Manipulating the optical and electronic properties of MoO3 films through electric-field-induced ion migration
Xiaoxia
Wang
ab,
Fanfan
Du
ab,
Yingmei
Zhang
ab,
Jie
Yang
ab,
Xiaoli
Li
 *ab and
Xiaohong
Xu
*ab and
Xiaohong
Xu
 *ab
*ab
aKey Laboratory of Magnetic Molecules & Magnetic Information Materials of Ministry of Education and School of Chemistry and Materials Science, Shanxi Normal University, Linfen, 041004, P. R. China. E-mail: lixli@sxnu.edu.cn; xuxh@sxnu.edu.cn
bResearch Institute of Materials Science of Shanxi Normal University and Collaborative Innovation Center for Shanxi Advanced Permanent Magnetic Materials and Technology, Linfen, 041004, P. R. China
First published on 7th December 2021
Abstract
MoO3 films with a (0k0) orientation were deposited by pulsed laser deposition on a glass substrate and lithium conducting ceramic substrate. Hydrogen or lithium evolution was realized in the MoO3 thin films by ionic liquid gating due to the existence of hydrogen ions and lithium ions in the ionic liquid and the substrate, respectively. The intercalation of hydrogen ions or lithium ions causes the reduction of the Mo element and the resultant transformation of the electronic and optical properties of the MoO3 films. A nearly five orders of magnitude modulation of the MoO3 conductivity is obtained. And the pristine transparent film is changed to blue. Thus, the migration of ions driven by the ionic liquid gating can trigger the reduction of Mo in the MoO3 films, resulting in a large change in the electronic and optical properties. This study shows potential in the field of the emulation of the artificial synapses, and can supply a novel method for designing multifunctional devices.
1. Introduction
The use of electric fields to modify the microstructure and properties of various materials is a powerful tool to probe their fundamental nature and even develop novel multifunctional devices. The process often requires large electric fields, which are beyond those achievable by conventional dielectric materials.1,2 Ionic liquids, as gate dielectric materials, can induce large electric fields due to the formation of electric double layers at the interface between the material and the electrolyte.1–3 Thus, ionic liquid gating (ILG) has attracted a wide range of research attention in the last two decades,3,4 leading to the discovery of a plethora of exotic phenomena, such as the electrical control of electronic, magnetic and optical properties of materials in various material systems.1,2,5–15 Contrary to initial expectations of a purely electrostatic response based on electron or hole doping, electrochemical mechanisms based on ionic motion are now understood to be common, suggesting new promising electrical control concepts.15 Electrochemical mechanisms based on ionic motion not only include oxygen ion evolution,1,2,6,7,12 but also the intercalation and extraction of some cations, such as lithium and hydrogen ions, etc.7,11,14,16–18 As typical systems, the insulator–metal transition and structural deformation of VO2 and WO3 thin films have been induced by ILG due to oxygen ion evolution driven by electrochemical processes.1,2,6 Furthermore, it has recently been reported that electrochemical protonation can also emerge in VO2 and WO3 systems with ILG.11,16,17 Further research into ILG-induced hydrogen evolution can extend this approach to a large variety of functional materials, which will give a big boost to the development of related fields, such as the discovery of novel crystalline structures and exotic electronic states, energy storage applications, neuromorphic computing etc.Stoichiometric MoO3 phases show a wealth of diverse structures which are constructed in different ways based on the MoO6 octahedron building block.19 They have garnered attention in sensors20 and energy storage applications,21 and have potential in optoelectronic,22 resistive switching,23 and artificial synapse devices.24,25 Among them, orthorhombic α-MoO3 possesses the well-known layered crystal structure of MoO3,26 which offers the possibility of creating two-dimensional morphologies.19 The layered structure of α-MoO3 facilitates the intercalation of ions into the layer spacing, which may give rise to a multitude of interesting properties. Stoichiometric MoO3 is insulating, and no insulator to metal transition with temperature has been reported.25
