Investigating students’ argumentation when judging the plausibility of alternative reaction pathways in organic chemistry
Leonie
Lieber
 and
Nicole
Graulich
and
Nicole
Graulich
 *
*
Justus-Liebig University Giessen, Institute of Chemistry Education, Heinrich-Buff-Ring 17, 35392 Giessen, Germany. E-mail: nicole.graulich@dc.jlug.de
First published on 23rd August 2021
Abstract
Building scientific arguments is a central ability for all scientists regardless of their specific domain. In organic chemistry, building arguments is a necessary skill to estimate reaction processes in consideration of the reactivities of reaction centres or the chemical and physical properties. Moreover, building arguments for multiple reaction pathways might help students overcome the tendency toward one-reason decision-making and offer them an authentic perspective on organic processes. Reasoning about multiple alternative organic reaction pathways requires students to build arguments and then judge and weigh the plausibility of these pathways. However, students often struggle to build strong arguments and use scientific principles appropriately to justify their claims. In the present study, the argumentation patterns of 29 chemistry majors students were analysed using a simplified version of Toulmin's argumentation model (claim–evidence–reasoning). The students solved various tasks related to alternative reaction pathways of a substitution reaction. They supported their claims with evidence and justified the evidence through reasoning. We investigated (a) the extent to which the students use evidence and reasoning in their argumentation (referred to as their argumentation approach), (b) how students with different argumentation approaches rationalised changes in their initial claims, and (c) how students used reasoning to justify their arguments. The results indicate that students need further support to appropriately use evidence and reasoning and to apply conceptual knowledge to build well-grounded arguments.
Introduction
In scientific research, sequencing a complex problem into manageable pieces simplifies the problem, and this approach is often referenced in the scientific method described by Francis Bacon (Bacon, 1878; Franck, 2012). The scientific method involves the formulation of a hypothesis, followed by testing it to verify or falsify the hypothesis (Turro, 1986). Almost 60 years ago, Platt (1964) emphasised the importance of considering alternatives as part of the scientific method. In Platt's perspective on the scientific method, which is called strong inference, the aforementioned steps of the scientific method are enhanced through the creation of alternative hypotheses. Platt (1964) claimed that the explicit and regular use of alternatives at each step of the research process offers additional power. Turro (1986) reported that considering alternatives allows a researcher to stay open-minded to deviations or unexpected findings rather than focused exclusively on one hypothesis and possibly missing interesting findings. However, incorporating alternatives into decision-making can be challenging. One way to support this decision-making process is to build well-grounded arguments for various alternatives.Building arguments or explanations is a core ability that must be learned by science students. This process includes evaluating claims and weighing evidence (Driver et al., 2000). Erduran (2019) purposefully linked these two aspects (claims and evidence), which were previously considered separate, by stating that claims need to be justified with evidence. Justifying and refuting claims using evidence is part of a scientific mode of thinking.
In organic chemistry, a student's success is often linked to the ability to build arguments (Bodé et al., 2019; Cruz-Ramirez de Arellano and Towns, 2014; Deng and Flynn, 2021) and reason about reaction mechanisms (Grove et al., 2012; Becker et al., 2016; Caspari et al., 2018; Watts et al., 2021). More explicitly, this means that students who solve tasks related to reaction mechanisms need to be able to argue and reason about the structural changes that occur and the causes of these changes. To explain these causes, students need to justify their evidence with reasoning (Cooper et al., 2016; Watts et al., 2020). However, students show various problems when building arguments and using reasoning. Students often struggle to consider alternative pathways since they tend to focus on one reaction product instead of the underlying processes (Popova and Bretz, 2018a). Thus, students are often triggered by single clues such as the presence of a leaving group, which often leads them to neglect other reactivities or multiple reaction pathways (Kraft et al., 2010). With regard to argumentation structures, forming a claim is often the easiest component for students (McNeill and Krajcik, 2012). Problems occur when students try to justify claims as they experience uncertainties regarding what counts as evidence (Sadler, 2004). Thereby, students explicitly struggle with the appropriate use of scientific principles while explaining why their given evidence supports a claim (McNeill et al., 2006; McNeill and Krajcik, 2012; Walker et al., 2019). Problems in students’ understanding of chemical concepts such as nucleophilicity and electrophilicity or acidity and basicity are well documented (Cartrette and Mayo, 2011; Anzovino and Bretz, 2015; DeFever et al., 2015; Akkuzu and Uyulgan, 2016). To avoid these problems in chemical concepts, students rely on personal views when judging the plausibility of a given context (Hogan and Maglienti, 2001). Even when students justify their claims, they often rely on one-reason decision-making based on single pieces of evidence when multiple supporting pieces are needed (McNeill et al., 2006; Talanquer, 2006; Kraft et al., 2010). This is primarily observed when students must argue about data that contradict their own ideas (Chinn and Brewer, 2001).
Use of argumentation models in chemistry education research
Research in the field of chemistry education has provided information on how to support students’ argumentation skills through assessments, activities, or teaching approaches (Cooper, 2015; Talanquer, 2018; Bodé et al., 2019; Luo et al., 2020). Studies on the formation of arguments by students have been conducted in the fields of natural science (Abi-El-Mona and Abd-El-Khalick, 2011; Luo et al., 2020), laboratory work (Hand and Choi, 2010; Walker et al., 2019; Hosbein et al., 2021; Petritis et al., 2021), physical chemistry (Becker et al., 2013; Towns et al., 2019), and organic chemistry (Cruz-Ramirez de Arellano and Towns, 2014; Bodé et al., 2019; Deng and Flynn, 2021). Some studies purposefully used contrasting cases to capture students’ argumentation abilities in terms of weighing properties, reaction centres, or reaction conditions (Caspari et al., 2018; Bodé et al., 2019; Galloway et al., 2019; Graulich et al., 2019; Watts et al., 2021).Many chemistry education researchers have specifically used Toulmin's argumentation model to analyse or support argumentation. For instance, Becker et al. (2013) analysed classroom discourse in which students made claims and particulate-level justifications about thermodynamic topics such as enthalpy and heat capacity. The authors suggested that while a conceptual understanding of the particular nature of matter is important, students must to be able to apply this knowledge about chemical and physical properties to build arguments (Becker et al., 2013). Lazarou and Erduran (2021) analysed science teachers’ instructional adaptions of Toulmin's argumentation pattern in the classroom and the quality of students’ written arguments. They revealed that some elements of Toulmin's argumentation pattern cause ambiguity and interpretative difficulties for students and science teachers (Lazarou and Erduran, 2021).
The claim–evidence–reasoning (CER) model, a simplified version of Toulmin's argumentation model, is currently the prevalent argumentation model used in chemistry education research. Walker et al. (2019) conducted a study in a general chemistry laboratory course based on the argument-driven inquiry instructional model. The participants worked in groups and collected data in the laboratory to build arguments that included claims, evidence, and reasoning as justifications. The authors found that students had problems changing their claims and considering alternatives. Instead, they stuck with their initial claims despite critique. Moreover, students struggle to justify their claims with scientific principles. Walker et al. (2019) reported that students may need additional guidance to revise or change their claims and to use scientific principles in their justification. Luo et al. (2020) investigated the effect of inquiry on students’ argumentation skills. They examined the influence of a reasoning flow scaffold in comparison to a control group that received conventional argumentation training. The students in the intervention group showed significant improvement in certain argumentation elements compared to the control group. Luo et al. (2020) concluded that dividing a claim into two parts (one at the beginning and one at the end as an opportunity for falsification) may assist students in building arguments by causing them to reflect on their decision more carefully. In organic chemistry, Bodé et al. (2019) investigated how students build scientific arguments when comparing two reaction mechanisms for nucleophilic substitution. They concluded that practicing argumentation skills are essential to make expectations clear for the students. These practices may include debating about justifications or comparing with prior knowledge.
Overall, the above studies indicate that students (1) struggle to revise their initial, possibly erroneous claims, (2) rarely consider alternatives in their argumentation, and (3) struggle to build well-grounded arguments.
Clarifying how students use evidence and reasoning in their arguments and rationalise their claims when assessing the plausibility of multiple alternative reaction pathways may provide further insights into their argumentation behaviours.
Theoretical framework
Toulmin's argumentation pattern
Toulmin can be considered as one of the “founding fathers of modern argumentation theory” because of the impact his ideas have on everyday reasoning (Van Eemeren et al., 2014: p. 204).Toulmin determined that an argument consists of six elements, as represented in Fig. 1, which also illustrates the commonalities between Toulmin's argumentation pattern and the simplified CER model (Toulmin, 2003). More generally, Kuhn (1991) claimed that an argument is an assertion with justification, while Osborne and Patterson (2011) stated that an argument is a justified claim.
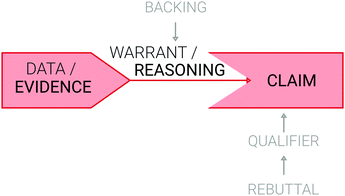 | ||
| Fig. 1 Coherence of Toulmin's argumentation pattern (2003) and the CER model often used in chemistry education research. | ||
The following descriptions of the elements of an argument are taken from Toulmin's book The Uses of Argument (Toulmin, 2003).
