Qualifying domains of student struggle in undergraduate general chemistry laboratory
Clarissa
Keen
 * and
Hannah
Sevian
* and
Hannah
Sevian
 *
*
Department of Chemistry, University of Massachusetts Boston, Boston, Massachusetts, USA. E-mail: clarissa.keen001@umb.edu; hannah.sevian@umb.edu
First published on 9th August 2021
Abstract
Learning and learning goals in undergraduate chemistry laboratory have been a popular research topic for the past three decades due to calls for curriculum reform, cost justification, and overall efficacy of necessary skill development. While much work has been done to assess curricular interventions on students’ learning and attitudes towards lab, few have discussed the increased difficulties of these non-traditional laboratory activities or the obstacles students must overcome in the laboratory setting. The work presented here focuses on student struggles in undergraduate general chemistry laboratory activities, the source of these struggles, and the actions students take to overcome them. Using an activity theoretical lens and multiple domains (cognitive, epistemological, socioemotional, and psychomotor), we developed a domains-of-struggle framework which encompasses how struggles emerge through contradictions within the laboratory activity system. This framework was extended and refined through iterative analysis of two consecutive semesters of undergraduate general chemistry laboratory (GC1 and GC2) video (n = 51), survey (n = 327), and interview (n = 44) data. In this paper, we model the activity system of the general chemistry laboratory, define the domains of struggle observed, and present actions the students took to move past these obstacles, while illustrating the interconnected complexity of the activity system. We then discuss how this framework may be used in future curriculum design or teacher training, as well as potential for future research on the learning outcomes associated with moments of struggle.
Introduction
Since the inception of chemistry as an academic subject, the laboratory has been a crucial part of learning. Historically, chemistry is regarded for its apprenticeship education involving practical laboratory training to varying degrees (Morris, 2015). This tradition continues in chemistry education today through laboratory components of chemistry courses in both secondary and tertiary education. While the structure and role of the chemistry laboratory have been much debated, it is still believed to be a crucial component of hands-on learning for students (Hofstein, 2004; Hofstein and Lunetta, 2004; Smith and Alonso, 2020) and, at the university level, key to training for practical skills needed in chemistry careers (Galloway and Bretz, 2016; Bretz, 2019).This career training objective has led to much debate regarding the best ways to bring chemistry laboratory into the 21st century (Hofstein and Lunetta, 2004). In K-12 education in the US, the National Research Council recommended developing 21st century skills in three domains – cognitive, interpersonal, and intrapersonal (National Research Council, 2012) – and has worked with other national organizations to develop the practice-focused Next Generation Science Standards (National Research Council, 2015). The United Nations has formulated sustainable development goals for science (UN Department of Economic and Social Affairs, 2015), and many countries in the EU and beyond have responded with focuses on responsible research and innovation (e.g., for chemistry, Apotheker et al., 2017). These reforms encompass much of education's response to new demands within the workforce, particularly an increased focus on skills such as problem solving, critical thinking, teamwork, etc. (Kondo and Fair, 2017; Yasin and Yueying, 2017). The National Research Council (2010) has also identified crucial skills needed in STEM careers such as adaptability, coping with uncertainty, and learning from failure. Yet, little research has been contributed about the opportunities for students to learn these skills in undergraduate chemistry laboratory courses, thus identifying a gap in our understanding of how laboratory training may continue to meet the demands of the 21st century workforce.
Additionally, as laboratory curricula and assessments have been adapted and updated to meet 21st century standards, new challenges have emerged. It has been reported that conflicting laboratory goals (DeKorver and Towns, 2015; Santos-Díaz et al., 2019) and the increased difficulty of unstructured inquiry tasks (McDonnell et al., 2007; Kelly and Finlayson, 2009; Sandi-Urena et al., 2011; Ural, 2016; Chopra et al., 2017) can hinder student engagement in laboratory activities. Furthermore, researchers perceive that undergraduate chemistry students lack the problem-solving skills to deal with laboratory struggles and failures (Yuriev et al., 2017; Owens et al. 2020). These reported difficulties present barriers to students gaining necessary skills for the 21st century workforce and a career in science.
Nevertheless, some literature suggests that students grappling with struggles, conflict, and challenges during laboratory activity may present opportunities to learn these crucial skills (Miller, 2020). This led us to question if/how these opportunities arise during laboratory activity and whether or not these opportunities are acted upon. Therefore, in line with productive struggle literature, we conceptualize moments struggle as opportunities to learn (Roth, 2019; Baker et al., 2020) and seek to better understand the ways these struggles arise from and shape the laboratory environment. We posit that by observing the types of struggles that occur, we can identify the learning opportunities that are available to students and observe how students and teaching assistants (TAs) act upon them. Therefore, this work seeks to add to the existing literature by offering a framework to describe the difficulties that students face in undergraduate general chemistry laboratory activities. In this paper, we explore the struggles that occurred through analysis of students’ actions and interactions with the activity system and probe their experiences with these struggles through interview and survey data. Our analysis grapples with the difficulties of understanding struggle and accounting for the learning opportunities they present. While this work relies on foundations of previous productive struggle literature, we are focused specifically on how student struggle emerges from the complex context of the laboratory in order to elucidate a broader and multi-dimensional view of the challenges students face. Our findings hope to support chemistry instructors and researchers in attending to different types of student struggles and facilitating actions to overcome these obstacles.
Struggles that promote learning
Struggle and learning are related, according to findings from both education and psychology literature. Researchers and practitioners in mathematics education often refer to the terms productive struggle or productive failure to describe the phenomenon of students engaging in struggles and learning from mistakes. In his work on implementing productive failure in math education, Kapur (2014) found significant gains in students’ retention of math content and application to novel contexts similar to the proposed benefits of desirable difficulties (Bjork, 1994) and impasse-driven learning (VanLehn et al., 2003). In this literature, researchers have presented frameworks for identifying moments of struggle and assigning levels of productivity (Pathak et al., 2011; Warshauer, 2015; Sengupta-Irving and Agarwal, 2017). This literature shows that if struggle is framed and acted upon correctly, it presents opportunities for deeper conceptual understanding and development of problem solving skills (National Council of Teachers of Mathematics, 2014).Research has highlighted the benefits of productive struggle and productive failure in science education, demonstrating conceptual gains and increased transfer similar to findings in math education (Schwartz et al., 2011; Trueman, 2014; Song, 2018). Furthermore, research has been carried out on how students grapple with conflict, failure, and resistance and the skills that may be developed through these struggles (Manz, 2015; Sohr et al., 2018; Henry et al., 2019). Manz (2015) presented how elementary school science students were able to adopt scientific practices by grappling with difficult data and evidence. At the undergraduate level, Henry et al. (2019) argued that failure promotes learning key skills for the STEM workforce and Sohr et al. (2018) showed students in physics developing tools for addressing collaborative conflict. In light of this research, we believe the difficulties students face in both traditional and inquiry-based laboratory activities provide learning opportunities for both scientific content and scientific practice. Yet, to our knowledge, there is no current research in chemistry education that has focused on how these struggles manifest in a laboratory environment, and how they differ from those identified in math education.
Multiple domains of learning and interactions
Chemistry education research often utilizes domains or dimensions to account for the myriad of learning outcomes and interactions that can occur in a laboratory setting. Therefore, we believe that categorizing struggles by domains will help us see the type of learning opportunities they present as well as look beyond the cognitive focus of previous struggle literature (e.g., Kapur, 2014) and incorporate categories relevant to chemistry. For instance, researchers have employed the domains of conceptual, experimental, and analytical when describing the nature of teaching assistant and student verbal interactions (Velasco et al., 2016) and cognitive, affective, and psychomotor when defining areas of STEM literacy (Zollman 2012). In chemistry education research, these domains were incorporated into Galloway and Bretz (2015) Meaningful Learning in the Laboratory Instrument in order to capture the wide range of outcomes unique to the laboratory environment. The authors argue that meaningful learning in the chemistry laboratory occurs at the intersection of conceptual understanding of chemistry content (cognitive), interest or motivation in inquiry (affective), and proper technique with tools and instrumentation (psychomotor) (Galloway and Bretz, 2015). While this framework goes a long way in investigating these multi-dimensional objectives, it fails to account for the sociocultural complexity of the laboratory environment (Holbrook and Rannikmae, 2007). When describing barriers to scientific literacy, Holbrook and Rannikmae (2007) argue that this frame does not encompass “a wider view of educational components” (p. 1351). Duschl (2008) offers a broader framework claiming, “New perspectives and understandings in the learning sciences about learning and learning environments, and in science studies about knowing and inquiring, highlight the importance of science education teaching and learning harmonizing conceptual, epistemological, and social learning goals.” (p. 268–269) Duschl's (2008) description of conceptual, epistemological, and social domains has been adopted in the science education literature specifically when categorizing dimensions of argumentation and conflict. Sohr et al. (2018) presented conceptual, epistemological, social, and emotional categories as interaction domains which arise during student conflict in a collaborative problem-solving space. In chemistry specifically, Walker et al. (2019) qualified scientific argumentation in laboratory activities by categorizing student actions within cognitive and conceptual, epistemic, and social domains. These studies demonstrate the power of using domain categories (e.g. cognitive, epistemological, socioemotional, etc.) when observing a complex, sociocultural context and provide the empirical basis for our work. Following these examples, this work seeks to categorize the types of struggles students face using the domains of cognitive, epistemological and socioemotional. This categorization allows us to understand the domains in which struggle and learning occur within the situated context of the undergraduate general chemistry laboratory.Theoretical framework
The chemistry laboratory at any educational level is a complex and diverse environment where student learning is affected by many variables such as task design (Domin, 1999; Xu and Talanquer, 2013; Laverty et al., 2016; Moon et al., 2017), instructor and student goals (DeKorver and Towns, 2015; Santos-Díaz et al., 2019), and interaction with peers and TAs (Krystyniak and Heikkinen, 2007; Sund, 2016; Jobér, 2017). In the undergraduate general chemistry laboratory specifically, this complexity is increased due to the variety of activities and diversity of student backgrounds. These factors produce unconformity in the laboratory tasks students experience within and across institutions making it difficult for researchers and educators to measure and compare chemistry laboratory learning. Research and student assessment are further complicated by the social and collaborative nature of chemistry lab; students working with partners or small groups and frequently interacting with a teaching assistant (TA) or instructor obstructs individual assessment (Sund, 2016; Jobér, 2017). Furthermore, general chemistry is a prerequisite for many STEM degrees and thus includes students at different levels of education (first-year through final year of undergraduate), who come from different secondary school backgrounds, and who are pursuing various STEM majors. Because of this diversity, the general chemistry laboratory presents a complex environment full of barriers and opportunities for productive learning outcomes.Therefore, it is clear that chemistry students do not learn in isolation; the social nature of the laboratory exemplifies situated learning (Greeno, 2005) that is highly complex, collaborative, and context dependent. We advance the argument that before testing curriculum reform or instructional methods, we must seek to understand the social complexity of the laboratory environment and its impact on student struggles (and therefore learning). This understanding can be beneficial in providing a holistic approach to laboratory design and student assessment specific to the university, the laboratory course, and the students themselves. This paper uses sociocultural activity theory (also called cultural historical activity theory) to examine the components of the chemistry laboratory and to develop a domains-of-struggle framework rooted in these components. The goal being to elucidate the complex, multi-domain struggles students face in chemistry laboratory learning.
Sociocultural activity theory
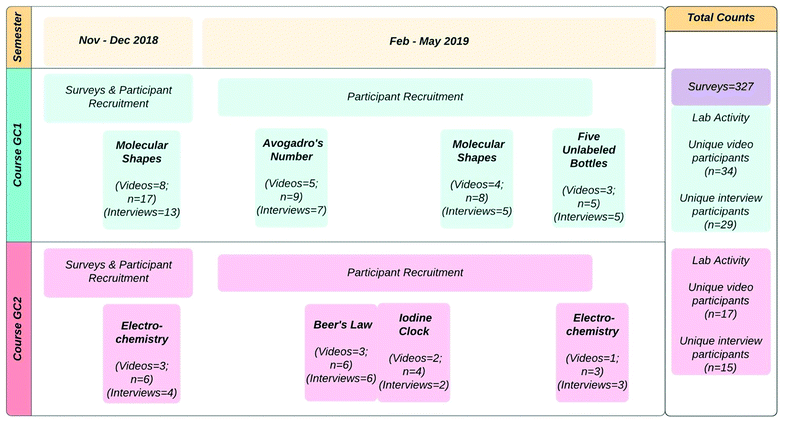
The social complexity of the chemistry laboratory and the contextual nature of struggle are framed in this endeavor by the lens of sociocultural activity theory, which is hereafter referred to as activity theory. Activity theory is based on the theoretical foundation of Vygotsky and Leontyev which claims that knowledge acquisition and human development occur in the social plane through interactions with mediating artifacts (Leontyev, 1978; Wertsch, 1985; Lantolf, 2000). Activity theory is often used in education research to capture the sociocultural components involved in learning by analyzing the situated activity systems of classrooms (Greeno, 2005; Gedera and Williams, 2016). An activity system is comprised of the subjects who utilize tools and mediating artifacts to complete the object of the activity. Engeström (1999) proposed a second-generation model of this system by adding the rules, community, and division of labor of the activity to the original subjects, tools, and object of the activity system triangle (Fig. 1). The second generation activity system triangle was used for this work due to its ability to “explicate the societal and collaborative nature of [students’] actions” (Engeström, 1999, p. 30).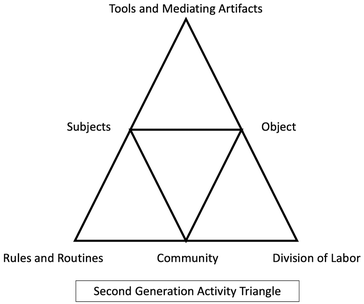 | ||
| Fig. 1 Components of the second-generation activity system triangle (Engeström 1999). | ||
This compartmentalization of sociocultural variables, while acknowledging their continuously dialectic nature, allows for a holistic analysis of the learning process and makes clear the connections and contradictions between components which produce certain outcomes (Gedera and Williams, 2016). In science education, activity theory has been proposed as a framework for incorporating socio-scientific issues into the classroom and increasing the relevance of chemistry in students’ lives (Van Aalsvoort, 2004; Burmeister et al., 2012) as well as analyzing pedagogical contradictions (Russ and Berland, 2019). Moreover, Engeström and others have proposed broader educational applications by observing the process of expansive learning which emerges from contradiction resolution (Engeström, 1999; Engeström and Sannino, 2010; Gedera, 2016).