To sum up, taking into account the ILG-induced protonation of various materials to realize their functionality and the layered structural characteristics of α-MoO3, we used ILG to drive hydrogen intercalation in α-MoO3 films grown on amorphous glass substrates and correspondingly modulate their optical and electronic properties. The choice of amorphous glass substrates is to avoid the strain and the clamping effect due to the lattice difference between the single-crystal substrate and MoO3 films. In previous reports on ILG-driven protonation, various ionic liquids, including DEME-TFSI, EMIM-TFSI, HMIM-TFSI, and EMIM-BF4etc. were used.7,11,17,24 The most likely source for the hydrogen ions may be the water content inside the ionic liquid. Lu et al.7 and Wang et al.17 noted that water inevitably existed inside the ionic liquid, and provided solid evidence to attribute the origin of hydrogen ions to the water electrolysis effect in studying the protonation of SrCoO2.5 and WO3. Yang et al.24 observed the humidity dependence of gate responses in modifying the conductance of MoO3. They suggested that more water molecules were adsorbed with the increase of the relative humidity level, which resulted in the increase of the proton concentration in the ionic liquid gate, that was, the increase of the ionic liquid acidity, and correspondingly larger modulations were induced.24 Thus, increasing the ionic liquid acidity may be a significant way to induce protonation of the materials and correspondingly modulate their properties. So, in this work, an acidic ionic liquid, [BHSO3MIm]HSO4, is chosen to modulate MoO3 films in order to avoid the dependency of the modulation on the ambient environment and the water content in the ionic liquid. Moreover, we also grew a MoO3 film on a lithium conducting ceramic substrate and induced the intercalation of lithium ions in it. It is found that the optical and electronic properties of MoO3 films can be dramatically modulated by the two modes. The related mechanism is also discussed.
2. Experimental section
A MoO3 film with a thickness of about 400 nm was deposited on a glass substrate using pulsed laser deposition from a MoO3 target in a 14 Pa oxygen environment at a substrate temperature of 600 °C. A KrF excimer laser (λ = 248 nm) with a 5 Hz repetition rate and 200 mJ energy was focused on the target to obtain the films. The microstructures of the sample were analyzed by X-ray diffraction (XRD) and transmission electron microscopy (TEM). The chemical states of Mo in the sample were analyzed by X-ray photoelectron spectroscopy (XPS). The dependence of the resistance on the temperature was measured by a physical property measurement system. The optical transmission properties were measured using an ultraviolet–visible spectrophotometer.The acidic ionic liquid [BHSO3MIm]HSO4 was used as an electrolyte to apply gate voltages on the MoO3 films. Firstly, a layer of aluminum foil was set up above the sample and the three side edges of the aluminum foil were wrapped and pasted on the sample with double-sided tape, leaving about a 2 mm distance between the aluminum foil and the sample surface. Subsequently, the aluminum foil and the surface of the MoO3 film were bonded to two electrodes of the chip with Au wires as the gate electrode and the bottom electrode, respectively.27–29 Then, we used a small syringe to inject a certain amount of ionic liquid in the space between the aluminum foil and the sample from the unwrapped side of the sample.29 Finally, the DC gate biases were applied between the gate electrode and bottom electrode by a Keithley 2400 semiconductor characterization system in open air at room temperature. After gating, the ionic liquid was removed by washing the sample with acetone and then alcohol for the ex situ measurements.
