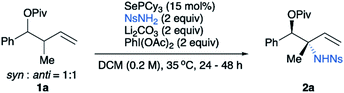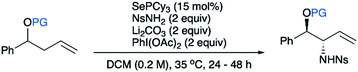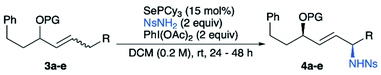Open Access Article
Open Access ArticleDiastereoconvergent synthesis of anti-1,2-amino alcohols with N-containing quaternary stereocenters via selenium-catalyzed intermolecular C–H amination†
Tianyi
Zheng
,
Janna L.
Berman
and
Forrest E.
Michael
 *
*
Department of Chemistry, University of Washington, Box 351700, Seattle, Washington 98195-1700, USA. E-mail: michael@chem.washington.edu
First published on 3rd August 2022
Abstract
We report a diastereoconvergent synthesis of anti-1,2-amino alcohols bearing N-containing quaternary stereocenters using an intermolecular direct C–H amination of homoallylic alcohol derivatives catalyzed by a phosphine selenide. Destruction of the allylic stereocenter during the selenium-catalyzed process allows selective formation of a single diastereomer of the product starting from any diastereomeric mixture of the starting homoallylic alcohol derivatives, eliminating the need for the often-challenging diastereoselective preparation of starting materials. Mechanistic studies show that the diastereoselectivity is controlled by a stereoelectronic effect (inside alkoxy effect) on the transition state of the final [2,3]-sigmatropic rearrangement, leading to the observed anti selectivity. The power of this protocol is further demonstrated on an extension to the synthesis of syn-1,4-amino alcohols from allylic alcohol derivatives, constituting a rare example of 1,4-stereoinduction.
Introduction
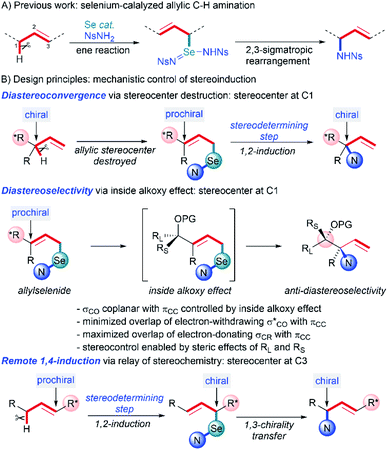
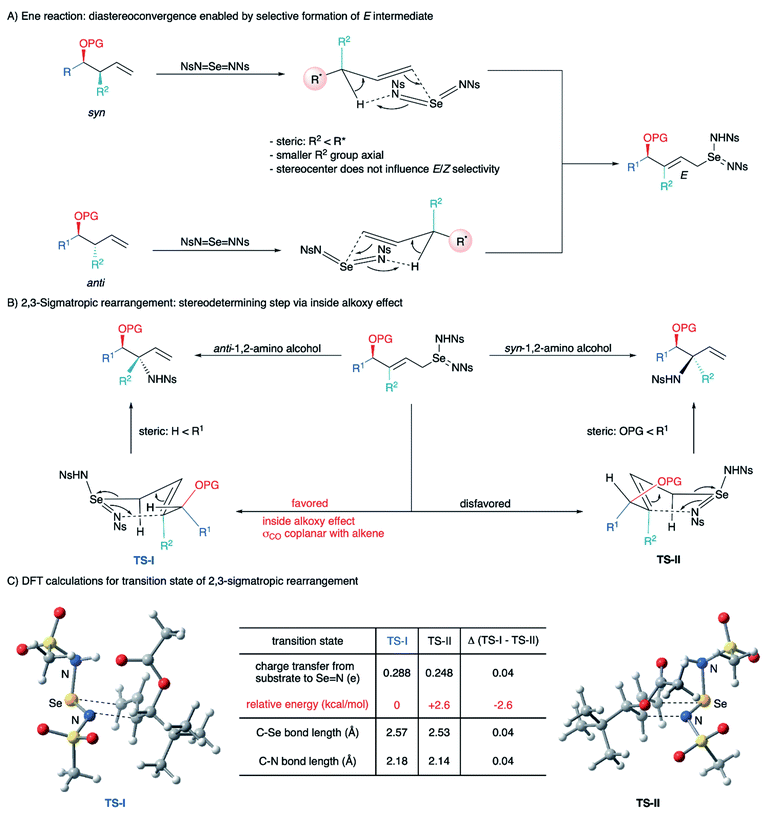
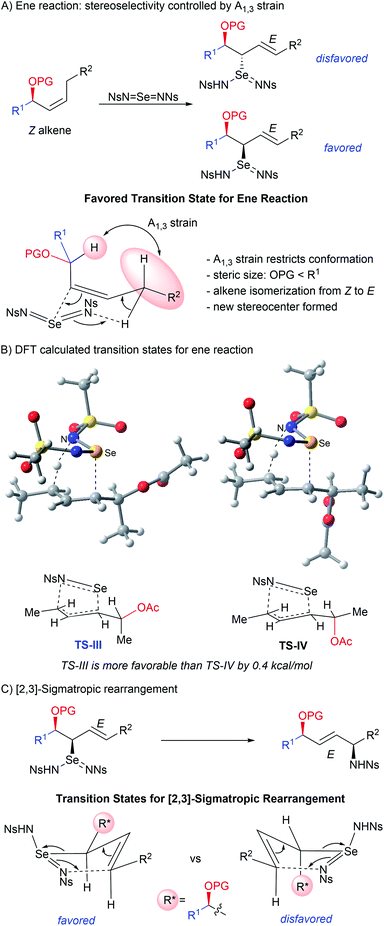
The prevalence of stereodefined 1,2- and 1,4-amino alcohol motifs in pharmaceuticals and asymmetric catalysts has generated a need for stereocontrolled synthesis of these structures.1 Direct C–H functionalization is now a state-of-the-art strategy for the synthesis of 1,2-amino alcohols, allowing introduction of new C–N or C–O bonds without prefunctionalization at that carbon atom.2 In this context, a variety of transition-metal catalyzed3 and radical-mediated4 C–H amination approaches to 1,2-amino alcohols have been reported. Although these methods provide efficient access to these compounds, little attention has been paid to the diastereoselectivity1,3a–c,5 of these transformations, especially intermolecular aminations, or to the construction of quaternary