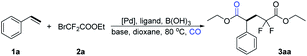Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium-catalyzed difluoroalkylative carbonylation of styrenes toward difluoropentanedioates†
Zhi-Peng
Bao
ab,
Youcan
Zhang
a and
Xiao-Feng
Wu
 *ab
*ab
aDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 116023 Dalian, Liaoning, China. E-mail: xwu2020@dicp.ac.cn
bLeibniz-Institut für Katalyse e.V., Albert-Einstein-Straße 29a, 1, 8059 Rostock, Germany. E-mail: Xiao-Feng.Wu@catalysis.de
First published on 3rd August 2022
Abstract
The introduction of fluorine atoms into organic molecules is an attractive but challenging topic. In this work, an interesting palladium-catalyzed difluoroalkylative carbonylation of aryl olefins has been developed. A wide range of aryl olefins were transformed into the corresponding difluoropentanedioate compounds with good functional-group tolerance and excellent regioselectivity. Inexpensive ethyl bromodifluoroacetate acts both as a difluoroalkyl precursor and a nucleophile here. Additionally, a scale–up reaction was also performed successfully, and further transformations of the obtained product were shown as well.
Introduction
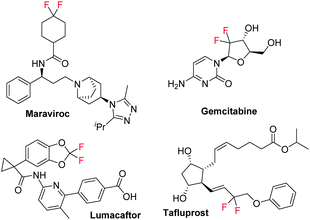
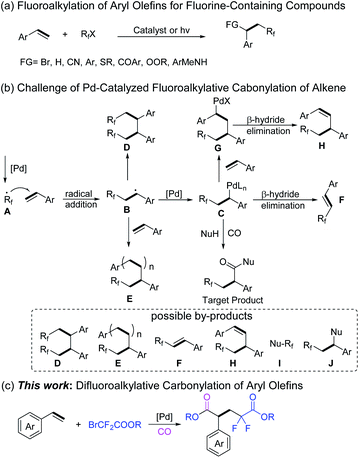
Organic fluorides play an important role in organic synthesis, medicinal chemistry, and materials science due to their special physical and chemical properties.1 The introduction of a fluorine atom into an organic molecule can often change the biological activity and physical properties of the compound. Among the fluorine containing moieties, the difluoromethylene group has good metabolic stability, and its electron-withdrawing character can affect the electronic properties, chemical properties, and biological reactivity of the adjacent functional groups among the fluorine-containing compounds, thus it exists in diverse drug molecules.2 For example, Maraviroc is a CCR5 co-receptor antagonist used for treating CCR5-tropic HIV-1 infection together with other antiretroviral medications.3 Gemcitabine, a nucleoside metabolic inhibitor, is used as an adjunct therapy in the treatment of certain types of ovarian cancer, non-small cell lung carcinoma, metastatic breast cancer, and as a single agent for pancreatic cancer.4 Lumacaftor is a protein chaperone, used for the treatment of cystic fibrosis in patients who are homozygous for the F508del mutation in the CFTR gene by combining with ivacaftor.5 Tafluprost, an ophthalmic prostaglandin analogue, has been used to lower intraocular pressure in patients with ocular hypertension or open-angle glaucoma (Scheme 1).6Due to the double bond of aryl alkenes conjugated with an aromatic ring, they are quite activated and will usually lead to selectivity and reactivity issues in organic transformations. On the other hand, it represents an attractive route to construct fluorinated compounds by using aryl olefins and commercially available fluoroalkyl halides as the starting materials.7 Various transition-metal-catalyzed-,8 photoinduced-9 or N-heterocyclic carbene (NHC) catalyzed-10 fluoroalkylation reactions of aryl alkenes with fluoroalkyl halides have been developed for the preparation of difunctional saturated fluorine-containing compounds (Scheme 2a).
Transition metal-catalyzed carbonylation is an effective strategy for preparing various functionalized carbonyl-containing compounds.11 In recent years, more and more procedures for fluoroalkylative carbonylations of olefins have been developed for the construction of fluorine-containing carbonylated compounds. For example, Liu's group reported a novel cooperative strategy based on palladium-catalyzed and iodine(III)-mediated β-fluorocarboxylation of alkenes;12 palladium-catalyzed multi-component perfluoroalkylative carbonylation for the synthesis of β-perfluoroalkyl esters and amides was also realized;13 and a copper-catalyzed 1,2-trifluoromethylation carbonylation of unactivated alkenes to get β-trifluoromethylated aliphatic carboxylic acid derivatives has been reported recently.14 However, most of them are based on unactivated aliphatic olefins, and the transformation of aryl olefins in the fluoroalkylative carbonylation reaction to give the corresponding ester product is still not reported.
Under all the above discussed backgrounds, we became interested in developing a new fluoroalkylative carbonylation procedure for aryl olefins to construct fluorine-containing carbonylated compounds. However, this strategy faces several challenges. As depicted in Scheme 2b, palladium-mediated single-electron reduction of fluoroalkyl halide forms radical A, which subsequently adds to the aryl olefins to generate a new benzylic carbon radical B. The intermediate B recombines with palladium to form a new active benzylic intermediate C. Meanwhile, B may dimerize to form D, which may even continue to polymerize with other aryl olefins to form product E. Intermediate C can give product F after β-hydride elimination.7a Intermediate C can also continue to add to another olefin to form G, and then β-hydride elimination takes place to give product H.15 Theoretically, fluoroalkyl halide can also react directly with a nucleophile to give compound I, and C may be quenched by a nucleophile to form J. Hence, a selective and efficient procedure for fluoroalkylative carbonylation of aryl olefins to construct fluorine-containing carbonylated compounds is a challenging topic.
After systematic optimizations, herein, we developed an efficient palladium-catalyzed difluoroalkylative carbonylation reaction for aryl olefins. Ethyl bromodifluoroacetate acts both as a difluoroalkyl precursor and a nucleophile in this system. A wide range of aryl olefins were transformed into the corresponding difluoropentanedioate compounds in good yields with broad functional group tolerance and excellent regioselectivity (Scheme 2c).
Results and discussion
Initially, styrene 1a and cheap ethyl bromodifluoroacetate 2a were chosen as the model substrates to evaluate the feasibility of this difluoroalkylation carbonylation reaction. To our delight, with DiPEA as the base and assisted by B(OH)3 in dioxane at 80 °C, the desired product 3aa was obtained in 57% yield in the presence of PdCl2 and using Xantphos as the ligand (Table 1, entry 1). Subsequently, various bases were studied, and a lower yield was observed with Na2CO3 (Table 1, entry 2). The desired product 3aa was not detected when using NaOtBu as the base (Table 1, entry 3). We then studied the effect of palladium pre-catalysts, but unfortunately, reduced yields were obtained when Pd(OAc)2, Pd(PPh3)4, or Pd(TFA)2 were tested (Table 1, entries 4–6). Further screening showed that 0.4 mmol of boric acid was proven to be the best for the target transformation (Table 1, entry 7 vs. 6). The yield of 3aa dropped to 17% in the absence of boric acid (Table 1, entry 8). And the yield of 3aa increased to 66% when employing MeCN as the solvent (Table 1, entry 9). Then we studied the effect of ligands, but reduced yields were obtained with the tested ligands (Table 1, entries 10–13). Interestingly, the yield of 3aa was almost not changed when we decreased the pressure of CO to 5 bar (Table 1, entry 14). To our satisfaction, 3aa was obtained in 81% yield when CsF was used as an additive (Table 1, entry 15).| Entry | [Pd] | Ligand | Base | B(OH)3 (mmol) | Yield [%]b |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (0.9 mmol), [Pd] (10 mol%), monodentate ligand (20 mol%) or bidentate ligand (10 mol%), B(OH)3, base (1.2 mmol) in dioxane (1.5 mL) at 80 °C for 18 h under CO (10 bar). b Yields were determined by GC-FID analysis using n-hexadecane as an internal standard. c MeCN as solvent. d CO (5 bar). e CsF (0.3 mmol) as an additive. f Yield of the isolated product. | |||||
| 1 | PdCl2 | Xantphos | DiPEA | 0.2 | 57 |
| 2 | PdCl2 | Xantphos | Na2CO3 | 0.2 | 19 |
| 3 | PdCl2 | Xantphos | NaOtBu | 0.2 | N.D. |
| 4 | Pd(OAc)2 | Xantphos | DiPEA | 0.2 | 50 |
| 5 | Pd(PPh3)4 | Xantphos | DiPEA | 0.2 | 46 |
| 6 | Pd(TFA)2 | Xantphos | DiPEA | 0.2 | 46 |
| 7 | PdCl2 | Xantphos | DiPEA | 0.4 | 60 |
| 8 | PdCl2 | Xantphos | DiPEA | 0 | 17 |
| 9c | PdCl2 | Xantphos | DiPEA | 0.4 | 66 |
| 10c | PdCl2 | PPh3 | DiPEA | 0.4 | 50 |
| 11c | PdCl2 | DPEphos | DiPEA | 0.4 | 18 |
| 12c | PdCl2 | Nixantphos | DiPEA | 0.4 | 46 |
| 13c | PdCl2 | DPPP | DiPEA | 0.4 | Trace |
| 14c,d | PdCl2 | Xantphos | DiPEA | 0.4 | 67 |
| 15c,d,e | PdCl2 | Xantphos | DiPEA | 0.4 | 81 (78)f |
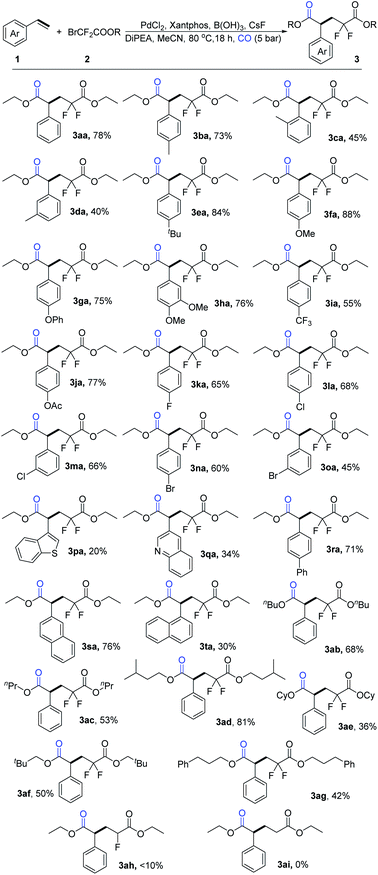
With the best reaction conditions in hand, we conducted our investigation into substrate scope, and a variety of aryl olefins were tested (Scheme 3). Aryl olefins with electron-donating groups, such as methyl, tert-butyl, methoxy, benzeneoxy, and 3, 4-dimethoxy groups were tolerated well to give the desired difluoropentanedioate products in moderate to high isolated yields (3ba-3ha), Notably, the yield can reach up to 88% when the olefin bears a methoxy group at the para-position. It should be mentioned that ortho-substituted styrene provided the corresponding product in lower yield compared with para-substituted styrene probably due to the steric hindrance (3cavs3ba). Aryl olefins bearing electron-withdrawing groups, such as trifluoromethyl, acetate, can afford the target products in moderate to good yields as well (3ia-3ja). For those substrates with halogen groups, including fluoro, chloro, and bromo substituents, the desired products were isolated in good yields (3ka-3oa). To our delight, 3-vinylbenzo thiophene and 3-vinylquinoline were also tolerable under our standard conditions (3pa-3qa). Moreover, substrates with 1-biphenyl and 2-naphthalene moieties could also work well to give the corresponding products in good yields (3ra-3sa). 1-Vinylnaphthalene gave the corresponding product in lower yield compared with 2-vinylnaphthalene probably because of the steric hindrance (3tavs.3sa). Moreover, six additional examples of bromodifluoroacetates were examined and the desired products 3ab-3ag were all isolated in moderate to good yields. However, when ethyl 2-bromo-2-fluoroacetate and ethyl 2-bromoacetate were tested, very low or no yield of the desired product could be detected (3ah, 3ai).
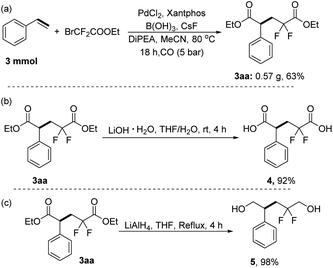
To demonstrate the scalability and utility of this method, we conducted a scale-up reaction and further transformations of the obtained product 3aa. The desired diethyl 2, 2-difluoro-4-phenylpentanedioate 3aa can still be obtained in 63% yield when we expanded the reaction by 10 times (Scheme 4a). Then 2, 2-difluoro-4-phenylpentanedioic acid was obtained with 92% yield by alkaline hydrolysis of the product 3aa in THF/H2O with LiOH as the base (Scheme 4b). Subsequently, 98% yield of 2,2-difluoro-4-phenylpentane-1,5-diol was achieved from the product 3aa by using lithium aluminum hydride as the reductant (Scheme 4c).
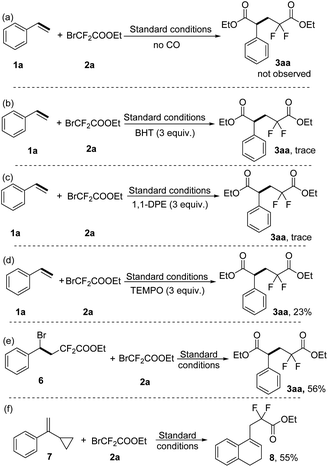
In order to gain some insight into the reaction mechanism, several control experiments were performed. Firstly, the target product 3aa was not observed in the absence of carbon monoxide gas under the standard conditions (Scheme 5a). Secondly, only a trace amount of the target product 3aa was detected when the radical inhibitor BHT (2,4-di-tert-butyl-4-methylphenol, 3 equiv.) or 1, 1-DPE (1,1-diphenylethylene, 3 equiv.) was added to our model reaction under the standard conditions (Scheme 5b and 5c). Similarly, the yield of 3aa was decreased to 23% when the radical inhibitor TEMPO (3 equiv.) was added (Scheme 5d). Furthermore, radical inhibitors 1, 1-DPE and TEMPO both trapped the difluoroacetate radical and detected it in GC-MS (see the ESI†), which indicates that this reaction involves radical intermediates. Moreover, the possible intermediate 6 was prepared and then reacted with ethyl bromodifluoroacetate 2a under standard conditions, and the desired product 3aa was obtained in 56% yield (Scheme 5e). The radical nature of this reaction was also proven by the ring-opening radical clock reaction and 55% of the corresponding product 8 was obtained under our standard conditions (Scheme 5f).
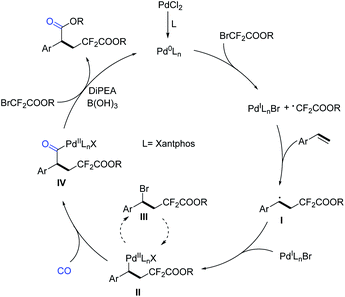
Based on the above results and literature studies,13,16 a plausible reaction mechanism is proposed (Scheme 6). The catalytic cycle starts from the active catalyst Pd0Ln species, which was generated from the PdCl2 pre-catalyst. Then, the Pd0Ln complex induced a SET (single-electron transfer) process of bromodifluoroacetate to give the corresponding difluoroacetate radical and a PdILnX species, followed by the addition of the difluoroacetate radical to aryl olefin to give a new secondary benzylic radical I. Subsequently, the PdILnX species was reincorporated with the carbon radical I to afford the key intermediate II. It is important to mention that the complex II can be converted into III through reductive elimination. However, the reaction is reversible and compound III can react with the reactive Pd0Ln species and be reconverted into II. After the insertion of carbon monoxide, complex II will be transformed into intermediate IV. Finally, intermediate IV reacts with another molecule of bromodifluoroacetate and gives the desired final product after the reductive elimination procedure. Meanwhile, in the presence of DiPEA, Pd0Ln will be regenerated for the next catalytic cycle. Although their roles are not very clear, we believe that B(OH)3 can promote the decomposition of bromodifluoroacetate for alcohol release and CsF is more like a buffer here.
Conclusions
In summary, a novel palladium-catalyzed procedure for difluoroalkylative carbonylation of aryl olefins has been developed. We have overcome the previous inability to take advantage of aryl olefins in difluoroalkylative carbonylation. A variety of aryl olefins were transformed into the corresponding difluoropentanedioate compounds in good yields with broad functional group tolerance and excellent selectivity. Additionally, the scaled-up reaction to a 3 mmol scale can be performed smoothly with a similar yield. Furthermore, the produced difluoropentanedioate product can be efficiently converted to the corresponding diacid and diol in a facile manner.Author contributions
XFW surprised this project and revised the manuscirpt. ZPB and YZ performed all the experiments. ZPB prepared the first version of this manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful for the financial support from DICP and the K. C. Wong Education Foundation (GJTD-2020-08).Notes and references
- (a) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422–518 CrossRef CAS PubMed; (b) M. Salwiczek, E. K. Nyakatura, U. I. M. Gerling, S. Ye and B. Koksch, Chem. Soc. Rev., 2012, 41, 2135–2171 RSC; (c) W. Zhang, Chem. Rev., 2009, 109, 749–795 CrossRef CAS PubMed; (d) W. Zhou, W.-J. Pan, J. Chen, M. Zhang, J.-H. Lin and W. C. J.-C. Xiao, Chem. Commun., 2021, 57, 9316–9329 RSC.
- (a) P. Kirsch, Modern fluoroorganic chemistry: synthesis, reactivity, applications, John Wiley & Sons, 2013 CrossRef; (b) N. A. Meanwell, J. Med. Chem., 2018, 61, 5822–5880 CrossRef CAS PubMed; (c) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS PubMed; (d) Z. Feng, Y.-L. Xiao and X. Zhang, Acc. Chem. Res., 2018, 51, 2264–2278 CrossRef CAS PubMed; (e) X. Ma and Q. Song, Chem. Soc. Rev., 2020, 49, 9197–9219 RSC.
- N. J. Carter and G. M. Keating, Drugs, 2007, 67, 2277–2288 CrossRef CAS PubMed.
- P. Shweta, V. Juhi, D. D. Ravindra, P. D. Rikeshwer, M. Nagashekhara, A. B. Ranjeet, K. S. Pravat and K. Prashant, Int. J. Pharm., 2021, 592, 120043 CrossRef PubMed.
- E. D. Deeks, Drugs, 2016, 76, 1191–1201 CrossRef CAS PubMed.
- M. B. Pantcheva, L. K. Seibold, N. S. Awadallah and M. Y. Kahook, Adv. Ther., 2011, 28, 707 CrossRef CAS PubMed.
- (a) Z. Feng, Q.-Q. Min, H.-Y. Zhao, J.-W. Gu and X. Zhang, Angew. Chem., Int. Ed., 2015, 127, 1286–1290 CrossRef; (b) N. J. W. Straathof, S. E. Cramer, V. Hessel and T. Noël, Angew. Chem., Int. Ed., 2016, 55, 15549–15553 CrossRef CAS PubMed; (c) X. Wang, S. Zhao, J. Liu, D. Zhu, M. Guo, X. Tang and G. Wang, Org. Lett., 2017, 19, 4187–4190 CrossRef CAS PubMed; (d) D. P. Tiwari, S. Dabral, J. Wen, J. Wiesenthal, S. Terhorst and C. Bolm, Org. Lett., 2017, 19, 4295–4298 CrossRef CAS PubMed; (e) L. Zhao, Y. Huang, Z. Wang, E. Zhu, T. Mao, J. Jia, J. Gu, X.-F. Li and C.-Y. He, Org. Lett., 2019, 21, 6705–6709 CrossRef CAS PubMed; (f) K. Li, X. Zhang, J. Chen, Y. Gao, C. Yang, K. Zhang, Y. Zhou and B. Fan, Org. Lett., 2019, 21, 9914–9918 CrossRef CAS PubMed; (g) C. Yu, N. Iqbal, S. Park and E. J. Cho, Chem. Commun., 2014, 50, 12884–12887 RSC; (h) W.-W. Xu, L. Wang, T. Mao, J. Gu, X.-F. Li and C.-Y. He, Molecules, 2020, 25, 508 CrossRef CAS PubMed.
- For selected examples on transition-metal catalyzed radical fluoroalkylation of aryl olefins, see: (a) W. Kong, C. Yu, H. An and Q. Song, Org. Lett., 2018, 20, 4975–4978 CrossRef CAS PubMed; (b) X. Wang, Y. Li, H.-C. Liu, B.-S. Zhang, X.-Y. Gou, Q. Wang, J.-W. Ma and Y.-M. Liang, J. Am. Chem. Soc., 2019, 141, 13914–13922 CrossRef CAS PubMed; (c) B. Gao, Y. Ni, X. Liu, T. Jiang, Q. Yan, R. Yang and X. Zhang, Eur. J. Org. Chem., 2021, 1913–1918 CrossRef CAS; (d) W.-Y. Shi, Y.-N. Ding, C. Liu, N. Zheng, X.-Y. Gou, M. Li, Z. Zhang, H.-C. Liu, Z.-J. Niu and Y.-M. Liang, Chem. Commun., 2020, 56, 12729–12732 RSC; (e) Y. Chen, L. Li, Y. Ma and Z. Li, J. Org. Chem., 2019, 84, 5328–5338 CrossRef CAS PubMed.
- For selected examples on photoinduced radical fluoroalkylation of aryl olefins, see: (a) P. Zhang, W. Li, W. Qu, Z. Shu, Y. Tao, J. Lin and X. Gao, Org. Lett., 2021, 23, 9267–9272 CrossRef CAS PubMed; (b) M. Zhang, J.-H. Lin, C.-M. Jin and J.-C. Xiao, Chem. Commun., 2021, 57, 2649–2652 RSC; (c) X.-L. Lv, C. Wang, Q.-L. Wang and W. Shu, Org. Lett., 2019, 21, 56–59 CrossRef CAS PubMed; (d) R. Xu and C. Cai, Chem. Commun., 2019, 55, 4383–84386 RSC; (e) R. Xu and C. Cai, Org. Biomol. Chem., 2019, 17, 8541–8545 RSC; (f) Y.-F. Yang, J.-H. Lin and J.-C. Xiao, Org. Lett., 2021, 23, 9277–9282 CrossRef CAS PubMed; (g) M. Zhang, J.-H. Lin and J.-C. Xiao, Org. Lett., 2021, 23, 6079–6083 CrossRef CAS PubMed; (h) M. Zhang, J.-H. Lin and J.-C. Xiao, Angew. Chem., Int. Ed., 2019, 58, 6079–6083 CrossRef CAS PubMed.
- For selected examples on N-heterocyclic carbene (NHC) catalysed radical fluoroalkylation of aryl olefins, see: (a) J.-L. Li, Y.-Q. Liu, W.-L. Zou, R. Zeng, X. Zhang, Y. Liu, B. Han, Y. He, H.-J. Leng and Q.-Z. Li, Angew. Chem., Int. Ed., 2020, 59, 1863–1870 CrossRef CAS PubMed; (b) H.-B. Yang, Z.-H. Wang, J.-M. Lia and C. Wu, Chem. Commun., 2020, 56, 3801–3804 RSC.
- (a) J.-B. Peng, F.-P. Wu and X.-F. Wu, Chem. Rev., 2019, 119, 2090–2127 CrossRef CAS PubMed; (b) H.-Y. Zhao, M. Zhou and X. Zhang, Org. Lett., 2021, 23, 9106–9111 CrossRef CAS PubMed; (c) M. Zhou, H.-Y. Zhao, S. Zhang, Y. Zhang and X. Zhang, J. Am. Chem. Soc., 2020, 142, 18191–18199 CrossRef CAS PubMed; (d) Q. Wang, Y.-T. He, J.-H. Zhao, Y.-F. Qiu, L. Zheng, J.-Y. Hu, Y.-C. Yang, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2016, 18, 2664–2667 CrossRef CAS PubMed; (e) F.-P. Wu, Y. Yuan, J. Liu and X.-F. Wu, Angew. Chem., Int. Ed., 2021, 60, 8818–8822 CrossRef CAS PubMed; (f) H.-J. Ai, W. Lu and X.-F. Wu, Angew. Chem., Int. Ed., 2021, 60, 17178–17184 CrossRef CAS PubMed; (g) X.-P. Fu, X.-S. Xue, X.-Y. Zhang, Y.-L. Xiao, S. Zhang, Y.-L. Guo, X. Leng, K. N. Houk and X. Zhang, Nat. Chem., 2019, 11, 948–956 CrossRef CAS PubMed; (h) R. Cheng, Y. Sang, X. Gao, S. Zhang, X.-S. Xue and X. Zhang, Angew. Chem., Int. Ed., 2021, 60, 12386–12391 CrossRef CAS PubMed; (i) B. Tian, X. Li, P. Chen and G. Liu, Angew. Chem., Int. Ed., 2021, 60, 14881–14886 CrossRef CAS PubMed; (j) Carbon monoxide in organic synthesis-Carbonylation chemistry, ed., B. Gabriele, Wiley-VCH, 2021 Search PubMed; (k) L. Kollär, in Modern Carbonylation Methods, Wiley-VCH, Weinheim, 2008 CrossRef.
- X. Qi, F. Yu, P. Chen and G. Liu, Angew. Chem., Int. Ed., 2017, 56, 12692–12696 CrossRef CAS PubMed.
- (a) Y. Zhang, H.-Q. Geng and X.-F. Wu, Angew. Chem., Int. Ed., 2021, 60, 24292–24298 CrossRef CAS PubMed; (b) Y. Zhang, H.-Q. Geng and X.-F. Wu, Chem. – Eur. J., 2021, 27, 17682–17687 CrossRef CAS PubMed.
- F.-P. Wu, Y. Yuan and X.-F. Wu, Angew. Chem., Int. Ed., 2021, 60, 25787–25792 CrossRef CAS PubMed.
- H.-J. Ai, C.-X. Cai, X. Qi, J.-B. Peng, F. Zheng and X.-F. Wu, Tetrahedron Lett., 2017, 58, 3846–3850 CrossRef CAS.
- (a) H. Yin and T. Skrydstrup, J. Org. Chem., 2017, 82, 6474–6481 CrossRef CAS PubMed; (b) S. Domański, O. Staszewska-Krajewska and W. Chaładaj, J. Org. Chem., 2017, 82, 7998–8007 CrossRef PubMed; (c) B. Gatlik and W. Chaładaj, ACS Catal., 2021, 11, 6547–6559 CrossRef CAS; (d) Q. Wang, X. Yu, J. Jin, Y. Wu and Y. Liang, Chin. J. Chem., 2018, 36, 223–226 CrossRef CAS; (e) Q. Wang, L. Zheng, Y.-T. He and Y.-M. Liang, Chem. Commun., 2017, 53, 2814–2817 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: general comments, general procedure, analytic data, and NMR spectra. See https://doi.org/10.1039/d2sc02665a |
| This journal is © The Royal Society of Chemistry 2022 |