3. Results and discussion
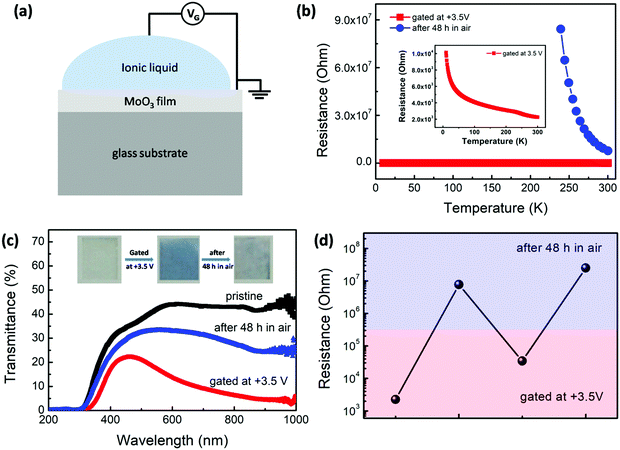
Fig. 1(a) shows the XRD pattern of the pristine MoO3 film, in which (0k0) peaks of the α-MoO3 phase, including the (020), (040) and (060) peaks, are clearly observed. The high-resolution TEM image shown in Fig. 1(b) and the corresponding fast Fourier transformed (FFT) image, as shown in the inset of Fig. 1(b), also reveal MoO3 lattice fringes. Thus, it is indicated from the XRD and TEM results that the α-MoO3 phase with high quality is formed in the present work.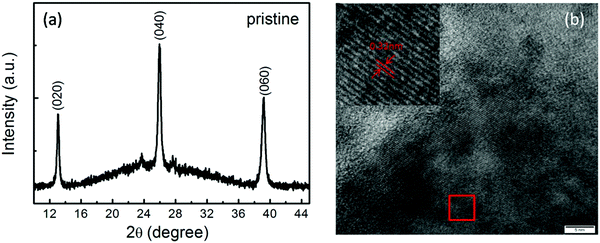 | ||
| Fig. 1 The XRD pattern (a) and the high resolution TEM image (b) of the pristine α-MoO3 film. The inset of (b) shows the corresponding fast Fourier transformed image. | ||
So, we turn toward the hydrogenation process in the α-MoO3 film by ILG and its influence on the corresponding conductivity and optical properties, as shown in Fig. 2(a–d). The pristine MoO3 film is transparent and behaves as a semiconductor with a resistance of more than 108 Ohm, which is too large to be measured. By designing the ILG device, as shown in Fig. 2(a), the MoO3 film becomes blue and its resistance falls rapidly to 2.25 × 103 Ohm when a gating voltage (VG) of +3.5 V is applied on the MoO3 film for 60 min, as shown in Fig. 2(b and c) and their insets. A modulation of about 5 orders of magnitude of the MoO3 conductivity is obtained. The gated film varies from blue to almost transparent and its resistance recovers to 7.73 × 106 Ohm after the gated film is placed in open air for 48 h. The change of the color of the films can also be clearly observed from their optical transmission spectra in Fig. 2(c). The values of the band gap of the MoO3 film in the three states are also roughly estimated from the transmission spectra. The band gap of the pristine film is about 3.20 eV, which is agreement with previous reports.30,31 It is reduced to about 2.61 eV right after gating. And then it recovers to 3.07 eV after placing in open air for 48 h. The results will be discussed in details later. The reproducibility of the gating progress is also studied. Fig. 2(d) shows that the resistance can be reduced again and, correspondingly, the sample can change to blue when it is gated at +3.5 V for 60 min again. Therefore, the above results imply that ILG can dramatically modulate the optical and electronic properties of the MoO3 film. The properties of the gated state can recover to a certain extent when it is placed in open air. However, it cannot completely recover to the pristine state. Thus, it is a partially reversible process.
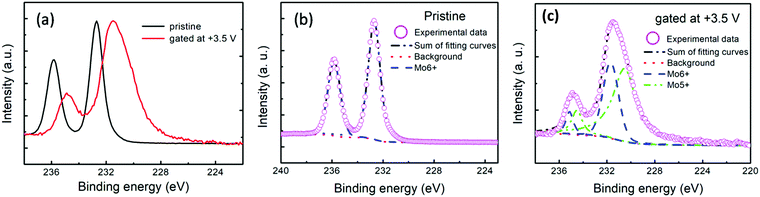
XPS is well known to be sensitive to the chemical environment, which might provide information on the change of the chemical states of the MoO3 films before and right after gating. A comparison of the Mo 3d core-level XPS spectra of the MoO3 films before and right after gating is displayed in Fig. 3(a–c). In the figures, the Mo 3d spectrum of the pristine MoO3 film exhibits primary Mo 3d3/2 and 3d5/2 peaks at 235.98 and 232.68 eV, respectively, confirming the Mo6+ valence state.32 Remarkably, the positions of both Mo 3d3/2 and 3d5/2 peaks of the right gated MoO3 film shift toward lower binding energies of 234.58 eV and 231.28 eV, respectively, compared to those of the pristine MoO3 film, suggesting a subsequent change of the stoichiometry and less oxygen ions binding with Mo ions at this time. By fitting the XPS spectra, as shown in Fig. 3(b and c), it is found that Mo in the pristine MoO3 film exists as Mo6+ ions, whereas Mo5+ and Mo6+ ions coexist in the gated one. This implies the reduction of Mo ions during the +3.5 V gating progress. It is well known that an electric double layer is formed at the interface of the ionic liquid and MoO3 films during the +3.5 V ILG progress. This may drive the directional migration of the hydrogen ions or oxygen ions in the MoO3 films. The reduction of Mo may be due to the ILG inducing the extraction of oxygen ions or the intercalation of hydrogen ones in the materials with a positive bias VG.
