Metal–organic frameworks based on infinite secondary building units: recent progress and future outlooks
Jun
Guo
 *a,
Xiaomin
Xue
a,
Haibin
Yu
b,
Yulong
Duan
a,
Fangfang
Li
a,
Ye
Lian
a,
Yi
Liu
*a,
Xiaomin
Xue
a,
Haibin
Yu
b,
Yulong
Duan
a,
Fangfang
Li
a,
Ye
Lian
a,
Yi
Liu
 *a and
Meiting
Zhao
*a and
Meiting
Zhao
 *c
*c
aState Key Laboratory of Separation Membranes and Membrane Processes, School of Chemistry, Tiangong University, Tianjin 300387, China. E-mail: junguo@tiangong.edu.cn; yiliuchem@whu.edu.cn
bCenerTech Tianjin Chemical Research & Design Institute Co., Ltd, Tianjin 300131, China
cTianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, Institute of Molecular Aggregation Science, Tianjin University, Tianjin 300072, China. E-mail: mtzhao@tju.edu.cn
First published on 27th June 2022
Abstract
Metal–organic frameworks (MOFs) are a type of inorganic–organic hybrid connected by organic ligands and discrete metal ions/metallic secondary building units (SBUs). Recently, more and more MOF examples based on infinite SBUs (ISBUs) including one-dimensional (1D) rods, two-dimensional (2D) sheets, and even three-dimensional (3D) nets have emerged and exhibited unique physical and chemical properties (e.g., excellent stability, strong light absorption, and fast charge carrier mobility). Hence, rapidly increasing endeavors have been devoted to the structure design, synthesis methodology, property exploration and performance evaluation of novel ISBU MOFs. In this timely review, we will first summarize the established synthesis methods and resolved crystallographic structures of various types of ISBU MOFs. At the same time, the distinct physical and chemical properties resulting from corresponding ISBUs deserve to be highlighted and compared with traditional MOFs consisting of discrete metal ions/SBUs. Moreover, recent progress of those ISBU MOFs achieved in fields of gas adsorption and separation, energy conversion, photocatalysis, etc. is also introduced. We hope it is constructive and attractive for further advancement achieved in this new but intriguing research field.
1. Introduction
Metal–organic frameworks (MOFs) have become a new class of porous materials and have attracted substantial attention in various fields1–6 thanks but not limited to their large surface area, high porosity, and versatile tunability.7–11 In most cases, the metal ions or metallic secondary building units (SBUs) are considered as discrete zero-dimensional (0D) nodes that are further linked by organic ligands for the construction of frameworks.12–15 Distinctly, infinite SBUs (ISBUs) like one-dimensional (1D) chains,16–18 two-dimensional (2D) sheets,19–21 and even three-dimensional (3D) nets22–24 have also emerged in MOFs and been rapidly developed recently. Consequently, MOFs can also be categorized into OD, 1D, 2D, and 3D subclasses according to the ISBU types (Scheme 1). On account of the pure inorganic metal-oxo nature in one dimension at least, ISBU MOFs resemble the corresponding metal oxides/metal hydroxides in terms of physical and chemical properties (e.g., excellent stability, high carrier mobility and dense unit package).21,25–27 To a certain degree, ISBU MOFs can be even considered as special assemblies of metal-oxide/hydroxide nanostructures (e.g. nanowires, nanosheets and nano-frames) and organic linkers. As a result, exceptional performances of ISBU MOFs have been achieved in application fields of adsorption,3,28,29 gas separation,30,31 energy conversion,27,32 biomaterial33 and photocatalysis.34,35 For instance, the 1D chain-like Al-MOF reported the recent state-of-the-art water-harvesting uptake, thanks to its unique channels embraced by the hydrophilic Al–O chains along with polar linkers.3 Moreover, the densely stacking of linkers enforced by 1D MOFs is beneficial for commanding multivariate active sites working in a synergistic way, which is essential to achieve excellent catalytic activity32 and even specific selectivity.30 Analogous to inorganic semiconductor nanomaterials, ISBU MOFs can even exhibit nearly identical energy band structure and comparable even surpassed carrier mobility, as demonstrated in the case of some Ti-MOFs constituted of infinite Ti–O chains18,35–37 and sheets.19 Last but not least, ISBU MOFs are expected to inherit the robust physical and chemical stabilities of corresponding metal inorganics, which are crucial for maintaining long-term performances in various application fields, especially in catalysis.17,38In this review, we will first comprehensively introduce the synthesis and crystal structure of ISBU MOFs according to their central metallic valence states. In order to give a clear and straightforward illustration, many crystallographic structures of ISBU MOFs have been redepicted on basis of the reported crystallographic information files.39,40 However, considering the enormous number of examples, especially 1D MOFs reported so far, only the recently developed or prototype ones are presented in this review, readers who are interested in a more comprehensive summary of 1D MOFs reported before 2017 can refer to another review.41 Secondly, we are going to discuss the performances of ISBU MOFs in versatile fields including gas adsorption, gas separation, energy-related applications and catalysis with attempts to highlight the roles of corresponding ISBU structures. Last, the existing challenges and future outlooks in this infancy but intriguing research field are also proposed based on our personal insights. It is hoped that this review will be constructive and attractive for achieving further advancement for ISBU MOFs in the near future.
2. ISBU MOFs
According to the basic principle of coordination chemistry, the structure type of ISBU among MOFs depends on the coordination mode (e.g., coordination number, geometry pattern and bonding strength) of the central metal ion crucially, which is also related to its bonding orbitals, carried charges, ionic radius and so on. Attempt to give a clear representation to authors, the introduction of each type of ISBU (e.g., 1D, 2D and even 3D) MOFs in this review has been further arranged by the valent state of central metals mainly.2.1 ISBU MOFs constituted of metal(I) central ions
In 2019, Davies et al.42 reported a series of 1D MOFs based on Li+ and Na+ metal centers using silane-based organic linkers. For example, the 1D Li-MOF [IMP-24Li, Li4L1·DMF, in which L1 = 4,4′,4''-(methylsilanetriyl)tribenzoate] was synthesized by mixing lithium nitrate (LiNO3) and H3L1 in DMF at the reaction temperature of 170 °C. IMP-24Li crystallized in a unique chiral space group of P3121, wherein each unit cell contained one L1 ligand, four lithium cations, and one coordination DMF molecule. Caused by the positional disorders within the unit cell, the detailed coordination of IMP-24Li was unable to be accurately measured. Nevertheless, the 1D helical Li–O chain with a pitch of 1.64 nm (Fig. 1a) has been unambiguously observed, which was further linked with each other by L1 bridging linkers to form the open 3D IMP-24 framework (Fig. 1b). Furthermore, the 1D Na-MOF named as IMP-27Na [Na4(L2)(H2O)4(DMF)4, in which L2 = 4,4′,4′′,4′′′-silanetetrayltetrabenzoate] was synthesized by mixing NaNO3 and H4L2 in DMF at 170 °C. Differently, IMP-27Na crystallized in a triclinic system with the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, in which there were four sodium ions, one L1 ligand, four coordinated DMF molecules, and additional two water molecules. Fortunately, the 1D Na–O chain in IMP-27Na has been successfully resolved by single-crystal X-ray diffraction measurement, which was composed of repeat asymmetric units containing four types of Na+ ions (Fig. 1c). Specifically, the 6-coordinated Na1 and Na3 as well as the 7-coordinated Na2 and Na4, were bridged by carboxylate and water oxygens to form the 1D Na–O chain along the c-axis. After linking the neighboring chains together by L2 linkers, the final IMP-27Na with a potential void space of 39% was constructed if the complete removal of all solvents (Fig. 1d).
space group, in which there were four sodium ions, one L1 ligand, four coordinated DMF molecules, and additional two water molecules. Fortunately, the 1D Na–O chain in IMP-27Na has been successfully resolved by single-crystal X-ray diffraction measurement, which was composed of repeat asymmetric units containing four types of Na+ ions (Fig. 1c). Specifically, the 6-coordinated Na1 and Na3 as well as the 7-coordinated Na2 and Na4, were bridged by carboxylate and water oxygens to form the 1D Na–O chain along the c-axis. After linking the neighboring chains together by L2 linkers, the final IMP-27Na with a potential void space of 39% was constructed if the complete removal of all solvents (Fig. 1d).
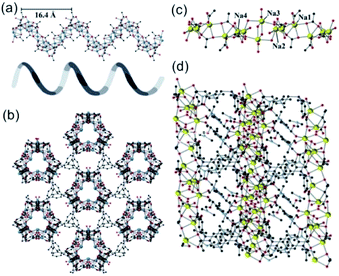 | ||
| Fig. 1 (a) The 1D helical Li–O chain with a pitch of 16.4 Å observed in IMP-24Li; (b) the projection view of the formed 1D Li–O chain along the [001] direction; (c) the structure presentation of 1D Na–O chain among IMP-27Na; (d) the projection view of the solvent-filled IMP-27Na along the [110] direction. Element color: C is black; O is red; Li is blue; Na is yellow. Reprinted with permission from ref. 42. Copyright 2018, American Chemical Society. | ||
Moreover, 2D MOFs based on alkali metal have also been reported.26,43–45 For examples, ULMOF-1 [UL = ultralight, Li2(2,6-NDC), 2,6-NDC = naphthalene-2,6-dicarboxylate]26 and ULMOF-2 [Li2(4,4-BPDC), 4,4-BPDC = 4,4-bisphthalboxylate]43 both with lithium ions as metal centers were constructed by using 2,6-NDC and 4,4-BPDC as the organic ligand, respectively. ULMOF-1 was synthesized by heating LiNO3, 2,6-H2NDC, and ammonia fluoride (NH4F) as an additive in the mixture of DMF and ethanol at 180 °C. According to the crystallographic data, the structural unit of ULMOF-1 was composed of a novel type of 2D Li–O inorganic sheet bridged by aromatic 2,6-NDC linkers. In which, the lithium center was coordinated with four carboxylate oxygens from 2,6-NDC (Fig. 2a). Subsequently, as-resultant Li–O tetrahedrons in neighboring formed a polyhedral dimer via sharing one tetrahedron edge, which was further connected with each other via sharing the remaining oxygen corners to extend to be an infinite 2D LiO2 sheet (Fig. 2c). As building pillars, 2,6-NDC were further spaced at the adjacent LiO2 layers to construct the final 3D ULMOF-1 skeleton. ULMOF-2 presented an isoreticular structure and was synthesized under other identical reaction conditions except for using 4,4-BPDC as the organic linker (Fig. 2e). Thanks to the larger dimensionality of 4,4-BPDC spaced at adjacent LiO2 layers, ULMOF-2 (Fig. 2d) exhibited more open channels along the a-axis in comparison to ULMOF-1.
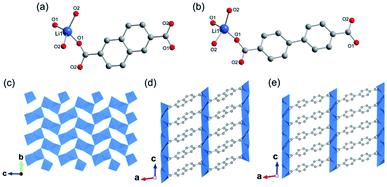 | ||
| Fig. 2 (a and b) Local coordination environment of the lithium-ion in ULMOF-1 and ULMOF-2, respectively. Reproduced with permission from ref. 26. Copyright 2009, American Chemical Society. (c) An [100] orientation view of the 2D Li–O inorganic sheet. (d and e) The [010] projection view of ULMOF-1 and ULMOF-2, respectively. Element color: C is grey; O is red; Li is blue. Reproduced with permission from ref. 43. Copyright 2009, American Chemical Society. | ||
The first 2D Na-MOF MOF-705 [Na4(BDA)(CH3OH)(H2O), BDA = (2S,2′S)-2,2′-(terephthaloylbis(azanediyl))disuccinate] was reported by Yaghi et al.45 MOF-705 was synthesized by heating the methanolic solution involving sodium hydroxide and H4BDA at 50 °C. The obtained product belonged to the chiral monoclinic P21 space group and featured a unique 2D sodium oxide layer in infinite extensions along the [100] and [010] directions. Through lengthening the dimensionality of organic ligand, isoreticular MOF-706 [Na4(BPDA)(CH3OH)(H2O), BPDA = (2S,2′S)-2,2′-(1,1′-biphenyl)-4,4′(dicarbonyl)bis(azanediyl)disuccinate] could be further obtained.
Apart from the common Li+- and Na+-based ISBU MOF examples,42,46,47 heavier alkaline cations like K+ and Rb+ have also been utilized for ISBU MOF constructions.44 A redox tetracarboxylic acid H4(TTF-TC) (Fig. 3a) was reported to react with corresponding alkali chloride via the hydrothermal method for constructions of 1D K2H2(TTF-TC) namely MIL-132(K), and 2D M2H2(TTF-TC) (M = K+ or Rb+) namely MIL-133(K, Rb). Taking the 1D MIL-132(K) for representation, H4(TTF-TC), KCl were mixed in the alkaline water containing KOH as the first step. Due to the thermal susceptibility of H4(TTF-TC), the resultant solution was then heated at a relatively lower reaction temperature (70 °C) to obtain the MIL-132(K) product. According to the resolved crystal structure, there was a novel K–O chain composed of edge-sharing [KO5] polyhedron found along the [010] direction. Bridged by the carboxylate group in a bidentate fashion, two neighboring single chains are linked together in parallel to form the dimerized K–O chain shown in Fig. 3b. Finally, an open framework belonged to the monoclinic system and a P21/c space group was built after TTF-TC linkers being installed within the a–c plane (Fig. 3d). More interestingly, another novel MIL-133(K) phase characteristic of distinct 2D K–O inorganic sheet structures was obtained via simply changing the added amount of KOH (i.e. the pH value) in the hydrothermal system. Under other same conditions, MIL-133(K) crystallized in the orthorhombic system and a higher symmetric Pnmm group, in which the central K+ adopted a tetrahedron coordination mode. After being bridged by carboxylate groups in a bidentate fashion, the isolated [KO4] tetrahedron was compressed into a novel 2D K–O inorganic sheet within the a–b plane. An isoreticular MIL-133(Rb) with slightly expanded cell parameters due to the larger radius of Rb+ could be further obtained by replacing RbCl with KCl simply.
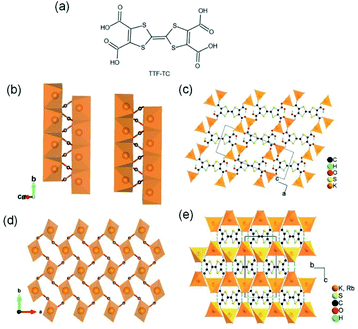 | ||
| Fig. 3 (a) The chemical structure of tetrathiafulvalene tetracarboxylic acid [H4(TTF-TC)]; (b) the dimerized 1D K–O inorganic chain among MIL-132(K) which are bundled with carboxylate group; (c) the structure of MIL-132(K) projected view along the [010] direction; (d) the 2D K–O inorganic sheet among the polymorphic MIL-133(K, Rb); (e) the structure of MIL-133(K, Rb) projected view along the [100] direction. Reproduced with permission from ref. 44. Copyright 2010, American Chemical Society. | ||
For transition metal(I)-based ISBU MOFs,48–50 Bai et al.48 reported the [Cu6I6(L3)3, L3 = 1,4-bis(phenylthio)but-2-yne] with 1D [CuI]n ladders by the acetonitrile-based solvothermal synthesis method. According to the resolved single-crystal structure, the Cu(I) cation was coordinated with three μ3-iodide ions (each was connected with three Cu(I) cations in a bridged way) and hence formed an [CuI]n ladder along the a-axis. In addition, each [CuI]n ladder was linked to four adjacent ones to construct the final 3D network by sulfur donors on L3.
2.2 ISBU MOFs constituted of metal(II) central ions
Yaghi et al.10 reported a series of 1D Mg-MOFs dating back to 2012. Taking the famed Mg-MOF-74 for representation, the reaction of Mg(NO3)2·6H2O and H2L4 (3,3′-dihydroxy-[1,1′-biphenyl]-4,4′-dicarboxylic acid) in the mixed solvent of DMF, H2O and ethanol at 120 °C produced colorless needle-shaped microcrystals after 24 h. The obtained Mg-MOF-74 crystallized in a trigonal system with the R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group, where Mg2+ adopted a 6-coordinated mode with oxygens (three from carboxylate oxygens, two from phenolic hydroxyls and one water oxygen) to form an octahedron (Fig. 4a). These MgO6 octahedrons were further packed into a triple-helical 1D Mg–O chain with a pitch of 6.8 Å along the crystallographic c-axis via edge-sharing (Fig. 4b). Finally, a 3D framework with regular honeycomb channels (1.8 nm, Fig. 4c) was constructed after the installation of organic linkers. Noteworthily, half of the chiral Mg–O chains among the whole 3D framework spiraled clockwise while the others spiraled counterclockwise, thereby eventually behaving a racemic character for the overall framework. Interestingly, the chiral Mg-MOF [Mg2(DOBPTC), DOBPTC4− = 4,4′-di-oxidobiphenyl-3,3′-dicarboxylate] with the same Mg–O chain to Mg-MOF-74 has been further successfully developed by Long.28 Distinct from the centrosymmetric Mg-MOF-74, as-obtained R-Mg2(DOBPTC) crystallized in a trigonal system with the chiral P3221 space group, in which the triple-helical Mg–O chains followed a clockwise spiral homogeneously. Vice versa, the R-Mg2(DOBPTC) crystallized in the chiral P3121 space group and followed a counterclockwise spiral for its Mg–O chains. Moreover, another kind of chiral Mg-MOF structure, which was named as CPF-1, has been synthesized by Feng and his colleagues in 2011.51 The formula of CPF-1 was [Mg2(H2O)2(BPTC), BPTC = 3,3′,5,5′-tetracarboxylate], which was obtained by the hydrothermal reaction of Mg(NO3)2·6H2O and H4BPTC in the water and N-ethylformamide (NEF) mixed solvent at 120 °C for 72 h. Differently, CPF-1 crystallized in a tetragonal system with the chiral I4122 space group, in which Mg2+ was coordinated by four carboxylate oxygens from four BPTC ligands and two water oxygens to form a MgO6 octahedron (Fig. 4d). Furthermore, these octahedrons were connected along the crystallographic c-axis via octahedron corner-sharing, which was twisted into a 1D quadruple spiral with a pitch of 12.3 Å (Fig. 4e). After being linked by BPTC likers, as-formed 1D chiral chains were further assembled into a highly opened 3D framework with ordered cylinder channels (1.3 nm, Fig. 4f).
space group, where Mg2+ adopted a 6-coordinated mode with oxygens (three from carboxylate oxygens, two from phenolic hydroxyls and one water oxygen) to form an octahedron (Fig. 4a). These MgO6 octahedrons were further packed into a triple-helical 1D Mg–O chain with a pitch of 6.8 Å along the crystallographic c-axis via edge-sharing (Fig. 4b). Finally, a 3D framework with regular honeycomb channels (1.8 nm, Fig. 4c) was constructed after the installation of organic linkers. Noteworthily, half of the chiral Mg–O chains among the whole 3D framework spiraled clockwise while the others spiraled counterclockwise, thereby eventually behaving a racemic character for the overall framework. Interestingly, the chiral Mg-MOF [Mg2(DOBPTC), DOBPTC4− = 4,4′-di-oxidobiphenyl-3,3′-dicarboxylate] with the same Mg–O chain to Mg-MOF-74 has been further successfully developed by Long.28 Distinct from the centrosymmetric Mg-MOF-74, as-obtained R-Mg2(DOBPTC) crystallized in a trigonal system with the chiral P3221 space group, in which the triple-helical Mg–O chains followed a clockwise spiral homogeneously. Vice versa, the R-Mg2(DOBPTC) crystallized in the chiral P3121 space group and followed a counterclockwise spiral for its Mg–O chains. Moreover, another kind of chiral Mg-MOF structure, which was named as CPF-1, has been synthesized by Feng and his colleagues in 2011.51 The formula of CPF-1 was [Mg2(H2O)2(BPTC), BPTC = 3,3′,5,5′-tetracarboxylate], which was obtained by the hydrothermal reaction of Mg(NO3)2·6H2O and H4BPTC in the water and N-ethylformamide (NEF) mixed solvent at 120 °C for 72 h. Differently, CPF-1 crystallized in a tetragonal system with the chiral I4122 space group, in which Mg2+ was coordinated by four carboxylate oxygens from four BPTC ligands and two water oxygens to form a MgO6 octahedron (Fig. 4d). Furthermore, these octahedrons were connected along the crystallographic c-axis via octahedron corner-sharing, which was twisted into a 1D quadruple spiral with a pitch of 12.3 Å (Fig. 4e). After being linked by BPTC likers, as-formed 1D chiral chains were further assembled into a highly opened 3D framework with ordered cylinder channels (1.3 nm, Fig. 4f).
