Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium-catalyzed/copper-mediated carbon–carbon cross-coupling reaction for synthesis of 6-unsubstituted 2-aryldihydropyrimidines†
Yoshio Nishimura *ab,
Takanori Kubo
*ab,
Takanori Kubo b,
Saho Takayamab,
Hanako Yoshidab and
Hidetsura Choc
b,
Saho Takayamab,
Hanako Yoshidab and
Hidetsura Choc
aSchool of Pharmaceutical Sciences, Ohu University, 31-1 Misumido, Tomita-machi, Koriyama, Fukushima 963-8611, Japan. E-mail: y-nishimura@pha.ohu-u.ac.jp
bFaculty of Pharmacy, Yasuda Women's University, 6-13-1, Yasuhigashi, Asaminami-ku, Hiroshima 731-0153, Japan
cGraduate School of Pharmaceutical Sciences, Tohoku University, 6-3 Aoba, Aramaki, Aoba-ku, Sendai 980-8578, Japan
First published on 3rd October 2022
Abstract
Dihydropyrimidines (DPs) show a wide range of biological activities for medicinal applications. Among the DP derivatives, 2-aryl-DPs have been reported to display remarkable pharmacological properties. In this work, we describe a method for the synthesis of hitherto unavailable 6-unsubstituted 2-aryl-DPs by Pd-catalyzed/Cu-mediated carbon–carbon cross-coupling reaction of 1-Boc 2-methylthio-DPs with organostannane reagents. The Boc group of the substrate significantly increases the substrate reactivity. Aryl tributylstannanes having various substituents such as MeO, Ph, CF3, CO2Me, and NO2 groups smoothly afforded the corresponding products in high yields. Various heteroaryl tributylstannanes having 2-, or 3-thienyl, 2-, or 3-pyridinyl groups were also applicable to the reaction. Regarding the substituents at the 4-position, the reactions of DPs bearing various aryl and alkyl substituents proceeded smoothly to give the desired products. The Boc group of the products was removed under a standard acidic condition to produce N-unsubstituted DP as a mixture of the tautomers in quantitative yields. The synthetic procedure was also applied to 4,4,6-trisubstituted 2-methylthio-DP to give novel 2,4,4,5,6-pentasubstituted DP. Therefore, the Pd-catalyzed/Cu-mediated reaction should help expand the DP-based molecular diversity, which would impact biological and pharmacological studies.
Introduction
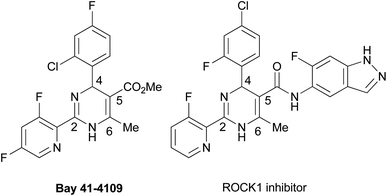
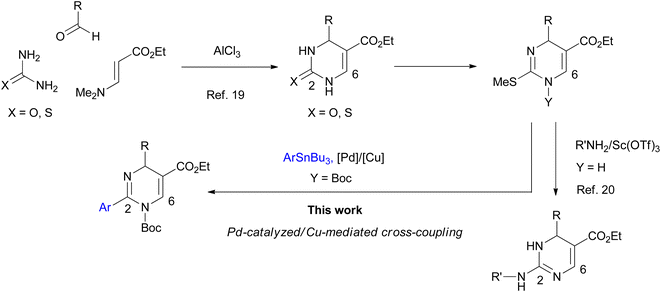
Dihydropyrimidines (DPs) show a wide range of biological activities for medicinal applications. They display calcium channel inhibitory,1 anticancer,2 antibacterial,3 antifungal,4 anti-HIV,5 antimalarial,6 anti-inflammatory,7 and antioxidation8 activities. Many reviews on synthetic methods developed for the heterocycles and their biological activities published thus far suggest their great potential as leading compounds for developing medicines.9 Among the DP derivatives, tautomeric 2-aryl-DPs have been reported to display remarkable pharmacological properties (Fig. 1). In 2003, Bay 41-4109 was shown to exhibit highly potent anti-hepatitis B virus (HBV) replication activity in vitro and in vivo.10 As a Bay 41-4109 analog with good water solubility, 6-morpholinylmethyl DP hydrochloride salt was reported as a HBV capsid assembly inhibitor.11 In 2008, another tautomeric 2-aryl-DP was also developed as a Rho-associated kinase isoform 1 (ROCK1) inhibitor, which may be a potential therapeutic agent for cardiovascular diseases.12 Recently 2-arylethenyl DP was reported as a potent heat shock protein 90 (Hsp90) C-terminal inhibitor, which may be a drug candidate for cancer therapeutics.13The biologically important tautomeric 2-aryl-DPs shown in Fig. 1 have four substituents at the 2-, 4-, 5-, and 6-positions. In general, these derivatives and related compounds were synthesized by three-component cyclocondensation reaction such as Biginelli reaction,9–12 or a transition-metal-catalyzed arylation reaction from 2-thioxo-DPs prepared in advance.13,14 Recently a one-pot synthetic method for tetrasubstituted 2-aryl-DPs from α-azidocinnamates by irradiation of LED light and base-catalyzed isomerization was also reported.15 Development of synthetic methods to access tautomeric 2-aryl-DPs with different substituent patterns expands their structural diversity, which impacts the DP-based drug discovery program. For example, a conventional cyclocondensation reaction of arylamidine with α,β-unsaturated aldehydes gives simple 2-aryl-DPs with fewer substituents.16 We previously reported the cyclization–elimination sequential reactions of 1,3-diazabuta-1,3-diene with electron-deficient olefins to give hitherto unavailable 4,6-unsubstituted 2-phenyl-DPs and related analogs.17 With our continuing interest in developing efficient methods of synthesizing DPs with fewer or more substituents,18 we have recently developed a general synthetic method for 6-unsubstituted DPs (Scheme 1). The 2-oxo- and 2-thioxo-DPs were synthesized by an AlCl3-mediated Biginelli-type three-component cyclocondensation reaction involving urea, aldehyde, and aminoacrylate.19 The 2-thioxo-DPs were stepwise converted into hitherto unavailable 2-amino-DPs via Sc(OTf)3-mediated nucleophilic substitution of 2-methylthio-DPs with amines.20 The proliferative effect of these 6-unsubstituted 2-oxo-, 2-thioxo-, and 2-amino-DPs on the human promyelocytic leukemia cell line HL-60 was also accessed, which led to the discovery of a highly active 2-benzylamino-DP with IC50 of <100 nM.20 In this study, we planned that 2-methylthio-DPs or 2-thioxo-DPs were used as precursors for the synthesis of hitherto unavailable 6-unsubstituted 2-aryl-DPs by a transition-metal-catalyzed 2-arylation reaction, Liebeskind–Srogl-type cross-coupling reaction.21 As a result, we realized the Pd-catalyzed/Cu-mediated 2-arylation reaction of 1-Boc 2-methylthio-DPs with arylstannane reagents.22 The Boc group significantly increases reactivity of DPs. This protocol enables the synthesis of 6-unsubstituted 2-aryl-DPs using various substituents at the 2- and 4-positions; to the best of our knowledge, the general formula of the 2-aryl-DPs has not been reported. Owing to our results, a series of 6-unsubstituted 2-oxo-, 2-thioxo-, and 2-amino-, and 2-aryl-DPs becomes available, which would impact DP-based biological and pharmacological studies.
Results and discussion
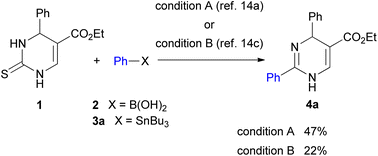
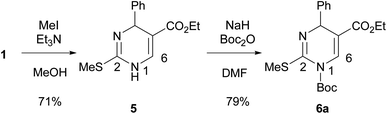
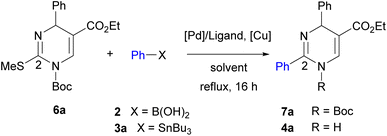
Initial studies of 2-thioxo-DP 1 were carried out under reaction condition A reported by Kappe14a [Pd(PPh3)4 (3.0 mol%), Cu(I)-thiophene-2-carboxylate (CuTC, 3.0 equiv.), PhB(OH)2 2 (1.5 equiv.) in THF at reflux for 18 h] and condition B reported by Suzenet14c [Pd(PPh3)4 (5.0 mol%), CuBr·Me2S (2.2 equiv.), PhSnBu3 3a (2.2 equiv.) in THF at reflux for 24 h]. These reactions gave 2-phenyl-DP 4a in moderate yields of 47% under condition A and 22% under condition B (Scheme 2).To increase the yield of the 2-arylation product, DP 1 was converted into 2-methylthio-DP 5 because the methylthio group is a typical substrate for the Liebeskind–Srogl reaction (Scheme 3).21 Our previous studies on the substitution reaction of DPs showed that a Boc group increased the electrophilicity of DPs.23 Therefore, 1-Boc 2-methylthio DP 6a was prepared by incorporating the Boc group into 5. The reaction occurred preferentially at the 1-position of 5 to give 6a in 79% yield. The position of the Boc group of 6a was determined; as for 1-Boc 2-phenyl DP 7a shown in Table 1, a significant heteronuclear multiple bond correlation (HMBC) was observed between the 6-proton and the carbonyl carbon of the Boc group at the 1-position. Therefore, the Boc groups of 7a and 6a were determined to be located at the 1-position. To determine a suitable substrate for the cross-coupling reaction, the reactivity of 6a was examined and compared with those of 1 and 5.
