. J., 2020, 26, 4850 CrossRef CAS PubMed; (c) D. Pucci, A. Crispini, M. Ghedini, M. La Deda, P. F. Liguori, C. Pettinari and E. I. Szerb, RSC Adv., 2012, 2, 9071 RSC.
- (a) L. Soria, P. Ovejero, M. Cano, J. A. Campo, M. R. Torres, C. Núñez and C. Lodeiro, Dyes Pigm., 2014, 110, 159 CrossRef CAS; (b) D. Pucci, A. Crispini, M. Ghedini, E. I. Szerb and M. La Deda, Dalton Trans., 2011, 40, 4614 RSC.
- C. Cretu, A. A. Andelescu, A. Candreva, A. Crispini, E. I. Szerb and M. La Deda, J. Mater. Chem. C, 2018, 6, 10073 RSC.
- (a) G. Zou, L. Zhao, L. Zeng, K. Luo, H. Ni, H. Wang, Q. Li, W. Yu and X. Li, Inorg. Chem., 2019, 58(1), 861 CrossRef CAS PubMed; (b) A. M. Prokhorov, A. Santoro, J. A. G. Williams and D. W. Bruce, Angew. Chem., Int. Ed., 2012, 51, 95 CrossRef CAS PubMed; (c) E. I. Szerb, A. M. Talarico, I. Aiello, A. Crispini, N. Godbert, D. Pucci and T. Pugliese, Eur. J. Inorg. Chem., 2010, 3270 CrossRef CAS.
- (a) H. Ishida, M. Handa, I. Hiromitsu, S. Ujiie, D. Yoshioka, R. Mitsuhashi and M. Mikuriya, New J. Chem., 2019, 43, 1134 RSC; (b) D. Pucci, A. Bellusci, A. Crispini, M. Ghedini, N. Godbert, E. I. Szerb and A. M. Talarico, J. Mater. Chem., 2009, 19, 7643 RSC.
- L. Rossi, C. Huck-Iriart, M. A. Castro and F. D. Cukiernik, J. Coord. Chem., 2017, 70(21), 3633 CrossRef CAS.
- P. Alam, C. Climent, P. Alemany and I. R. Laskar, J. Photochem. Photobiol., C, 2019, 41, 100317 CrossRef CAS.
- T. Shu, J. Wang, L. Su and X. Zhang, Crit. Rev. Anal. Chem., 2018, 48(4), 330 CrossRef CAS PubMed.
- P. Peng, Y. Li, W. Song and X. Yu, Colloids Surf., A, 2020, 589, 124439 CrossRef CAS.
- A. Agosti, E. Kuna and G. Bergamini, Eur. J. Inorg. Chem., 2019, 577 CrossRef CAS.
- (a) A. Winter and U. S. Schubert, ChemCatChem, 2020, 12, 2890 CrossRef CAS; (b) C. Wei, Y. He, X. Shi and Z. Song, Coord. Chem. Rev., 2019, 385, 1 CrossRef CAS PubMed; (c) Y. H. Budnikova, D. A. Vicic and A. Klein, Inorganics, 2018, 6(1), 18 CrossRef.
- (a) S. Furumi, D. Janietz, M. Kidowaki, M. Nakagawa, S. Morino, J. Stumpe and K. Ichimura, Chem. Mater., 2001, 13, 1434 CrossRef CAS; (b) S. Zimmermann, J. H. Wendorff and C. Weder, Chem. Mater., 2002, 14, 2218 CrossRef CAS.
- The Cambridge Structural Database. Z. Ma, W. Lu, B. Liang and A. J. L. Pombeiro, New J. Chem., 2013, 37, 1529 RSC.
- D. Myśliwiec, B. Donnio, P. J. Chmielewski, B. Heinrich and M. Stępień, J. Am. Chem. Soc., 2012, 134, 4822 CrossRef PubMed.
- (a) X. Chen, Q. Zhou, Y. Cheng, Y. Geng, D. Ma, Z. Xie and L. Wang, J. Lumin., 2007, 126, 81 CrossRef CAS; (b) W. Goodall and J. A. G. Williams, Chem. Commun., 2001, 2514 RSC.
- X. Bi and Y. Pang, J. Phys. Chem. B, 2016, 120(13), 3311 CrossRef CAS PubMed.
- (a) A. Winter, C. Friebe, M. Chiper, M. D. Hager and U. S. Schubert, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4083 CrossRef CAS; (b) I. Eryazici, C. N. Moorefield and G. R. Newkome, Chem. Rev., 2008, 108, 1834 CrossRef CAS PubMed; (c) M. Humbert-Droz, C. Piguet and T. A. Wesolowski, Phys. Chem. Chem. Phys., 2016, 18, 29387 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, synthesis and characterization, FT-IR spectra of complexes Zn_1-3, 1H and 13C NMR spectra of complexes Zn_1-3, mass spectra of complexes Zn_1-3, TGA traces of precursor complexes Zn_P1-3 and complexes Zn_1-3, DSC traces of complexes Zn_1-3, S/WAXS patterns of complexes Zn_1-3, photophysical data of complexes Zn_1-3, and computational studies. See DOI: 10.1039/d1tc05059a |
| This journal is © The Royal Society of Chemistry 2022 |