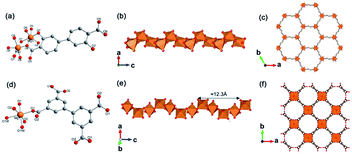 | ||
| Fig. 4 (a) The coordination environment of Mg(II) ions in Mg-MOF-74; (b) the 1D chain structure of Mg-MOF-74 extending along the c-axis; (c) the corresponding 3D framework of Mg-MOF-74 view down the [001] direction. Reproduced with permission from ref. 10. Copyright 2012, AAAS. (d) The coordination environment of Mg(II) ions in CPF-1; (e) the 1D chain structure of CPF-1 extending along the c-axis; (f) the corresponding 3D framework of the CPF-1 view down the [001] direction. Reproduced with permission from ref. 51. Copyright 2017, American Chemical Society. | ||
In addition, 1D MOFs based on other alkaline earth metals have been reported.52–56 For example, Martínez et al.53 synthesized two types of 1D Ca-MOF. The formula of Ca-MOF-1 was [Ca3(BTB)2(DMF)3, BTB = 1,3,5-tris(4-carboxyphenyl)benzene], which was obtained by the hydrothermal reaction of Ca(NO3)2·4H2O and BTB in the water and DMF mixed solvent at 70 °C for 72 h. As obtained Ca-MOF-1 crystallized in a tetragonal crystal system with the chiral space group of R3. In which, there were three kinds of coordination modes for Ca2+ centers (Fig. 5a). Namely, Ca1 and Ca2 were both coordinated by five carboxylate oxygens from four BTB ligands and one amide oxygen from DMF. While Ca3 was coordinated by six carboxylate oxygens from three BTB ligands and additional one amide oxygen from DMF. Interestingly, the edge-shared octahedral dimer of Ca1O6 and Ca2O6 was further connected to two distorted Ca3O7 pentagonal bipyramids at the two heads by corner oxygen-sharing, eventually forming a robust helical Ca–O chain along the [001] direction by unit repeat (Fig. 5b). Furthermore, the as-formed 1D Ca–O chain was linked with each other by BTB linkers to form the final 3D structure with a porosity of 43% (Fig. 5c). The formula of Ca-MOF-2 was [Ca(BDC)(DMF)(OH2), BDC = 1,4-benzenedicarboxylate], which was obtained by heating Ca(NO3)2·4H2O and H2BDC in the water and DMF mixed solvent at 80 °C for 72 h. The obtained Ca-MOF-2 crystallized in a monoclinic crystal system with the space group of P21/c. In which, each Ca2+ was coordinated by six carboxylate oxygens from two distinct BDC ligands, one water oxygen, and one DMF oxygen. Distinct from Ca-MOF-1, all the Ca centers in Ca-MOF-2 adopted an 8-coordination mode by chelated carboxylate groups. The resultant CaO8 polyhedrons were further corner-connected with each other to form the 1D Ca–O chain along the [010] direction. Since larger ionic radius, Sr2+ (ref. 54 and 57) and Ba2+ (ref. 58–60) usually manifested higher coordination numbers among MOFs than the cognate Ca2+. For instance, Wang et al.57 synthesized a 1D Sr-MOF-1 [Sr(BDC)(H2O)(DMF)] by the reaction of Sr(NO3)2 and H2BDC in the H2O and DMF mixture at 100 °C for three days. The obtained Sr-MOF-1 crystallized in a monoclinic system with the P21/c space group, where the Sr2+ exhibited an 8-coordinated environment with six carboxylate oxygens from four BDC ligands, one DMF molecule oxygen and one water molecule oxygen (Fig. 5d). In this MOF, the SrO8 polyhedrons were bridged by carboxylate groups to form a 1D chain along the crystallographic b-direction, which were further linked by BDC linkers to form a 3D framework with rhombic open channels (Fig. 5e and f). What's more, Zhu et al.58 recently constructed a 1D Ba-MOF-1 via reacting BaCl2·2H2O and 1,3,5-tri(2-carboxymethyltetrazol-5-yl)benzene (L5) in the water and DMF mixture at 120 °C. As-obtained Ba-MOF-1 with the formula of [Me2NH2][Ba(L5)(H2O)] crystallized in a monoclinical crystallographic system and a C2/c space group. In which, each Ba2+ was coordinated by nine carboxylate oxygens from six distinct ligands and one water oxygen. Meanwhile, each carboxylate group from L5 chelated to two Ba2+via a tridentate coordination mode (Fig. 5g). As shown in Fig. 5h, the resultant BaO10 sphenocorona polyhedrons were further packed into a 1D infinite [Ba(–CO2)3Ba] chain along the b-axis via sharing three bridging carboxylates (namely sharing one face of the polyhedron). Finally, each 1D chain was linked to four adjacent chains nearby within the a–c plane by four L5 linkers for the construction of a negative-charged 3D network ([Ba(L4)(H2O)]−). To balance the charge, additional [Me2NH2]+ ions produced from the in situ decomposition of DMF were trapped into channels (7 Å) of the network to form the 3D [Me2NH2] [Ba(L4)(H2O)] framework (Fig. 5i). Topologically, the L5 linker was derived as the 3-connected node simply. Meanwhile, the [Ba(–CO2)3Ba] could be simplified to the 1D face-sharing octahedron chain via connecting two types of 7-connected carboxylate C atoms. Hence, a trinodal 3,7,7-connected net with the Schläfli symbol (37·46·52·62·74)(37·46·52·63·73)2(63) could be assigned to the resultant 1D Ba-MOF-1. Martínez et al.53 synthesized another type of 1D Ba-MOF-2 in 2018 using Ba(NO3)2 and 2-amino-1,4-benzenedicarboxylic acid (NH2–H2BDC) in a water–DMF mixed solvent at 80 °C for 72 h. It was formulated as [Ba(NH2–BDC)(DMF)] and crystallized in a trigonal crystallographic system with the chiral P31 space group for Ba-MOF-2. The Ba2+ was an 8-atom coordination mode by seven oxygen atoms from five distinct NH2–BDC linkers and one DMF oxygen. In detail, Ba2+ were connected by NH2–BDC in the tridentate and bidentate bridging way to form a spiral 1D Ba–O chain along the crystallographic c-axis via edge-sharing.
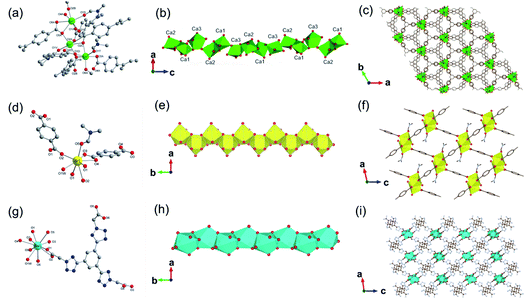 | ||
| Fig. 5 (a) The coordination environment of Ca(II) ions in Ca-MOF-1; (b) the 1D chain structure of Ca-MOF-1 projected along the b-axis; (c) corresponding 3D skeleton structure of Ca-MOF-1 projected along the c-axis. Reproduced with permission from ref. 53. Copyright 2018, Royal Society of Chemistry. (d) The coordination environment of Sr(II) ion in Sr-MOF-1; (e) the 1D chain structure formed by Sr(II) ions; (f) the corresponding 3D porous framework of Sr-MOF-1 after removal of coordinated solvent molecules. Reproduced with permission from ref. 57. Copyright 2021, WILEY-VCH Verlag GmbH. (g) The coordination environment of Ba(II) ion in Ba-MOF-1; (h) the 1D infinite [Ba(–CO2)3Ba] chain projected along the c-axis; (i) the anionic 3D framework ([Ba(L5)(H2O)]−) of Ba-MOF-1. Reproduced with permission from ref. 58. Copyright 2021, Royal Society of Chemistry. | ||
Up to now, there are a few reports of alkaline earth metal (Ca, Sr and Ba)-based 2D MOFs.61–68 In 2008, Loiseau et al.66 synthesized a 2D Ca-MOF-3 named [Ca2(OH)2(NDC), NDC = 2,6-naphathalenedicarboxylate] by reacting Ca(OH)2 and H2NDC in the dilute HNO3 solution at 180 °C for 24 h. The obtained Ca-MOF-3 crystallized in the monoclinic and P21/c space group. Each Ca2+ was the 7-atom coordination with four carboxylate oxygen atoms from four NDC ligands and three hydroxyl groups (Fig. 6a). In detail, the carboxylate oxygens (O1 and O3) of NDC adopted the μ3-bridging fashion with two Ca2+ and one C, as same as the μ3-hydroxyl group (O2) but with three Ca2+ ions. After edge-sharing each CaO7 mono-capped trigonal prism, a 2D lamellar Ca2(OH)2 structure was formed within the b–c plane (Fig. 6b), which was further pillared by NDC linkers along the a-direction to form the 3D framework (Fig. 6c). Chen et al.67 synthesized a 2D Sr-MOF formulated as [Me2NH2]2[Sr5(BTC)4(H2O)5] (BTC = 1,3,5-benzenetricarboxylate) by using Sr(NO3)2 and H3BTC in the DMF-water mixed solution. The Sr-MOF-2 crystallized in the orthorhombic system and the space group of C2221, in which Sr2+ behaved three distinct coordination modes. That was, Sr1 was coordinated by seven carboxylic oxygen atoms from five distinct BTC linkers and one water oxygen, and Sr2 was also coordinated by seven carboxylate oxygen atoms from six distinct BTC linkers and one water oxygen. Moreover, Sr3 was 10-coordinated by eight carboxylate oxygen atoms from four distinct BTC linkers and two water oxygens (Fig. 6d). A crown-like tetranuclear cluster [Sr4(COO)6(O1w)] was formed by two symmetrically related Sr1O8 and Sr2O8 pairs, which were connected with each other via the μ2-oxygens to form a 1D chain. As-formed 1D SrO chains were further bridged by the Sr3O10 polyhedrons to construct a 2D inorganic Sr–O layer (Fig. 6e). Finally, a unique 3D pillar-layered structure was built after linking those 2D Sr–O layers by BTC linkers (Fig. 6f).
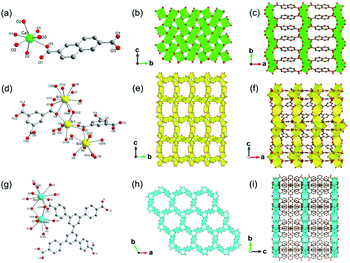 | ||
| Fig. 6 (a) The coordination environment of Ca(II) ion in Ca-MOF-3. O2 were oxygen atoms from the hydroxyl groups; (b) the 2D lamellar structure of the formed Ca–O sheet; (c) the 3D structure of Ca-MOF-3 projected along the c-axis. Reproduced with permission from ref. 66. Copyright 2008, American Chemical Society. (d) The coordination environment of Sr(II) ions in Sr-MOF-2; (e) the 2D inorganic Sr–O layer of Sr-MOF-2; (f) the 3D cylindrical framework of Sr-MOF-2 view down the b-axis. Reproduced with permission from ref. 67. Copyright 2013, Elsevier Inc. (g) The coordination environment of Ba(II) ions in Ba-MOF-3; (h) the 2D inorganic Ba–O–Ba layer view down the c-axis; (i) the 3D open structure of formed Ba-MOF-3 view down the a-axis. Reproduced with permission from ref. 68. Copyright 2014, Royal Society of Chemistry. | ||
Feng et al.68 reported a 2D Ba-MOF-3 with the formula of [Me2NH2+]2 [Ba5(L6)3(H2O)6] (L6 = 3,3′,5,5′-biphentetrolate) by using Ba(NO3)2 and H4L6 in the DMF-water mixed solution at 140 °C for 48 h. The Ba-MOF-3 crystallized in the tetragonal system and the P6/m space group. In this framework, Ba2+ had two coordination modes including the 10-coordinated Ba1 with four chelated carboxylate groups from four distinct ligands and two coordinated water oxygens, and the 9-coordinated Ba2 with six monodentate carboxylate oxygen atoms from six distinct ligands and three terminal water oxygens (Fig. 6g). Interestingly, each Ba1O10 polyhedron was connected to two Ba2O9 polyhedrons, and meanwhile, each Ba2O9 polyhedron was connected to three neighboring Ba1O10 polyhedrons via face-sharing, forming a 2D honeycomb Ba–O inorganic layer (Fig. 6h) consequently. Furthermore, these lamellar structures were connected by the tetratopic ligands along the crystallographic c-axis to form the final 3D framework with the charge neutralization of Me2NH2+ (Fig. 6i).
One can easily find that there are more examples of alkali earth metal-based ISBU MOFs thanks to their higher coordination numbers and stronger coordination strength in comparison to the group I metals. Even more, unique and rare MOF examples characteristic of the corresponding 3D metal-oxo net were also reported.24,69–72 For example, Henderson and his colleagues71 synthesized a type of 3D Mg-MOF-1 [Mg3(O2CH)6] by using Mg(NO3)2·H2O and formic acid in DMF at 110 °C for 40 h. The Mg-MOF-1 product crystallized in the monoclinic crystallographic system and the P21/c space group, in which Mg2+ had four kinds of coordination environments. Namely, Mg1 was coordinated by six μ2-O, both Mg2 and Mg4 were coordinated by four monodentate oxygens and two μ2-O, while Mg3 was coordinated by two monodentate oxygens and four μ2-O, noting that all oxygen atoms came from formates (Fig. 7a). Then, the formed Mg3O6 was connected to Mg1O6 by edge-sharing, while Mg2O6 and Mg4O6 were both connected to Mg1O6 by corner-sharing, thus forming a complex 3D Mg–O inorganic net (Fig. 7b and c) with exposing open 1D channels view down the b-axis (Fig. 7b). Ok et al.72 synthesized a type of 3D Sr-MOF named CAUMOF-8 [Sr2(BTC)(NO3)] by using Sr(NO3)2, N(CH3)4Cl, and H3BTC in the HNO3 and DMF mixture at 180 °C for 72 h. The CAUMOF-8 crystallized in the hexagonal crystallographic system and the P![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 2c space group. In which, two types of Sr2+ with different coordination modes were observed according to the single-crystal diffraction characterization. In detail, Sr1 was a 9-coordination and formed a Sr1O9 polyhedron, while Sr2 was an 8-coordination and formed a Sr2O8 polyhedron (Fig. 7d). Within the a–b plane, there were three Sr2O8 polyhedrons packed together to form a trimer via edge-sharing, which was further bridged by face-shared Sr1O9 polyhedrons to construct an infinite Sr–O inorganic sheet with exposure of 1D open channels (Fig. 7e). Along the c-axis, as Sr1O9 polyhedrons were further connected with each other via edge-sharing, a unique 3D Sr–O inorganic net was obtained (Fig. 7f). Thanks to the pure inorganic skeleton, CAUMOF-8 has exhibited excellent thermal stability up to 520 °C according to the thermogravimetric analysis (TGA) result.
2c space group. In which, two types of Sr2+ with different coordination modes were observed according to the single-crystal diffraction characterization. In detail, Sr1 was a 9-coordination and formed a Sr1O9 polyhedron, while Sr2 was an 8-coordination and formed a Sr2O8 polyhedron (Fig. 7d). Within the a–b plane, there were three Sr2O8 polyhedrons packed together to form a trimer via edge-sharing, which was further bridged by face-shared Sr1O9 polyhedrons to construct an infinite Sr–O inorganic sheet with exposure of 1D open channels (Fig. 7e). Along the c-axis, as Sr1O9 polyhedrons were further connected with each other via edge-sharing, a unique 3D Sr–O inorganic net was obtained (Fig. 7f). Thanks to the pure inorganic skeleton, CAUMOF-8 has exhibited excellent thermal stability up to 520 °C according to the thermogravimetric analysis (TGA) result.
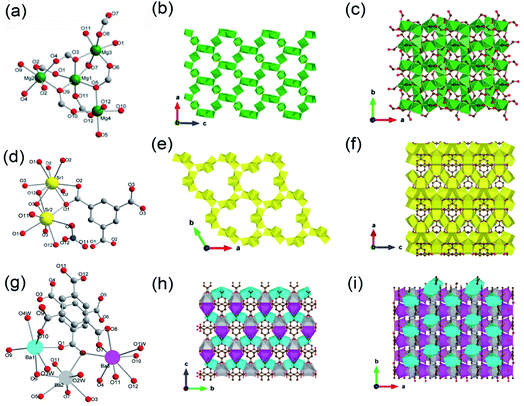 | ||
| Fig. 7 (a) The coordination pattern diagram of the Mg center among Mg-MOF-1; (b) A 2D surface map formed among the Mg-MOF-1 projected along the b-direction; (c) the local view of the Mg-MOF-1 projected along the c-direction. Reproduced with permission from ref. 71. Copyright 2006, American Chemical Society. (d) The coordination pattern diagram of the Sr center among CAUMOF-8; (e) a 2D surface map formed by the CAUMOF-8 projected along the c-direction; (f) the local view of the CAUMOF-8 projected along the b-direction. Reproduced with permission from ref. 72. Copyright 2011, American Chemical Society. (g) The coordination pattern diagram of the Ba center among Ba-MOF-4; (h) a 2D surface map formed by Ba-MOF-4 projected along the a-direction; (i) the local view of the Ba-MOF-4 projected along the c-direction. Reproduced with permission from ref. 70. Copyright 2012, American Chemical Society. | ||
The first 3D Ba-MOF-4 structure was reported by Yao and his colleagues,70 who carried out the synthesis by dissolving Ba(NO3)2 and H3BTC in the mixed water and DMF solution at room temperature for 30 minutes, and then reacted at 110 °C for 4 days. The Ba-MOF-4 product with the molecular formula of [Ba3(BTC)2(H2O)4] crystallized in the orthorhombic crystal system and Pna21 space group. In a cell unit, there were three types of crystallographic independent Ba(II) ions (Ba1, Ba2 and Ba3), two BTC linkers and four coordinated water molecules. Ba1 was coordinated to four carboxylate oxygens from four independent BTC ligands and three hydroxyl oxygens, forming a slightly distorted Ba1O7 triangular prism. Ba2 was eight-coordinated with seven oxygen atoms from seven different BTC linkers and one terminal water oxygen, forming a Ba2O8 square anti-prismatic structure. While Ba3 was nine-coordinated by eight carboxylate oxygens from five different BTC linkers and one terminal water ligand, thereby adopting a Ba3O9 single-capped square anti-prismatic geometry (Fig. 7g). Furthermore, each Ba1O7 polyhedron was linked to one Ba2O8 and one Ba3O9via edge-sharing, and to another Ba3O9via corner-sharing. At the same time, the Ba2O8 polyhedron was linked to one corner-shared Ba3O9 additionally (Fig. 7h). As a result, the accumulation of these polyhedrons formed a unique 3D inorganic Ba–O framework finally (Fig. 7i).
Transition metals have additional d-orbitals available for bonding with linkers, therefore transition metal-based ISBU MOFs exerted better stability normally and have been widely studied.73–79 He and his colleagues76 synthesized a series of 1D Fe(II)-MOF characteristic of excellent thermal stability. Taking the Fe(II)-MOF-1 [Fe(6-H2MNA)2, 6-H2MNA = 6-mercaptonicotinic acid] for representation, it was produced by the reaction of [CpFe(CO)2, Cp = cyclopentadienyl] and 6-H2MNA at 160 °C for 2 days in a mixed solvent of toluene and water. Fe(6-H2MNA)2 crystallized in a monoclinic system with the P21/c space group, wherein Fe2+ was 6-atom coordinated by four S atoms and two carboxylate oxygens from two different 6-H2MNA linkers, thereby forming a FeS4O2 octahedron (Fig. 8a). After bridging by μ2-S atoms, the polyhedron was packed into an infinite [FeO2S2]n chain along the a-axis, which was further connected with four neighboring ones by the 6-H2MNA linker to form the final 3D framework (Fig. 8b and c). Ji et al.79 used 1,4-bis(2-methylbenzimidazol-1-methylmethyl)benzene (BMB), H4BPTC and Cd(NO3)2·4H2O to obtain a 1D Cd-MOF-1 [Cd2(BMB)0.5(BPTC)] in water at 160 °C for 4 days. The obtained Cd-MOF-1 crystallized in the triclinic system and the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, in which Cd2+ had two types of coordination modes. Namely, Cd1 was coordinated by seven carboxylate oxygens from five different BPTC linkers and Cd2 was coordinated by five carboxylate oxygens from four different BPTC and one N atom from the BMB (Fig. 8d). Two edge-sharing Cd1O7 polyhedrons and two edge-sharing Cd2O5N polyhedrons were joined as the repeat unit by corner-sharing, which additionally shared head and tail corners to form the corresponding 1D chain (Fig. 8e). Finally, those 1D chains were connected by BPTC linkers to construct a 3D framework (Fig. 8f). Moreover, Pan et al.75 reported a novel 1D heterometallic MOF [Pb4Cu2I2(PDC)4(DMF)6, PDC = 3,5-pyridinedicarboxylate] recently, which was synthesized by mixing CuI, PbI2, and H2PDC in the DMF solution at room temperature for 4 days. According to the single-crystal X-ray characterization, the obtained PbCu-MOF-1 crystallized in the monoclinic system and the P21/c space group. In detail, there were two kinds of Pb coordinations in the repeated unit. That was, the 8-coordinated Pb1 by oxygens from 5 distinct PDC linkers and one DMF molecule, and the 5-connected Pb2 by three bridging carboxylate oxygens and two DMF oxygens (Fig. 8g). Correspondingly, the formed Pb1O8 polyhedron and Pb2O5 bipyramid were bridged by shared carboxylate groups from PDC, resulting in the 1D infinite Pb–O chain along the [100] direction (Fig. 8h). Furthermore, discrete Cu2I2 SBU as additional joint sites via binding to the pyridyl nitrogens of PDC, linked four adjacent 1D chains to construct the final 3D framework (Fig. 8i). Guo et al.80 synthesized a novel 2D PbAg-MOF-1 [AgPb(BTC)(H2O)] by the hydrothermal reaction of Pb(OAc)2·3H2O, AgNO3, H3BTC and NaOH in water at 170 °C for 3 d. As-obtained PbAg-MOF-1 crystallized in a triclinic crystal system with the space group of P
space group, in which Cd2+ had two types of coordination modes. Namely, Cd1 was coordinated by seven carboxylate oxygens from five different BPTC linkers and Cd2 was coordinated by five carboxylate oxygens from four different BPTC and one N atom from the BMB (Fig. 8d). Two edge-sharing Cd1O7 polyhedrons and two edge-sharing Cd2O5N polyhedrons were joined as the repeat unit by corner-sharing, which additionally shared head and tail corners to form the corresponding 1D chain (Fig. 8e). Finally, those 1D chains were connected by BPTC linkers to construct a 3D framework (Fig. 8f). Moreover, Pan et al.75 reported a novel 1D heterometallic MOF [Pb4Cu2I2(PDC)4(DMF)6, PDC = 3,5-pyridinedicarboxylate] recently, which was synthesized by mixing CuI, PbI2, and H2PDC in the DMF solution at room temperature for 4 days. According to the single-crystal X-ray characterization, the obtained PbCu-MOF-1 crystallized in the monoclinic system and the P21/c space group. In detail, there were two kinds of Pb coordinations in the repeated unit. That was, the 8-coordinated Pb1 by oxygens from 5 distinct PDC linkers and one DMF molecule, and the 5-connected Pb2 by three bridging carboxylate oxygens and two DMF oxygens (Fig. 8g). Correspondingly, the formed Pb1O8 polyhedron and Pb2O5 bipyramid were bridged by shared carboxylate groups from PDC, resulting in the 1D infinite Pb–O chain along the [100] direction (Fig. 8h). Furthermore, discrete Cu2I2 SBU as additional joint sites via binding to the pyridyl nitrogens of PDC, linked four adjacent 1D chains to construct the final 3D framework (Fig. 8i). Guo et al.80 synthesized a novel 2D PbAg-MOF-1 [AgPb(BTC)(H2O)] by the hydrothermal reaction of Pb(OAc)2·3H2O, AgNO3, H3BTC and NaOH in water at 170 °C for 3 d. As-obtained PbAg-MOF-1 crystallized in a triclinic crystal system with the space group of P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . According to the well-resolved crystallographic structure, each Ag+ was a 5-atom coordination fashion with four carboxylate oxygens from four inequivalent BTB linkers and one water oxygen. While, each Pb2+ was a 7-atom coordination fashion with seven carboxylate oxygens from five inequivalent BTB linkers to form a PbO7 polyhedron. Interestingly, the 1D Ag–O chain formed via edge-shared AgO5 polyhedrons and the 1D Pb–O chain connected via edge-shared PbO7 polyhedrons were parsed in the cell. Moreover, those chains were connected with each other via SBUs corner-sharing to form a condensed 2D Ag–O–Pb plate within the a–c plane. Finally, the 2D structure was further linked by BTC to form the final 3D framework.