| Entry | DP/arylating reagenta | [Pd]/ligand/[Cu]a | Solvent/temp./time | Combined yield (%) (7a + 4a) | Recovery (%) of DP |
|---|---|---|---|---|---|
| a Reaction conditions: 6a (0.25 mmol), 3a (0.50 mmol), Pd catalyst (5.0 mol%), ligand (20 mol%), and Cu reagent (0.50 mmol) in solvent (3 mL) were reacted under Ar.b Pd2dba3 (1.0 mol%) and (2-furyl)3P (8.0 mol%) were used. | |||||
| 1 | 6a/3a | Pd(PPh3)4/none/CuTC | THF/reflux/16 h | 65 (55 + 10) | 8 |
| 2 | 6a/3a | Pd(PPh3)4/none/CuBr·Me2S | THF/reflux/16 h | 58 (16 + 42) | 35 |
| 3 | 6a/3a | PdCl2(PPh3)2/none/CuTC | THF/reflux/16 h | 74 (64 + 10) | 13 |
| 4 | 6a/3a | Pd(OAc)2/none/CuTC | THF/reflux/16 h | 54 (49 + 5) | 33 |
| 5 | 6a/3a | Pd2dba3/(2-furyl)3P/CuTC | THF/reflux/16 h | 80 (70 + 10) | 7 |
| 6 | 6a/2 | Pd2dba3/(2-furyl)3P/CuTC | THF/reflux/16 h | 65 (56 + 9) | 24 |
| 7 | 6a/3a | Pd2dba3/none/CuTC | THF/reflux/16 h | 55 (45 + 10) | 43 |
| 8 | 6a/3a | Pd2dba3/Ph3P/CuTC | THF/reflux/16 h | 63 (58 + 5) | 27 |
| 9 | 6a/3a | Pd2dba3/(2-thienyl)3P/CuTC | THF/reflux/16 h | 63 (56 + 7) | 31 |
| 10 | 6a/3a | Pd2dba3/(2-MeOC6H4)3P/CuTC | THF/reflux/16 h | 16 (16 + 0) | 76 |
| 11 | 6a/3a | Pd2dba3/(cyclo-C6H11)3P/CuTC | THF/reflux/16 h | 11 (11 + 0) | 86 |
| 12 | 6a/3a | Pd2dba3/dppm/CuTC | THF/reflux/16 h | 21 (21 + 0) | 68 |
| 13 | 6a/3a | Pd2dba3/dppe/CuTC | THF/reflux/16 h | 17 (17 + 0) | 70 |
| 14 | 6a/3a | Pd2dba3/dppp/CuTC | THF/reflux/16 h | 22 (22 + 0) | 65 |
| 15 | 6a/3a | Pd2dba3/dppb/CuTC | THF/reflux/16 h | 49 (41 + 8) | 48 |
| 16 | 6a/3a | Pd2dba3/dppf/CuTC | THF/reflux/16 h | 51 (44 + 7) | 44 |
| 17 | 6a/3a | Pd2dba3/rac-BINAP/CuTC | THF/reflux/16 h | 26 (26 + 0) | 58 |
| 18 | 6a/3a | None/none/CuTC | THF/reflux/16 h | 3 (3 + 0) | 95 |
| 19 | 6a/3a | Pd2dba3/(2-furyl)3P/none | THF/reflux/16 h | 0 | 96 |
| 20 | 6a/3a | Pd2dba3/(2-furyl)3P/CuTC | Dioxane/70 °C/16 h | 74 (66 + 8) | 22 |
| 21 | 6a/3a | Pd2dba3/(2-furyl)3P/CuTC | DMF/70 °C/16 h | 78 (62 + 16) | 18 |
| 22 | 6a/3a | Pd2dba3/(2-furyl)3P/CuTC | Toluene/70 °C/16 h | 66 (63 + 3) | 27 |
| 23 | 6a/3a | Pd2dba3/(2-furyl)3P/CuTC | 1,2-DCE/70 °C/16 h | 78 (72 + 6) | 20 |
| 24 | 6a/3a | Pd2dba3/(2-furyl)3P/CuTC | CH2Cl2/reflux/16 h | 81 (79 + 2) | 18 |
| 25 | 6a/3a | Pd2dba3/(2-furyl)3P/CuTC | CH2Cl2/reflux/30 h | 93 (91 + 2) | 2 |
| 26 | 1/3a | Pd2dba3/(2-furyl)3P/CuTC | CH2Cl2/reflux/30 h | 24 (only 4a) | 0 |
| 27 | 5/3a | Pd2dba3/(2-furyl)3P/CuTC | CH2Cl2/reflux/30 h | 55 (only 4a) | 15 |
| 28b | 6a/3a | Pd2dba3/(2-furyl)3P/CuTC | CH2Cl2/reflux/30 h | 82 (80 + 2) | 10 |
The optimized reaction conditions for 6a are summarized in Table 1. The effect of two Cu sources was examined under the same reaction condition, and results showed that CuTC worked better than CuBr·Me2S to give a combined yield of 65% for a desired 2-phenyl-DP 7a and 4a (entries 1 and 2). In all reactions using 3 in this study, the DPs 7a and 4a were purified by column chromatography using silica gel–K2CO3 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to prevent mixing with degradation product from 3.24 Among the Pd catalysts tested, tris(dibenzylideneacetone)dipalladium (Pd2dba3) with (2-furyl)3P used in the reaction gave a good combined yield of 80% for 7a and 4a (entries 1, 3–5). As an arylation reagent, PhSnBu3 3a showed a higher reactivity than PhB(OH)2 2 (entries 5 and 6). Subsequently, the effect of phosphine ligands was examined; only a few monodentate ligands, such as (2-furyl)3P, (2-thienyl)3P, and triphenylphosphine (Ph3P), increased the yields compared with the reaction without phosphine (entries 5, 7–9). The reactions using other monodentate ligands such as (2-MeOC6H4)3P and (cyclo-C6H11)3P resulted in low yields (entries 10 and 11). All bidentate ligands including 1,1-bis(diphenylphophino)methane (dppm), 1,2-bis(diphenylphophino)ethane (dppe), 1,3-bis(diphenylphophino)propane (dppp), 1,1-bis(diphenylphophino)butane (dppb), 1,1′-bis(diphenylphophino)ferrocene (dppf), and racemic BINAP (rac-BINAP) gave low yields (entries 12–17). As a result, the best ligand was determined to be (2-furyl)3P (entry 5). We confirmed that either reaction in the absence of Pd2dba3/(2-furyl)3P or CuTC hardly proceeded with the recovery of only 6a (entries 18 and 19); therefore, the addition of these reagents was essential for the reaction. To examine the effect of solvents, several polar and nonpolar solvents, such as dioxane (1,4-dioxane), DMF, toluene, 1,2-DCE (1,2-dichloroethane), and CH2Cl2, were used (entries 20–24). Although a small effect on the yields was observed, the reaction in CH2Cl2 showed a superior result and good mass balance to give a combined yield of 81% for 7a and 4a with 18% recovery of 6a (entry 24). When the reaction was conducted for a longer time of 30 h, the combined yield of 7a and 4a was increased to 93% (entry 25). When the optimized reaction condition was applied to the reactions using 1 or 5 as a substrate, the desired 4a was obtained in lower yields of 24% and 55%, respectively (entries 26 and 27). Therefore, the best substrate among 1, 5, and 6a for the reaction was determined to be 6a. The Boc group in 6a had a significant effect on the reactivity of 6a probably owing to its high electrophilicity being further increased by the group. When lower amount of Pd2dba3 (1 mol%) and (2-furyl)3P (8 mol%) were used, the combined yield slightly decreased to 82% (entry 28).
1) to prevent mixing with degradation product from 3.24 Among the Pd catalysts tested, tris(dibenzylideneacetone)dipalladium (Pd2dba3) with (2-furyl)3P used in the reaction gave a good combined yield of 80% for 7a and 4a (entries 1, 3–5). As an arylation reagent, PhSnBu3 3a showed a higher reactivity than PhB(OH)2 2 (entries 5 and 6). Subsequently, the effect of phosphine ligands was examined; only a few monodentate ligands, such as (2-furyl)3P, (2-thienyl)3P, and triphenylphosphine (Ph3P), increased the yields compared with the reaction without phosphine (entries 5, 7–9). The reactions using other monodentate ligands such as (2-MeOC6H4)3P and (cyclo-C6H11)3P resulted in low yields (entries 10 and 11). All bidentate ligands including 1,1-bis(diphenylphophino)methane (dppm), 1,2-bis(diphenylphophino)ethane (dppe), 1,3-bis(diphenylphophino)propane (dppp), 1,1-bis(diphenylphophino)butane (dppb), 1,1′-bis(diphenylphophino)ferrocene (dppf), and racemic BINAP (rac-BINAP) gave low yields (entries 12–17). As a result, the best ligand was determined to be (2-furyl)3P (entry 5). We confirmed that either reaction in the absence of Pd2dba3/(2-furyl)3P or CuTC hardly proceeded with the recovery of only 6a (entries 18 and 19); therefore, the addition of these reagents was essential for the reaction. To examine the effect of solvents, several polar and nonpolar solvents, such as dioxane (1,4-dioxane), DMF, toluene, 1,2-DCE (1,2-dichloroethane), and CH2Cl2, were used (entries 20–24). Although a small effect on the yields was observed, the reaction in CH2Cl2 showed a superior result and good mass balance to give a combined yield of 81% for 7a and 4a with 18% recovery of 6a (entry 24). When the reaction was conducted for a longer time of 30 h, the combined yield of 7a and 4a was increased to 93% (entry 25). When the optimized reaction condition was applied to the reactions using 1 or 5 as a substrate, the desired 4a was obtained in lower yields of 24% and 55%, respectively (entries 26 and 27). Therefore, the best substrate among 1, 5, and 6a for the reaction was determined to be 6a. The Boc group in 6a had a significant effect on the reactivity of 6a probably owing to its high electrophilicity being further increased by the group. When lower amount of Pd2dba3 (1 mol%) and (2-furyl)3P (8 mol%) were used, the combined yield slightly decreased to 82% (entry 28).
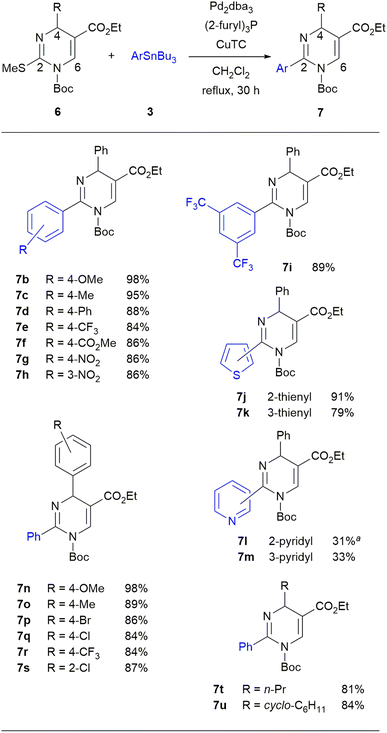
With the optimized condition in hand, we examined the scope of the Pd-catalyzed/Cu-mediated reaction using diverse aryl tributylstannanes 3 and DP derivatives 6 (Scheme 4). Regarding 3, we found no clear preference for either electron-donating or electron-withdrawing substituents of the phenyl group. When 6a (R = Ph) was reacted with p-methoxyphenyl- or p-tolyl tributylstannanes, the desired DPs 7b and 7c were produced in high yields of 98% and 95%, respectively. Aryl tributylstannanes having other substituents such as Ph, CF3, CO2Me, and NO2 groups at the para position smoothly afforded to give the products 7d–7g in 84–88% yields. The reactions using m-nitrophenyl or 3,5-bis(trifluoromethyl)phenyl tributylstannanes also proceeded smoothly to afford the products 7h and 7i in 86% and 89% yields, respectively. Various heteroaryl tributylstannanes having 2-thienyl, 3-thienyl, 2-pyridinyl, and 3-pyridinyl groups also reacted with 6a to give 7j–7m, albeit with low yields of 31–33% in the case of pyridine. We next examined the reaction scope for 6 using different substituents at the 4-position. We prepared seven 4-aryl-DPs 6a–6g having substituents such as H, OMe, Me, Br, Cl, and CF3 groups at the para position and Cl group at the ortho position. 4-n-Propyl-DP 6h and 4-cyclohexyl-DP 6i were also prepared. The synthetic procedure and the characteristic data of these DPs 6a–6i were shown in the experimental section. Regarding the aryl group of 6 at the 4-position, the reactions of DPs bearing substituents at the para position, proceeded smoothly to give the desired products 7n–7r in 84–98% yields. The reaction of the DP with the ortho-chlorophenyl group at the 4-position gave a DP 7s in 87% yield. Alkyl substituents such as n-propyl and cyclohexyl groups were also tolerated in the reaction to afford 7t and 7u in good yields.
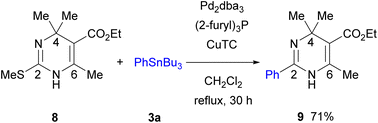
The Pd-catalyzed/Cu-mediated reaction was applied to 4,4,6-trisubstituted 2-methylthio-DP 8 (Scheme 5).18a An attempt to incorporate a Boc group to N-unsubstituted 8 using NaH/Boc2O failed owing to the steric congestion around the nitrogen atom. However, the reaction of 8 under the optimized conditions in Table 1 proceeded smoothly to give 2,4,4,5,6-pentasubstituted DP 9 in 71% yield. Such fully substituted 2-aryl-DP 9 has not been found in literature. Further optimization of the reaction condition for the synthesis of related pentasubstituted DPs is in progress.