The term contradiction has a specific and technical definition in activity theory, as a relational process to “overcome and transcend dichotomies” (Engeström, 1999, p. 21) that emerge from the “evolving cell concept of activity” (Engeström, 1999, p. 21). Engeström identifies six dichotomies whose tensions, when unpacked, illuminate how activity functions toward the achievement of outcomes. Gedera (2016) provides a more concrete definition of contradictions as “obstacles, interruptions, conflicts and gaps” (p. 56) within (and between) the activity system(s). Considering the ways in which different components of the system contradict each other elucidates “the driving force of transformation” (Engeström and Sannino, 2010, p. 5) or expansive learning.
This work pursued a similar interpretation using an operationalized definition of struggle from mathematics education: an obstacle which impedes forward progress in the task and requires effort to overcome (Pasquale, 2015; Warshauer, 2015; Sengupta-Irving and Agarwal, 2017). Integrating this definition with activity theory allowed us to locate the obstacle within the activity system and assign the interactions of specific sociocultural components responsible for the struggle. This specificity illuminated how and why that struggle occurred in this situated context and accounted for many of the variables within this complex environment. Similar to the signs-of-struggle framework presented by Sengupta-Irving and Argawal (2017), our framework encompasses the in situ struggle of collaborative problem solving in chemistry, because it provides “opportunities to support perseverance (i.e., Are they persisting?) rather than relying on retrospective accounts to assess its occurrence (i.e., Did they persist?)[…] moving in the direction of assisting teachers or researchers in anticipating productive struggle, which in turn provides opportunities to support or advance students developing this capacity together.” (Sengupta-Irving and Argawal, 2017, pp. 116, 122).
Research questions
The overall research questions that guided this work were (1) What is the source of students’ struggles in the undergraduate general chemistry laboratory? and (2) What subsequent actions do students employ when seeking resolution? The research presented here contributes to the current literature by constructing an activity system perspective for examining activity in the undergraduate general chemistry laboratory and developing a chemistry-specific, domains-of-struggle framework.Research methods
In order to pinpoint the source of students’ struggle within the laboratory activity system, we modeled the laboratory learning environments in two general chemistry laboratory courses using the second-generation activity system triangle (Fig. 1) at multiple levels of analysis (explained in detail in the section titled general chemistry laboratory activity system triangles). A major mechanism of organizing and making sense of the data was to build activity system triangles using different data streams and then comparing/contrasting categories of components. The resulting activity system triangles revealed the sociocultural components that interact with struggles that arose. Due to the situated nature of the laboratory context (Greeno, 2005), it was important to build the activity system triangles from our data to ensure the components comprised the variables students experienced. Through observation of the activity system components, we explored sociocultural causes of student struggle in the laboratory and used multiple domains to classify them. Once struggles were identified and categorized, we observed the students’ subsequent actions in order to understand how they worked towards overcoming the obstacle.Context and participants
This study was conducted at a large, highly diverse, public university in the northeastern US. Participants were recruited from the two lab courses in the undergraduate general chemistry sequence (GC1 and GC2) during two consecutive semesters (both GC1 and GC2 are offered every semester). Students enrolled in the general chemistry courses represent a wide range of majors (though mostly science related), academic years, and demographic identities. Surveys were administered and collected from students (n = 327) during the GC1 or GC2 laboratory sections offered in the first semester of data collection. Students were also recruited throughout the academic year to participate in the video recording of the laboratory (n = 51) and follow-up interviews (n = 44). Fig. 2 shows the timeline of data collection as well as total participant and data item counts for the research project. For all recorded activities, participants were asked to provide both written and verbal consent. TAs (n = 11) in the recorded laboratory sections also provided consent to use their interactions with participants captured during the recorded activity. These general chemistry TAs were a mixture chemistry graduate students and upper-level undergraduate chemistry majors with research experience. All research methods were approved by the University's Institutional Review Board (protocol #2012-102).Laboratory routine. In the GC1 and GC2 courses, students were obliged to complete a pre-lab notebook assignment prior to arriving to lab each week. This routine directed them to record the purpose of the lab, key concepts, procedure/methods, and necessary data tables in their lab notebooks. Students were also expected to have read the procedure before coming to lab and to have watched a video lecture (called a VoiceThread) which reviewed the procedure and concepts required for the activity. Lastly, students completed an online prelab quiz of two randomized questions which tested them on this knowledge.
Upon entering the lab, the students found their stations and partners while the TA collected and graded students’ pre-lab notebook entries. Most students worked with one other partner, though in our recordings one video had a group of 3 and two videos had solo participants. The TA then gave a lecture of similar content to the VoiceThread, with elaboration demonstrating calculations and equipment depending on what the TA deemed necessary. The teaching labs contained large white boards in the front of the room on which the TAs usually wrote important notes, safety guidelines, and tips/tricks for completing the lab. In our data, the boards functioned as a crucial tool for both students and TAs during all lab activities. Furthermore, the teaching labs are connected via an open walkthrough in the back of the lab providing students and TAs easy access to other lab sections. This connection allowed some TAs to combine sections for the lab lectures and facilitated interactions among students in different sections during the lab itself. After the lab lecture, the students gathered the necessary personal protective equipment and proceeded with the lab activity. Some TAs circulated and asked questions, while others stayed near the front of the room. Once students had completed the procedure, they cleaned up their bench before engaging in any data analysis, calculations, or discussion questions. After they had completed the requirements of a lab, students wrote a summary of their performance and results in a small paragraph at the end of that lab in the lab notebooks that all students were required to use. This summary was checked by the TA before students left the lab.
Data sources
In order to ensure credibility of our data, three streams of data were collected: surveys, video-recorded laboratory activities (videos), and video-recorded individual participant interviews (interviews) (Kyngäs et al., 2020). Surveys were designed to capture students’ perceptions of success in the chemistry laboratory, goals and daily routines, general facts about the environment (such as lab partner and TA), and an account of “a time when they struggled and how they overcame it.” The survey data were collected across two courses in a general chemistry sequence during September to December 2018 (Fig. 2), thus offering a representative description of both general chemistry laboratory environments within this University. These surveys were used to extend our analysis of the undergraduate general chemistry activity system beyond only the lab sections that were recorded and so that we could compare the video-recorded struggles to students’ self-reported struggles to establish a more representative framework. The videos served as the primary source of data for developing our struggle analysis. The video data provided direct evidence of struggles students faced and enabled observation of participants’ interactions with the task, tools, peers, and their TA. The interviews added an individual perspective of the activity system as well as a form of member checking (Miles et al., 2014) and deepened our initial interpretations of video data through video-stimulated recall (VSR) (DeKorver and Towns, 2015; Galloway and Bretz, 2016).Utilization of these three sources of data was crucial in our construction of activity system triangles and assigning domains of struggle due to their role in mitigating (to the extent possible) researcher bias and assumptions. Since the first author has taught these laboratory activities and was one of the TAs during the time of data collection, we hoped to challenge preconceived notions of students’ experiences and TA behaviors. These streams of data showed how activity systems were perceived by the students (through surveys and interviews) and how struggles played out in specific laboratory sections (through the videos). However, it must be acknowledged (as with all research) that the insider perspective of the first author has influenced, both positively and negatively, the interviews with participants and inferences made from the data (Chavez, 2008). Nevertheless, appropriate trustworthiness measures (described in this section) were taken to reduce negative effects on data analysis and findings.
Three lab activities were selected from each of the two laboratory courses for video recording, resulting in six activities in total. The videoed lab activities were selected because students completed discussion questions prior to leaving the lab in lieu of completing a lab report at home. These worksheets gave us access to collaborative problem solving that otherwise would (or might not) have happened outside of the laboratory setting. This also allowed collection of the participants’ written work to use during stimulated recall interviews. Descriptions of the six laboratory activities are provided in Tables 1 and 2. As part of the description of each lab activity, we assigned levels of inquiry using classifications (confirmation, structured, guided, and open inquiry) from Bruck et al. (2008), but the degree of inquiry in the activity was not the focus of our analysis. The level of inquiry is offered in the tables as a powerfully descriptive tool to characterize the range of lab activities included in the data. However, the type of task was only one of many variables in the activity system and was not a specific focus of this work.
| Activity name | Description of laba | Learning objectivesb |
|---|---|---|
| a Level of inquiry for each lab activity was characterized using the inquiry levels proposed by Bruck et al. (2008). The range of inquiry is given for some activities due to the differences seen between the task as designed and as implemented by the TAs (Kang et al., 2016). The effect of TAs on lab pedagogy has been previously studied in Sandi-Urena and Gatlin (2012). b Learning objectives for the lab activities were taken directly from the GC1 and GC2 laboratory manuals. | ||
| Estimating Avogadro's numbers | A confirmation lab that requires students to calculate Avogadro's number from both a monolayer of steric acid and a piece of aluminum foil. Students then determine the accuracy of their calculation by comparing it to the given value (6.022 × 1023) | • Estimate Avogadro's number using different known or measured values through a series of calculations and conversions |
| • Become familiar with the process of estimation, especially on a molecular scale | ||
| • Gain an appreciation for the applications and enormity of Avogadro's number | ||
| • Gain practice in deductive reasoning and problem solving | ||
| Molecular shapes | A confirmation to structured inquiry lab in which students work with model kits to determine and explain the 3D molecular structure of a series of molecules. Students are also asked to provide Lewis structures and compare bond angles | • Practice the application of Valance Shell Electron Pair Repulsion (VSEPR) theory for predicting the 3D shapes of molecules and complex ions |
| • Use a molecular model kit to help visualize the shapes predicted by VSEPR theory | ||
| • Learn how electron pair repulsion from lone pairs impacts bond angles | ||
| Five unlabeled bottles | A structured/guided inquiry lab where students mix together salt solutions from two sets of 5 unknowns. Then, given a list of possible choices, deduce the ionic compound in each bottle based on the reactions that occur | • Become familiar with applying the solubility rules to predict whether a precipitate forms in a mixture of ionic solutions |
| • Experience common reactions such as gas formations, precipitations, and dissolutions | ||
| • Practice writing net ionic equations and identifying common ions | ||
| • Learn how deductive reasoning is used to determine unknowns in an experiment | ||
| Activity name | Description of laba | Learning objectivesb |
|---|---|---|
| a Level of inquiry for each lab activity was characterized using the inquiry levels proposed by Bruck et al. (2008). The range of inquiry is given for some activities due to the differences seen between the task as designed and as implemented by the TAs (Kang et al., 2016). The effect of TAs on lab pedagogy has been previously studied in Sandi-Urena and Gatlin (2012). b Learning objectives for the lab activities were taken directly from the GC1 and GC2 laboratory manuals. | ||
| Dilutions, spectroscopy, and Beer's law | A structured inquiry lab which asks student to determine the concentration of red dye in an unknown beverage by creating a Beer's Law plot. Students use serial dilutions of the known concentration standard to make the plot and then calculate the beverage concentration by measuring the absorbance of the diluted unknown | • Understand the basic properties of light and how it interacts with matter |
| • Practice making precise dilutions and build a Beer's Law plot | ||
| • Use the calibration plot to determine the concentration of an unknown solution | ||
| Iodine clock kinetics | A confirmation to structured inquiry lab in which students run multiple trials of an iodine oxidation reaction in order to determine the rate equation. The reaction is performed with varying concentrations in part 1, and varying temperatures in part 2. By graphing the data, students determine the order of the reaction as well as the activation energy | • Investigate the effect of reactant concentration on the rate of a chemical reaction |
| • Investigate the effect of temperature on the rate and rate constant of a chemical reaction | ||
| • Become familiar with manipulating rate equations | ||
| • Apply the Arrhenius Equation. | ||
| • Learn the utility of linearizing exponential functions | ||
| Electrochemistry | A guided and open inquiry lab consisting of four parts: (1) create a galvanic cell which can power a 2 V LED using some combination of given salt solutions and their half-cell reduction potentials. (2) Determine the concentration of an unknown copper nitrate solutions using a concentration cell made with a known solution. (3) Dissect a common AA battery and determine the function of each component. (4) Design a galvanic cell using the AA battery components which can produce 2 V and power the LED | • Learn about redox reactions |
| • Practice writing and balancing half reactions | ||
| • Become familiar with standard half-cell potentials | ||
| • Use the Nernst equation in a lab setting | ||
| • Justify why one electrochemical cell could be more “green” than another | ||
Prior to data collection, the interview protocol and process were reviewed by researchers outside of this project, and two trial interviews were conducted with undergraduate chemistry students to improve credibility of the data collected (Kyngäs et al., 2020). As shown in Fig. 2, during the first semester of data collection, only the Molecular Shapes (in GC1) and Electrochemistry (in GC2) labs were recorded. This was intended as a pilot run of the video data collection system (Fig. 3) and interview protocols. In the second semester of data collection, the process and protocols were streamlined but otherwise unchanged thus allowing all data collected across both semesters to be analyzed. For participants who completed more than one interview (i.e., participated in more than one activity recording), the interview protocol was adjusted to omit some of the general questions the second time and to add further reflection questions.
Video recording equipment was installed in the ceiling of one of the teaching laboratories (Fig. 3a). Two cameras were used; one to capture students’ faces and the other to capture the bench top and equipment manipulation (Fig. 3c and d). To capture audio, participating students wore lapel microphones throughout the lab (Fig. 3b). Since all equipment was hands-free and unobtrusive, it did not hinder the safety of the lab and, as seen in the data, students often forgot they were being recorded.