. According to the well-resolved crystallographic structure, each Ag+ was a 5-atom coordination fashion with four carboxylate oxygens from four inequivalent BTB linkers and one water oxygen. While, each Pb2+ was a 7-atom coordination fashion with seven carboxylate oxygens from five inequivalent BTB linkers to form a PbO7 polyhedron. Interestingly, the 1D Ag–O chain formed via edge-shared AgO5 polyhedrons and the 1D Pb–O chain connected via edge-shared PbO7 polyhedrons were parsed in the cell. Moreover, those chains were connected with each other via SBUs corner-sharing to form a condensed 2D Ag–O–Pb plate within the a–c plane. Finally, the 2D structure was further linked by BTC to form the final 3D framework.
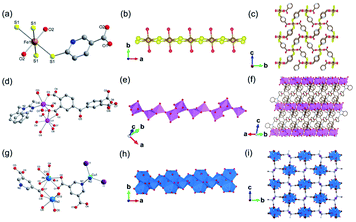 | ||
| Fig. 8 (a) The coordination mode of Fe(II) ion among the Fe(II)-MOF-1; (b) the 1D chain structure of [FeO2S2]n; (c) the 3D framework structure of Fe(II)-MOF-1 projected along the a-axis. Reproduced with permission from ref. 76. Copyright 2021, American Chemical Society. (d) The coordination modes of Cd(II) ions among Cd-MOF-1; (e) the 1D chain structure of Cd-MOF-1; (f) the 3D framework structure of Cd-MOF-1 projected along the a-axis. Reproduced with permission from ref. 79. Copyright 2017, Elsevier Inc. (g) The coordination modes of Pb(II) and Cu(I) among the 1D PbCu-MOF-1; (h) An infinite 1D chain formed by the connection of Pb(II)-oxo polyhedrons; (i) the 3D framework structure of PbCu-MOF-1 view down the [100] direction. Reproduced with permission from ref. 75. Copyright 2021, American Chemical Society. | ||
With concerns on 3D examples,22,23 Lin et al.23 constructed a 3D [Cd3Na6(BTC)4(H2O)12] via reacting Cd(CH3COO)2·2H2O, H3BTC and NaOH in the water–dimethylacetamide mixture at 130 °C for 5 days. The obtained CdNa-MOF-1 crystallized in the tetragonal system and the P42/n space group. In one unit, Cd2+ had two kinds of coordination modes. Namely, Cd1 was coordinated by six carboxylate oxygens from four distinct BTC linkers to form a distorted octahedron, and Cd2 was rarely coordinated by eight carboxylate oxygens from four distinct BTC linkers to form a polyhedron of Cd2O8. Thanks to the bridging of BTC linkers, a 3D single Cd3(BTC)4 framework whose building unit was composed of two Cd1O6 octahedrons, one Cd2O8 polyhedron and four BTC linkers was formed. Interestingly, the potential cavities of a single [Cd3(BTC)4]n framework were interpenetrated with another set of identical framework for energy minimization in thermodynamics. As the secondary metallic central, Na+ had two kinds of coordination modes. That was, Na1 was coordinated by four carboxylate oxygens from four distinct BTC linkers and two μ2-H2O oxygens to form a distorted octahedron, and Na2 was coordinated by four oxygens from four μ2-H2O and two terminal water oxygens to form a distorted octahedron (Fig. 9a). Those polyhedrons including two Na1O6 and one Na2O6 formed a Na3O12 trimer via face-sharing, which further acted as the bridge across the two sets of interpenetrated [Cd3(BTC)4]n frameworks to construct the final 3D MOF (Fig. 9b and c).
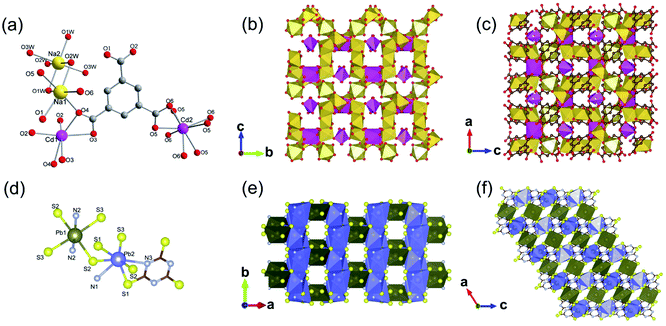 | ||
| Fig. 9 (a) The coordination environment of Cd(II) ions and Na(I) ions among the 3D CdNa-MOF-1; (b and c) the polyhedral structure diagram of CdNa-MOF-1 within the b–c plane and a–b plane, respectively. Reproduced with permission from ref. 23. Copyright 2011, American Chemical Society. (d) The coordination environment of Pb(II) ions among KGF-1; (e and f) the polyhedral structure diagram of KGF-1 within the a–b plane and a–c plane. Reproduced with permission from ref. 22. Copyright 2019, American Chemical Society. | ||
In 2019, there was a type of 3D Pb(II) MOF (KGF-1) reported by Tanaka et al.22 KGF-1 with the empirical formula of Pb3TTC2‧2H2O (TTC = trithiocyanuric acid) was obtained by reacting Pb(NO3)2 and TTC in the mixed DMSO and water solution at 100 °C for 24 h. In this framework, there were two coordination environments for central Pb2+, which gave rise to corresponding Pb1S4N2 and Pb2S5N2 polyhedrons as shown in Fig. 9d. Accordingly, a 1D chain-like SBU extended infinitely along the b-axis via edge-shared packing of Pb2S5N2 polyhedrons (Fig. 9e). As the joint, the 0D Pb1S4N2 polyhedron was connected to four neighboring 1D chains via corner-sharing, as illustrated by the 2D projection view of the constructed framework along the b-axis (Fig. 9f).
2.3 ISBU MOFs constituted of metal(III) central ions
1D Al-MOFs have been substantially found and investigated thanks to their unique breathing dynamics, outstanding stabilities and abundant resource on the earth.3,81–88 For example, Yaghi et al.3 synthesized the 1D Al-MOF through the reaction of AlCl3 and 1H-pyrazole-3,5-dicarboxylic acid (H2PZDC) in an alkaline aqueous solvent at 100 °C. The obtained MOF-303 with the molecular formula [Al(OH)(PZDC)] belonged to the monoclinic crystal system and P21/c space group. Among it, Al3+ centers were all coordinated by four carboxylate oxygens from four different ligands and two hydroxyl ions (Fig. 10a). The resulting AlO6 octahedrons were arranged into 1D infinite helical [Al(–OH)Al] chains by sharing polyhedron corners along the a-axis (Fig. 10b). Moreover, adjacent [Al(–OH)Al] chains were linked by PZDC linkers to construct the open 3D network with a xhh topology (Fig. 10c). By simply replacing H2PZDC with the H2FDC (furan-2,4-dicarboxylic acid), isoreticular MOF-333 [Al(OH)(FDC)] with nearly identical crystallographic structures was obtained as well. Reinsch and his colleagues86 constructed another 1D Al-MOF example by allowing Al2(SO4)3·18H2O and citraconic acid to react in an alkaline aqueous solution at 100 °C. The produced CAU-15-Cit with the molecular formula of [Al2(OH)4(O2C–C3H4–CO2)] was ascribed to the monoclinic system and the C2/c space group. Among CAU-15-Cit, each Al3+ was coordinated by four oxygen atoms from hydroxyl ions and two carboxylate oxygens coming from two different citraconates (Fig. 10d). Similarly, the resultant AlO6 octahedrons were packed into 4-fold helical Al–O chains along the c-axis by sharing polyhedron edges (Fig. 10e). Moreover, adjacent chains were interconnected by citraconate linkers together to form 2D infinite layers which were further stacked in an AAA fashion to build the 3D framework finally (Fig. 10f). Such 1D Al–O ISBU has also been observed elsewhere88 and not described duplicately herein.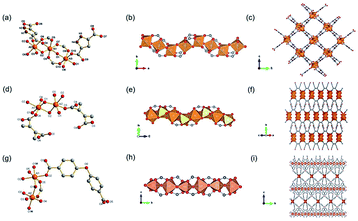 | ||
| Fig. 10 (a) The coordination environment of Al(III) ions among MOF-303. O1, O2 were both oxygen atoms from the hydroxyl groups; (b) the 1D infinite [Al(–OH)Al] chain extending along the a-axis; (c) the 3D framework structure of MOF-303. Reproduced with permission from ref. 3. Copyright 2021, AAAS. (d) The coordination environment of Al(III) ions among CAU-15. O1, O2 were both oxygen atoms from the hydroxyl groups; (e) the 1D chain structure condensed by AlO6 octahedrons; (f) the 3D porous framework of CAU-15. Reproduced with permission from ref. 86. Copyright 2018, Royal Society of Chemistry. (g) The coordination environment of Al(III) ions among CAU-8-OBD; (h) the 1D chain structure pattern of CAU-8-OBD running along the b-axis; (i) the 3D framework of CAU-8-OBD. Reproduced with permission from ref. 84. Copyright 2017, American Chemical Society. | ||
One more example of Al-MOFs needs to be mentioned, which was synthesized by the reaction between 4,4′-oxydibenzoic acid (H2OBD) with Al(NO3)3·9H2O in a water–DMF mixture at 120 °C.84 The produced 1D CAU-8-OBD with the formula of [Al(HODB)2(OH)] crystallized in the tetragonal crystal system and the I41/a space group. Specifically, each Al3+ was coordinated by four carboxylate oxygens and two aqueous oxygens (Fig. 10g). Unusually, the resulting AlO6 octahedrons formed two types of 1D Al–O chains by corner-sharing that extended independently along the a- and b-axis, respectively (Fig. 10h). Furthermore, those mutually perpendicular chains were connected with each other by the V-shaped ODB linkers, thus constructing a 3D framework with spherical cavities of a diameter of 6.4 Å (Fig. 10i).
Among the group III elements, Ga3+ (ref. 89–91) and In3+(ref. 92–94) are rarely reported as corresponding ISBU MOFs. Regarding In-MOF, Reinsch et al.93 obtained 1D CAU-43 In(OH)[Fe(C5H4)2(COO)2] via using the conventional solvothermal method. In detail, CAU-43 was synthesized by the reaction of InCl3 and 1,1′-ferrocenedicarboxlic acid (H2FcDC) in a water–DMF mixture at 80 °C. As-produced CAU-43 crystallized in the monoclinic crystal system and the C2/c space group. In which, In3+ also adopted a 6-connected mode with five carboxylate oxygen atoms from FcDC and the remaining one from water (Fig. 11a). The resulting InO6 octahedra were further packed into 1D quadruple chiral helical In–O chains along the c-axis via shared corners (Fig. 11b), which were further connected by FcDC linkers to construct a 3D framework with tetragonal channels (Fig. 11c). In addition to CAU-43, another MOF product with similar 1D In–O inorganic chains was designed and synthesized by changing the metal source to In(NO3)3 and reaction solvent to the water/methanol/acetic acid-based ternary mixture. As-produced In-MIL-53-FcDC_a with the molecular formula of In(OH)[Fe(C5H4)2(COO)2] crystallized in the same space group and also adopted the 6-coordination for central In3+. However, InO6 octahedrons were found packed into a straight achiral In–O chain via the co-occupation of para-corners. Furthermore, adjacent chains were connected by FcDC linkers to assemble a 3D framework with narrower pores than CAU-43. As a rare example, Stock and his colleagues91 report a 1D Ga-MOF [Ga(OH)(TDC), CAU-51] synthesized from Ga(NO3)3 and 2,5-thiophenedicarboxylic acid (H2TDC) in an acetic acid and water (v/v, 5/1) mixture at 120 °C. CAU-51 was assigned to the monoclinic crystal system and the P21/c space group, where Ga3+ was coordinated by four carboxylate oxygens from four different linkers and two oxygens from water (Fig. 11d). The resulting GaO6 octahedra formed a 1D quadruple chiral helical chain extending infinitely along the a-axis by sharing the edges of the octahedra (Fig. 11e). These chains were further interconnected by TDC linkers and formed square 1D channels with a diameter of about 6.2 Å along the [100] direction (Fig. 11f). Note that, this structure was isoreticular to the previously described MOF-303 in fact. Bi(III) normally exhibits similar coordination fashions89,95,96 to group III elements and therefore was introduced herein by exemplifying one Bi-MOF example. Stock et al.96 used Bi(NO3)3·5H2O and 1,3,5-triazine-2,4,6-tribenzoate (TATB) in the DMF/CH3OH mixed solution to construct corresponding Bi-MOF [Bi2(O)(OH)(TATB), CAU-35] which crystallized in the orthorhombic crystal system and the Pna21 space group. Among it, Bi3+ were all 7-coordinated but with two different coordination environments. Specifically, Bi1 was linked to three carboxylate oxygens of three different ligands, three μ4-oxo ions and one μ3-OH−. While Bi2 was also linked to four carboxylate oxygens of three different ligands but one μ4-oxo ion and two μ3-OH− ions (Fig. 11g). Interestingly, the Bi1O7 polyhedrons were interconnected into a chain by sharing edges, while the Bi2O7 polyhedron also formed a 1D chain but by sharing corners. Moreover, both two Bi1O and Bi2O chains were bundled to give a 1D chain tetramer via sharing corners (Fig. 11h). After the connection of tridentate TATB linkers, the final 3D framework was built with rhombic 1D channels view down the c-axis (Fig. 11i).
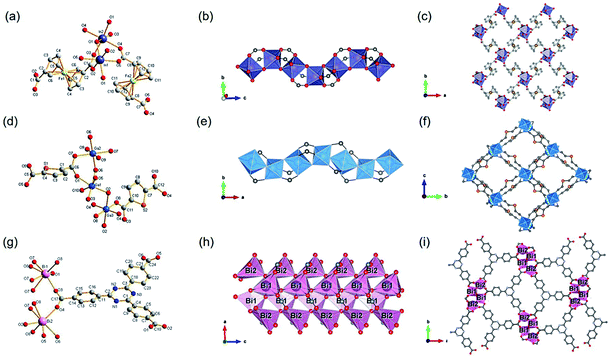 | ||
| Fig. 11 (a) The coordination environment of In(III) ions among CAU-43; (b) the 1D infinite InO6 octahedral chain running along the c-axis; (c) the 3D framework structure of CAU-43 vie down the c-axis. Reproduced with permission from ref. 93. Copyright 2020, American Chemical Society. (d) The coordination environment of Ga(III) ions among Ga-CAU-51. O2, O6 were both hydroxyl oxygen atoms; (e) the 1D chain structure of Ga–O running along the a-axis; (f) the 3D porous framework of CAU-51 projected at the a-axis. Reproduced with permission from ref. 91. Copyright 2021, American Chemical Society. (g) The coordination environment of Bi(III) ions among CAU-35. O7 was from μ4-O2− ions and O8 was from μ3-OH− ions; (h) the 1D chain ISBU pattern of CAU-35; (i) the projected view of 3D framework of CAU-35 along the c-axis. Reproduced with permission from ref. 96. Copyright 2017, Royal Society of Chemistry. | ||
Besides, ISBU MOFs based on transition metals(III) have also been prevailingly studied.21,31,97–100 The recently emerged CAU-50 (ref. 99) [Sc2(FcDC)3] was a 1D Sc-MOF constructed by reacting Sc(NO3)3·5H2O with H2FcDC in a mixture of water and DMF at 95 °C. CAU-50 crystallized in the orthorhombic crystal system and the Pnna space group, where the Sc3+ center was attached by six carboxylate oxygens from different linkers (Fig. 12a). An infinite ScO6 octahedral chain along the a-axis was formed by the μ2-bridged carboxylate group (Fig. 12b). Because of the rotatable conformation of FcDC linker, each chain was connected to four adjacent chains via FcDC linkers of the antiperiplanar (177.1°, a torsion angle between two carboxylate groups on FcDC) and the anticlinical (136.1°) conformations, thus constructing a 3D framework with relatively narrow cavities (Fig. 12c). What's more, FcDC linkers of the synclinical conformations (44.2°) were also observed as the capping modulator attached to the 1D chain. Recently, Schröder et al.31 constructed a 1D Cr-MOF [Cr2(OH)2(L7), denoted as MFM-300(Cr)] by the reaction of CrCl3·6H2O and biphenyl-3,3′,5,5′-tetracarboxylic acid (H4L7) in dilute chloride acid aqueous solution. Among MFM-300(Cr), each Cr3+ was also 6-coordinated by four carboxylate oxygens and two hydroxyl oxygens (Fig. 12d). The formed CrO4(OH)2 octahedron further extended its dimension along the c-axis to form a 1D polyhedral chain bridged by carboxylate and μ2-OH groups (Fig. 12e). Finally, adjacent 1D chains were connected by L7 among them to assemble into a highly open 3D framework with ordered cylindrical channels (Fig. 12f). Öhrström et al.21 reported a type of 1D Gd-MOF from the reaction of Gd(NO3)3·6H2O with benzene-1,2,4,5-tetracarboxylate (H4BTEC), 4,4′-azopyridine in a mixture of DMF and acetic acid. The central Gd3+ among the obtained CTH-16 [Gd2(BTEC)2] was coordinated by nine carboxylate oxygens from six different BTEC ligands (Fig. 12g), and the resulting GdO9 polyhedron formed corresponding 1D Gd–O chain extending indefinitely along the a-direction through sharing polyhedron edges (Fig. 12h). Furthermore, adjacent chains were bridged by BTEC ligands to form a bi-nodal htp net with square-like open channels (Fig. 12i). Moreover, the same research group reported the 2D Ce(III)-MOF by reacting Ce(NO3)3·6H2O with H4BTEC in a mixture of DMF and acetic acid.21 The obtained product (denoted as CTH-15 thereafter) had the molecular formula of [Ce3(BTEC)(HBTEC)(OAc)(HCO2)] and crystallized in the monoclinic crystal system and the P21/c space group. As shown in Fig. 13a, three symmetry-independent Ce(III) ions were observed with 10–12 oxygens-coordinated modes. With the Ce1–O polyhedra as the center, a polyhedral trimer was formed via the combination of one Ce2–O and Ce3–O polyhedron through sharing faces (Fig. 13b). As-formed polyhedral trimer was sequentially connected with each other via a face-to-face shared fashion along the b-axis and via an edge-by-edge shared fashion along the c-axis, thereby forming an infinite 2D Ce–O mesh structure within the b–c plane (Fig. 13c). Finally, adjacent Ce–O layers were connected by carboxylate groups of BTEC linkers to assembly into the 3D CTH-15 (Fig. 13d).