The first step in building an argument is the expression of a claim. Asserting something is the key component of making a claim (Van Eemeren et al., 2014). The foundation of the claim is called data. Data are initiated by the question “What have you got to go on?”. Therefore, the claim based on the given data is the first step to justify the claim (and the second step of an argument). The given data consist of rules or principles and show that the claim is appropriate. The third step of an argument is called the warrant, which is the justification of the given data. The warrant is a general statement based on rules or principles. Since a warrant is a bridge between the data and the claim, the initiating question is “How do you get from your data to your claim?”. On the basis of data, the warrant gives permission to make a claim. The fourth element of an argument is the backing, which is based on the question “What entitles you to conclude from… to…?” and refers to the warrant. A backing is required when the warrant is not immediately accepted. Thus, the backing relies on the principles of ethics, values, or general norms. The fifth element of an argument is called the qualifier and is initiated by the question “Is that necessarily so?”. The qualifier provides information about the obligation of the claim and thus possibly weakens the claim. Words such as likely, probably, or necessarily can be applied to introduce a qualifier. The sixth and last element of an argument is the rebuttal. The rebuttal describes the circumstances under which the claim is not valid or only valid to a certain extent. A rebuttal is an exception of rules and is initiated with the question “When does the rule not apply?”. As soon as a rebuttal appears in an argument, the claim must be weakened with a qualifier. Conversely, just because there is a qualifier, there is not necessarily the need for a rebuttal (Toulmin, 2003).
Claim–evidence–reasoning (CER) model
Toulmin noted that of the six aforementioned elements, only the claim, data, and warrant are present in every argument, Thus, these three elements form the core of the argument. The rebuttal, qualifier, and backing are only present when they are needed (Toulmin, 2003).Accordingly, CER is frequently used as a simplified version of Toulmin's argumentation pattern based on the terms “claim,” “evidence” (data), and “reasoning” (warrant).
In both the original and CER models, the main goal of an argument is the justification of a claim with the use of evidence and reasoning (Toulmin, 2003; Osborne and Patterson, 2011). The CER model consists of three parts (McNeill and Krajcik, 2012).The first part in both the CER and original models is the claim, which acts as a statement or a conclusion to a problem or question (McNeill et al., 2006; McNeill and Krajcik, 2012). Similar to Toulmin's argumentation pattern, the claim in the CER model is always in doubt and requires a justification based on known data and an explanation (Osborne and Patterson, 2011). The claim is supported with evidence based on scientific data (McNeill et al., 2006; McNeill and Krajcik, 2012). Thus, evidence in den CER model is similar to Toulmin's data (Van Eemeren et al., 2014). As scientific data contain information from various resources, multiple pieces of evidence can support a single claim (McNeill and Krajcik, 2012). Equally important is the reasoning (in the CER model) as it provides the bridge between the claim and the evidence; reason in the CER model combines Toulmin's warrant and backing (McNeill et al., 2006; McNeill and Krajcik, 2012). Reasoning indicates how the link between a claim and the given data can be justified and why they are justified by providing a logical connection (warrant) (McNeill and Krajcik, 2012; Toulmin, 2003; Van Eemeren et al., 2014). As this connection may need backing up, scientific principles should be included (backing) (McNeill and Krajcik, 2012). Reasoning is considered to be the most difficult step of an argument because a justification requires the bridging of the claim and evidence (McNeill and Krajcik, 2012).
Research questions
Given the need to diagnose how students build arguments in organic chemistry, our goal was to investigate students’ use of evidence and reasoning (i.e., the CER argumentation pattern) when forming arguments about the plausibility of alternative reaction pathways. We separated our overarching question into three research questions:(1) To what extent do students use evidence and reasoning in their argumentation (which we refer to as the argumentation approach)?
(2) How do students with different argumentation approaches rationalise changes in their initial claims?
(3) How do students use reasoning to justify their arguments while assessing the plausibility of alternative reaction products?
To answer these three research questions, we conducted qualitative interviews with students majoring in chemistry prompting them to elaborate on serial tasks related to the plausibility of reaction products.
Methods
Context and study setting
The study was conducted at a German university in October and November of 2019. Twenty-nine participants were recruited on a voluntary basis at the beginning of the course “Organic Chemistry 3 – Catalysis and Synthesis” (OC3), which is part of the bachelor of science chemistry major programme in the fifth of six semesters. Before students can attend Organic Chemistry 3, they must pass the Organic Chemistry 1 (OC1) and Organic Chemistry 2 (OC2) courses. Both courses consist of a lecture, a seminar, and a laboratory section. These two organic chemistry courses cover topics such as the reactivities of functional groups, structure–property relationships, organic reaction mechanisms (e.g., nucleophilic substitutions, rearrangements, and carbonyl reactions), hard and soft acid and base (HSAB) theory, and transition state theory. To successfully complete these two courses, students must pass a written exam. The aforementioned course topics covered in OC1 and OC2 were considered to be prior knowledge for the OC3 students as the study was conducted during the first four weeks of the OC3 course.Eleven female and 18 male chemistry major students participated on a voluntary basis. Students were recruited at the beginning of the OC3 course via an announcement in the lecture. The students were between 20 and 27 years old (average 22 years). While Institutional Review Board is not required at German universities, interviews followed ethical guidelines, and the students had the opportunity to terminate their interview at any point. All recruited students were provided with information about their rights and the manner of data collection and gave their written permission for the following: (1) the questionnaire can be evaluated by the research team, (2) the transcripts, drawings, video data, and audio data can be used by the research team; and (3) the collected data can be analysed and published by the research team. All students created a pseudonym, and the video data only included voice and drawings. As all students were native German speakers, the interviews were conducted in German, and direct quotes were translated into English for publication.
Data collection
During the interviews, the students were encouraged to draw products and reaction mechanisms to support their statements. Most of the students used drawings to solve the tasks.The students completed a demographic questionnaire at the beginning of the interview. The data also included pseudonymised scans of the students’ work sheets as well as audio and video recordings. Verbal utterances were transcribed verbatim. Individual think-aloud interviews were led by the first author using a semi-structured protocol (Bernard and Bernard, 2013). After their interviews, all students were asked not to talk about the tasks with their colleagues to minimise the chances of other students preparing beforehand. After the interviews, the first author provided sample solutions of the task during the lecture.
The quotes used in this publication were checked multiple times by the research group and a German English student teacher to guarantee the validity of the translations.
Research instrument
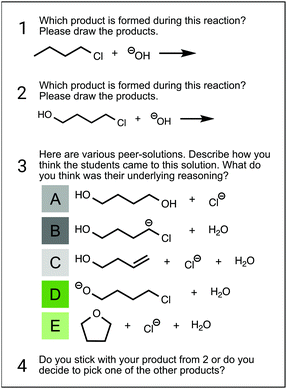
The research instrument used in the interviews consisted of four subtasks (see Fig. 2); a more detailed description of the task design can be found in Lieber and Graulich (2020).The first subtask contained a typical reaction that the students had experienced several times throughout the organic chemistry courses and laboratories (see Fig. 2, task 1). This reaction was chosen to provoke the use of intuitive heuristics since nucleophilic substitution and elimination were familiar to the students. The second subtask (Fig. 2, task 2) contained a reaction that – at first sight – seems similar to the reaction from subtask 1 as it only differs in one surface feature. The aim was for the students to use the same problem-solving approach as in subtask 1; however, in this case, the same problem-solving approach would not be productive. In subtask 3 (Fig. 2, task 3), students were provided with alternative products for the reaction given in subtask 2. This step was intended to challenge students’ problem-solving approaches as the confrontation with alternative products might initiate a more analytical thinking process. This stepwise process of first generating possibly erroneous solutions by themselves and then comparing their solutions with comparable or different solutions (Loibl and Leuders, 2019) has been shown to have a beneficial effect on students’ reasoning. In the last subtask (Fig. 2, task 4), students had the opportunity to defend or revise their generated product from subtask 2 (i.e., revise their initial claim).
Data analysis
All 29 interview transcripts were analysed using the software MAXQDA. All parts of the data analysis were discussed multiple times with the authors and the research group. The data were analysed in three consecutive steps to answer the research questions.Step I: Identifying students’ argumentation patterns
In the first step, we created argumentation pattern maps based on students’ interview transcripts to analyse students’ argumentation patterns. As students were not familiar with building arguments or the CER pattern, we did not provide them with specific prompts to form evidence and reasoning statements, we rather paraphrased it with why-questions and prompts to explain their reasoning throughout the interview. Prompts were therefore verbalised in a way that students’ answers could be assigned to claim, evidence, and reasoning without using the explicit terms. First, we identified the claim as the position being argued for each reaction product card and each student in subtask 4. Two different claims were possible: plausible or implausible. On the basis of the claim, evidence and reasoning were ascertained. To determine what counted as evidence and reasoning, the structure of the statements is important, since a statement can be both evidence and reasoning. Therefore, we prompted the students to provide evidence by having the interviewer ask why the students think something is plausible/implausible. Students responses that were coded as evidence included statements such as “chloride is a good leaving group” or “the H atoms are not acidic”. Reasoning was coded when the statement given by the student was a justification of evidence they mentioned before. This was prompted, for example, with the question why a certain leaving group is good or bad. Students answers for this question were, for instance, “chloride is stable” or “C–Cl bond is weaker compared to C–O bond”. As students’ argumentation patterns differed for each reaction product card, the instances of evidence and reasoning varied for each student and each product card (see Appendix 1).Step II: building reasoning categories
To obtain insights into the concepts students used while justifying the reaction product cards, we analysed the concepts students referred to in their reasoning. Towards this end, we created eight inductive reasoning categories. Table 1 shows an overview of the final coding rubric for the reasoning codes. We did not consider whether the given statements were technically correct because the aim was to analyse students’ argumentation patterns rather than their correctness. Thus, it was possible for technically incorrect statements to be part of the argumentation pattern. A sample solution of all product cards and the corresponding mechanisms has been published by Lieber and Graulich (2020).| Category | Description of the code | Student example |
|---|---|---|
| Electronics | Students describe electronic aspects like polarisation, electronegativity, or charge | “This bond is more polarised.” |
| Energetics | Students describe energetic aspects like bonds, energies, or thermodynamics | “The C–H bond is stronger. You have to spend energy to cleave the bond.” |
| Kinetics | Students describe kinetic aspects like rates or statistics | “… that acid–base reactions react very fast.” |
| Spatial arrangement | Students describe spatial aspects like steric effects, sizes, distances, or angles | “… because the groups are close to each other.” |
| Analogies | Students describe examples that are not part of the task but compare or demonstrate aspects | “You can deprotonate phenols with hydroxide.” |
| Strength | Students describe, for instance, the quality or strength of acids/bases, leaving groups, or nucleophiles | “… that this part is more acidic.” |
| Conditions | Students describe aspects like concentrations, temperatures, or pressures | “It depends on how much base I add.” |
| Stability | Students describe aspects of stability without an explicit explanation of the term (e.g., in terms of energetics) even after being prompted to do so | “… this product would be definitely not stable.” |
A special note is required for the coding of the Stability category. This code was only assigned if the students could not explain the term ‘stability’ after being prompted and relied on judgments such as “the reaction product is stable”.
Step III: determining students’ argumentation approaches
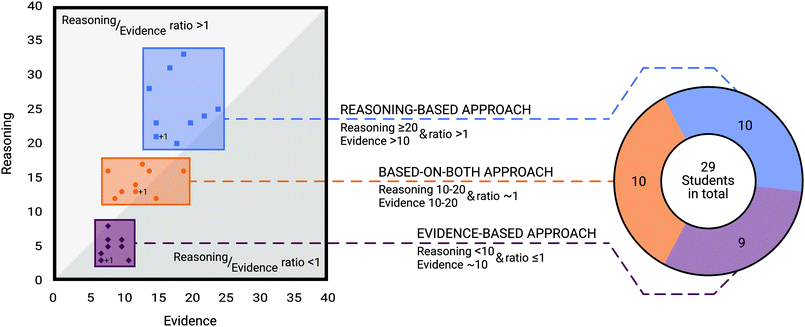
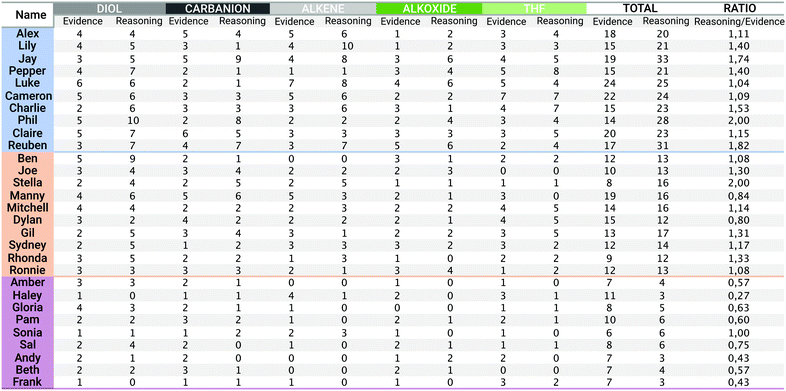
We then examined the frequency of evidence and reasoning as well as the ratio between them to identify different argumentation approaches. As the claim only consisted of stating if the product card was plausible or implausible, the ratio was calculated separately from the claim. For this reason, claims were omitted from this part of the analysis. To determine the argumentation approaches, both the frequency of statements and the ratio of reasoning to evidence were considered. Therefore, all evidence and reasoning statements across all product cards were aggregated for each student. Based on the results, we grouped the students into three categories according to their argumentation approaches. Group 1 contained students for which the ratio of reasoning to evidence statements was greater than 1 (i.e., the students used more reasoning than evidence in their argumentation pattern). Moreover, the frequency of evidence statements in this group was more than 10, while the frequency of reasoning statements was equal to or greater than 20.Students were placed into group 2 when the ratio of reasoning to evidence statements was close to 1, and each of the frequencies of evidence and reasoning was between 10 and 20.
Lastly, group 3 contained students for which the ratio of reasoning to evidence was less than or equal to 1 (i.e., the students mostly used evidence than reasoning). For these students, the frequency of evidence statements was nearly 10, while that of reasoning statements was less than 10.
Results and discussion
We analysed students’ interview data and their written work to (1), investigate the extent to which students used evidence and reasoning in their argumentation, (2) analyse how students with different argumentation approaches rationalised changes in their claims, and (3) identify how students used reasoning to justify their arguments while assessing the plausibility of reaction products.To what extent do students use evidence and reasoning in their argumentation?
To answer this question, we analysed the frequencies of evidence and reasoning statements along with their ratio for each student. We then identified three argumentation approaches that differ based on the evidence and reasoning frequencies and ratio. A detailed description of the students’ evidence and reasoning statements can be found in Appendix 1. Fig. 3 illustrates the distribution of students among the three groups of argumentation approaches based on the frequencies of reasoning and evidence. The diagonal bisecting line in Fig. 3 represents a ratio of 1. In addition to the description in the Data analysis section, the classification of students into the three argumentation approaches are detailed as follows.Argumentation approach 1 (reasoning-based approach): students’ argumentation patterns were classified as argumentation approach 1 when the ratio of reasoning to evidence statements was above 1. Additionally, the frequency of evidence statements had to be more than 10, and the frequency of students’ reasoning had to be equal to or greater than 20. Both the frequencies of students’ evidence and reasoning statements and the ratio has to be accomplished to be assigned to the approach. Ten out of the 29 students were assigned to the reasoning-based argumentation approach.
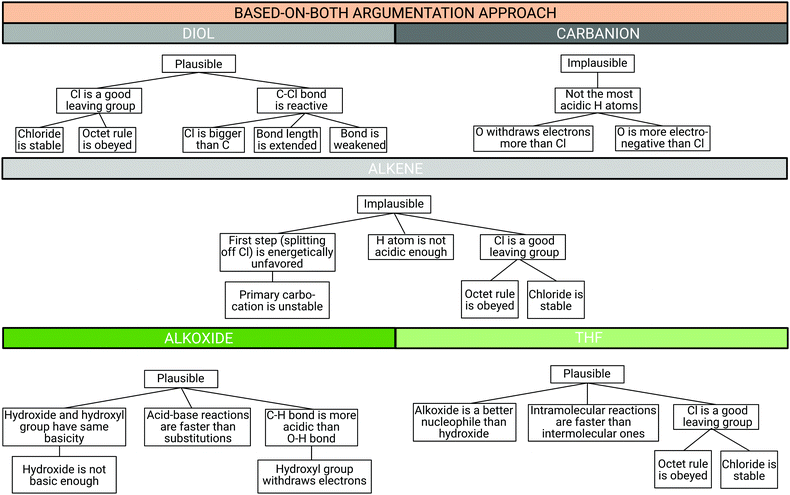
Argumentation approach 2 (based-on-both approach): students were assigned to argumentation approach 2 when the ratio of reasoning to evidence statements was nearly 1. Moreover, each of the frequencies of evidence and reasoning statements had to be between 10 and 20. The frequencies of evidence and reasoning statements as well as the ratio has to be accomplished to be assigned to the second argumentation approach. Ten out of the 29 students were assigned to this group.
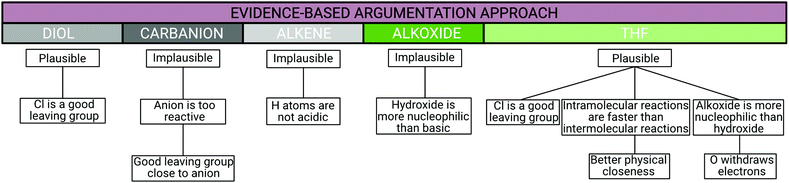
Argumentation approach 3 (evidence-based approach): students were assigned to approach 3 when the frequency of evidence in students’ argumentation was nearly 10 while the number of reasoning was lower. The ratio of reasoning to evidence was less than or equal to 1. In contrast to the based-on-both argumentation approach, the frequency of reasoning statements must be lower than 10 and the ratio of reasoning to evidence statements must not be greater than 1 to be assigned to the evidence-based argumentation approach. Thus, nine out of the 29 students were assigned to this approach.