The participants were recorded for the duration of the lab activity, lasting between 1.5 to 4 hours. Participants completed their follow-up interviews with the first author, which included two parts. The first consisted of semi-structured questions focused on establishing activity system components and individual participant histories (Jonassen and Rohrer-Murphy, 1999). The second utilized VSR during which students were shown salient video clips and asked to explain their feelings and experiences (DeKorver and Towns, 2015; Galloway and Bretz, 2016). Selection of video clips for VSR also served as the preliminary coding, in that clips were chosen based on apparent moments of struggle using previous signs-of-struggle frameworks (see Appendix B). The VSR offered a form of member checking by providing an opportunity for the interviewer to probe if students were actually struggling in these moments, and if so, to explore these struggles more deeply (Lichtman, 2013).
Data analysis
All survey data were compiled by question and analyzed using content analysis (Hsieh and Shannon, 2005). Video data from recorded laboratory activities and interviews were transcribed (transcript conventions in Appendix A), though coding was always done in conjunction with the video in order to maintain level of detail (Chan and Clarke, 2018). The goals of analysis were twofold: (1) to construct second-generation activity system triangles for this undergraduate general chemistry laboratory environment (process presented in Fig. 4; findings in Fig. 5 and 6), and (2) to explore how students’ struggles emerge from this activity system within multiple domains (process presented in Appendix B, findings in Table 3) and the actions they used to overcome these struggles. | ||
| Fig. 4 Construction and comparison process of general chemistry laboratory activity system comprised of components from Engeström's (1999) second-generation activity system triangle. The grey circles contain the data used to construct the general, course-level activity system (navy blue triangle; findings in Fig. 5) and the specific participant-level activity system (light blue triangle; findings in Fig. 6). The arrow represents the interconnected nature of these two activity systems and the tertiary contradictions that may arise between them. | ||
 | ||
| Fig. 5 Generalized course-level activity system triangle for the undergraduate general chemistry laboratory courses. | ||
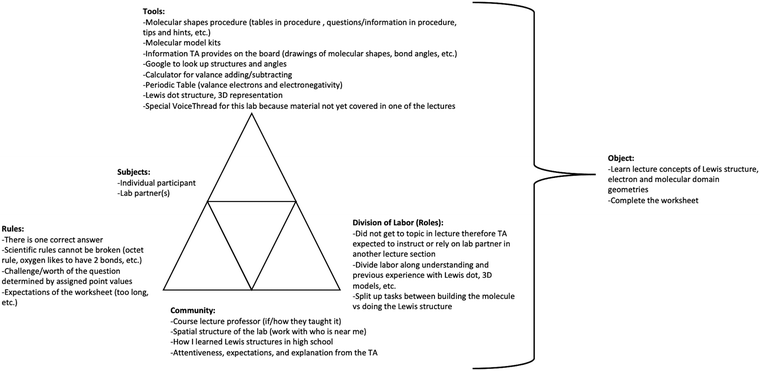 | ||
| Fig. 6 Example of a lab section/task-specific activity system triangle for the Molecular Shapes lab in GC1 for one lab pair. | ||
| Domain | Activity system definitiona | Examples from data |
|---|---|---|
| a This table refers to the components of the activity system, beginning each time with the component of the subject. | ||
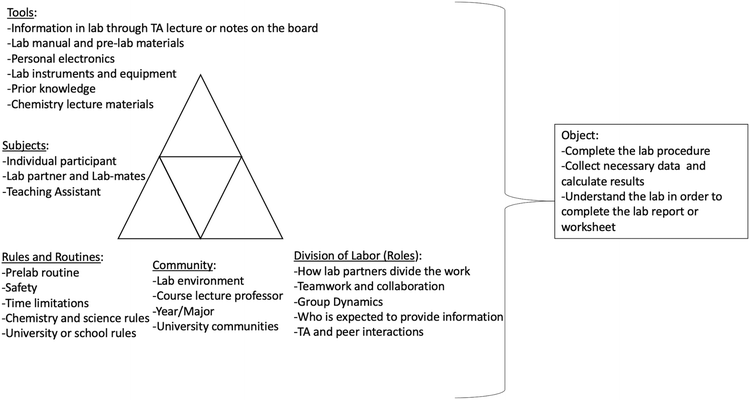
| Cognitive | Conceptual or operational struggles emerged through contradictions centered around the top portion of the activity system triangle: subjects using tools and mediating artifacts to complete the object (Fig. 7). These contradictions can lie within one of these components (e.g., information provided in the worksheet (tool) contradicts information provided on the internet (tool)) or between the components (e.g., the students (subject) do not know how to input calculations into excel (tool)) | Struggles with: |
| Calculations or calculator issues | ||
| Concepts and conceptual applications | ||
| Chemistry equations/formulas/representations | ||
| Math equations/formulas/representations | ||
| Operation of instrumentation due lack of connection to conceptual understanding | ||
| Lack of or contradicting prior knowledge | ||
| Unclear language or unknown vocabulary | ||
| Physical or psychomotor | Psychomotor or physical struggles emerged between subjects and physical requirements of the tools or between the tools ability to complete the object (Fig. 7). The struggles emerged due to physical constraints of the subject (e.g., someone who is color blind trying to read a spectrometer) or issues with the tools (e.g., the pH meter was broken) | Physical impairments |
| Lack of psychomotor skills | ||
| Non-functioning equipment | ||
| Lack of precision or accuracy due to physical constraints or instrument issues | ||
| Epistemological | These struggles emerged from contradictions within the bottom, left half of the triangle between the subject's framing of the object or the implicit and explicit rules of the system or the community in which the activity takes place (Fig. 8). These contradictions can lie within one of these components (e.g., The students expect to get the same answer as their peers, but the TA does not (subject's framing)) or between the components (e.g., procedure directs students to throw waste down the sink (rule specific contradicts rule general/science community)) | Unclear expectations |
| Deviations from routine | ||
| Waste of time/not enough time | ||
| Scientific standards/expectations | ||
| School expectations | ||
| Perceived knowledge, ability, or agency | ||
| Method or approach to problem solving | ||
| Socioemotional | Social struggles emerged from contradictions within the bottom right, half of the triangle among the subjects and the community's division of labor to complete the object (Fig. 9). These contradictions can lie within one of these components (e.g., the subjects have different ideas for how to divide up the task) or between the components (e.g., the subjects want to work independently but the community requires they work collaboratively). This domain also includes emotional struggles which emerge from contradictions (e.g., the student becomes too frustrated to complete object) | Social conflict or mismatch role |
| Disagreements around how to divide labor | ||
| Lack of communication or guidance | ||
| Social distraction | ||
| Emotions/feelings | ||
Responses were analyzed using content analysis (Hsieh and Shannon, 2005) producing descriptive code. The descriptive codes were then further categorized into activity system components (example in Appendix C). Video data from activities and interviews were analyzed for the activity system components (both general and specific) using the same process. Findings from each data set were compared to ensure representation and triangulation of general categories presented in the general laboratory activity system triangle (Fig. 5). The general activity triangle established the sociocultural components which affect the laboratory environment and was used to consistently categorize and compare findings at the specific, participant level.
Second, activity triangles were built to contextualize each specific laboratory activity (example for Molecular Shapes lab in Fig. 6) which embodied the activity system components for that activity, section, and participant pair (or triad or single student in a few cases). The specific lab triangle served to contextualize the struggles observed in the video data. Generation of both the generalized and specific triangles allowed us to situate the components of the specific lab within the course as well as uncover inconsistencies between the general system and specific lab activities (e.g., divergence from normal lab routine).
From this first round of analysis, we recognized that many of the struggles students faced were a result of contradicting activity system components. For example, Nutella and Eygever paused when drawing a Lewis structure during the Molecular Shapes lab because they were unsure of what atom to put in the middle. Upon closer observation of this moment, we recognized that this was due to a contradiction in their approaches; Eygever states that “the least electronegative atom should be in the center” while Nutella says “No, there are two Cls right? So I thought it would be something like (draws Cl on the outside with Be in the center)”. An outsider can see that Eygever has the trend for electronegativity backwards which causes them to arrive at different answers, but from the students’ perspective, they are experiencing a struggle that arises from a contradiction between these two methods. Since our research focuses on ways students experience and overcome struggles, we found the consideration of contradictions as sources of struggle promising since they directly connect the struggle to the components of the activity system and student actions to the “expansive transformation” of activity (Engeström, 2001, p. 137). Examples of these contradictions are presented in detail in the findings section. Struggles in our data arose from three different levels of contradiction (Engeström and Sannino, 2010):
(1) Primary contradictions within the component such as inconsistencies between different tools at the specific level of activity,
(2) Secondary contradictions between the components such as inconsistency between the tool and the object at the specific level of activity, and
(3) Tertiary contradictions between activities/systems such as inconsistency between labs, the general lab activity system and the specific lab activity system, or quaternary contradictions such as inconsistency between lab and lecture.
The identified moments of struggle were deductively coded as referring to cognitive, epistemological, and socioemotional domains, using the definitions provided by Sohr et al. (2018, p. 891): “[…] describing any extended opposition/decoherence in how people are relationally involved in the interaction (social), how knowledge is being enacted or constructed (epistemological) and the content of the interaction (conceptual).” In the second round of analysis, we recognized that, in our data, the domains of struggle appeared to correlate consistently with the components in contradiction within the activity system. This resulted in revised domain-of-struggle definitions. Revisiting the survey and video data using this combined framework revealed a fourth domain: physical/psychomotor. The emergence of this domain was unsurprising based on previously identified domains involved in chemistry laboratory learning (DeKorver and Towns, 2015), but was not accounted for initially as it had yet to be included in a sociocultural framework within chemistry education literature.
We defined these four domains of struggle based on their associated contradictions within the activity system and incorporated broader literature definitions (see Appendix B); they were supported with examples from the data to create the general chemistry laboratory domains-of-struggle framework presented in Table 3. Dependability of the final version of this framework was tested through independent coding and peer examination of the framework by the second author and other researchers outside of the project (Kyngäs et al., 2020). Discrepancies in coding were discussed until consensus was reached resulting in refinement of the domain definitions and producing 97% agreement. Our final round of coding involved using the domains-of-struggle framework (coding example presented in Appendix C) to qualify the moments of struggle identified in the videos and to observe the actions which led to the resolution of the contradiction or some other method of moving forward with the task. Common actions observed are discussed in the findings.
Quality of data and unit of analysis
Only one of the 29 lab activities recorded did not result in any participant interviews. This video was still analyzed for struggles, though any moments that were unclear were left uncoded. Additionally, only the first part (Part A out of A–D) of the Electrochemistry laboratory activity was analyzed for two out of four of these recordings since the later parts of the activity were compromised due to uncontrollable circumstances (e.g. participants leaving due to illness). Lastly, two videos contained a single consenting participant. These videos were still coded as the student interacted with their peers and TA.It is important to note that though course-wide and individual data was collected and analyzed, the unit of analysis for this work was a single lab activity for a consenting participant pair. This means the majority of the contradictions arose from specific activity triangles situated for that lab activity and those participants. The highest level of activity system that could be directly analyzed from the survey and interview data was the general lab level. As previously mentioned, the broader, course view and individual histories/perspectives provided by the surveys and interviews (respectively) were used to supplement and extend our understanding (Kyngäs et al., 2020) and are discussed as findings. However, we did not attempt to infer higher levels of contradiction beyond that which we had direct evidence for. While we acknowledge that roles or knowledge can produce contradictions with a higher level of activity (i.e., society), we focused on what was immediately observable in our data and the components instructors could control. For example, in instances where a student's high school teaching contradicts another student's understanding, we coded this as a contradiction between the prior knowledge of the subjects (cognitive) even though there may be an inferred societal level contradiction between the students’ high school experiences (community).
Findings
The analysis resulted in the domains-of-struggle framework. This analytical framework emerged from the two levels of activity systems (general lab course and specific lab activity, represented in Fig. 5 and 6, respectively) that were characterized. We begin with a brief description of the different items that arose from our data which comprised the general chemistry laboratory activity system (subjects, tools, rules, community, division of labor, and objects). Following this, we present the domains-of-struggle framework based on the activity system structures, common patterns of struggle which emerged, and observed participant actions towards overcoming these struggles.General chemistry laboratory activity systems
From all three streams of data, we identified and refined the items within the components of the system(s) we were observing. We used survey and interview data to further describe the interconnected nature of these components, which are presented here.The subjects of the undergraduate general chemistry activity system (Fig. 5) consisted of the students, their peers, and their TA because all of these individuals worked together towards the object of completing the lab. However, for the lab specific activity system triangle (Fig. 6), the subjects component was narrowed to the specific lab pair (or group) which constitutes the unit of analysis for this work. In the surveys and during the participant interviews, students were asked about their relationships with their partners and their TAs in order to explore the subjects’ relationships. The data showed that lab partner compositions included both randomly chosen and/or assigned partners and partners who knew each other prior to this lab course. Similarly, we observed a spectrum of students’ dependence on their TAs. Some students checked their progress with their TA after every step while others barely spoke to their TA. Students who did not like or trust their TA (stated explicitly in the interview data) spoke about utilizing peers or the TA in other sections for help when needed. Some of this data was categorized in the division of labor component, where we captured the social dynamics of the lab and the participants. As may be expected, we witnessed a plethora of roles played by partners, peers, and TAs during the laboratory activity. The division of labor component emerged from the survey and interview data explicitly when students talked about dividing up the tasks between the pair or asking someone else to contribute to the work. The division of labor was also affected by the task itself. For example, the Iodine Clock activity was designed for students to analyze whole class data requiring them to wait for other groups to finish before moving on to data analysis.
At both the general and specific levels, the community component represented the role of the university, chemistry lecture course, as well as the culture of the lab itself (collaborative, fun, etc.). Whereas, the rules and routines component of the activity system centered around implicit and explicit rules, and fulfilment of perceived expectations. In the data, students discussed the routines of the lab, such as prelab work or the TA lecture. They also frequently mentioned general lab rules such as safety routines, writing in pen, and cleaning up after the experiment.