heterosubstituted stereocenters.6 Additionally, diastereoselective synthesis of 1,4-amino alcohols via direct C–H functionalization has not been reported.We previously reported a metal-free allylic amination catalyzed by phosphine selenides that proceeds via a mechanism distinct from most other C–H functionalizations.7 Initial ene reaction between an imidoselenium species cleaves the C–H bond and transposes the alkene, and a subsequent [2,3]-sigmatropic rearrangement delivers the new C–N bond (Scheme 1A). This “flip-flop” mechanism has several distinct properties that enable new modes of diastereocontrol without requiring intramolecular delivery of the nitrogen group: (i) C–H bond cleavage and C–N bond formation occur in separate steps, (ii) both steps are concerted and proceed suprafacially, (iii) initial activation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond by Se takes place distal to the C–H bond being functionalized, and (iv) there is no allylic transposition of the intermediate, allowing either step to be stereodetermining depending on the substrate. Careful analysis of these key elements reveals that the presence of an oxygen-substituted stereocenter attached to either C1 or C3 of the allyl system would enable diastereoselective synthesis of 1,2- and 1,4-amino alcohols that are difficult to access using current C–H amination methods.
C bond by Se takes place distal to the C–H bond being functionalized, and (iv) there is no allylic transposition of the intermediate, allowing either step to be stereodetermining depending on the substrate. Careful analysis of these key elements reveals that the presence of an oxygen-substituted stereocenter attached to either C1 or C3 of the allyl system would enable diastereoselective synthesis of 1,2- and 1,4-amino alcohols that are difficult to access using current C–H amination methods.
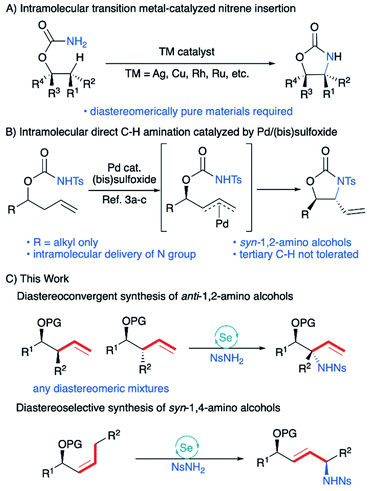
When the stereocenter is attached to C1 (Scheme 1B), the allylic stereocenter in starting material is destroyed in the ene reaction, and then formed anew in the stereodetermining [2,3]-sigmatropic rearrangement, thus enabling a diastereoconvergent process whereby a single diastereomer can be accessed from diastereomeric mixtures of the starting material. In contrast, the stereospecificity of most C–H nitrenoid insertion approaches3d–o would require diastereomerically pure starting materials that are often challenging to access8 (Scheme 2A).