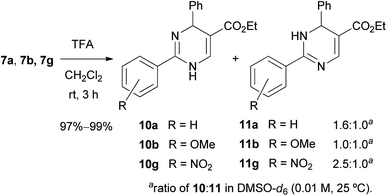
The Boc group of 7 was removed under a standard acidic condition (TFA in CH2Cl2) to produce N-unsubstituted 1,4-DP 10 and 1,6-DP 11 as a mixture of the tautomers (Scheme 6). To analyze the tautomeric behavior of 10 and 11, 1H NMR spectra of a mixture of 10a/11a, 10b/11b, and 10g/11g were measured in CD3OD and DMSO-d6, respectively (0.01 M, 25 °C). In CD3OD, only average spectra of 10/11 were observed because of the relatively fast tautomerization in the protic solvent. On the other hand, two individual tautomers of 10/11 were observed in the ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0–2.5
1.0–2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 in DMSO-d6. The ratio of 10/11 in DMSO-d6 was affected by substituents at the para position of the 2-phenyl group; the ratios were 1.0
1.0 in DMSO-d6. The ratio of 10/11 in DMSO-d6 was affected by substituents at the para position of the 2-phenyl group; the ratios were 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 for 10b/11b (R = OMe), 1.6
1.0 for 10b/11b (R = OMe), 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 for 10a/11a (R = H), and 2.5
1.0 for 10a/11a (R = H), and 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 for 10g/11g (R = NO2). These results indicate that the electron-donating property of the MeO group stabilized 1,6-DP 11b and increased the ratio of 11b owing to the resonance effect from the MeO group to the carbonyl group at the 5-position. In contrast, the electron-withdrawing property of the NO2 group weakens the effect and destabilizes 1,6-DP 11g. The thermodynamic preference of 1,4-DPs such as 10a and 10g over 11a and 11g was supported by our previous experimental and theoretical studies on 2-substituted DP tautomers.25
1.0 for 10g/11g (R = NO2). These results indicate that the electron-donating property of the MeO group stabilized 1,6-DP 11b and increased the ratio of 11b owing to the resonance effect from the MeO group to the carbonyl group at the 5-position. In contrast, the electron-withdrawing property of the NO2 group weakens the effect and destabilizes 1,6-DP 11g. The thermodynamic preference of 1,4-DPs such as 10a and 10g over 11a and 11g was supported by our previous experimental and theoretical studies on 2-substituted DP tautomers.25
In summary, we have developed a Pd-catalyzed/Cu-mediated cross-coupling reaction for the synthesis of 6-unsubstituted 2-aryl-DPs 7 from 1-Boc 2-methylthio-DP 6. The incorporation of the Boc group at the nitrogen atom of 6 significantly increased the reactivity of 6. The method is compatible with diverse DP substrates and aryl tributylstannane reagents. The method is also applicable to the reaction using 8 for the synthesis of highly pentasubstituted 2-aryl-DP 9. The Boc group of 7 was removed quantitatively to obtain a tautomeric mixture of 10/11. The synthetic procedure should help expand the DP-based molecular diversity, which would impact biological and pharmacological studies.
Experimental section
General information
Melting points were determined with an AS ONE melting point apparatus ATM-02 (AS ONE Corporation, Japan) or Yanaco melting point apparatus MP-J3 without correction. 1H NMR spectra were recorded on a Bruker AVANCE™ III 600 (600 MHz, Bruker Japan K.K., Japan) or JEOL JNM-ECZ500R (500 MHz, JEOL Ltd., Japan) with tetramethylsilane (δ 0 ppm) in CDCl3 or dimethylsulfoxide (δ 2.49 ppm) in DMSO-d6, or methanol (δ 3.30 ppm) in CD3OD as internal standards. 13C NMR spectra were recorded on a Bruker AVANCE™ III 600 (150 MHz) or JEOL JNM-ECZ500R (125 MHz) with chloroform (δ 77.0 ppm) in CDCl3 or dimethylsulfoxide (δ 39.7 ppm) in DMSO-d6 or methanol (δ 49.0 ppm) in CD3OD as internal standards. Multiplicities for 1H NMR were designated as s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, dt = doublet of triplets, dq = doublet of quartets, tt = triplet of triplets, ddd = doublet of doublets of doublets, m = multiplet, and br = broad. Infrared spectra (IR) were measured on a JASCO FT/IR-6100 or JASCO FT/IR-4100 Fourier transform infrared spectrophotometer (JASCO Corporation, Japan). Mass spectra were recorded on a JEOL JMS-700 mass analyzer (JEOL Ltd., Japan). High-resolution spectroscopy (HRMS) was performed using a JEOL JMS-700 mass analyzer.Synthesis of starting materials 6
Following the literature procedure,19 1-tert-butyl 5-ethyl 2-methylthio-4-phenyl-1,4-dihydropyrimidine-1,5-dicarboxylate (6a),19 1-tert-butyl 5-ethyl 4-(4-methoxyphenyl)-2-methylthio-1,4-dihydropyrimidine-1,5-dicarboxylate (6b), 1-tert-butyl 5-ethyl 4-(4-methylphenyl)-2-methylthio-1,4-dihydropyrimidine-1,5-dicarboxylate (6c), 1-tert-butyl 5-ethyl 4-(4-bromophenyl)-2-methylthio-1,4-dihydropyrimidine-1,5-dicarboxylate (6d), 1-tert-butyl 5-ethyl 4-(4-chlorophenyl)-2-methylthio-1,4-dihydropyrimidine-1,5-dicarboxylate (6e), 1-tert-butyl 5-ethyl 4-[4-(trifluoromethyl)phenyl]-2-methylthio-1,4-dihydropyrimidine-1,5-dicarboxylate (6f), 1-tert-butyl 5-ethyl 4-(2-chlorophenyl)-2-methylthio-1,4-dihydropyrimidine-1,5-dicarboxylate (6g), 1-tert-butyl 5-ethyl 2-methylthio-4-propyl-1,4-dihydropyrimidine-1,5(4H)-dicarboxylate (6h),19 1-tert-butyl 5-ethyl 4-cyclohexyl-2-methylthio-1,4-dihydropyrimidine-1,5-dicarboxylate (6i) were prepared.General procedure for synthesis of 2-aryl-DPs 7 and 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1;24 eluent: n-hexane–EtOAc, 11
1;24 eluent: n-hexane–EtOAc, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 6
1 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give 7a (93.0 mg, 0.229 mmol, 91%) as colorless crystals. Mp 139–141 °C (n-hexane–EtOAc). 1H NMR (CDCl3, 600 MHz): δ = 1.18 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.28 (t, J = 7.8 Hz, 1H), 7.32–7.44 (m, 7H), 7.47 (d, J = 7.8 Hz, 2H), 8.13 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.3, 58.7, 60.7, 84.6, 114.2, 127.0, 127.2, 127.5, 128.1, 128.7, 129.7, 133.6, 136.7, 141.0, 149.5, 151.3, 165.0. IR (KBr): 2981, 1726, 1709, 1673, 1353, 1267, 1243, 1154, 1070, 754, 703 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H27N2O4: 407.1971; found: 407.1975.
1) to give 7a (93.0 mg, 0.229 mmol, 91%) as colorless crystals. Mp 139–141 °C (n-hexane–EtOAc). 1H NMR (CDCl3, 600 MHz): δ = 1.18 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.28 (t, J = 7.8 Hz, 1H), 7.32–7.44 (m, 7H), 7.47 (d, J = 7.8 Hz, 2H), 8.13 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.3, 58.7, 60.7, 84.6, 114.2, 127.0, 127.2, 127.5, 128.1, 128.7, 129.7, 133.6, 136.7, 141.0, 149.5, 151.3, 165.0. IR (KBr): 2981, 1726, 1709, 1673, 1353, 1267, 1243, 1154, 1070, 754, 703 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H27N2O4: 407.1971; found: 407.1975.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 98%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.23 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 3.83 (s, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.92 (s, 1H), 6.89 (d, J = 9.0 Hz, 2H), 7.27 (t, J = 7.8 Hz, 1H), 7.33 (t, J = 7.8 Hz, 2H), 7.37 (d, J = 7.8 Hz, 2H), 7.43 (d, J = 9.0 Hz, 2H), 8.09 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 55.4, 58.5, 60.7, 84.3, 113.4, 114.6, 126.9, 127.4, 128.6, 128.8, 128.9, 133.7, 141.0, 149.6, 151.1, 161.0, 165.0. IR (neat): 2980, 1733, 1711, 1669, 1609, 1514, 1354, 1250, 1152, 1025 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H29N2O5: 437.2076; found: 437.2094.
1. Yield: 98%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.23 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 3.83 (s, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.92 (s, 1H), 6.89 (d, J = 9.0 Hz, 2H), 7.27 (t, J = 7.8 Hz, 1H), 7.33 (t, J = 7.8 Hz, 2H), 7.37 (d, J = 7.8 Hz, 2H), 7.43 (d, J = 9.0 Hz, 2H), 8.09 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 55.4, 58.5, 60.7, 84.3, 113.4, 114.6, 126.9, 127.4, 128.6, 128.8, 128.9, 133.7, 141.0, 149.6, 151.1, 161.0, 165.0. IR (neat): 2980, 1733, 1711, 1669, 1609, 1514, 1354, 1250, 1152, 1025 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H29N2O5: 437.2076; found: 437.2094.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 6
1 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 95%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.20 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 2.38 (s, 3H), 4.19 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.93 (s, 1H), 7.17 (d, J = 8.4 Hz, 2H), 7.27 (t, J = 7.2 Hz, 1H), 7.33 (t, J = 7.2 Hz, 2H), 7.35–7.40 (m, 4H), 8.10 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 21.3, 27.4, 58.6, 60.6, 84.4, 114.3, 126.9, 127.2, 127.4, 128.6, 128.7, 133.6, 133.7, 139.8, 141.0, 149.5, 151.4, 165.0. IR (neat): 2980, 1734, 1712, 1670, 1615, 1354, 1315, 1246, 1152, 1028 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H29N2O4: 421.2127; found: 421.2135.
1. Yield: 95%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.20 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 2.38 (s, 3H), 4.19 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.93 (s, 1H), 7.17 (d, J = 8.4 Hz, 2H), 7.27 (t, J = 7.2 Hz, 1H), 7.33 (t, J = 7.2 Hz, 2H), 7.35–7.40 (m, 4H), 8.10 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 21.3, 27.4, 58.6, 60.6, 84.4, 114.3, 126.9, 127.2, 127.4, 128.6, 128.7, 133.6, 133.7, 139.8, 141.0, 149.5, 151.4, 165.0. IR (neat): 2980, 1734, 1712, 1670, 1615, 1354, 1315, 1246, 1152, 1028 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H29N2O4: 421.2127; found: 421.2135.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 88%; pale yellow amorphous. 1H NMR (CDCl3, 600 MHz): δ = 1.21 (s, 9H), 1.29 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.25 (dq, J = 10.8, 7.2 Hz, 1H), 5.95 (s, 1H), 7.29 (t, J = 7.2 Hz, 1H), 7.35 (t, J = 7.2 Hz, 2H), 7.37 (t, J = 7.2 Hz, 1H), 7.40 (d, J = 7.2 Hz, 2H), 7.45 (t, J = 7.2 Hz, 2H), 7.54 (d, J = 8.4 Hz, 2H), 7.60 (d, J = 7.2 Hz, 2H), 7.61 (d, J = 8.4 Hz, 2H), 8.14 (d, 1H, J = 1.2 Hz). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 58.7, 60.7, 84.6, 114.3, 126.7, 127.0, 127.1, 127.5, 127.70, 127.73, 128.6, 128.8, 133.6, 135.5, 140.3, 141.0, 142.6, 149.4, 151.0, 165.0. IR (KBr): 2980, 1734, 1711, 1669, 1370, 1355, 1246, 1152, 754 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C30H31N2O4: 483.2284; found: 483.2292.
1. Yield: 88%; pale yellow amorphous. 1H NMR (CDCl3, 600 MHz): δ = 1.21 (s, 9H), 1.29 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.25 (dq, J = 10.8, 7.2 Hz, 1H), 5.95 (s, 1H), 7.29 (t, J = 7.2 Hz, 1H), 7.35 (t, J = 7.2 Hz, 2H), 7.37 (t, J = 7.2 Hz, 1H), 7.40 (d, J = 7.2 Hz, 2H), 7.45 (t, J = 7.2 Hz, 2H), 7.54 (d, J = 8.4 Hz, 2H), 7.60 (d, J = 7.2 Hz, 2H), 7.61 (d, J = 8.4 Hz, 2H), 8.14 (d, 1H, J = 1.2 Hz). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 58.7, 60.7, 84.6, 114.3, 126.7, 127.0, 127.1, 127.5, 127.70, 127.73, 128.6, 128.8, 133.6, 135.5, 140.3, 141.0, 142.6, 149.4, 151.0, 165.0. IR (KBr): 2980, 1734, 1711, 1669, 1370, 1355, 1246, 1152, 754 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C30H31N2O4: 483.2284; found: 483.2292.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 6
1 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 79%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.21 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.93 (s, 1H), 7.30 (tt, J = 6.6, 1.8 Hz, 1H), 7.33–7.39 (m, 4H), 7.58 (d, J = 8.4 Hz, 2H), 7.64 (d, J = 8.4 Hz, 2H), 8.12 (d, J = 0.6 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 58.9, 60.8, 85.1, 114.2, 123.8 (q, J = 271.5 Hz), 125.1 (q, J = 3.5 Hz), 127.0, 127.6, 127.8, 128.8, 131.6 (q, J = 33.0 Hz), 133.3, 140.2, 140.6, 149.0, 149.9, 164.8. IR (neat): 2981, 1739, 1713, 1673, 1326, 1247, 1154, 1068, 1025, 851 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H26F3N2O4: 475.1845; found: 475.1855.
1. Yield: 79%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.21 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.93 (s, 1H), 7.30 (tt, J = 6.6, 1.8 Hz, 1H), 7.33–7.39 (m, 4H), 7.58 (d, J = 8.4 Hz, 2H), 7.64 (d, J = 8.4 Hz, 2H), 8.12 (d, J = 0.6 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 58.9, 60.8, 85.1, 114.2, 123.8 (q, J = 271.5 Hz), 125.1 (q, J = 3.5 Hz), 127.0, 127.6, 127.8, 128.8, 131.6 (q, J = 33.0 Hz), 133.3, 140.2, 140.6, 149.0, 149.9, 164.8. IR (neat): 2981, 1739, 1713, 1673, 1326, 1247, 1154, 1068, 1025, 851 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H26F3N2O4: 475.1845; found: 475.1855.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 86%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.19 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 3.94 (s, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.30 (tt, J = 7.2, 1.8 Hz, 1H), 7.35 (t, J = 7.2 Hz, 2H), 7.38 (dd, J = 7.2, 1.8 Hz, 2H), 7.54 (d, J = 8.4 Hz, 2H), 8.05 (d, J = 8.4 Hz, 2H), 8.12 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 52.2, 58.9, 60.8, 85.0, 114.2, 127.0, 127.3, 127.7, 128.7, 129.4, 131.0, 133.3, 140.7, 141.0, 149.1, 150.3, 164.8, 166.4. IR (neat): 2980, 1723, 1671, 1355, 1280, 1247, 1152 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C26H29N2O6: 465.2026; found: 465.2025.
1. Yield: 86%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.19 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 3.94 (s, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.30 (tt, J = 7.2, 1.8 Hz, 1H), 7.35 (t, J = 7.2 Hz, 2H), 7.38 (dd, J = 7.2, 1.8 Hz, 2H), 7.54 (d, J = 8.4 Hz, 2H), 8.05 (d, J = 8.4 Hz, 2H), 8.12 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 52.2, 58.9, 60.8, 85.0, 114.2, 127.0, 127.3, 127.7, 128.7, 129.4, 131.0, 133.3, 140.7, 141.0, 149.1, 150.3, 164.8, 166.4. IR (neat): 2980, 1723, 1671, 1355, 1280, 1247, 1152 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C26H29N2O6: 465.2026; found: 465.2025.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 86%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.26 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.29–7.39 (m, 5H), 7.63 (d, J = 8.4 Hz, 2H), 8.10 (s, 1H), 8.24 (d, J = 8.4 Hz, 2H). 13C NMR (CDCl3, 150 MHz): δ = 14.1, 27.5, 59.1, 60.9, 85.4, 114.3, 123.3, 127.0, 127.9, 128.2, 128.8, 133.0, 140.3, 142.7, 148.3, 148.8, 149.1, 164.6. IR (neat): 2980, 1739, 1712, 1672, 1600, 1524, 1348, 1246, 1152 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H26N3O6: 452.1822; found: 452.1831.
1. Yield: 86%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.26 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.29–7.39 (m, 5H), 7.63 (d, J = 8.4 Hz, 2H), 8.10 (s, 1H), 8.24 (d, J = 8.4 Hz, 2H). 13C NMR (CDCl3, 150 MHz): δ = 14.1, 27.5, 59.1, 60.9, 85.4, 114.3, 123.3, 127.0, 127.9, 128.2, 128.8, 133.0, 140.3, 142.7, 148.3, 148.8, 149.1, 164.6. IR (neat): 2980, 1739, 1712, 1672, 1600, 1524, 1348, 1246, 1152 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H26N3O6: 452.1822; found: 452.1831.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 86%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.26 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.25 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (d, J = 1.2 Hz, 1H), 7.29–7.40 (m, 5H), 7.57 (t, J = 7.8 Hz, 1H), 7.83 (ddd, J = 7.8, 1.8, 1.2 Hz, 1H), 8.12 (d, J = 1.2 Hz, 1H), 8.28 (ddd, J = 7.8, 2.4, 1.2 Hz, 1H), 8.31 (dd, J = 2.4, 1.8 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.1, 27.5, 59.0, 60.9, 85.4, 114.5, 122.3, 124.3, 127.0, 127.9, 128.8, 129.2, 133.15, 133.22, 138.3, 140.3, 147.8, 148.8, 148.9, 164.6. IR (neat): 2979, 1738, 1712, 1674, 1616, 1533, 1348, 1318, 1245, 1152, 1024, 752 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H26N3O6: 452.1822; found: 452.1825.
1. Yield: 86%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.26 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.25 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (d, J = 1.2 Hz, 1H), 7.29–7.40 (m, 5H), 7.57 (t, J = 7.8 Hz, 1H), 7.83 (ddd, J = 7.8, 1.8, 1.2 Hz, 1H), 8.12 (d, J = 1.2 Hz, 1H), 8.28 (ddd, J = 7.8, 2.4, 1.2 Hz, 1H), 8.31 (dd, J = 2.4, 1.8 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.1, 27.5, 59.0, 60.9, 85.4, 114.5, 122.3, 124.3, 127.0, 127.9, 128.8, 129.2, 133.15, 133.22, 138.3, 140.3, 147.8, 148.8, 148.9, 164.6. IR (neat): 2979, 1738, 1712, 1674, 1616, 1533, 1348, 1318, 1245, 1152, 1024, 752 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H26N3O6: 452.1822; found: 452.1825.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 89%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.24 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.19 (dq, J = 10.8, 7.2 Hz, 1H), 4.25 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.29–7.40 (m, 5H), 7.90 (s, 2H), 7.93 (s, 1H), 8.12 (d, J = 0.6 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.1, 27.4, 59.2, 60.9, 85.6, 114.6, 122.98 (q, J = 271.5 Hz), 123.04 (q, J = 2.7 Hz), 127.1, 127.5, 128.0, 128.9, 131.7 (q, J = 33.0 Hz), 133.0, 138.8, 140.2, 148.4, 148.7, 164.5. IR (neat): 2982, 1743, 1714, 1675, 1341, 1280, 1244, 1150 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C26H25F6N2O4: 543.1719; found: 543.1704.
1. Yield: 89%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.24 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.19 (dq, J = 10.8, 7.2 Hz, 1H), 4.25 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.29–7.40 (m, 5H), 7.90 (s, 2H), 7.93 (s, 1H), 8.12 (d, J = 0.6 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.1, 27.4, 59.2, 60.9, 85.6, 114.6, 122.98 (q, J = 271.5 Hz), 123.04 (q, J = 2.7 Hz), 127.1, 127.5, 128.0, 128.9, 131.7 (q, J = 33.0 Hz), 133.0, 138.8, 140.2, 148.4, 148.7, 164.5. IR (neat): 2982, 1743, 1714, 1675, 1341, 1280, 1244, 1150 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C26H25F6N2O4: 543.1719; found: 543.1704.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 6
1 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 91%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.28 (t, J = 7.2 Hz, 3H), 1.32 (s, 9H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.03 (dd, J = 4.8, 3.6 Hz, 1H), 7.23 (dd, J = 3.6, 1.2 Hz, 1H), 7.26 (t, J = 7.2 Hz, 1H), 7.32 (t, J = 7.2 Hz, 2H), 7.35 (d, J = 7.2 Hz, 2H), 7.38 (dd, J = 4.8, 1.2 Hz, 1H), 8.01 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.5, 58.6, 60.7, 84.6, 115.6, 126.7, 126.8, 127.5, 127.8, 128.0, 128.6, 133.7, 138.4, 140.4, 146.6, 149.4, 164.8. IR (neat): 2978, 1735, 1711, 1664, 1340, 1245, 1151 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C22H25N2O4S: 413.1535; found: 413.1534.
1. Yield: 91%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.28 (t, J = 7.2 Hz, 3H), 1.32 (s, 9H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.03 (dd, J = 4.8, 3.6 Hz, 1H), 7.23 (dd, J = 3.6, 1.2 Hz, 1H), 7.26 (t, J = 7.2 Hz, 1H), 7.32 (t, J = 7.2 Hz, 2H), 7.35 (d, J = 7.2 Hz, 2H), 7.38 (dd, J = 4.8, 1.2 Hz, 1H), 8.01 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.5, 58.6, 60.7, 84.6, 115.6, 126.7, 126.8, 127.5, 127.8, 128.0, 128.6, 133.7, 138.4, 140.4, 146.6, 149.4, 164.8. IR (neat): 2978, 1735, 1711, 1664, 1340, 1245, 1151 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C22H25N2O4S: 413.1535; found: 413.1534.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 4
1 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 79%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.276 (t, J = 7.2 Hz, 3H), 1.281 (s, 9H), 4.19 (dq, J = 10.8, 7.2 Hz, 1H), 4.23 (dq, J = 10.8, 7.2 Hz, 1H), 5.90 (s, 1H), 7.16 (dd, J = 4.8, 1.2 Hz, 1H), 7.26–7.30 (m, 2H), 7.33 (t, J = 7.8 Hz, 2H), 7.37 (d, J = 7.8 Hz, 2H), 7.54 (dd, J = 3.0, 1.2 Hz, 1H), 8.07 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 58.5, 60.7, 84.5, 114.4, 125.2, 125.8, 126.8, 126.9, 127.5, 128.6, 133.5, 137.5, 140.8, 147.0, 149.4, 164.9. IR (neat): 2980, 1733, 1711, 1669, 1371, 1342, 1245, 1151, 1025 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C22H25N2O4S: 413.1535; found: 413.1527.
1. Yield: 79%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.276 (t, J = 7.2 Hz, 3H), 1.281 (s, 9H), 4.19 (dq, J = 10.8, 7.2 Hz, 1H), 4.23 (dq, J = 10.8, 7.2 Hz, 1H), 5.90 (s, 1H), 7.16 (dd, J = 4.8, 1.2 Hz, 1H), 7.26–7.30 (m, 2H), 7.33 (t, J = 7.8 Hz, 2H), 7.37 (d, J = 7.8 Hz, 2H), 7.54 (dd, J = 3.0, 1.2 Hz, 1H), 8.07 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 58.5, 60.7, 84.5, 114.4, 125.2, 125.8, 126.8, 126.9, 127.5, 128.6, 133.5, 137.5, 140.8, 147.0, 149.4, 164.9. IR (neat): 2980, 1733, 1711, 1669, 1371, 1342, 1245, 1151, 1025 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C22H25N2O4S: 413.1535; found: 413.1527.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 20
1 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 31%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.22 (s, 9H), 1.26 (t, J = 7.2 Hz, 3H), 4.17 (dq, J = 10.8, 7.2 Hz, 1H), 4.23 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.29 (t, J = 7.2 Hz, 1H), 7.33–7.38 (m, 3H), 7.41 (d, J = 7.2 Hz, 2H), 7.68 (d, J = 7.8 Hz, 1H), 7.75 (ddd, J = 7.8, 7.8, 1.8 Hz, 1H), 8.17 (d, J = 1.2 Hz, 1H), 8.55–8.57 (m, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 58.9, 60.6, 84.2, 112.5, 123.1, 124.3, 127.1, 127.6, 128.7, 133.6, 136.8, 141.0, 148.0, 149.4, 150.4, 154.2, 165.0. IR (neat): 2980, 2932, 1741, 1711, 1671, 1362, 1321, 1244, 1155, 1075, 1025, 750 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C23H26N3O4: 408.1923; found: 408.1927.
1. Yield: 31%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.22 (s, 9H), 1.26 (t, J = 7.2 Hz, 3H), 4.17 (dq, J = 10.8, 7.2 Hz, 1H), 4.23 (dq, J = 10.8, 7.2 Hz, 1H), 5.94 (s, 1H), 7.29 (t, J = 7.2 Hz, 1H), 7.33–7.38 (m, 3H), 7.41 (d, J = 7.2 Hz, 2H), 7.68 (d, J = 7.8 Hz, 1H), 7.75 (ddd, J = 7.8, 7.8, 1.8 Hz, 1H), 8.17 (d, J = 1.2 Hz, 1H), 8.55–8.57 (m, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.4, 58.9, 60.6, 84.2, 112.5, 123.1, 124.3, 127.1, 127.6, 128.7, 133.6, 136.8, 141.0, 148.0, 149.4, 150.4, 154.2, 165.0. IR (neat): 2980, 2932, 1741, 1711, 1671, 1362, 1321, 1244, 1155, 1075, 1025, 750 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C23H26N3O4: 408.1923; found: 408.1927.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Yield: 33%; colorless crystals, mp 107–108 °C (n-hexane–EtOAc). 1H NMR (CDCl3, 600 MHz): δ = 1.24 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.95 (s, 1H), 7.28–7.39 (m, 6H), 7.80 (dt, J = 7.8, 1.8 Hz, 1H), 8.13 (d, J = 1.2 Hz, 1H), 8.65 (dd, J = 4.8, 1.8 Hz, 1H), 8.69 (d, J = 1.8 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.5, 58.9, 60.8, 85.2, 114.3, 123.0, 127.0, 127.8, 128.8, 132.7, 133.2, 134.8, 140.6, 148.1, 148.7, 149.0, 150.4, 164.7. IR (KBr): 2980, 1726, 1711, 1673, 1356, 1312, 1244, 1154, 1071 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C23H26N3O4: 408.1923; found: 408.1918.
2. Yield: 33%; colorless crystals, mp 107–108 °C (n-hexane–EtOAc). 1H NMR (CDCl3, 600 MHz): δ = 1.24 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.95 (s, 1H), 7.28–7.39 (m, 6H), 7.80 (dt, J = 7.8, 1.8 Hz, 1H), 8.13 (d, J = 1.2 Hz, 1H), 8.65 (dd, J = 4.8, 1.8 Hz, 1H), 8.69 (d, J = 1.8 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.5, 58.9, 60.8, 85.2, 114.3, 123.0, 127.0, 127.8, 128.8, 132.7, 133.2, 134.8, 140.6, 148.1, 148.7, 149.0, 150.4, 164.7. IR (KBr): 2980, 1726, 1711, 1673, 1356, 1312, 1244, 1154, 1071 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C23H26N3O4: 408.1923; found: 408.1918.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 92%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.18 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 3.80 (s, 3H), 4.19 (dq, J = 10.8, 7.2 Hz, 1H), 4.23 (dq, J = 10.8, 7.2 Hz, 1H), 5.86 (s, 1H), 6.87 (d, J = 9.0 Hz, 2H), 7.30 (d, J = 9.0 Hz, 2H), 7.36 (t, J = 7.2 Hz, 2H), 7.41 (tt, J = 7.2, 1.8 Hz, 1H), 7.45 (dd, J = 7.2, 1.8 Hz, 2H), 8.12 (d, J = 0.6 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.3, 55.2, 58.1, 60.6, 84.5, 114.0, 114.3, 127.2, 128.05, 128.10, 129.6, 133.28, 133.33, 136.7, 149.5, 150.9, 159.0, 165.0. IR (neat): 2980, 1734, 1712, 1670, 1610, 1511, 1354, 1317, 1247, 1153, 1035 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H29N2O5: 437.2076; found: 437.2081.
1. Yield: 92%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.18 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 3.80 (s, 3H), 4.19 (dq, J = 10.8, 7.2 Hz, 1H), 4.23 (dq, J = 10.8, 7.2 Hz, 1H), 5.86 (s, 1H), 6.87 (d, J = 9.0 Hz, 2H), 7.30 (d, J = 9.0 Hz, 2H), 7.36 (t, J = 7.2 Hz, 2H), 7.41 (tt, J = 7.2, 1.8 Hz, 1H), 7.45 (dd, J = 7.2, 1.8 Hz, 2H), 8.12 (d, J = 0.6 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.3, 55.2, 58.1, 60.6, 84.5, 114.0, 114.3, 127.2, 128.05, 128.10, 129.6, 133.28, 133.33, 136.7, 149.5, 150.9, 159.0, 165.0. IR (neat): 2980, 1734, 1712, 1670, 1610, 1511, 1354, 1317, 1247, 1153, 1035 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H29N2O5: 437.2076; found: 437.2081.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 89%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.17 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 2.34 (s, 3H), 4.19 (dq, J = 10.8, 7.2 Hz, 1H), 4.23 (dq, J = 10.8, 7.2 Hz, 1H), 5.89 (s, 1H), 7.15 (d, J = 8.4 Hz, 2H), 7.27 (d, J = 8.4 Hz, 2H), 7.36 (t, J = 7.2 Hz, 2H), 7.41 (tt, J = 7.2, 1.8 Hz, 1H), 7.46 (dd, J = 7.2, 1.8 Hz, 2H), 8.12 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 21.1, 27.3, 58.5, 60.6, 84.4, 114.3, 126.9, 127.2, 128.1, 129.3, 129.6, 133.4, 136.7, 137.2, 138.1, 149.5, 151.1, 165.0. IR (KBr): 2979, 1734, 1712, 1669, 1354, 1245, 1151 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H29N2O4: 421.2127; found: 421.2131.
1. Yield: 89%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.17 (s, 9H), 1.28 (t, J = 7.2 Hz, 3H), 2.34 (s, 3H), 4.19 (dq, J = 10.8, 7.2 Hz, 1H), 4.23 (dq, J = 10.8, 7.2 Hz, 1H), 5.89 (s, 1H), 7.15 (d, J = 8.4 Hz, 2H), 7.27 (d, J = 8.4 Hz, 2H), 7.36 (t, J = 7.2 Hz, 2H), 7.41 (tt, J = 7.2, 1.8 Hz, 1H), 7.46 (dd, J = 7.2, 1.8 Hz, 2H), 8.12 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 21.1, 27.3, 58.5, 60.6, 84.4, 114.3, 126.9, 127.2, 128.1, 129.3, 129.6, 133.4, 136.7, 137.2, 138.1, 149.5, 151.1, 165.0. IR (KBr): 2979, 1734, 1712, 1669, 1354, 1245, 1151 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H29N2O4: 421.2127; found: 421.2131.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 86%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.17 (s, 9H), 1.29 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.88 (s, 1H), 7.26 (d, J = 8.4 Hz, 2H), 7.38 (t, J = 7.2 Hz, 2H), 7.41–7.46 (m, 3H), 7.47 (d, J = 8.4 Hz, 2H), 8.14 (d, J = 0.6 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.3, 58.1, 60.8, 84.8, 113.5, 121.5, 127.2, 128.1, 128.7, 129.8, 131.7, 133.8, 136.5, 140.1, 149.3, 151.5, 164.8. IR (neat): 2980, 1737, 1711, 1671, 1371, 1353, 1245, 1152, 1011 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H2679BrN2O4: 485.1076; found: 485.1068.
1. Yield: 86%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.17 (s, 9H), 1.29 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.88 (s, 1H), 7.26 (d, J = 8.4 Hz, 2H), 7.38 (t, J = 7.2 Hz, 2H), 7.41–7.46 (m, 3H), 7.47 (d, J = 8.4 Hz, 2H), 8.14 (d, J = 0.6 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.3, 58.1, 60.8, 84.8, 113.5, 121.5, 127.2, 128.1, 128.7, 129.8, 131.7, 133.8, 136.5, 140.1, 149.3, 151.5, 164.8. IR (neat): 2980, 1737, 1711, 1671, 1371, 1353, 1245, 1152, 1011 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H2679BrN2O4: 485.1076; found: 485.1068.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 84%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.17 (s, 9H), 1.29 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.89 (d, J = 1.2 Hz, 1H), 7.32 (s, 4H), 7.38 (t, J = 7.2 Hz, 2H), 7.41–7.46 (m, 3H), 8.14 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.3, 58.0, 60.8, 84.8, 113.6, 127.2, 128.1, 128.3, 128.8, 129.8, 133.3, 133.8, 136.5, 139.5, 149.3, 151.5, 164.8. IR (neat): 2980, 1737, 1711, 1671, 1371, 1353, 1246, 1153, 1015 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H2635ClN2O4: 441.1581; found: 441.1575.
1. Yield: 84%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.17 (s, 9H), 1.29 (t, J = 7.2 Hz, 3H), 4.20 (dq, J = 10.8, 7.2 Hz, 1H), 4.24 (dq, J = 10.8, 7.2 Hz, 1H), 5.89 (d, J = 1.2 Hz, 1H), 7.32 (s, 4H), 7.38 (t, J = 7.2 Hz, 2H), 7.41–7.46 (m, 3H), 8.14 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.3, 58.0, 60.8, 84.8, 113.6, 127.2, 128.1, 128.3, 128.8, 129.8, 133.3, 133.8, 136.5, 139.5, 149.3, 151.5, 164.8. IR (neat): 2980, 1737, 1711, 1671, 1371, 1353, 1246, 1153, 1015 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H2635ClN2O4: 441.1581; found: 441.1575.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 84%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.18 (s, 9H), 1.29 (t, J = 7.2 Hz, 3H), 4.21 (dq, J = 10.8, 7.2 Hz, 1H), 4.25 (dq, J = 10.8, 7.2 Hz, 1H), 5.98 (s, 1H), 7.39 (t, J = 7.8 Hz, 2H), 7.44 (tt, J = 7.8, 1.2 Hz, 1H), 7.46 (dd, J = 7.8, 1.2 Hz, 2H), 7.51 (d, J = 7.8 Hz, 2H), 7.61 (d, J = 7.8 Hz, 2H), 8.16 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.3, 58.3, 60.9, 84.9, 113.3, 124.1 (J = 271.5 Hz), 125.6 (J = 3.8 Hz), 127.2, 127.3, 128.2, 129.8 (q, J = 31.5 Hz), 129.9, 134.0, 136.4, 144.9, 149.3, 151.8, 164.8. IR (neat): 2982, 1738, 1711, 1672, 1618, 1354, 1326, 1245, 1152, 1125, 1067 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H26F3N2O4: 475.1845; found: 475.1850.
1. Yield: 84%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.18 (s, 9H), 1.29 (t, J = 7.2 Hz, 3H), 4.21 (dq, J = 10.8, 7.2 Hz, 1H), 4.25 (dq, J = 10.8, 7.2 Hz, 1H), 5.98 (s, 1H), 7.39 (t, J = 7.8 Hz, 2H), 7.44 (tt, J = 7.8, 1.2 Hz, 1H), 7.46 (dd, J = 7.8, 1.2 Hz, 2H), 7.51 (d, J = 7.8 Hz, 2H), 7.61 (d, J = 7.8 Hz, 2H), 8.16 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 27.3, 58.3, 60.9, 84.9, 113.3, 124.1 (J = 271.5 Hz), 125.6 (J = 3.8 Hz), 127.2, 127.3, 128.2, 129.8 (q, J = 31.5 Hz), 129.9, 134.0, 136.4, 144.9, 149.3, 151.8, 164.8. IR (neat): 2982, 1738, 1711, 1672, 1618, 1354, 1326, 1245, 1152, 1125, 1067 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C25H26F3N2O4: 475.1845; found: 475.1850.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 87%; colorless crystals, mp 135–136 °C (n-hexane–EtOAc). 1H NMR (CDCl3, 600 MHz): δ = 1.17 (s, 9H), 1.22 (t, J = 7.2 Hz, 3H), 4.14 (dq, J = 10.8, 7.2 Hz, 1H), 4.17 (dq, J = 10.8, 7.2 Hz, 1H), 6.27 (s, 1H), 7.20–7.25 (m, 3H), 7.33 (t, J = 7.2 Hz, 2H), 7.35–7.40 (m, 3H), 7.42–7.46 (m, 1H), 8.29 (s, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.1, 27.3, 56.5, 60.7, 84.6, 112.3, 127.1, 127.2, 128.0, 128.7, 128.9, 129.5, 130.1, 134.1, 134.9, 136.9, 138.4, 149.5, 150.6, 164.7. IR (KBr): 2978, 1728, 1711, 1665, 1350, 1262, 1249, 1156 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H2635ClN2O4: 441.1581; found: 441.1588.
1. Yield: 87%; colorless crystals, mp 135–136 °C (n-hexane–EtOAc). 1H NMR (CDCl3, 600 MHz): δ = 1.17 (s, 9H), 1.22 (t, J = 7.2 Hz, 3H), 4.14 (dq, J = 10.8, 7.2 Hz, 1H), 4.17 (dq, J = 10.8, 7.2 Hz, 1H), 6.27 (s, 1H), 7.20–7.25 (m, 3H), 7.33 (t, J = 7.2 Hz, 2H), 7.35–7.40 (m, 3H), 7.42–7.46 (m, 1H), 8.29 (s, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.1, 27.3, 56.5, 60.7, 84.6, 112.3, 127.1, 127.2, 128.0, 128.7, 128.9, 129.5, 130.1, 134.1, 134.9, 136.9, 138.4, 149.5, 150.6, 164.7. IR (KBr): 2978, 1728, 1711, 1665, 1350, 1262, 1249, 1156 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H2635ClN2O4: 441.1581; found: 441.1588.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 5
1 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 84%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 0.98 (t, J = 7.2 Hz, 3H), 1.18 (s, 9H), 1.32 (t, J = 7.2 Hz, 3H), 1.43–1.70 (m, 4H), 4.23 (dq, J = 10.8, 7.2 Hz, 1H), 4.26 (dq, J = 10.8, 7.2 Hz, 1H), 4.80 (t, J = 6.0 Hz, 1H), 7.36 (t, J = 7.2 Hz, 2H), 7.40 (tt, J = 7.2, 1.8 Hz, 1H), 7.43 (dd, J = 7.2, 1.8 Hz, 2H), 8.02 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.1, 14.2, 18.4, 27.3, 38.0, 55.0, 60.5, 84.2, 115.1, 127.1, 128.0, 129.4, 133.7, 137.0, 149.6, 150.6, 165.2. IR (neat): 2960, 2935, 1733, 1712, 1670, 1370, 1351, 1245, 1153 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C21H29N2O4: 373.2127; found: 373.2135.
1. Yield: 84%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 0.98 (t, J = 7.2 Hz, 3H), 1.18 (s, 9H), 1.32 (t, J = 7.2 Hz, 3H), 1.43–1.70 (m, 4H), 4.23 (dq, J = 10.8, 7.2 Hz, 1H), 4.26 (dq, J = 10.8, 7.2 Hz, 1H), 4.80 (t, J = 6.0 Hz, 1H), 7.36 (t, J = 7.2 Hz, 2H), 7.40 (tt, J = 7.2, 1.8 Hz, 1H), 7.43 (dd, J = 7.2, 1.8 Hz, 2H), 8.02 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.1, 14.2, 18.4, 27.3, 38.0, 55.0, 60.5, 84.2, 115.1, 127.1, 128.0, 129.4, 133.7, 137.0, 149.6, 150.6, 165.2. IR (neat): 2960, 2935, 1733, 1712, 1670, 1370, 1351, 1245, 1153 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C21H29N2O4: 373.2127; found: 373.2135.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 6
1 to 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 81%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.02–1.45 (m, 5H), 1.18 (s, 9H), 1.32 (t, J = 7.2 Hz, 3H), 1.60–1.89 (m, 6H), 4.22 (dq, J = 10.8, 7.2 Hz, 1H), 4.26 (dq, J = 10.8, 7.2 Hz, 1H), 4.73 (d, J = 5.4 Hz, 1H), 7.36 (t, J = 7.2 Hz, 2H), 7.40 (t, J = 7.2 Hz, 1H), 7.45 (d, J = 7.2 Hz, 2H), 8.03 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 26.3, 26.4, 27.4, 27.7, 29.2, 44.1, 60.2, 60.5, 84.0, 113.9, 127.1, 128.0, 129.4, 133.9, 137.0, 149.6, 150.6, 165.5. IR (neat): 2928, 2853, 1731, 1713, 1670, 1371, 1351, 1318, 1244, 1154, 1012 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H33N2O4: 413.2440; found: 413.2446.
1. Yield: 81%; pale yellow oil. 1H NMR (CDCl3, 600 MHz): δ = 1.02–1.45 (m, 5H), 1.18 (s, 9H), 1.32 (t, J = 7.2 Hz, 3H), 1.60–1.89 (m, 6H), 4.22 (dq, J = 10.8, 7.2 Hz, 1H), 4.26 (dq, J = 10.8, 7.2 Hz, 1H), 4.73 (d, J = 5.4 Hz, 1H), 7.36 (t, J = 7.2 Hz, 2H), 7.40 (t, J = 7.2 Hz, 1H), 7.45 (d, J = 7.2 Hz, 2H), 8.03 (d, J = 1.2 Hz, 1H). 13C NMR (CDCl3, 150 MHz): δ = 14.2, 26.3, 26.4, 27.4, 27.7, 29.2, 44.1, 60.2, 60.5, 84.0, 113.9, 127.1, 128.0, 129.4, 133.9, 137.0, 149.6, 150.6, 165.5. IR (neat): 2928, 2853, 1731, 1713, 1670, 1371, 1351, 1318, 1244, 1154, 1012 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C24H33N2O4: 413.2440; found: 413.2446.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 100
1 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 71%; colorless crystals, mp 86–88 °C (n-hexane-Et2O). 1H NMR (CD3OD, 500 MHz): δ = 1.31 (t, J = 7.5 Hz, 3H), 1.47 (s, 6H), 2.09 (s, 3H), 4.20 (q, J = 7.5 Hz, 2H), 7.45 (t, J = 7.0 Hz, 2H), 7.51 (t, J = 7.0 Hz, 1H), 7.67 (d, J = 7.0 Hz, 2H). 13C NMR (CD3OD, 125 MHz): δ = 14.6, 19.9, 30.2, 54.9, 61.1, 109.7, 128.6, 129.5, 131.9, 135.7, 146.4 (br), 155.8 (br), 169.3. IR (neat): 2969, 1690, 1644, 1478, 1459, 1268, 1225, 1166, 1109, 1073, 1055, 770, 693 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C16H21N2O2: 273.1603; found: 273.1602.
1. Yield: 71%; colorless crystals, mp 86–88 °C (n-hexane-Et2O). 1H NMR (CD3OD, 500 MHz): δ = 1.31 (t, J = 7.5 Hz, 3H), 1.47 (s, 6H), 2.09 (s, 3H), 4.20 (q, J = 7.5 Hz, 2H), 7.45 (t, J = 7.0 Hz, 2H), 7.51 (t, J = 7.0 Hz, 1H), 7.67 (d, J = 7.0 Hz, 2H). 13C NMR (CD3OD, 125 MHz): δ = 14.6, 19.9, 30.2, 54.9, 61.1, 109.7, 128.6, 129.5, 131.9, 135.7, 146.4 (br), 155.8 (br), 169.3. IR (neat): 2969, 1690, 1644, 1478, 1459, 1268, 1225, 1166, 1109, 1073, 1055, 770, 693 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C16H21N2O2: 273.1603; found: 273.1602.General procedure for synthesis of tautomeric 2-aryl-DPs 10 and 11
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 100
1 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give a tautomeric mixture of 10a and 11a (249 mg, 0.813 mmol, 99%) as yellow crystals. Mp 152–153 °C (n-hexane–EtOAc). 1H NMR of the mixture of tautomers, 10a
1) to give a tautomeric mixture of 10a and 11a (249 mg, 0.813 mmol, 99%) as yellow crystals. Mp 152–153 °C (n-hexane–EtOAc). 1H NMR of the mixture of tautomers, 10a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11a = 1.6
11a = 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (DMSO-d6, 500 MHz): δ = 1.147 (10a, t, J = 7.0 Hz, 3H), 1.152 (11a, t, J = 7.0 Hz, 3H), 3.98–4.12 (10a, m, 2H + 11a, m, 2H), 5.45 (11a, d, J = 3.5 Hz, 1H), 5.57 (10a, s, 1H), 7.16–7.56 (10a, m, 8H + 11a, m, 8H), 7.38 (10a, d, J = 5.5 Hz, 1H), 7.66 (11a, s, 1H), 7.80 (10a, d, J = 8.5 Hz, 2H), 7.88 (11a, d, J = 8.5 Hz, 2H), 9.28 (11a, d, J = 3.5 Hz, 1H), 9.88 (10a, d, J = 5.5 Hz, 1H). 1H NMR, average spectrum of the tautomers (CD3OD, 500 MHz): δ = 1.21 (t, J = 7.0 Hz, 3H), 4.10 (dq, J = 10.5, 7.0 Hz, 1H), 4.13 (dq, J = 10.5, 7.0 Hz, 1H), 5.58 (s, 1H), 7.25 (t, J = 7.5 Hz, 1H), 7.33 (t, J = 7.5 Hz, 2H), 7.39 (d, J = 7.5 Hz, 2H), 7.45 (t, J = 7.5 Hz, 2H), 7.53 (t, J = 7.5 Hz, 1H), 7.58 (s, 1H), 7.69 (d, J = 7.5 Hz, 2H). 13C NMR, average spectrum of the tautomers (CD3OD, 125 MHz): δ = 14.6, 56.6, 61.3, 107.5 (br), 128.1, 128.3, 128.8, 129.6, 129.8, 132.5, 135.0, 140.7 (br), 146.2, 156.8 (br), 168.0. IR (neat): 2974, 1694, 1684, 1620, 1478, 1393, 1299, 1228, 1095, 756, 713, 698 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C19H19N2O2: 307.1447; found: 307.1444.
1 (DMSO-d6, 500 MHz): δ = 1.147 (10a, t, J = 7.0 Hz, 3H), 1.152 (11a, t, J = 7.0 Hz, 3H), 3.98–4.12 (10a, m, 2H + 11a, m, 2H), 5.45 (11a, d, J = 3.5 Hz, 1H), 5.57 (10a, s, 1H), 7.16–7.56 (10a, m, 8H + 11a, m, 8H), 7.38 (10a, d, J = 5.5 Hz, 1H), 7.66 (11a, s, 1H), 7.80 (10a, d, J = 8.5 Hz, 2H), 7.88 (11a, d, J = 8.5 Hz, 2H), 9.28 (11a, d, J = 3.5 Hz, 1H), 9.88 (10a, d, J = 5.5 Hz, 1H). 1H NMR, average spectrum of the tautomers (CD3OD, 500 MHz): δ = 1.21 (t, J = 7.0 Hz, 3H), 4.10 (dq, J = 10.5, 7.0 Hz, 1H), 4.13 (dq, J = 10.5, 7.0 Hz, 1H), 5.58 (s, 1H), 7.25 (t, J = 7.5 Hz, 1H), 7.33 (t, J = 7.5 Hz, 2H), 7.39 (d, J = 7.5 Hz, 2H), 7.45 (t, J = 7.5 Hz, 2H), 7.53 (t, J = 7.5 Hz, 1H), 7.58 (s, 1H), 7.69 (d, J = 7.5 Hz, 2H). 13C NMR, average spectrum of the tautomers (CD3OD, 125 MHz): δ = 14.6, 56.6, 61.3, 107.5 (br), 128.1, 128.3, 128.8, 129.6, 129.8, 132.5, 135.0, 140.7 (br), 146.2, 156.8 (br), 168.0. IR (neat): 2974, 1694, 1684, 1620, 1478, 1393, 1299, 1228, 1095, 756, 713, 698 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C19H19N2O2: 307.1447; found: 307.1444.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 75
1 to 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150
150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 98%; pale yellow amorphous. 1H NMR of the mixture of tautomers, 10b
1. Yield: 98%; pale yellow amorphous. 1H NMR of the mixture of tautomers, 10b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11b = 1
11b = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (DMSO-d6, 500 MHz): δ = 1.11–1.18 (10b, t, J = 7.0 Hz, 3H + 11b, t, J = 7.0 Hz, 3H), 3.76–3.81 (10b, s, 3H + 11b, s, 3H), 3.97–4.12 (10b, m, 2H + 11b, m, 2H), 5.41 (11b, d, J = 3.5 Hz, 1H), 5.54 (10b, s, 1H), 6.95–7.90 (10b, m, 9H + 11b, m, 9H), 7.37 (10b, d, J = 5.5 Hz, 1H), 7.64 (10b, s, 1H), 9.16 (11b, d, J = 3.5 Hz, 1H), 9.79 (10b, d, J = 5.5 Hz, 1H). 1H NMR, average spectrum of the tautomers (CD3OD, 500 MHz): δ = 1.21 (t, J = 7.0 Hz, 3H), 3.83 (s, 3H), 4.10 (dq, J = 10.5, 7.0 Hz, 1H), 4.13 (dq, J = 10.5, 7.0 Hz, 1H), 5.55 (s, 1H), 6.98 (d, J = 8.5 Hz, 2H), 7.25 (t, J = 7.0 Hz, 1H), 7.32 (t, J = 7.0 Hz, 2H), 7.37 (d, J = 7.0 Hz, 2H), 7.60 (s, 1H), 7.66 (d, J = 8.5 Hz, 2H). 13C NMR, average spectrum of the tautomers (CD3OD, 125 MHz): δ = 14.6, 55.9, 56.1, 61.2, 107.7 (br), 115.0, 126.9, 128.0, 128.8, 129.5, 130.1, 142.1 (br), 146.3, 157.2 (br), 164.0, 168.0. IR (neat): 1691, 1670, 1605, 1480, 1251, 1225, 1173, 1097, 1075, 1029, 838, 754, 697 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C20H21N2O3: 337.1552; found: 337.1568.
1 (DMSO-d6, 500 MHz): δ = 1.11–1.18 (10b, t, J = 7.0 Hz, 3H + 11b, t, J = 7.0 Hz, 3H), 3.76–3.81 (10b, s, 3H + 11b, s, 3H), 3.97–4.12 (10b, m, 2H + 11b, m, 2H), 5.41 (11b, d, J = 3.5 Hz, 1H), 5.54 (10b, s, 1H), 6.95–7.90 (10b, m, 9H + 11b, m, 9H), 7.37 (10b, d, J = 5.5 Hz, 1H), 7.64 (10b, s, 1H), 9.16 (11b, d, J = 3.5 Hz, 1H), 9.79 (10b, d, J = 5.5 Hz, 1H). 1H NMR, average spectrum of the tautomers (CD3OD, 500 MHz): δ = 1.21 (t, J = 7.0 Hz, 3H), 3.83 (s, 3H), 4.10 (dq, J = 10.5, 7.0 Hz, 1H), 4.13 (dq, J = 10.5, 7.0 Hz, 1H), 5.55 (s, 1H), 6.98 (d, J = 8.5 Hz, 2H), 7.25 (t, J = 7.0 Hz, 1H), 7.32 (t, J = 7.0 Hz, 2H), 7.37 (d, J = 7.0 Hz, 2H), 7.60 (s, 1H), 7.66 (d, J = 8.5 Hz, 2H). 13C NMR, average spectrum of the tautomers (CD3OD, 125 MHz): δ = 14.6, 55.9, 56.1, 61.2, 107.7 (br), 115.0, 126.9, 128.0, 128.8, 129.5, 130.1, 142.1 (br), 146.3, 157.2 (br), 164.0, 168.0. IR (neat): 1691, 1670, 1605, 1480, 1251, 1225, 1173, 1097, 1075, 1029, 838, 754, 697 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C20H21N2O3: 337.1552; found: 337.1568.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 100
1 to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Yield: 97%; orange amorphous. 1H NMR of the mixture of tautomers, 10g
1. Yield: 97%; orange amorphous. 1H NMR of the mixture of tautomers, 10g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11g = 2.5
11g = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (DMSO-d6, 500 MHz): δ = 1.14 (11g, t, J = 7.0 Hz, 3H), 1.16 (11g, t, J = 7.0 Hz, 3H), 3.97–4.12 (10g, m, 2H + 11g, m, 2H), 5.49 (11g, d, J = 3.0 Hz, 1H), 5.62 (10g, s, 1H), 7.16–7.44 (10g, m, 5H + 11g, m, 5H), 7.41 (10g, d, J = 5.0 Hz, 1H), 7.67 (11g, s, 1H), 8.05 (10g, d, J = 8.5 Hz, 2H), 8.12 (11g, d, J = 8.5 Hz, 2H), 8.30 (10g, d, J = 8.5 Hz, 2H), 8.32 (11g, d, J = 8.5 Hz, 2H), 9.54 (11g, d, J = 3.0 Hz, 1H), 10.15 (10g, d, J = 5.0 Hz, 1H). 1H NMR, average spectrum of the tautomers (CD3OD, 500 MHz): δ = 1.21 (t, J = 7.0 Hz, 3H), 4.10 (dq, J = 10.5, 7.0 Hz, 1H), 4.13 (dq, J = 10.5, 7.0 Hz, 1H), 5.63 (s, 1H), 7.26 (t, J = 7.5 Hz, 1H), 7.34 (t, J = 7.5 Hz, 2H), 7.40 (d, J = 7.5 Hz, 2H), 7.44–7.70 (brs, 1H), 7.92 (d, J = 9.0 Hz, 2H), 8.30 (d, J = 9.0 Hz, 2H). 13C NMR, average spectrum of the tautomers (CD3OD, 125 MHz): δ = 14.5, 57.4, 61.4, 105.5–108.5 (br), 124.7, 128.2, 128.9, 129.5, 129.7, 137.0–141.0 (br), 140.8, 146.1, 150.8, 153.0–156.0 (br), 167.7. IR (neat): 1695, 1674, 1600, 1521, 1487, 1344, 1297, 1242, 1190, 1097, 1072, 851, 752, 698 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C19H18N3O4: 352.1297; found: 352.1305.
1 (DMSO-d6, 500 MHz): δ = 1.14 (11g, t, J = 7.0 Hz, 3H), 1.16 (11g, t, J = 7.0 Hz, 3H), 3.97–4.12 (10g, m, 2H + 11g, m, 2H), 5.49 (11g, d, J = 3.0 Hz, 1H), 5.62 (10g, s, 1H), 7.16–7.44 (10g, m, 5H + 11g, m, 5H), 7.41 (10g, d, J = 5.0 Hz, 1H), 7.67 (11g, s, 1H), 8.05 (10g, d, J = 8.5 Hz, 2H), 8.12 (11g, d, J = 8.5 Hz, 2H), 8.30 (10g, d, J = 8.5 Hz, 2H), 8.32 (11g, d, J = 8.5 Hz, 2H), 9.54 (11g, d, J = 3.0 Hz, 1H), 10.15 (10g, d, J = 5.0 Hz, 1H). 1H NMR, average spectrum of the tautomers (CD3OD, 500 MHz): δ = 1.21 (t, J = 7.0 Hz, 3H), 4.10 (dq, J = 10.5, 7.0 Hz, 1H), 4.13 (dq, J = 10.5, 7.0 Hz, 1H), 5.63 (s, 1H), 7.26 (t, J = 7.5 Hz, 1H), 7.34 (t, J = 7.5 Hz, 2H), 7.40 (d, J = 7.5 Hz, 2H), 7.44–7.70 (brs, 1H), 7.92 (d, J = 9.0 Hz, 2H), 8.30 (d, J = 9.0 Hz, 2H). 13C NMR, average spectrum of the tautomers (CD3OD, 125 MHz): δ = 14.5, 57.4, 61.4, 105.5–108.5 (br), 124.7, 128.2, 128.9, 129.5, 129.7, 137.0–141.0 (br), 140.8, 146.1, 150.8, 153.0–156.0 (br), 167.7. IR (neat): 1695, 1674, 1600, 1521, 1487, 1344, 1297, 1242, 1190, 1097, 1072, 851, 752, 698 cm−1. HRMS-FAB: m/z [M + H]+ calcd for C19H18N3O4: 352.1297; found: 352.1305.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Y. N. thanks financial support from the Foundation for Japanese Chemical Research [No. 505 (R)]. This work was also financially supported by JSPS KAKENHI Grant Number 19K05703.References
- (a) H. Cho, M. Ueda, K. Shima, A. Mizuno, M. Hayashimatsu, Y. Ohnaka, Y. Takeuchi, M. Hamaguchi, K. Aisaka, T. Hidaka, M. Kawai, M. Takeda, T. Ishihara, K. Funahashi, F. Satoh, M. Morita and T. Noguchi, J. Med. Chem., 1989, 32, 2399 CrossRef CAS; (b) K. S. Atwal, B. N. Swanson, S. E. Unger, D. M. Floyd, S. Moreland, A. Hedberg and B. C. O'Reilly, J. Med. Chem., 1991, 34, 806 CrossRef CAS PubMed.
- (a) T. U. Mayer, T. M. Kapoor, S. J. Haggarty, R. W. King, S. L. Schreiber and T. J. Mitchison, Science, 1999, 286, 971 CrossRef CAS PubMed; (b) Z. Maliga, T. M. Kapoor and T. J. Mitchison, Chem. Biol., 2002, 9, 989 CrossRef CAS.
- V. Ramachandran, K. Arumugasamy, S. K. Singh, N. Edayadulla, P. Ramesh and S.-K. Kamaraj, J. Chem. Biol., 2016, 9, 31 CrossRef.
- A. K. Chhillar, P. Arya, C. Mukherjee, P. Kumar, Y. Yadav, A. K. Sharma, V. Yadav, J. Gupta, R. Dabur, H. N. Jha, A. C. Watterson, V. S. Parmar, A. K. Prasad and G. L. Sharma, Bioorg. Med. Chem., 2006, 14, 973 CrossRef CAS PubMed.
- B. N. Naidu, M. E. Sorenson, M. Patel, Y. Ueda, J. Banville, F. Beaulieu, S. Bollini, I. B. Dicker, H. Higley, Z. Lin, L. Pajor, D. D. Parker, B. J. Terry, M. Zheng, A. Martel, N. A. Meanwell, M. Krystal and M. A. Walker, Bioorg. Med. Chem. Lett., 2015, 25, 717 CrossRef CAS.
- N. October, N. D. Watermeyer, V. Yardley, T. J. Egan, K. Ncokazi and K. Chibale, ChemMedChem, 2008, 3, 1649 CrossRef CAS PubMed.
- H. J. M. Gijsen, D. Berthelot, M. A. J. De Cleyn, I. Geuens, B. Brône and M. Mercken, Bioorg. Med. Chem. Lett., 2012, 22, 797 CrossRef CAS PubMed.
- D. L. da Silva, F. S. Reis, D. R. Muniz, A. L. T. G. Ruiz, J. E. de Carvalho, A. A. Sabino, L. V. Modolo and Â. de Fátima, Bioorg. Med. Chem., 2012, 20, 2645 CrossRef CAS PubMed.
- (a) C. O. Kappe, Eur. J. Med. Chem., 2000, 35, 1043 CrossRef CAS; (b) C. O. Kappe and A. Stadler, Org. React., 2004, 63, 1 CAS; (c) H. Cho, Heterocycles, 2013, 87, 1441 CrossRef CAS; (d) H. Nagarajaiah, A. Mukhopadhyay and J. N. Moorthy, Tetrahedron Lett., 2016, 57, 5135 CrossRef CAS; (e) R. Kaur, S. Chaudhary, K. Kumar, M. K. Gupta and R. K. Rawal, Eur. J. Med. Chem., 2017, 132, 108 CrossRef CAS PubMed; (f) L. H. S. Matos, F. T. Masson, L. A. Simeoni and M. Homem-de-Mello, Eur. J. Med. Chem., 2018, 143, 1779 CrossRef CAS; (g) L. V. Chopda and P. N. Dave, ChemistrySelect, 2020, 5, 5552 CrossRef CAS.
- K. Deres, C. H. Schröder, A. Paessens, S. Goldmann, H. J. Hacker, O. Weber, T. Krämer, U. Niewöhner, U. Pleiss, J. Stoltefuss, E. Graef, D. Koletzki, R. N. A. Masantschek, A. Reimann, R. Jaeger, R. Groβ, B. Beckermann, K.-H. Schlemmer, D. Haebich and H. Rübsamen-Waigmann, Science, 2003, 299, 893 CrossRef CAS PubMed.
- X.-y. Yang, X.-q. Xu, H. Guan, L.-l. Wang, Q. Wu, G.-m. Zhao and S. Li, Bioorg. Med. Chem. Lett., 2014, 24, 4247 CrossRef CAS PubMed.
- C. A. Sehon, G. Z. Wang, A. Q. Viet, K. B. Goodman, S. E. Dowdell, P. A. Elkins, S. F. Semus, C. Evans, L. J. Jolivette, R. B. Kirkpatrick, E. Dul, S. S. Khandekar, T. Yi, L. L. Wright, G. K. Smith, D. J. Behm, R. Benthley, C. P. Doe, E. Hu and D. Lee, J. Med. Chem., 2008, 51, 6631 CrossRef CAS PubMed.
- S. Teracciano, M. G. Chini, M. C. Vaccaro, M. Strocchia, A. Foglia, A. Vassallo, C. Saturnino, R. Riccio, G. Bifulco and I. Bruno, Chem. Commun., 2016, 52, 12857 RSC.
- (a) A. Lengar and C. O. Kappe, Org. Lett., 2004, 6, 771 CrossRef CAS; (b) H. Prokopcová and C. O. Kappe, J. Org. Chem., 2007, 72, 4440 CrossRef; (c) Q. Sun, F. Suzenet and G. Guillaumet, Tetrahedron Lett., 2012, 53, 2694 CrossRef CAS.
- T. K. Nguyen, G. D. Titov, O. V. Khoroshilova, M. A. Kinzhalov and N. V. Rostovskii, Org. Biomol. Chem., 2020, 18, 4971 RSC.
- (a) A. Weis, F. Frolow, D. Zamir and M. Bernstein, Heterocycles, 1984, 22, 657 CrossRef CAS; (b) A. Weis, Synthesis, 1985, 528 CrossRef CAS.
- (a) H. Cho, Y. Nishimura, Y. Yasui and M. Yamaguchi, Tetrahedron Lett., 2012, 53, 1177 CrossRef CAS; (b) Y. Nishimura, Y. Yasui, S. Kobayashi, M. Yamaguchi and H. Cho, Tetrahedron, 2012, 68, 3342 CrossRef CAS; (c) Y. Nishimura and H. Cho, Tetrahedron Lett., 2014, 55, 411 CrossRef CAS; (d) Y. Nishimura, T. Kubo, Y. Okamoto and H. Cho, Tetrahedron Lett., 2016, 57, 4492 CrossRef CAS.
- (a) Y. Nishimura, Y. Okamoto, M. Ikunaka and Y. Ohyama, Chem. Pharm. Bull., 2011, 59, 1458 CrossRef CAS PubMed; (b) Y. Nishimura and H. Cho, Synlett, 2015, 26, 233 CrossRef CAS; (c) Y. Nishimura, T. Kubo, Y. Okamoto and H. Cho, Tetrahedron Lett., 2017, 58, 4236 CrossRef CAS; (d) Y. Nishimura, H. Kikuchi, T. Kubo, R. Arai, Y. Toguchi, B. Yuan, K. Sunaga and H. Cho, Chem. Pharm. Bull., 2022, 70, 111 CrossRef CAS PubMed.
- Y. Nishimura, H. Kikuchi, T. Kubo, Y. Gokurakuji, Y. Nakamura, R. Arai, B. Yuan, K. Sunaga and H. Cho, Tetrahedron Lett., 2020, 61, 151967 CrossRef CAS.
- Y. Nishimura, H. Kikuchi, T. Kubo, I. Nakakita, M. Oguni, M. Ohta, R. Arai, B. Yuan, K. Sunaga and H. Cho, Tetrahedron Lett., 2021, 65, 152760 CrossRef CAS.
- (a) L. S. Liebeskind and J. Srogl, J. Am. Chem. Soc., 2000, 122, 11260 CrossRef CAS; (b) C. Savarin, J. Srogl and L. S. Liebeskind, Org. Lett., 2001, 3, 91 CrossRef CAS PubMed; (c) C. L. Kusturin, L. S. Liebeskind and W. L. Neumann, Org. Lett., 2002, 4, 983 CrossRef CAS PubMed; (d) M. Egi and L. S. Liebeskind, Org. Lett., 2003, 5, 801 CrossRef CAS PubMed; (e) H. Prokopcová and C. O. Kappe, Angew. Chem., Int. Ed., 2009, 48, 2276 CrossRef PubMed; (f) V. Hirschbeck, P. H. Gehrtz and I. Fleischer, Chem.–Eur. J., 2018, 24, 7092 CrossRef CAS.
- Recent examples of Pd-catalyzed/Cu-mediated oxidative cross-coupling reactions of 2-thioxo-DPs, see: (a) N. H. T. Phan, H. Kim, H. Shin, H.-S. Lee and J.-H. Sohn, Org. Lett., 2016, 18, 5154 CrossRef; (b) H. Kim, N. H. T. Phan, H. Shin, H.-S. Lee and J.-H. Sohn, Tetrahedron, 2017, 73, 6604 CrossRef CAS; (c) H. Kim, J. Lee, H. Shin and J.-H. Sohn, Org. Lett., 2018, 20, 1961 CrossRef CAS PubMed; (d) N. S. L. Phan, H. Shin, J. Y. Kang and J.-H. Sohn, J. Org. Chem., 2020, 85, 5087 CrossRef PubMed; (e) T. N. H. Phan, J. Lee, H. Shin and J.-H. Sohn, J. Org. Chem., 2021, 86, 5423 CrossRef CAS.
- H. Cho, Y. Nishimura, Y. Yasui, S. Kobayashi, S. Yoshida, E. Kwon and M. Yamaguchi, Tetrahedron, 2011, 67, 2661 CrossRef CAS.
- D. C. Harrowven, D. P. Curran, S. L. Kostiuk, I. L. Wallis-Guy, S. Whiting, K. J. Stenning, B. Tang, E. Packard and L. Nanson, Chem. Commun., 2010, 46, 6335 RSC.
- H. Cho, Y. Nishimura, H. Ikeda, M. Asakura and S. Toyota, Tetrahedron, 2018, 74, 2405 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra05155a |
| This journal is © The Royal Society of Chemistry 2022 |