Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enabling Suzuki–Miyaura coupling of Lewis-basic arylboronic esters with a nonprecious metal catalyst†
Michael C.
Haibach
 *a,
Andrew R.
Ickes
a,
Sergei
Tcyrulnikov
b,
Shashank
Shekhar
a,
Sebastien
Monfette
b,
Rafal
Swiatowiec
a,
Brian J.
Kotecki
a,
Jason
Wang
a,
Amanda L.
Wall
a,
Rodger F.
Henry
a and
Eric C.
Hansen
b
*a,
Andrew R.
Ickes
a,
Sergei
Tcyrulnikov
b,
Shashank
Shekhar
a,
Sebastien
Monfette
b,
Rafal
Swiatowiec
a,
Brian J.
Kotecki
a,
Jason
Wang
a,
Amanda L.
Wall
a,
Rodger F.
Henry
a and
Eric C.
Hansen
b
aProcess Research and Development, AbbVie Inc., North Chicago, Illinois 60064, USA. E-mail: michael.haibach@abbvie.com
bPfizer Chemical Research and Development, Pfizer Inc., Groton, Connecticut 06340, USA
First published on 21st October 2022
Abstract
The high cost and negative environmental impact of precious metal catalysts has led to increased demand for nonprecious alternatives for widely practiced reactions such as the Suzuki–Miyaura coupling (SMC). Ni-catalyzed versions of this reaction have failed to achieve high reactivity with Lewis-basic arylboron nucleophiles, especially pinacolboron esters. We describe the development of (PPh2Me)2NiCl2 as an inexpensive and air-stable precatalyst that addresses this challenge. Under activation by n-BuMgCl, this complex can catalyze the coupling of synthetically important heteroaryl pinacolborons with heteroaryl halides. Mildly basic conditions (aqueous K3PO4) allow the reaction to tolerate sensitive functional groups that were incompatible with other Ni-SMC methods. Experimental and computational studies suggest that catalyst inhibition by substitution of PPh2Me from Ni(II) intermediates by Lewis basic reactants and products is disfavored relative to more commonly employed ligands in the Ni-SMC, which allows it to operate efficiently in the presence of Lewis bases such as unhindered pyridines.
Introduction
The Pd-catalyzed Suzuki–Miyaura Coupling (Pd-SMC) of aryl halides and arylboron reagents is one of the most practical catalytic methods for C(sp2)–C(sp2) bond formation.1–3 In the pharmaceutical industry, it ranks among the top 2–3 most widely practiced reactions in both medicinal and process chemistry groups.4,5 Many ligands have been identified for the Pd-SMC; however, fewer ligands for catalysis by earth-abundant metals (Ni, Cu, Co and Fe) have been developed. Given the increased emphasis on greener and more sustainable catalysis, non-precious metal alternatives for the Pd-SMC are being actively sought.6The Ni-catalyzed Suzuki–Miyaura Coupling (Ni-SMC), known since the mid-1990s, is a promising alternative to Pd-SMC and even has its advantages (Scheme 1).7–9 For example, aryl electrophiles normally unreactive under Pd catalysis, such as carbamates, can be coupled with Ni.10 A key challenge to the wider application of the Ni-SMC, however, is the significantly reduced scope of several key heteroarylboron nucleophiles with Ni vs. Pd.11 Lewis-basic heteroarylborons such as pyridines are particularly problematic for Ni, possibly due to their coordinating ability.12–14 Furthermore, aryl BPins were the least reactive of the common SMC nucleophiles examined in a study by Percec.15
Complexes of other nonprecious metals besides Ni have also been reported as catalysts for the SMC. Fe16,17 and Co-catalyzed18,19 couplings of arylboronic esters with alkyl halides have recently been developed. Co catalysts have also been reported for the coupling of arylboronic esters with aryl halides.20–23 These examples rely on strong bases (alkyllithiums, lithium amides, or lithium alkoxides) to activate the arylboron reagent, limiting their application with base or nucleophile-sensitive functional groups. An interesting exception to the strong base requirement is the Cu-SMC reported by Giri, which can couple arylboronic esters in the presence of CsF at 120 °C.24 In the Fe, Co, and Cu systems, heterocyclic or Lewis-basic arylboron nucleophiles are also not well-precedented.
For these reasons, nonprecious metal catalyzed analogues of the Pd-SMC remain mostly limited to the preparation of biaryl compounds with limited functional groups. The Ni-SMC has rarely been used to prepare heterobiaryls, which are highly relevant in the synthesis of bioactive molecules.6 Given our interest in more cost-effective and sustainable catalysts for cross-coupling reactions,25–27 the authors at AbbVie and Pfizer desired to develop a general and economical Ni-SMC that addressed these drawbacks. Here we report the identification of a simple inexpensive ligand, PPh2Me, that enables the Ni-SMC of an unprecedented range of heterocyclic and Lewis-basic BPins and trifluoroborate salts. The reaction conditions tolerate base-sensitive functional groups such as methyl esters, arylsulfonamides, and unprotected indoles. For several substrates, we observe catalyst performance on par with the best reported Pd catalysts. The origin of the high activity of this catalytic system was also investigated by experimental and computational approaches.
Results and discussion
Catalyst optimization
At the outset of our research, Ni-SMCs of the important pyridine-3-BPin 1 were unknown with (hetero)aryl halides, and had only been reported in a few cases with specialized electrophiles including vinyl sulfones and amides.28–30 The absence of successful precedents and the limited mechanistic understanding of the Ni-SMC of pyridylborons make rational ligand design challenging. Hence, we turned to high-throughput experimentation (HTE)31–33 to identify potential leads for a model reaction (Scheme 2).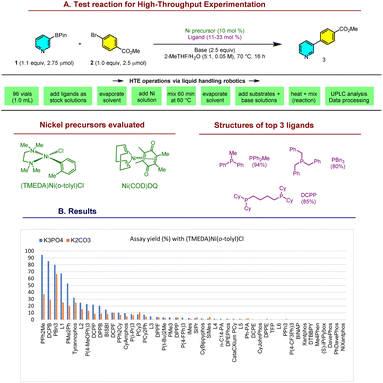 | ||
| Scheme 2 Catalyst discovery by HTE on a bespoke ligand library. See ESI† for structures of all ligands. | ||
Screening the coupling of 1 with methyl 4-bromobenzoate 2 against a bespoke library of 48 phosphine, nitrogen, and NHC ligands with (TMEDA)Ni(o-tolyl)Cl34,35 as the Ni precursor afforded promising leads: PBn3 (80% yield), DCPB36 (85%) and PPh2Me (94%). Other interesting trends were apparent from the data. Reactions with K3PO4 were typically more effective than K2CO3, although this difference was smaller with PBn3. Commonly applied ligands for the Ni-SMC were ineffective for this coupling, highlighting the challenge posed by Lewis-basic arylborons such as 1 for Ni:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPF (<5%), DPEPhos (<5%), PCy3 (11%), and PPh3 (<5%). The same screen was also conducted with Ni(COD)DQ37 as the Ni precursor, however this resulted in much lower assay yields (0–17%). We selected the highest yielding, readily available PPh2Me for further development.
DPPF (<5%), DPEPhos (<5%), PCy3 (11%), and PPh3 (<5%). The same screen was also conducted with Ni(COD)DQ37 as the Ni precursor, however this resulted in much lower assay yields (0–17%). We selected the highest yielding, readily available PPh2Me for further development.
Based on the reported reactivity of (TMEDA)Ni(o-tolyl)Cl,34,35 we expected that ligand substitution with PPh2Me was initially generating (PPh2Me)2Ni(o-tolyl)Cl in the HTE experiment. Indeed, when used as the catalyst for the coupling of 1 and 2, 3 mol% (PPh2Me)2Ni(o-tolyl)Cl38,39 afforded promising conversion (82%) after 16 h (Table 1, entry 2). While both (PPh2Me)2Ni(o-tolyl)Cl and (TMEDA)Ni(o-tolyl)Cl are commercially available, they are currently more costly than common Pd sources such as Pd(OAc)2 and Pd2(dba)3. Hence, we pursued the inexpensive and easily prepared (PPh2Me)2NiCl2 (Ni-1) as a catalyst precursor. The catalyst generated from Ni-1, PPh2Me and n-BuMgCl as an activator afforded 95% conversion of 2 to 3 after 16 h (entry 1). Omitting the in situ activation leads to lower conversion and the observation of significant amounts of byproducts (entries 3–4). Zn and n-BuLi could also be employed as in situ activators40–42 of Ni-1, though conversion was slightly lower than with n-BuMgCl (entries 5–6).
| Entry | Change from conditions shown | %Convb |
|---|---|---|
| a Reactions were conducted on 1.0 mmol scale, 0.4 M in the organic solvent. [Ni-1], PPh2Me and n-BuMgCl were added to 2-MeTHF prior to addition of water and substrates. b LC area %conversion to 3, 100 *area product/(total area of product, limiting reagent and relevant impurities). c Catalyst activation carried out in 2-MeTHF (0.1 v/v vs. t-AmOH). d Isolated yield. | ||
| 1 | None | 95 |
| 2 | (PPh2Me)2Ni(o-tolyl)Cl (3 mol%) as catalyst | 82 |
| 3 | No added PPh2Me | 22 |
| 4 | No added PPh2Me, no n-BuMgCl | 38 |
| 5 | Zn instead of n-BuMgCl | 86 |
| 6 | n-BuLi instead of n-BuMgCl | 86 |
| 7 | 2-MeTHF/H2O 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v 1 v/v |
92 |
| 8 | 2-MeTHF/H2O 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v 1 v/v |
93 |
| 9 | 2-MeTHF solvent (no added H2O) | 97 |
| 10 | Dioxane instead of 2-MeTHF | 98 |
| 11c | t-AmOH instead of 2-MeTHF | 87 |
| 12 | Toluene instead of 2-MeTHF | 15 |
| 13 | K2HPO4 instead of K3PO4 | 2 |
| 14 | K2CO3 instead of K3PO4 | 6 |
| 15 | 1.4 equiv. 1 | >99 (91)d |
Many reported Ni-SMC methods are either sensitive to adventitious moisture or require careful control of H2O stoichiometry. Gratifyingly, the reaction proceeded efficiently with no added H2O through 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ratio of 2-MeTHF/H2O (entries 7–9). We chose 5
1 v/v ratio of 2-MeTHF/H2O (entries 7–9). We chose 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v 2-MeTHF/H2O for subsequent evaluation due to the slightly higher conversion obtained at this ratio. The conditions with less H2O may be useful for specific applications (i.e., a water-sensitive substrate). Dioxane and t-AmOH were also effective solvents (entries 10 and 11). Weaker bases K2CO3 and K2HPO4 were less effective than K3PO4 (entries 13 and 14).
1 v/v 2-MeTHF/H2O for subsequent evaluation due to the slightly higher conversion obtained at this ratio. The conditions with less H2O may be useful for specific applications (i.e., a water-sensitive substrate). Dioxane and t-AmOH were also effective solvents (entries 10 and 11). Weaker bases K2CO3 and K2HPO4 were less effective than K3PO4 (entries 13 and 14).
Scope of the reaction
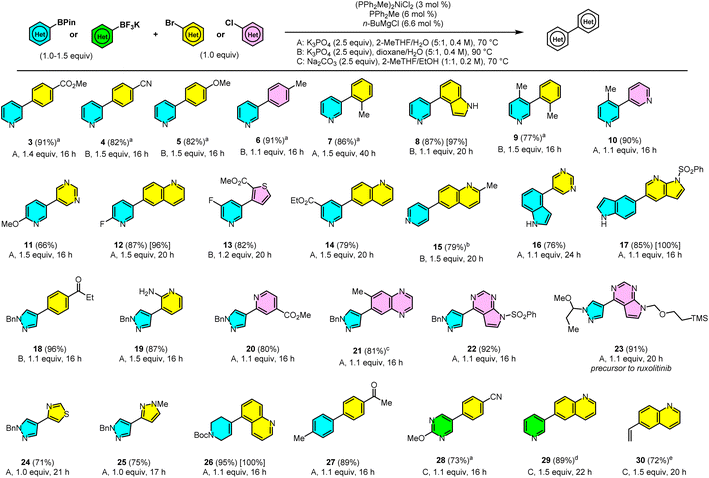
With optimized conditions in hand, we explored the scope of the reaction catalyzed by 3 mol% Ni-1 (Scheme 3). The coupling of 1 proceeds efficiently with a range of aryl halides at 70 °C (in 2-MeTHF, bp = 80 °C) or 90 °C (in dioxane, bp = 101 °C). Electron-poor (3 and 4), electron-rich (5 and 6), and ortho-substituted (7 and 9) aryl bromides afforded the coupled products in excellent yields. Impressively, an electronically neutral aryl chloride (6), an unprotected bromoindole (8), and Lewis-basic 3-chloropyridine (10) also coupled in high yield.In addition to the electron-rich pyridine-3-BPin (11), the previously unreported electron-poor pyridine-3-BPins coupled with heteroaryl halides (12–14). Even the less nucleophilic pyridine-4-BPin, an unknown substrate for the Ni-SMC and a challenging one even for the Pd-SMC, coupled efficiently at 90 °C using 5 mol% catalyst (15). A functionalized chlorothiophene was well tolerated as an electrophile (13).
Unprotected indoles are another class of heterocycles previously unreported in the Ni-SMC and were shown to be problematic in Watson's study of DPPF-Ni.11 Examples 8, 16 and 17 show the efficient coupling of unprotected indoles as both nucleophile and electrophile components. Compound 17 also demonstrates the viability of a derivatized 7-azaindole as an electrophile, a motif found in several pharmaceuticals.43 Methyl/ethyl esters (3, 13, 14, and 20), 2-fluoropyridine (12) and heterocyclic sulfonamides (17 and 22) were well-tolerated, due to the mildly basic conditions of the reaction.
Five-membered Lewis-basic arylborons represent another important class of nucleophiles that is typically difficult for the Ni-SMC. The Ni-SMC of (hetero)aryl halides with pyrazoleborons was previously unreported. Examples 18–25 show the coupling of a pyrazole-4-BPin with functionalized heteroaryl halides at 70 °C, including an unprotected aminopyridine (18). The pyrazolodiazaindole core of several classes of kinase inhibitors was efficiently generated in 22 and 23.44,45 Diazaindole 22 is an intermediate in an enantioselective synthesis of ruxolitinib reported by Lin and coworkers at Incyte.46 Examples 24 and 25 are notable examples of coupling two five-membered rings, which is typically more challenging than 6-membered rings for both Pd and Ni.47,48 An N-Boc containing vinyl BPin coupled to form 26 in excellent yield at 70 °C. This type of Suzuki coupling is used extensively in pharma to access 4-arylpiperidine derivatives yet was previously unknown with Ni.49 Heteroaryl or Lewis-basic substrates are not required for the catalyst to operate efficiently, as shown by the coupling to form 27.
Excitingly, Ni-1 could also efficiently couple potassium organotrifluoroborates under slightly modified conditions. Both Lewis-basic heteroaryl and vinyl BF3K derivatives coupled efficiently at 70–90 °C in the presence of Na2CO3/EtOH/2-MeTHF50 (26–28). These examples further highlight the flexibility of this catalytic system to proceed under mild conditions.
While the scope of the reaction was explored with 3 mol% Ni-1, examples 8, 12, 17 and 26 were also conducted on gram scale with 1 mol% Ni-1/2 mol% PPh2Me/2.2 mol% n-BuMgCl. The reactions afforded excellent assay yields in 16–24 h without any modification of the general reaction conditions, demonstrating the amenability of this system to large-scale applications. For general couplings of the arylboron nucleophile in 24, the lowest reported catalyst loading with Pd was 1 mol%.51,52 In the case of the arylboron in 11, it was 2 mol% with Pd.53 Thus, our Ni catalyst can achieve efficiency on par with Pd catalysts for these more challenging substrates! Overall, the scope of the Ni-SMC catalyzed by Ni-1 shows broad Lewis base and functional group tolerance, while in many cases requiring only 1.1 equiv. of the aryl-BPin to afford high yield.
Characterization of the activated precatalyst
As noted earlier, Ni-1/PPh2Me/n-BuMgCl showed activity comparable to (PPh2Me)2Ni(o-tolyl)Cl or PPh2Me/(TMEDA)Ni(o-tolyl)Cl in our optimization studies. NMR and X-ray analysis of Ni-1/PPh2Me/n-BuMgCl confirmed clean and rapid formation of (PPh2Me)4Ni, a compound known for over 50 years54 but with few prior catalytic applications.55–57 Isolated (PPh2Me)4Ni was found to be a competent precatalyst in the Ni-SMC but had limited stability on the bench, thus in situ generation from Ni-1 is preferred.Mechanistic studies
We were intrigued by the high activity afforded by the relatively simple PPh2Me ligand in the Ni-SMC of Lewis-basic arylboron esters, a reaction where many well-established ligands for Ni did not perform well (see Scheme 2). The most obvious challenge posed by the coupling of substrates such as 1 is their ability to coordinate to Ni, potentially displacing phosphine ligands. Indeed phosphine substitution by Lewis basic amines, including pyridine, was studied by Mizoroki and Nakamura using the related (PPh3)2Ni(o-tolyl)Br complex over forty years ago.58–60 Given this precedent, we wondered if the unique ability of (PPh2Me)2NiCl2 in the SMC stems from a reduced tendency of the PPh2Me ligand to be substituted by Lewis basic donors.To test this hypothesis, we first investigated the non-heterocyclic Ni-SMC shown in Scheme 4A. With PCy3, PPh3, PPh2Me, or PBn3, the reaction proceeded to 59–98% assay yield after 16 h. When 100 mol% pyridine was added, however, the reactions catalyzed by PCy3 and PPh3 (inactive with 1) were significantly inhibited, as expected, while PPh2Me and PBn3 (active with 1) were unaffected.61 While consistent with our hypothesis that catalyst derived from (PPh2Me)2NiCl2 could be resistant to phosphine displacement, we explored this in more detail through 31P NMR studies.
 | ||
| Scheme 4 Mechanistic experiments on the role of Lewis bases in the Ni-SMC. aSee ESI† for details. | ||
Thus we prepared oxidative addition complexes (PPh2Me)2Ni(o-tolyl)Br and (PPh3)2Ni(o-tolyl)Br to probe the extent of phosphine substitution. We chose these complexes as (1) the ortho substituent renders them isolable as well as air-stable, and (2) 2-bromotoluene is a catalytically relevant aryl halide with PPh2Me (Scheme 3, compounds 7 and 9). Complex (PPh3)2Ni(o-tolyl)Br reacted with 2 equiv. 1 at 50 °C to generate a new downfield species in 31P NMR that is consistent with previously reported (PPh3)(pyridine)Ni(o-tolyl)Br complexes (Scheme 4B).60 By contrast, heating (PPh2Me)2Ni(o-tolyl)Br with 2 equiv. 1 at 70 °C for 24 h produced no new 31P NMR peaks. Hence, substitution of PPh2Me by pyridines is disfavored relative to PPh3. The extent of substitution of PPh3 is significant even with only 2 equiv. of 1 relative to the Ni complex. Under typical catalytic conditions (37–47 equiv. 1 per Ni) the substitution would be expected to proceed much further.62 Taken together, these experiments show that addition of a Lewis basic donor such as 1 or pyridine leads to significant substitution of the phosphine ligands, which could lead to off-cycle species and thus slow or inhibit the desired SMC.
In Mizoroki and Nakamura's ligand substitution studies, sterics were the primary determinant of substitution equilibrium between PPh3 and other ligands. Less-hindered amines and smaller cone angle phosphines displaced PPh3 to a greater extent than larger or more hindered ones. When steric effects were held constant, however, higher basicity of the incoming ligand led to greater displacement of PPh3. Thus, under typical reaction conditions for the SMC, we should expect a potentially different degree of substitution of the phosphine ligand as BPin 1 is converted into the biaryl product 3. Indeed, the Lewis basicity of 1 and 3, estimated using computed descriptors and experimental values for related pyridines (Scheme 5) show that 1 is 0.70 units more Lewis basic than 3, which corresponds to a 5-fold greater Keq in coordinating to a Lewis acid. If correct, we would expect the reaction to show a sigmoidal reaction profile consistent with conversion-dependent relief of inhibition. Thus, we profiled the coupling of 1 and 2 by Ni-1 over time (Scheme 4C) and did observe the expected conversion profile. When the same reaction was conducted with a greater excess 1 (3.0 equiv. vs. 1.4 equiv.), the reaction showed overall zero-order behavior over the first 16 h, further evidence of inhibition by 1.63 An aliquot of the reaction with 1.4 equiv. 1 at 80 min (5.5% yield) was analyzed by 31P NMR, and showed 96% uncoordinated PPh2Me, 1% (PPh2Me)4Ni, and 3% of a PPh2Me–Ni complex.64
 | ||
| Scheme 5 Summary of the procedure used to estimate computed Lewis basicities of pyridines 1 and 3. See ESI† for computational details, calibration of linear models and thermodynamic data. | ||
The evidence described above implies that the Lewis basic Ni-SMC is governed by substantial pre-equilibrium steps involving ligand substitution between PPh2Me, 1 and 3 (eqn (1)). While under catalytic conditions ([1] > [PPh2Me]) most of the equilibrium favors the off-cycle species, enough of the on-cycle intermediates are retained with PPh2Me to allow the reaction to proceed efficiently. Indeed, our experiments show that the commonly employed ligands PPh3 and PCy3 are more susceptible to substitution by pyridines than PPh2Me and PBn3. Mizoroki and Nakamura's studies imply that this is primarily a steric effect.
 | (1) |
Because they are minor species in eqn (1), the PPh2Me–Ni intermediates must also be able to promote very efficient on-cycle steps, including the potentially difficult65,66 transmetallation. One implication of this result for future research is to avoid using a large excess of Lewis-basic arylboron reagents when screening Ni-SMCs.
Conclusions
We have developed an effective and inexpensive catalyst system, in situ activated (PPh2Me)2NiCl2, for the Suzuki–Miyaura coupling of Lewis basic heteroaryl BPins and BF3Ks with (hetero)aryl bromides and chlorides. The scope and functional group tolerance of the reaction allow important Lewis-basic and biheteroaryl compounds to be prepared with a nonprecious metal catalyst for the first time. Ligand substitution of PPh2Me by Lewis basic heterocycles is disfavored relative to more commonly studied phosphines, a key reason for its unique catalytic activity. Future research on the precise balance of steric and electronic factors that contribute to this property should lead to the development of even more highly active ligands for Ni-catalyzed cross couplings of Lewis-basic substrates.Author contributions
M. C. H. conceived and directed the study with contributions from S. S. and S. M. M. C. H., A. R. I., and S. S. conducted synthetic experiments. S. T. conducted the computational study. R. S., B. J. K., and J. W. conducted high-throughput experiments. A. L. W. and R. F. H. conducted HRMS and X-ray crystallography, respectively. M. C. H. and S. T. wrote the final draft manuscript. All authors contributed to the data interpretation, manuscript review, and have approved of the final version of the manuscript.Conflicts of interest
AbbVie contributed to the design, approval, and execution of this study. M. C. H., A. R. I., S. S., R. S., B. J. K., J. W., A. L. W., and R. F. H. are current or former AbbVie employees and may own AbbVie stocks.Acknowledgements
The authors thank the participants in the Pharma Alliance at AbbVie, Pfizer and Boehringer Ingelheim for their helpful discussions.Notes and references
- N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437–3440 CrossRef
.
- N. Miyaura, T. Yanagi and A. Suzuki, Synth. Commun., 1981, 11, 513–519 CrossRef CAS
.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS
.
- J. Boström, D. G. Brown, R. J. Young and G. M. Keserü, Nat. Rev. Drug Discovery, 2018, 17, 709–727 CrossRef PubMed
.
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC
.
- M. C. Bryan, P. J. Dunn, D. Entwistle, F. Gallou, S. G. Koenig, J. D. Hayler, M. R. Hickey, S. Hughes, M. E. Kopach, G. Moine, P. Richardson, F. Roschangar, A. Steven and F. J. Weiberth, Green Chem., 2018, 20, 5082–5103 RSC
.
- V. Percec, J.-Y. Bae and D. H. Hill, J. Org. Chem., 1995, 60, 1060–1065 CrossRef CAS
.
- S. Saito, S. Oh-tani and N. Miyaura, J. Org. Chem., 1997, 62, 8024–8030 CrossRef CAS PubMed
.
- A. F. Indolese, Tetrahedron Lett., 1997, 38, 3513–3516 CrossRef CAS
.
- F.-S. Han, Chem. Soc. Rev., 2013, 42, 5270–5298 RSC
.
- M. J. West and A. J. B. Watson, Org. Biomol. Chem., 2019, 17, 5055–5059 RSC
.
- A. J. J. Lennox and G. C. Lloyd-Jones, Chem. Soc. Rev., 2014, 43, 412–443 RSC
.
- S. Ge and J. F. Hartwig, Angew. Chem., 2012, 124, 13009–13013 CrossRef
.
- R. S. Sawatzky and M. Stradiotto, Synlett, 2018, 29, 799–804 CrossRef CAS
.
- N. Zhang, D. J. Hoffman, N. Gutsche, J. Gupta and V. Percec, J. Org. Chem., 2012, 77, 5956–5964 CrossRef CAS PubMed
.
- M. P. Crockett, C. C. Tyrol, A. S. Wong, B. Li and J. A. Byers, Org. Lett., 2018, 20, 5233–5237 CrossRef CAS PubMed
.
- M. P. Crockett, A. S. Wong, B. Li and J. A. Byers, Angew. Chem., Int. Ed., 2020, 59, 5392–5397 CrossRef CAS PubMed
.
- J. R. Ludwig, E. M. Simmons, S. R. Wisniewski and P. J. Chirik, Org. Lett., 2021, 23, 625–630 CrossRef CAS PubMed
.
- L. R. Mills, D. Gygi, J. R. Ludwig, E. M. Simmons, S. R. Wisniewski, J. Kim and P. J. Chirik, ACS Catal., 2022, 12, 1905–1918 CrossRef CAS PubMed
.
- S. Asghar, S. B. Tailor, D. Elorriaga and R. B. Bedford, Angew. Chem., Int. Ed., 2017, 56, 16367–16370 CrossRef CAS PubMed
.
- J. M. Neely, M. J. Bezdek and P. J. Chirik, ACS Cent. Sci., 2016, 2, 935–942 CrossRef CAS PubMed
.
- S. B. Tailor, M. Manzotti, S. Asghar, B. J. S. Rowsell, S. L. J. Luckham, H. A. Sparkes and R. B. Bedford, Organometallics, 2019, 38, 1770–1777 CrossRef CAS
.
- S. B. Tailor, M. Manzotti, G. J. Smith, S. A. Davis and R. B. Bedford, ACS Catal., 2021, 11, 3856–3866 CrossRef CAS
.
- S. K. Gurung, S. Thapa, A. Kafle, D. A. Dickie and R. Giri, Org. Lett., 2014, 16, 1264–1267 CrossRef CAS PubMed
.
- E. C. Hansen, D. J. Pedro, A. C. Wotal, N. J. Gower, J. D. Nelson, S. Caron and D. J. Weix, Nat. Chem., 2016, 8, 1126–1130 CrossRef CAS PubMed
.
- A. F. Baldwin, M. A. Caporello, G. Chen, A. E. Goetz, W. Hu, C. Jin, K. M. Knopf, Z. Li, C. V. Lu and S. Monfette, Org. Process Res. Dev., 2021, 25, 1065–1073 CrossRef CAS
.
- A. Modak, A. J. Nett, E. C. Swift, M. C. Haibach, V. S. Chan, T. S. Franczyk, S. Shekhar and S. P. Cook, ACS Catal., 2020, 10, 10495–10499 CrossRef CAS
.
- L. Gong, H.-B. Sun, L.-F. Deng, X. Zhang, J. Liu, S. Yang and D. Niu, J. Am. Chem. Soc., 2019, 141, 7680–7686 CrossRef CAS PubMed
.
- T. B. Boit, N. A. Weires, J. Kim and N. K. Garg, ACS Catal., 2018, 8, 1003–1008 CrossRef CAS PubMed
.
- J. Zhou, J. H. J. Berthel, M. W. Kuntze-Fechner, A. Friedrich, T. B. Marder and U. Radius, J. Org. Chem., 2016, 81, 5789–5794 CrossRef CAS PubMed
.
- J. R. Coombs, R. A. Green, F. Roberts, E. M. Simmons, J. M. Stevens and S. R. Wisniewski, Organometallics, 2019, 38, 157–166 CrossRef CAS
.
- S. M. Mennen, C. Alhambra, C. L. Allen, M. Barberis, S. Berritt, T. A. Brandt, A. D. Campbell, J. Castañón, A. H. Cherney, M. Christensen, D. B. Damon, J. Eugenio de Diego, S. García-Cerrada, P. García-Losada, R. Haro, J. Janey, D. C. Leitch, L. Li, F. Liu, P. C. Lobben, D. W. C. MacMillan, J. Magano, E. McInturff, S. Monfette, R. J. Post, D. Schultz, B. J. Sitter, J. M. Stevens, I. I. Strambeanu, J. Twilton, K. Wang and M. A. Zajac, Org. Process Res. Dev., 2019, 23, 1213–1242 CrossRef CAS
.
- M. J. Goldfogel, X. Guo, J. L. Meléndez Matos, J. A. Gurak, M. V. Joannou, W. B. Moffat, E. M. Simmons and S. R. Wisniewski, Org. Process Res. Dev., 2022, 26(3), 785–794 CrossRef CAS
.
- J. D. Shields, E. E. Gray and A. G. Doyle, Org. Lett., 2015, 17, 2166–2169 CrossRef CAS PubMed
.
- J. Magano and S. Monfette, ACS Catal., 2015, 5, 3120–3123 CrossRef CAS
.
- X. Guo, H. Dang, S. R. Wisniewski and E. M. Simmons, While this manuscript was being prepared, Guo and coworkers at BMS reported a Ni-SMC of arylboronic acids using the structurally related DPPB as the optimal ligand:, Organometallics, 2022, 41(11), 1269–1274 CrossRef CAS
.
- V. T. Tran, Z.-Q. Li, O. Apolinar, J. Derosa, M. V. Joannou, S. R. Wisniewski, M. D. Eastgate and K. M. Engle, Angew. Chem., Int. Ed., 2020, 59, 7409–7413 CrossRef CAS PubMed
.
- E. A. Standley and T. F. Jamison, J. Am. Chem. Soc., 2013, 135, 1585–1592 CrossRef CAS PubMed
.
- E. A. Standley, S. J. Smith, P. Müller and T. F. Jamison, Organometallics, 2014, 33, 2012–2018 CrossRef CAS PubMed
.
- K. Inada and N. Miyaura, Tetrahedron, 2000, 56, 8657–8660 CrossRef CAS
.
- J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1997, 119, 6054–6058 CrossRef CAS
.
- B. H. Lipshutz, S.-K. Kim, P. Mollard, P. A. Blomgren and K. L. Stevens, Tetrahedron, 1998, 54, 6999–7012 CrossRef CAS
.
- T. Irie and M. Sawa, Chem. Pharm. Bull., 2018, 66, 29–36 CrossRef CAS PubMed
.
-
J. D. Rodgers, S. Shepard, T. P. Maduskuie, H. Wang, N. Falahatpisheh, M. Rafalski, A. G. Arvanitis, L. Storace and R. K. Jalluri, US Pat., 8415362B2, 2013 Search PubMed
.
-
M. E. Kobierski, M. E. Kopach, J. R. Martinelli, D. L. Varie and T. M. Wilson, US Pat., 20180134713A1, 2018 Search PubMed
.
- Q. Lin, D. Meloni, Y. Pan, M. Xia, J. Rodgers, S. Shepard, M. Li, L. Galya, B. Metcalf, T.-Y. Yue, P. Liu and J. Zhou, Org. Lett., 2009, 11, 1999–2002 CrossRef CAS PubMed
.
- A. S. Guram, A. O. King, J. G. Allen, X. Wang, L. B. Schenkel, J. Chan, E. E. Bunel, M. M. Faul, R. D. Larsen, M. J. Martinelli and P. J. Reider, Org. Lett., 2006, 8, 1787–1789 CrossRef CAS PubMed
.
- T. Kinzel, Y. Zhang and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 14073–14075 CrossRef CAS PubMed
.
- P. S. Campbell, C. Jamieson, I. Simpson and A. J. B. Watson, Chem. Commun., 2018, 54, 46–49 RSC
.
- G. A. Molander, B. Canturk and L. E. Kennedy, J. Org. Chem., 2009, 74, 973–980 CrossRef CAS PubMed
.
- J. Yang, S. Liu, J.-F. Zheng and J. Zhou, Eur. J. Org. Chem., 2012, 2012, 6248–6259 CrossRef CAS
.
- K. Wilson, J. Murray, C. Jamieson and A. Watson, Synlett, 2017, 29, 650–654 Search PubMed
.
-
Q. Ye, Y. Liang, S. Liu, K. S. Chichak and J. Liu, Intl. Pat. App., WO2009142870A1, 2009 Search PubMed
.
- C. A. Tolman, W. C. Seidel and L. W. Gosser, J. Am. Chem. Soc., 1974, 96, 53–60 CrossRef CAS
.
- L.-B. Han, C. Zhang, H. Yazawa and S. Shimada, J. Am. Chem. Soc., 2004, 126, 5080–5081 CrossRef CAS PubMed
.
- C. A. Malapit, J. R. Bour, C. E. Brigham and M. S. Sanford, Nature, 2018, 563, 100–104 CrossRef CAS PubMed
.
- S. W. Reilly, Y.-h. Lam, S. Ren and N. A. Strotman, J. Am. Chem. Soc., 2021, 143, 4817–4823 CrossRef CAS PubMed
.
- Y. Nakamura, K.-I. Maruya and T. Mizoroki, J. Organomet. Chem., 1976, 104, C5–C8 CrossRef CAS
.
- Y. Nakamura, K.-I. Maruya and T. Mizoroki, Nippon Kagaku Kaishi, 1978, 1978, 1486–1491 CrossRef
.
- Y. Nakamura, K.-i. Maruya and T. Mizoroki, Bull. Chem. Soc. Jpn., 1980, 53, 3089–3092 CrossRef CAS
.
- Pyridine did not inhibit a non-Lewis basic Ni-SMC with DPPF in Ref 11. It seems that a different mechanistic step is problematic with this ligand.
- We also conducted the ligand substitution experiments in the presence of water (20% v/v in 2-MeTHF) in an attempt to more closely mimic the catalytic system. Under these conditions, transmetalation of 1 with the Ni oxidative addition complexes and reductive elimination of 3-(2-tolyl)pyridine was observed. We thank a reviewer for this suggestion.
- The analogous reaction of 2 with 1.4 equiv. PhBPin showed first-order dependence on 2, pointing to a different rate limiting step for the Ni-SMC of non-Lewis basic arylborons. See the ESI† for details.
- By contrast, 31P NMR of the coupling of PhBPin and 2 showed the expected mixture of PPh2Me-Ni complexes (52–85%) and free PPh2Me (48–15%) depending on the reaction progress. See the ESI† for details.
- A. H. Christian, P. Müller and S. Monfette, Organometallics, 2014, 33, 2134–2137 CrossRef CAS
.
- P.-A. Payard, L. A. Perego, I. Ciofini and L. Grimaud, ACS Catal., 2018, 8, 4812–4823 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2109558 and 2109557. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc03877c |
| This journal is © The Royal Society of Chemistry 2022 |