Second, the stereodetermining [2,3]-sigmatropic rearrangement now takes place in close proximity to the controlling stereocenter, allowing unprecedented diastereoselectivity via steric and/or electronic differentiation of the prochiral faces of the alkene (Scheme 1B). We hypothesized that an analog of the “inside alkoxy effect”9 might control the orientation of the C–O stereocenter in the transition state. Minimizing overlap of the 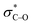 orbital with the π system would stabilize the relatively electron-poor transition state, allowing delivery of the nitrogen to the less sterically hindered face and thereby giving high anti selectivity. This is complementary to the syn selectivity observed for known methods3a–c,10 resulting from intramolecular delivery of a tethered nitrogen group (Scheme 2B).
orbital with the π system would stabilize the relatively electron-poor transition state, allowing delivery of the nitrogen to the less sterically hindered face and thereby giving high anti selectivity. This is complementary to the syn selectivity observed for known methods3a–c,10 resulting from intramolecular delivery of a tethered nitrogen group (Scheme 2B).
Alternately, when the stereocenter is attached to C3, the stereodetermining step switches to the initial ene reaction. In this step, attack of the selenium now occurs alpha to the stereocenter, allowing diastereoselectivity via 1,2-induction (Scheme 1B). This stereochemistry is then selectively transferred to the distal center in the suprafacial [2,3]-rearrangement. In this scenario, initial 1,2-stereoselection is efficiently relayed into remote 1,4-induction. Remote stereoinduction of this type is difficult to achieve.11
Herein, we disclose a diastereoconvergent synthesis of anti-1,2-amino alcohols bearing N-containing quaternary stereocenters via intermolecular direct C–H amination of homoallylic alcohol derivatives under metal-free conditions. We show that high diastereoselectivity is observed in the formation of 1,2-amino alcohols without quaternary centers as well. We also demonstrate the application of this protocol to remote 1,4-induction, resulting in the synthesis of syn-1,4-amino alcohols from (Z)-allylic alcohol derivatives (Scheme 2C).
Results and discussion
To test the feasibility of this approach, we started with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers of homoallylic ester 1a as the model substrate. Our previously reported standard conditions for allylic amination gave the desired amination product, but in a disappointing 15% yield (Table 1, entry 1). Both conversion and overall mass recovery were low. Notably, we found that addition of an insoluble base is crucial to the success of the reaction. The role of this base is still unclear, but we hypothesize that trace acids formed under these conditions may partially decompose the starting material and/or product. A screen of basic additives revealed that Li2CO3 was optimal, giving product 2a in 79% yield (Table 1, entry 2). Conversely, an acid additive lowers the conversion (Table 1, entry 5). Importantly, the starting 1
1 mixture of diastereomers of homoallylic ester 1a as the model substrate. Our previously reported standard conditions for allylic amination gave the desired amination product, but in a disappointing 15% yield (Table 1, entry 1). Both conversion and overall mass recovery were low. Notably, we found that addition of an insoluble base is crucial to the success of the reaction. The role of this base is still unclear, but we hypothesize that trace acids formed under these conditions may partially decompose the starting material and/or product. A screen of basic additives revealed that Li2CO3 was optimal, giving product 2a in 79% yield (Table 1, entry 2). Conversely, an acid additive lowers the conversion (Table 1, entry 5). Importantly, the starting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture in substrate 1a was converted to a 10
1 diastereomeric mixture in substrate 1a was converted to a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomeric products 2a. The steric size of the protecting group has a minor effect on the diastereoselectivity, with acetate giving somewhat lower selectivity than pivalate (Table 1, entry 3).
1 mixture of diastereomeric products 2a. The steric size of the protecting group has a minor effect on the diastereoselectivity, with acetate giving somewhat lower selectivity than pivalate (Table 1, entry 3).
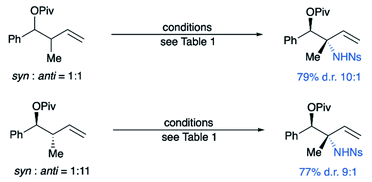
Importantly, as hypothesized, the relative configuration of the starting material has no impact on the diastereoselectivity or yield of the reaction (Scheme 3). Even substrate 1a enriched in the anti-diastereomer gives inversion of stereochemistry of the allylic stereocenter to give the same diastereomeric ratio with nearly identical yields. This highlights the advantage of this reaction in eliminating the need for preparing a single diastereomer of the substrate.
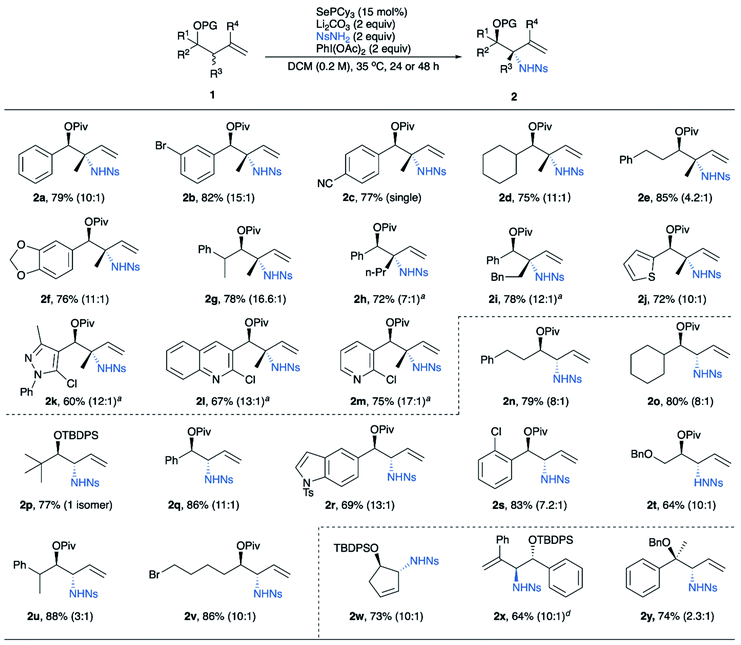
Next, we examined homoallylic alcohol substrates without substitution at the allylic position to see if our conditions would also give stereoselectivity for these substrates. A screen of common alcohol protecting groups showed that most give good yields and diastereoselectivities, including acetyl (Ac), pivaloyl (Piv), benzyl (Bn), and t-butyldiphenylsilyl (TBDPS) (Table 2). For the best combination of yield and selectivity we chose to continue with pivaloyl protected substrates. Thus, we obtained an optimal unified protocol for amination of substrates both with and without allylic substituents.
The optimized conditions were applied to a variety of homoallylic alcohol derivatives (Table 3). The reaction displays excellent compatibility with many functional groups including ester, alkyl halides, nitrile, aryl halides, ether, silyl ether, etc. Notably, our reaction tolerates a variety of heterocycles, especially nitrogen-containing heteroaromatics, including thiophene (2j), indole (2r), pyrazole (2k), quinoline (2l) and pyridine (2m). Additional substitution at the allylic position can be tolerated, giving aminated quaternary stereocenters in good yields and diastereoselectivities (2h, 2i). The reaction also tolerates substituents with a wide range of steric demands at the homoallylic position, including primary, secondary, and tertiary alkyl groups, as well as aryl groups. Notably, diastereoselectivities are high even with minimal steric difference between the R group and OPiv group (2n, 2v, 2t), though diastereoselectivity increases as the homoallylic group becomes larger. These trends apply to both substrates without (2n, 2o, 2q and 2p) and with allylic substituents (2e, 2a, and 2d). Furthermore, the reaction is also effective for 1,1-disubstituted alkenes (2x) and cyclic alkenes (2w).
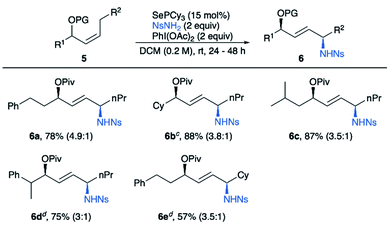
We then investigated the diastereoselective synthesis of 1,4-amino alcohols starting from internal allylic alcohol derivatives. We started our exploration by subjecting (E)-alkene substrates 3a–d to our previously published allylic amination conditions. Promisingly, the desired product was obtained in high yields, but no diastereoselectivity was observed using acetate as protecting group (Table 4, entry 1). A screen of protecting groups showed that larger groups gave only modest increases in diastereoselectivity (Table 4). We hypothesized that changing the alkene geometry to Z could improve the stereoselectivity by introducing 1,3-allylic strain,12 thereby reducing the conformational flexibility at the allylic position (see Scheme 8A). To our delight, amination of (Z)-alkene 3e gave a marked increase in diastereoselectivity to 4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 4, entry 5). Though starting alkene 3e had the Z configuration, product 4e was found to be exclusively E, which was confirmed by observation of a coupling constant of 15.5 Hz between the alkene protons. The substrate scope for this 1,4-amino alcohol synthesis is given in Table 5. The reaction tolerates both primary and secondary groups on both sides of the alkene.
1 (Table 4, entry 5). Though starting alkene 3e had the Z configuration, product 4e was found to be exclusively E, which was confirmed by observation of a coupling constant of 15.5 Hz between the alkene protons. The substrate scope for this 1,4-amino alcohol synthesis is given in Table 5. The reaction tolerates both primary and secondary groups on both sides of the alkene.
| Entry | Substrate | PGb and R | E or Z | Yielda (%) | d.r.a |
|---|---|---|---|---|---|
| a Yields and diastereomeric ratio (d.r.) determined by 1H NMR spectroscopy using 1,3-dinitrobenzene as internal standard. b PG = protecting group. | |||||
| 1 | 3a | Ac, Me | E | 75 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 3b | Bz, Me | E | 75 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 3c | TBDPS, Me | E | 62 | 1.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 3d | Piv, Me | E | 76 | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 3e | Piv, n-Pr | Z | 78 | 4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
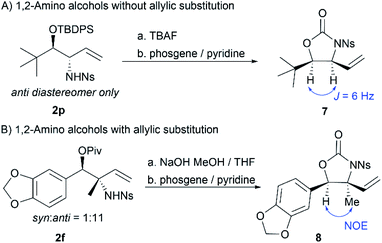
To probe the stereochemistry of the 1,2-amino alcohol products, 2p and 2f were deprotected and cyclized to 7 and 8, respectively (Scheme 4). Comparison of the coupling constant in 7 to the known literature value10b reveals that the protons are cis, indicating that the reaction affords anti-amino alcohols when there are no allylic substituents. For substituted product 8, an NOE was observed between the proton alpha to oxygen and the allylic methyl group, indicating that the major product bearing allylic substituents is also the anti-amino alcohol.
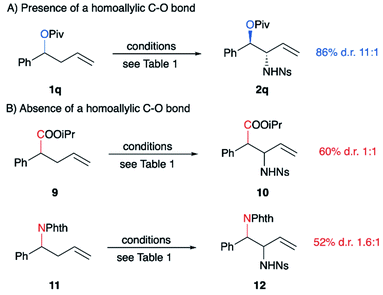
To explain the diastereoselectivity, a stereochemical model was built based on the proposed mechanism (Scheme 5), namely that the reaction proceeds via an ene reaction between a selenium bis(imide) and the alkene, followed by a [2,3]-sigmatropic rearrangement. The diastereoconvergence is explained by the fact that the allylic stereocenter is destroyed in the initial ene reaction, resulting in preferential formation of the E alkene intermediate from both diastereomers of the starting material (Scheme 5A). The allylic amine stereocenter is then formed in the [2,3]-sigmatropic rearrangement step. Importantly, the relative stereochemistry of the C–N and C–O bonds is the same for both R2 = H and R2 = alkyl, indicating that the stereochemistry is being controlled only by the stereocenter attached to oxygen. To further test the importance of the inside alkoxy effect in controlling selectivity, ester 9 and homoallylic amine 11 were treated under our optimal reaction conditions, respectively (Scheme 6). Though the reactions proceeded in moderate yields, poor diastereoselectivity was observed, strongly supporting our hypothesis that the inside alkoxy effect9 is the primary factor responsible for the high diastereoselectivity we observe for homoallylic alcohol derivatives.
To gain further insights into the stereochemistry-determining [2,3]-sigmatropic rearrangement, a DFT computational study was performed (Scheme 5C).13 Transition states for the [2,3]-sigmatropic rearrangement step leading to each diastereomeric product were found. The one leading to the anti product (TS-I) was lower in energy by 2.6 kcal mol−1 than the one leading to the syn product (TS-II), consistent with our experimental results. Careful examination of the two transition states did not reveal any obvious steric clashes. In both, approach of the nitrogen takes place on the opposite face of the bulky alkyl substituent. Interestingly, we observed that TS-I had notably longer C–Se and C–N distances than TS-II (Δ ∼ 0.04 Å), indicating a looser transition state. This was accompanied by a greater degree of charge transfer from the allyl fragment to the selenium bis(imide) fragment (Δq = 0.04). These results are consistent with a stereoelectronic effect on the transition state similar to the known inside alkoxy effect9 described for cycloaddition reactions. NBO calculations show that the π system becomes electron-deficient during the rearrangement and that the orientation of the oxygen substituent affects the degree of charge transfer. In the most stable transition state (TS-I), the protected hydroxyl group is coplanar with the alkene, thereby minimizing the overlap of the electron-withdrawing 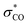 orbital with the π system. Simultaneously, this orientation maximizes the overlap of the electron-donating σC–H and σC–C bonds with the π system, enabling greater charge transfer and thereby stabilizing the transition state.
orbital with the π system. Simultaneously, this orientation maximizes the overlap of the electron-donating σC–H and σC–C bonds with the π system, enabling greater charge transfer and thereby stabilizing the transition state.
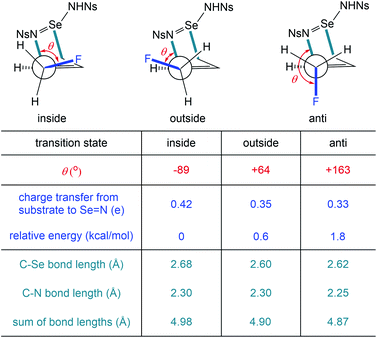
To isolate this stereoelectronic effect and more closely examine it, transition states for the [2,3]-sigmatropic rearrangement on a simple homoallyl fluoride substrate were found for three conformations about the C–C bond: anti, inside, and outside (Scheme 7). The inside fluoro transition state was again found to be the lowest in energy. As before, there is a clear correlation between the dihedral angle of the C–F bond and the amount of charge transfer from the substrate to the Se–N fragment, with the greatest charge transfer occurring in the inside fluoro transition state. Similarly, the transition states with more charge transfer were found to be looser, as indicated by the sum of the C–Se and C–N bond lengths, and had substantially more charge transfer to the selenium imide fragment. These calculated transition states confirm the importance of stereoelectronic control in the stereochemistry-determining [2,3]-sigmatropic rearrangement.
The stereoselectivity in the 1,4-amino alcohols was explored through DFT calculations using a similar stereochemical model to that developed for the anti-1,2-amino alcohols.13 Unlike for 1,2-amino alcohols, however, the stereochemistry of this product is determined in the initial ene reaction. Avoidance of 1,3-allylic strain fixes the orientation of the allylic stereocenter as in Scheme 8A. DFT calculations reveal that the most stable transition state has the selenium bis(imide) approach from the same face of the alkene as the acyloxy group (TS-III), rather than adjacent to the alkyl group (TS-IV). Though small, the preference for TS-III over TS-IV of 0.4 kcal mol−1 is consistent with the relative steric size of these two groups and the observed diastereomeric ratio. The requirement for conformational restriction using 1,3-allylic strain explains the improved performance of Z alkenes over E alkenes.
In the subsequent [2,3]-sigmatropic rearrangement, orientation of the R* group in the equatorial position is favored, leading to the formation of the syn-1,4-amino alcohol bearing an E alkene as the major diastereomer (Scheme 8C). This is consistent with the observation that only E alkenes are formed, regardless of the initial configuration of the starting alkenes.
Conclusions
In summary, we have developed a diastereoconvergent synthesis of anti-1,2-amino alcohols bearing quaternary N-containing stereocenter via intermolecular direct allylic C–H amination catalyzed by phosphine selenides, complementary to existing approaches that give syn-1,2-amino alcohols. Experimental and computational studies reveal that the diastereoselectivity is controlled by a stereoelectronic effect similar to the inside alkoxy effect, in which the protected hydroxyl group avoids withdrawing electron density from the transition state of the [2,3]-sigmatropic rearrangement. The diastereoconvergent ene reaction step eliminates the need to prepare a single diastereomer of homoallylic alcohol derivatives to achieve high diastereoselectivity. We have also developed a synthesis of syn-1,4-amino alcohols via direct C–H amination, constituting a rare example of remote stereochemical induction in the preparation of these compounds.Data availability
Data for this paper, including characterization data for all new compounds and structures and energies for all DFT computations, are provided in the ESI.†Author contributions
T. Z. conducted all experiments. J. L. B. performed all DFT calculations. F. E. M. directed the research. T. Z. and F. E. M. wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the University of Washington and the National Science Foundation (CHE-2102267) for funding. Computations were facilitated through the use of advanced computational, storage, and networking infrastructure provided by the Hyak supercomputer system at the University of Washington, funded by the Student Technology Fee.Notes and references
- For selected reviews on amino alcohols, see: (a) H.-S. Lee and S. H. Kang, Synlett, 2004, 1673–1685 CrossRef CAS and references therein.; (b) L. Pu and H.-B. Yu, Chem. Rev., 2001, 101, 757–824 CrossRef CAS PubMed; (c) D. J. Ager, I. Prakash and D. R. Schaad, Chem. Rev., 1996, 96, 835–876 CrossRef CAS PubMed.
- For selected reviews on C–H functionalization, see: (a) H. M. L. Davies and J. R. Manning, Nature, 2008, 451, 417–424 CrossRef CAS PubMed; (b) W. R. Gutekunst and P. S. Baran, Chem. Soc. Rev., 2011, 40, 1976–1991 RSC; (c) L. McMurray, F. O'Hara and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885–1898 RSC.
- Synthesis of 1,2-amino alcohols via direct C–H amination. For intramolecular transformations, see: (a) T. J. Osberger and M. C. White, J. Am. Chem. Soc., 2014, 136, 11176–11181 CrossRef CAS PubMed; (b) K. J. Fraunhoffer and M. C. White, J. Am. Chem. Soc., 2007, 129, 7274–7276 CrossRef CAS PubMed; (c) I. I. Strambeanu and M. C. White, J. Am. Chem. Soc., 2013, 135, 12032–12037 CrossRef CAS PubMed; (d) H. Lebel, K. Huard and S. Lectard, J. Am. Chem. Soc., 2005, 127, 14198–14199 CrossRef CAS PubMed; (e) H. Lebel, L. Mamani Laparra, M. Khalifa, C. Trudel, C. Audubert, M. Szponarski, C. Dicaire Leduc, E. Azek and M. Ernzerhof, Org. Biomol. Chem., 2017, 15, 4144–4158 RSC; (f) C. G. Espino and J. Du Bois, Angew. Chem., Int. Ed., 2001, 40, 598–600 CrossRef CAS; (g) G. Grelier, R. Rey-Rodriguez, B. Darses, P. Retailleau and P. Dauban, Eur. J. Org. Chem., 2017, 2017, 1880–1883 CrossRef CAS; (h) R. D. Grigg, J. W. Rigoli, S. D. Pearce and J. M. Schomaker, Org. Lett., 2012, 14, 280–283 CrossRef CAS PubMed; (i) M. Ju, M. Huang, L. E. Vine, M. Dehghany, J. M. Roberts and J. M. Schomaker, Nat. Catal., 2019, 2, 899–908 CrossRef CAS; (j) Y. Cui and C. He, Angew. Chem., Int. Ed., 2004, 43, 4210–4212 CrossRef CAS PubMed; (k) E. Milczek, N. Boudet and S. Blakey, Angew. Chem., Int. Ed., 2008, 47, 6825–6828 CrossRef CAS PubMed; (l) J.-L. Liang, S.-X. Yuan, J.-S. Huang, W.-Y. Yu and C.-M. Che, Angew. Chem., Int. Ed., 2002, 41, 3465–3468 CrossRef CAS; (m) D. N. Barman and K. M. Nicholas, Eur. J. Org. Chem., 2011, 2011, 908–911 CrossRef; (n) Y. Liu, X. Guan, E. L.-M. Wong, P. Liu, J.-S. Huang and C.-M. Che, J. Am. Chem. Soc., 2013, 135, 7194–7204 CrossRef CAS PubMed; (o) H. Jung, H. Keum, J. Kweon and S. Chang, J. Am. Chem. Soc., 2020, 142, 5811–5818 CrossRef CAS PubMed . For intermolecular transformations, see: ; (p) Y. Dong, J. Chen and H. Xu, Chem. Commun., 2018, 54, 11096–11099 RSC; (q) Y. Dong and G. Liu, J. Org. Chem., 2017, 82, 3864–3872 CrossRef CAS PubMed; (r) L. Jin, X. Zeng, S. Li, X. Hong, G. Qiu and P. Liu, Chem. Commun., 2017, 53, 3986–3989 RSC; (s) T. Kang, H. Kim, J. G. Kim and S. Chang, Chem. Commun., 2014, 50, 12073–12075 RSC.
- For examples of radical-mediated C–H amination for synthesis of 1,2-amino alcohols, see: (a) X.-Q. Mou, X.-Y. Chen, G. Chen and G. He, Chem. Commun., 2018, 54, 515–518 RSC; (b) K. M. Nakafuku, Z. Zhang, E. A. Wappes, L. M. Stateman, A. D. Chen and D. A. Nagib, Nat. Chem., 2020, 12, 697–704 CrossRef PubMed; (c) E. A. Wappes, K. M. Nakafuku and D. A. Nagib, J. Am. Chem. Soc., 2017, 139, 10204–10207 CrossRef CAS PubMed; (d) A. F. Prusinowski, R. K. Twumasi, E. A. Wappes and D. A. Nagib, J. Am. Chem. Soc., 2020, 142, 5429–5438 CrossRef CAS PubMed; (e) D. Kurandina, D. Yadagiri, M. Rivas, A. Kavun, P. Chuentragool, K. Hayama and V. Gevorgyan, J. Am. Chem. Soc., 2019, 141, 8104–8109 CrossRef CAS PubMed.
- For selected reviews on diastereoselective C–H amination, see: (a) P. Herrmann and T. Bach, Chem. Soc. Rev., 2011, 40, 2022–2038 RSC; (b) F. Collet, C. Lescot and P. Dauban, Chem. Soc. Rev., 2011, 40, 1926–1936 RSC; (c) T. A. Ramirez, B. Zhao and Y. Shi, Chem. Soc. Rev., 2012, 41, 931–942 RSC; (d) B. Darses, R. Rodrigues, L. Neuville, M. Mazurais and P. Dauban, Chem. Commun., 2017, 53, 493–508 RSC.
- N. Mateu, S. L. Kidd, L. Kalash, H. F. Sore, A. Madin, A. Bender and D. R. Spring, Chem.–Eur. J., 2018, 24, 13681–13687 CrossRef CAS PubMed and references therein..
- (a) W. P. Teh, D. C. Obenschain, B. M. Black and F. E. Michael, J. Am. Chem. Soc., 2020, 142, 16716–16722 CrossRef CAS PubMed; (b) T. P. Maloney, A. F. Dohoda, A. C. Zhu and F. E. Michael, Chem. Sci., 2022, 13, 2121–2127 RSC.
- For selected reviews on diastereoselective synthesis of homoallylic alcohols, see: (a) M. Xiang, D. E. Pfaffinger and M. J. Krische, Chem.–Eur. J., 2021, 27, 13107–13116 CrossRef CAS PubMed; (b) J. M. Ketcham, I. Shin, T. P. Montgomery and M. J. Krische, Angew. Chem., Int. Ed. Engl., 2014, 53, 9142–9150 CrossRef CAS PubMed; (c) J. W. J. Kennedy and D. G. Hall, Angew. Chem., Int. Ed. Engl., 2003, 42, 4732–4739 CrossRef CAS PubMed; (d) M. Yus, J. C. González-Gómez and F. Foubelo, Chem. Rev., 2013, 113, 5595–5698 CrossRef CAS PubMed; (e) G. Zanoni, A. Pontiroli, A. Marchetti and G. Vidari, Eur. J. Org. Chem., 2007, 2007, 3599–3611 CrossRef; (f) S. E. Denmark and J. Fu, Chem. Rev., 2003, 103, 2763–2794 CrossRef CAS PubMed.
- (a) J. K. Cha and N.-S. Kim, Chem. Rev., 1995, 95, 1761–1795 CrossRef CAS; (b) K. N. Houk, S. R. Moses, Y. D. Wu, N. G. Rondan, V. Jager, R. Schohe and F. R. Fronczek, J. Am. Chem. Soc., 1984, 106, 3880–3882 CrossRef CAS; (c) S. Hatakeyama, K. Saijo and S. Takano, Tetrahedron Lett., 1985, 26, 865–868 CrossRef CAS.
- For selected examples of diastereoselective synthesis of 1,3-amino alcohols via direct C–H amination, see: (a) S. M. Paradine and M. C. White, J. Am. Chem. Soc., 2012, 134, 2036–2039 CrossRef CAS PubMed; (b) G. T. Rice and M. C. White, J. Am. Chem. Soc., 2009, 131, 11707–11711 CrossRef CAS PubMed; (c) S. M. Paradine, J. R. Griffin, J. Zhao, A. L. Petronico, S. M. Miller and M. C. White, Nat. Chem., 2015, 7, 987–994 CrossRef CAS PubMed; (d) R. Ma, J. Young, R. Promontorio, F. M. Dannheim, C. C. Pattillo and M. C. White, J. Am. Chem. Soc., 2019, 141, 9468–9473 CrossRef CAS PubMed; (e) P. M. Wehn, J. Lee and J. Du Bois, Org. Lett., 2003, 5, 4823–4826 CrossRef CAS PubMed.
- For selected examples of 1,4-stereoinduction, see: (a) K. Tomooka, P.-H. Keong and T. Nakai, Tetrahedron Lett., 1995, 36, 2789–2792 CrossRef CAS; (b) T. Kobayakawa, T. Narumi and H. Tamamura, Org. Lett., 2015, 17, 2302–2305 CrossRef CAS PubMed and references therein..
- R. W. Hoffmann, Chem. Rev., 1989, 89, 1841–1860 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gom-perts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Rana-singhe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. To-masi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. For-esman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2sc02648a |
| This journal is © The Royal Society of Chemistry 2022 |