Open Access Article
Open Access ArticleAn ESIPT-based fluorescent probe for the detection of multiple analytes and a facile approach to discriminate between arsenate and pyrophosphate in water†
Srushti
Gadiyaram
 ,
Vikas D.
Ghule
,
Vikas D.
Ghule
 ,
Amrita
Ghosh
,
Amrita
Ghosh
 and
D. Amilan
Jose
and
D. Amilan
Jose
 *
*
Department of Chemistry, National Institute of Technology (NIT) Kurukshetra, Kurukshetra – 136119, Haryana, India. E-mail: amilanjosenit@nitkkr.ac.in
First published on 14th September 2022
Abstract
A simple ESIPT-based fluorescent probe for the detection of the multiple analytes such as chromium (Cr3+), arsenate (HAsO42−), and pyrophosphate (PPi, P2O74−) has been reported. The probe HJ-DNP displayed weak emission in organic solvents owing to excited-state intramolecular proton transfer (ESIPT) and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization. However, upon the addition of water, the fluorescence emission could be increased to a maximum through the aggregation-induced emission (AIE) behavior of HJ-DNP. The combined effects of ESIPT and AIE in the DMSO–water mixture were used to sense multiple analytes. The probe HJ-DNP could selectively detect Cr3+ through the CHEF mechanism with a turn-on fluorescence response. The limit of detection (LOD) for Cr3+ ions was estimated to be as low as 3.9 μM. The probe HJ-DNP was also used to detect Cr3+ ions in tomato extract. The probe HJ-DNP also binds preferentially with arsenate and pyrophosphate (PPi) in the presence of various anions. The sensing of arsenate and PPi could be carried out through naked-eye detection with a color change from orange to red; the LOD values are calculated to be 3.9 μM and 0.19 mM for arsenate and PPi, respectively. It was found that arsenate detection is more sensitive than PPi detection. Moreover, the different emission behavior of HJ-DNP in the presence of arsenate or PPi was used to easily discriminate them. The probe HJ-DNP was used to detect arsenate in real water samples. Arsenate was also detected and adsorbed from water samples using HJ-DNP incorporating alginate polymer beads.
N isomerization. However, upon the addition of water, the fluorescence emission could be increased to a maximum through the aggregation-induced emission (AIE) behavior of HJ-DNP. The combined effects of ESIPT and AIE in the DMSO–water mixture were used to sense multiple analytes. The probe HJ-DNP could selectively detect Cr3+ through the CHEF mechanism with a turn-on fluorescence response. The limit of detection (LOD) for Cr3+ ions was estimated to be as low as 3.9 μM. The probe HJ-DNP was also used to detect Cr3+ ions in tomato extract. The probe HJ-DNP also binds preferentially with arsenate and pyrophosphate (PPi) in the presence of various anions. The sensing of arsenate and PPi could be carried out through naked-eye detection with a color change from orange to red; the LOD values are calculated to be 3.9 μM and 0.19 mM for arsenate and PPi, respectively. It was found that arsenate detection is more sensitive than PPi detection. Moreover, the different emission behavior of HJ-DNP in the presence of arsenate or PPi was used to easily discriminate them. The probe HJ-DNP was used to detect arsenate in real water samples. Arsenate was also detected and adsorbed from water samples using HJ-DNP incorporating alginate polymer beads.
1. Introduction
Fluorescence-based sensors of anions and cations are of great interest to researchers due to their high sensitivity and fluorescence bio-imaging abilities.1–4 Several fluorescent chemical sensors, working on the principles of photoinduced electron transfer (PET), internal charge transfer (ICT), chelation-enhanced fluorescence (CHEF), excited-state intramolecular proton transfer (ESIPT), aggregation-induced emission (AIE), and aggregation-induced quenching (AIQ), are known for a variety of anions and cations that are biologically and environmentally relevant.5Aggregation-induced emission (AIE) is a photophysical phenomenon associated with chromophore aggregation.6,7 In the AIE process, non-emissive luminogens are induced to emit via aggregate formation.8 In general, a molecule can exhibit ESIPT fluorescence if its structure incorporates an intramolecular hydrogen bonding interaction between –OH/NH2 hydrogen bond donor and a hydrogen bond acceptor (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and –C
N– and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).9 ESIPT fluorescence probes have the advantage of showing very significant Stokes shifts. Generally, ESIPT-based fluorescent probes are designed in a way in which the H-bond is initially blocked, inhibiting the ESIPT process; consequently, only enol emission is possible.10 However, exposure to a specific analyte can allow a H-bond and the keto form, unlocking the ESIPT process.
O).9 ESIPT fluorescence probes have the advantage of showing very significant Stokes shifts. Generally, ESIPT-based fluorescent probes are designed in a way in which the H-bond is initially blocked, inhibiting the ESIPT process; consequently, only enol emission is possible.10 However, exposure to a specific analyte can allow a H-bond and the keto form, unlocking the ESIPT process.
Most AIE- and ESIPT-based fluorescent sensors are known to detect either one individual analyte or they act as dual-responsive probes for two analytes, rather than sensing multiple analytes in parallel.11 Dual-responsive probes contain a distinct sensing group for each analyte.12 Therefore, developing an ESIPT-based multi-analyte chemosensor is crucial for environmental and biological applications.13–17 Different interaction modes need to be considered to design a multianalyte probe, which increases the design difficulty. From a practical point of view, designing such molecular probes with differential responses towards multiple analytes is cost-effective and highly desirable. They can detect more than one analyte based on creating different output signal responses to different analytes.
Cr3+ is a heavy metal ion and a nutrient necessary for balancing human and animal diets. However, excessive consumption of Cr3+ is known to negatively affect human health. Thus, the selective and sensitive detection of Cr3+ ions in environmental and biological samples is required.18,19 Although many chemical sensors for Cr3+ have been designed, most of them are unsuccessful in detecting Cr3+ ions in an aqueous medium, making them unsuitable for practical applications.20–29 Therefore, it is essential to design new water-friendly chemical sensors for the sensitive detection of Cr3+.
Phosphorus- and arsenic-derived oxyanions exhibit similar properties, notably inorganic phosphate (Pi) and arsenate. Their physiochemical similarities make them difficult to differentiate when they co-exist in water.30 Arsenate is considered to be highly toxic to humans compared with phosphates. Arsenate is also a critical chemical pollutant in water. Several fluorescent chemical sensors related to the detection of phosphates are known but reports of fluorescent chemical sensors for arsenate are rare.31,32 Therefore, the development of new, simple, sensitive, and reliable fluorescence-based methods for arsenate ion recognition is highly desired.
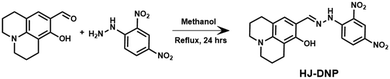
In this work, a multianalyte-responsive probe, HJ-DNP, was developed via conjugating 8-hydroxyjulolidine-9-carboxaldehyde and 2,4-dinitrophenylhydrazine through a Schiff base reaction (Scheme 1). The probe HJ-DNP displayed aggregation-induced emission (AIE) behaviour in a DMSO–water mixture. The probe HJ-DNP was also studied for the fluorescent detection of Cr3+ and discrimination between PPi and arsenate in aqueous solution.
2. Results and discussion
The probe HJ-DNP was prepared via a condensation reaction between 8-hydroxyjulolidine-9-carboxaldehyde and 2,4-dinitrophenyl hydrazine in a single-step reaction (Scheme 1). The compound HJ-DNP was isolated in pure form with 80% yield, and the characterization data matched well with the desired product (see the experimental details below and ESI† Fig. S1–S3).2.1. Photophysical and water-dependent aggregation studies
The UV-vis spectrum of HJ-DNP (0.05 mM) in dimethyl sulphoxide (DMSO) displayed two peaks at 365 nm (ε = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 M−1 cm−1) and 470 nm (ε = 7240 M−1 cm−1). The fluorescence emission spectrum of HJ-DNP in DMSO exhibits weak broad emission at 420 nm and 585 nm upon excitation at 365 nm (Fig. S4†). Almost all solvents result in weak fluorescence emission (Fig. S5†). The large Stokes shift of around 585 nm was assigned to proton-transfer tautomer emission. The weak emission of HJ-DNP is owing to the excited-state intramolecular proton-transfer (ESIPT) process (the phenolic proton transfers to the imine nitrogen atom) and C
400 M−1 cm−1) and 470 nm (ε = 7240 M−1 cm−1). The fluorescence emission spectrum of HJ-DNP in DMSO exhibits weak broad emission at 420 nm and 585 nm upon excitation at 365 nm (Fig. S4†). Almost all solvents result in weak fluorescence emission (Fig. S5†). The large Stokes shift of around 585 nm was assigned to proton-transfer tautomer emission. The weak emission of HJ-DNP is owing to the excited-state intramolecular proton-transfer (ESIPT) process (the phenolic proton transfers to the imine nitrogen atom) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization.33
N isomerization.33
The photosensitivity of the probe HJ-DNP was explored, as it has two nitro groups that are sensitive to UV exposure. This implies that under strong UV light irradiation, the probe can be degraded. To avoid such a situation, the experiments were performed with fresh stock solutions and were carried out mostly via avoiding direct contact with UV light. Experiments were monitored for up to 90 min (Fig. S6†).
The formation of an intramolecular hydrogen bond between hydrogen-donor and hydrogen-acceptor groups is a prerequisite for ESIPT. The compound HJ-DNP bears a 2,4-dinitrophenylhydrazine N atom as a proton acceptor and a julolidine hydroxyl group as the proton donor.
To confirm the ESIPT process in HJ-DNP, the ground state (S0) and first excited-state (S1) energies for both the enol and keto tautomer forms of HJ-DNP were calculated using the respective optimized geometries (Fig. 1a). The molecular geometries of the receptor keto–enol tautomer and the transition state have been optimized using the B3LYP/6-311G(d,p) level of theory and are shown in Fig. 1b. A transition state via direct H-transfer bonding between the oxygen atom (–OH) and the azomethine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) nitrogen atom (see Fig. 1) was determined. The C
N) nitrogen atom (see Fig. 1) was determined. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O⋯H, and H–N bond distances in the keto form are 1.2671, 1.6763, and 1.0471 Å, respectively. In the enol form, the C–O, O–H, and H⋯N distances are 1.3512, 0.9804, and 1.7973 Å, respectively. As can be seen, strong H-bonding exists in the tautomer, and H-transfer from O to N is established. In the transition state, the C–O, N–H, and O–H distances increase, indicating proton transfer. The C–O, O⋯H, and H⋯N distances in the transition state are 1.2886, 1.4002, and 1.1297 Å, respectively.
O, O⋯H, and H–N bond distances in the keto form are 1.2671, 1.6763, and 1.0471 Å, respectively. In the enol form, the C–O, O–H, and H⋯N distances are 1.3512, 0.9804, and 1.7973 Å, respectively. As can be seen, strong H-bonding exists in the tautomer, and H-transfer from O to N is established. In the transition state, the C–O, N–H, and O–H distances increase, indicating proton transfer. The C–O, O⋯H, and H⋯N distances in the transition state are 1.2886, 1.4002, and 1.1297 Å, respectively.
 | ||
| Fig. 1 (a) The optimised keto–enol molecular geometries of the probe HJ-DNP using the B3LYP/6-311G(d,p) level of theory. (b) The keto–enol tautomer and transition-state energy levels of HJ-DNP. | ||
The HOMO–LUMO energy difference was reduced from 2.63 eV for the enol form to 2.42 eV for the keto form. This observation indicates that the probe HJ-DNP undergoes ESIPT. Consequently, the tautomer forms in the excited state, which is responsible for the emission properties.
The probe HJ-DNP shows enhancement in emission at 585 nm with an increasing amount of water in a DMSO mix (Fig. 2). The intensity of the emission peak at ca. 585 nm increased continuously, showing a ten-fold increase with 90% water in DMSO (v/v) compared with 0% water. Up to 60% water, almost no change in emission was obtained, but emission enhancement was observed beyond that. The enhancement of emission in the presence of water was due to the formation of aggregates and AIE. It is already known from the literature that ESIPT and AIE occur in hydroxy aromatic imines, and the emission is dependent on the proportion of water in the solvent.34–37
Therefore, it is proposed that the red emission that occurred at 585 nm upon the addition of water is due to a combined effect of ESIPT and AIE. Further, to confirm the formation of aggregates in the presence of water, the particle size of HJ-DNP was studied via the dynamic light scattering (DLS) method in DMSO and a DMSO–water mixture (Fig. S8†). The average particle size of HJ-DNP in the DMSO–water mixture increased to 1268 nm compared to 232 nm in DMSO alone. The results suggest that the compound HJ-DNP tends to form aggregates in DMSO–water. Further, it validates the tendency of HJ-DNP to show aggregation-induced emission (AIE) behaviour (Scheme 2).
2.2. Chromium-ion selectivity
The binding properties of HJ-DNP towards different transition-metal and heavy-metal ions, such as Ni2+, Ag+, Fe2+, Fe3+, Cr3+, Cd2+, Cu2+, Mn2+, Na+, Mg2+, Ca2+, Co2+, and Hg2+, were investigated by fluorescence experiments. All the experiments were carried out in a DMSO–water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) mixture, where weak monomer emission was predominant. The fluorescence emission spectra of HJ-DNP (0.1 mM) with excess amounts (5 equivalents, 0.5 mM) of different metal ions were measured. A considerable enhancement in emission was observed at 420 nm (λex = 365 nm) along with a decrease in emission at 585 nm only in the presence of Cr3+. This enhancement (at 420 nm) was not found in the case of any other metals (Fig. 3a). Systematic emission titration was used to determine the sensitivity of Cr3+ binding with HJ-DNP. As shown in Fig. 3b, with the addition of Cr3+, emission at 585 nm continuously decreased and an increase in the emission peak at 420 nm was observed, with a clear isosbestic point at 540 nm. The binding constant for Cr3+ was calculated to be 4.81 × 104 M−1. The limit of detection (LOD) was estimated to be 3.9 × 10−6 M. 1
20) mixture, where weak monomer emission was predominant. The fluorescence emission spectra of HJ-DNP (0.1 mM) with excess amounts (5 equivalents, 0.5 mM) of different metal ions were measured. A considerable enhancement in emission was observed at 420 nm (λex = 365 nm) along with a decrease in emission at 585 nm only in the presence of Cr3+. This enhancement (at 420 nm) was not found in the case of any other metals (Fig. 3a). Systematic emission titration was used to determine the sensitivity of Cr3+ binding with HJ-DNP. As shown in Fig. 3b, with the addition of Cr3+, emission at 585 nm continuously decreased and an increase in the emission peak at 420 nm was observed, with a clear isosbestic point at 540 nm. The binding constant for Cr3+ was calculated to be 4.81 × 104 M−1. The limit of detection (LOD) was estimated to be 3.9 × 10−6 M. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric binding between HJ-DNP and Cr3+ was established based on a Job plot (Fig. S9†).
1 stoichiometric binding between HJ-DNP and Cr3+ was established based on a Job plot (Fig. S9†).
The increase in the fluorescence intensity at 420 nm and the decrease at 585 nm in the presence of Cr3+ ions may be due to the inhibition of H-bonding; therefore, the ESIPT process was blocked (Scheme 3). The observed emission at 420 nm in the DMSO–water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) mixture corresponds to the standard form of HJ-DNP. In contrast, emission at 585 nm arises due to intramolecular H-bonding between the julolidine OH group and the hydrazine N atom of HJ-DNP. When a Cr3+ ion binds with the julolidine OH group and the hydrazine N atom of HJ-DNP, it does not allow the ESIPT process (Scheme 3) and the emission at 420 nm is recovered.
20) mixture corresponds to the standard form of HJ-DNP. In contrast, emission at 585 nm arises due to intramolecular H-bonding between the julolidine OH group and the hydrazine N atom of HJ-DNP. When a Cr3+ ion binds with the julolidine OH group and the hydrazine N atom of HJ-DNP, it does not allow the ESIPT process (Scheme 3) and the emission at 420 nm is recovered.
Further, the Cr3+ ion binding behaviour of HJ-DNP was also tested via UV-vis experiments. The systematic titration of HJ-DNP (0.05 mM) with Cr3+ (0–0.145 mM) showed a decrease in absorbance at 470 nm and a shift in the absorbance maximum from 365 nm to 340 nm (Fig. S10†). Based on the absorbance titration studies, the binding constant (Ka) was calculated to be 4.34 × 104 M−1, closely matching the value obtained from emission titration experiments. Time-resolved fluorescence lifetime measurements were also performed to confirm the turn-on sensor response of HJ-DNP in the presence of Cr3+. The average fluorescence lifetime of the probe HJ-DNP in the absence of analyte was measured to be 0.82 ns. However, in the presence of Cr3+, it was increased to 2.07 ns (Fig. S11†). The emission quantum yield (Φ) calculated for HJ-DNP (Φ = 0.00031) was increased almost three-fold in the presence of Cr3+ (Φ = 0.00081) ions.
The proposed binding mode of HJ-DNP with Cr3+ is shown in Scheme 3. It can be presumed that the chelating mode of HJ-DNP binding to Cr3+ restricts the molecule, which extends the π-electron conjugation throughout the molecule. The extension in conjugation triggers a switch-ON response in the fluorescence spectrum via a chelation-enhanced fluorescence (CHEF) mechanism. The sensing mechanism of the probe toward Cr3+ is also confirmed with ESI-mass analysis. As shown in mass experiments, in the presence of Cr3+, the probe displayed a peak at an m/z value of 449.24 for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of Cr3+ with HJ-DNP, and the [2M + H]+ peak is also observed at an m/z value of 898.5608. If the analyte is prone to complex formation or is highly concentrated, [2M + H]+ ions could also be observed in the full mass spectrum (Fig. S12†).
1 complex of Cr3+ with HJ-DNP, and the [2M + H]+ peak is also observed at an m/z value of 898.5608. If the analyte is prone to complex formation or is highly concentrated, [2M + H]+ ions could also be observed in the full mass spectrum (Fig. S12†).
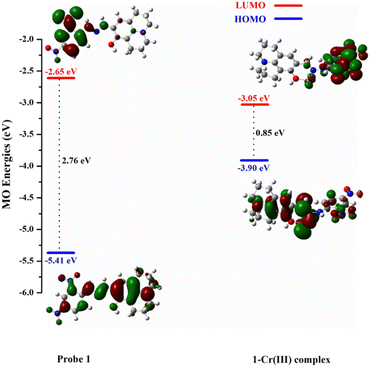
To gain more insight into the sensing behaviour of HJ-DNP toward Cr3+, DFT calculations were carried out using the Gaussian 09 program package. The geometries of HJ-DNP and HJ-DNP with Cr3+ were fully optimized and investigated using the density functional theory method (B3LYP) with the 6-311g(d,p) and LANL2DZ basis sets. The HOMO, LUMO, and bandgap energy values were calculated using DFT studies. The optimized structures of the probe HJ-DNP and the corresponding Cr3+ adduct are shown in Fig. 4.
 | ||
| Fig. 4 The optimized structures of the probe HJ-DNP and the HJ-DNP–Cr3+ complex obtained using DFT theory at the B3LYP level with the 6-311g(d,p) and LANL2DZ basis sets. | ||
The HOMO is delocalized over the whole molecule, while the LUMO is mainly delocalized on 2,4-dinitrophenylhydrazine. In the HJ-DNP–Cr3+ complex, the HOMO is delocalized over the whole molecule, whereas the LUMO is strongly delocalized on (2,4-dinitrophenyl)hydrazine and slightly on the Cr3+ part. The calculated data show that the HJ-DNP–Cr3+ complex has significantly reduced HOMO energy (−3.9 eV) compared to HJ-DNP. The computed energy gaps for the HJ-DNP and HJ-DNP–Cr3+ complexes are 2.76 and 0.85 eV, suggesting that the binding of Cr3+ significantly reduces the energy gap and favours an electron transition. The lowering of the HOMO–LUMO energy gap in the HJ-DNP–Cr3+ complex compared to free HJ-DNP probably causes a slight red shift in the emission spectrum of HJ-DNP at 420 nm when Cr3+ is added. The substantial decrease in the total energy of HJ-DNP upon complexation with Cr3+ suggests that the HJ-DNP–Cr3+ complex is highly stable, probably initiating the spectral outcome through the CHEF mechanism.
2.3. Chromium detection in real-life samples
Although chromium(III) ions are an essential element in the human body, excess intake can harm the stomach and blood glucose levels. Recent studies showed that compounds containing Cr3+ probably provoke toxicity, leading to carcinogenicity.38,39Vegetables are essential components of the human diet, but studies have demonstrated the presence of trace metals within them. Vegetables grown in heavily polluted water may show heavy-metal contamination.40
These metals cannot be removed by washing the vegetables or cooking, even at high temperatures. Hence, we have explored the possible detection of Cr3+ in tomatoes (Solanum lycopersicon) using the probe HJ-DNP. Several studies have described the toxic effects of Cr3+ in tomatoes and other plants.41 Chromium detection experiments were performed with tomato extract spiked with known concentrations of Cr3+ (see the ESI†). HJ-DNP emission spectra were recorded from Cr3+-spiked tomato extract. As shown in Fig. 5, good linearity (R2 = 0.982) was observed in a plot of the concentration of Cr3+ in tomato extract and the emission intensity at 420 nm. This also provided a LOD of 22.4 nm. Therefore, the probe HJ-DNP can be used to detect and quantify Cr3+ ions in tomatoes. This could prove to be a powerful tool to quantify an unknown amount of Cr3+ in various food samples.
 | ||
| Fig. 5 A plot of the linear relationship between the emission intensity of HJ-DNP and the concentration of Cr3+ in tomato extract. | ||
2.4. Binding studies with anions
The binding abilities of HJ-DNP with various anions are also monitored via UV-vis and fluorescence spectrophotometry. As shown in Fig. 6, in the presence of excess anions (10 equivalents), only PPi and arsenate42 induced distinct spectral and colour changes. Other relevant anions, such as F−, PO43−, AcO−, Cl−, Br−, I−, NO3−, H2PO4−, and HSO4−, did not show any noticeable changes in terms of their UV–vis and fluorescence spectra (Fig. 6). The probe HJ-DNP immediately changed from dark yellow to red in the presence of PPi and arsenate (Fig. 6a). Anions such as S2−, HS−, CN−, CO32−, and HCO3− also showed definite changes in terms of their UV-vis absorbance. However, the changes are mainly due to deprotonation of the –OH moiety in HJ-DNP. As the probe is sensitive to basic pH, these highly basic anions drive the probe HJ-DNP to be deprotonated (Fig. S13†).The sensitivity of the probe HJ-DNP toward arsenate and PPi was calculated based on systematic titration studies. As shown in Fig. 7a, a decrease in the emission band at 585 nm and an increase in the emission band at 510 nm were observed upon adding arsenate ions to HJ-DNP. The stoichiometry of HJ-DNP binding with arsenate was calculated through a Job's plot, and the results confirmed the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (anion
2 (anion![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand) complex (Fig. S14†). In the UV-vis spectrum, with an increase in the arsenate concentration, a decrease in the absorbance band at 365 nm and a simultaneous increase at 410 nm were observed (Fig. S15†).
ligand) complex (Fig. S14†). In the UV-vis spectrum, with an increase in the arsenate concentration, a decrease in the absorbance band at 365 nm and a simultaneous increase at 410 nm were observed (Fig. S15†).
 | ||
| Fig. 7 The emission spectra of the probe HJ-DNP (0.1 mM) with various concentrations of (a) arsenate (0–1.17 mM) and (b) PPi (P2O74−) (0–1.33 mM) ions (λex = 365 nm, slit width: 20/20). | ||
The binding constant (4.81 × 104 M−1) and LOD (3.9 μM) values were calculated for arsenate detection via the emission method.43–45 Further, the sensitivity of HJ-DNP toward PPi was also determined via systematic fluorescence and UV-vis absorbance titration. As shown in Fig. 7b, upon the addition of PPi, the emission band intensity at 505 nm was increased. In the UV-vis spectra, the sensor HJ-DNP exhibited ratiometric changes when detecting PPi at 340 nm and 425 nm (Fig. S16†). A Job plot confirmed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (PPi
2 (PPi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HJ-DNP) stoichiometry of HJ-DNP binding with PPi (Fig. S17†). The LOD and Ka values for PPi were calculated to be 0.19 mM and 8.77 × 102 M−1, respectively. These values revealed that the binding of PPi is very weak and less sensitive compared to arsenate. The sensitivity of arsenate binding is 55 times higher than that of PPi binding.
HJ-DNP) stoichiometry of HJ-DNP binding with PPi (Fig. S17†). The LOD and Ka values for PPi were calculated to be 0.19 mM and 8.77 × 102 M−1, respectively. These values revealed that the binding of PPi is very weak and less sensitive compared to arsenate. The sensitivity of arsenate binding is 55 times higher than that of PPi binding.
Furthermore, time-resolved fluorescence lifetime measurements were performed to reconfirm the turn-on sensor responses of HJ-DNP towards PPi and arsenate ions. It was observed that the fluorescence decay constants (s) of HJ-DNP were affected, as summarized in Table S2.† The average lifetime values of HJ-DNP increased to 1.77 ns and 3.24 ns, respectively, for arsenate and PPi (Fig. 8). The relative quantum yield values calculated for HJ-DNP with PPi (Φ = 0.0022) and arsenate (Φ = 0.0012) are more than five times that of HJ-DNP (Φ = 0.00031), which confirms the turn-on fluorescence response toward PPi and arsenate ions.
 | ||
Fig. 8 Lifetime measurements of the probe HJ-DNP (0.1 mM) with arsenate (1 mM) and PPi (9.45 mM) in DMSO–water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). 20). | ||
Phosphorus- and arsenic-derived oxyanions display similar properties, making them difficult to distinguish when they co-exist. Interestingly, the natures of the emission spectrum changes toward PPi and arsenate are different (Fig. 7), making PPi and arsenate distinguishable.46–48 PPi displayed emission enhancement at 543 nm, whereas arsenate showed ratiometric changes in emission and enhancement at 523 nm. This spectral difference can be used to identify and discriminate between PPi and arsenate in solution easily. The acidic protons of the probe HJ-DNP are involved in binding with the –OH groups of arsenate and pyrophosphate ions, as shown in the proposed mechanism (ESI† Scheme S1).
FTIR analysis was performed to understand the binding modes of PPi and arsenate. HJ-DNP was mixed with different concentrations of PPi and arsenate. These solutions were evaporated, and the solid product was analysed via FT-IR using the KBr method. The IR spectra of HJ-DNP with and without PPi and arsenate were compared. The IR spectrum of HJ-DNP shows a strong band observed at 1604 cm−1, which can be assigned to the stretching vibrations of the –NH group, and the moderately intense bands observed around 2985–3005 cm−1 are due to azomethine (–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) stretching. The shifts in the IR bands of –NH, –CH
N) stretching. The shifts in the IR bands of –NH, –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and –OH in the presence of PPi and arsenate confirm the involvement of these groups during the binding process (Fig. S18†).
N, and –OH in the presence of PPi and arsenate confirm the involvement of these groups during the binding process (Fig. S18†).
Further, 1H-NMR spectra of HJ-DNP were also obtained in the presence of arsenate and PPi to confirm the binding mode of HJ-DNP with these anions. The interaction of PPi and arsenate caused significant changes in the chemical shifts in the 1H-NMR spectra of HJ-DNP. The protons of –OH (15.3 ppm) and –NH (9.9 ppm) disappeared due to hydrogen bonding between PPi/arsenate and HJ-DNP. 1H-NMR experiments confirm the involvement of –OH and –NH groups in binding with arsenate (Fig. S19†). Based on IR and 1H-NMR analysis, the mechanism of the binding of PPi and arsenate with HJ-DNP is proposed in ESI† Scheme S1.
The binding behaviour of HJ-DNP with arsenate and PPi was also studied via quantum mechanical calculations using Gaussian 09. Calculations of frontier molecular orbital HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital) energies and band gaps were performed in the gas phase. The structure was optimized using the B3LYP theoretical method with the 6-311g(d,p) and LANL2DZ basis sets (Fig. 9). The calculations predicted that the bandgaps between the HOMOs and LUMOs of HJ-DNP with arsenate and PPi were found to be 1.49 eV and 1.39 eV, respectively (Fig. S20†). These results confirm a decrease in energy compared to free HJ-DNP, which validates the spectral shifts in the experimental absorption spectra.
 | ||
| Fig. 9 Optimized structures of (a) HJ-DNP–arsenate and (b) HJ-DNP–PPi at the B3LYP/LANL2DZ level of theory. | ||
The binding energies (ΔE) for probe HJ-DNP–arsenate and probe HJ-DNP–PPi complexes were computed via calculating the differences between the energies of the isolated receptor and anions and their complexes (Table 1). The calculated binding energies for the corresponding arsenate and PPi complexes are −351 and −228 kJ mol−1, respectively, revealing the higher affinity of HJ-DNP for arsenate than PPi. These results are well in line with the experimental results.
| Compound | Total energy (a.u.) | ΔEbind (kJ mol−1) | ||
|---|---|---|---|---|
| HJ-DNP–anion | HJ-DNP only | Anion only | ||
| Arsenate | −3077.23271 | −1384.52253 | −308.05396 | −351 |
| PPi | −3310.95527 | −1384.52253 | −541.82046 | −228 |
The primary source of binding energy in the HJ-DNP–arsenate and HJ-DNP–PPi complexes was hydrogen bonding between –OH, –NH, and –NO2 groups present in the framework of HJ-DNP and the anions.
2.5. pH-Dependent studies
The effects of pH on the sensing behaviour of the probe HJ-DNP were studied based on changes in the UV-vis spectrum. Three different pH levels, pH 5.0, 7.0, and 9.0, were chosen in order to explore acidic, neutral, and basic pH conditions. The experiments were performed using acetate buffer, water, and borax buffer to maintain the respective pH levels. From a comparative table of LOD values (Table S3†), it is evident that the probe HJ-DNP can efficiently detect analytes only at neutral pH (Fig. S21 and S22†). At acidic pH (pH = 5), changes in UV-vis absorbance and colour are very minimal or negligible, so LOD calculations are not feasible. However, at pH 9.0, in basic medium, only Cr3+ effectively binds with HJ-DNP, as the probe deprotonates in a basic medium and binding sites are not available for the binding of anions such as arsenate and PPi. Thus, pH studies confirm that acidic pH is not suitable for the binding of analytes. However, interestingly, at basic pH the probe is selective for Cr3+.2.6. Arsenate detection in real samples
Arsenate detection was carried out using three natural water samples (groundwater, pond water, and tap water) and washed rice water (WRW). Rice is a significant contributor of inorganic arsenic in the human diets. Rice accumulates a higher amount of arsenic than any other grain crop. Arsenic-containing irrigation water can lead to long-term soil contamination with arsenic.49,50 In soil, arsenic is present mainly as inorganic species (arsenate As(V) and arsenic As(III)).43,51 Therefore, we have selected a water sample obtained from rice washing. It is known that 50–60% of arsenic impurities are removed upon soaking rice in water before cooking. Arsenate-spiked water samples were used for recovery analysis. Fluorescence spectra were recorded, and the emission intensity at 522 nm was monitored. The response of HJ-DNP increased linearly with an increase in the arsenate concentration in spiked water.Based on emission changes, the arsenate % recovery values were estimated, and acceptable average recovery (95.0–105%) and RSD (2.0–4.0%) values were obtained (Table 2). The recovery of arsenate from wastewater samples was statistically close to 100%. This result indicates the validation of this method for the detection of arsenate in different water samples and washed rice water. In addition, the concentration of arsenate in these samples detected via the proposed approach agreed well with ICP-MS experiments. These studies implied that the sensor HJ-DNP could be a promising tool for monitoring arsenate in water and grains like rice.
| Water sample | HAsO42− spiked (μM) | Found (μM) | Recovery % | RSD% (n = 3) |
|---|---|---|---|---|
| Ground | 40 | 38.63 | 96.57 | 3.03 |
| 80 | 75.67 | 94.58 | 1.86 | |
| Pond | 20 | 20.08 | 100.0 | 2.13 |
| 80 | 76.02 | 95.02 | 2.47 | |
| 100 | 96.08 | 96.08 | 2.58 | |
| Tap | 20 | 19.78 | 98.90 | 3.15 |
| 60 | 60.03 | 100.0 | 3.09 | |
| 100 | 98.08 | 98.08 | 2.36 | |
| Washed rice | 80 | 76.79 | 95.98 | 3.91 |
| 100 | 100.03 | 100.13 | 3.43 |
2.7. Detection of arsenate ions using sodium alginate beads
The use of alginate beads (biopolymer) as a sorbent to detect and remove arsenate from contaminated water has also been investigated. The probe HJ-DNP was incorporated with polymeric sodium alginate beads52,53 for ready-to-use applications. HJ-DNP-incorporating alginate hydrogel beads (HJ-DNP@alginate) were prepared using distilled water via a well-known method described previously (see the Experimental details below).HJ-DNP@alginate was characterized via scanning electron microscopy (SEM) and EDX analysis. As shown in Fig. 10A, the beads display an uneven polymer surface, indicating the incorporation of HJ-DNP on the surface of the polymer.54HJ-DNP@alginate was immersed in a sample of arsenate-contaminated water. An immediate change in the colour of the polymer beads from dark yellow to red confirms the detection of arsenate and its uptake by the polymer beads (Fig. 10).
SEM–EDX analysis of red-coloured HJ-DNP@alginate confirmed the detection and adsorption of arsenate ions from water samples (Fig. 10B). Arsenic peaks were observed in the SEM–EDX spectra only after arsenate adsorption by the alginate beads. This phenomenon shows the effective adsorption of arsenate on the surface of HJ-DNP@alginate. Thus, these experiments confirm the advantage of using HJ-DNP@alginate for the detection of μM-levels of arsenate in water.
2.8. Multianalyte detection based on the CIE chromaticity diagram
The CIE 1931 chromaticity parameters (x, y) from the luminescence data of HJ-DNP, HJ-DNP with Cr3+, and HJ-DNP with anions were calculated, and the chromaticity diagram was plotted.55–57Fig. 11 depicts the changes in the CIE coordinates in response to various analytes. The CIE diagram shows the luminescence of probe HJ-DNP in the presence of arsenate, PPi, and Cr3+. The probe HJ-DNP (0.36, 0.35) lies in the centre of the red colour space, showing no specific fluorescence. But with analytes, this changes and moves to different regions.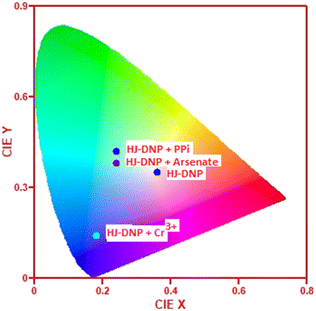 | ||
| Fig. 11 The CIE chromaticity diagram of the probe HJ-DNP with the analytes pyrophosphate (PPi), arsenate, and Cr3+. | ||
In the presence of arsenate, the probe displayed chromaticity in the blue region, and this moved towards the green region upon the addition of PPi. Again, the chromaticity shifted towards the blue-purple region in the presence of Cr3+, unlike PPi and arsenate. Therefore, it is evident that the probe HJ-DNP could be used to detect and distinguish between the three analytes of Cr3+, arsenate, and PPi.
3. Experimental section
General information and materials
All the chemicals used were purchased from commercial sources and used without further purification. 8-Hydroxyjulolidine-9-carboxaldehyde was purchased from TCI Chemicals. 2,4-Dinitrophenylhydrazine, tetrabutylammonium salts of different anions (such as Cl−, I−, Br−, CH3COO−, PO43−, NO3−, F−, OH−, P2O74−, and HSO4−), and the sodium salt of arsenate (HAsO42−) were purchased from Sigma-Aldrich. Perchlorate salts of various metal ions, such as Mn2+, Mg2+, Ca2+, Na+, Fe2+, Fe3+, Cu2+, Ni2+, Al3+, Co3+, Cr3+, Hg2+, and Ag+, were purchased from Sigma-Aldrich. Solvents were obtained from Loba Chemie and S. D. fine chemical and were used following further purification via distillation. 1H-NMR spectra were recorded using a Bruker Avance (II) 600 MHz NMR instrument with TMS as an internal standard. Mass spectra were obtained using a XEVO G2-XS QTOF (LC-MS) spectrometer. An Agilent Technologies Cary Eclipse fluorescence spectrophotometer was used to obtain fluorescence spectra. Absorption spectra were recorded using an Agilent Technology UV-vis spectrophotometer, using quartz cells with a path length of 1.0 cm.Synthesis of the probe HJ-DNP
250 mg of 8-hydroxyjulolidine-9-carboxaldehyde (1.15 mmol) and 228 mg of 2,4-dinitrophenylhydrazine (1.15 mmol) were dissolved in distilled methanol (30 mL) and allowed to react with each other for 24 h under N2 to form an orange precipitate. The reaction was then stopped, and the precipitate was filtered and washed with cold methanol. The solid product was further precipitated in a warm methanol–ethanol mixture and collected as a dark orange-coloured solid, yielding the pure compound HJ-DNP. Yield = 79.6%. 1H-NMR (600 MHz, DMSO-d6): δ (ppm) 15.13 (s, 1H), 9.89 (s, 1H), 9.33 (s, 1H), 8.31 (d, J = 6 Hz, 1H), 8.04 (d, J = 12 Hz, 2H), 7.89 (d, J = 6 Hz, 2H), 7.59 (m, 4H), 7.24 (d, J = 12 Hz, 1H), 7.08 (d, J = 12 Hz, 1H), 6.97 (d, J = 6 Hz, 1H). 13C-NMR (150 MHz, DMSO-d6): δ (ppm) 167.50, 160.04, 152.06, 148.45, 143.67, 142.25, 131.56, 130.59, 129.39, 128.77, 127.38, 125.35, 122.09, 119.32, 118.42, 116.04, 115.83. MS-ES (m/z) C19H19N5O5: calculated, 397.14; found, 398.8412.Binding constant determination
The binding constant (Ka) values of HJ-DNP with different analytes in DMSO–water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) were determined based on Hill plots, using the formula Ka = 1/slope. The slope was obtained via the linear fitting of a plot of the emission or intensity vs. the concentration of analyte.
20) were determined based on Hill plots, using the formula Ka = 1/slope. The slope was obtained via the linear fitting of a plot of the emission or intensity vs. the concentration of analyte.
The calculation of the limit of detection (LOD)
LOD was calculated using the equation (3.3σ/slope), where σ is the standard deviation of the λmax intensity value of the blank probe HJ-DNP. The slope was obtained via the linear fitting of a plot of intensity vs. the analyte concentration.DFT studies
All calculations were carried out using the Gaussian 09 program package.58 The geometries of all compounds were fully optimized and investigated using the density functional theory method (B3LYP) with the 6-311g(d,p) and LANL2DZ basis sets.Conclusions
In summary, a new fluorescent and colorimetric sensor, HJ-DNP, was developed to detect the multiple analytes of Cr3+, arsenate, and PPi ions in aqueous solution. The probe HJ-DNP displayed aggregation-induced emission (AIE) behaviour at 585 nm, with a 10-fold increase in the emission intensity upon increasing the proportion of water in DMSO. The ESIPT mechanism of the probe HJ-DNP was explored via DFT calculations and studying the optimized geometries of HJ-DNP in both keto and enol forms. The probe HJ-DNP could preferentially detect Cr3+ ions over various metal ions via the CHEF mechanism with a turn-on response. The LOD towards Cr3+ ions was calculated to be 3.9 μM. The probe HJ-DNP was also used to detect Cr3+ in the food product tomatoes. Among various anions/oxyanions, HJ-DNP could detect arsenate and pyrophosphate ions via a colour change from pale orange to red. Also, there were changes in the emission spectra. The LOD values toward arsenate and PPi were calculated to be as low as 3.9 μM and 0.19 mM, respectively. The different emission behaviour of HJ-DNP in the presence of PPi and arsenate enabled us to distinguish between these ions in water. The probe HJ-DNP is also incorporated with sodium alginate polymer beads for ready-to-use detection and arsenate removal from water. Lifetime measurements and 1H-NMR titration studies were performed to confirm the proposed binding modes and mechanisms. Furthermore, arsenate detection was carried out in real-life water samples, such as groundwater, pond water, tap water, and rice washing water. It is also evident from the CIE chromaticity plot that the probe HJ-DNP can act as an efficient probe for detecting the multiple analytes of Cr3+, arsenate, and PPi.Author contributions
Srushti Gadiyaram: methodology, investigation, validation. Amrita Ghosh: investigation, validation. Vikas D. Ghule: investigation, validation. D. Amilan Jose: conceptualization, methodology, resources, supervision, funding acquisition, writing: review and editing.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
DAJ thanks DST-SERB, Core Research Grant no. EMR/2017/004085 for financial help.Notes and references
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Fluorescent chemosensors: the past, present and future, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- K. P. Carter, A. M. Young and A. E. Palmer, Fluorescent Sensors for Measuring Metal Ions in Living Systems, Chem. Rev., 2014, 114, 4564–4601 CrossRef CAS PubMed.
- D. Cao, Z. Liu, P. Verwilst, S. Koo, P. Jangjili, J. S. Kim and W. Lin, Coumarin-Based Small-Molecule Fluorescent Chemosensors, Chem. Rev., 2019, 119, 10403–10519 CrossRef CAS.
- T. L. Mako, J. M. Racicot and M. Levine, Supramolecular Luminescent Sensors, Chem. Rev., 2019, 119, 322–477 CrossRef CAS PubMed.
- A. C. Sedgwick, L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents, Chem. Soc. Rev., 2018, 47, 8842–8880 RSC.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: Together We Shine, United We Soar!, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- Y. Chen, J. W. Y. Lam, R. T. K. Kwok, B. Liu and B. Z. Tang, Aggregation-induced emission: fundamental understanding and future developments, Mater. Horiz., 2019, 6, 428–433 RSC.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun., 2001, 1740–1741 RSC.
- J. Zhao, S. Ji, Y. Chen, H. Guo and P. Yang, Excited state intramolecular proton transfer (ESIPT): from principal photophysics to the development of new chromophores and applications in fluorescent molecular probes and luminescent materials, Phys. Chem. Chem. Phys., 2012, 14, 8803–8817 RSC.
- J. E. Kwon and S. Y. Park, Advanced Organic Optoelectronic Materials: Harnessing Excited-State Intramolecular Proton Transfer (ESIPT) Process, Adv. Mater., 2011, 23, 3615–3642 CrossRef CAS.
- V. Bhardwaj, L. Hindocha, S. K. Ashok Kumar and S. K. Sahoo, An aggregation-induced emissive pyridoxal derived tetradentate Schiff base for the fluorescence turn-off sensing of copper(ii) in an aqueous medium, New J. Chem., 2022, 46, 3248–3257 RSC.
- Z. Zhou, Y. Li, W. Su, B. Gu, H. Xu, C. Wu, P. Yin, H. Li and Y. Zhang, A dual-signal colorimetric and near-infrared fluorescence probe for the detection of exogenous and endogenous hydrogen peroxide in living cells, Sens. Actuators, B, 2019, 280, 120–128 CrossRef CAS.
- J. L. Kolanowski, F. Liu and E. J. New, Fluorescent probes for the simultaneous detection of multiple analytes in biology, Chem. Soc. Rev., 2018, 47, 195–208 RSC.
- M. Chhatwal, A. Kumar, V. Singh, R. D. Gupta and S. K. Awasthi, Addressing of multiple-metal ions on a single platform, Coord. Chem. Rev., 2015, 292, 30–55 CrossRef CAS.
- Y. Tu, Y. Yu, D. Xiao, J. Liu, Z. Zhao, Z. Liu, J. W. Y. Lam and B. Z. Tang, An Intelligent AIEgen with Nonmonotonic Multiresponses to Multistimuli, Adv. Sci., 2020, 7, 2001845 CrossRef CAS PubMed.
- V. Kumar, H. Rana, G. Raviraju, P. Garg, A. Baghel and A. K. Gupta, Chromogenic and fluorogenic multianalyte detection with a tuned receptor: refining selectivity for toxic anions and nerve agents, RSC Adv., 2016, 6, 59648–59656 RSC.
- R. Kaushik, R. Sakla, N. Kumar, A. Ghosh, V. D. Ghule and D. A. Jose, Multianalytes Sensing Probe: Fluorescent Moisture Detection, Smartphone Assisted Colorimetric Phosgene recognition and Colorimetric Discrimination of Cu2+and Fe3+ ions, Sens. Actuators, B, 2021, 328, 129026 CrossRef CAS.
- F. Ali, S. Saha, A. Maity, N. Taye, M. K. Si, E. Suresh, B. Ganguly, S. Chattopadhyay and A. Das, Specific Reagent for Cr(III): Imaging Cellular Uptake of Cr(III) in Hct116 Cells and Theoretical Rationalization, J. Phys. Chem. B, 2015, 119, 13018–13026 CrossRef CAS PubMed.
- P. Mahato, S. Saha, E. Suresh, R. Di Liddo, P. P. Parnigotto, M. T. Conconi, M. K. Kesharwani, B. Ganguly and A. Das, Ratiometric Detection of Cr3+ and Hg2+ by a Naphthalimide-Rhodamine Based Fluorescent Probe, Inorg. Chem., 2012, 51, 1769–1777 CrossRef CAS PubMed.
- V. Raju, R. Selva Kumar, S. K. Ashok Kumar, Y. Tharakeswar and S. K. Sahoo, Development of highly selective chemosensor for chomium(III) estimation in aqueous environment, Inorg. Chem. Commun., 2019, 101, 74–80 CrossRef CAS.
- V. K. Gupta, N. Mergu and A. K. Singh, Rhodamine-derived highly sensitive and selective colorimetric and off–on optical chemosensors for Cr3+, Sens. Actuators, B, 2015, 220, 420–432 CrossRef CAS.
- S. Dutta, S. Let, M. M. Shirolkar, A. V. Desai, P. Samanta, S. Fajal, Y. D. More and S. K. Ghosh, A luminescent cationic MOF for bimodal recognition of chromium and arsenic based oxo-anions in water, Dalton Trans., 2021, 50, 10133–10141 RSC.
- Q.-Q. He, S.-L. Yao, T.-F. Zheng, H. Xu, S.-J. Liu, J.-L. Chen, N. Li and H.-R. Wen, A multi-responsive luminescent sensor based on a stable Eu(III) metal-organic framework for sensing Fe3+, MnO4-, and Cr2O72- in aqueous solutions, CrystEngComm, 2022, 24, 1041–1048 RSC.
- T. Hu, L. Wang, J. Li, Y. Zhao, J. Cheng, W. Li, Z. Chang and C. Sun, A new fluorescent sensor L based on fluorene-naphthalene Schiff base for recognition of Al3+ and Cr3+, Inorg. Chim. Acta, 2021, 524, 120421 CrossRef CAS.
- M.-J. Tsai, K.-S. Liao and J.-Y. Wu, A Water-Stable 2-Fold Interpenetrating cds Net as a Bifunctional Fluorescence-Responsive Sensor for Selective Detection of Cr(III) and Cr(VI) Ions, Nanomaterials, 2022, 12, 158 CrossRef CAS PubMed.
- C. Wang, S. Ma, L. Pan, W. Wu, Y. Wei and J. Ou, Cyclized conjugated microporous polymer-coated silica nanospheres as fluorescent sensors for iron (III) and chromium (III), Chem. Eng. J., 2022, 435, 134368 CrossRef CAS.
- X. Wang, Y. Zhang, Z. Shi, T. Lu, Q. Wang and B. Li, Multifunctional Zr-MOF Based on Bisimidazole Tetracarboxylic Acid for pH Sensing and Photoreduction of Cr(VI), ACS Appl. Mater. Interfaces, 2021, 13, 54217–54226 CrossRef CAS PubMed.
- H. Zhao, J. Zhang, X. Lin, H. Li and Q. Pan, Synthesis of 4-dimethylaminobenzyl chrysin ester-Zn fluorescent chemical sensor for the determination of Cr(VI) in water, Luminescence, 2022, 37, 72–81 CrossRef CAS PubMed.
- X. Zheng, S. Ren, L. Wang, Q. Gai, Q. Dong and W. Liu, Controllable functionalization of carbon dots as fluorescent sensors for independent Cr(VI), Fe(III) and Cu(II) ions detection, J. Photochem. Photobiol., A, 2021, 417, 113359 CrossRef CAS.
- N. Mekjinda, S. Phunnarungsi, V. Ruangpornvisuti, R. J. Ritchie, I. Hamachi, A. Ojida and J. Wongkongkatep, Masking Phosphate with Rare-Earth Elements Enables Selective Detection of Arsenate by Dipycolylamine-ZnII Chemosensor, Sci. Rep., 2020, 10, 2656 CrossRef CAS PubMed.
- J. Ma, M. K. Sengupta, D. Yuan and P. K. Dasgupta, Speciation and detection of arsenic in aqueous samples: A review of recent progress in non-atomic spectrometric methods, Anal. Chim. Acta, 2014, 831, 1–23 CrossRef CAS PubMed.
- D. Banik, S. K. Manna and A. K. Mahapatra, Recent development of chromogenic and fluorogenic chemosensors for the detection of arsenic species: Environmental and biological applications, Spectrochim. Acta, Part A, 2021, 246, 119047 CrossRef CAS PubMed.
- W. Paisuwan, T. Lertpiriyasakulkit, V. Ruangpornvisuti, M. Sukwattanasinitt and A. Ajavakom, 8-Hydroxyjulolidine aldimine as a fluorescent sensor for the dual detection of Al3+ and Mg2+, Sens. Bio-Sens. Res., 2020, 29, 100358 CrossRef.
- J. Du, B. Zhao, W. Kan, H. Yin, T. Song, L. Wang, L. Sun, X. Wang, G. Yin and J. Wang, A phenanthrene[9,10-d]imidazole-phenol-based fluorescent probe combining ESIPT and AIE for the “turn-on” detection of Cu2+ with green-emission and improved Stokes' shift, and its application, New J. Chem., 2021, 45, 15415–15422 RSC.
- H. Liu, X. Wang, Y. Xiang and A. Tong, Fluorescence turn-on detection of cysteine over homocysteine and glutathione based on “ESIPT” and “AIE”, Anal. Methods, 2015, 7, 5028–5033 RSC.
- Y. Chen, T. Wei, Z. Zhang, T. Chen, J. Li, J. Qiang, J. Lv, F. Wang and X. Chen, A Benzothiazole-Based Fluorescent Probe for Ratiometric Detection of Al3+ in Aqueous Medium and Living Cells, Ind. Eng. Chem. Res., 2017, 56, 12267–12275 CrossRef CAS.
- J.-C. Qin, B.-D. Wang, Z.-Y. Yang and K.-C. Yu, A ratiometric fluorescent chemosensor for Zn2+ in aqueous solution through an ESIPT coupled AIE process, Sens. Actuators, B, 2016, 224, 892–898 CrossRef CAS.
- X. Wan, H. Liu, S. Yao, T. Liu and Y. Yao, A stimuli-responsive nanogel-based sensitive and selective fluorescent sensor for Cr(3+) with thermo-induced tunable detection sensitivity, Macromol. Rapid Commun., 2013, 35, 323–329 CrossRef PubMed.
- B. Janani, K. M. Alarjani, L. L. Raju, A. M. Thomas, A. Das and S. S. Khan, A potent multifunctional Ag/Co-polyvinylpyrrolidone nanocomposite for enhanced detection of Cr(III) from environmental samples and its photocatalytic and antibacterial applications, Spectrochim. Acta, Part A, 2020, 243, 118766 CrossRef CAS PubMed.
- O. O. Olayinka and A. R. Ipeaiyeda, Chromium and Lead Concentrations in Tomatoes (Solanum Lycopersicum) and Red Peppers (Capsicum Frutescens) Cultivated in Roadside Farmland around High Traffic Density Area of Ibadan, Nigeria, Soil Sediment Contam., 2011, 20, 1–11 CrossRef CAS.
- D. Romero-Estévez, G. S. Yánez-Jácome, K. Simbaña-Farinango, P. Y. Vélez-Terreros and H. Navarrete, Determination of cadmium and lead in tomato (Solanum lycopersicum) and lettuce (Lactuca sativa) consumed in Quito, Ecuador, Toxicol. Rep., 2020, 7, 893–899 CrossRef PubMed.
- S. Dey, S. Sarkar, D. Maity and P. Roy, Rhodamine based chemosensor for trivalent cations: Synthesis, spectral properties, secondary complex as sensor for arsenate and molecular logic gates, Sens. Actuators, B, 2017, 246, 518–534 CrossRef CAS.
- A. S. M. Islam, R. Alam, A. Katarkar, K. Chaudhuri and M. Ali, Di-oxime based selective fluorescent probe for arsenate and arsenite ions in a purely aqueous medium with living cell imaging applications and H-bonding induced microstructure formation, Analyst, 2015, 140, 2979–2983 RSC.
- S. Nandi, A. Sahana, B. Sarkar, S. K. Mukhopadhyay and D. Das, Pyridine Based Fluorescence Probe: Simultaneous Detection and Removal of Arsenate from Real Samples with Living Cell Imaging Properties, J. Fluoresc., 2015, 25, 1191–1201 CrossRef CAS.
- L. Tong, X. Wang, W. Gao, Z. Liu, Z. Chen, G. Cheng, W. Cao, M. Sui and B. Tang, CeO2 Nanowire-BODIPY-Adenosine Triphosphate Fluorescent Sensing Platform for Highly Specific and Sensitive Detection of Arsenate, Anal. Chem., 2018, 90, 14507–14513 CrossRef CAS PubMed.
- S. Lee, K. K. Y. Yuen, K. A. Jolliffe and J. Yoon, Fluorescent and colorimetric chemosensors for pyrophosphate, Chem. Soc. Rev., 2015, 44, 1749–1762 RSC.
- J. Wongkongkatep, A. Ojida and I. Hamachi, Fluorescence Sensing of Inorganic Phosphate and Pyrophosphate Using Small Molecular Sensors and Their Applications, Top. Curr. Chem., 2017, 375, 30 CrossRef PubMed.
- S. Anbu, A. Paul, G. J. Stasiuk and A. J. L. Pombeiro, Recent developments in molecular sensor designs for inorganic pyrophosphate detection and biological imaging, Coord. Chem. Rev., 2021, 431, 213744 CrossRef CAS.
- K. Pramanik, P. Sarkar and D. Bhattacharyay, 3-Mercapto-propanoic acid modified cellulose filter paper for quick removal of arsenate from drinking water, Int. J. Biol. Macromol., 2019, 122, 185–194 CrossRef CAS PubMed.
- J. Das and P. Sarkar, A new dipstick colorimetric sensor for detection of arsenate in drinking water, Environ. Sci.: Water Res. Technol., 2016, 2, 693–704 RSC.
- B. Yuan, J. Qu and Q. Lin, Fluorimetric Determination of Arsenite and Arsenate in Water Using Fluorescein and Iodine, Int. J. Environ. Anal. Chem., 2002, 82, 31–36 CrossRef CAS.
- I. Jung, H. B. Seo, J.-E. Lee, B. Chan Kim and M. B. Gu, A dip-stick type biosensor using bioluminescent bacteria encapsulated in color-coded alginate microbeads for detection of water toxicity, Analyst, 2014, 139, 4696–4701 RSC.
- H. Zhu, Q. Zhang and S. Zhu, Alginate Hydrogel: A Shapeable and Versatile Platform for in Situ Preparation of Metal–Organic Framework–Polymer Composites, ACS Appl. Mater. Interfaces, 2016, 8, 17395–17401 CrossRef CAS PubMed.
- P. Kumar and D. A. Jose, Naked eye detection of moisture in organic solvents and development of alginate polymer beads and test cassettes as a portable kit, Anal. Chim. Acta, 2020, 1136, 178–186 CrossRef CAS PubMed.
- F. Arshad and M. P. Sk, Aggregation-induced red shift in N,S-doped chiral carbon dot emissions for moisture sensing, New J. Chem., 2019, 43, 13240–13248 RSC.
- P. Kumar, S. Gadiyaram and D. A. Jose, Simple Iron(III) Complex Based Highly Sensitive Fluorescent Off-On Sensor for the Detection of Trace Amount of Water in Organic Solvents and Edible Oilseeds, ChemistrySelect, 2020, 5, 10648–10655 CrossRef CAS.
- J.-S. Kim, H.-B. Oh, A. H. Kim, J.-S. Kim, E.-S. Lee, J.-Y. Baek, K. S. Lee, S.-C. Chung and J.-H. Jun, A study on detection of glucose concentration using changes in color coordinates, Bioengineered, 2017, 8, 99–104 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Rev. B.01, Wallingford, CT, 2016 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) availabl. See DOI: https://doi.org/10.1039/d2sd00157h |
| This journal is © The Royal Society of Chemistry 2022 |