Open Access Article
Open Access ArticleDiffuse reflectance-based spectroscopic technique for real-time estimation of localized blood oxygenation parameters from human fingertips: a preliminary study†
Ajay
Kumar
 ab,
Kalaivani
Chellappan
ab,
Kalaivani
Chellappan
 c,
Aulia
Nasution
d,
Dnyandeo
Pawar
c,
Aulia
Nasution
d,
Dnyandeo
Pawar
 e,
Manoj Kumar
Patel
bf and
Rajesh
Kanawade
e,
Manoj Kumar
Patel
bf and
Rajesh
Kanawade
 *ab
*ab
aCSIR-National Chemical Laboratory, Dr. Homi Bhabha Road, Pune – 411008, India. E-mail: rv.kanawade@ncl.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad – 201002, India
cFaculty of Engineering & Built Environment, Universiti Kebangsaan Malaysia, UKM, Bangi, Selangor – 43600, Malaysia
dDepartment of Engineering Physics, Sepuluh Nopember Institute of Technology, Kampus ITS Sukolilo, Surabaya – 60111, Indonesia
eCollege of Materials Science and Engineering, Shenzhen University, 518060, China
fCSIR-Central Scientific Instruments Organisation, Sector 30-C, Chandigarh – 160030, India
First published on 27th September 2022
Abstract
Non-invasive and real-time measurement of localized blood oxygenation parameters such as reduced hemoglobin, oxyhemoglobin, and oxygen saturation is regularly required by emergency & rescue teams as well as by intensive care units (ICUs). These parameters vary with gender and age. Therefore, the aim of this study is to investigate the ability of diffuse reflectance spectroscopy (DRS) to measure localized blood oxygenation parameters from the fingertips of human subjects and their variation with gender and age. 91 healthy subjects (male = 55 and female = 36) aged between 22 to 51 years were selected. The subjects were categorized into 5 age groups (20–24 years, 25–29 years, 30–34 years, 35–39 years, and above 40 years). DRS experiments were performed on the fingertips of each subject to record three sets of 150 diffuse reflectance spectra. The localized blood oxygenation parameters were derived from the recorded spectra. To compare gender and age-based variations in the relative change in reduced hemoglobin (ΔRHb), oxyhemoglobin (ΔHbO2), and oxygen saturation (ΔSO2), the Mann–Whitney U test and Kruskal–Wallis ANOVA test were performed, respectively. We found in the gender difference study that the female subjects have a significantly higher ΔRHb and ΔSO2, but lower ΔHbO2 than the male subjects (p < 0.001). The age variation study concludes that with the increase in age, ΔRHb is found to significantly increase, while ΔHbO2 and ΔSO2 are found to significantly decrease (p < 0.05). Thus, the preliminary investigation suggests that DRS has potential for real-time estimation of localized blood oxygenation parameters from the fingertips of human subjects which may be used to improve medical diagnosis and therapeutic assessment in ICU patients.
Introduction
Real-time monitoring of localized blood oxygenation parameters such as reduced hemoglobin (RHb), oxyhemoglobin (HbO2), and oxygen saturation (SO2) is critically important for clinical monitoring purposes such as COVID-19 treatment and assessment, fluid replacement therapy, suspected hemorrhaging, diabetic foot ulcer treatment and its assessment, photodynamic therapy (PDT), hemoglobin-based blood substitution and resuscitation.1–3 Furthermore, blood oxygenation parameters are regularly required by surgical departments, emergency & rescue teams and intensive care units (ICUs) for bedside continuous monitoring of patients. Studies reported that gender and age affect these blood oxygenation parameters. It has been reported that adult males and females have different hemoglobin concentration levels in their bodies.4 The normal range of hemoglobin for men is 13.5 to 17.5 grams per deciliter and for women is 12.0 to 15.5 grams per deciliter. Studies also reported that with an increase in age, the reduced hemoglobin and oxyhemoglobin concentration levels also alter.5 Therefore, the age and gender factors affect effective diagnosis, treatment planning, and accuracy of blood oxygenation parameter monitoring.Currently, multiple optical techniques like pulse oximetry,6 spectrophotometry,7,8 near-infrared spectroscopy (NIR),9–11 photoacoustic imaging,12 thermal imaging,13 and hyperspectral imaging14,15 are used for non-invasive blood oxygenation measurement. The existing techniques are inexpensive, portable, and not technically demanding, and do an excellent job of determining the average blood oxygenation state in tissue. However, these techniques cannot provide information on reduced hemoglobin and are also not able to resolve the relative local blood volume fraction of reduced hemoglobin/oxyhemoglobin within the bulk of highly scattering tissue media. Therefore, there remains a need for a technique that can overcome this limitation and provide continuous, real-time, age and gender-based accurate measurements of blood oxygenation parameters.
In this regard, diffuse reflectance spectroscopy (DRS) is probably the best option among the available optical methods. This is because of its ability to investigate structures and functions of tissue or even whole organism levels.16–19 DRS signals are sensitive to the absorption and scattering properties of tissue. The absorption is directly related to the concentration of chromophores such as reduced hemoglobin and oxyhemoglobin, and the scattering coefficient reflects the size and density of scattering structures such as cellular nuclei, collagen fibers, and the surrounding components of the tissue.20 Studies reported that DRS has been used for patient monitoring in ICUs for various applications like monitoring oxygen saturation changes in the brain,21 evaluation of the severity of psoriasis,22 and photodynamic therapy and its assessment.23 In addition, DRS has also been explored to distinguish the normal and malignant states of different types of tissues by measuring the oxygenation level.24 DRS can investigate tissues up to a depth of several millimeters and can be used to study various aspects of pathophysiological functions by measuring the absorption of blood.25,26 The applicability of DRS techniques to detect real-time oxygenation changes associated with tissue and blood vessel spatial patterns for early clinical shock detection has been reported elsewhere.27
Thus, considering the above advantages, the aim of the study is to prove the applicability of DRS to monitoring localized blood oxygenation parameters from the fingertips of male and female healthy subjects of various age groups. Effort was focused on improving the accuracy of the real-time reduced hemoglobin, oxyhemoglobin, and oxygen saturation monitoring techniques for intensive care units and for emergency & rescue teams.
Materials and methods
Experimental setup and diffuse reflectance spectra measurement procedure
A reflection-backscattering bifurcated fiber probe (FCB-UVIR600-2, Avantes, The Netherlands) was used to deliver and collect the incident and diffusely reflected light, respectively. It consists of six illumination fibers (0.22 NA, 600 μm core, multimode) and a single collection fiber at the centre of the probe. A white light source (HL-2000-FHSA, Ocean Optics, USA) was connected to the illumination fiber to illuminate the index fingertip. The diffusely reflected light from the fingertip was collected using the collection fiber with the other end connected to a high-resolution spectrometer (Avaspec UL 3648, wavelength range: 200–1100 nm, Avantes, The Netherlands) for detection (Fig. 1). The measurements were performed at a 5 mm distance from the fingertip. Since the finger size of each subject varies, in order to maintain the 5 mm distance between the fiber probe and fingertip of each subject, the fiber probe was fixed on an adjustable stand (shown in Fig. 1). Furthermore, to avoid errors in the collection of diffuse reflected light due to the motion of a fingertip, the subjects remained at rest in a sitting position for 5 minutes and then the measurement was performed on the subjects’ index fingertip resting on a fixed table. In total, 91 healthy subjects of male and female genders having ages between 22 to 51 years old were selected and tested. Furthermore, for the age variation blood oxygenation monitoring study, these 91 healthy subjects were categorized into 5 age groups (20–24 years, 25–29 years, 30–34 years, 35–39 years, and above 40 years). Precautions for having male and female genders in each age group were taken for the age variation blood oxygenation monitoring study.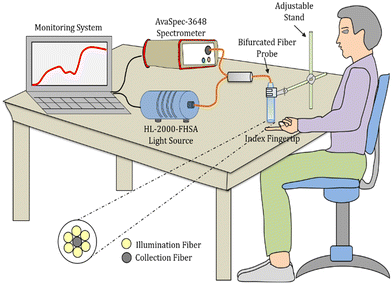 | ||
| Fig. 1 Schematics of the experimental setup used for diffuse reflectance measurement from the index fingertip of a healthy subject. | ||
The raw spectra were processed to compute the diffuse reflectance spectra corrected for stray light and light source emission spectra. The measurements were carried out under constant conditions of minimized environmental stray light, under normal physiological conditions. In order to get sufficient data for further statistical analysis, 150 diffuse reflectance spectra were collected from each subject under the same experimental conditions; however, the first 10 spectra were not considered during the data analysis. Each measurement was repeated three times (n = 3). This study was approved by the Institutional Ethical Committee (IEC) of CSIR-Central Scientific Instruments Organisation, India (approval no. IEC/CSIO/2020). The experiments were performed in accordance with relevant guidelines and regulations. Before measurement, health parameters such as heartbeat, oxygen saturation, and blood pressure of the subject were measured with the help of a pulse oximeter (Dr. Trust, Nureca Inc. USA) and blood pressure unit (HEM-8712, Omron, Kyoto, Japan), respectively.
Blood oxygenation calculation
The diffuse reflectance spectra of the subjects were measured as a function of visible wavelengths over a broad range from λ = 400 nm to λ = 700 nm. Three adjacent wavelengths λ0, λi, and λi+1 were selected to deduce equations that allowed us to retrieve the local volume fractions of reduced hemoglobin/oxyhemoglobin and oxygen saturation. The data processing method used for the reduced hemoglobin/oxyhemoglobin concentration and oxygen saturation determinations is explained elsewhere.16,27,28 Taylor series expansion is used to build three equations from the measured diffuse reflectance spectra for three adjacent wavelengths λ0, λ1, and λ2.16,27,28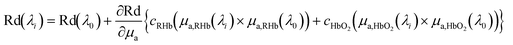 | (1) |
 | (2) |
 | (3) |
 | (4) |
 and
and  are the relative volume fractions of reduced hemoglobin and oxyhemoglobin; and SO2 is the oxygen saturation. Meanwhile, μa,RHb(λi) and μa,HbO2(λi) are the absorption coefficients of reduced and oxygenated hemoglobin at wavelength λi respectively. The terms Δμ1,RHb and Δμ2,RHb are used for the absorption difference between wavelengths (λ1, λ0), and (λ2, λ0) respectively, measured for reduced hemoglobin. Similarly, the terms Δμ1,HbO2 and Δμ2,HbO2 are used for the absorption difference for oxyhemoglobin. Three adjacent wavelengths λ0 = 618 nm, λ1 = 628 nm and λ2 = 638 nm were used for signal analysis.29,30
are the relative volume fractions of reduced hemoglobin and oxyhemoglobin; and SO2 is the oxygen saturation. Meanwhile, μa,RHb(λi) and μa,HbO2(λi) are the absorption coefficients of reduced and oxygenated hemoglobin at wavelength λi respectively. The terms Δμ1,RHb and Δμ2,RHb are used for the absorption difference between wavelengths (λ1, λ0), and (λ2, λ0) respectively, measured for reduced hemoglobin. Similarly, the terms Δμ1,HbO2 and Δμ2,HbO2 are used for the absorption difference for oxyhemoglobin. Three adjacent wavelengths λ0 = 618 nm, λ1 = 628 nm and λ2 = 638 nm were used for signal analysis.29,30
Statistical analysis
The local volume fractions of RHb, HbO2, and SO2 values were derived from each measured spectra using eqn (2)–(4), respectively. For the gender and age variation study, the ΔRHb, ΔHbO2, and ΔSO2 values were calculated from the obtained RHb, HbO2, and SO2, respectively, for each subject. To compare the ΔRHb, ΔHbO2, and ΔSO2 between the male and female groups, the Mann–Whitney U test was performed. Furthermore, the Kruskal–Wallis ANOVA test was carried out to assess differences in ΔRHb, ΔHbO2, and ΔSO2 among the age variation groups. Whenever differences were detected, Dunn's multiple comparison post hoc test was performed to identify the age groups that differed from each other. In all statistical tests, a ‘p-value’ less than 0.05 was considered statistically significant. The data were processed and analysed using OriginPro, Version 2020 software.Results
Blood oxygenation measured from a human fingertip
Fig. 2 shows the measured diffuse reflectance spectra and derived RHb, HbO2, and SO2 in arbitrary units measured from the diffuse reflectance spectra of subject number 12. The observed reduced hemoglobin/oxyhemoglobin concentrations show relative trends (Fig. 2b–d).28 The reproducibility of the reduced hemoglobin and oxyhemoglobin measurements (from 150 spectra of three sets of recordings (n = 3)) is shown with error bars in Fig. 2(b–d) for subject 12. Small deviations are observed in the reduced hemoglobin and oxyhemoglobin concentrations. This small deviation is likely from biological artefacts. Furthermore, similar trends are observed for the reduced hemoglobin/oxyhemoglobin concentration measurements from all the subjects. Relatively good oxyhemoglobin has been reported from the index fingertip as the palm of the hand's vascular system has a high content of capillary beds;31,32 thus, the overall perfusion and vascularization is high. Studies have reported that most of the blood (around 77%) is found in capillary beds and small venules.33,34 Therefore, the fingertip was selected for this measurement. Further, the measurements showed that the diffuse reflectance spectra of the subject may vary. The optical response of the fingertip/any tissue strongly depends upon the blood and its relevant parameters such as oxygenation and hematocrit values.11,26 Therefore, the perfusion rate and localized blood volume could be the reason for the variation in spectral behaviour.Gender analysis for the identified parameters
The ΔRHb, ΔHbO2, and ΔSO2 for the female (n = 36) and male (n = 55) healthy subject groups are shown in Fig. 3. We found that female subjects have a significantly larger ΔRHb than male subjects (Fig. 3a, medians = 0.360 and 0.126, for females and males respectively, U = 122, n1 = 36, n2 = 55, p < 0.001; Mann–Whitney U test). In contrast, male subjects have a significantly larger ΔHbO2 than female subjects (Fig. 3b, medians = 0.531 and 0.875, for females and males respectively, U = 1887, n1 = 36, n2 = 55, p < 0.001; Mann–Whitney U test). These observations are also supported by the literature; reported values show that adult females have an approximately 12% lower mean hemoglobin concentration in their body than adult males.4,35 This could be the reason for the lower oxyhemoglobin concentrations in the female subjects than in the male subjects. | ||
| Fig. 3 Box plots comparing the relative change in (a) reduced hemoglobin (ΔRHb), (b) oxyhemoglobin (ΔHbO2), and (c) oxygen saturation (ΔSO2) for female and male subjects, respectively. | ||
However, ΔSO2 values measured from female subjects are significantly higher than those from male subjects (Fig. 3c, medians = 0.642 and 0.604, for females and males respectively, U = 1204, n1 = 36, n2 = 55, p < 0.05; Mann–Whitney U test). The measured average SO2 values (%) with the pulse oximeter (Dr. Trust, Nureca Inc. USA) from the fingertip of the female and male subjects are 98.94 ± 0.67 and 98.40 ± 0.60, respectively (shown in Fig. S1 in the ESI†). The measured trend is in agreement with our obtained trend. Furthermore, this finding is also consistent with the literature, which reports that healthy young female adults have a higher (1.5%) SO2 than their male counterparts.36 The reason for the high SO2 value in females could be that females have smaller conducting airways than males. This may cause a reduction in dead space in female lungs as compared to male lungs of a matching size.36 Thus, lower dead space may support efficient oxygen exchange and higher SO2 concentration in females as compared to males.
Age analysis for the identified parameters
Table 1 shows the number of subjects for different age groups used in this study.| S. No. | Age group (years) | Number of subjects (n) |
|---|---|---|
| 1 | A = 20–24 | 14 |
| 2 | B = 25–29 | 32 |
| 3 | C = 30–34 | 22 |
| 4 | D = 35–39 | 11 |
| 5 | E = above 40 | 12 |
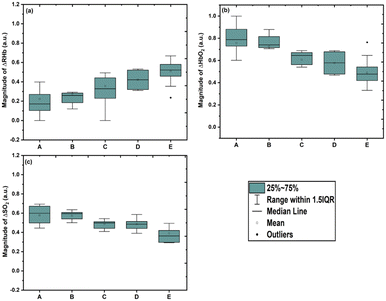
Age variation comparisons of the relative changes in reduced hemoglobin, oxyhemoglobin and oxygen saturation for the age groups A = 20–24 years, B = 25–29 years, C = 30–34 years, D = 35–39 years, and E = above 40 years are shown in Fig. 4. The Kruskal–Wallis ANOVA test was performed and significant differences in ΔRHb, ΔHbO2 and ΔSO2 were found with age variation (χ2 (4) = 39.78, n = 5, p < 0.001; χ2 (4) = 41.69, n = 5, p < 0.001; and χ2 (4) = 33.78, n = 5, p < 0.001 for ΔRHb, ΔHbO2, and ΔSO2, respectively). After confirmation of the statistical difference of ΔRHb, ΔHbO2 and ΔSO2 from the Kruskal–Wallis ANOVA test, Dunn's multiple comparison post hoc test was performed to identify the age groups that differed from each other. It was observed that ΔRHb shows a significantly increasing trend with age (Fig. 4a: n = 14, median = 0.172, IQR = 0.103–0.277; n = 32, median = 0.260, IQR = 0.182–0.285; n = 22, median = 0.329, IQR = 0.220–0.450; n = 11, median = 0.421, IQR = 0.321–0.522; and n = 12, median = 0.522, IQR = 0.457–0.583 for age groups A–E, respectively: Dunn's multiple comparison post hoc test p < 0.05). In contrast, ΔHbO2 shows a significantly decreasing trend with age (Fig. 4b: n = 14, median = 0.787, IQR = 722–0.887; n = 32, median = 0.740, IQR = 0.714–0.817; n = 22, median = 0.645, IQR = 0.556–0.672; n = 11, median = 0.578, IQR = 0.477–0.678 and n = 12, median = 0.477, IQR = 0.416–0.542 for age groups A–E, respectively: Dunn's multiple comparison post hoc test p < 0.05).
Furthermore, ΔSO2 shows a significantly decreasing trend with age; however, age groups A and B (p = 0.585) and C and D (p = 0.571) have shown no significant difference (Fig. 4c: n = 14, median = 0.599, IQR = 0.496–0.682; n = 32, median = 0.595, IQR = 0.540–0.608; n = 22, median = 0.498, IQR = 0.439–0.512; n = 11, median = 0.486, IQR = 0.436–0.514; and n = 12, median = 0.363, IQR = 0.296–0.420 for age groups A–E, respectively: Dunn's multiple comparison post hoc test p < 0.05). Dunn's multiple comparison post hoc test statistics for the age variation study of ΔRHb, ΔHbO2 and ΔSO2 are given in the ESI† (Table S1). In addition, the measured average SO2 values (%) with the pulse oximeter for the different age groups A–E are 99.07 ± 0.62; 98.56 ± 0.61; 98.50 ± 0.67; 98.45 ± 0.52 and 98.40 ± 0.66, respectively (shown in Fig. S2 in the ESI†), and in agreement with our obtained trend.
These observations are also in agreement with the literature which reports that with an increase in age, the hemoglobin concentration in subjects decreases.5,37 The reason for the decrease in hemoglobin could be reduced hematopoietic activity with an increase in age, a decrease in bone marrow cellularity of up to 50% in individuals beyond the age of 60 years, and a significant reduction in peripheral blood counts.5,38,39 Similarly, the decrease in oxygen saturation with increasing age can be associated with variation in the effect of aging on lung function.40 Chest wall compliance and increasing air trapping are associated with aging. Further, progressive reduction of vital capacity with an increase of residual volume, a reduction of pulmonary compliance, an increase in uneven ventilation, increasing arterial stiffness, and a reduced diffusing capacity may also affect the decrease in oxygen saturation with an increase in age.40,41 In addition, our obtained trend in Fig. 5(c) i.e., decrease in oxygen saturation with increase in age is also in agreement with the study of Sharma G. and Goodwin J.42 (1981).
Discussion
This study demonstrates the feasibility of diffuse reflectance spectroscopy for non-invasive optical monitoring of RHb, HbO2, and SO2 from the index fingertips of 91 human subjects. RHb and HbO2 are governed by different perfusion rates and localized blood volumes which are further used to deduce the SO2. Although the measurements showed that the diffuse reflectance spectra of each subject are different, a similar trend of reduced hemoglobin/oxyhemoglobin concentrations is observed from all 91 measured subjects. In gender analysis, female subjects (36 subjects) have shown a comparatively higher ΔRHb than male subjects (55 subjects) measured from the median values while male subjects have a higher ΔHbO2 than female subjects measured under normal physiological conditions. These observations are consistent with the literature; reported values show that adult females have approximately 12% lower mean hemoglobin concentrations in their body than adult males.4 The ΔSO2 measured from females is slightly higher than the values measured from males. This observation is consistent with the literature, which reports that healthy young female adults have a higher (1.5%) SO2 than their male counterparts.36,41 In the age variation study, we observed that with an increase in age, the trend of ΔRHb increases while that of ΔHbO2 decreases. These observations are consistent with the literature, which reports that with an increase in age, the hemoglobin concentrations in male and female subjects decrease.5 Furthermore, with an increase in age, the ΔSO2 also decreases.Researchers have used various DRS-based approaches for monitoring the average blood oxygenation state for various applications. Feather et al. used the logarithmic inverse of diffuse reflectance signals to monitor skin pigments.43 Stratonnikov et al. have used Taylor series expansion of DRS attenuated signals to monitor finger occlusion by oxygen saturation and hemoglobin concentration (HbT).44 Knoefel et al. used linear square fit approximation by converting DR signals into apparent absorbance to measure the oxygen saturation for monitoring the pancreatic microcirculation.45 Subash et al. used the diffuse reflectance intensity ratio of oxyhemoglobin bands (R540/R575) for determining malignant lesions and normal mucosa of the oral cavity.46 Anand et al. used a similar ratiometric approach with oxyhemoglobin bands (R542/R580) and oxy- and deoxy-hemoglobin bands (R580/R555) for the assessment of foot ulcer healing.24 Bachir et al. used the second derivative of diffuse reflectance to measure tissue oxygen saturation (StO2) for estimating tissue hypoxia.47 All these DRS-based approaches monitor the average blood oxygenation state based on the alterations in blood oxygenation level and perfusion. In the same line, the presented DRS method gives an alternative approach to monitor age- and gender-wise variations in blood oxygenation parameters. This method provides real-time information on reduced hemoglobin, oxyhemoglobin, and oxygen saturation from the localized vascular beds of the human fingertips. In addition, this method is also able to resolve the relative local blood volume fraction of reduced hemoglobin/oxyhemoglobin within the bulk of highly scattering tissue media.
The cognitions of the work could be the foundation for further research of clinical diagnostic tools related to tissue oxygenation. These findings could improve clinical diagnosis, treatment of COVID-19 patients, non-invasive rapid assessment of tissue oxyhemoglobin concentration during fluid replacement, assessment of haemorrhaging, and hemoglobin-based blood substitution. Furthermore, these findings and the proposed blood oxygenation monitoring approach can be used for the assessment of reconstituted blood supply in free and pedicle flaps (organ implantations), diabetic foot ulcer treatment and assessment, and cancer diagnostics as well as PDT treatments. Although the non-invasive broadband diffuse reflectance spectroscopy can monitor the relative change in reduced and oxygenated blood concentrations from human tissues, some study limitations should be noted. The current blood oxygenation monitoring prototype is limited to a single source-detector. Therefore, some measurements could be influenced by strong absorption (blood vessels) or scattering inhomogeneities. The oxygen saturation values measured from the skin are affected to some degree by a number of relative differences that might occur in the amount of vasodilation or vasoconstriction due to instantaneous physiological changes in the upper limb. Moreover, the lower limb was not included in this study. Further studies could be extended to the lower limb with the toe as the location for blood oxygenation measurement, which could help to understand the oxygen saturation in tissues of diabetes patients to estimate the potential of early ulcer development.
Conclusions
In conclusion, the preliminary result shows that the proposed diffuse reflectance spectroscopy-based approach can sensibly monitor relative changes in local blood oxygenation parameters such as RHb, HbO2, and SO2 from the fingertips of human subjects. The gender and age variation study found different concentration trends of blood oxygenation. In the gender study, the female subjects have shown a significantly higher ΔRHb than the male subjects (p < 0.001). However, the female subjects have a lower ΔHbO2 than the male subjects (p < 0.001). Furthermore, females have shown a slightly higher ΔSO2 than the male subjects (p < 0.05). The age variation study concludes that with an increase in age, ΔRHb is significantly found to increase, while ΔHbO2 and ΔSO2 gradually decrease (p < 0.05). The obtained results suggest that the proposed approach of diffuse reflectance spectroscopy could be used to improve non-invasive diagnosis, and rapid assessment of tissue oxyhemoglobin concentration required by emergency & rescue teams as well as by ICUs. However, further studies will need to confirm the potential clinical applicability and accuracy of the technique for various health complications that are related to blood oxygenation variations.Author contributions
A. K. and R. K. were responsible for conceptualization and design of the study, and carried out the experiment. A. K., R. K., and K. C. assisted with the analysis and writing of the first draft of the manuscript. A. K., A. N., D. P., M. K. P., and R. K. validated and interpreted the results. R. K. supervised the study. All authors read and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research work was supported by the DST-SERB (under ASEAN-India Collaborative research scheme, Grant No. CRD-2018-000034). The authors would like to thank the Director, CSIR-CSIO, Chandigarh and Director, CSIR-NCL, Pune for providing infrastructure. The authors are also thankful to Ms. Karvan Kaushal, Mr. Kunvar Kant Patel, Ms. Sarika Hinge, and Mr. Maruti Salve for their help during the measurements.References
- J. Lee, D. J. Saltzman, A. E. Cerussi, D. V. Gelfand, J. Milliken, T. Waddington, B. J. Tromberg and M. Brenner, Physiol. Meas., 2006, 27, 757 CrossRef
.
- J. Lee, A. E. Cerussi, D. Saltzman, T. Waddington, B. J. Tromberg and M. Brenner, J. Biomed. Opt., 2007, 12, 024001–024008 CrossRef
.
- R. Santora and F. Moore, J. Crit. Care, 2009, 13, S10 CrossRef
.
- W. G. Murphy, Blood Rev., 2014, 28, 41–47 CrossRef CAS PubMed
.
- U. Mahlknecht and S. Kaiser, Exp. Ther. Med., 2010, 1, 1019–1025 CrossRef PubMed
.
- A. Zourabian, A. Siegel, B. Chance, N. Ramanujam, M. Rode and D. A. Boas, J. Biomed. Opt., 2000, 5, 391–405 CrossRef CAS PubMed
.
- M. Shivaramakrishnan, M. K. Masalov, H. F. Zhang, G. Stoica and L. V. Wang, Phys. Med. Biol., 2007, 52, 1349–1361 CrossRef PubMed
.
- H. A. Rinia, M. Bonn, E. M. Vartiainen, C. B. Schaffer and M. Muller, J. Biomed. Opt., 2006, 11, 050502–050503 CrossRef PubMed
.
- P. G. Carlier, D. Bertoldi, C. Baligand, C. Wary and Y. Fromes, NMR Biomed., 2006, 19, 954–967 CrossRef CAS
.
- H. Liu, Y. Song, K. L. Worden, X. Jiang, A. Constantinescu and R. P. Mason, Appl. Opt., 2000, 39, 5231–5243 CrossRef CAS PubMed
.
- M. Ferrari, L. Mottola and V. Quaresima, Can. J. Appl. Physiol., 2004, 29, 463–487 CrossRef PubMed
.
- M. Li, Y. Tang and J. J. P. Yao, J. Photoacoust., 2018, 10, 65–73 CrossRef
.
- M. Tepper, R. Neeman, Y. Milstein, M. B. David and I. Gannot, J. Biomed. Opt., 2009, 14, 054048 CrossRef
.
- M. A. Calin, I. C. Boiangiu, S. V. Parasca, S. Miclos, D. Savastru and D. Manea, Spectrosc. Lett., 2017, 50, 150–155 CrossRef CAS
.
- C. Sicher, R. Rutkowski, S. Lutze, S. V. Podewils, T. Wild, M. Kretching and G. Daeschlein, Biomedical Engineering, 2018, 63, 609–616 CrossRef CAS
.
- R. Kanawade, G. Saiko and A. Douplik, Monitoring of Epithelial Capillary Density, in Novel Optical Instrumentation for Biomedical Applications IV, ed. C. Depeursinge and I. Vitkin, Vol. 7371 of Proceedings of SPIE-OSA Biomedical Optics, Optica Publishing Group, 2009, paper 7371_1L Search PubMed.
- G. Zonios, J. Bykowski and N. Kollias, J. Invest. Dermatol., 2001, 117, 1452–1457 CrossRef CAS PubMed
.
- M. J. van Gemert, J. S. Nelson, T. E. Milner, D. J. Smithies, W. Verkruysse, J. F. de Boer, G. W. Lucassen, D. M. Goodman, B. S. Tanenbaum, L. T. Norvang and L. O. Svaasand, Phys. Med. Biol., 1997, 42, 937–950 CrossRef CAS PubMed
.
-
L. L. Randeberg, Fakultet for informasjonsteknologi, matematikk og elektroteknikk, 2005 Search PubMed
.
- B. LaRiviere, N. L. Ferguson, K. S. Garman, D. A. Fisher and N. M. Jokerst, Biomed. Opt. Express, 2019, 10, 5703–5715 CrossRef CAS
.
- P. Rejmstad, J. D. Johansson, N. Haj-Hosseini and K. Wårdell, J. Biophotonics, 2017, 10, 446–455 CrossRef CAS PubMed
.
- S. Y. Tzeng, J. Y. Guo, C. C. Yang, C. K. Hsu, H. J. Huang, S. J. Chou, C. H. Hwang and S. H. Tseng, Biomed. Opt. Express, 2016, 7, 616–628 CrossRef CAS PubMed
.
- J. C. Finlay, T. C. Zhu, A. Dimofte, J. S. Friedberg and S. M. Hahn, Proc. SPIE, 2006, 6139, 61390O CrossRef PubMed
.
- S. Anand, N. Sujatha, V. B. Narayanamurthy, V. Seshadri and R. Poddar, Opt. Lasers Eng., 2014, 53, 1–5 CrossRef
.
- R. J. Hunter,
et al.
, Phys. Med. Biol., 2002, 47, 193 CrossRef CAS PubMed
.
- N. Rajaram, A. Gopal, X. Zhang and J. W. Tunnell, Lasers Surg. Med., 2010, 42, 680–688 CrossRef PubMed
.
- R. Kanawade, F. Klämpfl, M. Riemann, C. Knipfer, K. Tangermann-Gerk, M. Schmidt and F. Stelzle, J. Biophotonics, 2014, 7, 841–849 CrossRef CAS PubMed
.
-
A. Kumar and R. Kanawade, Progress in Optomechatronics, ed. I. Bhattacharya, Y. Otani, P. Lutz and S. Cherukulappurath, Springer, Singapore, 2020, vol. 249, pp. 25–29 Search PubMed
.
- R. Kanawade, G. Saiko and A. Douplik, Phys. Procedia, 2010, 5, 659–664 CrossRef CAS
.
- R. Kanawade, F. Stelzle and M. Schmidt, 2012 Asia Communications and Photonics Conference (ACP), 2012, AS2E4, DOI:10.1364/ACP.2012.AS2E.4.
- R. R. Anderson and J. A. Parrish, J. Invest. Dermatol., 1981, 77, 13–19 CrossRef CAS
.
- S. L. Jacques, Skin Optics, 1998, https://omlc.org/ Search PubMed.
-
B. D. D. Gerard and J. Tortora, Principles of Anatomy and Physiology chapter 21: The Cardiovascular Systeam: Blood Vessels and Hemodynamics, Wiley, 11th edn, 2007 Search PubMed
.
-
Physiologie, ed. R. Klinke, H.-C. Pape, A. Kuntz and S. Silbernagl, Thieme, 6. Auflage, 2010 Search PubMed
.
- B. Vahlquist, Blood, 1950, 5, 874–875 CrossRef CAS
.
- S. Levental, E. Picard, F. Mimouni, L. Joseph, T. Y. Samuel, R. Bromiker, D. Mandel, N. Arish and S. Goldberg, Clin. Respir. J., 2018, 12, 1900–1904 CrossRef CAS
.
- W. W. Hawkins, E. Speck and V. G. Leonard, Blood, 1954, 9, 999–1007 CrossRef CAS
.
- J. G. Baldwin, Arch. Intern. Med., 1988, 148, 2544–2546 CrossRef
.
-
L. Berkahn and A. Keating, Hematology, Amsterdam, Netherlands, 2004, vol. 9, pp. 159–163 Search PubMed
.
- C. A. Sorbini, V. Grassi, E. Solinas and G. Muiesan, Respiration, 1968, 25, 3–13 CrossRef CAS PubMed
.
- E. Zahedi, K. Chellappan, M. A. M. Ali and H. Singh, Cardiovascular Engineering, 2007, 7, 172–181 CrossRef
.
- G. Sharma and J. Goodwin, Clin. Interventions Aging, 2006, 1, 253–260 CrossRef CAS
.
- J. Feather, M. Hajizadeh-Saffar, G. Leslie and J. B. Dawson, Phys. Med. Biol., 1989, 34, 807 CrossRef CAS
.
- A. A. Stratonnikov and V. B. Loschenov, J. Biomed. Opt., 2001, 6, 457–467 CrossRef CAS PubMed
.
- W. T. Knoefel, N. Kollias, D. W. Rattner, N. S. Nishioka and A. L. Warshaw, J. Appl. Physiol., 1996, 80, 116–123 CrossRef CAS
.
- N. Subhash, J. Mallia, S. S. Thomas, A. Mathews, P. Sebastian and J. Madhaven, J. Biomed. Opt., 2006, 11, 014018 CrossRef CAS
.
- W. Bachir and O. Hamadah, OSA Continuum, 2021, 4, 650–664 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sd00126h |
| This journal is © The Royal Society of Chemistry 2022 |