The tools encompassed the lab manual/procedure, information written on the board, chemistry equipment and instruments, and computational machines (computers, phones, calculators, etc.). Students sometimes revisited the VoiceThread or introduction section of the procedure if they got confused or had questions. Prior knowledge played an important role in many of the labs, with students frequently comparing what they were doing/learning to labs they had done in high school or concepts they had learned in lecture. A finding that emerged specifically in the video and interview data was the great quantity of resources students were navigating for each activity and the contradictions that emerged from them (discussed in detail in the Discussion section). At the general level, the object was to simply complete the lab. However, in the specific-level activity system, the object of the lab was defined as the task or question at hand (i.e., solving for Avogadro's number, determining the unknown solution, etc.). This allowed us to identify what the students were working towards and when progress was impeded.
Domains of struggle in general chemistry laboratory
In this section, we present the domains of struggles we observed in our data using the activity system definitions presented in Table 3. It is important to acknowledge that our focus on struggles resulting from contradictions revealed this relationship between our domain codes and the components of the activity system triangle. That is to say, throughout the second round of analysis, we repeatedly found a correlation between the components involved in the contradiction and the domain definitions. This finding led to redefining the domains of struggle using this system relationship and revisiting the data to see what this combined framework revealed about students’ struggles. Therefore, the findings presented here are the result of our final round of analysis and provide examples of these specific domain definitions and moments where the domains overlapped or shifted. It is also important to note that due to the interconnected nature of the activity system, all components of the system (and thus all domains) are present in every moment of struggle. However, our definition of struggle focuses on what system components are in contradiction; these components determine the domain of the struggle. This coding sequence is illustrated in the Appendix (C).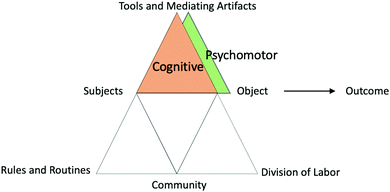 | ||
| Fig. 7 Activity system definition of cognitive and psychomotor/physical domains representing contradictions between and within subject, tools, and object. | ||
These struggles often involved primary contradictions among tools within the specific lab activity. A common example of a cognitive struggle from the Molecular Shapes lab involved contradictions between different tools for representing molecular structure. In the example below, Star and Planet were working on representing and interpreting the molecular structure of carbon dioxide. Star had built the structure using the molecular model with the tetrahedral (rather than the linear) configuration of carbon. This caused their model to have a bond angle of 109.5 degrees, which contradicted with their Lewis diagram and the image they found on the internet (both linear). In the excerpt below, the students were trying to decide whether this conflict arose due to the difference between the two structures the question asked for: “For CO2: Draw the Lewis dot models for both molecules (1 pt each) and draw the structural representation of their molecular shapes (1 pt each).”
Star: Cause that's what I was mentioning if we draw that ((points to board)) or do we draw this? ((points to worksheet))
Planet: What I know is that is the electron, electron geometry. Molecular geometry… let me see ((picks up phone and googles structure for CO2))
Star: Because over here I’m just like over there wouldn’t this one be considered bent? ((picks up model and puts it down))
Planet: Um:: yeah I think it would be bent
Star: And then over here ((points to phone in Planet's hand)) it's um linear cause over here you can’t make it be straight like even if we put it ((starts moving bonds around on the model to try to make it linear))
Planet: Yeah.
Star: Wherever I put it will be bent it in any part it will be bent so I don’t know if…
Planet: This, this is the structure formula ((points to image on her phone)) I’m just not sure. But I’m sure it's bent.
This struggle presented a cognitive struggle resulting from a primary contradiction between mediating artifacts of the tetrahedral molecular model and the linear structure drawn in their Lewis diagram and confirmed on the internet. Star and Planet struggled to account for this contradiction when drawing the Lewis dot model and structural representation and thus could not move forward in the task. This type of cognitive struggle occurred in the majority of molecular shapes labs recorded and was often resolved with clarification from the TA on the different meanings of the representations. We found it interesting that, among themselves, the TAs did not have consistent answers to this question, indicating that “structural representation” in this task was subjective. Similarly, some students did not ask the TA and instead invented a meaning of their own. Regardless, establishing a definition of structural representation allowed students to move forward.
As shown in this example, students used not only the tools provided but also outside resources (i.e., internet, class notes, high school chemistry experiences, etc.). Though the answers to the worksheet questions are often in one of the provided tools, these students chose to search the internet. This may be in part due to the common inconsistencies within the provided material which students reported about in their survey responses; “The lab manual is very inconsistent, meaning that at many points during the lab, I was stuck because the instructions were poor”, “The Voicethread confused me at first because it said this lab was going to be divided up into 2 parts, so we would do the second part next week. My TA later told me that was not the case.” The contradictions between tools (primary) or between subjects and tools (secondary) often led to cognitive struggles and resulted in students ignoring information or equipment because they did not know what to do with it or how it fit into the activity. In some instances, repeated inconsistency among the tools forced students to rely on the TA to provide clarity and instruction rather than following the procedure.
The domain of physical and psychomotor struggle first emerged when coding the open-ended survey question which asked, “Reflect on a time when you were struggling with an activity. What was the activity and why was it difficult? Did you ask for help – from whom? Did you overcome the challenge – how?” While many of the responses fell into the other three domains of struggles, responses such as “The spectra lab was one I found trouble with. My lab partner and I both have vision problems making the spectroscope hard to detect” and “We were struggling to light up the burner, so we asked the TA. It was difficult because our lighter was not working eventually getting another one we tried it and it worked” proved difficult to code. These struggles involved issues with the subjects and the tools, however they did not seem to arise from any conceptual or procedural contradiction, but rather a physical one. Once the psychomotor domain was incorporated, reapplying the updated framework to the video data illuminated new examples. A common example was the difficulty in dissecting an AA battery during the Electrochemistry lab activity. In the excerpt below, Pineapple and Z were beginning their battery dissection:
Pineapple: Ok. And then you’re going to go open it that way kind of ((showing Z how to twist the pliers))
Z: What am I… I’m making the incision with (?)
Pineapple: So incision with the wire cutters here and here ((points to the battery)) and you got to like when you open it it's gonna spray, ok? So that's when you want the gloves on its gonna spray it's gonna start getting hot but then you open it, it should like spray or whatever and then you’re going like into the incision this way and trying to open up that way.
Z: Mhm. ((P puts down pliers and steps back to let Z into the hood)) Ok you’re going to be right here?
Pineapple: I haven’t done this before either Z, so I really don’t know, that's just that's like what TA just showed me that 30 second diagram and I’m just showing it to you now.
Z : ((Laughing)) Alright. Oh my god… ((tries to start cutting into the battery with the wire cutters))
Pineapple: I don’t know if you don’t want to, if you want to stop or whatever, just say so.
Z: ((Repositions herself)) How do I… Oh.
Pineapple: yeah, yeah they’re a little tricky if they don’t have a latch. (referring to the pliers)
Z: Ok hold on, how do I work this?
In this example, Pineapple explained to Z how to dissect the battery, demonstrating that there was no cognitive struggle. However, Z struggled to operate the pliers in order to cut the battery. This struggle continued throughout the dissection as Pineapple and Z took turns trying to cut it open and exclaiming that the battery was too hot to hold. This interaction reveals a contradiction between the ability to use the pliers or hold the battery and the physical capacity of the participants. These students were able to move forward through a division of labor by taking turns and working together.
As others who have studied the psychomotor learning domain (Hofstein, 2004; DeKorver and Towns, 2015), we believe these types of psychomotor struggles are unique to a chemistry laboratory learning environment. The underlying, secondary contradiction with these struggles appeared as an impairment of the subject's physical ability or an impairment of a tool. However, these struggles were important in the overall trajectory of the lab because (1) they hindered forward progress, and (2) they often resulted in emotional outcomes such a fatigue or frustration which led to other domains of struggle (e.g., not being able to fulfil their role resulting in a socioemotional struggle). These struggles were usually resolved quickly by the TA granting accommodations (e.g., providing data for the student) or replacing the equipment. While psychomotor struggles are highly individualistic, the sociocultural implications of these struggles are expanded upon in the Discussion section.
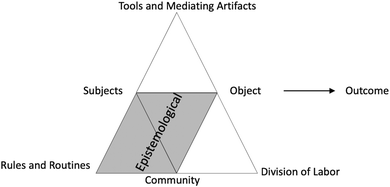 | ||
| Fig. 8 Activity system definition of the epistemological domain representing contradictions between and within subject, rules and routines, community, and object. | ||
In the survey data, the most common epistemological struggle involved a secondary contradiction between the laboratory timeframe (rule) and students wanting to get the lab done so they could leave (subject's expectations), similar to the ‘get an A and get out early’ student goal presented by DeKorver and Towns (2015). In interviews, epistemological struggles were also attached to students’ self-identity. These struggles arose from a secondary contradiction between the expectation of performance (rule) and the students’ perception of not being able to meet that expectation (subject). For example, this is evident in Universe's interview statement, “Like in myself, I kind of don't know the material well and then I'm like, I'm not confident in myself to enough to, you know, go ahead by myself and do things.” Here, Universe evaluates herself as a student/scientist/chemist. Her lack of confidence in the material creates a contradiction with what she believes is necessary to “go ahead and do things” in the lab. Universe seems to resolve this contradiction later in the interview when she says that she feels like a scientist:
Interviewer: And you said when we were watching this that you kind of feel like a scientist now. What made you feel like that?
Universe: I don't know, I just see the gloves, like the lab coat and I feel like I know what I'm talking about. And then, you know, interact with other people. It's just great. Cause I would look at this (the lab procedure) like five years ago, like before and I'd be like, wait, what is this? Like how do you do this? And now I'm just like, yeah, yeah, yeah.
When reflecting on this moment of struggle, Universe sees herself as a scientist potentially transforming her expectation and sense of self.
Epistemological struggles were also present in the activities themselves, especially when the lab procedure asked students to go against a lab norm (a tertiary contradiction between the general lab activity system and the specific lab activity system). For instance, the Beer's Law activity directed students to throw all of the waste down the sink instead of in the waste container. The instructions clarified that this was due to the greenness of the activity since the only solution used in this lab is a mixture of a red commercial beverage. Nevertheless, this instruction (specific rule) contradicted the general laboratory rule of ‘waste goes in the waste container and never down the sink’ and often caused confusion among students as well as TAs.
Positioning the procedure as an external authority or demand (usually connoted as “they” or “them”) tended to be a marker associated with epistemological struggles. These findings are reminiscent of beliefs about learning from an authority in physics education research (Hammer, 1994; Elby and Hammer, 2001). Students often grappled with what “they” would want or whether the effort of doing the lab was worthwhile; a contradiction between the subjects’ and the community's value of the object. We also observed many instances of confusion when students were presented with a question worth zero points in the Molecular Shapes lab:
GT: I know but part 2's the hard one though.
Shelby: Really? ((looks at part 2)) [Oh man…
GT: //Yeah no look at part 2 though like this, zero points, so I don’t know if that counts. Like these ones have points --
Shelby: I think it's 10.
GT: Ah sh*t then I don’t even want to look at that then.
This exchange between GT and Shelby illustrates the students’ epistemological struggle with an implicit rule (questions are worth points) and the explicit listing in the worksheet of zero points for the first question of part two. GT and Shelby rationalized this contradiction by assuming the zero was a typo. In other videos where this struggle occurred, students chose to skip the question entirely (not worth it), to ask the TA to clarify why it is only worth zero points, or to justify it by realizing they had already drawn the molecule in an earlier question.
Socioemotional struggles. Socioemotional struggles are characterized by social conflict or concerns and/or emotional barriers (Table 3) (Isohätälä et al., 2018; Sohr et al., 2018). Socioemotional struggle emerged mainly through contradictions in who should do the work (division of labor), the lab community, and the subjects’ reactions to the object (Fig. 9). These struggles were often indicated by emotional responses, miscommunication, and/or a misunderstanding of who should do what in the activity system.
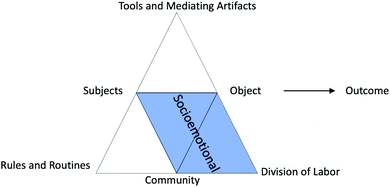 | ||
| Fig. 9 Activity system definition of the socioemotional domain representing contradictions between and within subject, community, division of labor (roles), and object. | ||
Socioemotional struggles occurred frequently when partners did not discuss who should do what before starting a task. For example, in the Iodine Clock lab, Dolphin and Aretha struggled to work together to start the lab:
((Dolphin starts looking at the labels on the provided flasks.))
Aretha: Oh we need test tubes ((reaches for test tubes in box)) how many?
Dolphin: I don’t know give me a second. ((keeps looking at the flasks and reading the procedure))
Aretha: ((reads the procedure)) I think it's just one.
Dolphin: So there's HI, HO2, then what's the last one? ((picks up the third flask))
Aretha: Do you want to get it?
Dolphin: Get what?
Aretha: H2O2.
Dolphin: Yeah I just want to make sure that all of them are labeled. So this one would be the SO [The S2O3 --
Aretha: //I’ll just label them.
Dolphin: No cause these two are labeled I just don’t know what the third thing is. I think the S2 --
Aretha: This one? ((holds up flask))
Dolphin: No this one. ((holds up other flask)) This one isn’t labeled.
Aretha: OK I’ll label that one if you get that. ((takes flask from Dolphin))
In this example, Dolphin and Aretha struggled to communicate clearly what they were doing or trying to accomplish with their actions. Both students were attempting to orient themselves to the first step of the activity by reading the instructions. Aretha was focused on collecting materials like test tubes for the reactions and stock solutions of hydroiodic acid (HI), hydrogen peroxide (H2O2), and thiosulfate (S2O32−). Dolphin was focused on whether or not the three flasks provided for the stock solutions had been labeled correctly. This mismatch in labor created a primary contradiction within the division of labor between Dolphin's role and Aretha's role which continued throughout the first trial of the lab until each of them had redone each other's work. Once both were ready to run the first sample, the contradiction of individual roles created confusion and caused them to start over, now working together, in order to make sure they had done everything correctly and not forgotten a step.
The survey data also revealed socioemotional struggles that emerged from a perceived asymmetry in partners’ division of labor: “I was struggling on one of the labs and my partner was more focused on getting the answers from other people while I was more concerned with figuring out how to get the answers. There was a lot of conflict and I asked my TA for help to get through it” and “I feel like most activities aren’t difficult in themselves but I get very frustrated because I felt like I was doing all of the work for 2 people which always is more difficult and took longer.” These survey responses tell of the conflict and frustration associated with socioemotional struggles that arose from contradictions surrounding the division of labor. Additionally, these struggles focused on social dynamics of the lab community. For example, several students expressed concern about asking too many questions because they felt they were “bugging the TA” or avoiding the TA because the TA “seemed annoyed”.
Socioemotional struggles also emerged when students struggled to complete a task or fulfill their role due to some emotional barrier. For example, during the Five Unlabeled Bottles lab, Star expressed her concern about mixing the unknown substances:
Planet: So we start to mix?
Star: I’m like so scared to mix them.
Planet: ((Laughs)) Me too.
Star: I feel like we just leave it like that… ((starts reading procedure again))
((TA comes over and tells them what to do.))
Star: I’m like so scared. Imagine it like explodes in my hand ((Star laughs; Planet starts to pour the solution into the test tube Star is holding)) I’m just kidding.
Though Star laughed off her fear, this emotion caused her to pause in the activity and revisit the procedure before carrying on. Often times, students moved forward as long as their emotional struggle was acknowledged. For instance, if a lab partner or TA acknowledged the emotions and offered encouragement, then participants were more likely to move beyond socioemotional struggles. When socioemotional struggles originated from other domains (e.g. frustration from an unresolved cognitive struggle), addressing or affirming the student's emotions and offering support enabled students to grapple with the other domain struggle. When support was not available, socioemotional struggle led to disengagement from collaborative behavior—lab partners stopped working with each other, they ignored their TA, one partner took over if the other was too scared, etc.
Embedded domains of struggles
A significant challenge when assigning domains to moments of struggle was that often times struggles emerged from some combination or layering of domains. In these cases, the struggles were double-coded (e.g., cognitive/epistemological). Two reoccurring patterns emerged from these embedded domains: (1) the overlap of cognitive and epistemological domains when students grappled with a tool that did not function as expected (“rules of tools”); and (2) the overlap of socioemotional and epistemological domains when students experienced a contradiction in expected roles (“rules of roles”). We further explore these combinations below to shed light on complicated moments of struggle. In the final part of this section, we explore a moment where all domains are intertwined to exemplify the complexity of these struggles.Like I asked a lot of questions more just to like verify because I feel like the TA will tell us one thing and that'll really be another thing. So like most of my questions aren't because I'm actually confused. It's just like I want to make sure before I do something and then have to restart. […] And then the TA will go ask like another TA and then come back and tell us, actually it's this. But then like at that point we would have already started, we'd have to like restart or redo the calculations just gets super frustrating, especially when you're, I want to leave.
In this quote, Dolphin voiced a contradiction between the TAs actions, “the TA will tell us one thing and that'll really be another thing,” and the implied, expected role of the TA (that the TA should know and/or tell them the answer). Within all data streams, socioemotional struggles were commonly voiced through value-laden qualifiers such as good/bad lab partner or good/bad TA, suggesting some evaluation of the actions of the subject vs. the expected rules of their roles.
Hummus: ((Leans in to look at the absorbance reading on the spectrometer)) Oh:: (pause while they both watch the instrument. H Whispers.)) What I do is I like I stop ((laughs then in a normal voice)), this is so weird. Whenever I’m measuring something and it keeps fluctuating I like stop breathing and I stay really still for a while to see if [I’m like messing with it. Especially when we’re measuring thing (?) ((laughing))
Felix: //It will stop. Oh my god analytical balance. I hate those things.
Hummus: Yeah I mean like if I move the table it’ll ((gestures towards instrument)) it’ll sense it.
Felix: Yeah yeah [You know
Hummus: //It's going up and then down and then up and then down.
Felix : Sigh it's not happy. Well it keep, eh no, it keeps going back to like 3.177… 76?
Hummus: No it's 7, no it's --
Felix: Oh god. I mean it keeps going back to 7. It will go like up then it will go back to 7 then down back to 7.
Hummus: Why don’t we open it up and go again?
((Felix runs the sample again while Hummus goes back to her computer and continues doing calculations.))
Felix: Oh it stopped!
Hummus: It stopped because I stopped looking at it.
This moment begins with an apparent cognitive and epistemological struggle; the students are struggling to understanding why the instrument is fluctuating (cognitive) and expect the instrument to produce a stable reading (epistemological) indicating a rules of tools type struggle. However, Hummus's reaction to the struggle implies she has framed this as a psychomotor struggle (i.e., the instrument is not working properly because we are moving too much). She tries to stand very still and whispers. She laughs about this solution, citing the analytical balance for this learned behavior which seems to have become a general rule of instruments (i.e., don’t move or touch the bench while measuring). This could imply the contradiction lies between this general rule of tools and the actual operation of the spectrometer. However, the pair agrees to rerun the sample resulting in a division of labor; Felix runs the sample again and Hummus returns to her computer at the other end of the bench. When the instrument finally produces a stable reading, Hummus attributes this to her disengagement with the instrument, “It stopped because I stopped looking at it”, implying a psychomotor and/or socioemotional resolution to the struggle. This example illustrates how a single moment in the lab can include struggles in multiple domains, and that these domains are complex and interconnected. In many instances, we witnessed struggles that resulted in semi-resolutions which addressed one domain but not the other. Hummus and Felix are able to move forward because their solution (Hummus disengaging in the task) results in a stable reading. However, the worksheet requires them to grapple with the cognitive/epistemological portion of this struggle by asking them to identify the upper limit of the instrument. This struggle was then overcome completely when the TA explained to them why the instrument began to fluctuate.
Shifts in domain and subsequent actions
Once the domains of struggle were identified, we focused on following the actions towards resolving the contradictions in order to understand the actions students used to proceed forward in the task. Many of these actions are address above in their respective domain section examples. In addition, a common subsequent action that emerged, from both student and TA actions, was to shift the domain of the struggle in order to provide an escape hatch (Sohr et al., 2018). For instance, rather than engage in resolving a difficult cognitive struggle, the students and TA shifted their activity into the epistemological domain, dismissing the effort as not worth the time or not within the scope of the lab activity which implies a contradiction between the perceived value or framing of the activity. An example of this occurred when Pineapple and Z were working on the Electrochemistry lab activity. At this point in the activity, Pineapple and Z were selecting which metal ion pair to use in a galvanic cell in order to produce 2 V to light an LED. The TA arrived to watch the voltmeter as they tested their first choice. The voltmeter was presently reading 1.7 V:((P switches the clip and TA leans in again over the voltmeter.))
TA: Oo:: you’re getting close, you’re getting close ((said in a singing voice))
Pineapple: Psh:: ((laughing)) How do we get it up?
TA: Well again don’t forget that this is all [experimental --
Pineapple: //Yeah right
TA: So and this isn’t a perfect system ((TA gets called away))
((P and Z lean over the bench, P moves the alligator clips in the wells.))
Pineapple : 1.74, I want more!
In this example, Pineapple was asking a cognitive question, ‘How can we get the voltage higher?’ Instead of engaging in this cognitive struggle, the TA shifted to the epistemological domain, judging the lab as not “a perfect system”. In this moment, the students do not take up the TA's framing and continue to figure out how to get more voltage. However, later in the lab when Pineapple and Z were rebuilding the AA battery, Pineapple judged their voltage (which was less than expected) as good enough citing the imperfect system and proceeded past this potential struggle with no engagement.
Epistemological shifts often accompanied cognitive struggles, especially when students perceived risk (e.g., it will take too much time, we’ll have to start over, etc.) or questioned ‘is it worth it?’ (see Shelby and GT example above). Additionally, epistemological struggles often resulted in shifting the domain to the socioemotional realm through changes in the division of labor. When partners held different framings of the task, they often split the work between them or deferred to one partner as the leader rather than engaging in the task collaboratively, which allowed them to ignore their epistemological differences.
For example, during the Electrochemistry lab Pixel and Universe struggled to reconstruct the AA battery due to a contradiction in their framing of the task. Pixel framed the task as applying conceptual understanding of how batteries work in order to accomplish the object of connecting the cells in series, whereas Universe believed they should change each variable in a systematic trial and error method. While this appeared initially as a cognitive struggle (a contradiction between object of getting the battery to produce 2 V and the students use of the tools provided to create a battery in series), Pixel's interview illuminated the epistemological struggle:
I don't like randomly like throwing things in a bucket and be like, maybe it'll work. So by the end I like couldn't figure out how to like to make a series work. […] And so Universe was like, do you want to try this? And I was like sure. Like why not use aluminum instead? Like why not use silver and said like go ahead. But I was like kind of over it just because I felt like there was like a barrier in my understanding that like wasn't going to be like bridged by like doing things on the table. […] And so like, because I felt like we weren't going to get there, I was like, what's the point anymore then? […] Um, but yeah, so I, I did kind of like disengage a little bit. Um, I tried to be present enough to like respond to anything that Universe said, like if she would ask me a question and I don't want to be like, awful person and like just ignore her. Yeah. But like I would only like respond. I wasn't like trying to think of anything anymore cause I couldn't, I couldn't think of like how to do it.
We see from this example that, while Pixel was having difficulty understanding how to connect cells in series, the barrier to his engagement was in fact Universe's method. The contradiction lay between Pixel's need to understand the theory and Universe's trial and error. Additionally, Pixel admits avoiding a socioemotional conflict by deferring to Universe for the rest of the lab activity.
Secondly, although cognitive and epistemological struggles were almost always followed by the participants explicitly requesting help from another person (peer or TA), this action was not observed when students were grappling with socioemotional struggles. Although students did not explicitly ask for help for their socioemotional struggles, we observed many examples in our data of both partners and TAs cheering students on and offering socioemotional support. For example, Flower cheered on Red as she grew frustrated with the long and confusing worksheet of the Molecular Shapes lab:
Red: Like I feel like we’ve already done so much but we haven’t. ((Audibly groans))
Flower: ((whispering)) We still have 10 more [questions]. ((In a normal voice)) Come on let's do it! Let's go! ((claps hands together and moves back from the bench)) You have to study for your exam. ((Laughs))
In this moment, we see Flower providing socioemotional support to Red by taking on the role of a cheerleader and reminding her of why they need to get this lab done (so she can go study). Red then reread the problem they were working on and continued forward in the task.
Similarly, when V7 expressed that she did not understand anything during the Avogadro's Number lab, her partner, NDA, held V7's hand to help her continue to engage in their work.
V7: ((Shakes her head)) I’m completely lost on this thing.
NDA: ((Looking around then back at V7)) Well what are you getting yourself confused about?
V7: Everything. ((laughs a lot))
NDA: Oh well I am very sorry.
V7: No I just mean I’m bad with calculations that's it. ((shakes head))
NDA: Oh ((looks down at the worksheet)) Oh sh*t man… you are so – ((laughs))
V7: Screwed.
NDA: On this one at least. Well it's just kind of the like a weird puzzle I guess, cause I feel like if we could just grab different markers and make a line to what goes where ((gesturing to the board)) cause you’re just using what you find out what is given to you already.
V7: mhm ((hand still on her head))
NDA: Sooo like this one here I’m just confused because my brain started to poop out on me. Maybe your brain pooped out on you
((V7 looks upset.))
NDA: now ((reaches for V7's hand which is still on her head)) let's hold hands and we’ll figure this out ((both start laughing as NDA holds V7s hand)). Moral support. Alright so I got the V atoms-
V7: ((still holding hands and looking NDAs worksheet)) mhm
NDA : Cause I got that ((points to worksheet)) by she [the TA] told me the diameter in this case is the same as the height. And we got height before in trial 2 –
((V7 leans in and looks at the calculations NDA is pointing to.))
NDA: Your hands are really cold dude, I’m gonna let go.
((V7 laughs again and they let go of each other's hands.))
NDA: Moral support is over.
Although NDA let go of V7's hand, V7 picked up her own notebook and began to work on the calculation with her calculator showing that this moment of levity allowed her to reengage in the task.
These instances of socioemotional support addressed the socioemotional struggle which demonstrates the importance of attending to these types of obstacles. In moments where a socioemotional struggle arose that was not addressed, students struggled to move forward in the task. For example, in the Iodine Clock lab, Dolphin struggled greatly with her emotions, repeatedly saying things like, “I’m done. I give up on chemistry” or “I’m so depressed my ears are ringing.” In response to these statements, the TA always addressed the assumed cognitive struggle by providing answers or explanations to the task. While the TA was trying to make the task easier for Dolphin, these actions did not recognize or address Dolphin's socioemotional struggle and thus did not result in her reengagement in the task. Instead, the subjects shifted the division of labor so that Aretha (Dolphin's partner) continued the work alone. From these examples, we can see that recognizing and attending to socioemotional struggles in chemistry laboratory is crucial to maintaining student engagement in the task.
Additionally, students often implemented the “trick” (term common to many students) of moving forward (jumping to a new problem) or backward (checking how they did an older problem) in the task in order to glean hints or figure out what they were supposed to do. When asked how he dealt with confusion during lab activities, participant M&M spoke directly to this tactic:
M&M: It's sometimes it involves, rereading the introduction and methods section as much like starting from the back and just reading it until it clicks, which sometimes can be like multiple readings, you know? Um, other times it's seeing if there's anything that's in the future that makes sense that I can be like, okay, so like for this is going to be like a real bad example. But like if you had ((starts writing on worksheet)), um, kilograms times, meters over seconds and you had like three things over here, like kilograms, meters, second, like I saw this and this was like in the future this I was, I have these three things, I'd be like, Oh well obviously I'm going to need the kilograms in mass over a second. So that's what it's going to wind up being. So then I could start there and be like, okay, what is it asking me in terms of this and then go from there.
In this interview quote, M&M explains the process of re-reading the introduction or searching for hints (in this case required units) in questions later on in the procedure. Examining previous and forthcoming information and problems in the procedure was a common subsequent action associated with all domains of struggles.
Discussion and implications for future research
This study examined and characterized the struggles that groups of students faced when engaging in GC1 and GC2 laboratory activities. A robust definition of struggle emerged as an obstacle created by a contradiction(s) within the activity system that students must overcome in order to continue forward in the task. Applying an activity theoretical lens to analyzing three streams of data (surveys, interviews, and videos of students engaged in the lab activities), we identified four domains of struggle—cognitive, psychomotor, episteme-ological, and socioemotional—associated with specific components of the activity system of the undergraduate general chemistry laboratory. These comprise a domains-of-struggle framework for unpacking how students experience struggle in these chemistry laboratory activities and enabled the identification of layered and associated struggles in which students engaged. Below we situate our findings within the areas of curricular design, TA training, and the productive struggle literature. Finally, we discuss the perspective afforded by utilizing a sociocultural lens and think ahead to how we may assess productivity of student struggles in this unique learning environment.Implications for curricular design and TA training
Reflection on the components of the activity systems offers practical implications for chemistry laboratory task and curriculum design. In the productive struggle literature, struggle is believed to be highly task-dependent, favoring designing ill-structured tasks to produce more moments of struggle (Pathak et al., 2011; Kapur and Bielaczyc, 2012; Sengupta-Irving and Agarwal, 2017). Chemistry education literature has reported the intentional incorporation of multiple representations as a pedagogical tool to generate cognitive dissonance in order to prompt students to adjust their understanding (Linenberger and Bretz, 2012; Corradi et al., 2015). We saw moments in laboratory tasks analogous to this theory of learning when inconsistent tools or representations provided constraints to students’ prior knowledge or other tools. For example, in the Molecular Shapes activity, students were challenged to grapple with differences between Lewis dot models and the 3D geometries of molecules. Reconciling these representations was intended to promote the learning of VSPER theory and how electron pair repulsion from lone pairs impacts bond angles. Similarly, in the Beer's Law activity, students were required to take measurements beyond the upper limit of the spectrometer (when the instrument cannot read the sample due to high solution concentration), a designed discrepancy intended to produce reasoning around data quality.It could be argued that a goal of lab is to learn which tools are useful for a specific task and to allow students to encounter the constraints required for learning (Russ and Berland, 2019). Russ and Berland (2019) attribute scientific learning to the repeated incorporation of feedback that students receive, either through directed feedback from a teacher or feedback from nature and society, on whether a tool is useful/used correctly. This feedback creates conceptual, experiential, and epistemological constraints; encounters with these constraints refine students’ scientific knowledge. Thus, justifying the number of resources students must negotiate could be a design consideration.
However, our findings show that engagement with the pedagogical dissonance in these laboratory activities was heavily dependent on the how often these moments occurred, and the perceived level of importance given by the authority (TA and procedure) and community/subjects (TA, partner, peers). For example, some students became accustomed to grappling with contradictions among tools that were not pedagogically intentional (e.g., inconsistencies between the procedure and VoiceThread) in addition to moments of misguidance from TAs or following unclear tasks. The repetition of these struggles resulted in students avoiding engagement with other discrepancies and/or relying heavily on their TAs or peers for instruction.
Though students were challenged to use novel tools in all lab activities recorded, and to reconcile multiple sources of instruction, we found that students abandoned necessary tools if their utility was not made clear. This occurred, for example, in the Molecular Shapes lab when many students stopped using the molecular models and instead relied on the internet for structures and bond angles. Yet, we do not believe that students lacked problem-solving skills; in fact, students demonstrated many problem-solving techniques in their subsequent actions (see examples above). Instead, we propose that students utilized the resources available to them in order to complete the task within the constraints of the system. For example, if they were running out of time and/or in a community that did not value sense-making, the best solution may be to rely on an outside authority for the answer.
As curriculum designers and educators, we must be aware of and intentional about what purposes the provided tools play in the activity system overall and anticipate the contradictions that can arise from their use. Because of this, we suggest laboratory instructors clearly present the purposes of tools to students or incorporate questions which explicitly guide students through the discovery of a tool's use during the laboratory process. One method which may be helpful in achieving these goals is the TILT (Transparency in Learning and Teaching) method (Winkelmes, 2014). This method applies a Transparency Framework to all levels of curricular materials enabling instructors and students to quickly identify the purpose, task, and criteria for learning activities. Furthermore, instructors must consider any outside tools the students may access and determine their purpose in the lab. For example, if students have internet access in the lab, what websites are helpful and what potential skills are they learning from accessing them? When designing a task, instructors should identify the contradictions which are likely to arise from the use of different tools and examine how these struggles may or may not lead to desired learning outcomes. Simply reviewing lab manuals and lecture materials for unintentional inconsistencies and coaching TAs on how to frame pedagogical discrepancies will help students trust the provided tools and may increase student engagement in struggles.
Additionally, TAs would benefit from greater familiarity with and practice in recognizing the four domains of struggle in order to assist students in their resolution and the learning process. Previous literature has focused specifically on TA-student interactions during chemistry and physics laboratory activities (Velasco et al., 2016; Wan et al., 2020). These studies identified a number of actions taken by students and TAs during their time in the lab and showed correlation between patterns of action and nature of the lab activity and instructional environment. Our domains-of-struggle framework adds to these findings by demonstrating that student action was prompted by struggles which arose from not only the nature of the activity but also the interactions of the system components at multiple levels of activity. Furthermore, Velasco et al. (2016) found wide variations between TAs’ implementation of the same curriculum and reported that if an activity did not explicitly address conceptual understanding, TAs and students did not discuss it. In our findings, TAs’ actions varied greatly as well (e.g., subjective definitions of ‘structural representation’ in the Molecular Shapes lab) and sometimes did not address specific issues regardless of whether they were explicit in the procedure or not (for example, the TA's dismissal of Pineapple and Z's engagement in the cognitive struggle of producing the correct voltage for the Electrochemistry laboratory activity). This implies that, if TAs actions do not identify the contradiction during a moment of struggle, students may miss crucial learning opportunities or internalize unproductive actions leading to a futile framing of chemistry or science overall.
This recognition may be difficult for TAs depending on their prior teaching experience (both as a teacher and as a student); Sandi-Urena and Gatlin (2012) showed that graduate teaching assistants tend to reenact the instructional methods they experienced as students. Therefore, utilizing the domains-of-struggle framework during TA training may help TAs better identify the sociocultural components of student struggles in the chemistry laboratory and provide guidance towards supporting students in learning from these struggles. Introducing this multi-domain framework to TAs would expand their view from transfer of knowledge and enforcing rules (Sandi-Urena and Gatlin, 2012) to seeing themselves as a subject in the activity system with their own knowledge, beliefs, and emotions that can affect students’ experiences. From the sociocultural perspective, this is justified as TAs shifting away from the “banking model” of teaching towards more humanizing and supportive interactions (Freire, 2000).
Domains vs. signs of struggle
The domains-of-struggle framework proved successful in elucidating the struggles embedded in the situated activity of chemistry laboratory. Previous literature has found that identifying the source of the struggle is a key action in making that struggle productive (Warshauer, 2015; Miller 2020). Our sociocultural framework goes beyond previous research in demonstrating a method for identifying sources of struggle beyond the cognitive domain and separating the components of the struggle from the resulting actions and interactions. For example, in math education literature, signs of struggle have been identified as students failing to get started or to carry out a process, expressing uncertainty or misconceptions, seeking support, clarifying the task, or arguing over a declared solution or strategy (Warshauer, 2015; Sengupta-Irving and Agarwal, 2017). While many of these signs occurred in our data, our analysis focused on the source of these moments within the activity system. For example, one common sign of struggle is “failing to start an activity” (Warshauer, 2015). In chemistry lab, there are many reasons students may fail to start an activity: it may be unclear what tool to use (cognitive) or what is expected of students (epistemological), students may grapple with a broken piece of equipment (psychomotor), or they may be scared to start the task (socioemotional). Assigning these domains allowed us to examine the sociocultural components involved in the phenomenon of struggle in order to address contradictions within the system and identify the subsequent actions that led towards resolution.Furthermore, the domains-of-struggle framework allowed us to account for a wide range of student behaviors and actions (e.g. emotional response, escape hatches, etc.) beyond those included in previously reported math education frameworks. The domain framework presented here differs also in the categorization of the moment of struggle which is separate from the subsequent actions. We distinguish previously published signs of struggle, such as students seeking support or asking the TA to clarify (Sengupta-Irving and Agarwal, 2017), as subsequent actions. This distinction clarified the obstacle, the contradiction which must be resolved, as well as actions taken towards resolution. By clarifying how and why these moments occur, connecting them to the activity system components, and separating struggle from subsequent action, our framework allows researchers and practitioners alike to understand and address the specific barriers students face in a chemistry laboratory context and suggest actions to overcome them. The students’ and TAs’ actions presented in this paper have practical implications for supporting continued engagement in struggle (e.g., validating experiences and emotions) and creating opportunities to learn other skills (e.g., socioemotional struggle presenting an opportunity to learn collaboration and teamwork as in the Aretha and Dolphin example). More work must be done to explore the learning outcomes produced through students interactions with these systems in order to capitalize on these complex moments of struggle.
Adding a sociocultural perspective
Other frameworks have been applied to studying students’ performance in the chemistry laboratory and have similar categories of relevant domains. For the most part, these frameworks have been situated in a constructivist perspective (Walker and Sampson, 2013; DeKorver and Towns, 2015; Galloway and Bretz, 2016). Our work adds to the previous literature by providing a sociocultural perspective and an understanding of how the domains interact, overlap, and shift through the course of students’ struggles. Though the combination and shifting of domains added some difficulty to our coding, this complexity was endemic to our choice of a sociocultural perspective because the domains were defined within the activity system. As is commonly represented using arrows, the activity triangle depicts all components connected and related to each other (Engeström, 1999).This complexity is recognized by others. In their work on group conflict, Sohr et al. (2018) pointed out the entangled and intertwined nature of these domains (which they refer to as dimensions) and state that instructors should be attentive to the shifting and embedding of domains throughout an argument. While they acknowledge the instructor's role in supporting students during conflict, our research demonstrates that students and the instructors are both subjects in the activity system; thus, instructors’ interventions also add complexity (and sometimes confusion) as all parties work together towards completing the lab. As shown in our data, whether they realized it or not, the instructors (TAs) had immense control over the struggles that emerged and how students engaged with them, and they either helped or hindered the struggles by shifting the domains (see Pineapple and TA example). The role of instructor interventions on collaborative student work has also been studied. Gonzales et al. (2019) argues that teachers who focused only on the cognitive domain were able to manage the struggle and frustration of students. However, our findings showed that students were better able to re-engage if the instructor or a peer acknowledged their emotions and offered socioemotional support. The ability to attend to the socioemotional domain while engaging in high-level cognitive tasks (e.g., argumentation) has been examined as a crucial skill for collaborative learning and instruction (Isohätälä et al., 2018).
The entanglement of domains prompts further speculation on a sociocultural definition of psychomotor struggles. Psychomotor struggles often emerge and are framed as highly individual. However, as we analyzed psychomotor struggles through a sociocultural lens, we recognized an implied requirement of labor – something akin to the rules of roles. While ableism is an acknowledged problem in STEM education (Prema and Dhand, 2019; Peterson, 2021), this sociocultural perspective offers evidence for its systematism in the laboratory activity. For example, if we view psychomotor struggles as a contradiction between the subject's physical (as opposed to cognitive) abilities and the physical requirements of the tools, psychomotor struggles are contradictions between the tools required to do science and the physical labor required to be a scientist. This expanded view can be understood as an overlap of the cognitive and sociocultural domains creating a contradiction between the scientific community's tools required to the complete the object and the subjects’ labor. Extending the definition of a psychomotor struggle to encompass the division of labor, we see the expectations the scientific community has for who can or cannot be a scientist, an arguably epistemological evaluation of the subjects’ labor, revealing roots in all domains. More research must be done to examine psychomotor struggle through a sociocultural lens as it may be a valuable way of identifying ableism inherent in chemistry and science laboratory and offer means for addressing equity in STEM.
Productivity in general chemistry laboratory
We aim to apply what we have learned about the connection between the activity system and the domains of struggle in order to foster desired learning outcomes in general chemistry laboratory activities. As other researchers have observed (DeKorver and Towns, 2015; Walker et al., 2019), these domains can lead to different learning outcomes and can be used to understand student argumentation, goals, motivations, etc. Our framework adds the connection of the domains to the components of the laboratory activity system, thereby revealing linkages between the environmental components of the lab and the outcomes produced. This analysis then prompts a question that is crucial to address in order to advance equitable student success: What is considered a productive outcome in undergraduate general chemistry laboratory?In STEM education research, productivity varies widely depending on the orientation of the teacher or the researchers. Productivity has variously been defined as achieving the correct answer (Kapur, 2014), engaging in greater or sustained levels of cognitive demand (Warshauer, 2015; Moon et al., 2017), exhibiting collaborative behaviors (Sengupta-Irving and Agarwal, 2017), applying the concept to novel contexts (Kapur, 2014), or developing a scientific identity (Levrini et al., 2015). Sohr et al. (2018) identified escape hatches which were considered productive by allowing students to reengage in a task, avoid social conflict, or reframe the problem. Similarly, in our analysis, we repeatedly witnessed students participating in social breaks after they became frustrated or felt defeated. While many instructors would see this as off-task behavior, it often led to students being able to reengage with the problem soon thereafter. A similar rethinking of off-task behavior has been explored in math education research (Langer-Osuna, 2018).
Furthermore, in chemistry education research, a diversity of instructional goals for chemistry laboratory have been documented (e.g., Bruck et al., 2010; Bruck and Towns, 2013). One definition of productivity could be made in relation to these goals, identifying if the learning outcomes of the struggle fulfill these purposes of chemistry laboratory. Through further analysis of students’ subsequent actions, we may be able to identify what learning outcomes are achieved and in which domains. However, any claim of productivity depends on the values of the stakeholders (e.g., students, instructors, the university, chemical industry, etc.) and the lens of the researchers.
Limitations
Positionality of the first author was a potential limitation to this work which we attempted to mitigate through rigorous credibility (member checking, triangulation) and dependability (constant comparison and consistent operationalization) measures (Kyngäs et al., 2020). Nevertheless, we acknowledge that this insider positionality may have affected interviews due to presumptions in what questions to ask and inferences derived from students’ answers. In two sets of data collected, the first author was the TA in the lab and interviewed her own students. However, this relationship affected only four participants out of 51, and thus likely had minimal effect.Another limitation is the generalizability of our findings to the overall chemistry laboratory experience within this and other universities. Some of the findings may be relevant to worksheet labs and may not capture student struggles in other activities, such as completion of the lab report (which also was reported as a major source of struggle in the surveys). Furthermore, while the students in this university are highly diverse and non-traditional, there are some ways that the diversity of our sample was constrained. In particular, the majority (>60%) of the participants in this study were within their first year of college, female, biology majors. As it is expected that students’ goals, motivations, and personal histories affect the struggles that emerge in the activity systems, these findings likely are most representative of our participants/student body and further data would be needed to increase generalizability. Thus, research could be done to compare these struggles to those experienced by undergraduate chemistry students at a variety of institutions.
Lastly, there are two methodological limitations. First, the activities recorded were not distributed proportionally across the data set. The majority of the videos collected were of the Molecular Shapes lab (41% of the total video recorded activities) followed by Avogadro's Number (17%), Electrochemistry (14%), Five Unlabeled Bottles and Beer's Law (10%), and Iodine Clock (7%). Due to this uneven distribution, 69% of the video data collected were from GC1 while only 31% of the video data collected were from GC2. Mitigating this in part, however, we did receive a >50% return rate from the written surveys from both GC1 and GC2 labs which allowed us to check the representative nature of our findings against students’ self-reported experiences. Furthermore, DeKorver and Towns (2016) showed that there is little change in perceptions and behaviors as students progress from GC1 to GC2. Second, the research was carried out in an iterative process. For example, the survey analysis was used to build the structure of the activity system for a chemistry laboratory activity in general. While this has the benefit of building a model that fits the data, it also makes it difficult to examine the potential that the model may not fit laboratory activities in other settings. As is the case for grounded theory, addressing this constraint would require further studies that attempt to apply our model to data collected in a different setting, such as general chemistry labs at a more traditional university.
Conflicts of interest
There are no conflicts of interest to declare.Appendix
Appendix A: transcript conventions
The transcript excerpts presented in this paper use the following symbols (Jefferson, 2004)::: Elongated words or vowels
[ Start of overlapping speech of first speaker is shown with open bracket
//Start of overlapping speech of second speaker
– Turns that are cut off by other speakers or end abruptly are marked with a hyphen
… Speaker turns that trail off are marked with an ellipsis
((![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) )) actions other than speech, including gestures, are represented in italics and surrounded by double parentheses
)) actions other than speech, including gestures, are represented in italics and surrounded by double parentheses
(?) Pieces of speech that are difficult to discern are preceded or replaced
(#) Length of a pause
Appendix B: iterative process of analysis
Table 4 provides a description of the iterative process of the qualitative analysis used to construct the domains-of-struggle framework.| Data stream | Surveys | Videos | Interviews | Literature |
|---|---|---|---|---|
| Preliminary coding | Holistic analysis for moments of struggle using previous signs-of-struggle framework and unclear moments | Confirmed moments of struggle with participants and explored feelings, thoughts, experiences | Warshauer (2015) and Sengupta-Irving and Agarwal (2017) math education signs-of-struggle frameworks. DeKorver and Towns (2015) video stimulated recall | |
| First round coding | Descriptively coded struggles in one video from each lab activity and sorted into domains | Incorporated relevant interview data to assign domains of struggle. | Sohr et al. (2018) definitions of interaction domains. | |
| Resulting in first iteration of struggle framework (V1FW) | ||||
| Second round coding | Applied V1FW to 8 more videos. Analyzed how V1 struggles emerged from activity system and identified domain overlap with contradictions. Refined domains | Incorporated relevant interview data to assign domains of struggle | Engeström and Sannino (2010) and Gedera, (2016) work with contradictions and learning. Extended epistemic and socioemotional domain definitions using additional literature (Hammer, 1994; Chinn et al., 2011; Berland et al., 2016; Isohätälä et al., 2018) | |
| Resulting in second iteration of struggle framework (V2FW) | ||||
| Third round coding | Descriptively coded students self-reported struggles and sorted into domains using V2 definitions. Identified unaccounted for psychomotor/physical domain | Compared V2FW struggles to self-reported struggles identified in surveys. Incorporated new examples and 4th domain. Applied to 8 more videos | Incorporated relevant interview data to assign domains of struggle | Incorporation of DeKorver and Towns (2015) psychomotor domain in chemistry laboratory |
| Resulting in final iteration of struggle framework | ||||
Appendix C: example of metadata generated during activity system and domain-of-struggle coding process
Below we present examples of coding for a moment of struggle excerpted from a recording of the Iodine Clock Kinetics laboratory activity. First, we provide the portion of transcript for this moment of struggle example, in order to establish context for the coding. Then, Table 5 is presented to exemplify the activity system coding of components identified in this moment, and Table 6 depicts the domain-of-struggle coding for this specific struggle.| Component | Description | Level | Notes |
|---|---|---|---|
| Subjects | A and B | Specific | Usual lab partners, have worked together all semester and knew each other from last semester |
| Tools | Micropipettes/tips | Specific | |
| Test tubes/wrack | Specific | ||
| DI water squeeze bottle | General | Each bench always has a communal squeeze bottle of DI water | |
| Lab manual (printed and on laptop) | Specific/general | ||
| Laptop | General | B brings her laptop to every lab | |
| Stopwatch | Specific | ||
| Container/flask | General | All glassware is stored in cabinets at the benches and around the lab | |
| Division of labor | Observe what others do in lab to figure out what to do | General | |
| Each of us need to add different solutions at the same time | Specific | The procedure calls for the addition of the reactive chemicals at the same time | |
| Rule | Do not contaminate stock solutions | General | |
| Do not take glassware not provided on bench | General | TAs set out all of the glassware students should need on the bench top at each station; enforcement of this rule varies by lab section |
| Descriptions of struggle | A&B struggle to figure out how to measure out the DI water into the reaction test tubes because they don’t have a “stock” of DI like the other solutions, they only have a squeezy bottle of DI water. The tools provided are limited to 3 flasks which are filled with the other solutions and a beaker which is presumably for waste (they eventually use it for dirty pipette tips). A tries to overcome tool issue by just using the squeezy bottle as the stock solution of DI, but she worries about contaminating the DI water with the pipette tip. |
| Contradiction between what and what | Tool: Not provided a container for DI water like they are for the other solutions |
| Rule: Don’t contaminate the DI bottle | |
| VS | |
| Object: Need to use a measured amount of DI water in reaction | |
| Domain of struggle | Cognitive/epistemic |
| Subsequent actions | In the first part of the struggle A suggests they just wait to see what other groups do (socioemotional solution – Community/Division of Labor); later she goes for the DI bottle and B stops her and gets a new beaker from a drawer and fills it up with water (cognitive solution – incorporation of a new tool). B's actions break the lab rule of not getting out additional glassware, but it is unclear whether either student recognizes this; it was not discussed in the interview. |
Iodine Clock Kinetics Laboratory Activity Transcript 03210827: Participants: Ant (A) & Batman (B), TA Consent
((B goes to get pipette tips. A reads the procedure and fidgets with her sweatshirt and hair. B comes back to the bench.))
00:24:26
B: OK ((puts pipette tips down on bench and reaches for test tubes)).
A: What about DI water?
B: Right here ((points to the squeezy bottle of DI water on bench))
A: But how do we measure it, what are we supposed to put it in ((gestures a container with hand))
B: I think just open the bottle. (5) ((A looks at the procedure)) Common sense, you know.
A: ((A laughs)) We’re contaminating the whole thing who cares ((A grabs DI bottle))
B: Is it? So do you want to get a new thing?
A: ((A stops reaching for bottle and looks around the lab)) Let's just wait until we’ll see what people do ((A looks back at the procedure)).
B: Ok
((A and B continue on with the lab, reading procedure and setting the micropipettes to their appropriate volumes.))
00:25:21
A: Ok so you want to do –
B: 400 of 0.20 (3) H2O[2
A://2. You do that. ((continues twisting pipette to set volume)).
B: To where? In the tube? ((points to the test tube in the wrack with pipette)).
A: Mhm- Wait wait! We need to time ((reaches for stopwatch)) when we put it in together.
B: You need the time of this one? I don’t think we need to time this one, do you?
A: Wait…
((They both read procedure. B reads out loud from laptop. A holds stopwatch and follows along in her packet.))
B: So I think you put in the 400 first and then you start, and then you start doing (2) the timer when we start the (1) you know what I’m talking about?
A: Mhm ((Grabs the DI bottle and starts screwing off the top))
B: ok I’m gonna put the –
A: ((whispers)) Wait wait, what about the DI water? I just -
B: No you just have to put that first ((both look back at the procedure on B's laptop)), [that's it.
A: //We’re putting those two in first but I mean am I really just gonna like get it out of here? ((finishes taking cap off of the DI bottle))
B: Oh:: you need 400 DI and then 400 of…
A: ((holding pipette tip over open DI bottle)) This is so unsanitary.
B: Ok ready? Wait. ((grabs pipette out of A's hand and puts both of pipettes down on the bench. B steps back and looks at the drawers in the bench)) Where's the… what? ((B walks off screen))
((A laughs while watching B walk away and screw cap back on DI bottle.)) (24)
B: ((coming back on screen with flask full of DI water)). There you go.
A: Good job B. ((B puts flask on bench in front of them and picks up her pipette.)) Should we label the tubes?
B: Yeah…
00:27:00
Acknowledgements
We thank the students and teaching assistants who participated in this study, as well as Prof. Mridula Satyamurti, who oversees the general chemistry laboratory courses. We gratefully acknowledge the IT and AV department for their tremendous support in installing and maintaining the recording equipment used for this study. Special thanks to Miyah Rivera, an undergraduate researcher who compiled the survey results for analysis and Daniliz Capellán Pichardo, an undergraduate researcher who assisted with data collection. We are grateful to the National Science Foundation (DRL-1621228) for funding. We thank Oracle for fellowship support to CK for this project.References
- Apotheker J., Blonder R., Akaygun S., Reis P., Kampschulte L. and Laherto A., (2017), Responsible research and innovation in secondary school science classrooms: Experiences from the project Irresistible, Pure Appl. Chem., 89(2), 211–219.
- Baker K., Jessup N. A., Jacobs V. R., Empson S. B. and Case J., (2020), Productive struggle in action, Math. Teach.: Learn. Teach., 113(5), 361–367.
- Berland L. K., Schwarz C. V., Krist C., Kenyon L., Lo A. S. and Reiser B. J., (2016), Epistemologies in practice: Making scientific practices meaningful for students, J. Res. Sci. Teach., 53(7), 1082–1112.
- Bjork R. A., (1994), Memory and metamemory considerations in the training of human beings, in Metacognition: Knowing about Knowing, Cambridge, MA: The MIT Press, pp. 185–206.
- Bretz S. L., (2019), Evidence for the importance of laboratory courses, J. Chem. Educ., 96(2), 193–195.
- Bruck L. B. and Towns M. H., (2013), Development, implementation, and analysis of a national survey of faculty goals for undergraduate chemistry laboratory, J. Chem. Educ., 90(6), 685–693.
- Bruck L. B., Bretz S. L. and Towns M. H., (2008), Characterizing the level of inquiry in the undergraduate laboratory, J. Coll. Sci. Teach., 38(1), 52–58.
- Bruck L. B., Towns M. H. and Bretz S. L., (2010), Faculty perspectives of undergraduate chemistry laboratory: Goals and obstacles to success, J. Chem. Educ., 87(12), 1416–1424.
- Burmeister M., Rauch F. and Eilks I., (2012), Education for sustainable development (ESD) and chemistry education, Chem. Educ. Res. Pract., 13(2), 59–68.
- Chan M. C. E. and Clarke D., (2018), Video-based research in a laboratory classroom, in Xu L., et al. (ed.), Video-based Research in Education, London: Routledge, pp. 107–123.
- Chavez C., (2008), Conceptualizing from the inside: Advantages, complications, and demands on insider positionality, Qual. Rep., 13(3), 474–494.
- Chinn C. A., Buckland L. A. and Samarapungavan A., (2011), Expanding the dimensions of epistemic cognition: Arguments From philosophy and psychology, Educ. Psychol., 46(3), 141–167.
- Chopra I., O’Connor J., Pancho R., Chrzanowski M. and Sandi-Urena S., (2017), Reform in a general chemistry laboratory: How do students experience change in the instructional approach? Chem. Educ. Res. Pract., 18(1), 113–126.
- Corradi D., Clarebout G. and Elen J., (2015), Cognitive dissonance as an instructional tool for understanding chemical representations, J. Sci. Educ. Technol., 24(5), 684–695.
- Dalgety J., Coll R. K. and Jones A., (2003), Development of chemistry attitudes and experiences questionnaire (CAEQ), J. Res. Sci. Teach., 40(7), 649–668.
- DeKorver B. K. and Towns M. H., (2015), General chemistry students’ goals for chemistry laboratory coursework, J. Chem. Educ., 92(12), 2031–2037.
- DeKorver B. K. and Towns M. H., (2016), Upper-level undergraduate chemistry students’ goals for their laboratory coursework, J. Res. Sci. Teach., 53(8), 1198–1215.
- Domin D. S., (1999), A review of laboratory instruction styles, J. Chem. Educ., 76(4), 543–547.
- Duschl R., (2008), Science education in three-part harmony: Balancing conceptual, epistemic, and social learning goals, Rev. Res. Educ., 32(1), 268–291.
- Elby A. and Hammer D., (2001), On the substance of a sophisticated epistemology, Sci. Educ., 85(5), 554–567.
- Engeström Y., (1999), Activity theory and individual and social transformation, in Perspectives on Activity Theory, Cambridge, UK: Cambridge University Press, pp. 19–38.
- Engeström Y., (2001), Expansive learning at work: Toward an activity-theoretical conceptualization, J. Educ. Work, 14(1), 133–156.
- Engeström Y. and Sannino A., (2010), Studies of expansive learning: Foundations, findings and future challenges, Educ. Res. Rev., 5(1), 1–24.
- Freire P., (2000), (30th anniversary ed.), Pedagogy of the Oppressed, Translated by Bergman Ramos M. New York: Bloomsbury.
- Galloway K. R. and Bretz S. L., (2015), Development of an assessment tool to measure students’ meaningful learning in the undergraduate chemistry laboratory, J. Chem. Educ., 92(7), 1149–1158.
- Galloway K. R. and Bretz S. L., (2016), Video episodes and action cameras in the undergraduate chemistry laboratory: Eliciting student perceptions of meaningful learning, Chem. Educ. Res. Pract., 17(1), 139–155.
- Gedera D. S. P., (2016), The application of activity theory in identifying contradictions in a university blended learning course, in Activity Theory in Education: Research and Practice, Boston, MA: Sense Publishers, pp. 53–70.
- Gedera D. S. P. and Williams J., (2016), Activity Theory in Education: Research and Practice, Boston, MA: Sense Publishers.
- Glaser B. and Strauss A., (1967), The discovery of grounded theory, London: Weidenfield & Nicolson.
- Gonzales A. C., Purington S., Robinson J. and Nieswandt M., (2019), Teacher interactions and effects on group triple problem solving space, Int. J. Sci. Educ., 41(13), 1744–1763.
- Greeno J. G., (2005), Learning in activity, in Sawyer R. K. (ed.), The Cambridge Handbook of the Learning Sciences, Cambridge: Cambridge University Press, pp. 61–78.
- Hammer D., (1994), Epistemological beliefs in introductory physics, Cognit. Instruct., 12(2), 151–183.
- Henry M. A., Shorter S., Charkoudian L., Heemstra J. M., Corwin L. A. and Gardner S., (2019), FAIL is not a four-letter word: A theoretical framework for exploring undergraduate students approaches to academic challenge and responses to failure in STEM learning environments, CBE—Life Sci. Educ., 18(11), 1–17.
- Hofstein A., (2004), The laboratory in chemistry education: Thirty years of experience with developments, implementation, and research, Chem. Educ. Res. Pract., 5(3), 247–264.
- Hofstein A. and Lunetta V. N., (2004), The laboratory in science education: Foundations for the twenty-first century, Sci. Educ., 88(1), 28–54.
- Holbrook J. and Rannikmae M., (2007), The nature of science education for enhancing scientific literacy, Int. J. Sci. Educ., 29(11), 1347–1362.
- Hsieh H.-F. and Shannon S. E., (2005), Three approaches to qualitative content analysis, Qual. Health Res., 15(9), 1277–1288.
- Isohätälä J., Näykki P., Järvelä S. and Baker M. J., (2018), Striking a balance: Socio-emotional processes during argumentation in collaborative learning interaction, Learn. Cult. Soc. Interact., 16, 1–19.
- Jefferson G., (2004), Glossary of transcript symbols with an introduction, in Lerner G. H. (ed.), Conversation analysis: Studies from the first generation, Amsterdam, The Netherlands and Philadelphia, PA: John Benjamins, pp. 13–31.
- Jobér A., (2017), Revising laboratory work: Sociological perspectives on the science classroom, Cult. Stud. Sci. Educ., 12(3), 615–635.
- Jonassen D. H. and Rohrer-Murphy L., (1999), Activity theory as a framework for designing constructivist learning environments, Educ. Tech. Res. Dev., 47(1), 61–79.
- Kang H., Windschitl M., Stroupe D. and Thompson J., (2016), Designing, launching, and implementing high quality learning opportunities for students that advance scientific thinking: Instructional tasks and opportunity to learn, J. Res. Sci. Teach., 53(9), 1316–1340.
- Kapur M., (2014), Productive failure in learning math, Cognit. Sci., 38(5), 1008–1022.
- Kapur M. and Bielaczyc K., (2012), Designing for productive failure, J. Learn. Sci., 21(1), 45–83.
- Kelly O. and Finlayson O., (2009), A hurdle too high? Students’ experience of a PBL laboratory module, Chem. Educ. Res. Pract., 10(1), 42–52.
- Kondo A. E. and Fair J. D., (2017), Insight into the chemistry skills gap: The duality between expected and desired skills, J. Chem. Educ., 94(3), 304–310.
- Krystyniak R. A. and Heikkinen H. W., (2007), Analysis of verbal interactions during an extended, open-inquiry general chemistry laboratory investigation, J. Res. Sci. Teach., 44(8), 1160–1186.
- Kyngäs H., Kääriäinen M. and Elo S., (2020), The trustworthiness of content analysis, in Kyngäs H., Mikkonen K. and Kääriäinen M. (ed.), The Application of Content Analysis in Nursing Science Research. Cham: Springer International Publishing, pp. 41–48.
- Langer-Osuna J. M., (2018), Productive disruptions: Rethinking the role of off-task interactions in collaborative mathematics learning, Educ. Sci., 8(2), 1–11.
- Lantolf J. P., (2000), Introducing sociocultural theory, in Sociocultural theory and second language learning, Oxford: Oxford University Press, pp. 1–26.
- Laverty J. T., Underwood S. M., Matz R. L., Posey L. A. and Carmel J. H., (2016), Characterizing college science assessments: The three-dimensional learning assessment protocol, PLoS One, 11(9), 1–21.
- Leontyev A. N., (1978) Activity and consciousness, in Hall M.J. (trans.), Philosophy in the USSR, problems of dialectical materialism, Moscow: Progress, pp. 180–202.
- Levrini O., Fantini P., Tasquier G., Percori B. and Levin M., (2015), Defining and operationalizing appropriation for science learning, J. Learn. Sci., 24(1), 93–136.
- Lichtman, M., (2013), Qualitative research in education: A user's guide, 3rd edn, Thousand Oaks, CA: SAGE Publications, Inc.
- Linenberger K. J. and Bretz S. L., (2012), Generating cognitive dissonance in student interviews through multiple representations, Chem. Educ. Res. Pract., 13(3), 172–178.
- Manz E., (2015), Resistance and the development of scientific practice: Designing the mangle into science instruction, Cognit. Instruct., 33(2), 89–124.
- McDonnell C., O’Connor C. and Seery M. K., (2007), Developing practical chemistry skills by means of student-driven problem based learning mini-projects, Chem. Educ. Res. Pract., 8(2), 130–139.
- Miles M. B., Huberman A. M. and Saldaña J., (2014), Qualitative data analysis: a methods sourcebook, 3rd edn, Los Angeles: SAGE Publications, Inc.
- Miller M. S., (2020), The impact of productive struggle support on student mindset in a high school technology and engineering class: A case study, Dissertation, University of Pittsburgh.
- Moon A., Stanford C., Cole R. and Towns M., (2017), Analysis of inquiry materials to explain complexity of chemical reasoning in physical chemistry students’ argumentation: Chemical reasoning in physical chemistry, J. Res. Sci. Teach., 54(10), 1322–1346.
- Morris P. J. T., (2015), The Matter Factory: A history of the chemistry laboratory. London: Reaktion Books Ltd.
- National Council of Teachers of Mathematics, (2014), Principles to actions: Ensuring mathematical success for all. Reston: National Council of Teachers of Mathematics, Inc.
- National Research Council, (2010), Exploring the Intersection of Science Education and 21st Century Skills: A Workshop Summary, Washington, DC: The National Academies Press.
- National Research Council, (2012), Education for life and work: Developing transferable knowledge and skills in the 21st century, Washington, DC: National Academies Press.
- National Research Council, (2015), Guide to implementing the next generation science standards, Washington DC: The National Academies Press.
- Owens D. C., Sadler T. D., Barlow A. T. and Smith-Walters C., (2020), Student motivation from and resistance to active learning rooted in essential science practices, Res. Sci. Educ., 50(1), 253–277.
- Pasquale M., (2015), Productive struggle in mathematics: Interactive technologies in STEM teaching and learning research brief, Educ. Dev. Cent., 1–5.
- Pathak S. A., Kim B., Jacobson M. J. and Zhang B., (2011), Learning the physics of electricity: A qualitative analysis of collaborative processes involved in productive failure, Int. J. Comp.-Support. Collab. Learn., 6(1), 57–73.
- Peterson R. J., (2021), We need to address ableism in science, Mol. Bio. Cell, 32(7), 507–510.
- Prema D. and Dhand R., (2019), Inculsion and accessibility in STEM education: Navigating the duty to accommodate and disability rights, Can. J. Dis. Stud., 8(3), 121–141.
- Roth J. A., (2019), Making the struggle productive: Conceptualizing the role and impact of the mathematics teacher in episodes of productive struggle, Dissertation, Keenesaw State University.
- Russ R. S. and Berland L. K., (2019), Invented science: A framework for discussing a persistent problem of practice, J. Learn. Sci., 28(3), 279–301.
- Sandi-Urena S. and Gatlin T. A., (2012), Experimental chemistry teaching: Understanding teaching assistants’ experience in the academic laboratory’, Educ. Quím., 23, 141–148.
- Sandi-Urena S., Cooper M. M., Gatlin T. A. and Bhattacharyya G., (2011), Students’ experience in a general chemistry cooperative problem based laboratory, Chem. Educ. Res. Pract., 12(4), 434–442.
- Santos-Díaz S., Hensiek S., Owings T. and Towns M. H., (2019), Survey of undergraduate students’ goals and achievement strategies for laboratory coursework, J. Chem. Educ., 96(5), 850–856.
- Schwartz D. L., Chase C. C., Oppezzo M. A. and Chin D. B., (2011), Practiving versus inveting with contrasting cases: The effects of telling first on learning and transfer, J. Educ. Psychol., 103(4), 759–776.
- Sengupta-Irving T. and Agarwal P., (2017), Conceptualizing perseverance in problem solving as collective enterprise, Math. Think. Learn., 19(2), 115–138.
- Smith K. C. and Alonso V., (2020), Measuring student engagement in the undergraduate general chemistry laboratory’, Chem. Educ. Res. Pract., 21(1), 399–411.
- Sohr E. R., Gupta A. and Elby A., (2018), Taking an escape hatch: Managing tension in group discourse, Sci. Educ., 102(5), 883–916.
- Song Y., (2018), Improving primary students’ collaborative problem solving competency in project-based science learning with productive failure instructional design in a seamless learning environment, Educ. Tech. Res. Dev., 66(4), 979–1008.
- Sund P., (2016), Science teachers’ mission impossible?: A qualitative study of obstacles in assessing students’ practical abilities’, Int. J. Sci. Educ., 38(14), 2220–2238.
- Trueman R. J., (2014), Productive failure in STEM education, J. Educ. Tech. Sys. 42(3), 199–214.
- UN Department of Economic and Social Affairs, (2015), The 17 sustainable development goals, available at: https://sdgs.un.org/goals (accessed: 11 August 2020).
- Ural E., (2016), The effect of guided-inquiry laboratory experiments on science education students’ chemistry laboratory attitudes, anxiety and achievement, J. Educ. Train. Stud., 4(4), 217–227.
- Van Aalsvoort J., (2004), Activity theory as a tool to address the problem of chemistry's lack of relevance in secondary school chemical education, Int. J. Sci. Educ., 26(13), 1635–1651.
- VanLehn K., Siler S., Murray C., Yamauchi T. and Baggett W. B., (2003), Why do only some events cause learning during human tutoring?, Cognit. Instruct., 21(3), 209–249.
- Velasco J. B., Knedeisen A., Xue D., Vickrey T. L., Abebe M. and Stains M., (2016), Characterizing instructional practices in the laboratory: The laboratory observation protocol for undergraduate STEM, J. Chem. Educ., 93(7), 1191–1203.
- Walker J. P. and Sampson V., (2013), Learning to argue and arguing to learn: Argument-driven inquiry as a way to help undergraduate chemistry students learn how to construct arguments and engage in argumentation during a laboratory course, J. Res. Sci. Teach., 50(5), 561–596.
- Walker J. P., Van Duzor A. G. and Lower M. A., (2019), Facilitating argumentation in the laboratory: The challenges of claim change and justification by theory, J. Chem. Educ., 96(3), 435–444.
- Wan T., Geraets A. A., Doty C. M., Saitta E. K. H. and Chini J. J., (2020), Characterizing science graduate teaching assistants’ instruction practices in reformed laboratories and tutorials, Int. J. STEM Educ., 7(1), 1–21.
- Warshauer H. K., (2015), Productive struggle in middle school mathematics classrooms, J. Math. Teach. Educ., 18(4), 375–400.
- Wertsch J. V., (1985), Vygotsky's genetic method, in Vygotsky and the Social Formation of Mind, Cambridge, MA: Harvard University Press, pp. 17–57.
- Winkelmes M., (2014), TILT higher ed: Transparency in learning and teaching. Available at: http://titlhighered.com (accessed: 9 February 2021).
- Xu H. and Talanquer V., (2013), Effect of the level of inquiry on student interactions in chemistry laboratories’, J. Chem. Educ., 90(1), 29–36.
- Yasin N. Y. B. M. and Yueying O., (2017), Evaluating the relevance of the chemistry curriculum to the workplace: Keeping tertiary education relevant, J. Chem. Educ., 94(10), 1443–1449.
- Yuriev E., Naidu S., Schembri L. S. and Short J. L., (2017), Scaffolding the development of problem-solving skills in chemistry: Guiding novice students out of dead ends and false starts, Chem. Educ. Res. Pract., 18(3), 486–504.
- Zollman A., (2012), Learning for STEM literacy: STEM literacy for learning. Sch. Sci. Math., 112(1), 12–19.
| This journal is © The Royal Society of Chemistry 2022 |