Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly improved performance of a film-based fluorescent sensor via a nanomesh scaffold strategy†
Quan
Liu
 *ab,
Meng
Liu
a,
Dong
Li
a,
Kun
Li
a,
Haitao
Xu
a,
Jiufu
Lu
a,
Xianzhao
Shao
a and
Taihong
Liu
*ab,
Meng
Liu
a,
Dong
Li
a,
Kun
Li
a,
Haitao
Xu
a,
Jiufu
Lu
a,
Xianzhao
Shao
a and
Taihong
Liu
 c
c
aShaanxi Province Key Laboratory of Catalytic Foundation and Applications, School of Chemical and Environmental Science, Shaanxi University of Technology, Hanzhong 723001, P. R. China. E-mail: liuq@sunt.edu.cn
bKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, P. R. China
cKey Laboratory of Applied Surface and Colloid Chemistry of Ministry of Education, School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an 710062, P. R. China
First published on 1st November 2021
Abstract
Public safety and environmental monitoring are of great significance to maintain human health. Therefore, a simple and effective strategy for preparing the substrate layer of a film-based fluorescent sensor is developed by a nanomesh scaffold technology. The limit of detection of the nanomesh scaffold-modified sensor ranges from 4.8 ppb to 3750 ppm. This can provide an effective method for detecting potential biological warfare agent, 2,6-dicarboxyridine.
Film-based fluorescent sensing technology is mainly realized through the interaction between the analyte and fluorescent molecules in the excited state.1 It has attracted wide attention due to its high sensitivity, good selectivity, easy device, miniaturization, and reusability.2 More importantly, film-based fluorescent sensors can also be manufactured in a roll-to-roll manner, which enables the combination of different sensing modes to be used in one array. Film-based fluorescent sensors are a promising candidate to meet the requirements of complex samples and high-throughput detection.3 These sensors have potential application value in the fields of complex samples and high-throughput detection, such as breath diagnosis, explosive detection, drug detection, indoor environment monitoring and precision agriculture.4
Using sensing molecules with specific response is one of the main strategies to prepare new film-based fluorescent sensors. A large number of research studies have focused on the structure and property study of fluorescent molecules.5 The other important strategy is to change or modify the substrate structure used in the film-based fluorescent sensing. Fang et al. found that the sensing performance could be adjusted by changing the substrate of different materials. They used a pyrene bisimide (PBI) derivative as a fluorescent active substance to detect illegal drugs.6 They found that the fluorescence intensity significantly quenched when using an ordinary smooth glass plate and polyvinyl chloride plate as the substrate. When a silica gel plate was used as the substrate, the fluorescent intensity of the film hardly changed in the presence of drug vapor. This shows that different substrate structures have huge impact on the sensing performance. Cheng et al. used ordered assembly arrays of zinc oxide (ZnO) nanorods as a film-based fluorescent sensing substrate to detect triacetone triperoxide (TATP) explosives.7 Compared with the planar substrate, the ordered assembly arrays of ZnO nanorods improve the mass transfer of the analyte, resulting in significantly enhanced fluorescence emission, improved quenching efficiency and extended service life.
The above-mentioned examples clearly illustrate that the diffusion and adsorption speed of the analyte in the substrate directly affect the interaction between the analyte and the fluorescent molecule. The specific mass transfer process can be simulated by the theoretical model shown in Scheme 1a. In the process of film-based fluorescent sensing, VOCs will first diffuse to the surface of the film, and then adsorb on the surface of the film, affecting the fluorescence properties of fluorescent active substances. VOC molecular diffusion flux plays a major role in the sensing process. Larger the diffusion flux is, higher the mass transfer efficiency will be, which indicates that the contact probability between fluorescent molecules and VOCs will be significantly improved, thus improving the sensing performance.8 Referring to the Fang's group previous research reports by constructing non-planar porous structures to improve the diffusion flux.9 We consider designing of the substrate layer has a bowl cavity can effectively to support fluorescent molecules on the surface (Scheme 1b), the maximum enhances fluorescence molecular contact area, at the same time ensure VOCs in longitudinal diffusion under the condition of constant flux, using fluorescent molecules at the bottom of the cavity further upward diffusion flux formation, the overall ascension VOCs response sensitivity.
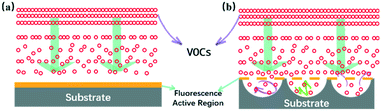 | ||
| Scheme 1 Schematic of the VOC mass transfer process at the interface of the ground glass-based sensor (a) and the nanomesh scaffold modified sensor (b). | ||
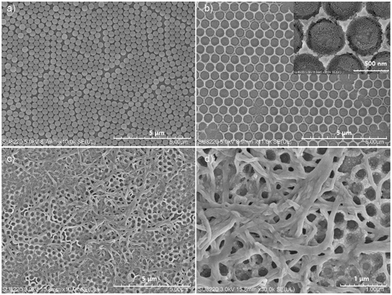
In this report, the nanomesh scaffold technology was used to prepare a film-based fluorescence sensing substrate with an ordered bowl cavity substrate layer.10 The nanomesh scaffold-modified film-based fluorescent sensor can be fabricated as per Scheme 2. The specific preparation steps can be seen in the Supporting Information. First, polystyrene (PS) spheres with a diameter of 300 nm were dispersed on the liquid surface to obtain PS colloidal sphere monolayer templates. The PS monolayer was transferred to a round glass substrate (Fig. 1a), and then the spin-coating method was used to fill silica gel into the void of the glass substrate covered by a PS sphere monolayer on the surface. It is worth noting that the bowl cavity with different hole depths can be prepared by selecting different concentrations of silica gel. Then, the substrate was immersed in a toluene solution to dissolve the PS sphere templates and endow the substrate with bowl-like cavity (Fig. 1b, S1 and S2†). Compared with the traditional ground glass-base substrate (Fig. S6†), the nanomesh scaffold-modified substrate with a neat and orderly microstructure is conducive to the mass transfer process of VOCs.
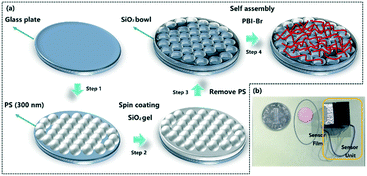 | ||
| Scheme 2 (a) Schematic of the procedures for fabricating nanomesh scaffold-modified film-based fluorescent sensor; (b) physical drawing of the sensor and sensing unit. | ||
Due to the good photophysical properties of PBI, N,N′-bis(2-ethylhexyl)-1-bromoperylene-3,4,9,10-tetracarboxylic acid diimide (PBI–Br) was synthesized and selected as the fluorescent active material.11 The synthesis and preparation of one-dimensional assembly of PBI–Br can be seen in the Supporting Information. The assembled PBI–Br was transferred to the nanomesh scaffold substrate surface to obtain the film-based fluorescent sensor. As shown in Fig. 1c and d, it is apparent that the PBI–Br assembly is evenly distributed on the surface of the nanomesh scaffold-modified substrate.
Pyridine derivatives, common environmental pollutants in daily life, were selected as the detection object to evaluate the sensing performance.12 The home-made sensing platform was used according to the protocol reported previously.13 The detailed experimental procedures can be seen in the ESI.†
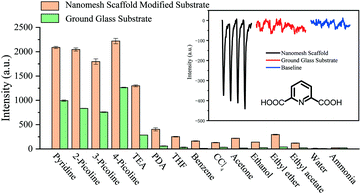
As we all know, the film thickness has a great influence on the sensing performance. Two kinds of the nanomesh scaffold-modified substrate with different hole depths (200 nm and 300 nm) were obtained by optimizing the concentration of silica gel. Pyridine and its derivatives (2-picoline, 3-picoline and 4-picoline) were diluted to the same concentration for the sensing test. The sensing results show that the substrate layer with deeper holes has higher response intensity, which may be due to the deeper holes more effectively improving the diffusion flux of VOCs (Fig. S9†). Therefore, the nanomesh scaffold-modified substrate with a greater hole depth (300 nm) was selected for further sensing experiments. Also, it was found that the nanomesh scaffold-modified sensor had more sensitive response intensity than the ground glass-based sensor (Fig. S7 and S8†). Specifically, the responses intensity of the nanomesh scaffold-modified sensor towards pyridine and its derivatives increased by 180% to 250% (Fig. 2).
Notably, the nanomesh scaffold-modified sensor can sense biomarker of anthrax spores (2,6-dicarboxyridine, PDA), which is a highly lethal agent to human beings and animals and also potential biological warfare agents.14 The nanomesh scaffold-modified sensor has a significant response towards saturated vapor of PDA (cPDA = 2.8 ppt), and the response intensity is seven times stronger than that of the ground glass-based sensor (inset of Fig. 2). The simulated field tests were carried out for evaluating the practical applicability of the nanomesh scaffold-modified sensor (Fig. S10†). The results show that the sensor can detect PDA accurately.
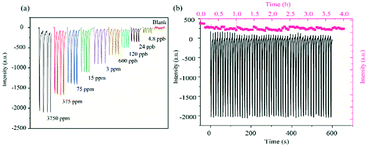
The limit of detection (LOD) and stability of the nanomesh scaffold-modified sensor were further tested (Fig. 3a). The pyridine saturated vapor was diluted to different concentrations and injected to the sensor chamber to test the response intensity. It can be seen that the LOD of the nanomesh scaffold-modified sensor for pyridine ranges from 4.8 ppb to 3750 ppm. The nanomesh scaffold-modified sensor can detect the concentration of pyridine to as low as 4.8 ppb, which is lower than that of the glass-based sensor (24 ppb) (Fig. S11a†). By comparing with the reported literature (Table S1†), it is found that the sensor reported in this study has the lowest detection limit for pyridine.
The excellent re-usability of the nanomesh scaffold-modified sensor was proved by the results from more than 50 cycles of continuous tests with pyridine (cpyridine = 3750 ppm) (Fig. 3b). In addition, the signal strength of the nanomesh scaffold-modified sensor did not show significant attenuation after 4 h of continuous testing. This shows that the nanomesh scaffold-modified sensor has good photochemical stability. Compared with the glass substrate sensor, the nanomesh scaffold-modified sensor has less observable attenuation for both the reversibility and signal intensity (Fig. S11b†).
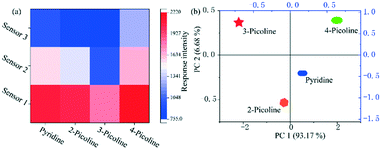
To distinguish the pyridine and its derivatives is of great practical significance for the specific detection of a certain target. Based on this, the sensors based on different substrates (with 200 nm and 300 nm hole depths and glass) are combined into the sensing array, and the distinguish detection can be realized through pattern recognition algorithm (PCA) of the response signal difference. As shown in Fig. 4a, the hot plots of the response intensities of the film-based devices towards pyridine and its derivatives resulted in an easily distinguishable pattern. Further, PCA is used to analyze the response signals of pyridine and its derivatives in different sensors (Fig. 4b). The PCA score plot as generated shows a clear clustering of the data using the first principal component (PC1) and the second principal component (PC2), where PC1 carries about 93.17% of the variance and PC2 carries 6.68%, demonstrating the strong discriminating capability of the nanomesh scaffold-modified sensor array.
Conclusions
A new type of film-based fluorescent sensor was constructed by the nanomesh scaffold technology. This technology is conducive to the contact between analytes and fluorescent molecules, which greatly improves the diffusion flux. The LOD of the nanomesh scaffold-modified sensor for pyridine ranges from 4.8 ppb to 3750 ppm. In particular, the nanomesh scaffold-modified sensor can be applied to detect PDA, a high-risk biological warfare agent. More importantly, this strategy can easily form a sensing array to realize distinguish detection of pyridine and its derivatives. It is expected to be an ideal choice for the detection and analysis of trace chemicals in public safety and environmental monitoring in the future.We thank the Natural Science Basic Research Program of Shaanxi (2021KJXX-52), the Open Fund of Key Laboratory of Organic Synthesis of Jiangsu Province (KJS2010), the Key Research and Development Program of Shaanxi (2020FP-026) and the Scientific Research Plan Projects of Shaanxi Education Department (20JS014).
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Chen, P. Slattum, C. Wang and L. Zang, Chem. Rev., 2015, 115, 11967–11998 CrossRef CAS PubMed.
- R. Miao, J. Peng and Y. Fang, Langmuir, 2017, 33, 10419–10428 CrossRef CAS PubMed.
- Q. Liu, T. Liu and Y. Fang, Langmuir, 2020, 36, 2155–2169 CrossRef CAS PubMed.
- (a) X. Jiang, H. Gao, X. Zhang, J. Pang, Y. Li, K. Li, Y. Wu, S. Li, J. Zhu, Y. Wei and L. Jiang, Nat. Commun., 2018, 9, 3799 CrossRef PubMed; (b) A. Jalal, F. Alam, S. Roychoudhury, Y. Umasankar, N. Pala and S. Bhansali, ACS Sens., 2018, 3, 1246–1263 CrossRef CAS PubMed; (c) Y. Fu, W. Xu, Q. He and J. Cheng, Sci. China: Chem., 2015, 35, 3–15 Search PubMed; (d) T. Minami, N. A. Esipenko, A. Akdeniz, B. Zhang, L. Isaacs and P. Anzenbacher, J. Am. Chem. Soc., 2013, 135, 15238–15243 CrossRef CAS PubMed; (e) Z. Jiao, Y. Zhang, W. Xu, X. Zhang, H. Jiang, P. Wu, Y. Fu, Q. He, H. Cao and J. Cheng, ACS Sens., 2017, 2, 687–694 CrossRef CAS PubMed; (f) D. Sun, L. Huang, H. Pu and J. Ma, Chem. Soc. Rev., 2021, 50, 1070–1110 RSC.
- F. Wurthner, C. R. Saha-Moller, B. Fimmel, S. Ogi, P. Leowanawat and D. Schmidt, Chem. Rev., 2016, 116, 962–1052 CrossRef PubMed.
- K. Liu, C. Shang, Z. Wang, Y. Qi, R. Miao, K. Liu, T. Liu and Y. Fang, Nat. Commun., 2018, 9, 1695 CrossRef PubMed.
- (a) C. He, D. Zhu, Q. He, L. Shi, Y. Fu, D. Wen, H. Caoand and J. Cheng, Chem. Commun., 2012, 48, 5739–5741 RSC; (b) D. F. Zhu, Q. Chen, Y. Y. Fu, C. He, L. Q. Shi, X. Meng, C. M. Deng, H. M. Cao and J. G. Cheng, ACS Nano, 2011, 5, 4293–4299 CrossRef CAS PubMed.
- K. Luan, R. Meng, C. Shan, J. Cao, J. Jia, W. Liu and Y. Tang, Anal. Chem., 2018, 90, 3600–3607 CrossRef CAS PubMed.
- (a) H. Peng, L. Ding, T. Liu, X. Chen, L. Li, S. Yin and Y. Fang, Chem. – Asian J., 2012, 7, 1576–1582 CrossRef CAS PubMed; (b) G. Wang, X. Chang, J. Peng, K. Liu, K. Zhao, C. Yu and Y. Fang, Phys. Chem. Chem. Phys., 2015, 17, 5441–5449 RSC; (c) M. He, H. Peng, G. Wang, X. Chang, R. Miao, W. Wang and Y. Fang, Sens. Actuators, B, 2016, 227, 255–262 CrossRef CAS; (d) J. Zhang, K. Liu, Z. Liu, Z. Wang, C. Hua, T. Liu and Y. Fang, ACS Appl. Mater. Interfaces, 2021, 13, 5625–5633 CrossRef CAS PubMed; (e) Y. Qi, W. Xu, N. Ding, X. Chang, C. Shang, H. Peng, T. Liu and Y. Fang, Mater. Chem. Front., 2019, 3, 1218–1224 RSC.
- (a) X. Zhong, E. Pavlica, S. Li, A. Klekachev, G. Bratina, T. Ebbesen, E. Orgiu and P. Samori, Nat. Nanotechnol., 2016, 11, 900–906 CrossRef PubMed; (b) L. Zhang, E. Pavlica, X. Zhong, F. Liscio, S. Li, G. Bratina, E. Orgiu and P. Samori, Adv. Mater., 2017, 29, 1605760 CrossRef PubMed; (c) C. Gu, N. Hosono, J. Zheng, Y. Sato, S. Kusaka, S. Sakaki and S. Kitagawa, Science, 2019, 363, 387–391 CrossRef CAS PubMed.
- Z. Zhou, W. Xiong, Y. Zhang, D. Yang, T. Wang, Y. Che and J. Zhao, Anal. Chem., 2017, 89, 3814–3818 CrossRef CAS PubMed.
- (a) G. G. Huang, C. J. Lee, J. Yang, C. H. Chang, M. Sathiyendiran, Z. Z. Lu and K. L. Lu, ACS Appl. Mater. Interfaces, 2016, 8, 35634–35640 CrossRef CAS PubMed; (b) W. Chen, S. A. Elfeky, Y. Nonne, L. Male, K. Ahmed, C. Amiable, P. Axe, S. Yamada, T. D. James, S. D. Bull and J. S. Fossey, Chem. Commun., 2011, 47, 253–255 RSC; (c) Y. Xie, W. Cheng, B. Jin, C. Liang, Y. Ding and W. Zhang, Analyst, 2018, 143, 5583–5588 RSC; (d) S. Soorkia, C. A. Taatjes, D. L. Osborn, T. M. Selby, A. J. Trevitt, K. R. Wilsone and S. R. Leonew, Phys. Chem. Chem. Phys., 2010, 12, 8750–8758 RSC.
- Z. Wang, G. Wang, X. Chang, K. Liu, Y. Qi, C. Shang, R. Huang, T. Liu and Y. Fang, Adv. Funct. Mater., 2019, 29, 1905295 CrossRef CAS.
- L. Liu, W. Xiong, L. Cui, Z. Xue, C. Huang, Q. Song, W. Bai, Y. Peng, X. Chen, K. Liu, S. Zhang, L. Wen, Y. Che and T. Wang, Angew. Chem., 2020, 59, 15953–15957 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sd00016k |
| This journal is © The Royal Society of Chemistry 2022 |