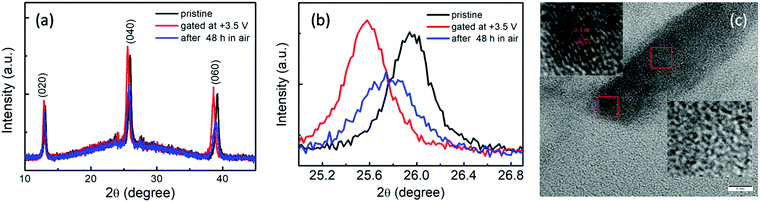
To further confirm that the gating mechanism is related to the extraction of oxygen ions or the intercalation of hydrogen ions, XRD patterns of the MoO3 films before, right after gating, and after gating for 48 h were recorded, as shown in Fig. 4(a). Almost the same diffraction peaks can be observed in them, which implies that the crystal structure of the MoO3 film has not been significantly changed during the gating progress. However, the differences among the three states is also clear from Fig. 4(a), in which all of the diffraction peaks shift a small angle. The point can be more clearly seen from the enlarged plots of the (040) peaks, as shown Fig. 4(b). Gating at +3.5 V for 60 min causes the shift of the diffraction peaks to lower angles, suggesting a clear expansion of the crystal lattice. After placing the gated sample in open air for 48 h, its diffraction peaks return to the large angles, signifying the shrinkage of the crystal lattice. However, they did not return to the original positions of the pristine state.
The expansion of the crystal lattice during the gating progress may be not a result of the extraction of oxygen ions, but the intercalation of the hydrogen ions in the acidic ionic liquid into α-MoO3, driven by the ILG. Gating for 60 min leads to the injection of a considerable amount of hydrogen ions into the MoO3 films, which results in the formation of HxMoO3 and the reduction of part of the Mo6+ ions to Mo5+ ones. Connecting the XPS and XRD results with the above band gap values, the intercalation of hydrogen ions into MoO3 may be the source of the changes of the electronic and optical properties. Intrinsic MoO3 is a semiconductor with a wide bandgap energy of about 3.20 eV. It consists of Mo6+ forming the conduction bands and O2− forming the valence bands.33 The doping electrons in MoO3 introduced by hydrogen intercalation occupy the electronic states at the bottom of the conduction band, and correspondingly reduce the band gap by about 0.60 eV, which is in agreement with the theoretical calculations by Huang et al.34 The narrowing of the fundamental band gap enhances the conductivity24 and leads to intense absorption in the visible range, ultimately causing the observed coloration.19 In brief, the gating progress gives rise to hydrogen ions intercalating into MoO3, resulting in the reduction of the band gap and, correspondingly, the color change from transparent to blue, the reduction of transmittance, the enhancement of conductivity, and the reduction of the resistance. Whereas when the gated sample is placed in open air for 48 h, most of the intercalated hydrogen ions may extract from the α-MoO3 lattice, which gives rise to the shrinkage of the lattice and the partial return of the diffraction peaks to higher angles. Simultaneously, the color, the band gap, the conductivity, and the resistance of the MoO3 film trend towards recovery to a certain extent. Anyway, not all of the hydrogen ions can be completely extracted, even after 48 h. And in the progress of the extraction of the hydrogen ions, some oxygen atoms alongside the hydrogen ions may be lost, resulting in reduced MoO3−y.19,35 So, the existence of the oxygen vacancies and residual hydrogen ions in the MoO3 sample means that the sample cannot be recovered to the pristine state and the diffraction peaks, transmittance, band gap and resistance could not completely return to the original states. Thus, the whole progress is a partially reversible one. Additionally, although the crystal structures have not be changed before and after gating, according to the XRD results shown in Fig. 4(a and b), the intercalation and the extraction of hydrogen ions in the MoO3 films may influence the microstructure to a certain extent and induce the appearance of some defects and disordering, which can be seen by comparing their TEM images before and after gating, as shown in Fig. 1(b) and Fig. 4(c). Quintana et al.36 observed the formation of highly nanostructured or even amorphous-like phases upon biasing when investigating an electrolyte-gated Co3O4 film. In addition, to confirm whether the acidic ionic liquid is universal, we extended our studies to modify WO3 films. It successfully induced the desired properties. The related investigation will be done in the future.