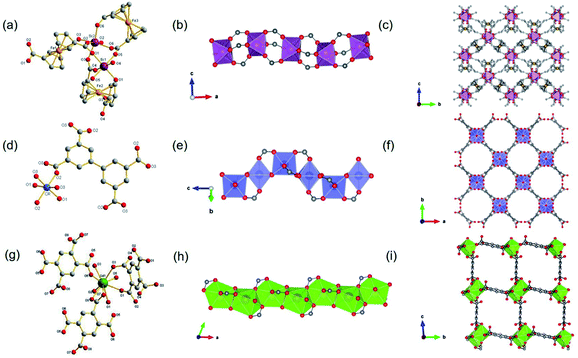 | ||
| Fig. 12 (a) The coordination environment of Sc(III) ions among CAU-50; (b) The 1D infinite Sc–O chain extending along the a-axis; (c) the 3D framework structure of CAU-50 view down the a-axis. Reproduced with permission from ref. 99. Copyright 2020, Royal Society of Chemistry. (d) The coordination environment of Cr(III) ions among MFM-300(Cr); (e) the 1D chain structure formed by CrO6 polyhedrons; (f) the 3D porous framework of MFM-300 projected down the c-axis. Reproduced with permission from ref. 31. Copyright 2014, Royal Society of Chemistry. (g) The coordination environment of Gd(III) ions among CTH-16; (h) the 1D chain ISBU pattern of CTH-16; (i) the [100] projection of 3D framework of CTH-16. Reproduced with permission from ref. 21. Copyright 2021, Elsevier Inc. | ||
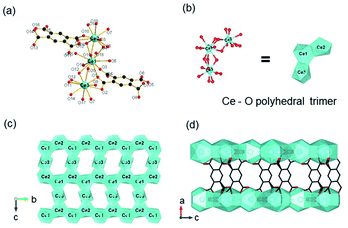 | ||
| Fig. 13 (a) The coordination environment of Ce(III) ions among CTH-15; (b) the structure of Ce–O polyhedral trimer via face-sharing; (c) the 2D structure of CTH-15 growing within the b–c plane; (d) the channel distribution of CTH-15 projected along the b-axis. Reproduced with permission from ref. 21. Copyright 2021, Elsevier Inc. | ||
2.4 ISBU MOFs constituted of metal(IV) central ions
According to the classical hard-soft acids and bases (HSAB) theory, MOFs composed of tetravalent metals ions normally enjoy outcomes of the strong M(IV)–carbonate coordination,12,101 like good thermal stability and chemical stability. To the best of our knowledge, ISBU MOFs based on metal(IV) elements are merely reported as examples of Ti(IV),18,19,27,35–37,102 Zr(IV)17,30,34,38,103–106 and Ce(IV),105,107,108 which have been surveyed and introduced in this section.In 2018, Serre et al.27 carried out the reaction between Ti(iPrO)4 (iPr = isopropoxide) and 5,5′-methylenediisophthalic acid (H4MDIP) under reflux of formic acid to obtain 0D Ti-oxo-based Ti12O15(MDIP)3(formate)6 (MIL-177-LT), which further underwent phase transformation at 280 °C to produce MIL-177-HT with novel 1D Ti-oxo chains. MIL-177-HT featured the formula of [(Ti6O9)2(MDIP)3] and crystallized in a hexagonal crystallographic system and the P6/mmm space group. Specifically, the 6-coordinated raw TiO6 polyhedrons were connected to the Ti6O9 secondary clusters via sharing edges (Fig. 14a), which were further assembled into 1D infinite [Ti6O9]n chain via corner-sharing (Fig. 14b). Noteworthily, such titanium-oxo hexamer was found identical to the unit structure of anatase TiO2. After the connection of neighboring [Ti6O9]n chains by MDIP linkers, a 3D network with honeycomb channels (0.9 nm) was constructed finally (Fig. 14c).
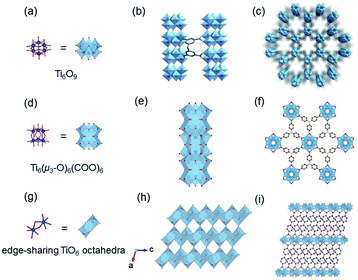 | ||
| Fig. 14 (a) The structure of Ti6O9 cluster; (b) adjacent infinite (Ti6O9)n 1D chain connected by MDIP linker; (c) the 3D structure of MIL-177-HT. Reproduced with permission from ref. 27. Copyright 2018, Springer Nature Limited. (d) The structure of Ti6(μ3-O)6(COO)6 unit; (e) the 1D infinite chain of ZSTU-1; (f) the open structure of ZSTU-1 projected at the [001] direction. Reproduced with permission from ref. 18. Copyright 2019, Royal Society of Chemistry. (g) The dimer of edge-sharing TiO6 octahedrons; (h) the 2D sheet structure of COK-47 growing within the a–c plane; (i) the side view of COK-47. Reproduced with permission from ref. 19. Copyright 2019, WILEY-VCH Verlag GmbH. | ||
After that, Chen et al.18 synthesized a series of ZSTU MOFs characteristic of another type of 1D Ti-oxo chain. Taking ZSTU-1 [Ti6(μ3-O)6(μ2-OH)6(TCA)2(H2O)(DMF)2] for the representation, it was synthesized by the solvothermal reaction of 4,4′,4′′-nitrilotribenzoic acid (H3TCA) and titanium isopropoxide in DMF at 180 °C for 24 h. The obtained ZSTU-1 crystallized in a hexagonal crystallographic system and P63/mcm space group. In which, the Ti(IV) cation adopted an unusual 7-coordination fashion and was sequentially packed into a Ti6(μ3-O)6(COO)6 subunit (Fig. 14d). Moreover, a 1D [Ti(μ3-O)6(μ3-OH)6(COO)6]n chain was formed along the c-axis by six μ3-OH-bridged connections of the hexamer subunits (Fig. 14e). In the a-b plane, the hexatopic Ti6(μ3-O)6(COO)6 was further linked by the triangular TCA linker to form a (3,6)-connected kgd network with elliptical channels of 0.61 × 0.34 nm in dimensions (Fig. 14f). Isoreticular ZSTU-2 [Ti6(μ3-O)6(μ2-OH)6(BTB)2(H2O)(DMF)2] and ZSTU-3 [Ti6(μ3-O)6(μ2-OH)6(BTCA)2(H2O)(DMF)2] can be facilely obtained by using H3BTB and H3BTCA [tris(4′-carboxybiphenyl)amine] as organic ligands, respectively. Accordingly, the pore dimensions of ZSTU-2 and ZSTU-3 were enlarged to 0.85 × 0.46 nm and 1.37 × 0.71 nm, respectively.
In addition, the first 2D Ti-MOF example (denoted as COK-47s) was reported by Bueken et al.19 COK-47s was synthesized by a microwave-assisted synthesis method involving heating H2BPDC with Cp2TiCl2 (Cp = cyclopentadienyl) at 185 °C in the anhydrous mixed solvent of DMF and propylene carbonate. Among COK-47s, each Ti(IV) was coordinated to two carboxylate oxygen atoms, three μ3-O oxygen atoms and one μ2-O oxygen atom, and therefore adopted a TiO6 octahedral mode, which was dimerized via sharing one edge (Fig. 14g). Furthermore, the dimer was condensed into infinite 2D Ti-oxo sheet structures by sharing edges along the c-axis and sharing corners along the a-axis, respectively (Fig. 14h). After the linkage of ditopic BPDC, adjacent sheets were assembled into the final 3D framework (Fig. 14i). The proposed 2D Ti-oxo sheet-based network was verified also applicable for terephthalic acid and 2,2′-bipyridine-5,5′-dicarboxylic acid, indications of the great potential for further modifications and functionalizations.
Thanks to the strong binding ability to carboxylate ligands, Zr(IV) MOFs usually exhibit robust thermal and chemical stabilities which are indispensable merits quested in versatile application fields.12,104 The classical and famous OD [Zr6(μ3-O)4(μ3-OH)4] node contributes almost Zr(IV) MOFs and very few examples based on infinite Zr-oxo units have been reported to the best of our knowledgement.105
In 2016, Stock and his colleagues38 used ZrOCl2·8H2O to react with 2,5-pyrazinedi-carboxylic acid (H2PzDC) in a mixture of H2O and formic acid at 120 °C for 24 h to produce the 1D CAU-22 with the formula of [Zr6(μ3-O)4(μ3-OH)4(μ2-OH)2(PzDC)3(OH)2(H2O)2(HCO2)2]. Among CAU-22, the 1D [Zr6O4(OH)4(μ2-OH)2]n rod was connected from discrete [Zr6O4(OH)4] octahedrons by μ2-OH bridging along the c-axis (Fig. 15a and b). Moreover, each 1D chain was interconnected with six adjacent chains via six ditopic PzDC edges to build the final 3D framework (Fig. 15c). Later, the same research group reported another type of 1D Zr-MOF named as CAU-27,17 which was obtained from phthalic acid and zirconium acetate under the acetic acid-based solvothermal conditions. Needle-like CAU-27 crystals with the formula of [Zr5O4(OH)4(OAc)4(BDC)2] belonged to the tetragonal system and I4/mcm space group. In which, the 1D Zr-oxo chain grew from the infinite condensation of Zr-corner-shared [Zr6O4(OH)4] octahedrons along the c-axis (Fig. 15a and d). After linking by BDC at the sides, those chains were ultimately assembled into the corresponding tetragonal cell (Fig. 15e) with part of the metal sites occupied by acetate terminals.
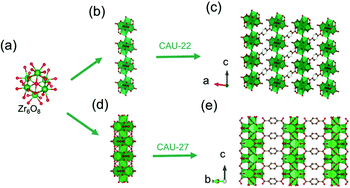 | ||
| Fig. 15 (a) The structure of initial OD [Zr6(μ3-O)4(μ3-OH)4] cluster; (b) the infinite 1D Zr-oxo chain structure bridged by μ2-OH; (c) corresponding framework structure of CAU-22. Reproduced with permission from ref. 38. Copyright 2016, Royal Society of Chemistry. (d) The infinite 1D Zr-oxo chain structure via sharing Zr corners; (e) corresponding framework structure of CAU-27. Reproduced with permission from ref. 17. Copyright 2019, WILEY-VCH Verlag GmbH. | ||
Distinct from the Zr6O8-condensed 1D chain introduced above, Devic et al.106 reported a 1D Zr-MOF based on the novel Zr-oxo chain. The so-called MIL-163 [Zr(H2-TzGal)] was synthesized from the reaction of 5,5′-(1,2,4,5-tetrazine-3,6-diyl)bis(benzene-1,2,3-triol) (H6TzGal) and ZrCl4 in DMF at 130 °C. Among MIL-163, the central Zr4+ adopted an 8-coordinated fashion with four carboxylate oxygens and four phenolic oxygens, and the as-formed ZrO8 polyhedron (Fig. 16a) grew indefinitely along the c-axis to form corresponding Zr-oxo chains with shared edges (Fig. 16b). Together with the linkage of TzGal extending along both a- and b-axis (Fig. 16b), the open network with exposure of square channels was constructed as the final product. By replacing the ditopic H6TzGal with tetratopic THPP (5,10,15,20-tetrakis(3,4,5-trihydroxyphenyl)porphyrin), Lin et al.34 constructed novel ZrPP-1 [Zr2(THPP)] featuring identical 1D Zr-oxo chains to MIL-163 in the water–DMF (v/v, 4/5) mixture at 140 °C. Distinctly, those 1D chains among ZrPP-1 were connected by the square-shaped THPP linkers and therefore formed a 4-connected nbo network (Fig. 16c).
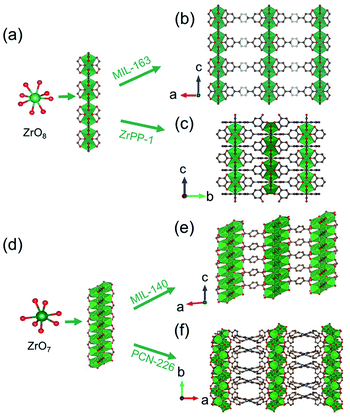 | ||
| Fig. 16 (a) The infinite 1D Zr(IV)-oxo chain assembled from ZrO8 polyhedrons; (b) corresponding framework structure of MIL-163. Reproduced with permission from ref. 106. Copyright 2015, WILEY-VCH Verlag GmbH. (c) The corresponding framework structure of ZrPP-1. Reproduced with permission from ref. 34. Copyright 2018, WILEY-VCH Verlag GmbH. (d) The infinite zigzag 1D Zr(IV)-oxo chain assembled from ZrO7 polyhedrons; (e) corresponding framework structure of MIL-140. Reproduced with permission from ref. 30. Copyright 2021, WILEY-VCH Verlag GmbH. (f) The corresponding framework structure of PCN-226. Reproduced with permission from ref. 32. Copyright 2020, American Chemical Society. | ||
In addition, Zhao et al.30 reported the synthesis of Zr(IV)-MIL-140-4F reacted via heating ZrCl4 and tetrafluoro-terephthalic acid (H2TFBDC) in acetonitrile at 120 °C. Isoreticular to the well-known MIL-140 series,105,108,109 the Zr(IV) cation adopted rare 7-connected coordination with four carboxylate groups and three μ3-bridged oxygens atoms to form a ZrO7 polyhedron (Fig. 16d). Sequentially, an infinitely Zr–O chain was obtained via edge-sharing those ZrO7 polyhedrons in a zigzag way. The 3D network with 1D triangular channels (Fig. 16e) was constructed after the linkers installation in the a–b plane. More significantly, Ce(IV)-MIL-140-4F107 with a similar [CeO7]n zigzag chain was also obtained by simpling varying the metal precursor type. Recently, Huang et al.32 further expanded such 1D chain-connected networks feasible for the tetra-connected tetrakis(4-carboxyphenyl)porphyrin (H2TCPP) linker. The obtained PCN-226 [Zr3O3(TCPP)(benzoate)2] crystallized in a higher symmetric orthorhombic crystallographic system and the Ibam space group due to the square geometry of TCPP. Among PCN-226, the edge-sharing zigzag ZrO7 chain ran along the b-axis (Fig. 16d) and was further linked with metalloporphyrin linkers each other to form the final 3D framework of a new ztt topology (Fig. 16f).
3 Applications of ISBU MOFs
MOFs have drawn extensive application studies in fields of adsorption, gas separation, catalysis, energy storage and others, owing to their large surface area, tunable pore size, versatile functionalization ability, etc.9,25,110–115 In this section, it is beyond our intention to summarize those application performances even based on ISBU MOFs, considering the innumerable examples reported so far. Rather, we have introduced and discussed their application performances by presenting some purposely selected ISBU MOFs, in order to highlight the unique physical and chemical properties enabled by corresponding ISBU structures.3.1 Gas absorption
As a highly porous absorbent for gas uptake, the surface area and pore size of MOFs are well-known key factors determining their performances.116–118 Besides, the recently developed series of 1D Al-MOFs featuring high gas uptake indicated the non-negligible function of unique 1D chain structures.3,85,86 For example, MOF-303 featured with the 1D Al–O chain was reported as the world-record high uptake toward water adsorption (Fig. 17a).3 The in-depth mechanism according to single-crystal X-ray diffraction (SXCRD) and theoretic simulation results deciphered that the unique 1D chain structure together with polar organic linkers played a major role. Enforced by densely packing of the 1D Al–O chain, adjacent pyrazole linkers, therefore, pointed toward each other closely accompanied by forming a hydrophilic pocket involved with three μ2-OH dangling on the 1D chain and two N(H) on linkers. As the red region shown in Fig. 17a, a step adsorption isotherm was observed at very low pressure, corresponding to the formation of a four-water-assembled cluster inside such pocket by multiple and strong hydrogen bonding (Fig. 17b). Moreover, this water cluster acted as the seed for binding many more water molecules, which was ascribed to the large water uptake of MOF-303 within a very small pressure range (yellow region shown in Fig. 17a). It is hence noteworthy to highlight that the dense layout of the hydrogen-bonding sites exposed on the infinite 1D Al–O chains together with adjacent pyrazole linkers cooperatively resulted in the hydrophilic pocket available for water cluster seeding and subsequent condensation. Full water adsorption of MOF-303 was accomplished at the saturation pressure with a maximum uptake of 0.45 g g−1, which was a state-of-the-art performance in 2019. However, the strong hydrogen bonding on the other hand cast not easy desorption and regeneration of the harvested water, especially for waters captured before the S-step indicated in Fig. 17a. Hence, an isoreticular MOF-333 [Al(OH)(FDC)] with identical 1D Al–O chains but a less hydrophilic FDC linker instead, was constructed to control the hydrogen bonding toward water molecules. Compared with pyrazole, the less hydrophilic FDC made it weaker in hydrogen bonding with water molecules, as demonstrated by the absence of the corresponding S-step in the adsorption isotherm of MOF-333 but at a cost of much higher onset pressure (Fig. 17c). To achieve two birds with one stone, mixed PZDC and FDC ligands were adopted to construct the multivariate MOF for making a balance between the working capacity and regeneration ability. An optimized ‘4/4’ (the molar ratio of PZDC to FDC) multivariate MOF exhibited excellent isobaric desorption performance at water vapor pressures of 0.85 to 1.70 kPa (corresponding to 20 to 40% RH at 30 °C), which was very practical for the water-harvesting system (Fig. 17d).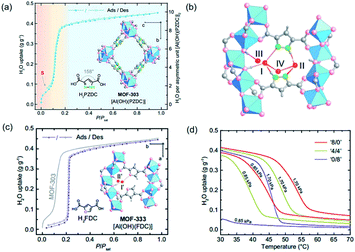 | ||
| Fig. 17 (a) Water adsorption and desorption isotherms of MOF-303 at 25 °C. The three adsorption steps were shown in red, yellow, and blue in the background. Insets correspond to the structures of H2PZDC and MOF-303; (b) the adsorption sites (I to IV) for the first to four water molecules inside the hydrophilic pocket; (c) water adsorption and desorption isotherms of MOF-333 at 25 °C. Insets showed H2FDC structure and the first two adsorption sites inside the pocket of MOF-333; (d) the water vapor isotherms of homo- and mixed-ligand MOFs (‘8/0’, ‘4/4’ and ‘0/8’) at 0.85 to 1.70 kPa. Reprinted with permission from ref. 3. Copyright 2021, AAAS. | ||
Normally, MOFs have been popularly quested for large CO2 uptake.108,119 Distinctly, Long et al.28 reported appealing CO2 triggered enantio-recognitions enabled by 1D Mg2(DOBPTC) with helical Mg–O chain structures. With the assistance of chiral trans-1,2-diaminocyclohexane (dach), it was found that both the adsorption and desorption temperature of CO2 selectively varied with the chiral conformation of the used Mg2(DOBPTC) adsorbent. In combination with the experimental proofs and density functional theory (DFT) calculations, a unique chiral ammonium carbamate chain was formed via the co-condensation of the appended dach and the adsorbed CO2, which further formed corresponding diastereomers with Mg2(DOBPTC) of opposite chirality. Without double, the unique 1D Mg–O helical chain among Mg2(DOBPTC) was recognized as the chiral template to trigger the chirality transfer. As shown in Fig. 18a, the space-filling models indicated a much tighter contact for (R,R)-dach-R-Mg2(DOBPTC) in comparison to (S,S)-dach-R-Mg2(DOBPTC). In accordance, the ammonium among (R,R)-dach-R-Mg2(DOBPTC) also formed stronger hydrogen bonds with two different carboxylate oxygens with corresponding shorter N–H⋯O lengths than those among the (S,S)-dach-R-Mg2(DOBPTC), as a further consolidation of the more favourable (R,R)-dach-R-Mg2(DOBPTC) in thermodynamics (Fig. 18b). Experimentally, (R,R)-dach grafted racemic Mg2(DOBPTC) presented two CO2 desorption steps with distinct onset temperate, which could be assigned to the (R,R)-dach-R-Mg2(DOBPTC) of higher desorption temperature and (R,R)-dach-S-Mg2(DOBPTC) of relatively lower desorption temperature. By contrast, when the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (R,R)
50 (R,R)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (S,S) dach was used, racemic Mg2(DOBPTC) presented only a single-step desorption curve with corresponding desorption temperature in between (Fig. 18c and d).
(S,S) dach was used, racemic Mg2(DOBPTC) presented only a single-step desorption curve with corresponding desorption temperature in between (Fig. 18c and d).
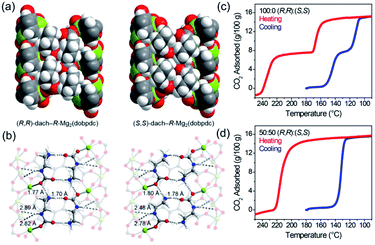 | ||
| Fig. 18 (a) The space-filling models of (R,R)-dach-R-Mg2(DOBPTC) and (S,S)-dach-R-Mg2(DOBPTC); (b) the DFT-calculated structure parameters of the CO2-insert (R,R)-dach-R-Mg2(DOBPTC) and (S,S)-dach-R-Mg2(DOBPTC). Element color: Mg is light-green, N is blue, O is red, C is gray, and H is white; (c and d) the thermogravimetric analyses of pure CO2 isobars of Mg2(DOBPTC) functionalized with different chiral dach isomers. Reprinted with permission from ref. 28. Copyright 2012, AAAS. | ||
3.2 Gas separation
Thanks to the large specific surface area and adjustable pore size, MOFs have become one of the best promising materials for gas separation.120–122 Isolation of CO2 from acetylene (C2H2) is a long-standing challenge in the petrochemical industry arising from the nearly identical size, shape and physicochemical properties between the two gases. It was completely differentiated from the C2H2-preferred adsorption capabilities of conventional absorbents, inverse adsorption behavior was obtained by the 1D Ce(IV)-MIL-140-4F of trigonal-shaped ultramicroporous channels along with the exceptionally high CO2/C2H2 selectivity.30 There were electron-rich site I and electron-poor site II shown in the electrostatic potential distributions on the Hirshfeld surface of the Ce-MIL-140-4F (Fig. 19a) and the Zr-MIL-140-4F as a reference (Fig. 19b). In comparison to the Zr-MIL-140-4F counterpart, Ce(IV) with additional unoccupied 4f orbitals extracted electrons from the organic linker to the metal node more efficiently and thus offered a greater polarity on site I. Consequentially, a favorable uptake toward CO2 (110.3 cm3 cm−3 at 298 K, Fig. 19c) and inversed CO2/C2H2 selectivity were conferred by Ce(IV)-MIL-140-4F via the strong host–guest interaction occurring at the site I. As another reference, Ce(IV)-UiO-66-4F phase comprising the same Ce(IV) and organic linker to Ce(IV)-MIL-140-4F, however, merely presented conventional C2H2/CO2 separation selectivity (Fig. 19d), reflecting the potential contribution devoted by the unique 1D chain structure of Ce(IV)-MIL-140-4F. Likely, the condensed stacking fashion of polar organic linkers among 1D Ce(IV)-MIL-140-4F offered unique CO2-favoured electrostatic potential distribution throughout the whole framework, which was absent in the case of 0D Ce(IV)-UiO-66-4F.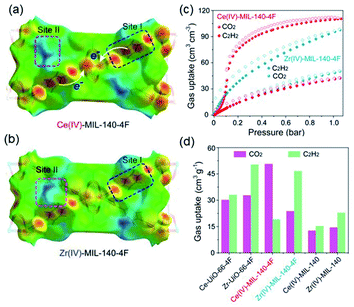 | ||
| Fig. 19 The electrostatic potential distributions on the Hirshfeld surface of (a) Ce-MIL-140-4F and (b) Zr-MIL-140-4F (red-to-blue color indicates the high-to-low transition of electron density); (c) the CO2 and C2H2 isotherms of Ce(IV)- and Zr(IV)-MIL-140-4F collected at 298 K; (d) the CO2 and C2H2 uptakes of different MOF samples at 298 K. Reprinted with permission from ref. 30. Copyright 2021, WILEY-VCH Verlag GmbH. | ||
The CO2 separation over N2, another important industrial process used in the treatment of fuel gas, was tested by a rare pure Na-based 2D MOF-705 [Na4(BDA)(CH3OH)(H2O), BDA = (2S,2′S)-2,2′-(terephthaloylbis (azanediyl))disuccinate].45 MOF-705 featured infinite 2D sodium oxide sheets extending along its crystallographic a–b plane and showed uptakes of 65.0 and 3.1 cm3 cm−3 toward adsorption of CO2 and N2, respectively, at 273 K and 800 Torr pressure. What's more, the breakthrough experiment was carried out in a mixture of 84% N2 and 16% CO2 to further show the practical potential of MOF-705. Promisingly, MOF-705 could adsorb CO2 specifically with the capture efficiency over 98% and at the same time interact with N2 negligibly. The adsorbed CO2 in pores of MOF-705 could be removed simply by flowing pure N2 at room temperature and the regenerated material could be repeatedly used for at least three cycles without obvious performance decline.
Metal-mixing is a common but effective strategy to enhance performances by combining both merits of the homometallic analogies.20,31,123 Schroder and his colleagues31 constructed a heterometallic 1D MFM-300(Al0.67Cr0.33) for SO2 separation from CO2, which was compared in parallel to its homometallic MFM-300(Al) and MFM-300(Cr). According to the experimental results, MFM-300(Al) showed both stronger adsorption toward SO2 and CO2 but at the compromise of lower SO2/CO2 selectivity than MFM-300(Cr). In between, heterometallic MFM-300(Al0.67Cr0.33) inherited high SO2 uptake up to 8.59 mmol g−1 at 273 K and 1 bar pressure (Fig. 20a), and meanwhile an appreciable SO2/CO2 selectivity (Fig. 20b). The overall superior performance achieved by MFM-300(Al0.67Cr0.33) has been attributed to the interaction strength between its 1D chain (Al-μ2OH–Cr) with adsorbents. A shorter binding length was found for μ2OH⋯OSO (3.163 Å) in the case of MFM-300(Al0.67Cr0.33) compared with the homometallic ones, meaning the stronger SO2 interaction of MFM-300(Al0.67Cr0.33) (Fig. 20c and d). In addition, the intermediate SO2/CO2 selectivity was clued by the guest–guest packing alongside the 1D chain, as one can see a minorly loose SO2⋯SO2 distance (3.477) found in the MFM-300(Al0.67Cr0.33) than that (3.45) of MFM-300(Cr) of the best selectivity.
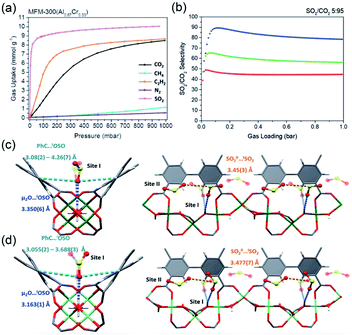 | ||
Fig. 20 (a) The adsorption isotherms toward CO2 (black), CH4 (aqua), C2H2 (orange), N2 (purple) and SO2 (pink) of MFM-300(Al0.67Cr0.33), at 273 K; (b) the ideal adsorbed solution theory (IAST) selectivity of SO2/CO2 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) achieved by MFM-300(Al) (red), MFM-300(Al0.67Cr0.33) (green) and MFM-300(Cr) (blue) up to 1 bar at 273 K. Views of binding of SO2I and of SO2I⋯SO2II interactions among (c) MFM-300(Cr) and (d) MFM-300(Al0.67Cr0.33). Element color: Cr is green, AlCr is light green, S is yellow, O is red, C is grey, H is white. Bond color: μ3-OH⋯OSOI is blue bond, Ph⋯SO2I is turquoise bond, SO2I⋯SO2II interactions is orange bond. Reprinted with permission from ref. 31. Copyright 2021, Royal Society of Chemistry. 95) achieved by MFM-300(Al) (red), MFM-300(Al0.67Cr0.33) (green) and MFM-300(Cr) (blue) up to 1 bar at 273 K. Views of binding of SO2I and of SO2I⋯SO2II interactions among (c) MFM-300(Cr) and (d) MFM-300(Al0.67Cr0.33). Element color: Cr is green, AlCr is light green, S is yellow, O is red, C is grey, H is white. Bond color: μ3-OH⋯OSOI is blue bond, Ph⋯SO2I is turquoise bond, SO2I⋯SO2II interactions is orange bond. Reprinted with permission from ref. 31. Copyright 2021, Royal Society of Chemistry. | ||
3.3 Energy-related application
By taking the advantages of rich active sites, low resistance of mass transport and easy alternation of functionality, MOFs are also promising candidates for energy storage,124 like being used as, the active electrodes for batteries32,44,125–128 and the proton-conductive membrane27,58,129–131 used in fuel cells.132,133 More than those, the unique ISBU structures can bring additional merits (e.g., higher energy density and improved performance stability), as compared with conventional OD MOFs. As a proof of concept, the ID MIL-132(K) and 2D MIL-133(K) both composed of redox-active TTF linkers (corresponding crystal structures have been specifically described in Fig. 21a) were used as cathode materials in Li-ion batteries.44 Based on the solid-state cyclic voltammetry at a scanning rate of 1 mV s−1, the 1D MIL-132K exhibited a reversible redox couple between 2.0 and 4.0 V vs. Li/Li+, giving rise to an initial charge capacity of nearly 110 mA h g−1 (Fig. 21b). Similar redox coupling was also observed for MIL-133K but corresponding to a much lower capacity of 55 mA h g−1, owing to the densely packed 2D K–O sheet unfavorable for Li-ions insertion and diffusion. But, a better cycling performance was achieved by the 2D MIL-133K on basis of its more robusticity, as seen from the nearly unchanged capacity after 6 concussive cycles (Fig. 21c). In sharp contrast, the charge capacity of the 1D MIL-132(K) declined to about half of the initial one after only 5 cycles (Fig. 21d). | ||
| Fig. 21 (a) The solid-state cyclic voltammetry of MIL-132K between the 2 and 4 V vs. Li/Li+ at 1 mV s−1; (b) the charge and discharge capacity of MIL-132K within 5 cycles; (c) the solid-state cyclic voltammetry of MIL-133K between the 2 and 4 V vs. Li/Li+ at 1 mV s−1; (d) the charge and discharge capacity of MIL-132K within 6 cycles. Reprinted with permission from ref. 44. Copyright 2010, American Chemical Society. | ||
MOFs of good proton conductivities have been nominated as promising membranes used in fuel cells. Mak et al.129 synthesized a 1D [Sr2(BPTC)(H2O)6, denoted as Sr-MOF-3] featuring hydrophilic channels (Fig. 22a). Water molecules were captured inside Sr-MOF-3 and self-assembled into heptamer-based networks via the interaction with ligated oxygen atoms on the 1D Sr–O chain and carboxylate oxygens from BPTC. Undoubtedly, the specific 1D Sr–O chain acted as the template for the construction of a straightforward hydrophilic path for acquiring good proton conductivity (Fig. 22b). Nyquist plots of Sr-MOF-3 showed an optimum proton conductivity of 2.7 × 10−4 S cm−1 at 98% and 90 °C (Fig. 22c). More recently, Yang et al.58 constructed a 1D Ba-MOF-1 (detailed crystal structure is available in Fig. 5) for proton-conducting. The densely packed Ba–O 1D chain together with the also closely spaced polar tetrazole rings triggered the formation of strong intermolecular hydrogen-bonding networks involved with Ow–H⋯O, Ow–H⋯N, N–H⋯O, C–H⋯N and C–H⋯O inside the hydrophilic 1D channel (Fig. 22d and e). The alternating current (AC) impedance spectrum (Fig. 22f) showed that a pronounced proton conductivity equal to 1.72 × 10−4 S cm−1 was acquired by robust moisture and thermal stabilities, and a further improved conductivity of 4.47 × 10−3 S cm−1 was achieved by Ba-MOF-1 Ba-MOF-1 at 98%RH and 25 °C. By fully taking advantage of once testing the sample at 85 °C.
 | ||
| Fig. 22 (a) The crystal structure of 1D [Sr2(BPTC)(H2O)6], H atoms are omitted for clarity; (b) polyhedral structure diagram of 1D Sr–O chain. Hydrogen bonding networks composed of water molecules (pink) and carboxylate oxygen atoms (red); (c) the impedance spectra of 1D [Sr2(BPTC)(H2O)6] from 25 to 75 °C at 98% RH. Reprinted with permission from ref. 129. Copyright 2014, American Chemical Society. (d) The 3D framework structure of Ba-MOF-1, the part marked is the hydrogen-bonding networks formed in the channel; (e) hydrogen-bonding networks formed in the 1D channels of Ba-MOF-1; (f) the impedance spectra of Ba-MOF-1 from 25 to 85 °C at 98% RH. Reprinted with permission from ref. 58. Copyright 2021, Royal Society of Chemistry. | ||
Metalloporphyrinic MOFs have been widely tested in hydrogen evolution reaction (HER),125,134 carbon dioxide reduction reaction (CO2RR),34 oxygen-involved reduction (ORR)32 and evolution reaction (OER).135 Nevertheless, challenges still stand in terms of acquiring high current density and long-term performance stability. As high surface area as well as large porosity offered by conventional OD MOFs are always compromised of the risk of framework collapses. Huang et al.32 recently reported a novel PCN-226 comprising 1D infinite [ZrO7]n chain and densely packed metalloporphyrinic linkers (TCPP M, M = Fe, Co, Ni, Cu, Zn, detailed structure description is available in Fig. 23a), which exhibited excellent electrocatalytic activity and good longevity in ORR by virtue of its unique 1D [ZrO7]n chain. After coating the PCN-226 catalyst on carbon cloth, the assembled cell exhibited an open-circuit voltage of 1.37 V, and discharge/charge voltages of 1.17/2.14 V, respectively (Fig. 23b). The corresponding specific capacity of PCN-226(Co) was calculated as 724 mA h g−1, comparable to 757 mA h g−1 for the commercially available Pt/C + RuO2 catalyst (Fig. 23c). More significantly, thanks to the robust 1D chain structure, the PCN-226-based cell maintained a no obvious performance degradation over 160 h, surpassing the 60 h of Pt/C + RuO2 by far (Fig. 23d).
 | ||
| Fig. 23 (a) The schematic of the mechanism of PCN-226(Co) as a redox electrocatalyst in ORR; (b) cycle curves of PCN-226 and commercial Pt/C + RuO2 for discharge and charge in the zinc–air cell. (c) The discharge curves of PCN-226(Co) and commercial Pt/C + RuO2 at the current density of 20 mA cm−2. (d) Long-term durability test of the Zn–air batteries at the current density of 2 mA cm−2 assembled with PCN-226(Co) and commercial Pt/C + RuO2 active electrode, respectively. Reprinted with permission from ref. 32. Copyright 2020, American Chemical Society. | ||
3.4 Photocatalysis
The versatile chemistries of both metal nodes and organic linkers enable MOFs as promising photocatalysts with tunable band structures.136–139 Nevertheless, further advancing MOFs in practice is hampered by their ultralow charge separation efficiencies and much inferior charge mobilities to commercial inorganic semiconductors. Distinct from majorities of OD samples, photoactive MOFs based on ISBUs are suggested to behave more like corresponding metal-oxide semiconductors.18,19,27,35 For instance, Serre et al.27 reported the exceptional photoconductivity of novel 1D titanium-oxo MOF (MIL-177-HT), which has exhibited comparably high charge mobility to semiconductive TiO2 in addition to its excellent stability and high porosity. MIL-177-HT was obtained from the thermal-induced phase transformation of 0D Ti12O15 cluster-based MIL-177-LT (Fig. 24a). Note that, the resulting 1D (Ti6O9)n chain among MIL-177-HT presented an unprecedented high condensation degree of 1.5 among all reported Ti-MOFs, an indictor of structure closing to pure inorganic TiO2 with a condensation degree of 2.0. In the flash photoluminescence time-resolved microwave conductivity (FP-TRMC) testing, MIL-177-HT exerted very significant signs up to 3 × 10−4 cm2 s−1 V−1 under ultraviolet light (266 nm) excitation, a comparable value as high as traditional TiO2 semiconductors.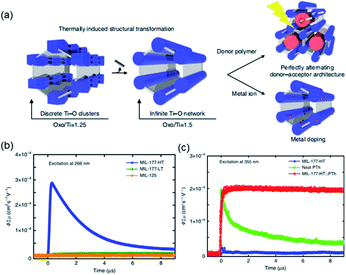 | ||
| Fig. 24 (a) The schematic of the phase transformation and modification of MIL-177 structure; (b) transient conductivity of FP-TRMC under 266 nm laser excitation; (c) transient conductivity of FP-TRMC under 355 nm laser irradiation. Reproduced with permission from ref. 27. Copyright 2018, Springer Nature Limited. | ||
In sharp contrast, OD MIL-177-LT and familiar MIL-125 with the condensation degree of 1.25 and 1.0, respectively, both gave negligible photoresponse due to the absence of related 1D conduction chains (Fig. 24b). Further performance improvement could be facilely achieved via doping electron-donative polymers or metal ions. Taking the former for representation, MIL-177-HT⊃PTh (PTh = polythiophene) composite exhibited remarkably improved photoconductivity under excitation of 355 nm than neat MIL-177-HT. More significantly, a much-prolonged decay lifetime was observed for MIL-177-HT⊃PTh compared with pure PTh, indicating the facilitated charge separation process (Fig. 24c). Recently, another Ti-MOF [TiO2(TBAPy), TBAPy = 4,4′,4′′,4′′′-(pyrene-1,3,6,8-tetrayl)tetrabenzonate], denoted as ACM-1,37 was reported with its energy band dispersion alongside the corner-shared 1D [TiO6]n octahedral chain. Hence, the high mobility of photoinduced electrons together with a long charge separation lifetime guaranteed the excellent performance of ACM-1 in both photocatalytic hydrogen evolution and selective oxidation of organics.
What's more, the easy tunability of organic linkers is also facile for performance regulation.18,35 Chen et al.18 used H3TCA, H3BTB and H3BTCA to construct 1D Ti-oxo ZSTU-1, ZSTU-2 and ZSTU-3 (corresponding crystal structures are described in Fig. 10), respectively, in the application of photo-induced hydrogen evolution (Fig. 25a). Using Pt nanoparticles (NPs) as the co-catalyst and triethanolamine (TEOA) as the sacrificial agent, ZSTU-1 and ZSTU-3 exhibited much higher performance than ZSTU-2 (Fig. 25b) thanks to the photoactive triphenylamine segment giving response to the visible light. In comparison to ZSTU-1, ZSTU-3 still showed slightly improved activity due to the extended conjugation system in H3BTCA. It deserves to highlight that the hydrogen evolution rate (1350 μmol g−1 h−1) achieved by 1D ZSTU-3 was more than double that of the OD MIL-125-NH2, mightily due to the much faster charge mobility of 1D ISBU. In addition, three MOF catalysts all exerted excellent performances after three concussive testing cycles thanks to their robust 1D inorganic chains (Fig. 25c). Moreover, Alshareef and his colleagues35 applied the 1D titanium-phosphonate MOF-based nanowires (TiPNW) for photocatalytic water splitting. Under light irradiation, the photoexcited electrons were transferred from organophosphorus linkers to 1D Ti-oxo chains via an efficient linker-to-metal node charge transfer (LCMT) path. With the aid of co-catalytic Pt NPs, electrons were further separated from the resulting Ti3+ intermediates and utilized for the reduction of water. Meanwhile, concurrent holes were located at the organic linker and quenched by TEOA. As shown in Fig. 25e, TiPNW with a rationally designed linker showed the highest photocatalytic hydrogen evolution rate up to 1260 μmol h−1 g−1, nearly five times the rate of commercial anatase.
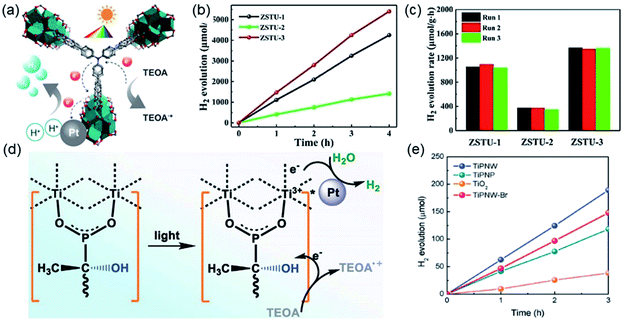 | ||
| Fig. 25 (a) Schematic diagram of photocatalytic hydrogen evolution mechanism of ZSTU MOFs; (b) the amount of H2 produced by ZSTU-1, ZSTU-2 and ZSTU-3 under visible light irradiation (λ > 420 nm); (c) recovery performance of ZSTU-1, ZSTU-2 and ZSTU-3 in the photocatalytic hydrogen evolution reaction under visible light irradiation for three times. Reprinted with permission from ref. 18. Copyright 2019, Royal Society of Chemistry. (d) Mechanism diagram of TiPNW photocatalytic hydrogen evolution reaction. (e) The photocatalytic hydrogen evolution activity under visible light irradiation. Reprinted with permission from ref. 34. Copyright 2020, WILEY-VCH Verlag GmbH. | ||
The dense spatial packing of active sites among ISBU MOFs is anticipated for the activation of substrate cooperatively in catalysis.32,34 In 2018, Lin et al.34 constructed a series of 1D phenolic porphyrinc ZrPP-1-M (M = Co, Fe, Cu, Zn, H2) for selective photoreduction of CO2 to CO. According to the CO2 adsorption isotherms (Fig. 26a), ZrPP-1-M (M = Co, Fe, Cu, Zn, H2) have presented high CO2 uptake of 87.7 88.4, 59.8, 77.5, 81.9 cm3 g−1, respectively, at 273 K. Such small adsorption disparency was ascribed to the atomic mass of installed metal types rather than their CO2 adsorption strength, as verified by their nearly identical isosteric heats. It was suggested that the strong CO2 adsorption among ZrPP-1-M resulted from the densely eclipsed stacking of parallel metalloporphyrins enforced by its unique 1D Zr-oxo chain, which anchored two oxygens of CO2 molecule via a pair of positively charged metal centers. Bearing this in mind, ZrPP-1-M was further tested in the photoreduction of CO2. ZrPP-1-Co with the narrowest band gap capable of efficient visible light absorption showed the highest CO production rate among tested samples (Fig. 26b).
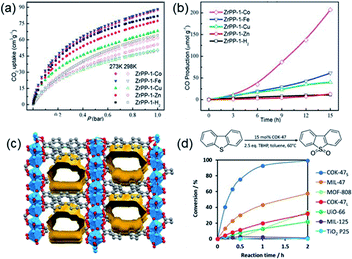 | ||
| Fig. 26 (a) CO2 adsorption of ZrPP-1-M (M = Co, Fe, Cu, Zn, H2) at different temperatures; (b) reduction process of CO2 to CO with ZrPP-1-M catalyst under visible light irradiation. Reprinted with permission from ref. 34. Copyright 2018, WILEY-VCH Verlag GmbH. (c) The visualized pore structure of COK-47, resulting in approximately 6 Å pores due to lack of connectives; (d) conversion rates of different catalysts in the photooxidation of DBT. Reprinted with permission from ref. 19. Copyright 2019, WILEY-VCH Verlag GmbH. | ||
By taking the robustness advantages, moreover, Bueken et al.19 successfully introduced substantial defects into 2D COK-47s for photooxidation of dibenzothiophene (DBT). It was found that there were two-fold functions of the introduced linker-missing defects in the catalysis. First, the pore size of COK-47s was enlarged to 6 Å (Fig. 26c) by missing organic linkers, which was favorable for substrate diffusion. Second, the open Ti sites as catalytical Lewis acids were competent for efficient substrate activations. Experiment results showed that COK-47s indeed exerted the highest conversion rate among all tested samples including the defect-free COK-47L reference, familiar Ti-MOFs, Zr-MOFs and commercial P25 TiO2 (Fig. 26d). Furthermore, the robust 2D inorganic sheet structure of COK-47s has taken charge of its stable catalytic performance within three cycling tests, which was keen merit rarely reported in the defect-based catalysts.
4. Conclusion and outlook
In this review, we have thoroughly introduced the recent development of MOFs of infinite secondary building units (ISBUs) including 1D chains, 2D sheets and 3D nets. On account of the unique inorganic nature resembling metal-oxides/hydroxides, ISBU MOFs have been conferred ultrahigh packing density of building blocks, robust physical/chemical stabilities, superior semiconductor-like conductivity and carrier mobility, and so on. Therefore, a blooming growth of applications of ISBU MOFs has been made in the fields of gas adsorption, gas separation, energy storage and catalysis. We have also summarized the prototypes and recently developed examples of ISBU MOFs for easy reference and direct comparison (Table 1).| No. | MOF | Metal | ISBU | ISBU composition | Formula | Coordination polyhedron | Crystallography system | Space group | Topology | Properties & applications | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | IMP-24Li | Li(I) | 1D | [LiO6]n | Li4L1(4,4′,4''-(methylsilanetriyl)tribenzoate)DMF | LiO6 | Trigonal | P3121 | CO2 uptake: 32.4 wt% at 500 °C | 42 | |
| 2 | ULMOF-1 | Li(I) | 2D | [LiO4]n | Li2(naphthalene-2,6-dicarboxylate) | LiO4 | Monoclinic | P21/c | Decomposition T = 610 °C | 26 | |
| 3 | ULMOF-2 | Li(I) | 2D | [LiO4]n | Li2(4,4-bisphthalboxylate) | LiO4 | Monoclinic | P21/c | Decomposition T = 575 °C | 43 | |
| 4 | IMP-27Na | Na(I) | 1D | [NaO6]n | Na4(4,4′,4′′,4′′′-silanetetrayltetrabenzoate)(H2O)4 | NaO6 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
42 | ||
| 5 | MOF-705 | Na(I) | 2D | [NaO]n | Na4[(2S,2′S)-2,2′-(terephthaloylbis(azanediyl))disuccinate](CH3OH)(H2O) | Na1O5, Na2O4, Na3O4, Na4O6 | Monoclinic | P21 | CO2/N2 separation, Sel. = 20.9 | 45 | |
| 6 | MOF-706 | Na(I) | 2D | [NaO]n | Na4[(2S,2′S)-2,2′-(1,1′-biphenyl)-4,4′(dicarbonyl)bis(azanediyl)disuccinate])(CH3OH)(H2O) | Na1O5, Na2O4, Na3O4, Na4O6 | Monoclinic | P21 | CO2/N2 separation, Sel. = 20.5 | 45 | |
| 7 | MIL-132 | K(I) | 1D | [KO5]n | K2H2(redox tetracarboxylic acid) | KO5 | Monoclinic | P21/c | Li-ion battery, initial charge capacity = 110 mA h g−1 | 44 | |
| 8 | MIL-133 | K(I) | 2D | [KO4]n | M2H2(redox tetracarboxylic acid) (M = K+ or Rb+) | KO4 | Orthorhombic | Pnmm | Li-ion battery, initial charge capacity = 55 mA h g−1 | 44 | |
| 9 | Cu(I)-MOF | Cu(I) | 1D | [CuI]n | Cu6I6(1,4-bis(phenylthio)but-2-yne)3 | CuI | Orthorhombic | Pbca | Thermoelectric composites | 48 | |
| 10 | PbCu-MOF-1 | Pb(II) | 1D | [PbO]n | Pb4Cu2I2(3,5-pyridinedicarboxylate)4(DMF)6 | Pb1O8, Pb2O5 | Monoclinic | P21/c | Luminescence ratiometric thermometer | 75 | |
| 11 | KGF-1 | Pb(II) | 3D | [PbS5N2]n | Pb3(Trithiocyanuric acid)2‧2H2O | Pb1S4N2, Pb2S5N2 | Monoclinic | P21/c | Decomposition T = 390 °C, conductivity = 7.4 × 10−5 cm2 V−1 s−1 | 22 | |
| 12 | AgPb-MOF | Pb(II) | 2D | [AgPbO]n | Ag2Pb2(1,3,5-benzenetricarboxylic acid)2 | AgO5, PbO7 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Decomposition T = 320 °C | 80 | |
| 13 | Pb-MOF | Pb(II) | 2D | Pb–O–Pb | Pb5(1,4-benzenedicarboxylate)4(OAc)2 | Pb1O6, Pb2O8, Pb3O8 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
CO2 uptake: 25.8 cm3 cm−1 at 298 K | 140 | |
| 14 | Mg-MOF-74 | Mg(II) | 1D | [MgO6]n | Mg2(3,3′-dihydroxy-[1,1′-biphenyl]-4,4′-dicarboxylic acid) | MgO6 | Trigonal |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
Adjustable ultra-large aperture: 14–98 Å | 10 | |
| 15 | Mg-MOF | Mg(II) | 1D | [MgO6]n | Mg2(4,4′-di-oxidobiphenyl-3,3′-dicarboxylate) | MgO6 | Trigonal | P3221 | CO2 enantioselective adsorption | 28 | |
| 16 | CPF-1 | Mg(II) | 1D | [MgO6]n | Mg2(H2O)2(3,3′,5,5′-tetracarboxylate) | MgO6 | Tetragonal | I4122 | 51 | ||
| 17 | Mg-MOF-1 | Mg(II) | 3D | [MgO6]n | Mg3(formic acid)6 | MgO6 | Monoclinic | P21/c | Decomposition T = 417 °C | 71 | |
| 18 | Mg-BPTC | Mg(II) | 1D | [MgO6]n | [Mg(2,2,6,6-tetracarboxybipheny)0.5(H2O)3]·5H2O | MgO6 | Monoclinic | C2/c | Proton conductivity = 2.6 × 10−4 S cm−1 at 100 °C and 98% RH | 129 | |
| 19 | Ca-MOF-1 | Ca(II) | 1D | [CaO]n | Ca3(1,3,5-tris(4-carboxyphenyl)benzene)2 | Ca1O6, Ca2O7 | Trigonal | R3 | Blue fluorescence | 53 | |
| 20 | Ca-MOF-2 | Ca(II) | 1D | [CaO8]n | Ca(1,4-benzenedicarboxylate)(DMF)(OH2) | CaO8 | Monoclinic | P21/c | Blue fluorescence | 53 | |
| 21 | Ca-MOF-3 | Ca(II) | 2D | [CaO7]n | Ca2(OH)2(2,6-naphathalenedicarboxylate) | CaO7 | Monoclinic | P21/c | 66 | ||
| 22 | Ca-MOF-4 | Ca(II) | 2D | [Ca3O19]n | Ca3(5-bromoisophthalic acid)3(H2O)3·H2O | Ca1O7, Ca2O7, Ca3O9 | Monoclinic | P21/c | Decomposition T = 240 °C | 62 | |
| 23 | Ca-MOF-5 | Ca(II) | 1D | [CaOCa]n | [H2N(CH3)2]Ca7(1,3,5-tris(4-carboxyphenyl)benzene)5-(H2O)8(DMF)4]·4H2O | Ca1O7, Ca2O9 | Monoclinic | C2/c | 54 | ||
| 24 | Sr-MOF-1 | Sr(II) | 1D | [SrO8]n | Sr(1,4-benzenedicarboxylate)(H2O)(DMF) | SrO8 | Monoclinic | P21/c | Decomposition T = 328 °C, C2H2 uptake: 185.50 cm3 g−1 at 298 K | 57 | |
| 25 | Sr-MOF-2 | Sr(II) | 2D | [SrO]n | Me2NH2]2[Sr5(1,3,5-benzenetricarboxylate)4(H2O)5 | Sr1O8, Sr2O8, Sr3O10 | Orthorhombic | C2221 | Decomposition T = 100 °C | 67 | |
| 26 | Sr-MOF-3 | Sr(II) | 1D | [SrO]n | [Sr2(2,2,6,6-tetracarboxybipheny)(H2O)6]·H2O | Sr1O8, Sr2O9 | Monoclinic | P21/c | Proton conductivity = 2.7 × 10−4 S cm−1 at 98% RH and 90 °C | 129 | |
| 27 | Sr-MOF-4 | Sr(II) | 1D | [Sr5O28]n | [H2N(CH3)2]2Sr5(H2O)6(1,3,5-tris(4-carboxyphenyl)benzene)4 | SrO8 | Monoclinic | C2/c | 54 | ||
| 28 | CAUMOF-8 | Sr(II) | 3D | [SrO]n | Sr2(1,3,5-benzenetricarboxylic)(NO3) | Sr1O9, Sr2O8 | Hexagonal | P-62/c | Decomposition T = 500 °C | 72 | |
| 29 | Ba-MOF-1 | Ba(II) | 1D | [BaO10]n | Me2NH2][Ba(1,3,5-tri(2-carboxymethyltetrazol-5-yl)benzene)(H2O) | BaO10 | Monoclinic | C2/c | Proton conductivity: 1.72 × 10−4 S cm−1 at 98% RH and 85 °C | 58 | |
| 30 | Ba-MOF-2 | Ba(II) | 1D | [BaO8]n | Ba(NH2–1,4-benzenedicarboxylate)(DMF) | BaO8 | Trigonal | P31 | Decomposition T = 300 °C | 53 | |
| 31 | Ba-MOF-3 | Ba(II) | 2D | [BaO]n | [Me2NH2+]2 [Ba5(3,3′,5,5′-biphentetrolate)3(H2O)6] | Ba1O10, Ba2O9 | Tetragonal | P6/m | Decomposition T = 540 °C | 68 | |
| 32 | Ba-MOF-4 | Ba(II) | 3D | [BaO]n | Ba3(1,3,5-benzenetricarboxylic)2(H2O)4 | Ba1O7, Ba2O8, Ba3O9 | Orthorhombic | Pna21 | Decomposition T = 500 °C | 70 | |
| 33 | Ba-MOF-6 | Ba(II) | 2D | [BaO]n | Ba2(3,6-dibromobenzene-1,2,4,5-tetracarboxylic acid)(H2O)2 | BaO10 | Orthorhombic | Cmce | Decomposition T = 370 °C | 65 | |
| 34 | Ba-MOF-7 | Ba(II) | 2D | [Ba3(μ4-NO3)2(μ3-Cl)]n | Ba3(μ5-2-(hydroxymethyl)-1H-imidazole-4,5-dicarboxylicacid)(μ5-2-(hydroxymethyl)-1H-imidazole-4,5-dicarboxylicacid)(μ4-NO3)2(μ3-Cl) | Ba1ClO8, Ba2ClNO8 | Orthorhombic | Fdd2 | Decomposition T = 330 °C | 64 | |
| 35 | Ba-MOF-8 | Ba(II) | 1D | [BaO9]n | [H2N(CH3)2]Ba(H2O)(1,3,5-tris(4-carboxyphenyl)benzene) | BaO9 | Orthorhombic | Pna21 | SHG response, ferroelectric and piezoelectric properties | 54 | |
| 36 | Ba-BPTC | Ba(II) | 1D | [BaO]n | [Ba6(2,2,6,6-tetracarboxybipheny)3(H2O)6]·11H2O | Ba1O7, Ba2O8, Ba3O9 | Monoclinic | P21/c | Water stability over one month | 129 | |
| 37 | Ba-MOF-9 | Ba(II) | 1D | [BaO9]n | [Ba2(N,N-di(2-carboxymethyltetrazol-5-yl)amine)2(H2O)5]·2H2O | BaO9 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Decomposition T = 260 °C | 58 | |
| 38 | Ba-MOF-10 | Ba(II) | 1D | [Ba(CO2)3Ba]n | (Me2NH2)[Ba(1,3,5-tri(2-carboxymethyltetrazol-5-yl)benzene)(H2O)]·3H2O | BaO10 | Monoclinic | P21/c | Proton conductivity = 4.47 × 10−3 S cm−1 at 85 °C and 98% RH | 58 | |
| 39 | Ba-MOF-11 | Ba(II) | 1D | [BaO9]n | [Ba3(μ2-H2O)(4,5-di(tetrazol-5-yl)imidazolylacetic acid)2(H2O)3]·2H2O | BaO9 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Decomposition T = 204 °C | 58 | |
| 40 | Ba-MOF-12 | Ba(II) | 1D | [BaO9]n | Ba(3,5-pyrazoledicarboxylic acid)H2O | BaO9 | Monoclinic | P21/c | Catalytic aldol condensation, yield = 96% | 141 | |
| 41 | CAUMOF-15 | Ba(II) | 1D | [BaO8]n | Ba(4,4′-sulfonyldibenzoic acid)(DMF)4 | BaO8 | Monoclinic | P21/n | Decomposition T = 400 °C | 142 | |
| 42 | CAUMOF-16 | Ba(II) | 1D | [BaO9]n | Ba2(1,2,4,5-benzenetetracarboxylic acid)(H2O) | BaO9, BaO10 | Monoclinic | C2/c | Decomposition T = 400 °C | 142 | |
| 43 | Fe(II)-MOF-1 | Fe(II) | 1D | [FeO2S2]n | Fe(6-mercaptonicotinic acid)2 | FeS4O2 | Monoclinic | P21/c | Antiferromagnetic behaviour at 20 K | 76 | |
| 44 | Fe(II)-MOF-2 | Fe(II) | 1D | [Fe3(CO2)8]n | [NH2(CH3)2][FeII5(1,3,5-benzenetricarboxylate)3(Ac)2(DMA)2] | FeO6 | Monoclinic | P21/c | Antiferromagnetic interaction at 3 K | 143 | |
| 45 | MIL-62 | Fe(II) | 1D | [FeO6]n | Fe2(2,4,5-benzenetetracarboxylates) | FeO6 | Monoclinic | C2/m | Antiferromagnetic below 25 K | 144 | |
| 46 | Cd-MOF-1 | Cd(II) | 1D | [CdON]n | Cd2(BMB)0.5(3,3′,5,5′-tetracarboxylate) | Cd1O7, Cd2O5N | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Purple fluorescent | 79 | |
| 47 | Cd-MOF-2 | Cd(II) | 1D | {[Cd(COO)]+}n{[Cd2(bptc)]3+}n | [Cd2(1,4-bis(2-methylbenzimidazol-1-ylmethyl)benzene)0.5(3,3′,4,4′-benzophenonetetracarboxylic acid)]n | Cd1O5, Cd2O8 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Decomposition T = 429 °C | 79 | |
| 48 | Cd-MOF-3 | Cd(II) | 1D | [CdO7]n | [Cd(3,6-di(3-imidazolyl)benzene-1,2-diamine)1.5(5-hydroxyisophthalic acid)]·(H2O) | CdO7 | Orthorhombic | Fdd2 | hxg-d | 145 | |
| 49 | Cd-MOF-4 | Cd(II) | 1D | [Cd4(CO2)8(OH)2]n | [Cd2(bis(4-carboxylphenyl)amine)2(H2O)]·(H2O)2 | Cd1O7, Cd2O8 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Disposition T = 300 °C | 146 | |
| 50 | Cd-MOF-5 | Cd(II) | 1D | [Cd–O–C]n | [Cd(L)0.5(isophthaloylbisglycine)] | CdO6 | Monoclinic | C2/c | hxg-d | 145 | |
| 51 | Cd-MOF-6 | Cd(II) | 2D | [Cd3(suc)2]n | [Cd3(succinate)2.5(4,4′-dipyridylamine)2](ClO4) | Cd1O6, Cd2N2O5, Cd3N2O5 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
145 | ||
| 52 | CdNa-MOF-1 | Cd(II) | 3D | [Cd3(BTC)4]n | Cd3Na6(1,3,5-benzenetricarboxylic)4(H2O)12 | Cd1O6, Cd2O8 | Tetragonal | P42/n | H2 uptake of 1.11 wt% at 77 K | 23 | |
| 53 | CAU-46 | Ni(II) | 1D | [Ni2O10]n | [Ni2(1,1,2,2-tetrakis[4-phosphonophenyl]ethylene)(H2O)6] | NiO6 | Monoclinic | C2/c | pts | Decomposition T = 550 °C | 73 |
| 54 | CAU-47 | Co(II) | 1D | [Co2O6]n | [Co2(1,1,2,2-tetrakis[4-phosphonophenyl]ethylene)(H2O)4] | CoO6 | Monoclinic | C2/c | pts | Decomposition T = 380 °C | 73 |
| 55 | Cu-MOF | Cu(II) | 1D | [CuO6]n | [Cu4(μ3-OH)4(4,4′-(phenylazanediyl)dibenzoic acid)2] | CuO6 | Orthorhombic | Pnna | Proton conductivity = 3.98 × 10−6 S cm−1 | 78 | |
| 56 | USTC-7 | Zn(II) | 1D | [ZnO6]n | Zn2(4'-(1H-tetrazol-5-yl)-[1,1′-biphenyl]-3,5-dicarboxylic acid)(μ3-OH)(H2O)2 | ZnO6 | Orthorhombic | Cmcm | 147 | ||
| 57 | MOF-303 | Al(III) | 1D | [Al(–OH)Al]n | Al(OH)(1H-pyrazole-3,5-dicarboxylic) | AlO6 | Monoclinic | P21/c | xhh | Water uptake of 0.45 g g−1 at 25 °C | 3 |
| 58 | CAU-15-Cit | Al(III) | 1D | [AlO6]n | Al2(OH)4(O2C–C3H4–CO2) | AlO6 | Monoclinic | C2/c | Decomposition T = 350 °C | 86 | |
| 59 | CAU-8-OBD | Al(III) | 1D | [AlO6]n | Al(4,4′-oxydibenzoic)2(OH) | AlO6 | Tetragonal | I41/a | CO2 adsorb = 70 mg g−1 at 298 K | 84 | |
| 60 | Al-CAU-13 | Al(III) | 1D | [AlO6]n | [Al(OH)(O2C–C6H10–CO2)]·H2O | AlO6 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
83 | ||
| 61 | CAU-51 | Ga(III) | 1D | [GaO6]n | Ga(OH)(2,5-thiophenedicarboxylic) | GaO6 | Monoclinic | P21/c | Water sorption = 370 mg g−1 under p/p0 = 0.38 | 91 | |
| 62 | CAU-43 | In(III) | 1D | [InO6]n | In(OH)[Fe(C5H4)2(COO)2] | InO6 | Monoclinic | C2/c | Decomposition T = 250 °C | 93 | |
| 63 | In-MIL-53 | In(III) | 1D | [InO6]n | In(OH)[Fe(C5H4)2(COO)2] | InO6 | Monoclinic | C2/c | Decomposition T = 350 °C | 93 | |
| 64 | La,Pr-MOF | Ln(III) | 1D | [LnO6]n | Ln(3,6-di(3-imidazolyl)benzene-1,2-diamine)(DMF)(H2O)(HCOO),Ln = La,Pr | LnO6 | Monoclinic | C2/c | 148 | ||
| 65 | Ln-MOF | Ln(III) | 1D | [Ln2O17]n | Ln2(Succ)3(H2O)2, Ln = La, Pr, Nd, Sm, Eu, Gd and Tb | Ln2O17 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
pcu | 149 | |
| 66 | CAU-35 | Bi(III) | 1D | [BiO]n | Bi2(O)(OH)(1,3,5-triazine-2,4,6-tribenzoate) | Bi1O7, Bi2O7 | Orthorhombic | Pna21 | 96 | ||
| 67 | CAU-50 | Sc(III) | 1D | [ScO6]n | Sc2(1,1′-ferrocenedicarboxlic)3 | ScO6 | Orthorhombic | Pnna | Decomposition T = 250 °C | 99 | |
| 68 | MFM-300 | Cr(III) | 1D | [CrO4(OH)2]n | Cr2(OH)2(biphenyl-3,3′,5,5′-tetracarboxylic) | CrO6 | Tetragonal | I4122 | SO2 uptake up to 8.59 mmol g−1 at 273 K | 31 | |
| 69 | CTH-16 | Gd(III) | 1D | [GdO9]n | Gd2(Benzene-1,2,4,5-tetracarboxylate)2 | GdO9 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
htp | Decomposition T = 350 °C | 21 |
| 70 | CTH-15 | Ce(III) | 2D | [CeO]n | Ce3(Benzene-1,2,4,5-tetracarboxylate)(benzene-1,2,4,5-tetracarboxylate acid)(OAc)(HCO2) | Ce1–O, Ce2–O,Ce3–O | Monoclinic | P21/c | Decomposition T = 350 °C | 21 | |
| 71 | Ce(III)-MOF | Ce(III) | 1D | [CeO9]n | [Ce2(3,6-Di(3-imidazolyl)benzene-1,2-diamine)3(DMA)3(H2O)4]n DMA | CeO9 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
sql | 148 | |
| 72 | Tb-MOF | Tb(III) | 1D | [TbO9]n | [Tb(3,6-di(3-imidazolyl)benzene-1,2-diamine)(DMA)2(NO3)]nH2O | TbO9 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
148 | ||
| 73 | La -MOF | La(III) | 1D | [LaO11]n | [La2(Succ)3(H2O)3]·2H2O | LaO11 | Orthorhombic | Pbnm | pcu | Decomposition T = 420 °C | 150 |
| 74 | MIL-177-HT | Ti(IV) | 1D | [Ti6O9]n | Ti12O15(5,5′-methylenediisophthalic)3(formate)6 | TiO6 | Hexagonal | P6/mmm | Photocarrier mobility = 4 × 10−4 cm2 s−1 V−1 | 27 | |
| 75 | ZSTU-1 | Ti(IV) | 1D | [Ti(μ3-O)6(μ3-OH)6(COO)6]n | Ti6(μ3-O)6(μ2-OH)6(4,4′,4′′-nitrilotribenzoic)2(H2O)(DMF)2 | Ti6(μ3-O)6(COO)6 | Hexagonal | P63/mcm | kgd | Hydrogen evolution rate: 1120 μmol g−1 h−1 | 18 |
| 76 | ZSTU-2 | Ti(IV) | 1D | [Ti(μ3-O)6(μ3-OH)6(COO)6]n | Ti6(μ3-O)6(μ2-OH)6(1,3,5-tris(4-carboxyphenyl)benzene)2(H2O) | Ti6(μ3-O)6(COO)6 | Hexagonal | P63/mcm | Hydrogen evolution rate: 390 μmol g−1 h−1 | 18 | |
| 77 | ZSTU-3 | Ti(IV) | 1D | [Ti(μ3-O)6(μ3-OH)6(COO)6]n | Ti6(μ3-O)6(μ2-OH)6(tris(4′-carboxybiphenyl)amine)2(H2O)(DMF)2 | Ti6(μ3-O)6(COO)6 | Hexagonal | P63/mcm | Hydrogen evolution rate: 1350 μmol g−1 h−1 | 18 | |
| 78 | COK-47 | Ti(IV) | 2D | [TiO6]n | Cp2TiCl2 | TiO6 | Triclinic |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
Photocatalytic dibenzothiophene oxidation (Con. = 99%) | 19 | |
| 79 | CAU-22 | Zr(IV) | 1D | [Zr6O4(OH)4(μ2-OH)2]n | Zr6(μ3-O)4(μ3-OH)4(μ2-OH)2(1H-pyrazole-3,5-dicarboxylic)3(OH)2(H2O)2(HCO2)2 | Zr6O4(OH)4 | Monoclinic | C2/m | Decomposition T = 270 °C | 38 | |
| 80 | CAU-27 | Zr(IV) | 1D | [Zr6O4(OH)4]n | [Zr5O4(OH)4(OAc)4(BDC)2] | Zr6O4(OH)4 | Tetragonal | I 4/mcm | Decomposition T = 470 °C | 17 | |
| 81 | MIL-163 | Zr(IV) | 1D | [ZrO8]n | [Zr(H2-TzGal)] | ZrO8 | Tetragonal | P42/mmc | CO2 uptake (6 mmol g−1) at 298 K and 25 bar | 106 | |
| 82 | ZrPP-1 | Zr(IV) | 1D | [ZrO8]n | Zr2(THPP) | ZrO8 | Tetragonal | P42/mmc | nbo | CO2 uptake = 87.7 cm3 g−1 at 273K, CO production rate = 210 μmol g−1 | 34 |
| 83 | Zr-MIL-140-4F | Zr(IV) | 1D | [ZrO7]n | [ZrO(BDC-F4)] | ZrO7 | Monoclinic | C21/c | C2H2/CO2 separation, Sel. = 2.7 | 30 | |
| 84 | PCN-226 | Zr(IV) | 1D | [ZrO7]n | Zr3O3(Tetrakis(4-carboxyphenyl)porphyrin)(benzoate)2 | ZrO7 | Orthorhombic | Ibam | ztt | Zn–air battery with specific capacity up to 724 mA h g−1 | 32 |
| 85 | Ce-MIL-140-4F | Ce(IV) | 1D | [CeO7]n | [CeO(BDC-F4)] | CeO7 | Monoclinic | C21/c | CO2/C2H2 separation, Sel. = 2.5 | 30 | |
| 86 | NSM | Np(V) | 1D | [NpO7]n | [(CH3)2NH2]4[(NpO2)4(C53O8H32)(HCOO)4] | NpO7 | Tetragonal | I41/a | 16 |
While, ISBU MOFs also have some disadvantages in comparison to conventional discrete node MOFs. Up till now, the development of ISBU MOFs is still in its infancy and most of the examples have been obtained by trial and error, without guidable principles and universe protocols. In spite of the robust stabilities, the formation of dense ISBU at certain dimensions somewhat decreases porosities and specific surface areas of resultant MOFs. Equally, it is more or less difficult to functionalize corresponding MOFs via modifying the pure inorganic ISBUs. Challenges are always with opportunities. Hence, some perspectives are also provided according to our personal insights.
(1) It is well known that reticular chemistry151,152 guided the structure design and deciphering of MOFs in virtue of topology. Unfortunately, the dense and complicated metal-oxo interconnection casts hardly unified standards for the topological decomposition of ISBU MOFs. Taking a scenario, the usually adopted “all-nodes” and “extension-of-points” approaches face troubles in connecting the simplified nodes of ISBU MOFs, which is totally different from the case of OD metal nodes only connected by organic edges. As a result, how to construct ISBU MOFs with ordered and well-defined structures and topologies is still an open question. Considering the pure inorganic ISBU nature resembling traditional metal oxides and metal hydroxides, we suggest that a fundamental crystallographic and property understanding of those inorganics might be essential for further development of ISBU MOFs with well-defined compositions and structures. As a proof-of-concept, the very recently reported metal hydroxide-organic frameworks153 have been successfully obtained from corresponding hydroxide precursors.
(2) Hydrothermal and solvothermal strategies are routinely utilized for the synthesis of various types of MOFs witnessed with great success in past decades. With regard to ISBU MOFs, the metal-oxo inorganic nature renders them forming corresponding metal-oxide/hydroxides byproduct easily in conventional solvent-involved conditions. Especially for alkali metal cations (e.g. Li+, Na+ and K+), the strong solvation effect results in an overwhelming competition with the organic linker coordination, and hence usual formations of unstable frameworks with excessive adsorbed solvents. Mechano-assisted syntheses like grinding and balling free of solvent are suggested as potential workable routes.
(3) Single-crystal X-ray diffraction (SCXRD) is widely accepted as the golden tool for characterization of emerged novel MOFs. However, powdery polycrystals with micrometer scales are insufficient for SCXRD characterization but are popularly obtained in examples of ISBU MOFs. Aims at obtaining accurate crystallographic information, low-dose transmission electron/high-resolution cryo-electron microscopy, continuous 3D electron diffraction together with theoretic modeling are suggested as the combinational standard.
(4) Though unique physical/chemical properties stemming from their inorganic character, the application scopes of ISBU MOFs are still very limited. In addition to achievements in adsorption, gas separation, energy storage and photocatalysis, opto- and electro-devices based on ISBU MOFs by fully exploiting their inorganic semiconductor-like and flexible organic characters are very appealing but still are virgin research fields. Besides, ISBU MOFs may become a very potential bridge across inorganic chemistry and biomaterials via selections of low toxic (e.g., alkali and alkali earth) metal-oxo building blocks and biocompatible organic linkers (e.g., amino acid and peptide derivates). For instance, chiral ISBU MOFs designed for drug loading, anti-tumor and/or bioimaging are welcome.
(5) Equally importantly, the fundamental mechanism study is essential to pursue better performances as well as expand application scope via the made-to-order structure redesign. Hence, much more effort attempting to in-depth understand the ISBU functions by the time-resolved and operando technologies is highly desirable to be devoted.
In spite of the standing challenges, the unique structure character and associated intriguing physicochemical properties of ISBU MOFs will attract much more effort devoted to this emerging research field. We can foresee that much more novel ISBU MOFs will be rationally designed, controllably synthesized and competently applied in versatile fields in the near future.
Abbreviations
| L1 | 4,4′,4′′-(Methylsilanetriyl)tribenzoate |
| L2 | 4,4′,4′′,4′′′-Silanetetrayltetrabenzoate |
| L3 | 1,4-Bis(phenylthio)but-2-yne |
| H2L4 | 3,3′-Dihydroxy-[1,1′-biphenyl]-4,4′-dicarboxylic acid |
| L5 | 1,3,5-Tri(2-carboxymethyltetrazol-5-yl)benzene |
| L6 | 3,3′,5,5′-Biphentetrolate |
| H4L7 | Biphenyl-3,3′,5,5′-tetracarboxylic acid |
| 4,4-BPDC | 4,4-Bisphthalboxylate |
| 6-H2MNA | 6-Mercaptonicotinic acid |
| BDA | (2S, 2′S)-2,2′-(Terephthaloylbis(azanediyl))disuccinate |
| BDC | 1,4-Benzenedicarboxylate |
| BMB | 1,4-Bis(2-methylbenzimidazol-1-methylmethyl)benzene |
| BPDA | (2S,2′S)-2,2′-(([1,1′-Biphenyl]-4,4′-dicarbonyl)bis(azanediyl)) di-succinate |
| BPTC | 1,1′-Biphenyl-2,2′,6,6′-tetracarboxylate |
| BTB | 1,3,5-Tris(4-carboxyphenyl)benzene |
| CB | Conduction band |
| Cp | Cyclopentadienyl |
| dach | trans-1,2-Diaminocyclohexane |
| DOBPTC | 4,4′-Di-oxidobiphenyl-3,3′-dicarboxylate |
| DFT | Density functional theory |
| DMF | N,N-Dimethylformamide |
| FP-TRMC | Flash photoluminescence time-resolved microwave conductivity |
| H4BPTC | 3,3′,4,4′-Benzophenone tetracarboxylic acid |
| H3BTC | 1,3,5-Benzenetricarboxylic acid |
| H3BTCA | Tris(4′-carboxybiphenyl)amine acid |
| H4BTEC | Benzene-1,2,4,5-tetracarboxylate acid |
| HER | Hydrogen evolution reaction |
| H2FcDC | 1,1′-Ferrocenedicarboxlic acid |
| H2FDC | Furan-2,4-dicarboxylic acid |
| H4MDIP | 5,5′-Methylenediisophthalic acid |
| H2OBD | 4,4′-Oxydibenzoic acid |
| H2PZDC | 1H-Pyrazole-3,5-dicarboxylic acid |
| H3TCA | 4,4′,4′′-Nitrilotribenzoic acid |
| H2TCPP | Tetrakis(4-carboxyphenyl)porphyrin acid |
| H2TDC | 2,5-Thiophenedicarboxylic acid |
| H2TFBDC | Tetrafluoro-terephthalic acid |
| H4(TTF-TC) | Tetrathiafulvalene tetracarboxylic acid |
| H6TzGal | 5,5′-(1,2,4,5-Tetrazine-3,6-diyl)bis(benzene-1,2,3-triol) acid |
| IAST | Ideal adsorbed solution theory |
| IPr | Isopropoxide |
| LCCT | Linker-to-cluster charge transfer |
| LMCT | Ligand-to-metal charge transfer |
| NH2–H2BDC | 2-Amino-1,4-benzenedicarboxylic acid |
| NDC | 2,6-Naphathalenedicarboxylate |
| OER | Oxygen-evolution reaction |
| ORR | Oxygen-involved reduction |
| PDC | 3,5-Pyridinedicarboxylate |
| TATB | 1,3,5-Triazine-2,4,6-tribenzoate |
| TEOA | Triethanolamine |
| THPP | 5,10,15,20-Tetrakis(3,4,5-trihydroxyphenyl)porphyrin |
| TiPNW | Titanium-phosphonate MOF based nanowires |
| TTC | Trithiocyanuric acid |
| UL | Ultralight |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by National Natural Science Foundation of China (No. 22103055, 21905195 and 22073070), Natural Science Foundation of Tianjin City (No. 20JCYBJC00800), Science and Technology Plans of Tianjin (21ZYJDJC00050) and PEIYANG Young Scholars Program of Tianjin University (No. 2020XRX-0023).Notes and references
- W. Cheng, H. Zhang, D. Luan and X. W. D. Lou, Sci. Adv., 2021, 7, eabg2580 CrossRef CAS PubMed.
- Y. Zhang, J. Guo, J. Zhang, X. Qiu, X. Zhang, J. Han, B. Zhang, C. Long, Y. Shi, Z. Yang, W. Zhao and Z. Tang, Chem, 2022, 8, 1688–1704 Search PubMed.
- N. Hanikel, X. Pei, S. Chheda, H. Lyu, W. Jeong, J. Sauer, L. Gagliardi and O. M. Yaghi, Science, 2021, 374, 454–459 CrossRef CAS PubMed.
- M. Zhao, K. Yuan, Y. Wang, G. Li, J. Guo, L. Gu, W. Hu, H. Zhao and Z. Tang, Nature, 2016, 539, 76–80 CrossRef CAS PubMed.
- L. Wang, H. Xu, J. Gao, J. Yao and Q. Zhang, Coord. Chem. Rev., 2019, 398, 213016 CrossRef.
- J. Guo, Y. Qin, Y. Zhu, X. Zhang, C. Long, M. Zhao and Z. Tang, Chem. Soc. Rev., 2021, 50, 5366–5396 RSC.
- M. J. Kalmutzki, N. Hanikel and O. M. Yaghi, Sci. Adv., 2018, 4, eaat9180 CrossRef CAS PubMed.
- J. Guo, Y. Zhang, Y. Zhu, C. Long, M. Zhao, M. He, X. Zhang, J. Lv, B. Han and Z. Tang, Angew. Chem., Int. Ed., 2018, 57, 6873–6877 CrossRef CAS PubMed.
- Z. H. Syed, F. Sha, X. Zhang, D. M. Kaphan, M. Delferro and O. K. Farha, ACS Catal., 2020, 10, 11556–11566 CrossRef CAS.
- H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gandara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O'Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Science, 2012, 336, 1018–1023 CrossRef CAS PubMed.
- X. Zhang, Z. Chen, X. Liu, S. L. Hanna, X. Wang, R. Taheri Ledari, A. Maleki, P. Li and O. K. Farha, Chem. Soc. Rev., 2020, 49, 7406–7427 RSC.
- Y. Bai, Y. Dou, L. H. Xie, W. Rutledge, J. R. Li and H. C. Zhou, Chem. Soc. Rev., 2016, 45, 2327–2367 RSC.
- M. J. Kalmutzki, N. Hanikel and O. M. Yaghi, Sci. Adv., 2018, 4, eaat9180 CrossRef CAS PubMed.
- W. Xu, B. Tu, Q. Liu, Y. Shu, C. Liang, C. S. Diercks, O. M. Yaghi, Y. Zhang, H. Deng and Q. Li, Nat. Rev. Mater., 2020, 5, 764–779 CrossRef CAS.
- H. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- S. E. Gilson, M. Fairley, S. L. Hanna, J. E. S. Szymanowski, P. Julien, Z. Chen, O. K. Farha, J. A. LaVerne and P. C. Burns, J. Am. Chem. Soc., 2021, 143, 17354–17359 CrossRef CAS PubMed.
- S. Leubner, H. Zhao, N. Van Velthoven, M. Henrion, H. Reinsch, D. E. De Vos, U. Kolb and N. Stock, Angew. Chem., Int. Ed., 2019, 58, 10995–11000 CrossRef CAS PubMed.
- C. Li, H. Xu, J. Gao, W. Du, L. Shangguan, X. Zhang, R.-B. Lin, H. Wu, W. Zhou, X. Liu, J. Yao and B. Chen, J. Mater. Chem. A, 2019, 7, 11928–11933 RSC.
- S. Smolders, T. Willhammar, A. Krajnc, K. Sentosun, M. T. Wharmby, K. A. Lomachenko, S. Bals, G. Mali, M. B. J. Roeffaers, D. E. De Vos and B. Bueken, Angew. Chem., Int. Ed., 2019, 58, 9160–9165 CrossRef CAS PubMed.
- H. Jia, Q. Han, W. Luo, H. Cong and H. Deng, Chem. Catal., 2022, 2, 84–101 CrossRef.
- F. M. Amombo Noa, M. Abrahamsson, E. Ahlberg, O. Cheung, C. R. Göb, C. J. McKenzie and L. Öhrström, Chem, 2021, 7, 2491–2512 CAS.
- Y. Kamakura, P. Chinapang, S. Masaoka, A. Saeki, K. Ogasawara, S. R. Nishitani, H. Yoshikawa, T. Katayama, N. Tamai, K. Sugimoto and D. Tanaka, J. Am. Chem. Soc., 2020, 142, 27–32 CrossRef CAS PubMed.
- Y. Fu, J. Su, Z. Zou, S. Yang, G. Li, F. Liao and J. Lin, Cryst. Growth Des., 2011, 11, 3529–3535 CrossRef CAS.
- M. K. Kim and K. M. Ok, CrystEngComm, 2011, 13, 4599–4603 RSC.
- N. Kolobov, M. G. Goesten and J. Gascon, Angew. Chem., Int. Ed., 2021, 60, 26038–26052 CrossRef CAS PubMed.
- D. Banerjee, S. J. Kim and J. B. Parise, Cryst. Growth Des., 2009, 9, 2500–2503 CrossRef CAS.
- S. Wang, T. Kitao, N. Guillou, M. Wahiduzzaman, C. Martineau Corcos, F. Nouar, A. Tissot, L. Binet, N. Ramsahye, S. Devautour Vinot, S. Kitagawa, S. Seki, Y. Tsutsui, V. Briois, N. Steunou, G. Maurin, T. Uemura and C. Serre, Nat. Commun., 2018, 9, 1660–1668 CrossRef PubMed.
- J. D. Martell, L. B. Porter Zasada, A. C. Forse, R. L. Siegelman, M. I. Gonzalez, J. Oktawiec, T. e. Runčevski, J. Xu, M. Srebro Hooper, P. J. Milner, A. Colwell, J. Autschbach, J. A. Reimer and J. R. Long, J. Am. Chem. Soc., 2017, 139, 16000–16012 CrossRef CAS PubMed.
- F. Fathieh, M. J. Kalmutzki, E. A. Kapustin, P. J. Waller, J. Yang and O. M. Yaghi, Sci. Adv., 2018, 4, eaat3198 CrossRef PubMed.
- Z. Zhang, S. B. Peh, R. Krishna, C. Kang, K. Chai, Y. Wang, D. Shi and D. Zhao, Angew. Chem., Int. Ed., 2021, 60, 17198–17204 CrossRef CAS PubMed.
- L. Briggs, R. Newby, X. Han, C. G. Morris, M. Savage, C. P. Krap, T. L. Easun, M. D. Frogley, G. Cinque, C. A. Murray, C. C. Tang, J. Sun, S. Yang and M. Schröder, J. Mater. Chem. A, 2021, 9, 7190–7197 RSC.
- M. O. Cichocka, Z. Liang, D. Feng, S. Back, S. Siahrostami, X. Wang, L. Samperisi, Y. Sun, H. Xu, N. Hedin, H. Zheng, X. Zou, H. C. Zhou and Z. Huang, J. Am. Chem. Soc., 2020, 142, 15386–15395 CrossRef CAS PubMed.
- Y. Pan, Q. Li, H. Li, J. Farmakes, A. Ugrinov, X. Zhu, Z. Lai, B. Chen and Z. Yang, Chem. Catal., 2021, 1, 146–161 CrossRef.
- E. X. Chen, M. Qiu, Y. F. Zhang, Y. S. Zhu, L. Y. Liu, Y. Y. Sun, X. Bu, J. Zhang and Q. Lin, Adv. Mater., 2018, 30, 1704388–1704395 CrossRef PubMed.
- Y. P. Zhu, J. Yin, E. Abou Hamad, X. Liu, W. Chen, T. Yao, O. F. Mohammed and H. N. Alshareef, Adv. Mater., 2020, 32, 1906368–1906375 CrossRef CAS PubMed.
- Y. Keum, S. Park, Y. P. Chen and J. Park, Angew. Chem., Int. Ed., 2018, 57, 14852–14856 CrossRef CAS PubMed.
- A. Cadiau, N. Kolobov, S. Srinivasan, M. G. Goesten, H. Haspel, A. V. Bavykina, M. R. Tchalala, P. Maity, A. Goryachev, A. S. Poryvaev, M. Eddaoudi, M. V. Fedin, O. F. Mohammed and J. Gascon, Angew. Chem., Int. Ed., 2020, 59, 13468–13472 CrossRef CAS PubMed.
- S. Waitschat, H. Reinsch and N. Stock, Chem. Commun., 2016, 52, 12698–12701 RSC.
- H. Putz and K. Brandenburg, Crystal Impact-GbR, Kreuzherrenstr, 2006, vol. 102, p. 53227 Search PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- A. Schoedel, M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2016, 116, 12466–12535 CrossRef CAS PubMed.
- D. Pugh, E. Ashworth, K. Robertson, L. C. Delmas, A. J. P. White, P. N. Horton, G. J. Tizzard, S. J. Coles, P. D. Lickiss and R. P. Davies, Cryst. Growth Des., 2018, 19, 487–497 CrossRef.
- D. Banerjee, L. A. Borkowski, S. J. Kim and J. B. Parise, Cryst. Growth Des., 2009, 9, 4922–4926 CrossRef CAS.
- A. Nguyen Tle, R. Demir Cakan, T. Devic, M. Morcrette, T. Ahnfeldt, P. Auban Senzier, N. Stock, A. M. Goncalves, Y. Filinchuk, J. M. Tarascon and G. Ferey, Inorg. Chem., 2010, 49, 7135–7143 CrossRef PubMed.
- P. Siman, C. A. Trickett, H. Furukawa and O. M. Yaghi, Chem. Commun., 2015, 51, 17463–17466 RSC.
- X. Zhao, M. S. Shimazu, X. Chen, X. Bu and P. Feng, Angew. Chem., Int. Ed., 2018, 57, 6208–6211 CrossRef CAS PubMed.
- J. H. Lee, B. Moon, T. K. Kim, S. Jeoung and H. R. Moon, Dalton Trans., 2015, 44, 15130–15134 RSC.
- S. Q. Bai, I. H. K. Wong, N. Zhang, K. Lin Ke, M. Lin, D. J. Young and T. S. A. Hor, Dalton Trans., 2018, 47, 16292–16298 RSC.
- F. Liu, P. Hao, T. Yu, Q. Guan and Y. Fu, J. Mol. Struct., 2016, 1119, 431–436 CrossRef CAS.
- S. Q. Bai, J. Y. Kwang, L. L. Koh, D. J. Young and T. S. Hor, Dalton Trans., 2010, 39, 2631–2636 RSC.
- Q. Lin, T. Wu, S. T. Zheng, X. Bu and P. Feng, Chem. Commun., 2011, 47, 11852–11854 RSC.
- R. K. Vakiti, B. D. Garabato, N. P. Schieber, M. J. Rucks, Y. Cao, C. Webb, J. B. Maddox, A. Celestian, W.-P. Pan and B. Yan, Cryst. Growth Des., 2012, 12, 3937–3943 CrossRef CAS.
- D. Briones, P. Leo, J. Cepeda, G. Orcajo, G. Calleja, R. Sanz, A. Rodríguez-Diéguez and F. Martínez, CrystEngComm, 2018, 20, 4793–4803 RSC.
- K. S. Asha, M. Makkitaya, A. Sirohi, L. Yadav, G. Sheet and S. Mandal, CrystEngComm, 2016, 18, 1046–1053 RSC.
- X. Chen, A. M. Plonka, D. Banerjee and J. B. Parise, Cryst. Growth Des., 2012, 13, 326–332 CrossRef.
- R. K. Birkedal Nielsen, K. O. Kongshaug and H. Fjellvåg, Solid State Sci., 2006, 8, 1237–1242 CrossRef CAS.
- D. Guo, C. M. Liang, K. Chiu and W. K. Wang, Z. Anorg. Allg. Chem., 2021, 647, 1193–1197 CrossRef CAS.
- J. Yang, S. Zhang, Z. Feng, Y. Cao and D. R. Zhu, Dalton Trans., 2021, 50, 11975–11985 RSC.
- F. Dai, X. Wang, Y. Wang, Z. Liu and D. Sun, Angew. Chem., Int. Ed., 2020, 59, 22372–22377 CrossRef CAS PubMed.
- Y. Sun, B.-X. Dong and W.-L. Liu, J. Solid State Chem., 2019, 278, 120897–120903 CrossRef CAS.
- W. z. Zhang, T. y. Lv, D. z. Wei, R. Xu, G. Xiong, Y. q. Wang, E. j. Gao and Y. g. Sun, Inorg. Chem. Commun., 2011, 14, 1245–1249 CrossRef CAS.
- P. Wang, G. Wu and X. Wang, J. Inorg. Organomet. Polym. Mater., 2012, 22, 1377–1383 CrossRef CAS.
- D. Saha, T. Maity, R. Sen and S. Koner, Polyhedron, 2012, 43, 63–70 CrossRef CAS.
- T. T. Li, S. L. Cai, R. H. Zeng and S. R. Zheng, Inorg. Chem. Commun., 2014, 48, 40–43 CrossRef CAS.
- S. Du, C. Ji, X. Xin, M. Zhuang, X. Yu, J. Lu, Y. Lu and D. Sun, J. Mol. Struct., 2017, 1130, 565–572 CrossRef CAS.
- C. Volkringer, J. Marrot, G. Férey and T. Loiseau, Cryst. Growth Des., 2008, 8, 685–689 CrossRef CAS.
- J. J. Niu, C. Chen, C. W. Ye, X. Zhang, Y. G. Yao and L. F. Chen, Inorg. Chem. Commun., 2013, 27, 149–151 CrossRef CAS.
- X. Chen, S. He, F. Chen and Y. Feng, CrystEngComm, 2014, 16, 8706–8709 RSC.
- D. T. Tran, D. Chu, A. G. Oliver and S. R. J. Oliver, Inorg. Chem. Commun., 2009, 12, 351–354 CrossRef CAS.
- X. Zhang, Y. Y. Huang, M. J. Zhang, J. Zhang and Y. G. Yao, Cryst. Growth Des., 2012, 12, 3231–3238 CrossRef CAS.
- J. A. Rood, B. C. Noll and K. W. Henderson, Inorg. Chem., 2006, 45, 5521–5528 CrossRef CAS PubMed.
- D. W. Lee, V. Jo and K. M. Ok, Cryst. Growth Des., 2011, 11, 2698–2701 CrossRef CAS.
- S. Wohlbrandt, C. Meier, H. Reinsch, E. Svensson Grape, A. K. Inge and N. Stock, Inorg. Chem., 2020, 59, 13343–13352 CrossRef PubMed.
- A. K. Singh, G. Pandey, K. Singh, A. Kumar, M. Trivedi and V. Singh, Dalton Trans., 2018, 47, 6386–6393 RSC.
- X. Y. Li, Y. L. Wang, Z. Xue, S. D. Han, Z. Z. Xue and J. Pan, Cryst. Growth Des., 2021, 21, 5261–5267 CrossRef CAS.
- S. Du, M. Cui and Z. He, Inorg. Chem., 2021, 60, 19053–19061 CrossRef CAS PubMed.
- J. Chen, X. Mu, M. Du and Y. Lou, Inorg. Chem. Commun., 2017, 84, 241–245 CrossRef CAS.
- B. Ay, O. Şahin and E. Yildiz, Solid State Sci., 2019, 96, 105958–105966 CrossRef CAS.
- C. Xu, B. M. Ji, S. B. Miao, D. S. Deng, H. w. Hou and M. h. Li, Polyhedron, 2017, 130, 160–164 CrossRef CAS.
- Q. Z. Sun, Y. B. Yin, L. Y. Chai, H. Liu, P. F. Hao, X. P. Yan and Y. Q. Guo, J. Mol. Struct., 2014, 1070, 75–79 CrossRef CAS.
- M. Wahiduzzaman, D. Lenzen, G. Maurin, N. Stock and M. T. Wharmby, Eur. J. Inorg. Chem., 2018, 2018, 3626–3632 CrossRef CAS.
- H. Reinsch, S. Waitschat and N. Stock, Dalton Trans., 2013, 42, 4840–4847 RSC.
- H. Reinsch, J. Benecke, M. Etter, N. Heidenreich and N. Stock, Dalton Trans., 2017, 46, 1397–1405 RSC.
- M. Kruger, A. K. Inge, H. Reinsch, Y. H. Li, M. Wahiduzzaman, C. H. Lin, S. L. Wang, G. Maurin and N. Stock, Inorg. Chem., 2017, 56, 5851–5862 CrossRef PubMed.
- N. Hermer, M. T. Wharmby and N. Stock, Z. Anorg. Allg. Chem., 2017, 643, 137–140 CrossRef CAS PubMed.
- N. Heidenreich, A. Lieb, N. Stock and H. Reinsch, Dalton Trans., 2018, 47, 215–223 RSC.
- E. Alvarez, N. Guillou, C. Martineau, B. Bueken, B. Van de Voorde, C. Le Guillouzer, P. Fabry, F. Nouar, F. Taulelle, D. de Vos, J. S. Chang, K. H. Cho, N. Ramsahye, T. Devic, M. Daturi, G. Maurin and C. Serre, Angew. Chem., Int. Ed., 2015, 54, 3664–3668 CrossRef CAS PubMed.
- C. Volkringer, T. Loiseau, M. Haouas, F. Taulelle, D. Popov, M. Burghammer, C. Riekel, C. Zlotea, F. Cuevas, M. Latroche, D. Phanon, C. Knöfelv, P. L. Llewellyn and G. Férey, Chem. Mater., 2009, 21, 5783–5791 CrossRef CAS.
- C. Hao, H. Ren, H. Zhu, Y. Chi, W. Zhao, X. Liu, W. J. S. Guo and P. Technology, Sep. Purif. Technol., 2022, 290, 120804 CrossRef CAS.
- R. Hajjar, C. Volkringer, T. Loiseau, N. Guillou, J. Marrot, G. Férey, I. Margiolaki, G. Fink, C. Morais and F. Taulelle, Chem. Mater., 2010, 23, 39–47 CrossRef.
- T. Rabe, E. S. Grape, H. Rohr, H. Reinsch, S. Wohlbrandt, A. Lieb, A. K. Inge and N. Stock, Inorg. Chem., 2021, 60, 8861–8869 CrossRef CAS PubMed.
- S. Hou, X. Zhang, W. Yuan, Y. Li and Z. Gu, Inorg. Chem., 2020, 59, 11298–11304 CrossRef CAS PubMed.
- J. Benecke, E. S. Grape, A. Fuss, S. Wohlbrandt, T. A. Engesser, A. K. Inge, N. Stock and H. Reinsch, Inorg. Chem., 2020, 59, 9969–9978 CrossRef CAS PubMed.
- R. J. Li, M. Li, X. P. Zhou, D. Li and M. O'Keeffe, Chem. Commun., 2014, 50, 4047–4049 RSC.
- M. Köppen, V. Meyer, J. Ångström, A. K. Inge and N. Stock, Cryst. Growth Des., 2018, 18, 4060–4067 CrossRef.
- M. Koppen, O. Beyer, S. Wuttke, U. Luning and N. Stock, Dalton Trans., 2017, 46, 8658–8663 RSC.
- P. Rönfeldt, H. Reinsch, M. P. M. Poschmann, H. Terraschke and N. Stock, Cryst. Growth Des., 2020, 20, 4686–4694 CrossRef.
- P. Rönfeldt, H. Reinsch, N. Faßheber, H. Terraschke and N. Stock, Eur. J. Inorg. Chem., 2020, 2020, 1147–1152 CrossRef.
- J. Benecke, E. Svensson Grape, T. A. Engesser, A. K. Inge and H. Reinsch, CrystEngComm, 2020, 22, 5569–5572 RSC.
- P. L. Llewellyn, P. Horcajada, G. Maurin, T. Devic, N. Rosenbach, S. Bourrelly, C. Serre, D. Vincent, S. Loera-Serna, Y. Filinchuk and G. Férey, J. Am. Chem. Soc., 2009, 131, 13002–13008 CrossRef CAS PubMed.
- Y. Yan, C. Li, Y. Wu, J. Gao and Q. Zhang, J. Mater. Chem. A, 2020, 8, 15245–15270 RSC.
- V. Benoit, R. S. Pillai, A. Orsi, P. Normand, H. Jobic, F. Nouar, P. Billemont, E. Bloch, S. Bourrelly, T. Devic, P. A. Wright, G. de Weireld, C. Serre, G. Maurin and P. L. Llewellyn, J. Mater. Chem. A, 2016, 4, 1383–1389 RSC.
- V. Trannoy, N. Guillou, C. Livage, C. Roch-Marchal, M. Haouas, A. Leaustic, C. Allain, G. Clavier, P. Yu and T. Devic, Inorg. Chem., 2019, 58, 6918–6926 CrossRef CAS PubMed.
- M. Taddei, F. Costantino, F. Marmottini, A. Comotti, P. Sozzani and R. Vivani, Chem. Commun., 2014, 50, 14831–14834 RSC.
- L. Samperisi, A. Jaworski, G. Kaur, K. P. Lillerud, X. Zou and Z. Huang, J. Am. Chem. Soc., 2021, 143, 17947–17952 CrossRef CAS PubMed.
- G. Mouchaham, L. Cooper, N. Guillou, C. Martineau, E. Elkaim, S. Bourrelly, P. L. Llewellyn, C. Allain, G. Clavier, C. Serre and T. Devic, Angew. Chem., Int. Ed., 2015, 54, 13297–13301 CrossRef CAS PubMed.
- J. Jacobsen, L. Wegner, H. Reinsch and N. Stock, Dalton Trans., 2020, 49, 11396–11402 RSC.
- R. D'Amato, A. Donnadio, M. Carta, C. Sangregorio, D. Tiana, R. Vivani, M. Taddei and F. Costantino, ACS Sustainable Chem. Eng., 2018, 7, 394–402 CrossRef.
- S. Leubner, R. Siegel, J. Franke, M. T. Wharmby, C. Krebs, H. Reinsch, J. Senker and N. Stock, Inorg. Chem., 2020, 59, 15250–15261 CrossRef CAS PubMed.
- M. Zhao, Y. Huang, Y. Peng, Z. Huang, Q. Ma and H. Zhang, Chem. Soc. Rev., 2018, 47, 6267–6295 RSC.
- Y. Qin, Y. Wan, J. Guo and M. Zhao, Chin. Chem. Lett., 2022, 33, 693–702 CrossRef CAS.
- J. Guo, Y. Duan, Y. Liu, H. Li, Y. Zhang, C. Long, Z. Wang, Y. Yang and S. Zhao, J. Mater. Chem. A, 2022, 10, 6463–6469 RSC.
- J. Guo, Y. Wan, Y. Zhu, M. Zhao and Z. Tang, Nano Res., 2021, 14, 2037–2052 CrossRef CAS.
- D. Gao, J. H. Chen, S. Fang, T. Ma, X. H. Qiu, J. G. Ma, Q. Gu and P. Cheng, Matter, 2021, 4, 1001–1016 CrossRef CAS.
- G. Zheng, I. Pastoriza Santos, J. Pérez Juste and L. M. Liz Marzán, SmartMat, 2021, 2, 446–465 CrossRef.
- X. Li, Y. Li, L. Ma, L. Hou, C. He, Y. Wang and Z. Zhu, J. Mater. Chem. A, 2020, 8, 5227–5233 RSC.
- M. F. Ghazvini, M. Vahedi, S. N. Nobar and F. Sabouri, J. Environ. Chem. Eng., 2021, 9, 104790 CrossRef.
- D. Wu, P. F. Zhang, G. P. Yang, L. Hou, W. Y. Zhang, Y. F. Han, P. Liu and Y. Y. Wang, Coord. Chem. Rev., 2021, 434, 213709 CrossRef CAS.
- N. Monni, E. Andres Garcia, K. Caamaño, V. García López, J. M. Clemente Juan, M. Giménez Marqués, M. Oggianu, E. Cadoni, G. Mínguez Espallargas, M. Clemente León, M. L. Mercuri and E. Coronado, J. Mater. Chem. A, 2021, 9, 25189–25195 RSC.
- R. Lin, S. Xiang, W. Zhou and B. Chen, Chem, 2020, 6, 337–363 CAS.
- Y. Li, G. Wang, L. Ma, L. Hou, Y. Wang and Z. Zhu, ACS Appl. Mater. Interfaces, 2021, 13, 4102–4109 CrossRef CAS PubMed.
- M. Xu, S. S. Yang and Z. Y. Gu, Chem.–Eur. J., 2018, 24, 15131–15142 CrossRef CAS PubMed.
- Z. Ji, T. Li and O. M. Yaghi, Science, 2020, 369, 674–680 CrossRef CAS PubMed.
- J. Zhu, X. Chen, A. Q. Thang, F. L. Li, D. Chen, H. Geng, X. Rui and Q. Yan, SmartMat, 2022 DOI:10.1002/smm2.1091.
- H. M. Mei, S. Li, J. R. Dong, L. Zhang and C. Y. Su, ChemistrySelect, 2020, 5, 10988–10995 CrossRef CAS.
- Z. Wu, J. Xie, Z. J. Xu, S. Zhang and Q. Zhang, J. Mater. Chem. A, 2019, 7, 4259–4290 RSC.
- Z. Wu, D. Adekoya, X. Huang, M. J. Kiefel, J. Xie, W. Xu, Q. Zhang, D. Zhu and S. Zhang, ACS Nano, 2020, 14, 12016–12026 CrossRef CAS PubMed.
- C. Li, K. Wang, J. Li and Q. Zhang, Nanoscale, 2020, 12, 7870–7874 RSC.
- X. Y. Dong, X. P. Hu, H. C. Yao, S. Q. Zang, H. W. Hou and T. C. Mak, Inorg. Chem., 2014, 53, 12050–12057 CrossRef CAS PubMed.
- J. Su, W. He, X. M. Li, L. Sun, H. Y. Wang, Y. Q. Lan, M. Ding and J. L. Zuo, Matter, 2020, 2, 711–722 CrossRef.
- Y. Guo, K. Wang, Y. Hong, H. Wu and Q. Zhang, Dalton Trans., 2021, 50, 11331–11346 RSC.
- Y. Dong, S. Li, S. Hong, L. Wang and B. Wang, Chin. Chem. Lett., 2020, 31, 635–642 CrossRef CAS.
- C. Wang, X. Li, W. Yang, Y. Xu and H. Pang, Chin. Chem. Lett., 2021, 32, 2909–2913 CrossRef CAS.
- Y. Pan, R. Abazari, Y. Wu, J. Gao and Q. Zhang, Electrochem. Commun., 2021, 126, 107024 CrossRef CAS.
- H. F. Wang, L. Chen, H. Pang, S. Kaskel and Q. Xu, Chem. Soc. Rev., 2020, 49, 1414–1448 RSC.
- X. P. Wu, L. Gagliardi and D. G. Truhlar, J. Am. Chem. Soc., 2018, 140, 7904–7912 CrossRef CAS PubMed.
- J. Gao, Q. Huang, Y. Wu, Y. Q. Lan and B. Chen, Adv. Energy Sustain. Res., 2021, 2, 2100033 CrossRef.
- A. de Oliveira, G. F. de Lima and H. A. De Abreu, Chem. Phys. Lett., 2018, 691, 283–290 CrossRef CAS.
- C. Y. Wang, C. C. Wang, X. W. Zhang, X. Y. Ren, B. Yu, P. Wang, Z. X. Zhao and H. f. Fu, Chin. Chem. Lett., 2022, 33, 1353–1357 CrossRef CAS.
- X. Y. Li, Y. B. Wang, Y. Song, D. Xiang and C. z. He, J. Inorg. Organomet. Polym. Mater., 2021, 31, 3823–3828 CrossRef CAS.
- T. Maity, D. Saha, S. Das and S. Koner, Eur. J. Inorg. Chem., 2012, 2012, 4914–4920 CrossRef CAS.
- S. Halake and K. M. Ok, J. Solid State Chem., 2015, 231, 132–137 CrossRef CAS.
- Y. W. Li, J. P. Zhao, L. F. Wang and X. H. Bu, CrystEngComm, 2011, 13, 6002–6006 RSC.
- M. Sanselme, J. M. Greneche, M. Riou-Cavellec and G. Ferey, Chem. Commun., 2002, 18, 2172–2173 RSC.
- X. Zhang, J. X. Chen and H. G. Zheng, Inorg. Chem. Commun., 2016, 69, 4–6 CrossRef CAS.
- M. Chen, Z. W. Wang, H. Zhao and C. S. Liu, Inorg. Chem. Commun., 2014, 45, 84–88 CrossRef CAS.
- Y. Hu, M. Ding, X. Q. Liu, L. B. Sun and H. L. Jiang, Chem. Commun., 2016, 52, 5734–5737 RSC.
- H. M. Titi, G. Nandi, R. Thakuria and I. Goldberg, Inorg. Chim. Acta, 2015, 426, 55–63 CrossRef CAS.
- G. E. Gomez, M. C. Bernini, E. V. Brusau, G. E. Narda, D. Vega, A. M. Kaczmarek, R. Van Deun and M. Nazzarro, Dalton Trans., 2015, 44, 3417–3429 RSC.
- R. F. D'Vries, I. Camps and J. Ellena, Cryst. Growth Des., 2015, 15, 3015–3023 CrossRef.
- M. O’Keeffe, M. A. Peskov, S. J. Ramsden and A. O. M. Yaghi, Acc. Chem. Res., 2008, 41, 1782–1789 CrossRef PubMed.
- O. M. Yaghi, Nano Lett., 2020, 20, 8432–8434 CrossRef CAS PubMed.
- S. Yuan, J. Peng, B. Cai, Z. Huang, A. T. Garcia Esparza, D. Sokaras, Y. Zhang, L. Giordano, K. Akkiraju, Y. G. Zhu, R. Hubner, X. Zou, Y. Roman Leshkov and Y. Shao Horn, Nat. Mater., 2022, 21, 673–680 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |