Open Access Article
Open Access ArticleUnderstanding the electrocatalytic mechanism of self-template formation of hierarchical Co9S8/Ni3S2 heterojunctions for highly selective electroreduction of nitrobenzene†
Xuanping
Wang‡
a,
Longbin
Li‡
ab,
Mingzhu
Shi
a,
Yiqi
Wang
c,
Guodong
Xu
a,
Kai
Yuan
 b,
Peipei
Zhu
*a,
Mengning
Ding
b,
Peipei
Zhu
*a,
Mengning
Ding
 *ac and
Yiwang
Chen
*ac and
Yiwang
Chen
 *ab
*ab
aNational Engineering Research Center for Carbohydrate Synthesis, Key Lab of Fluorine and Silicon for Energy Materials and Chemistry of Ministry of Education, College of Chemistry and Chemical Engineering, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang 330022, China. E-mail: ppzhu@jxnu.edu.cn; mding@nju.edu.cn; ywchen@ncu.edu.cn
bInstitute of Polymers and Energy Chemistry (IPEC), Nanchang University, 999 Xuefu Avenue, Nanchang 330031, China
cKey Laboratory of Mesoscopic Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, 163 Xianlin Avenue, Nanjing 210023, China
First published on 20th September 2022
Abstract
Aqueous electrochemical nitroarene reduction reaction using H2O as the sustainable hydrogen source is an emerging technology to produce functionalized anilines. However, the development of low-cost electrocatalysts and the fundamental mechanistic understanding of the selective NO-RR still remain challenging. Herein, self-supporting hierarchical nanosheets consisting of high-density Co9S8/Ni3S2 heterojunctions on Ni foam (Co9S8/Ni3S2-NF) are constructed via an in situ self-template strategy. With combined advantages of high-loading, high surface exposure, efficient conductivity and unique electronic structure of the Co9S8/Ni3S2 interface, the as-prepared Co9S8/Ni3S2-NF exhibits efficient electrocatalytic NO-RR performance, including up to 99.0% conversion and 96.0% selectivity towards aniline, and outstanding functional group tolerance. Mechanistic investigations and theoretical calculations reveal that electron transfer from Ni3S2 to Co9S8 is beneficial for the co-adsorption of H2O and nitrobenzene molecules at the interfacial sites, promoting the formation of active hydrogen and subsequent reduction of nitrobenzene. Additionally, the interfacial charge transfer breaks the symmetry of two active Co sites at the Co9S8/Ni3S2 interface, which markedly reduces the energy barrier for reduction of nitrobenzene to aniline. This work offers a successful example for the interfacial engineering of metal sulfide-based heterojunctions with excellent electrocatalytic nitroarene reduction performance, and also paves the way for the in-depth understanding of the corresponding mechanism.
Introduction
Functionalized aniline (PhNH2) is a group of vital chemical stocks in many industries, including dye, drug, conductive polymer, transparent electrode and pseudocapacitor.1–3 Traditional PhNH2 production via thermocatalytic or direct-amination endures the issues of high energy consumption, high carbon emission, high cost and unsafe proton donors (Table S1†).4–9 In addition, current nitroarene reduction reactions (NO-RR) typically utilize rare noble metals (Pt, Pd, Ru, Rh, etc.) as catalysts, which however face a significant challenge in achieving satisfactory chemoselectivity and are vulnerable to functional group poisoning.10–12 Moreover, the harsh reaction conditions lead to low compatibility with fragile groups (–OCH3, –OH, –Cl, –F, –Br, or/and –COCH3, etc.), which severely limits the scope of substrates for NO-RR.2,13–16 Therefore, it is crucial to develop noble-metal-free catalysts that enable selective reduction of functionalized nitroarenes with fragile side groups under mild conditions. In recent years, electrochemical organic synthesis has inspired the field with multiple advantages including ambient reaction conditions, utilization of sustainable electrical energy, and controllable selectivity.17–20 With continuous efforts, electrocatalytic nitroarene reduction has been greatly promoted, especially via the development of high-performance electrocatalysts (Table S2†) into the most promising technology for PhNH2 production.2,13,15,16,21,22 However, few studies uncover the active origins and fundamental mechanism of the selective NO-RR process under aqueous conditions.In view that reactive hydrogen (H*) participates and plays a crucial role in the aqueous electrochemical NO-RR, H2O activation performance of NO-RR electrocatalysts should be carefully considered for the modulation of their activities.13,15 Previous studies have confirmed that highly conductive transition metal sulfides (TMSs) with favorable H2O adsorption show promising potential in H2O splitting to replace precious metals.23–31 Nevertheless, pure TMSs exhibit weak adsorption on nitrobenzene (PhNO2), which leads to premature desorption of PhNO2 on the catalyst surface, leading to insufficient and incomplete reduction to PhNH2.15 To this end, interface engineering is an efficient strategy towards electronic structure regulation of interfacial active sites for designing efficient electrocatalysts.23,27,32–35 For example, Fu et al. successfully fabricated a MoO2–FeP heterojunction for efficient electrochemical hydrogen evolution and 5-HMF reaction, and interfacial electron redistribution between MoO2–FeP was confirmed as the origin of enhanced activity.23 In addition to the electronic structure, reasonable geometry regulations including the microstructure of electrocatalysts and selection of substrates are of vital importance.36–38 Construction of anisotropic nanosheets on conductive substrates can also increase active site exposure, accelerate mass transfer, and improve the electrical conductivity of electrocatalysts.39,40
Herein, self-supporting hierarchical nanosheets composed of Co9S8/Ni3S2 heterojunctions were constructed on Ni foam (Co9S8/Ni3S2-NF), with unique and abundant active interfaces that promote hydrogenation and conversion of nitroarenes into functionalized PhNH2. The as-prepared Co9S8/Ni3S2-NF shows efficient electrocatalytic reduction of PhNO2 to PhNH2 with up to 99.0% conversion and 96.0% selectivity under alkaline conditions, and demonstrates NO-RR favorable functional group tolerance. Both theoretical calculations and experimental characterization identify the critical role of electron transfer from Ni3S2 to Co9S8, which favors H2O activation for the formation of H* and adsorption of PhNO2 onto Co sites, thus improving the NO-RR activity. This work offers a design principle for NO-RR electrocatalysts via low-cost TMS-based heterojunction engineering, and also provides a systematic understanding of the electrocatalytic reduction mechanism during NO-RR.
Results and discussion
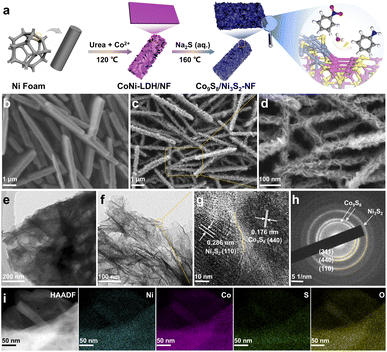
The construction of Co9S8/Ni3S2-NF was achieved by in situ sulfuration of Co–Ni layered-double-hydroxide (LDH) precursors pre-formed on the Ni foam (CoNi-LDH/NF) (Fig. 1a). First, CoNi-LDH/NF was prepared by a hydrothermal method with the Ni foam substrate placed in the Co salt and urea precursor solution at 120 °C. Subsequently, in situ sulfuration of self-sacrificing CoNi-LDH/NF was conducted by a second hydrothermal process with Na2S as the S source under 160 °C, and the final Co9S8/Ni3S2-NF was obtained. For better control of the morphology and catalytic performance of CoNi-LDH/NF precursors, the optimized hydrothermal time and the ratio of Co salts to urea is 8 hours and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. For comparison, catalysts obtained by in situ growth of cobalt sulfide on a titanium mesh (Co9S8/Ti) and nickel sulfide on Ni foam (Ni3S2/NF) were also successfully synthesized (see “Experimental section” for more details).
1, respectively. For comparison, catalysts obtained by in situ growth of cobalt sulfide on a titanium mesh (Co9S8/Ti) and nickel sulfide on Ni foam (Ni3S2/NF) were also successfully synthesized (see “Experimental section” for more details).
To reveal the morphology and microstructure features of the catalytic materials, field-emission scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used. CoNi-LDH shows a smooth surface and uniform growth on Ni-foam (see Fig. 1b and S1†). The Co9S8/Ni3S2-NF exhibits an obvious hierarchical nanosheet structure (i.e., nanosheets on the top of nanosheets), which implies that the sulfuration process takes place in situ on the CoNi-LDH/NF templates (Fig. 1c and d). Similarly, as shown in Fig. 1e and f, TEM images with different magnifications can more intuitively demonstrate the hierarchical nanosheets structure of Co9S8/Ni3S2-NF. High-resolution TEM (HRTEM) further reveals clear lattice fringes and intimate contact between Co9S8 (440) and Ni3S2 (110), confirming the formation of the heterojunction (see Fig. 1g). In addition, the selected area electron diffraction (SAED) pattern of Co9S8/Ni3S2-NF displays diffraction rings which can be satisfactorily ascribed to the (311) and (440) crystal planes of Co9S8, and the (110) crystal plane of Ni3S2 (Fig. 1h). EDS elemental maps (Fig. 1i and S2†) show the homogeneous distribution of Co, Ni, S and O elements, further supporting the successful synthesis of Co9S8/Ni3S2-NF, and the cobalt/nickel atomic ratio is about 3.48 (Table S3†). SEM images of Co9S8/Ti and Ni3S2/NF samples indicate that the monometallic TMS is spherical, and therefore it cannot grow evenly on the substrate (Fig. S3 and S4†). Further control of hydrothermal conditions can change the structure of the CoNi-LDH/NF templates (see SEM images in Fig. S5 and S6†) and hence the morphology of the catalysts. All these results confirm that the Co9S8/Ni3S2-NF with hierarchical nanosheets was successfully prepared, presumably with exposure of more active sites that benefits the mass diffusion during NO-RR.
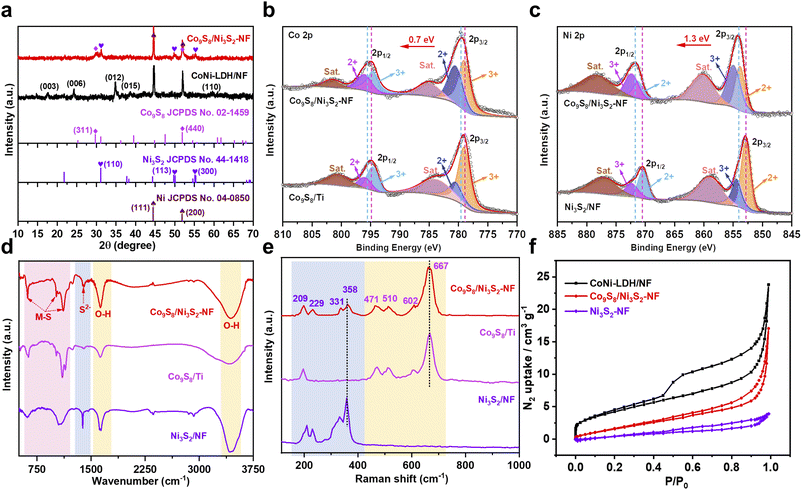
X-ray diffraction (XRD) patterns were obtained to verify the phase structure of the catalysts. In Fig. 2a, the XRD pattern of Co9S8/Ni3S2-NF exhibits characteristic diffraction signals ascribed to the (311) and (440) planes of Co9S8, as well as the (110) plane of Ni3S2 with (110) arising from the Ni foam. The XRD patterns of other samples (Co9S8 powder, Co9S8/Ti and Ni3S2/NF) are shown in Fig. S7–S9,† indicating that all samples were successfully synthesized. Additionally, CoNi-LDH/NF shows distinct diffraction characteristic signals for the hydrotalcite-like LDH phase, indicating that CoNi-LDH was successfully constructed on Ni foam.41–43 X-ray photoelectron spectroscopy (XPS) was performed to obtain further insight into the chemical states of each element and the electron effect between Co9S8 and Ni3S2 in Co9S8/Ni3S2-NF. The XPS survey spectra in Fig. S10a† reveal the coexistence of Ni, Co, and S signals in Co9S8/Ni3S2-NF, while no signal for S is observed in CoNi-LDH/NF, and the corresponding elemental quantifications are shown in Table S4.† Fig. S10b† shows that the proportions of Co and Ni in various Co9S8/Ni3S2-NF change with the addition of Co salts for CoNi-LDH/NF production, and the optimal Co9S8/Ni3S2-NF has the highest cobalt content among the control samples. To further figure out electron transfer between Co9S8 and Ni3S2, the high-resolution Co 2p, Ni 2p, and S 2p spectra were analyzed in detail.44 The Co 2p spectrum of Co9S8/Ni3S2-NF was deconvoluted into six peaks including two satellite peaks, and the others observed at 780.5 and 796.0 eV for Co2+ as well as 779.1 and 794.6 eV for Co3+ are ascribed to the spin–orbit doublets Co 2p1/2 and 2p3/2, respectively (Fig. 2b).45 Compared to Co9S8/Ti, the ratio of Co3+ to Co2+ in Co9S8/Ni3S2-NF is decreased, suggesting that the average valence state of Co is decreased.33,46 Moreover, the Ni 2p spectrum of Co9S8/Ni3S2-NF (Fig. 2c) can also be deconvoluted into six subpeaks, corresponding to Ni3+ (854.9/872.3 eV), Ni2+ (853.8/871.2 eV), and two satellite peaks of Ni 2p1/2 and Ni 2p3/2, severally.47 Interestingly, compared to Ni3S2/NF, the ratio of the peak area of Ni3+ to Ni2+ for Co9S8/Ni3S2-NF is higher than that for Ni3S2/NF, showing that the average valence state of Ni is raised.25,48 Therefore, it can be concluded that electrons transfer from Ni3S2 to Co9S8 through their interfaces. Moreover, the S 2p spectra in Fig. S11† demonstrate the presence of metal–sulfur bonds in Co9S8/Ni3S2-NF.40,49 Besides, the XPS survey spectra of the other control samples during optimization are detailed in Fig. S12–S14.† Notably, elemental oxygen is detected in almost all materials due to the large amount of hydroxyl or H2O molecules adsorbed on the catalyst surface during the hydrothermal reaction (Table S4†).
The Fourier transform infrared (FTIR) spectra were recorded to analyze the surface groups. Fig. 2d exhibits the FTIR spectra of Co9S8/Ni3S2-NF, Co9S8/Ti and Ni3S2/NF samples, and the two strong typical peaks at 3436 and 1631 cm−1 are ascribed to the stretching and bending vibration of O–H of surface-adsorbed H2O molecules.50,51 Besides, the obvious peaks at 1116 and 1020 cm−1 are attributed to the asymmetrical stretch of metal–sulfur, whereas the peak at 621 cm−1 corresponds to symmetrical vibrations. The presence of an additional band at 1381 cm−1 is due to the vibration of sulfide ions in the crystal in the TMS microstructures.52 Furthermore, compared with single metal analogues, the characteristic peaks at 194, 459, 507, 602 and 660 cm−1 are assigned to the vibrational modes of the Co9S8 phase in Co9S8/Ni3S2-NF, and the peaks in the 150–400 cm−1 region represent the phonon modes of heazlewoodite-phase Ni3S2 (Fig. 2e).53–56 In addition, the FTIR and Raman spectra of CoNi-LDH can be seen in Fig. S15 and S16,† illustrating the successful synthesis of the template.57,58 The Brunauer–Emmett–Teller (BET) surface area and pore texture of Co9S8/Ni3S2-NF were analyzed using N2 adsorption–desorption measurements. Compared with Ni3S2/NF, Co9S8/Ni3S2-NF results in a higher BET surface area of 7.37 m2 g−1 (Fig. 2f), and a higher average pore size of about 14.32 nm (Fig. S17†) due to the hierarchical nanosheet structure (Fig. 2f and Table S5†).33
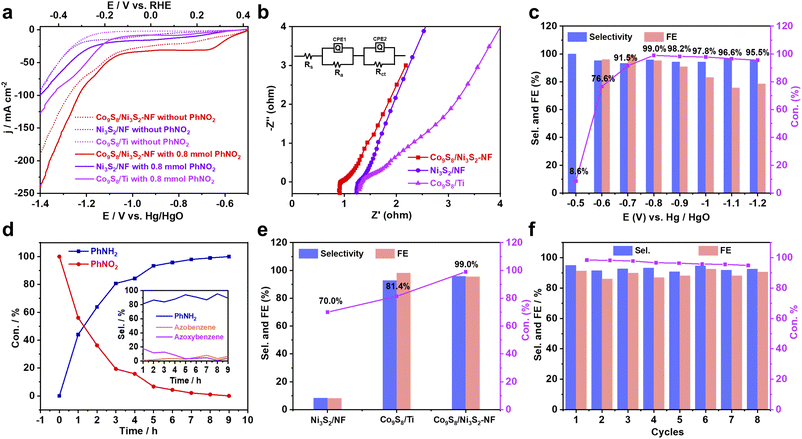
To explore the electrochemical NO-RR properties of the catalysts under ambient aqueous conditions, an H-type cell was constructed with a Pt plate counter electrode, an Hg/HgO reference electrode, and the self-supporting Co9S8/Ni3S2-NF as the working electrode (Fig. S18†). First, linear sweep voltammetry (LSV) curves of nitroarenes reduction and hydrogen evolution reaction (HER) were recorded in 1.0 M KOH solution using 1,4-dioxane as the co-solvent. In the presence of PhNO2 (0.8 mmol), Co9S8/Ni3S2-NF exhibits the lowest onset potential (Eonset) of 0.371 V versus the reversible hydrogen electrode (RHE) compared with Co9S8/Ti and Ni3S2/NF catalysts (Fig. 3a), indicating the best NO-RR performance. Besides, Co9S8/Ni3S2-NF also has the most positive Eonset for the HER (Fig. 3a), which suggests that Co9S8/Ni3S2-NF is favorable for H2O dissociation and H* generation. Previous studies have shown that such in situ generated H* is beneficial for subsequent protonation reactions of PhNO2.15 In addition, the Nyquist plots from electrochemical impedance spectroscopy (EIS) show that Co9S8/Ni3S2-NF shows a much lower internal resistance and smaller charge-transfer resistance compared with the control catalysts (Fig. 3b), implying better charge transfer capability of Co9S8/Ni3S2-NF. The solution resistance (Rs), electrode resistance (Ra), charge transfer resistance (Rct) and constant phase element (CPE) in the equivalent circuit model (Fig. 3b, inset) were extracted by fitting the experimental results and are listed in Table S6.† The smaller Rct of Co9S8/Ni3S2-NF than Ni3S2/NF and Co9S8/Ti reveals a faster charge transfer process in NO-RR.59 During the catalyst optimization, LSV curves (Fig. S19†) and Nyquist plots (Fig. S20†) of all samples were also recorded. In order to confirm the intrinsic activity of NO-RR of the obtained metal sulfides, electrochemical active surface areas (ECSA) and normalized current density by ECSA were also investigated.60–62 According to CV curves without faradaic processes (Fig. S21a–c†), the calculated capacitance and the ECSA are summarized in Table S7.† The ECSA of Co9S8/Ni3S2-NF is 3.0 times and 1.7 times larger than that of Ni3S2/NF and Co9S8/Ti respectively, indicating that the active sites on Co9S8/Ni3S2-NF are more accessible. Moreover, the normalized LSV curve of Co9S8/Ni3S2-NF exhibits a higher current density than Ni3S2/NF and Co9S8/Ti, showing the best intrinsic activity for NO-RR of Co9S8/Ni3S2-NF (Fig. S21e†).
To explore the potential-dependence of the selectivity of PhNH2, the electrocatalytic reduction of PhNO2 over Co9S8/Ni3S2-NF was carried out at various potentials from 0.421 to −0.279 V (vs. RHE) (Fig. 3c) by gas chromatography (GC) (Fig. S22–S23†). The wide Eonset gap between NO-RR and HER indicates efficient and highly selective reduction of PhNO2 to PhNH2 on the Co9S8/Ni3S2-NF electrode at different potentials from 0.321 to −0.079 V (vs. RHE). Specifically, the optimal catalytic performances for PhNO2 to PhNH2 were achieved at 0.121 V (vs. RHE) with up to 99.0% conversion, 96.0% selectivity and 95.3% faradaic efficiency (FE) (Table S8†). Nevertheless, PhNO2 cannot be reduced at potentials higher than 0.421 V (vs. RHE) and demonstrates low conversion and FE at potentials more negative than −0.079 V (vs. RHE) due to the dominant HER. In Fig. 3d, time-dependent transformations reveal that PhNO2 is almost completely converted to PhNH2 within ∼8 h at 0.121 V (vs. RHE), and the corresponding selectivity of NO-RR is depicted in the inset of Fig. 3d. By contrast, lower conversion and selectivity are obtained over Co9S8/Ti and Ni3S2/NF cathodes at 0.121 V (vs. RHE), showing the promotion effect of the Co9S8/Ni3S2 heterojunction in NO-RR (Fig. 3e). The other control catalysts were also evaluated to verify the more significant electrochemical performance of Co9S8/Ni3S2-NF (Fig. S24†).
Furthermore, the electrocatalytic stability of PhNO2 reduction is assessed over Co9S8/Ni3S2-NF. After 8 cycles (8 h per cycle) of electrolysis at 0.121 V vs. RHE, Co9S8/Ni3S2-NF maintained high selectivity and FE for PhNO2 to PhNH2 conversion (Fig. 3f and Table S9†). Furthermore, the current efficiency and Nyquist plots are almost consistent with the starting cycle (Fig. S25†). Negligible changes were discovered by further SEM, TEM, XRD and XPS characterization, implying the excellent durability of the Co9S8/Ni3S2-NF cathode for NO-RR (Fig. S26–S28 and Tables S10, S11†). All these electrochemical results demonstrate that Co9S8/Ni3S2-NF shows excellent catalytic activity and promising cycle stability for application in the electrocatalytic conversion of PhNO2 for PhNH2 production.
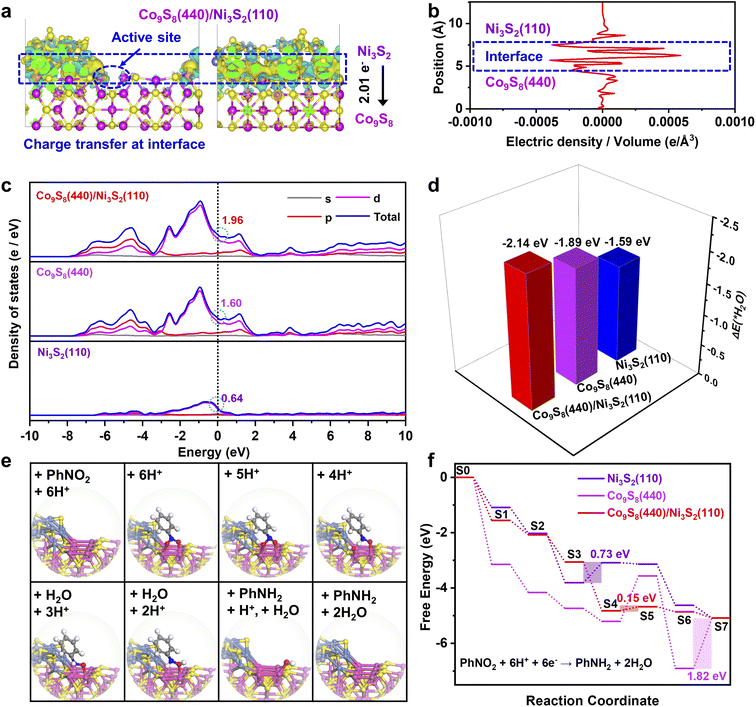
To understand the underlying origin of the excellent activity of the Co9S8/Ni3S2-NF heterostructure, density functional theory (DFT) calculations were further employed.2,16,63,64 According to the HRTEM and XRD results, Co9S8 (440) and Ni3S2 (110) lattice planes are preferentially exposed in Co9S8/Ni3S2-NF, and thus the models of the Co9S8 (440), Ni3S2 (110), and Co9S8 (440)/Ni3S2 (110) heterojunction surfaces were optimized (Fig. S29†). To evaluate the electronic interaction between Co9S8 and Ni3S2, the calculated charge density difference within the Co9S8/Ni3S2 heterojunction was analyzed. As displayed in Fig. 4a, charge density difference for the Co9S8/Ni3S2 model (front and left views) clearly shows a significant charge rearrangement occurring at the interface of Co9S8/Ni3S2.35 Additionally, electron accumulation is also observed on the Co atoms close the Co9S8/Ni3S2 interface (marked as a blue cycle) due to electron transfer (2.01 e−) from Ni3S2 to Co9S8, which was consistent with the XPS and Raman results. Such electron transfer at the Co9S8/Ni3S2 interface is more directly manifested in the corresponding radial distribution function (Fig. 4b). Furthermore, the calculated projected density of states reveals that the Co9S8/Ni3S2 heterostructure has a higher electron density of 1.96 at the Fermi level than single Co9S8 (1.60) and Ni3S2 (0.64) (Fig. 4c), which can facilitate electron transfer.65 Notably, all three models present no band gap crossing the Fermi level, indicating that the Co9S8/Ni3S2 heterostructure preserves the metallic nature of Ni3S2 and Co9S8 with high conductivity.
The adsorption energies of H2O (ΔEH2O) and the energy profile of the PhNO2 reduction were further studied. As shown in Fig. 4d, the Co9S8/Ni3S2 heterojunction has a more negative ΔEH2O value of −2.14 eV than Co9S8 (−1.89 eV) and Ni3S2 (−1.59 eV), which implies effective H2O activation for H* generation on the Co9S8/Ni3S2 interface.23 This calculation result is in line with the HER experimental measurements in alkaline medium (Fig. S19†), and the in situ formed active H* at the interface will participate in the transfer hydrogenation of PhNO2.
According to the structural optimization of intermediates in the aqueous electrochemical reduction of PhNO2, the corresponding energy profile of PhNO2 hydrogenation on the Co9S8/Ni3S2 surface along the pathway was calculated. The catalytic cycle is displayed in Fig. 4e, and specific elementary steps are as follows: (i) 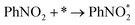 ; (ii)
; (ii) 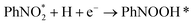 ; (iii) PhNOOH* + H + e− → PhNHO*OH; (iv) PhNHO*OH + H + e− → PhNHO* + H2O; (v) PhNHO* + H + e− → PhNHOH*; (vi) PhNHOH* + H + e− → PhNH2 + OH*; (vii) OH* + H + e− → H2O, in which * represents the adsorption site. Notably, the PhNO2 molecule will spontaneously adsorb on two Co atoms close to the Co9S8/Ni3S2 interface, such a diatomic metal-site adsorption mechanism has also been reported in thermal catalysis under high pressure.63 For comparison, both Co9S8 and Ni3S2 were also considered, and the corresponding mechanism process is shown in Fig. S30.†
; (iii) PhNOOH* + H + e− → PhNHO*OH; (iv) PhNHO*OH + H + e− → PhNHO* + H2O; (v) PhNHO* + H + e− → PhNHOH*; (vi) PhNHOH* + H + e− → PhNH2 + OH*; (vii) OH* + H + e− → H2O, in which * represents the adsorption site. Notably, the PhNO2 molecule will spontaneously adsorb on two Co atoms close to the Co9S8/Ni3S2 interface, such a diatomic metal-site adsorption mechanism has also been reported in thermal catalysis under high pressure.63 For comparison, both Co9S8 and Ni3S2 were also considered, and the corresponding mechanism process is shown in Fig. S30.†
Fig. 4f exhibits the computational energy profiles of the optimized intermediates for Co9S8 (440), Ni3S2 (110), and the Co9S8 (440)/Ni3S2 (110) heterojunction at U = 1.23 V vs. the reversible hydrogen electrode.66 Logically, Co9S8/Ni3S2 exhibits a lower energy absorption of 0.15 eV on the rate determining step (RDS) compared to Co9S8 of 1.82 eV and Ni3S2 of 0.73 eV, conforming to the unique catalytic activities of Co9S8/Ni3S2-NF again. Compared with pure Co9S8, the Co9S8/Ni3S2 model is more beneficial to the desorption of OH (S6 → S7), which is because the Co9S8/Ni3S2 interface breaks the charge symmetry of two Co atom sites (Fig. 4a).
Hence, the OH intermediate is prone to adhere to one Co atom at the Co9S8/Ni3S2 surface, but it exhibits strong bridging adsorption on symmetric two cobalt atoms at the Co9S8 surface (Fig. 4e and S29†). Overall, calculations demonstrate that the Co9S8/Ni3S2 heterojunction is beneficial for activation of H2O, reducing the energy barrier of subsequent PhNO2 hydrogenation and facilitating electron transfer at interfaces.
On the basis of DFT results, ultraviolet photoelectron spectroscopy (UPS) measurements were performed to gain further insights into interfacial electron transfer capacity. Fig. S31† shows that the work functions of Co9S8/Ni3S2-NF, Co9S8/Ti and Ni3S2/NF catalysts were calculated to be 6.77, 6.28 and 5.89 eV, severally. The low work function of Co9S8/Ni3S2-NF indicates a rapid electron donation capacity from the catalyst surface to the adsorbed nitro groups.67,68 Therefore, theoretical calculations and experiments together show that the construction of the Co9S8/Ni3S2 heterojunction facilitates the efficient reduction of PhNO2 to PhNH2.
The general applicability of the electrochemical selective hydrogenation of nitroarenes towards aminoarenes was extended with excellent performance over the Co9S8/Ni3S2-NF cathode (Table 1).2,69 Obviously, a series of functionalized aminoarenes with electro-donating groups of –OCH3 and –OH, and electron withdrawing groups of –Cl and –F on the ortho, meta or para positions can be obtained in excellent yields (entries 1–6). Nitroarenes with other fragile –Br and –COCH3 groups were also successfully converted with excellent yields (entries 7–10). Due to the two hexatomic rings of the 5-nitroquinoline substrate, the difficult adsorption of 5-nitroquinoline on the catalyst leads to a low conversion of NO-RR compared to other functionalized aminoarenes (entry 11). Overall, these results signify that the potential applicability of Co9S8/Ni3S2-NF provides a good opportunity for subsequent generation of more complex molecules, which are challenging in traditional synthesis methods.
| Entry | Reactant | Product | Con. (%) | Sel. (%) | FE (%) |
|---|---|---|---|---|---|
a Reaction conditions: nitro substrates (0.8 mmol), Co9S8/Ni3S2-NF (working area: 1 cm2), 1.0 M KOH solution (Diox/H2O, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v), room temperature, −0.421 ∼ −0.279 V (vs. RHE), 8 h. The conversion (Con.), selectivity (Sel.) and faradaic efficiency (FE) were determined by gas chromatography. 5 v/v), room temperature, −0.421 ∼ −0.279 V (vs. RHE), 8 h. The conversion (Con.), selectivity (Sel.) and faradaic efficiency (FE) were determined by gas chromatography.
|
|||||
| 1 |
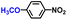
|
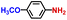
|
100.0 | 97.5 | 96.9 |
| 2 |
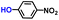
|
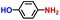
|
96.3 | 100.0 | 96.2 |
| 3 |
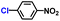
|
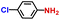
|
93.6 | 95.4 | 96.0 |
| 4 |
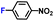
|
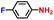
|
100.0 | 97.1 | 98.1 |
| 5 |

|
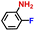
|
100.0 | 99.3 | 97.0 |
| 6 |
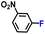
|
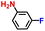
|
100.0 | 97.6 | 96.3 |
| 7 |
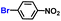
|
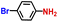
|
97.7 | 97.9 | 95.0 |
| 8 |
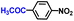
|
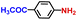
|
100.0 | 99.5 | 90.2 |
| 9 |
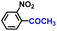
|
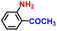
|
99.5 | 99.9 | 98.1 |
| 10 |
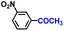
|
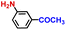
|
97.1 | 98.5 | 96.4 |
| 11 |
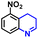
|
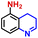
|
79.4 | 98.3 | 84.0 |
Conclusions
In summary, self-supporting hierarchical nanosheets composed of Co9S8/Ni3S2 heterojunctions were fabricated via an in situ sulphuration strategy using layered double hydroxide as a self-sacrificing template. Because of the integration of plentiful active Co9S8/Ni3S2 interfaces, open nanostructure, and high conductivity, the as-prepared Co9S8/Ni3S2-NF shows outstanding NO-RR performance in alkaline aqueous medium. Both the experimental results and theoretical calculations reveal electron transfer from Ni3S2 to Co9S8, which is identified by electron redistribution at interfaces. For this reason, the Co9S8/Ni3S2 heterojunction is more conducive to the adsorption of H2O and PhNO2 for the formation of H* and further protonation of PhNO2. Furthermore, interfacial charge transfer can break the symmetry of two active Co sites at the Co9S8/Ni3S2 interface to change the strong bridging adsorption of OH on Co sites, which markedly reduces the energy barrier. In addition, Co9S8/Ni3S2-NF also exhibits good functional group tolerance and excellent cycling stability for electrocatalytic NO-RR. This work reveals the electrocatalytic mechanism for NO-RR on metal sulfide-based heterojunctions, and reflects the advantage and great potential of interfacial engineering for green synthesis.Data availability
All data associated with this article have been included in the main text and ESI.†Author contributions
Y. C., M. D., and P. Z. conceived the concept and supervised the project. K. Y. contributed to valuable guidance and comments. X. W. and L. L. searched the literature and performed the synthesis, characterization and electrochemical experiments. G. X. and M. S. assisted with the HR-TEM testing and data analysis. Y. W. assisted with the XRD testing and data analysis. The manuscript was written through contributions of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (52073137, 22172075, and 92156024), the Education Department Science and Technology Research Foundation of Jiangxi Province (GJJ210318), the Natural Science Foundation of Jiangxi Province (20212BAB213018, 20203BDH80W011, and 20212BAB214028), and the Thousand Talents Plan of Jiangxi Province (jxsq2019102002).References
- K. M. R. V. Jagadeesh, A. S. Alshammari, H. Neumann, M.-M. Pohl, J. Radnik and M. Beller, Science, 2017, 358, 326–332 CrossRef PubMed
.
- J. L. Liang, Q. Q. Song, J. H. Wu, Q. Lei, J. Li, W. Zhang, Z. M. Huang, T. X. Kang, H. Xu, P. Wang, X. T. Zhou, P. K. Wong, H. M. Li, X. M. Meng, Z. F. Jiang and C. S. Lee, ACS Nano, 2022, 16, 4152–4161 CrossRef CAS PubMed
.
- K.-Y. Zhang, Z.-Y. Gu, E. H. Ang, J.-Z. Guo, X.-T. Wang, Y. Wang and X.-L. Wu, Mater. Today, 2022, 54, 189–201 CrossRef CAS
.
- J. Li, S. Song, Y. Long, L. Wu, X. Wang, Y. Xing, R. Jin, X. Liu and H. Zhang, Adv. Mater., 2018, 30, 1704416 CrossRef PubMed
.
- V. Babel and B. L. Hiran, Catal. Lett., 2020, 150, 1865–1869 CrossRef CAS
.
- Y. Xie, X. Shang, D. Liu, H. Zhao, Y. Gu, Z. Zhang and X. Wang, Appl. Catal., B, 2019, 259, 118087 CrossRef CAS
.
- J. Liu, R. Ye, J. Shi, H. Wang, L. Wang, P. Jian and D. Wang, Chem. Eng. J., 2021, 419, 129640 CrossRef CAS
.
- D. Formenti, F. Ferretti, F. K. Scharnagl and M. Beller, Chem. Rev., 2018, 119, 2611–2680 CrossRef PubMed
.
- S. Doherty, J. G. Knight, T. Backhouse, R. J. Summers, E. Abood, W. Simpson, W. Paget, R. A. Bourne, T. W. Chamberlain, R. Stones, K. R. J. Lovelock, J. M. Seymour, M. A. Isaacs, C. Hardacre, H. Daly and N. H. Rees, ACS Catal., 2019, 9, 4777–4791 CrossRef CAS
.
- F. Tong, X. Liang, F. Ma, X. Bao, Z. Wang, Y. Liu, P. Wang, H. Cheng, Y. Dai, B. Huang and Z. Zheng, ACS Catal., 2021, 11, 3801–3809 CrossRef CAS
.
- G. Portillo Perez and M.-J. Dumont, Chem. Eng. J., 2020, 382, 122766 CrossRef
.
- H. Wei, X. Liu, A. Wang, L. Zhang, B. Qiao, X. Yang, Y. Huang, S. Miao, J. Liu and T. Zhang, Nat. Commun., 2014, 5, 5634 CrossRef CAS PubMed
.
- X. Chong, C. Liu, Y. Huang, C. Huang and B. Zhang, Natl. Sci. Rev., 2020, 7, 285–295 CrossRef CAS PubMed
.
- C. Tang, Y. Zheng, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2021, 60, 19572–19590 CrossRef CAS PubMed
.
- Y. Zhao, C. Liu, C. Wang, X. Chong and B. Zhang, CCS Chem., 2021, 3, 507–515 CrossRef CAS
.
- M. Jin, Y. Liu, X. Zhang, J. Wang, S. Zhang, G. Wang, Y. Zhang, H. Yin, H. Zhang and H. Zhao, Appl. Catal., B, 2021, 298, 120545 CrossRef CAS
.
- X. Huang, Q. Zhang, J. Lin, K. Harms and E. Meggers, Nat. Catal., 2018, 2, 34–40 CrossRef
.
- Y. Yuan and A. Lei, Acc. Chem. Res., 2019, 52, 3309–3324 CrossRef CAS PubMed
.
- B. K. Peters, K. X. Rodriguez, S. H. Reisberg, S. B. Beil, D. P. Hickey, Y. Kawamata, M. Collins, J. Starr, L. Chen, S. Udyavara, K. Klunder, T. J. Gorey, S. L. Anderson, M. Neurock, S. D. Minteer and P. S. Baran, Science, 2019, 363, 838–845 CrossRef CAS PubMed
.
- P. Xiong and H.-C. Xu, Acc. Chem. Res., 2019, 52, 3339–3350 CrossRef CAS PubMed
.
- D. Carvajal, R. Arcas, C. A. Mesa, S. Giménez, F. Fabregat-Santiago and E. Mas-Marzá, Adv. Sustainable Syst., 2022, 6, 2100367 CrossRef CAS
.
- K. Iwase, N. Fujinami, K. Hashimoto, K. Kamiya and S. Nakanishi, Chem. Lett., 2018, 47, 304–307 CrossRef CAS
.
- G. Yang, Y. Jiao, H. Yan, Y. Xie, A. Wu, X. Dong, D. Guo, C. Tian and H. Fu, Adv. Mater., 2020, 32, 2000455 CrossRef CAS PubMed
.
- Y. Wu, Y. Liu, G.-D. Li, X. Zou, X. Lian, D. Wang, L. Sun, T. Asefa and X. Zou, Nano Energy, 2017, 35, 161–170 CrossRef CAS
.
- Y. Sun, J. Wu, Z. Zhang, Q. Liao, S. Zhang, X. Wang, Y. Xie, K. Ma, Z. Kang and Y. Zhang, Energy Environ. Sci., 2022, 15, 633–644 RSC
.
- A. Sivanantham, P. Ganesan and S. Shanmugam, Adv. Funct. Mater., 2016, 26, 4661–4672 CrossRef CAS
.
- J. Sun, H. Xue, N. Guo, T. Song, Y. r. Hao, J. Sun, J. Zhang and Q. Wang, Angew. Chem., Int. Ed., 2021, 60, 19435–19441 CrossRef CAS PubMed
.
- S. Chandrasekaran, L. Yao, L. Deng, C. Bowen, Y. Zhang, S. Chen, Z. Lin, F. Peng and P. Zhang, Chem. Soc. Rev., 2019, 48, 4178–4280 RSC
.
- Z. Zhang, L. Bian, H. Tian, Y. Liu, Y. Bando, Y. Yamauchi and Z. L. Wang, Small, 2022, 18, 2107450 CrossRef CAS PubMed
.
- Z. L. Wang, J. Choi, M. Xu, X. Hao, H. Zhang, Z. Jiang, M. Zuo, J. Kim, W. Zhou, X. Meng, Q. Yu, Z. Sun, S. Wei, J. Ye, G. G. Wallace, D. L. Officer and Y. Yamauchi, ChemSusChem, 2020, 13, 929–937 CrossRef CAS PubMed
.
- Y. Sun, H. Shin, F. Wang, B. Tian, C.-W. Chiang, S. Liu, X. Li, Y. Wang, L. Tang, W. A. Goddard and M. Ding, J. Am. Chem. Soc., 2022, 144, 15185–15192 CrossRef CAS PubMed
.
- Y.-T. Liao, V. C. Nguyen, N. Ishiguro, A. P. Young, C.-K. Tsung and K. C. W. Wu, Appl. Catal., B, 2020, 270, 118805 CrossRef CAS
.
- J. Zhang, J. Qian, J. Ran, P. Xi, L. Yang and D. Gao, ACS Catal., 2020, 10, 12376–12384 CrossRef CAS
.
- W. Fang, H. Hu, T. Jiang, G. Li and M. Wu, Carbon, 2019, 146, 476–485 CrossRef CAS
.
- S. Wang, L. Zhao, J. Li, X. Tian, X. Wu and L. Feng, J. Energy Chem., 2022, 66, 483–492 CrossRef
.
- M. Wang, C.-L. Dong, Y.-C. Huang and S. Shen, ACS Catal., 2020, 10, 1855–1864 CrossRef
.
- Z. Xiong, C. Hu, X. Luo, W. Zhou, Z. Jiang, Y. Yang, T. Yu, W. Lei and C. Yuan, Nano Lett., 2021, 21, 10486–10493 CrossRef CAS PubMed
.
- B. Yan, D. Krishnamurthy, C. H. Hendon, S. Deshpande, Y. Surendranath and V. Viswanathan, Joule, 2017, 1, 600–612 CrossRef CAS
.
- Y. Yan, S. Ding, X. Zhou, Q. Hu, Y. Feng, Q. Zheng, D. Lin and X. Wei, J. Alloys Compd., 2021, 867, 158941 CrossRef CAS
.
- S. Adhikari, Y. Kwon and D.-H. Kim, Chem. Eng. J., 2020, 402, 126192 CrossRef CAS
.
- X. Guan, M. Huang, L. Yang, G. Wang and X. Guan, Chem. Eng. J., 2019, 372, 151–162 CrossRef CAS
.
- M. Zhang, Y. Liu, B. Liu, Z. Chen, H. Xu and K. Yan, ACS Catal., 2020, 10, 5179–5189 CrossRef CAS
.
- R. Zhang, Z. Xue, J. Qin, M. Sawangphruk, X. Zhang and R. Liu, J. Energy Chem., 2020, 50, 143–153 CrossRef
.
- W. He, C. Wang, H. Li, X. Deng, X. Xu and T. Zhai, Adv. Energy Mater., 2017, 7, 1700983 CrossRef
.
- Y. Wang, J. Huang, Y. Xiao, Z. Peng, K. Yuan, L. Tan and Y. Chen, Carbon, 2019, 147, 146–153 CrossRef CAS
.
- K. Shah, R. Dai, M. Mateen, Z. Hassan, Z. Zhuang, C. Liu, M. Israr, W. C. Cheong, B. Hu, R. Tu, C. Zhang, X. Chen, Q. Peng, C. Chen and Y. Li, Angew. Chem., Int. Ed., 2021, 134, e202114951 Search PubMed
.
- K. Zhang, Y. Wei, J. Huang, Y. Xiao, W. Yang, T. Hu, K. Yuan and Y. Chen, Sci. China Mater., 2020, 63, 1898–1909 CrossRef CAS
.
- Y. Lu, C.-L. Dong, Y.-C. Huang, Y. Zou, Y. Liu, Y. Li, N. Zhang, W. Chen, L. Zhou, H. Lin and S. Wang, Sci. China: Chem., 2020, 63, 980–986 CrossRef CAS
.
- M. Verma, L. Sinha and P. M. Shirage, J. Mater. Sci.: Mater. Electron., 2021, 32, 12292–12307 CrossRef CAS
.
- D. Jiang, H. Liang, W. Yang, Y. Liu, X. Cao, J. Zhang, C. Li, J. Liu and J. J. Gooding, Carbon, 2019, 146, 557–567 CrossRef CAS
.
- J. Zhao, L. Song, X. Liang and Z. Zhao, Inorg. Chem. Commun., 2019, 107, 107469 CrossRef CAS
.
- S. Surendran, K. Vijaya Sankar, L. John Berchmans and R. Kalai Selvan, Mater. Sci. Semicond. Process., 2015, 33, 16–23 CrossRef CAS
.
- W. Li, Y. Li, H. Fu, G. Yang, Q. Zhang, S. Chen and F. Peng, Chem. Eng. J., 2020, 381, 122683 CrossRef CAS
.
- L. Zeng, K. Sun, X. Wang, Y. Liu, Y. Pan, Z. Liu, D. Cao, Y. Song, S. Liu and C. Liu, Nano Energy, 2018, 51, 26–36 CrossRef CAS
.
- C. Xia, P. Li, A. N. Gandi, U. Schwingenschlögl and H. N. Alshareef, Chem. Mater., 2015, 27, 6482–6485 CrossRef CAS
.
- H. Wang, Y. Yang, Q. Li, W. Lu, J. Ning, Y. Zhong, Z. Zhang and Y. Hu, Sci. China Mater., 2020, 64, 840–851 CrossRef
.
- T.-J. Wang, X. Liu, Y. Li, F. Li, Z. Deng and Y. Chen, Nano Res., 2019, 13, 79–85 CrossRef
.
- Y. Feng, X. Wang, J. Huang, P. Dong, J. Ji, J. Li, L. Cao, L. Feng, P. Jin and C. Wang, Chem. Eng. J., 2020, 390, 124525 CrossRef CAS
.
- P. Liu, J. Yan, J. Mao, J. Li, D. Liang and W. Song, J. Mater. Chem. A, 2020, 8, 11435–11441 RSC
.
- L. Li, S. Huang, R. Cao, K. Yuan, C. Lu, B. Huang, X. Tang, T. Hu, X. Zhuang and Y. Chen, Small, 2021, 18, 2105387 CrossRef PubMed
.
- S. He, H. Du, K. Wang, Q. Liu, J. Sun, Y. Liu, Z. Du, L. Xie, W. Ai and W. Huang, Chem. Commun., 2020, 56, 5548–5551 RSC
.
- Y. Liu, Q. Li, R. Si, G.-D. Li, W. Li, D.-P. Liu, D. Wang, L. Sun, Y. Zhang and X. Zou, Adv. Mater., 2017, 29, 1606200 CrossRef PubMed
.
- S. Tian, B. Wang, W. Gong, Z. He, Q. Xu, W. Chen, Q. Zhang, Y. Zhu, J. Yang, Q. Fu, C. Chen, Y. Bu, L. Gu, X. Sun, H. Zhao, D. Wang and Y. Li, Nat. Commun., 2021, 12, 3181 CrossRef CAS
.
- Y. Gu, A. Wu, L. Wang, D. Wang, H. Yan, P. Yu, Y. Xie, C. Tian, F. Sun and H. Fu, J. Mater. Chem. A, 2020, 8, 4807–4815 RSC
.
- J. Wu, R. Zhao, H. Xiang, C. Yang, W. Zhong, C. Zhang, Q. Zhang, X. Li and N. Yang, Appl. Catal., B, 2021, 292, 120200 CrossRef CAS
.
- Y. Li, R. Cao, L. Li, X. Tang, T. Chu, B. Huang, K. Yuan and Y. Chen, Small, 2020, 16, 1906735 CrossRef CAS PubMed
.
- Z. Wu, Y. Zhang, L. Li, Y. Zhao, Y. Shen, S. Wang and G. Shao, J. Mater. Chem. A, 2020, 8, 23248–23256 RSC
.
- B. Ni, R. Chen, L. Wu, P. Sun and T. Chen, Sci. China Mater., 2020, 64, 1159–1172 CrossRef
.
- M. Niakan and Z. Asadi, Catal. Lett., 2019, 149, 2234–2246 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2sc03585e |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |