A feasible strategy of coating CoMoO4 on Co11(HPO3)8(OH)6 nanorods for improved practical application in supercapacitors†
Wei
Guo
 a,
Tao
Yang
b,
Lianjie
Huang
b,
Wei
Hou
b and
Shuang
Wang
a,
Tao
Yang
b,
Lianjie
Huang
b,
Wei
Hou
b and
Shuang
Wang
 *bc
*bc
aCollege of Physics and Optoelectronics, Taiyuan University of Technology, Jinzhong, 030060, P. R. China
bCollege of Environmental Science and Engineering, Taiyuan University of Technology, Jinzhong, 030600, P. R. China. E-mail: wangshuang@tyut.edu.cn
cShanxi Key Laboratory of Gas Energy Efficient and Clean Utilization, Taiyuan University of Technology, Taiyuan, 030024, P. R. China
First published on 17th November 2021
Abstract
Cobalt hydrogen phosphate hydroxides are potential electrode materials for supercapacitors due to their various oxidation states and morphologies. However, their electrochemical performance and practical application are limited by the poor cycling stability during long charge/discharge cycles. To address this drawback, Co11(HPO3)8(OH)6 nanorods (CHPO NRs) are covered by CoMoO4 (CMO) with different microstructures using hydrothermal and reflux processes, respectively. The results indicate that the electrochemical performance of as-prepared CHPO@CMO is much better than that of individual CHPO and CMO. The specific capacities of CHPO@CMO obtained by hydrothermal and reflux processes are 495.1 and 837.1 C g−1 at 1 A g−1, respectively. More importantly, the electrochemical cycling performance of CHPO@CMO is significantly improved. In addition, a CHPO@CMO//AC hybrid device is assembled, and it shows improved electrochemical performance and good practical application, which come from the synergetic effect and high cycling stability of the covering CMO. The specific energies of the assembled devices are 23.8 W h kg−1 at the specific power of 849.9 W kg−1 (hydrothermal method) and 38.4 W h kg−1 at the specific power of 850.7 W kg−1 (reflux method). Thus, the strategy mentioned in this work gives the opportunity to improve the practical application of cobalt hydrogen phosphate hydroxides and other phosphates in energy conversion and storage, such as Co3(PO4)2, Co2(OH)PO4 and Co3P2O8.
Introduction
In the past 200 years, a large amount of fossil fuels has been consumed, causing depletion and serious environmental problems. It is urgent to find renewable energy sources and storage devices.1–3 As important energy storage devices, batteries and supercapacitors (SCs) have been intensively researched over the past several decades.4,5 Compared with traditional batteries, SCs have the advantages of low cost, environmental benignity, long lifespan and high power density.6–9 However, one visible drawback of SCs is lower energy density.10,11 The electrode material is an important aspect of SCs research, which its morphology is a key role for SCs. With the development of nanomaterial technology, one dimensional, two dimensional and three dimensional electrode materials with high specific surface areas have been developed.12–15 Generally, three types of electrode materials have been developed rapidly: carbonaceous materials, conducting polymers and transition metal oxides, which have their own limitations and are not adequate for application requirements.16–20 Naturally, some strategies have been provided to enhance their electrochemical performance, such as doping, defect engineering, composite formation, etc.21–23 Recently, more and more investigations have focused on transition metal salts due to their abundance in the earth, multiple microstructures, compositional diversity and application potential in energy storage. For example, Co(CO3)0.5(OH)/Ni2(CO3)(OH)2 with excellent specific capacitance and rate performance was prepared by G. Zhang et al.;24 G. Harichandran et al. prepared Cu3(MoO4)2(OH)2 NRs as electrode materials, and they had a specific capacitance of 532 F g−1 at 5 mV s−1.25As a common transition metal salt, cobalt hydrogen phosphate hydroxide has received considerable attention as an electrode material.26,27 On the one hand, abundant microstructures of cobalt hydrogen phosphate hydroxide (sphere, wire, rod, ribbon, flower etc.) can be easily obtained by simple methods.26,28–31 On the other hand, it possesses the advantages of good electrochemical performance, abundant active sites and pseudo-capacitance characteristics.32–34 In addition, it is eco-friendly and earth abundant.35 However, in order to improve its cycling performance, some research has been done by many researchers. For example, H. Dan et al. prepared Ni-doped CHPO with different morphologies, and they displayed an improved cycling performance of 71.1% after 8000 cycles at 8 A g−1;26 Y. Zhang et al. prepared a core–shell structure of CHPO–Co3O4, and it displayed excellent cycling performance of 98.7% after 3000 cycles.27 In our previous work, CHPO with various morphologies was prepared and the flower-like one had a specific capacity of 919.3 C g−1 at 1 A g−1; however, its cycling performances were unsatisfactory, 64% after 1000 cycles in a three-electrode system; in addition, the lower specific energy (14.5 W h kg−1) also needs to be improved.31 Therefore, we decided to try to improve the cycling performance and specific energy of CHPO after analyzing the advantages and disadvantages for their practical application.
In this work, a core–shell CHPO@CMO microstructure was designed and prepared by the hydrothermal method. CMO with a flaky shape was uniformly covered on CHPO NRs, and they showed improved electrochemical performance. To verify the effectiveness of the strategy proposed in this work, another simple method, a reflux method, was also used to obtain the CHPO@CMO microstructure. As expected, CMO nanoparticles formed on the CHPO surface, and the composite exhibited higher electrochemical performance than individual CHPO and CMO, respectively. The details of sample fabrication and structure, and electrochemical characterization are described in the experimental section in the ESI.†
Results and discussion
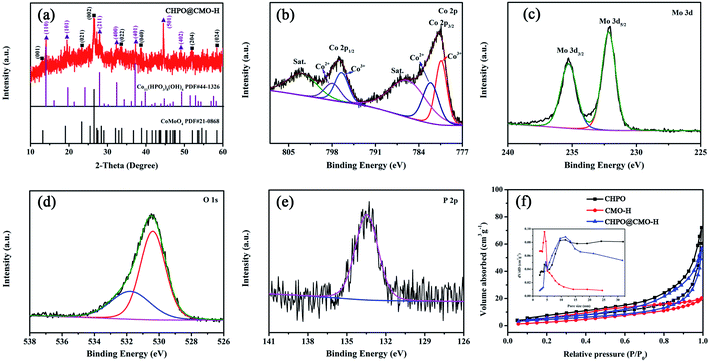
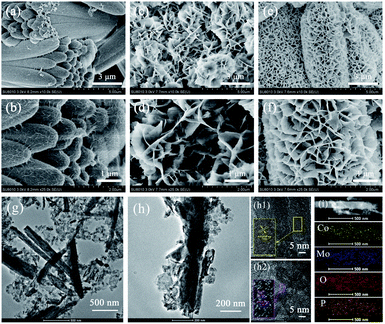
XRD patterns of as-prepared CHPO and CMO by the hydrothermal method (CMO obtained by the hydrothermal method is denoted as CMO-H) are shown in Fig. S1.† The major peaks located at 13.84°, 19.53°, 23.98°, 27.78°, 30.22° and 37.00° in Fig. S1a† and at 13.20°, 19.07°, 23.33°, 26.51°, 32.09°, 32.95° and 33.71° in Fig. S1b† are well matched to the standard peaks of CHPO (JCPDS #44-1326) and CMO (JCPDS #21-0868), respectively. In addition, no other additional peaks are observed, showing their high purity. The XRD pattern of the sample after CHPO was covered by CMO-H is shown in Fig. 1a. The peaks located at 13.84°, 19.53°, 27.78°, 32.22°, 37.01°, 44.60°, 49.10° and 13.20°, 23.33°, 26.51°, 32.95°, 38.87°, 52.07°, 58.44° correspond to (110), (101), (211), (400), (401), (501), (402) planes of CHPO and (001), (021), (002), (022), (040), (204), (024) planes of CMO-H, respectively. XPS measurement was further conducted to examine the chemical compositions and states of the as-prepared sample. The high resolution spectrum of Co 2p is shown in Fig. 1b. The peaks located at 780.41 and 796.46 eV correspond to Co 2p3/2 and Co 2p1/2, respectively. Two satellite peaks at 785.78 and 802.98 eV indicate the oxidation states of Co2+ and Co3+ in the sample. For Mo 3d, the two binding energy peaks of 232.15 and 235.27 eV are attributed to Mo 3d5/2 and Mo 3d3/2, respectively, implying the oxidation state of Mo6+ (Fig. 1c).36 The peak of O 1s located at 530.48 eV can be fitted into two peaks of 530.39 and 531.77 eV, which correspond to the bonding of metal-oxide (Co–O and Mo–O) and Co–OH (Fig. 1d).37,38 The binding energy peak of P 2p located at 133.40 eV belongs to the phosphate group (Fig. 1e).39 In addition, the results of N2 adsorption/desorption measurement of CHPO, CMO-H and CHPO@CMO-H indicate that they have a mesoporous characteristic verified by the typical type-IV isotherms with obvious hysteric loops (in Fig. 1f). The calculated specific surface area of CHPO@CMO-H is 17.14 m2 g−1, which is larger than that of CMO-H (14.03 m2 g−1) but lower than that of CHPO (21.76 m2 g−1). Moreover, the pore size of CHPO@CMO-H is mainly about 11.5 nm, which further confirms the mesoporous characteristic (Fig. 1f, inset). The large specific surface area and appropriate pore size of CHPO@CMO-H can facilitate electrolyte infiltration and provide more active sites, promoting the redox reaction during the charge and discharge process.The microstructure of CHPO@CMO-H was investigated by SEM and TEM (Fig. 2). The CHPO clusters made of CHPO NRs are distributed evenly on NF (Fig. 2a). These CHPO NRs with a smooth surface and round edges are around 10 μm in length and 2 μm in diameter (Fig. 2b). Meanwhile, the CMO-H nanoflowers composed of intersecting CMO nanosheets (NSs) can be obtained on NF by the hydrothermal method under appropriate conditions (Fig. 2c and d). Obviously, the morphology of CHPO@CMO-H preserves the rod-like and flaky features of CHPO and CMO, respectively. Compared with CHPO, the number of CHPO@CMO-H NRs decreases with an increase in diameter (Fig. 2e). We can conclude that the CHPO@CMO-H NRs with increased diameter are formed by coating CMO-H NSs on CHPO clusters (Fig. 2f). Meanwhile, the effects of growth temperature are considered and the morphologies of samples obtained at 120 and 160 °C for 5 h are shown in Fig. S2.† When the growth temperature is 120 °C, the morphology of the sample is similar to that of CHPO clusters, and many compact CMO-H NSs grow on the surface of CHPO clusters (Fig. S2a†). These CMO NSs are tiny and curly, indicating the insufficient growth of CMO-H (Fig. S2b†). When the growth temperature increases to 160 °C, the CHPO clusters disappear because of the complete coverage of CMO-H NSs (Fig. S2c and d†). The microstructure of CHPO@CMO-H was further investigated by TEM. In Fig. 2g, the CHPO NRs and exfoliated CMO-H NSs are observed distinctly. The images of individual CHPO@CMO-H NRs are shown in Fig. 2h. CHPO NRs are covered with many CMO-H NSs with an irregular shape, which is beneficial for increasing the active sites and contact area between the electrolyte and electrode. The HRTEM images of CHPO NRs and CMO-H NSs are shown in Fig. 2h1 and h2. The interlayer spacings of 0.639 and 0.465 nm framed in yellow and purple areas correspond to the (110) plane of CHPO and (−201) plane of CMO, respectively. In addition, the elemental mapping results clearly indicate that Co, Mo, O and P are evenly distributed in the as-prepared samples (Fig. 2i).
The growth mechanism of CHPO and CMO-H has been discussed in detail elsewhere, and the formation process of CHPO@CMO-H can be reasonably speculated, as follows:31,40
| 3H2PO2− + OH− = 2HPO32− + PH3↑ + H2O | (1) |
| 11Co2+ + 8HPO32− + 6OH− = Co11(HPO3)8(OH)6 | (2) |
| 2Na2+ + MoO42− + 2H2O + Co2+ + 2NO3− + 6H2O = CoMoO4 + 2Na2+ + 2NO3− + 8H2O | (3) |
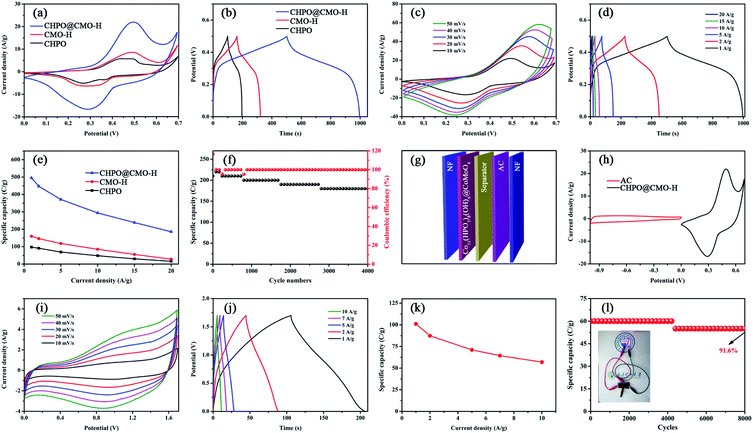
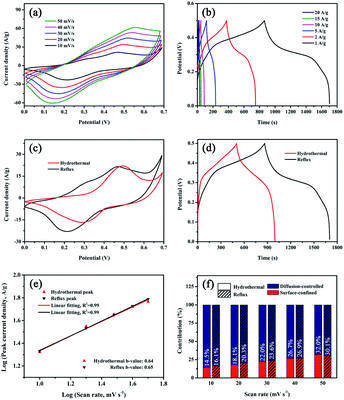
The electrochemical performance of CHPO@CMO-H was studied using a standard three electrode system in 2 M KOH solution. The CV and GCD curves of bare NF are shown in Fig. S3.† The calculated specific capacity of NF is 3.9 C g−1, indicating that NF contributes little to the capacity of the as-prepared sample. Fig. 3a shows the CV curves of CHPO, CMO-H and CHPO@CMO-H at 10 mV s−1. A pair of prominent redox peaks indicates that the redox reaction occurred during the charge/discharge process.41,42 It is speculated that the reversible redox reactions can be expressed by the following equations:43–45
| CoII11(HPO3)8(OH)6 + OH− ↔ CoIII11(HPO3)8(OH)7 + e− | (4) |
| CoIIMoO4 + OH− → CoIIIOOH + MoO3 + e− | (5) |
The negligible IR drop in GCD curves of CHPO, CMO-H and CHPO@CMO-H at 1 A g−1 indicates their lower internal resistance (Fig. 3b).46 In addition, the much larger area enclosed by the CV curve and longer discharge time in the GCD curve of CHPO@CMO-H than that of CHPO and CMO-H exhibit its much higher specific capacity. The CV curves of CHPO@CMO-H at different scan rates are shown in Fig. 3c. With the increase of scan rates from 10 to 50 mV, the shape of the CV curves hardly shows any change, revealing its high electrochemical reversibility. In addition, a slight shift of redox peaks owing to polarization indicates its lower resistance and fast ion/electron movement during the redox reaction.47 Similar results can also be verified by GCD curves at 1–20 A g−1 shown in Fig. 3d. The negligible IR drop and symmetrical charge and discharge curves at different current densities suggest its lower internal resistance and reversible redox reactions.48 The corresponding specific capacities of as-prepared CHPO@CMO-H can be calculated using formula (S1)† based on GCD curves (Fig. 3d and S4†), and the relevant results are shown in Fig. 3e and Table S1.† The specific capacities of CHPO@CMO-H are 495.1, 447.0, 371.0, 294.0, 238.5 and 186.0 C g−1 at 1, 2, 5, 10, 15 and 20 A g−1, respectively, which are much larger than those of CHPO and CMO-H. This indicates that the synergistic effect between CHPO and CMO-H occurs due to the suitable microstructure and abundant electrochemical active sites. Moreover, the cycling stability of CHPO@CMO-H has also been studied by GCD at 5 A g−1 and 0–0.5 V (Fig. 3f). After 4000 cycles, the capacity retention is 85.7%, showing its high cycling stability. The corresponding coulombic efficiency of the sample is nearly 100%, revealing its excellent electrochemical reversibility. This is consistent with the results of CV and GCD observations in Fig. 3c and d. The SEM images of CHPO@CMO-H after 4000 cycles are shown in Fig. S5.† After cycling, the morphology of the sample shows nearly no change, except for the partial collapse of CMO-H NSs. This proves that CMO-H covered on CHPO is very important for the structural preservation of CHPO. In addition, the effect of growth temperature on the electrochemical performance of CHPO@CMO-H was also studied. The CV, GCD curves and the corresponding specific capacities are shown in Fig. S6 and Table S1.† The results show that CHPO@CMO-H prepared at 140 °C exhibits better electrochemical performance. To further research on ion transfer kinetics and the resistance of the electrode, EIS measurement was conducted and the Nyquist impedance plots are shown in Fig. S7.† The solution resistance (Rs) values of CHPO, CMO-H and CHPO@CMO-H obtained by intersection of the plots and X-axis are 0.104, 0.125 and 0.086 Ω, respectively, indicating the lower intrinsic resistance of the active material and good conductivity of the electrolyte. Meanwhile, the charge transfer resistance (Rct) of CHPO@CMO-H obtained from the diameter of the plot in the high frequency region is 5.307 Ω, indicating quick electron transport and charge transfer in the redox reaction process.49 It can be seen that the Rct of CHPO@CMO-H is greater than that of CHPO due to the poor electrical conductivity of CMO and core/shell heterogeneous contact. Despite a low rate capability limited by higher Rct, the good structural stability features of CHPO@CMO-H can promote its electrochemical performance. The slopes of the straight lines of CHPO and CHPO@CMO-H are higher than 45°, revealing that the ion diffusion resistance (Rw) is not the decisive factor, and charge storage is more efficient. It may be attributed to the unique microstructure formed by the combination of one-dimensional CHPO and three-dimensional CMO-H. In addition, the Nyquist plots of CHPO@CMO-H before and after 4000 cycles are depicted in Fig. S8.† After the cycles, the Rs of CHPO@CMO-H is almost retained which benefits from the good conductivity of the electrolyte. The Rct of CHPO@CMO-H shows an obvious increase, which may be because of partial collapse of the structure during electrochemical cycling and increase of the internal resistance of the sample after 4000 cycles. Thus, the electrochemical performance will be partially restricted and affect the stability of the as-prepared device.
The potential application of CHPO@CMO-H was also studied. A hybrid supercapacitor (HSC) was assembled with CHPO@CMO-H and activated carbon (AC) as the positive and the negative electrodes, respectively (Fig. 3g). Before assembling, the properties of AC were checked and are shown in Fig. S9.† The AC electrode shows a typical CV curve with a rectangle and GCD curve with a symmetrical triangle, indicating its good electrical double-layer performance. The specific capacitance of AC is 141.2 F g−1. Charge balance should be done to evaluate the mass ratio of the two electrodes according to the formula:50,51m+/m− = (C− × ΔV−)/(C+ × ΔV+), where C+ and C− are specific capacities (C g−1), m+ and m− are mass loadings (g), and ΔV+ and ΔV− are potential windows (V). The optimum mass ratio of CHPO@CMO-H and AC is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, namely the mass loading of AC is 12.4 mg and the total mass loading of the HSC device is 16.5 mg, respectively. The CV curves of CHPO@CMO-H and AC at 10 mV s−1 indicate that the potential windows of CHPO@CMO-H and AC are −1–0 and 0–0.7 V, respectively (Fig. 3h). Moreover, the CV curves of the HSC device at different operating voltage windows are shown in Fig. S10.† The occurrence of polarization is observed up to a voltage window of 1.8 V. This means that the potential window of the HSC device is determined to be 1.7 V. The CV curves of the HSC device at 10–50 mV show the quasi-rectangular shape and good symmetry, revealing its fast redox reactions and high reversibility (Fig. 3i). Similarly, the GCD curves with a symmetric triangle and very small IR drop at different current densities indicate its low internal resistance (Fig. 3j). The calculated specific capacities of the HSC device are 101.0, 87.4, 71.1, 64.4 and 57.0 C g−1 at 1, 2, 5, 7 and 10 A g−1, respectively (Fig. 3k). Furthermore, 91.6% of the initial capacity is retained after 8000 cycles at 5 A g−1, proving the high cycling stability of the HSC device (Fig. 3l). And LEDs can be powered by the rechargeable HSC device, as shown in Fig. 3l (inset).
3, namely the mass loading of AC is 12.4 mg and the total mass loading of the HSC device is 16.5 mg, respectively. The CV curves of CHPO@CMO-H and AC at 10 mV s−1 indicate that the potential windows of CHPO@CMO-H and AC are −1–0 and 0–0.7 V, respectively (Fig. 3h). Moreover, the CV curves of the HSC device at different operating voltage windows are shown in Fig. S10.† The occurrence of polarization is observed up to a voltage window of 1.8 V. This means that the potential window of the HSC device is determined to be 1.7 V. The CV curves of the HSC device at 10–50 mV show the quasi-rectangular shape and good symmetry, revealing its fast redox reactions and high reversibility (Fig. 3i). Similarly, the GCD curves with a symmetric triangle and very small IR drop at different current densities indicate its low internal resistance (Fig. 3j). The calculated specific capacities of the HSC device are 101.0, 87.4, 71.1, 64.4 and 57.0 C g−1 at 1, 2, 5, 7 and 10 A g−1, respectively (Fig. 3k). Furthermore, 91.6% of the initial capacity is retained after 8000 cycles at 5 A g−1, proving the high cycling stability of the HSC device (Fig. 3l). And LEDs can be powered by the rechargeable HSC device, as shown in Fig. 3l (inset).
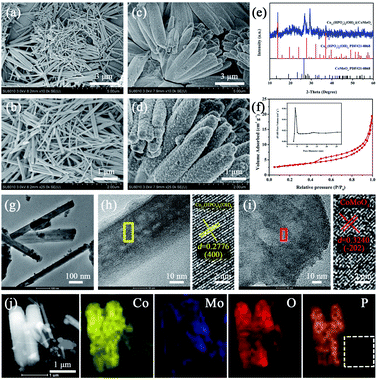
To verify the effectiveness of the strategy mentioned above and fine control of CMO growth on CHPO NRs, a simple reflux method under mild reaction conditions was also developed. CMO and CHPO@CMO composite obtained by the reflux method are denoted as CMO-R and CHPO@CMO-R, respectively. For comparison, CMO-R was synthesized by the reflux method and the XRD pattern is shown in Fig. S11.† All detected peaks are well matched with the standard of CMO (JCPDS #21-0868). In addition, the visible and increased diffraction peak intensities indicate that CMO-R has better crystallinity than that obtained by the hydrothermal method. Interestingly, the morphology of CMO-R changes obviously from nanosheets to nanorods, indicating that the morphology of CMO is sensitive to the preparation method and conditions. Many CMO NRs with round edges are stacked on NF, and some of them grow together (Fig. 4a and b). After coating CMO-R by the reflux method, the original morphology of CHPO is well maintained (Fig. 4c). It can be seen from the enlarged SEM image of the sample that many tiny CMO-R nanoparticles are evenly covered on CHPO NRs. It is obvious that the morphology of CMO obtained by the reflux method changes from nanorod to nanoparticle due to the presence of CHPO (Fig. 4d). Moreover, the main peaks located at 13.84°, 27.78°, 37.00° and 19.07°, 26.51° in the XRD pattern of the sample after CMO-R coverage correspond well to CHPO and CMO, respectively (Fig. 4e). In addition, the XPS spectra of CHPO@CMO-R show a variety of valence states of metal ions in different orbitals by fitting the peaks of Co and Mo elements, and the XPS spectra of O and P elements show that metal–oxygen (Co–O and Mo–O), Co–OH and phosphate groups exist in CHPO@CMO-R (Fig. S12†). The N2 adsorption/desorption measurement was also conducted and the BET surface area and pore distribution were calculated (Fig. 4f). The results show that the specific surface area of CHPO@CMO-R is 10.82 m2 g−1, which is a little smaller than that of the sample obtained by the hydrothermal method. However, the smaller average pore size of 4.10 nm is beneficial for electrolyte penetration and improving the electrochemical performance.52 The microstructure of CHPO@CMO-R was further studied by TEM. The sample with a rod-like shape can be observed in Fig. 4g, with many nanoparticles covered on it. Fig. 4h and i show the HRTEM images of rod and sheet regions in the as-prepared sample. Clear lattice fringes can be observed and the lattice spacings marked by yellow and red lines are 0.2776 and 0.3240 nm, respectively, which correspond to the (400) plane of CHPO and (−202) plane of CMO, respectively. The TEM elemental mappings of Co, Mo, O and P are shown in Fig. 4j, which indicate that all elements are evenly distributed in the samples. In addition, the lack of phosphorus in the white dotted box of P elemental mapping further indicates that the sheet fell off from CHPO@CMO-R rods is CMO. The above results prove that CHPO@CMO-R has been successfully prepared by the reflux method.
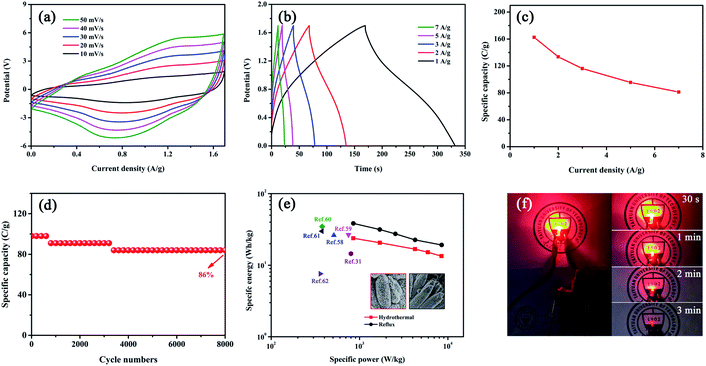
The electrochemical performances of as-prepared samples (including CHPO, CMO-R and CHPO@CMO-R) were studied (Fig. S13 and S14†). The largest enclosed area of the CV curve and longest discharge time of the GCD curve indicate the best electrochemical performance of the as-prepared CHPO@CMO-R. Furthermore, a pair of redox peaks and a charge/discharge plateau in all CV and GCD curves of the CHPO@CMO-R indicate its battery-type storage mechanism (Fig. 5a and b); with the increase of scan rates and current densities, the shape of the CV curves is slightly changed and the discharge time in GCD curves shows a decreasing trend, indicating the good reversibility and excellent ion transport capability of as prepared sample. The material still retains the cycling stability of 101.4% and coulomb efficiency of 100% after 4000 cycles of charging and discharging tests (Fig. S15†). In addition, the electrochemical performances of CHPO@CMO-H and CHPO@CMO-R were studied. As shown in Fig. 5c and d, it is found that the specific capacity of CHPO@CMO-R (837.1 C g−1 at 1 A g−1) is better than that of CHPO@CMO-H (495.1 C g−1 at 1 A g−1), judging by the bigger area enclosed by CV curves and longer discharge time in GCD curves. The details of the specific capacities of samples were calculated and are listed in Table S1.† It should be noted that the electrochemical performances of samples obtained by the two methods are not always directly comparable due to the different mass loadings of active materials on NF. However, it is obvious that the electrochemical performance of the composite is improved compared with individual CHPO and CMO. On the one hand, micro-/mesoporous CHPO contains abundant PO3− and HPO42−, and can provide more electrochemical active sites. It is a pity that the poor structural stability of CHPO hinders its electrochemical properties. This defect can be made up by coating CMO on the CHPO surface due to the high cycling stability of CMO.53,54 On the other hand, one dimensional CHPO NRs can provide a fast electron transfer pathway, and three dimensional interlaced CMO NSs affect the redox reactions. Thus, the synergistic effects of CHPO and CMO can enhance the electrochemical performance of the CHPO@CMO composite by the robust and hierarchical core–shell structure.
In order to study the effect of CHPO@CMO-H and CHPO@CMO-R on specific capacity in detail, the charge storage mechanism during the redox reaction was further studied, which comprised the contributions of the surface-controlled process related to surface adsorption and internal diffusion-controlled process related to pseudocapacitive redox processes, respectively. The linear function relationship between log(i) and log(v) is plotted by taking the logarithm of formulas (S5) and (S6),† respectively (R2 = 0.99). As shown in Fig. 5e, the slopes of b = 0.64 and 0.65 are extracted, which mainly shows the battery behavior of the materials and the existence of two processes, surface and diffusion controlled ones.55,56 After calculation, the comparison of the surface control contribution (red region) and diffusion control contribution (blue region) of CHPO@CMO-H (14.5% vs. 85.5%) at 10 mV s−1 is shown in Fig. S16a.† However, 83.9% (blue region) of the total contribution of CHPO@CMO-R comes from the diffusion controlled process (Fig. S16b†). Fig. S17 and S18† show the calculation results of CHPO@CMO-H and CHPO@CMO-R electrodes at 20–50 mV s−1, respectively. Based on the statistics of the results, we figure out the contributions of the two mechanisms in the electrodes prepared by the hydrothermal and reflux methods at different scan rates (Fig. 5f). At 10–50 mV s−1, the diffusion contribution decreases with increase of the scan rate, which is caused by the limited diffusion time at high scan rates. However, the surface contribution gradually increases, which is conducive to the rapid absorption of ions, thus improving the cycle stability and rate performance.57
Fig. 6 shows the electrochemical properties of the hybrid supercapacitor (HSC) assembled using CHPO@CMO-R and AC. The CV curves of the CHPO@CMO-R//AC device at different scan rates in the range of 0–1.7 V are shown in Fig. 6a. The shape of the curves shows no change with increase of the scan rate, which indicates its good rate capability. Fig. 6b shows the GCD curves of the HSC at different current densities. The quasi-triangular GCD curves indicate the pseudocapacity of the device. And the specific capacities of the HSC device are 162.5, 133.6, 116.1, 95.5 and 81.3 C g−1 at 1, 2, 3, 5 and 7 A g−1, respectively (Fig. 6c). In addition, the capacity retention of the HSC is 86% after 8000 cycles (Fig. 6d). The specific energy and specific power of the as-prepared HSC devices assembled with CHPO@CMO-H and -R as positive electrodes are compared. As shown in Fig. 6e, the specific energy values of CHPO@CMO-H//AC and CHPO@CMO-R//AC are 23.8 and 38.4 W h kg−1 at the specific power of 849.9 and 850.7 W kg−1, respectively, which is competitive with those reported in recent research studies (Table S2†), such as ZnCo2O4@NixCo2x(OH)6x//AC (26.2 W h kg−1 at 511.8 W kg−1),58 Co3(PO4)2//AC (26.66 W h kg−1 at 750 W kg−1),59 NiCo(PO4)3//AC (34.8 W h kg−1 at 377 W kg−1),60 NaNi0.33Co0.67PO4·H2O//G (29.85 W h kg−1 at 374.95 W kg−1),61 AC//Mn3(PO4)2/100 mg GF (7.6 W h kg−1 at 360.71 W kg−1)62 and Co11(HPO3)8(OH)6//AC (14.5 W h kg−1 at 799.4 W kg−1).31 After charging, the device assembled with CHPO@CMO-R could light a red LED for more than 3 min (Fig. 6f), which indicated the potential value of composite materials in the application of electrochemical energy storage.
Conclusions
In summary, CMO is used as a “shell” to cover CHPO through the different methods and is successfully synthesized. The electrochemical properties of the composites synthesized via hydrothermal and reflux methods are tested. It is found that the specific capacity of the composites prepared by the hydrothermal and reflux methods can reach 495.1 and 837.1 C g−1 at 1 A g−1, respectively. Moreover, the CHPO@CMO/NF//AC HSC device is assembled. The specific energy of the HSC synthesized by the reflux method is 38.4 W h kg−1 and the specific power is 8506 W kg−1, which are higher than that of the hydrothermal method (specific energy of 23.84 W h kg−1 and specific power of 8470.6 W kg−1). Impressively, the cycling performance of the as-prepared device is 91.6% (hydrothermal method) and 86% (reflux method) after 8000 charge/discharge cycles, which is higher than that of CHPO. This shows its potential application value in the field of supercapacitors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22078215), Shanxi Province Natural Science Foundation for Youths (No. 201901D211117) and Research Project by Shanxi Scholarship Council of China (No. 2021-055).Notes and references
- G. Liu, Y. Sheng, J. W. Ager, M. Kraft and R. Xu, EnergyChem, 2019, 1, 100014 CrossRef
.
- X. Li, X. Yang, H. Xue, H. Pang and Q. Xu, EnergyChem, 2020, 2, 100027 CrossRef
.
- H. Pang, Y. Z. Zhang, Z. Run, W. Y. Lai and W. Huang, Nano Energy, 2015, 17, 339–347 CrossRef CAS
.
- X. Guo, S. Zheng, Y. Luo and H. Pang, Chem. Eng. J., 2020, 401, 126005 CrossRef CAS
.
- C. Liu, Y. Bai, J. Wang, Z. Qiu and H. Pang, J. Mater. Chem. A, 2021, 9, 11201–11209 RSC
.
- C. Zhang, Z. Peng, C. Huang, B. Zhang, C. Xing, H. Chen, H. Cheng, J. Wang and S. Tang, Nano Energy, 2021, 81, 105609 CrossRef CAS
.
- Y. Z. Zhang, Y. Wang, T. Cheng, L. Q. Yao, X. Li, W. Y. Lai and W. Huang, Chem. Soc. Rev., 2019, 48, 3229–3264 RSC
.
- W. Raza, F. Ali, N. Raza, Y. Luo, K.-H. Kim, J. Yang, S. Kumar, A. Mehmood and E. E. Kwon, Nano Energy, 2018, 52, 441–473 CrossRef CAS
.
- C. V. V. M. Gopi, S. Sambasivam, K. V. G. Raghavendra, R. Vinodh, I. M. Obaidat and H.-J. Kim, J. Energy Storage, 2020, 30, 101550 CrossRef
.
- J. Wang, L. Zhang, X. Liu, X. Zhang, Y. Tian, X. Liu, J. Zhao and Y. Li, Sci. Rep., 2017, 7, 41088 CrossRef CAS PubMed
.
- J. Liu, X. Deng, S. Zhu, N. Zhao, J. Sha, L. Ma and F. He, Electrochim. Acta, 2021, 368, 137528 CrossRef CAS
.
- Y. Y. Liu, X. C. Li, S. Wang, T. Chen, H. Yang, C. Liu, Y. Gong, W. Y. Lai and W. Huang, Nat. Commun., 2020, 11, 5561 CrossRef CAS PubMed
.
- T. Chen, Y. W. Wu, Y. L. Chen, Y. Z. Zhang, W. Y. Lai and W. Huang, Small, 2019, 15, 1901830 CrossRef PubMed
.
- S. Zheng, Q. Li, H. Xue, H. Pang and Q. Xu, Nat. Sci. Rev., 2020, 7, 305–314 CrossRef CAS PubMed
.
- H. Pang, X. Li, Q. Zhao, H. Xue, W. Y. Lai, Z. Hu and W. Huang, Nano Energy, 2017, 35, 138–145 CrossRef CAS
.
- M. Li, M. F. EI-Kady, J. Y. Hwang, M. D. Kowal, K. Marsh, H. Wang, Z. Zhao and R. B. Kaner, Nano Res., 2018, 11, 2836–2846 CrossRef CAS
.
- C. Zhang, Y. Huang, S. Tang, M. Deng and Y. Du, ACS Energy Lett., 2017, 2, 759–768 CrossRef CAS
.
- X. Zhao, L. Mao, Q. Cheng, J. Li, F. Liao, G. Yang, L. Xie, C. Zhao and L. Chen, Chem. Eng. J., 2020, 387, 124081 CrossRef CAS
.
- H. Chen, H. Hu, F. Han, J. Liu, Y. Zhang and Y. Zheng, Dalton Trans., 2020, 49, 10799–10807 RSC
.
- Y. Z. Zhang, T. Cheng, Y. Wang, W. Y. Lai, H. Pang and W. Huang, Adv. Mater., 2016, 28, 5242–5248 CrossRef CAS PubMed
.
- K. S. Lee, I. Phiri, C. W. Park, S. Kim and J. M. Ko, J. Colloid Interface Sci., 2021, 592, 42–50 CrossRef CAS PubMed
.
- F. H. Hsu, S. Y. Hsu, C. W. Pao, J. L. Chen, C. L. Chen, J. M. Chen and K. T. Lu, Nanoscale, 2020, 12, 13388–13397 RSC
.
- D. P. Dubal, O. Ayyad, V. Ruiz and P. Gómez-Romero, Chem. Soc. Rev., 2015, 44, 1777–1790 RSC
.
- G. Zhang, P. Qin, R. Nasser, S. Li, P. Chen and J. Song, Chem. Eng. J., 2020, 387, 124029 CrossRef CAS
.
- G. Harichandran, S. Radha, J. Yesuraj and B. Muthuraaman, Chemistryselect, 2020, 5, 11037–11047 CrossRef CAS
.
- H. Dan, K. Tao, Q. Zhou, Y. Gong and J. Lin, ACS Appl. Mater. Interfaces, 2018, 10, 31340–31354 CrossRef CAS PubMed
.
- Y. Zhang, M. Zheng, M. Qu, M. Sun and H. Pang, J. Alloys Compd., 2015, 651, 214–221 CrossRef CAS
.
- Y. Ni, B. He, S. Luo, X. Wu, X. Feng, Y. Luo, J. Lin, J. Sun, K. Fan, Y. Ji, G. Zhang and H. Chen, Appl. Catal., B, 2019, 259, 118091 CrossRef CAS
.
- H. Pang, Y. Liu, J. Li, Y. Ma, G. Li, Y. Ai, J. Chen, J. Zhang and H. Zheng, Nanoscale, 2013, 5, 503–507 RSC
.
- R. C. Che, L. M. Peng and W. Z. Zhou, Appl. Phys. Lett., 2005, 87, 173122 CrossRef
.
- Y. Tian, X. Lian, Y. Wu, W. Guo and S. Wang, CrystEngComm, 2020, 22, 5218–5225 RSC
.
- B. A. Mahmoud, A. A. Mirghni, K. O. Oyedotun, D. Momodu, O. Fasakin and N. Manyala, J. Alloys Compd., 2020, 818, 153332 CrossRef CAS
.
- M. D. Marcos, P. Amorós, A. Beltrh-Porter, R. Martinez-Mfiez and J. P. Attfields, Chem. Mater., 1993, 5, 121–128 CrossRef CAS
.
- J. Xu, H. Fan, X. Tao, L. Guo and Z. Liu, Mater. Lett., 2020, 268, 127567 CrossRef CAS
.
- Y. Wang, W. Li, L. Zhang, X. Zhang, B. Tan, J. Hao, Z. Jian, X. Wang, Q. Hu and X. Lu, J. Power Sources, 2020, 449, 227487 CrossRef CAS
.
- F. Nti, D. A. Anang and J. I. Han, J. Alloys Compd., 2018, 742, 342–350 CrossRef CAS
.
- B. Liu, J. Hou, T. Zhang, C. Xu and H. Liu, J. Mater. Chem. A, 2019, 7, 16222–16230 RSC
.
- P. Sheng, S. Tao, X. Gao, Y. Tan, D. Wu, B. Qian and P. K. Chu, J. Mater. Sci., 2020, 55, 12091–12102 CrossRef CAS
.
- R. Ding, X. Li, W. Shi, Q. Xu and E. Liu, Chem. Eng. J., 2017, 320, 376–388 CrossRef CAS
.
- X. Xu, J. Shen, N. Li and M. Ye, J. Alloys Compd., 2014, 616, 58–65 CrossRef CAS
.
- S. C. Sekhar, G. Nagaraju, B. Ramulu, S. K. Hussain, D. Narsimulu and J. S. Yu, Nano Res., 2019, 12, 2597–2608 CrossRef
.
- X. Cao, J. He, H. Li, L. Kang, X. He, J. Sun, R. Jiang, H. Xu, Z. Lei and Z. Liu, Small, 2018, 14, 1800998 CrossRef PubMed
.
- Y. Gao, J. Zhao, Z. Run, G. Zhang and H. Pang, Dalton Trans., 2014, 43, 17000–17005 RSC
.
- W. Shaheen, M. F. Warsi, M. Shahid, M. A. Khan, M. Asghar, Z. Ali, M. Sarfraz, H. Anwar, M. Nadeem and I. Shakir, Electrochim. Acta, 2016, 219, 330–338 CrossRef CAS
.
- W. Li, X. Wang, Y. Hu, L. Sun, C. Gao, C. Zhang, H. Liu and M. Duan, Nanoscale Res. Lett., 2018, 13, 120 CrossRef PubMed
.
- Y. Yang, Y. Zhou, Y. An, Q. Zhang, X. Wang, X. Yang and Z. Hu, J. Phys. Chem. Solids, 2018, 119, 126–137 CrossRef CAS
.
- V. Elayappan, P. A. Shinde, G. K. Veerasubramani, S. C. Jun, H. S. Noh, K. Kim, M. Kim and H. Lee, Dalton Trans., 2020, 49, 1157–1166 RSC
.
- A. M. Elshahawy, C. Guan, X. Li, H. Zhang, Y. Hu, H. Wu, S. J. Pennycook and J. Wang, Nano Energy, 2017, 39, 162–171 CrossRef CAS
.
- G. Zhang, H. Yao, F. Zhang, Z. Gao, Q. Li, Y. Yang and X. Lu, Nano Res., 2019, 12, 1061–1069 CrossRef CAS
.
- V. Khomenko, E. Raymundo-Piñero, E. Frackowiak and F. Béguin, Appl. Phys. A, 2005, 82, 567–573 CrossRef
.
- D. Wang, Y. Xu, W. Sun, X. Guo, L. Yang, F. Wang and Z. Yang, Electrochim. Acta, 2020, 337, 135827 CrossRef CAS
.
- X. Lian, W. Guo, Y. Wu, Y. Tian and S. Wang, J. Alloys Compd., 2021, 865, 158296 CrossRef CAS
.
- D. Guo, H. Zhang, X. Yu, M. Zhang, P. Zhang, Q. Li and T. Wang, J. Mater. Chem. A, 2013, 1, 7247–7254 RSC
.
- Y. Wu, W. Guo, X. Lian, Y. Tian, W. Wang, J. Li and S. Wang, J. Alloys Compd., 2019, 793, 418–424 CrossRef CAS
.
- K. V. Sankar, Y. Seo, S. C. Lee, S. Liu, A. Kundu, C. Ray and S. C. Jun, Electrochim. Acta, 2018, 259, 1037–1044 CrossRef CAS
.
- D. Xu, C. Chen, J. Xie, B. Zhang, L. Miao, J. Cai, Y. Huang and L. Zhang, Adv. Energy Mater., 2016, 6, 1501929 CrossRef
.
- M. S. Javed, H. Lei, J. Li, Z. Wang and W. Ma, J. Mater. Chem. A, 2019, 7, 17435–17445 RSC
.
- W. Fu, Y. Wang, W. Han, Z. Zhang, H. Zha and E. Xie, J. Mater. Chem. A, 2016, 4, 173–182 RSC
.
- K. V. Sankar, S. C. Lee, Y. Seo, C. Ray, S. Liu, A. Kundu and S. C. Jun, J. Power Sources, 2018, 373, 211–219 CrossRef CAS
.
- A. A. Mirghni, K. O. Oyedotun, B. A. Mahmoud, A. Bello, S. C. Ray and N. Manyala, Composites, Part B, 2019, 174, 106953 CrossRef CAS
.
- M. Liu, N. Shang, X. Zhang, S. Gao, C. Wang and Z. Wang, J. Alloys Compd., 2019, 791, 929–935 CrossRef CAS
.
- A. A. Mirghni, M. J. Madito, T. M. Masikhwa, K. O. Oyedotun, A. Bello and N. Manyala, J. Colloid Interface Sci., 2017, 494, 325–337 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1se01349a |
| This journal is © The Royal Society of Chemistry 2022 |