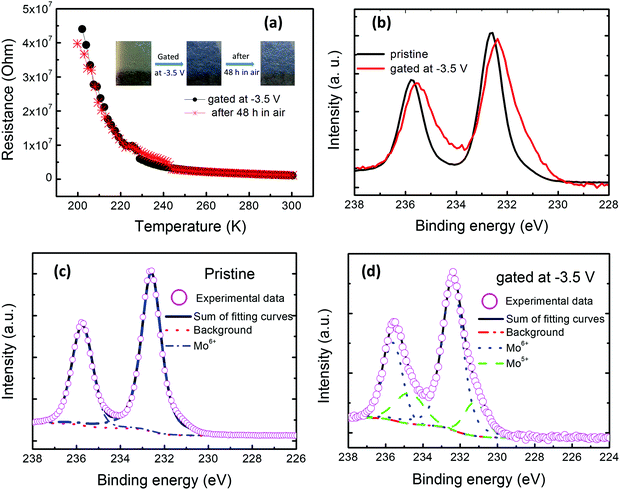
Besides hydrogen ions, lithium ions (Li+) also commonly intercalate into various oxides. Therefore, we also prepared a MoO3 film on a commercial Li+ conducting ceramic (Li1.5Al0.5Ge1.5P3O12) substrate with 14 Pa oxygen pressure at 600 °C in order to study the similarities and differences between lithium ions and hydrogen ones. The ionic liquid [BHSO3MIm]HSO4 is still used as an electrolyte to apply voltages on the MoO3 films. Different from the above gating process, a negative gating voltage of −3.5 V was applied on the gate electrode with the substrate being grounded. The results are shown in Fig. 5(a–d). The pristine MoO3 film is transparent. And its resistance is still too high to be measured. It also became blue and its resistance was decreased to about 106 Ohm after applying −3.5 V. Its color is not bleached and its resistance is almost unchanged after placing in open air for 48 h, as shown in Fig. 5(a) and its inset. Similar with the above results of the film prepared on the glass substrate, the XPS spectra of the one prepared on the Li+ conducting ceramic substrate, as shown in Fig. 5(b–d), also show that the Mo valence states vary from +6 to the mixed states of +5 and +6 during the gating progress. The similar trend with the above results about the intercalation of hydrogen ions implies the intercalation of lithium ones into the MoO3 film, driven by the electric field. Zhang et al.25 and Nishihaya et al.18 also observed the intercalation of lithium ions in MoO3 and WO3, respectively. The degree of the reduced resistance of the MoO3 films after gating and their retention are different for the intercalation of the hydrogen ions and lithium ones. This may be related to their kinetics essentially relying on long distance diffusion. Hydrogen ions are the lightest of all ions and present the smallest ionic radius, which may be more favorable for ion migration.37 To quantitatively determine the diffusion energy barrier is always an important issue and needs to addressed. Related experimental studies are rare.38,39 Many theoretical studies have been performed.25,40,41 The reported quantitative values of the diffusion energy barriers are controversial and depend on the different exchange–correlation functionals and parameters used, different doping positions and different doping concentrations etc.25,39 It is clear that there are two types of diffusion energy barriers governing the intercalation of hydrogen ions and lithium ones into α-MoO3, that is, the surface diffusion and bulk diffusion energy barriers, respectively.24 And in the entire migration pathway, the diffusion barrier is no more than the order of magnitude of 10−1 eV. So, the intercalation of hydrogen ions and lithium ones in the MoO3 films can be achieved.