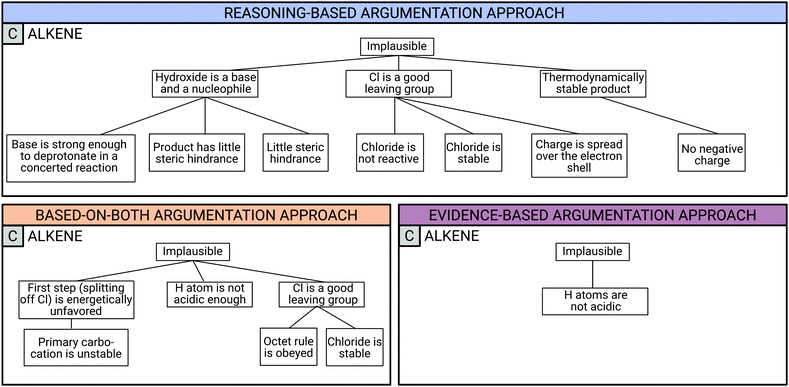
Fig. 4 illustrates example argumentation patterns for the alternative reaction product card C (“alkene”) in subtask 3 (see Fig. 2). A complete argumentation pattern map for each argumentation approach is shown in Appendix 2. In each argumentation pattern, the claim (plausible or implausible) and, when present, the support by evidence and the justification by reasoning are shown moving from top to bottom.
All three students whose argumentation patterns are shown in Fig. 4 for product card C initially provided the same correct claim: the alkene is implausible as a reaction product. However, the students differed in the way they supported the claim with evidence or justified it with reasoning. For instance, Reuben, who was assigned to the reasoning-based argumentation approach, supported his claim with three pieces of evidence and justified each piece of evidence with at least one reasoning statement. The reasoning statements were classified into different reasoning categories including Electronics, Spatial Arrangement, and Strength.
Sydney, who was classified into the based-on-both argumentation approach, supported her claim with three pieces of evidence, just as Reuben did. However, unlike Reuben, she did not support each of her evidence statements with reasoning statements.
Frank, who was classified into the evidence-based argumentation approach, provided the same claim as Reuben and Sydney. However, although he supported his claim with a piece of evidence, he was unable to justify it with reasoning even after being asked several times to do so. These three argumentation approaches are illustrated in more detail in the following section with examples provided for each approach.
Fig. 5 illustrates the reasoning-based argumentation approach with a quote from Phil. In general, Phil supported his five claims for the five reaction product cards with a total of 14 pieces of evidence and 28 reasoning statements (see Appendix 1). In the shown interview excerpt, he argued for reaction product card D, the reaction of 4-chlorobutanol and hydroxide to alkoxide and water, claiming correctly that the reaction product is plausible. He then supported his claim with two pieces of evidence (“If only a very small amount of base is added” and “the acid–base reaction is much faster [compared to the nucleophilic substitution]”). These two statements were categorised as evidence since they refer directly to the claim. Furthermore, he used reasoning to describe statements based on chemical concepts such as electronic or energetic aspects that support the evidence (e.g., why an acid–base reaction is faster compared to a SN reaction).
 | ||
| Fig. 5 Phil's argumentation for the alkoxide (product card D) as an example for the reasoning-based argumentation approach. | ||
To further justify the correct evidence “the acid–base reaction is much faster”, Phil provided four reasoning statements. First, Phil used the HSAB principle by reasoning that the hydroxide ion is a hard base, while the proton of the hydroxyl group is a hard acid (Strength category). Second, he emphasised that the energy gain is best for the reaction between a hard base and a hard acid (Energetics category). Third, Phil referred to the charge density that can be shifted into an empty orbital in the acid–base reaction instead of an antibonding orbital in the nucleophilic substitution (Electronics category). Finally, he referenced steric effects related to the ability of the hydroxide ion to attack from any direction in the acid–base reaction (Spatial Arrangement category). Overall, Phil used many reasoning statements to justify his evidence, which in turn supported his claim regarding the reaction product card. Thus, Phil was assigned to the reasoning-based argumentation approach.
Dylan serves as an example of the based-on-both argumentation approach. He gave 15 pieces of evidence in total and 12 reasoning statements (see Appendix 1). An interview excerpt of his argument regarding reaction product card E, the reaction of 4-chlorobutanol and hydroxide to tetrahydrofuran (THF) and water, is provided in Fig. 6. Dylan was asked if the reaction product card was plausible or implausible. He mentioned evidence five times correctly (“here [α-carbon atom] is a partial positive charge”, “there [hydroxide] is a negative charge”, “just a little ring strain”, “five-membered ring is quite stable”, and “chloride is a good leaving group”). However, only one piece of evidence (“chloride is a good leaving group”) was justified with three reasoning statements.
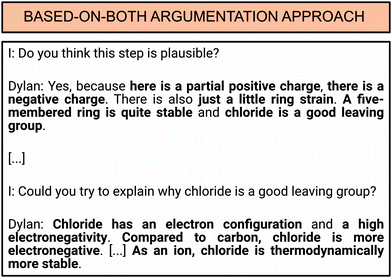 | ||
| Fig. 6 Dylan's argumentation for THF (product card E) as an example for the based-on-both argumentation approach. | ||
First, he used reasoning in the Electronics category when he said that chloride has an octet. The second reasoning statement is categorised in the Electronics category because he indicated that chlorine has a higher electronegativity than carbon. The final reasoning statement was related to the thermodynamic stability of chloride ion (Energetics category). Dylan was assigned to the based-on-both argumentation approach since he only supported one piece of evidence with reasoning.
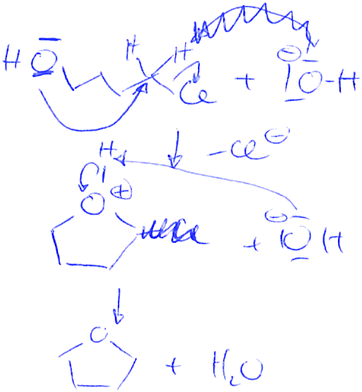
Amber serves as an example of the evidence-based argumentation approach. She used a total of seven pieces of evidence and four reasoning statements to support her claims (see Appendix 1). In the interview, she argued the plausibility of product card A, the reaction of 4-chlorobutanol and hydroxide to 1,4-butanediol and chloride. Here as well, Amber was asked if she thought the reaction product to be plausible or implausible. She answered erroneously that the reaction product is plausible and supported her claim with the evidence that chloride is a good leaving group. Fig. 7 shows an excerpt of Amber's explanation for why she thinks chloride is a good leaving group. At first, she answered that she has no idea why chloride is a good leaving group and that she learned or accepted the fact without questioning it. After the interviewer prompted her to try to explain her evidence, she was quiet for a while before responding incorrectly that chloride could be smaller than hydroxide ion but that she did not know the correct answer. This reasoning statement was assigned to the Spatial Arrangement category. Amber was one out of nine students assigned to the evidence-based argumentation approach because she hardly supported her claims with any evidence and barely used reasoning to justify her evidence.
 | ||
| Fig. 7 Amber's argumentation for the diol (product card A) as an example for the evidence-based argumentation approach. | ||
The three argumentation approaches identified illustrate that the students differed in the amount of evidence and reasoning statements used in their arguments. The more evidence and reasoning a student uses, the higher the probability that a large amount of influential variables are included in the decision-making process. This means that the probability of considering different chemical concepts such as kinetics, energetics, and energetic processes also increases. Since the course of a chemical reaction does not only depend on one factor but rather on various influencing factors, the inclusion of a larger number of evidence statements results in a more comprehensive decision-making process by increasing the breadth of the argumentation process. The number of reasoning statements can then influence the quality or depth of the argument. The more reasoning statements a student uses, the better the evidence can be justified. Reasoning serves to strengthen the claim through the justification of evidence; thus, the students’ arguments gain depth and are not just “empty envelopes”.
How do students with different argumentation approaches rationalise changes in their initial claims?
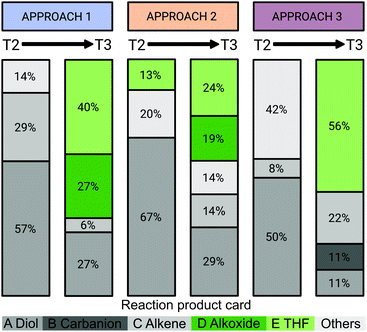
We were further interested in how students rationalise a change in their initial claim made in task 1 when prompted to reconsider it in task 4 and if this rationalisation is related to their argumentation approach.Each student made a claim about each of the five reaction product cards he or she discussed during the interview. Fig. 8 summarises “most plausible” claims made by the students; claims are given as percentages and separated into the three argumentation approaches. In task 2, students formed products independently as they only received the reactants. In contrast, in task 3, five cards with alternative reaction products were provided. All products that were claimed to be “most plausible” by the students in task 2 and task 3 were aggregated so that the total represents 100%. Since the students were able to claim more than one product as plausible, the number of products differs for the tasks and argumentation approaches. For example, 10 students in the reasoning-based argumentation approach claimed the formation of 14 products in task 2 and 15 products in task 3, which corresponded to product cards A-E. The 10 students with the based-on-both argumentation approach built 15 products in task 2 and 21 products in task 3. The nine students with the evidence-based argumentation approach claimed the formation of 12 products in task 2 and nine products in task 3. Regardless of the argumentation approach, most claimed incorrect products such as the diol (product card A) and the alkene (product card C) in task 2. This outcome is not surprising since tasks 1 and 2 were purposely designed to provoke the use of the same problem-solving approach used by students in task 1, leading to an SN2 reaction (Lieber and Graulich, 2020). After discussing the reaction product cards in task 3, an increase in claims for the correct product THF (product card E) and its precursor (alkoxide, product card D) along with a decrease in claims of the incorrect product diol (product card A) were observed for all argumentation approaches. Nevertheless, there were noticeable differences in how students with different argumentation approaches rationalised the changes in claims.
Considering only the percentage of THF (product card E) chosen as the most plausible product, it seems that the students who used an evidence-based argumentation approach claimed more correct products (56% THF) compared to those who used a reasoning-based argumentation approach (40% THF) or a based-on-both argumentation approach (24% THF). However, it should be noted that the alkoxide (product card D) is the precursor of THF. Many students who applied reasoning-based and based-on-both argumentation approaches recognised this fact and chose both THF and the alkoxide as the most plausible reaction products. When looking at the total of THF (product card E) and alkoxide (product card D), it is apparent that the students who used an evidence-based argumentation approach did not consider alkoxide as a plausible product or precursor. For example, Charlie who applied a reasoning-based argumentation approach chose THF and the precursor alkoxide as the most plausible products in task 3 when asked if he thought THF is plausible or implausible.
Charlie: “I don’t think it's not plausible (he refers to THF). I wouldn’t have intuitively guessed that something like this (THF) would happen if I am honest. But I would have taken an easier way and said it is a normal nucleophilic substitution, at this point, if I look at it that way I would not necessarily say it (THF) could be a by-product of what is being created. But the main product, the more I think about it, the more fascinating I think it is.”
Interviewer: “Why?”
Charlie: “Because I really didn't think that this (formation of THF) was a possibility. Well, I didn't think about it at all, I even think that what was there before (nucleophilic substitution) is wrong, because I just saw that I have a nucleophile and a good leaving group and nucleophilic substitution, but I didn't see that you can build another nucleophile (alkoxide).”
Charlie was one of the students who initially chose the diol (product card A) as the main product of the reaction of 4-chlorobutanol and hydroxide in task 2. When he received the alternative reaction product cards, he focused on THF as a reaction product. After he argued about both the alkoxide (product card D) and THF (product card E), he claimed both as the most plausible products of the reaction and explained that the alkoxide is the precursor of THF.
Among the students who applied evidence-based argumentation approaches, most did not provide a valid explanation for choosing THF as their most plausible product. Sonia, for instance, tried to build the mechanism for the formation of THF and could not find an acceptable explanation for her decision. As a result, the interviewer attempted to discuss the product THF in another way.
Interviewer: “We can do it differently. When you see the product, would you describe the product as plausible in principle?”
Sonia: “Yes, but I don't know why. My feeling tells me that again, I don't know. It looks so right somehow.”
Interviewer: “And are there any factors that help you determine why you think the product could be right?”
Sonia: “We once had a similar task in an exercise and I don't know, when I saw that, it kind of clicked in my head, somewhere in the back corner where I thought, I think that's it.”
Sonia later identified THF as the most plausible product of the reaction of 4-chlorobutanol and hydroxide. Nevertheless, she could not support her claim with any evidence or reasoning. She justified her claim based only on her feelings and on her memory of a similar task she completed sometime before. Like Sonia, no student with an evidence-based argumentation approach mentioned that the alkoxide (product card D) is the precursor for THF (product card E). Still, five out of nine students claimed THF as the most plausible product of the reaction with either little justification or without further justification. The following quote from Andy illustrates such a missing link from the precursor alkoxide to the reaction product THF. Andy built the diol (product card A) as a product in task 2 and chose the alkoxide as his first reaction product card to argue in task 3. He started to describe how the alkoxide was built.
Andy: “So here, the hydroxyl group was simply deprotonated.”
Interviewer: “How plausible do you think is this?”
Andy: “That's a good question. Actually, so it may happen. But a negative charge only on oxygen is very, I would say, unlikely.”
Interviewer: “Why?” […]
Andy: “So, that charge is on the oxygen and then is not somehow stabilised. That is a bit strange to me. Because, charge on an ester group would be kind of, I think, normal, because that's relatively stabilised by resonance. But only at the oxygen is a bit strange to me.”
Andy claimed incorrectly that the alkoxide is implausible as the reaction product. Since he could not explain why the charge on the oxygen atom seemed “strange” to him, he used an analogy with a molecule known to him, namely an ester, to support his claim. After Andy argued about the reaction product cards of the alkene (C) and the carbanion (B), he started to argue about THF (E) by drawing his idea of the reaction mechanism for the formation of THF (see Fig. 9).
Subsequently, the interviewer discussed the plausibility of the formation of THF (product card E) with Andy.
Interviewer: “How plausible do you think is it?”
Andy: “That might even be more plausible than this (diol).”
Interviewer: “Okay. Why?”
Andy: “The O has lone pairs, a five-membered ring is not as strained as a four-membered-ring, for example. I would say that could be possible. So, I would even consider it relatively plausible.”
Both in Andy's drawing of the reaction mechanism and his explanation of the plausibility, he did not consider the alkoxide (product card D) as a precursor of THF formation. Andy proposed falsely that the hydroxyl group of 4-chlorobutanol attacks intramolecularly to form a five-membered ring that is deprotonated by the hydroxide ion in the last step. Since Andy needed several attempts to complete the reaction mechanism to his satisfaction, the interviewer asked him how he proceeded in setting up the reaction mechanism.
Andy: “I noticed that the O has lone pairs. And then I thought, in order to reconstruct the five-membered-ring, it must attack here. That's where I first drew the Cl. But it would be a pentavalent carbon atom, which does not exist. So, the Cl has to get out somehow. And in the last step the H is taken by the OH. But only because the O is positive and it wants to become relatively neutral. And somehow furan (Andy confounded THF and furan) has to be the product.”
Interviewer: “That means, you basically made sure that you knew the product and how to put it together?”
Andy: “Yes, somehow. Exactly.”
Comparable statements to those made by Andy, are well known in the literature. Bhattacharyya and Bodner (2005) showed that students tend to postulate a mechanism with the goal of forming a given product. However, in doing so, students are likely to build intermediates that are implausible.
In summary, Andy was not the only student who did not associate the alkoxide (product card D) with THF (product card E). Nevertheless, while most students changed their initial claim, the rationale for the change varied greatly. In particular, students who used a reasoning-based argumentation approach identified the alkoxide as the precursor of THF and provided a rationale for it. Although five out of nine students who applied an evidence-based argumentation approach also chose THF as the most plausible product, they were not able to (sufficiently) justify their decision.
How do students use reasoning to justify their arguments while assessing the plausibility of alternative reaction products?
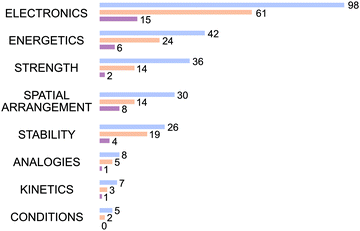
As described in the data analysis section, the analysis of students’ reasoning statements resulted in eight data-based reasoning categories, which are shown in Fig. 11. Examples of the four most frequently used reasoning categories illustrate which type of statements were assigned to the categories. The most frequently used category was Electronics, which refers to factors such as polarisation and electronegativity. Students’ statements coded in this category include “due to the positive inductive effect, which is an electron-pushing effect, more electrons would be distributed” (Ronnie, based-on-both argumentation approach) and “negative charge next to negative charge is unfavourable because there is a high excess of electrons” (Haley, evidence-based argumentation approach). The Energetics category covers energetic aspects such as bonds and energies. One example is the correct statement given by Pepper (reasoning-based argumentation approach), to justify the plausibility of THF (product card E): “that is because the entropy increases. The degeneracy in the system increases, since the energy can be distributed over more molecules.” Additional examples are the statements made by many students when talking about the plausibility of the carbanion (reaction product card B) or the alkene (reaction product card C) related to the high strength of the C–H bond because a lot of energy is required to cleave the bond. The Spatial Arrangement category includes statements that refer to aspects of sterics, distance, or size. Stella (based-on-both argumentation approach) justified her evidence incorrectly that chloride is a good leaving group as follows: “that chloride, as an ion, is smaller and can move easily compared to the hydroxide ion.” On the other hand, Sal (evidence-based argumentation approach) justified that the hydroxyl group is less reactive as follows: “because the carbon chain is quite long.” The last of the four most frequently used categories was Strength, which includes the quality or strength of leaving groups or acids and bases. Statements in this category include several students’ justification of good leaving groups by referring to the base strength and the justification for acids and bases based on the (estimated) pKa values, as cited by over a quarter of the students.Clear differences in the numbers of reasoning statements between argumentation approaches can be seen in Fig. 11. The reasoning-based argumentation approach (blue) was associated with the use of considerably more reasoning statements compared to the based-on-both argumentation approach (orange) and the evidence-based argumentation approach (purple), although a distinction is also visible between the latter two approaches. However, the percentages of reasoning statements in the different reasoning categories were similar across the three argumentation approaches (see Appendix 3). For example, the Electronics category accounted for 39% for the reasoning statements for the reasoning-based argumentation approach, 43% for the based-on-both argumentation approach, and 40% for the evidence-based argumentation approach. The percentages were also similar for the Energetics category (17% for the reasoning-based argumentation approach, 17% for the based-on-both argumentation approach, 16% for the evidence-based argumentation approach) and the Kinetics category (3% for the reasoning-based argumentation approach, 2% for the based-on-both argumentation approach, and 3% for the evidence-based argumentation approach). The students not only differed in their number of reasoning statements, but also in the way they justified their statements. For example, Cameron (reasoning-based argumentation approach) and Gloria (evidence-based argumentation approach) both mentioned the correct evidence that chloride is a good leaving group. However, these two students differed in how they justified this evidence.
Cameron: “Chloride is a good leaving group because the chlorocarbon bond is not very stable, unlike leaving groups such as OH - . Then it can be stabilised in an aqueous solution. […] There is also a high electronegativity. It's also very electron-withdrawing, chlorine as an atom.”
Interviewer: “Could you explain to me why chloride is a good leaving group and hydroxide is a weak leaving group?”
Cameron: “[…] So theoretically it should be that the electron that is added in chlorine is stored in an orbital, which is energetically higher than hydroxide. But I’m not really getting anywhere with it (laughs). So, I would say that this is not the cause because of the stability of the two ions, but because of the stability of the bond. That the splitting, here with chlorine and then there is another bond with carbon, that the splitting, if I were to occupy that as well, of the orbitals is lower with chlorine than with oxygen. And therefore, if this energy gain, through the bond or the energy loss through the cleavage of the bond, is then lower with chlorine than with OH.”
Cameron provided multiple reasoning statements for his evidence, including statements in different reasoning categories. In the Energetics category, for example, he stated, that the energy loss or energy gain in the case of the cleavage of the C–Cl bond is energetically more favourable than the cleavage of the C–O bond. Furthermore, Cameron stated that the orbital of the chlorine atom involved in the reaction is higher in energy than the hydroxide orbital. In general, few students included orbitals in their reasoning. Cameron also mentioned the stabilisation of the chloride ion in water (Stability) and the electronegativity of chlorine (Energetics) as factors that make chloride a better leaving group than hydroxide. Gloria also cited the fact that chloride is a good leaving group as evidence.
Gloria: “Halogens are generally a good leaving group. That's why I suspect a nucleophilic substitution.”
Interviewer: “Can you tell me why halogens are good leaving groups?”
Gloria: “Because Cl - in particular is relatively stable and obeys the octet rule.”
Gloria made two reasoning statements. On one hand, she described chloride as a relatively stable ion (Stability). On the other hand, she mentioned the obeyed octet rule (Electronics) as a factor to justify her evidence. In contrast to Cameron, Gloria did not provide any further information on the stabilisation of the chloride ion, even when asked. In addition, she did not draw a comparison to the hydroxide ion, which competes with the chloride ion as a possible leaving group. When prompted, she could not provide any reasoning for why hydroxide is a worse leaving group compared to the chloride. This is in line with the analysis of Popova and Bretz (2018b), who found that students often have problems when asked to justify why a certain leaving group is good or bad. Directly comparing the statements of Cameron and Gloria regarding the same evidence indicates that both the number of reasoning statements and the depth of the reasoning statements are important for substantiating an argument.
Conclusion and implications
The building of arguments in the field of organic chemistry is a central ability for students to make well-grounded decisions. Arguing about multiple reaction pathways requires the consideration of multiple chemical variables that influence the pathway of a reaction. Thus, principles that are often considered separately by students must be brought together in a meaningful way to weigh them against each other (Watts et al., 2021). Analytical thinking can be encouraged by providing students with the opportunity to include different aspects in their decision-making process, as has been reported for tasks based on alternative reaction pathways (Lieber and Graulich, 2020). In this study, we investigated how students argue when assessing the plausibility of alternative reaction pathways. We used a simplified version of Toulmin's argumentation model, which can be considered as the key components of an argument (Toulmin, 2003; Osborne and Patterson, 2011). In analysing students’ argumentation patterns, we specifically asked the following three questions. (1) To what extent do students make use of evidence and reasoning in their argumentation (i.e., argumentation approach)? (2) How do students with different argumentation approaches rationalise changes in their initial claims? (3) How do students use reasoning to justify their arguments while assessing the plausibility of alternative reaction pathways?The analysis revealed that students can be categorised into three argumentation approaches (reasoning-based, based-on-both, and evidence-based) based on the frequencies of evidence and reasoning statements and their ratio. There are different ways to form arguments, and it should be emphasised that there is no ideal argumentation approach. Instead, the numbers of evidence and reasoning statements were used in this study as an indicator of the potential quality of argumentation. A large number of evidence statements suggests that the student has recognised and included many different aspects in the decision-making process and considered various chemical variables influencing the reaction process. A large number of evidence statements can thus increase the quality and breadth of an argument since the course of a chemical reaction is influenced by a variety of factors. Recognising different influencing factors can also enhance the quality of the weighing process if the evidence is supported by reasoning. The number of reasoning statements is an indicator of how precise or elaborated the justification of evidence is. The more precise the justification, the greater the depth of the built argument. Proper justifying statements increase the quality of an argument by creating a stronger link between the components of the argument and the required conceptual understanding, which is a crucial aspect of the quality of an argument (Sandoval and Millwood, 2005; Choi et al., 2013). A well-grounded foundation of evidence and reasoning gives weight to an argument and should be an integral part of the classroom culture (Walker et al., 2012; Towns et al., 2019).
In addition to examining students’ use of evidence and reasoning, we assessed whether students changed their initial claims and their ability to rationalise these changes. Solving tasks that require the student to build multiple arguments can lead to more thorough decision-making, possibly enabling the student to justifiably defend a claim while also providing the opportunity to revise the claim. Based on the analysis of students’ argumentation patterns, 21 out of the 29 students in this study questioned their decisions and revised their claims during the argumentation process. Still, it seems that some students reached the limits of their conceptual understanding when rationalising their decisions; these students were unable to elaborate on their decisions beyond a certain individual point, even when specifically prompted. This issue was particularly noticeable for students who used the evidence-based argumentation approach, as these students were often unable to justify their claims. Luo et al. (2020) and Walker et al. (2019) found that students struggled to change their initial claims and thus stuck to their often erroneous claims. In the present study, the students were likely to question their initial claims after being specifically prompted to defend or revise them. Luo et al. (2020) analysed written arguments, whereas our study was based on interviews, which may lead students to be more willing to change their claim after prompting.
The findings of this study have some implications for teaching. In accordance with former studies, when introducing the building of arguments, teachers should ensure that students do not stop at supporting the claim with evidence, but also justify the evidence with reasoning (Bodé et al., 2019; Crandell et al., 2020). This part of argumentation is where students are likely to have the most problems; thus, it is important that teachers value justification through reasoning, even if it may be erroneous, to motivate students to build complete CER argumentation patterns. To help students learn to support and justify claims, argumentation patterns similar to concept maps can be used in teaching. As shown in Fig. 4 and Appendix 2, these argumentation structures can be used to create “argumentation trees” in the form of CER trees. This might help learners and teachers visualise both the depth and breadth of arguments and highlight gaps.
Furthermore, building arguments for alternative reaction pathways can be helpful as it helps students recognise that chemical reactions in the field of organic chemistry can lead to by-products (Popova and Bretz, 2018a) or may not form the desired product due to the choice of reaction conditions and reagents. In this study, we extended a common task format (i.e., comparing two reaction paths or reaction products) to the consideration of five alternative reaction pathways.
Students in this study were divided into three argumentation approaches based on the number of reasoning and evidence statements and their ratio. Based on the individual arguments formed by the students, the students need further support to develop strong arguments. Compared to the other two argumentation approaches, students who applied a reasoning-based argumentation approach provided a larger number of evidence and reasoning statements. To encourage this level of argumentation in future tasks, students should be supported in justifying their claims (e.g., by being trained to use several pieces of evidence for each claim and to justify each piece of evidence with several reasoning statements). In addition to the breadth of an argument, the depth of the argument is also of interest. In this regard, students could be provided with conceptual support to improve the quality of their evidence and reasoning statements. Additional support could focus on the weighing process through which students consider their formed evidence and reasoning statements. Students using the based-on-both argumentation approach supported their claims with multiple pieces of evidence and reasoning. However, the ratio of evidence to reasoning statements was close to 1, meaning that each evidence statement was justified on average with one reasoning statement. Thus, students in this group could receive targeted reasoning training to improve the breadth of their arguments and increase the number of reasoning statements for each piece of evidence. Students who used the evidence-based argumentation approach hardly provided any evidence or reasoning statements on their own and often failed to justify them, even after targeted prompting. On the one hand, this may be due to the fact that the students were conceptually unable to support their statements. Therefore, these students would benefit from additional conceptual support (e.g., providing information that they should include in their arguments). On the other hand, this group of students might benefit from examples highlighting the difference between evidence and reasoning statements or additional training on structuring an argument.
Scaffolds such as those used by Luo et al. (2020) and Caspari and Graulich (2019) to slow down students’ decision-making process (Caspari and Graulich, 2019) have proven to be helpful and could be adapted to help students build arguments for multiple alternative pathways. Students could be engaged to collect evidence as a first step and then be prompted to purposefully provide reasoning linked to the evidence. However, when developing evidence and reasoning to assess the plausibility of alternative reaction pathways, the quality of the evidence can vary, leading students to struggle in justifying their claims. Some students may profit from conceptual support in addition to support in building of arguments. An ongoing study is investigating whether students’ argumentation patterns in organic chemistry can be supported with an adaptive scaffold that targets the student's level of conceptual knowledge and argumentation skills (i.e., the use of evidence and reasoning).
Limitations
The conclusions drawn should be considered with caution given certain inherent limitations of this study. First, the students in this qualitative interview study voluntarily participated and may have been motivated to participate by different reasons. Nevertheless, 29 out of 40 students in the course agreed to participate in the study, which allowed this study to cover the spectrum of student performance in the course. Additionally, the interview format may also lead students to engage more intensively with the topic than when conducting tasks on their own. Second, the consideration of alternative reaction pathways may be a new concept for the students in this course, leading to cognitive overload. Moreover, considering the students’ previous course content, students were not used to building arguments or explaining the decisions that led to their solutions in detail. This may have resulted in students not expressing all their thoughts aloud, leading to individual cases of shortened argumentation being presented. The interviewer attempted to counteract this by asking specific questions, which were rephrased several times if necessary, to get each student to present their thoughts as precisely as possible. Third, the correctness of students’ statements was not considered since we wanted to assess the students’ thought processes in their entirety. Thus, erroneous statements that the student was not aware of were also considered. However, to determine what support is needed, it is essential to consider the complete thought process and not only erroneous or correct components. Lastly, our focus on the ratio of reasoning to evidence statements simplified the students’ argumentation processes and might not have captured subtle differences.Conflicts of interest
There are no conflicts to declare.Appendix 1: overview of the distribution of students’ evidence and reasoning statements
Fig. 11 shows the numbers of students’ evidence and reasoning statements for each alternative reaction product card (diol, alkene, carbanion, alkoxide, and THF), the total of all evidence and reasoning statements for each student, and the ratio of reasoning statements to evidence statements (on which the classification of students into the three argumentation approaches was based). The three argumentation approaches are colour-coded as follows in Fig. 11: the reasoning-based argumentation approach is highlighted in blue, the based-on-both argumentation approach is highlighted in orange, and the evidence-based argumentation approach is highlighted in purple.Appendix 2: example total argumentation patterns for the three argumentation approaches
Three example argumentation patterns for all product cards for each argumentation approach (reasoning-based, based-on-both, and evidence-based) are shown in the Fig. 12–14 below. Fig. 12 illustrates the argumentation patterns for Reuben (reasoning-based argumentation approach), Fig. 13 illustrates those for Sydney (based-on-both argumentation approach), and Fig. 14 shows those of Frank (evidence-based argumentation approach). All students built arguments for the plausibility of the five alternative reaction products. The arguments were analysed using the CER structure.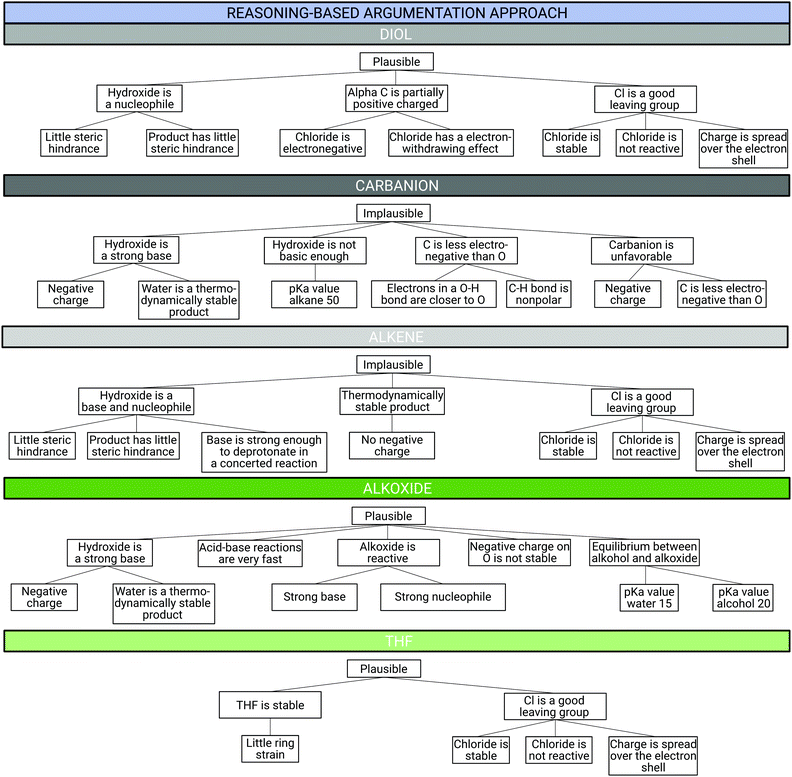 | ||
| Fig. 12 Example argumentation pattern (CER structure) for all product cards of the reasoning-based argumentation approach from Reuben. | ||
 | ||
| Fig. 13 Example argumentation pattern (CER structure) for all product cards of the based-on-both argumentation approach from Sydney. | ||
 | ||
| Fig. 14 Example argumentation patterns (CER structure) for all product cards of the evidence-based argumentation approach from Frank. | ||
Appendix 3: distribution of statements among the different reasoning categories
The total number of reasoning statements varied greatly among the three argumentation approaches (Fig. 10). Nevertheless, the percentage of reasoning statements in the different categories were similar among the three approaches. Table 2 illustrates the percentages of reasoning statements in each category for the three argumentation approaches.| Electronics (%) | Energetics (%) | Kinetics (%) | Spatial arrangement (%) | Analogies (%) | Strength (%) | Conditions (%) | |
|---|---|---|---|---|---|---|---|
| Approach 1 | 39 | 17 | 3 | 12 | 3 | 14 | 2 |
| Approach 2 | 43 | 17 | 2 | 10 | 4 | 10 | 1 |
| Approach 3 | 40 | 16 | 3 | 22 | 3 | 5 | 0 |
Acknowledgements
This publication is part of the first author's doctoral (Dr rer. nat.) thesis at the Faculty of Biology and Chemistry, Justus-Liebig-University Giessen, Germany. We are thankful for all students willing to participate in the study. Moreover, we thank all members of the Graulich group for fruitful discussions, Axel Langner for his support with graphics, and especially Krenare Ibraj. Leonie Lieber thanks the Verband der Chemischen Industrie (German Chemical Industry Association) for supporting her with the Kekulé Fellowship.References
- Abi-El-Mona I. and Abd-El-Khalick F., (2011), Perceptions of the nature and ‘goodness’ of argument among college students, science teachers, and scientists, Int. J. Sci. Educ., 33(4), 573–605.
- Akkuzu N. and Uyulgan M. A., (2016), An epistemological inquiry into organic chemistry education: Exploration of undergraduate students' conceptual understanding of functional groups, Chem. Educ. Res. Pract., 17(1), 36–57.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students' ideas about nucleophiles and electrophiles: The role of charges and mechanisms, Chem. Educ. Res. Pract., 16(4), 797–810.
- Bacon F., (1878), Novum organum, Clarendon Press.
- Becker N., Rasmussen C., Sweeney G., Wawro M., Towns M. and Cole R., (2013), Reasoning using particulate nature of matter: An example of a sociochemical norm in a university-level physical chemistry class, Chem. Educ. Res. Pract., 14(1), 81–94.
- Becker N., Noyes K. and Cooper M., (2016), Characterizing students' mechanistic reasoning about London dispersion forces, J. Chem. Educ., 93(10), 1713–1724.
- Bernard H. R. and Bernard H. R., (2013), Social research methods: Qualitative and quantitative approaches, Sage.
- Bhattacharyya G. and Bodner G. M., (2005), “It gets me to the product”: How students propose organic mechanisms, J. Chem. Educ., 82 (9), 1402–1407.
- Bodé N. E., Deng J. M. and Flynn A. B., (2019), Getting past the rules and to the WHY: Causal mechanistic arguments when judging the plausibility of organic reaction mechanisms, J. Chem. Educ., 96, 1068–1082.
- Cartrette D. P. and Mayo P. M., (2011), Students' understanding of acids/bases in organic chemistry contexts, Chem. Educ. Res. Pract., 12(1), 29–39.
- Caspari I. and Graulich N., (2019), Scaffolding the structure of organic chemistry students’ multivariate comparative mechanistic reasoning, Int. J. Phys. Chem. Educ., 11(2), 31–43.
- Caspari I., Kranz D. and Graulich N., (2018), Resolving the complexity of organic chemistry students' reasoning through the lens of a mechanistic framework, Chem. Educ. Res. Pract., 19 (4), 1117–1141.
- Chinn C. A. and Brewer W. F., (2001), Models of data: A theory of how people evaluate data, Cogn. Instruct., 19(3), 323–393.
- Choi A., Hand B. and Greenbowe T., (2013), Students' written arguments in general chemistry laboratory investigations, Res. Sci. Educ., 43(5), 1763–1783.
- Cooper M. M., (2015), Why ask why?, J. Chem. Educ., 92(8), 1273–1279.
- Cooper M. M., Kouyourndjian H. and Underwood S. M., (2016), Investigating students' reasoning about acid–base reactions, J. Chem. Educ., 93(10), 1703–1712.
- Crandell O. M., Lockhart M. A. and Cooper M. M., (2020), Arrows on the page are not a good gauge: Evidence for the importance of causal mechanistic explanations about nucleophilic substitution in organic chemistry, J. Chem. Educ., 97(2), 313–327.
- Cruz-Ramirez de Arellano D. and Towns M. H., (2014), Students' understanding of alkyl halide reactions in undergraduate organic chemistry, Chem. Educ. Res. Pract., 15(4), 501–515.
- DeFever R. S., Bruce H. and Bhattacharyya G., (2015), Mental rolodexing: Senior chemistry majors' understanding of chemical and physical properties, J. Chem. Educ., 92(3), 415–426.
- Deng J. M. and Flynn A. B., (2021), Reasoning, granularity, and comparisons in students' arguments on two organic chemistry items, Chem. Educ. Res. Pract.
- Driver R., Newton P. and Osborne J., (2000), Establishing the norms of scientific argumentation in classrooms, Sci. Educ., 84(3), 287–312.
- Erduran S., (2019), Argumentation in Chemistry Education, Royal Society of Chemistry, pp. 1–10.
- Franck G., (2012), Modern science: A case of collective intelligence? On the role of thought economy and gratifying attention in knowledge production, Angew. Chem., Int. Ed., 51(29), 7088–7092.
- Galloway K. R., Leung M. W. and Flynn A. B., (2019), Patterns of reactions: A card sort task to investigate students' organization of organic chemistry reactions, Chem. Educ. Res. Pract., 20(1), 30–52.
- Graulich N., Hedtrich S. and Harzenetter R., (2019), Explicit versus implicit similarity – Exploring relational conceptual understanding in organic chemistry, Chem. Educ. Res. Pract., 20(4), 924–936.
- Grove N. P., Cooper M. M. and Cox E. L., (2012), Does mechanistic thinking improve student success in organic chemistry?, J. Chem. Educ., 89(7), 850–853.
- Hand B. and Choi A., (2010), Examining the impact of student use of multiple modal representations in constructing arguments in organic chemistry laboratory classes, Res. Sci. Educ., 40(1), 29–44.
- Hogan K. and Maglienti M., (2001), Comparing the epistemological underpinnings of students' and scientists' reasoning about conclusions, J. Res. Sci. Teach., 38(6), 663–687.
- Hosbein K. N., Lower M. A. and Walker J. P., (2021), Tracking student argumentation skills across general chemistry through argument-driven inquiry using the assessment of scientific argumentation in the classroom observation protocol, J. Chem. Educ.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11 (4), 281–292.
- Kuhn D., (1991), The Skills of Argument, Cambridge University Press.
- Lazarou D. and Erduran S., (2021), “Evaluate what I was taught, not what you expected me to know”: Evaluating students' arguments based on science teachers' adaptations to toulmin's argument pattern, J. Sci. Teach. Educ., 32(3), 306–324.
- Lieber L. and Graulich N., (2020), Thinking in alternatives-A task design for challenging students' problem-solving approaches in organic chemistry, J. Chem. Educ., 97 (10), 3731–3738.
- Loibl K. and Leuders T., (2019), How to make failure productive: Fostering learning from errors through elaboration prompts, Learn. Instr., 62, 1–10.
- Luo X. L., Wei B., Shi M. and Xiao X., (2020), Exploring the impact of the reasoning flow scaffold (RFS) on students' scientific argumentation: based on the structure of observed learning outcomes (SOLO) taxonomy, Chem. Educ. Res. Pract., 21 (4), 1083–1094.
- McNeill K. L. and Krajcik J., (2012), Book study facilitator's guide: Supporting grade 5–8 students in constructing explanations in science: The claim, evidence and reasoning framework for talk and writing, Pearson Allyn & Bacon, New York.
- McNeill K. L., Lizotte D. J., Krajcik J. and Marx R. W., (2006), Supporting students' construction of scientific explanations by fading scaffolds in instructional materials, J. Learn. Sci, 15 (2), 153–191.
- Osborne J. F. and Patterson A., (2011), Scientific argument and explanation: A necessary distinction?, Sci. Educ., 95 (4), 627–638.
- Petritis S. J., Kelley C. and Talanquer V., (2021), Exploring the impact of the framing of a laboratory experiment on the nature of student argumentation, Chem. Educ. Res. Pract., 22 (1), 105–121.
- Platt J. R., (1964), Strong Inference: Certain systematic methods of scientific thinking may produce much more rapid progress than others, Science, 146 (3642), 347–353.
- Popova M. and Bretz S. L., (2018a), “It's only the major product that we care about in organic chemistry”: An analysis of students' annotations of reaction coordinate diagrams, J. Chem. Educ., 95 (7), 1086–1093.
- Popova M. and Bretz S. L., (2018b), Organic chemistry students' understandings of what makes a good leaving group, J. Chem. Educ., 95 (7), 1094–1101.
- Sadler T. D., (2004), Informal reasoning regarding socioscientific issues: A critical review of research, J. Res. Sci. Teach., 41 (5), 513–536.
- Sandoval W. A. and Millwood K. A., (2005), The quality of students' use of evidence in written scientific explanations, Cogn. Instruct., 23 (1), 23–55.
- Talanquer V., (2006), Commonsense chemistry: A model for understanding students' alternative conceptions, J. Chem. Educ., 83 (5), 811–816.
- Talanquer V., (2018), Importance of understanding fundamental chemical mechanisms, J. Chem. Educ., 95(11), 1905–1911.
- Toulmin S. E., (2003), The Uses of Argument, Updated Version, Cambridge University Press.
- Towns M. H., Cole R. S., Moon A. C. and Stanford C., (2019), Argumentation in Chemistry Education, Royal Society of Chemistry, pp. 247–274.
- Turro N. J., (1986), Geometric and topological thinking in organic-chemistry, Angew. Chem., Int. Ed. Engl., 25(10), 882–901.
- Van Eemeren F. H., Garssen B., Krabbe E. C. W., Francisca Snoeck Henkemans A., Verheij B. and Wagemans J. H. M., (2014), Handbook of Argumentation Theory, Dordrecht: Springer, pp. 203–256.
- Walker J. P., Sampson V., Grooms J., Anderson B. and Zimmerman C. O., (2012), Argument-driven inquiry in undergraduate chemistry labs: The impact on students’ conceptual understanding, argument skills, and attitudes toward science, J. Coll. Sci. Teach., 41(4), 74–81.
- Walker J. P., Van Duzor A. G. and Lower M. A., (2019), Facilitating argumentation in the laboratory: The challenges of claim change and justification by theory, J. Chem. Educ., 96 (3), 435–444.
- Watts F. M., Schmidt-McCormack J. A., Wilhelm C. A., Karlin A., Sattar A., Thompson B. C., Gere A. R. and Shultz G. V., (2020), What students write about when students write about mechanisms: Analysis of features present in students' written descriptions of an organic reaction mechanism, Chem. Educ. Res. Pract., 21 (4), 1148–1172.
- Watts F. M., Zaimi I., Kranz D., Graulich N. and Shultz G. V., (2021), Investigating students' reasoning over time for case comparisons of acyl transfer reaction mechanisms, Chem. Educ. Res. Pract., 22 (2), 364–381.
| This journal is © The Royal Society of Chemistry 2022 |