From the above analysis and discussion, both hydrogen and lithium ions can be inserted into the MoO3 films, driven by ILG, irrespective of the ions from the ionic liquid or solid substrate, as depicted schematically in Fig. 6(a and b). The intercalation of hydrogen and lithium ions causes a valence change from Mo6+ to Mo5+, and the formation of the AxMoO3 (A = H+, Li+) phase, which correspondingly leads to the change of the band gap, resistance and color of the MoO3 film. The intercalation of ions in various oxides, such as WO3, MoO3 and SrCoO2.5etc., has been widely studied through various methods. Hydrogen doping induced by ILG often relies on the water content in the ionic liquid or the relative humidity levels of the ambient atmosphere, due to the hydrogen ions originating from them.7,17,24 This work offers a readily available pathway to modulate the microstructural, optical and electronic transformation of the MoO3 material. Moreover, the modulation is independent of the environment around the ILG device, which removes the environmental dependence.
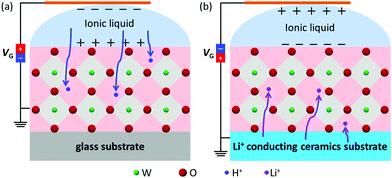 | ||
| Fig. 6 A schematic of the mechanism of electric-field modulation by ILG for the MoO3 films deposited on (a) a glass substrate and (b) a Li+ conducting ceramic substrate. | ||
4. Conclusions
In summary, transparent MoO3 films with (0k0) orientations were deposited on a glass substrate and a Li+ conducting ceramic one. ILG-induced hydrogen or lithium evolution was realized in the MoO3 thin films. The intercalation of hydrogen and lithium ions causes a valence change of the partial Mo ions from Mo6+ to Mo5+, which correspondingly results in the transformation of the electronic and optical properties of the MoO3 films. The decrease of the resistance of the gated MoO3 film by about five orders of magnitude compared with that of the pristine film was observed. And the transparent film was also changed to blue. These findings demonstrate that the ILG-induced evolution of small ions, such as hydrogen and lithium ions etc., can be a general tuning knob to achieve the modulation of microstructures and various properties in different degrees, and to realize the multi-functionalities of the materials, which is promising for potential applications in the field of emulating artificial synapses. Therefore, these results provide a new method for developing synaptic electrolyte-gated transistors, and are a sensible way of designing multifunctional devices.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Science Foundation of China (grant no. 61306109, 61434002, 51571136, 11611540333) the Natural Science Foundation of Shanxi Province (201901D111282), the Project of Returness Scholarship of Shanxi Province (grant no. 2014-044), and the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (grant no. [2014]779).References
- J. Jeong, N. Aetukuri, T. Graf, T. D. Schladt, M. G. Samant and S. S. P. Parkin, Science, 2013, 339, 1402–1405 CrossRef CAS.
- J. Jeong, N. B. Aetukuri, D. Passarello, S. D. Conradson, M. G. Samant and S. S. P. Parkin, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(4), 1013–1018 CrossRef CAS.
- T. Fujimoto and K. Awaga, Phys. Chem. Chem. Phys., 2013, 15, 8983–9006 RSC.
- S. Z. Bisri, S. Shimizu, M. Nakano and Y. Iwasa, Adv. Mater., 2017, 29(1607054), 1–48 Search PubMed.
- Y. Yamada, K. Ueno, T. Fukumura, H. T. Yuan, H. Shimotani, Y. Iwasa, L. Gu, S. Tsukimoto, Y. Ikuhara and M. Kawasaki, Science, 2011, 332, 1065–1067 CrossRef CAS PubMed.
- S. G. Altendorf, J. Jeong, D. Passarello, N. B. Aetukuri, M. G. Samant and S. S. P. Parkin, Adv. Mater., 2016, 28, 5284–5292 CrossRef CAS PubMed.
- N. Lu, P. Zhang, Q. Zhang, R. Qiao, Q. He, H.-B. Li, Y. Wang, J. Guo, D. Zhang, Z. Duan, Z. Li, M. Wang, S. Yang, M. Yan, E. Arenholz, S. Zhou, W. Yang, L. Gu, C.-W. Nan, J. Wu, Y. Tokura and P. Yu, Nature, 2017, 546, 124–128 CrossRef CAS PubMed.
- S. Zhao, Z. Zhou, B. Peng, M. Zhu, M. Feng, Q. Yang, Y. Yan, W. Ren, Z.-G. Ye, Y. Liu and M. Liu, Adv. Mater., 2017, 29(1606478), 1–8 Search PubMed.
- B. Cui, P. Werner, T. Ma, X. Zhong, Z. Wang, J. M. Taylor, Y. Zhuang and S. S. P. Parkin, Nat. Commun., 2018, 9(3055), 1–8 Search PubMed.
- L. Zhang, W. Hou, G. Dong, Z. Zhou, S. Zhao, Z. Hu, W. Ren, M. Chen, C.-W. Nan, J. Ma, H. Zhou, W. Chen, Z.-G. Ye, Z.-D. Jiang and M. Liu, Mater. Horiz., 2018, 5, 991–999 RSC.
- J.-T. Yang, C. Ge, J.-Y. Du, H.-Y. Huang, M. He, C. Wang, H.-B. Lu, G.-Z. Yang and K.-J. Jin, Adv. Mater., 2018, 30(1801548), 1–10 Search PubMed.
- H.-Y. Huang, C. Ge, Q.-H. Zhang, C.-X. Liu, J.-Y. Du, J.-K. Li, C. Wang, L. Gu, G.-Z. Yang and K.-J. Jin, Adv. Funct. Mater., 2019, 29(1902702), 1–8 CAS.
- M. S. Saleem, B. Cui, C. Song, Y. Sun, Y. Gu, R. Zhang, M. U. Fayaz, X. Zhou, P. Werner, S. S. P. Parkin and F. Pan, ACS Appl. Mater. Interfaces, 2019, 11, 6581–6588 CrossRef CAS PubMed.
- M. Wang, X. Sui, Y. Wang, Y.-H. Juan, Y. Lyu, H. Peng, T. Huang, S. Shen, C. Guo, J. Zhang, Z. Li, H.-B. Li, N. Lu, A. T. N’Diaye, E. Arenholz, S. Zhou, Q. He, Y.-H. Chu, W. Duan and P. Yu, Adv. Mater., 2019, 31(1900458), 1–8 CAS.
- C. Leighton, Nat. Mater., 2019, 18, 13–18 CrossRef CAS PubMed.
- K. Shibuya and A. Sawa, Adv. Electron. Mater., 2016, 2(1500131), 1–6 Search PubMed.
- M. Wang, S. Shen, J. Ni, N. Lu, Z. Li, H.-B. Li, S. Yang, T. Chen, J. Guo, Y. Wang, H. Xiang and P. Yu, Adv. Mater., 2017, 29(1703628), 1–7 Search PubMed.
- S. Nishihaya, M. Uchida, Y. Kozuka, Y. Iwasa and M. Kawasaki, ACS Appl. Mater. Interfaces, 2016, 8, 22330–22336 CrossRef CAS PubMed.
- I. A. de Castro, R. S. Datta, J. Z. Ou, A. Castellanos-Gomez, S. Sriram, T. Daeneke and K. Kalantar-zadeh, Adv. Mater., 2017, 29(1701619), 1–31 Search PubMed.
- M. Wang and K. J. Koski, ACS Nano, 2015, 9(3), 3226–3233 CrossRef CAS PubMed.
- P. Meduri, E. Clark, J. H. Kim, E. Dayalan, G. U. Sumanasekera and M. K. Sunkara, Nano Lett., 2012, 12, 1784–1788 CrossRef CAS PubMed.
- H. Kanno, R. J. Holmes, Y. Sun, S. Kena-Cohen and S. R. Forrest, Adv. Mater., 2006, 18, 339–342 CrossRef CAS.
- Z.-H. Tan, X.-B. Yin and X. Guo, Appl. Phys. Lett., 2015, 106(023503), 1–4 Search PubMed.
- C. S. Yang, D. S. Shang, N. Liu, G. Shi, X. Shen, R. C. Yu, Y. Q. Li and Y. Sun, Adv. Mater., 2017, 29(1700906), 1–10 Search PubMed.
- C. Zhang, P. R. Pudasaini, A. D. Oyedele, A. V. Ievlev, L. Xu, A. V. Haglund, J. H. Noh, A. T. Wong, K. Xiao, T. Z. Ward, D. G. Mandrus, H. Xu, O. S. Ovchinnikova and P. D. Rack, ACS Appl. Mater. Interfaces, 2018, 10, 22623–22631 CrossRef CAS PubMed.
- L. E. Firment and A. Ferretti, Surf. Sci., 1983, 129, 155–176 CrossRef CAS.
- J. Yang, X. Wang, X. Jia, Y. Zhang, X. Li, Z. Quan, Z. Zeng and X. Xu, J. Magn. Magn. Mater., 2020, 515(167261), 1–5 Search PubMed.
- Y. Gu, K. Xu, C. Song, X. Zhong, H. Zhang, H. Mao, M. S. Saleem, J. Sun, W. Liu, Z. Zhang, F. Pan and J. Zhu, ACS Appl. Mater. Interfaces, 2019, 11, 19584–19595 CrossRef CAS PubMed.
- J. Zhang, G. W. Zhou, Z. Yan, H. Ji, X. Li, Z. Quan, Y. Bai and X. Xu, ACS Appl. Mater. Interfaces, 2019, 11, 26460–26466 CrossRef CAS PubMed.
- S. Balendhran, S. Walia, H. Nili, J. Z. Ou, S. Zhuiykov, R. B. Kaner, S. Sriram, M. Bhaskaran and K. Kalantar-zadeh, Adv. Funct. Mater., 2013, 23, 3952–3970 CrossRef CAS.
- T. He and J. Yao, J. Photochem. Photobiol., C, 2003, 4, 125–143 CrossRef CAS.
- M. T. Greiner, L. Chai, M. G. Helander, W.-M. Tang and Z.-H. Lu, Adv. Funct. Mater., 2013, 23, 215–226 CrossRef CAS.
- A. Borgschulte, O. Sambalova, R. Delmelle, S. Jenatsch, R. Hany and F. Nüesch, Sci. Rep., 2017, 7, 40761 CrossRef CAS PubMed.
- P.-R. Huang, Y. He, C. Cao and Z.-H. Lu, Sci. Rep., 2014, 4, 7131 CrossRef CAS PubMed.
- J. Z. Ou, J. L. Campbell, D. Yao, W. Wlodarski and K. Kalantar-zadeh, J. Phys. Chem. C, 2011, 115, 10757–10763 CrossRef CAS.
- A. Quintana, E. Menéndez, M. O. Liedke, M. Butterling, A. Wagner, V. Sireus, P. Torruella, S. Estradé, F. Peiró, J. Dendooven, C. Detavernier, P. D. Murray, D. A. Gilbert, K. Liu, E. Pellicer, J. Nogues and J. Sort, ACS Nano, 2018, 12, 10291–10300 CrossRef CAS PubMed.
- H. Guo, D. Goonetilleke, N. Sharma, W. Ren, Z. Su, A. Rawal and C. Zhao, Cell Rep. Phys. Sci., 2020, 1, 100225 CrossRef CAS.
- C. Ritter, W. Müller-Warmuth and R. Schöllhorn, J. Chem. Phys., 1985, 83, 6130–6138 CrossRef CAS.
- W. Xie, M. Su, Z. Zheng, Y. Wang, L. Gong, F. Xie, W. Zhang, Z. Luo, J. Luo, P. Liu, N. Xu, S. Deng, H. Chen and J. Chen, ACS Nano, 2016, 10, 1662–1670 CrossRef CAS PubMed.
- H. Ding, H. Lin, B. Sadigh, F. Zhou, V. Ozoliņš and M. Asta, J. Phys. Chem. C, 2014, 118, 15565–15572 CrossRef CAS.
- L. Chen, A. C. Cooper, G. P. Pez and H. Cheng, J. Phys. Chem. C, 2008, 112, 1755–1758 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |