Open Access Article
Open Access ArticleA novel procedure for the synthesis of borylated quinolines and its application in the development of potential boron-based homeodomain interacting protein kinase 2 (HIPK2) inhibitors†
Bhaskar C. Das *ab,
Pratik Yadav
*ab,
Pratik Yadav a,
Sasmita Dasa and
John Cijiang Hebc
a,
Sasmita Dasa and
John Cijiang Hebc
aArnold and Marie Schwartz College of Pharmacy and Health Sciences, Long Island University, Brooklyn, NY 11201, USA. E-mail: Bhaskar.Das@liu.edu
bDivision of Nephrology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA
cRenal Section, James J. Peters Veterans Affairs Medical Center, Bronx, NY 10468, USA
First published on 25th August 2022
Abstract
Herein, we demonstrate a Pd catalyzed C-4 borylation of structurally complex chloroquinolines with bis(pinacolato)diboron under relatively simple and efficient conditions. Moreover, the borylated quinolines were converted into oxaborole, trifluoroborate salt and boronic acid and also rendered in the Suzuki reaction successfully. The method was also applied for the synthesis of potential boron-based homeodomain interacting protein kinase 2 (HIPK2) inhibitors. The strategy opens up new avenues for the functionalization of quinolines as potential probes and pharmacological agents for future biomedical research.
Homeodomain interacting protein kinase 2 (HIPK2) plays a vital role in kidney fibrosis and has been identified as a key regulator for various profibrosis pathways.1 In previous studies, it has been found that inhibition of HIPK2 might be a novel approach against fibrosis progression in kidney disease.2,3 However, HIPK2 inhibitors have not been well studied and are not available on a commercial scale. A recent report by Cozza et al.4 describes the discovery of a selective HIPK2 inhibitor, even though broad inhibition of HIPK2 function may not be beneficial in all cellular contexts.
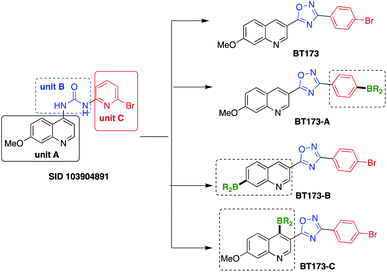
For our ongoing project to develop novel anti-fibrotic drugs for kidney fibrosis, we focused on HIPK2 as potential target.5a We performed a search for HIPK2 inhibitors and identified several potential compounds from a screening assay earlier studied by Abbott Labs.5b We executed the SAR (Structure Activity Studies) study on one of these compounds (PubChem SID 103904891), which comprises three units (Scheme 1): unit A, unit B and unit C. To explore the possibility of synthesizing a new class of HIPK2 inhibitors, we focused on the design and modification of each unit with suitable subunits. In our initial efforts, we successfully synthesized BT173 (Scheme 1),6 which was accomplished by the conversion of unit B into an oxadiazole subunit which serves as a bioisostere of guanidine structural motif. We found that the quinoline derived scaffold BT173 showed specific pharmacologic inhibition of HIPK2 for antifibrotic therapy.6 It strongly inhibited the ability of HIPK2 to potentiate the downstream transcriptional activity of Smad3 in kidney tubular cells.6 These data strongly support that BT173 is a “proof-of-concept compound” as HIPK2 inhibitor for treatment of kidney fibrosis. However, BT173 has low solubility and relatively low affinity. Therefore, we plan to use BT173 as our starting point to develop better compounds for lead optimization.
SAR study of BT173 revealed that by keeping quinoline and oxadiazole motifs intact in combination with boron at different positions of BT173 could lead to the potential HIPK2 inhibitors. We, therefore, turned our attention towards the design and synthesis of new boron-based potential HIPK2 inhibitors BT173-A, BT173-B and BT173-C (Scheme 1).
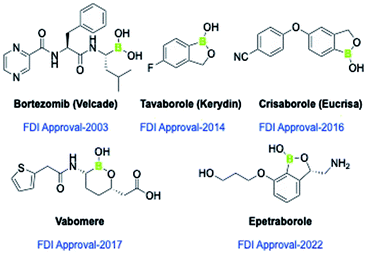
Boron-based scaffolds have recently gained considerable attention as biologically significant nucleuses as well as in material science.7 Examples of some clinically approved boron-containing therapeutics have been shown in Fig. 1. Due to vacant p-orbitals of boron, the boron-containing compounds are prone to accept electrons from electron-donating atoms/groups and thereby exhibit strong interaction at the active sites of enzymes. Moreover, the boron-based compounds have a tendency to form H-bond networks governing several biological phenomena and play a vital role in drug–receptor interaction. These properties make them potential therapeutic agents for the development of new drug candidates.7,8
We anticipated that the designed scaffolds BT173-A, BT173-B and BT173-C (Scheme 1) could be efficiently synthesized by catalytic borylation of suitably functionalized halo-quinolines. We first started investigating borylation reaction for the synthesis of challenging target BT173-C. The strategized reaction was challenging due to the following reasons; (1) the presence of a nitrogen heterocycle and a hindered C-4 position of quinoline, (2) catalytic borylation of heteroaromatic organohalides with B2(pin)2 are also very limited,9 (3) the probability of the formation of undesired N–B adduct, (4) rebellious nature of heteroaromatic organohalides coupling partners in metal-catalyzed cross coupling reactions.10
To our surprise, we could not find any previous report for the C-4 borylation of readily available chloroquinolines with bis(pinacolato)diboron (B2(pin)2). Very recently, during the course of our study, Kwong group demonstrated only two successful examples for the borylation of chloroquinolines with bis-(neopentyl glycolato)diborane.9a However, in this study a tailor-made ligand has been used at higher temperature with a relatively low yield with ester group at C-3 position. To address these challenges, we developed an economically viable, environmentally sustainable methodology to synthesize C-4 substituted borylated quinoline scaffolds. As a part of our ongoing research program on the development of boron-based therapeutics,6–8,11 herein, we wish to report a simpler, efficient and economical method for the borylation of chloroquinolines. This methodology allows the construction of boron-based potential first-in-class HIPK2 inhibitors in higher yields. The present strategy comprises a Pd-catalyzed borylation reaction12 of differently substituted quinolines and B2(pin)2 as a key step. To the best of our knowledge, this is the first report of Pd-catalyzed borylation of complex-chloroquinolines with readily available B2(pin)2 at C-4 position. Furthermore, the synthesized borylated quinolines were successfully used as starting materials for the construction of potential biologically active pharmacophore groups e.g. oxaboroles, trifluoroborate salts, boronic acids and synthetically useful intermediates using the Suzuki reaction.
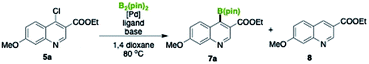
To achieve the synthesis of BT173-C, we started our optimization for the borylation reaction using ethyl 4-chloro-7-methoxyquinoline-3-carboxylate 5a and B2(pin)2 as a model substrate. We explored various reaction conditions and the results are summarized in Table 1. Our initial efforts with Pd2dba3 and Pd(dba)2 with XPhos using KOAc as a base did not yield the desired product ethyl 7-methoxy-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinoline-3-carboxylate 7a (Table 1, entry 1 and 2). Pd(OAc)2 was found to be a better Pd source to yield 35% and 32% of the desired product 7a with 2 mol% and 5 mol% catalyst loading respectively (Table 1, entry 3 and 4). In search of a superior catalytic system, we next tested the efficiency of Pd(PPh3)2Cl2 with XPhos as a ligand. To our delight, the desired product was obtained in 65% yields (Table 1, entry 5). To gauge the influence of a ligand, we performed the reaction in the absence of XPhos which also resulted in the desired product with a slight increase in the yield (Table 1, entry 6). This result showed that the reaction can be performed without the use of an external ligand. We also tested the outcome of the reaction at low catalyst loading (2 mol%) and found that there was no substantial loss in yield and 75% yield of desired product was obtained. It was noteworthy that compound 8 was not formed with Pd(PPh3)2Cl2 in the absence of ligand. Our attempt to convert ethyl 4-bromo-7-methoxyquinoline-3-carboxylate 6 into corresponding borylated product gave the desired product 7a but in relatively low yield (45%) under similar catalytic system (Table 1, entry 10). A competitive reaction was also observed and it was interesting to find out that the bromoquinoline 6 resulted dehalogenation/hydrogenation compound ethyl 7-methoxyquinoline-3-carboxylate 8 (Table 1).
| Entry | Pd (mol%) | Ligand | Base | Yieldsd (%) | |
|---|---|---|---|---|---|
| 7a | 8 | ||||
| a Reaction was performed using 5a (0.5 mmol), B2Pin2 (0.75 mmol), base (1 mmol), Pd (5 mol%) and ligand (10 mol%) using 1,4 dioxane (2 mL).b Pd (2 mol%) and ligand (5 mol% if required).c Reaction was performed using 6 (0.5 mmol), B2Pin2 (0.75 mmol), base (1 mmol), Pd (2 mol%) and 1,4 dioxane (2 mL).d Isolated yield. | |||||
| 1 | Pd2(dba)3 | XPhos | KOAc | Traces | 30 |
| 2 | Pd(dba)2 | XPhos | KOAc | Traces | 20 |
| 3 | Pd(OAc)2 | XPhos | KOAc | 35 | 10 |
| 4b | Pd(OAc)2 | XPhos | KOAc | 32 | 15 |
| 5 | Pd(PPh3)2Cl2 | XPhos | KOAc | 65 | 20 |
| 6 | Pd(PPh3)2Cl2 | — | KOAc | 72 | — |
| 7b | Pd(PPh3)2Cl2 | — | KOAc | 75 | — |
| 8b | Pd(PPh3)2Cl2 | — | K3PO4 | 55 | — |
| 9b | Pd(PPh3)2Cl2 | — | Cs2CO3 | 40 | — |
| 10c | Pd(PPh3)2Cl2 | — | KOAc | 45 | 35 |
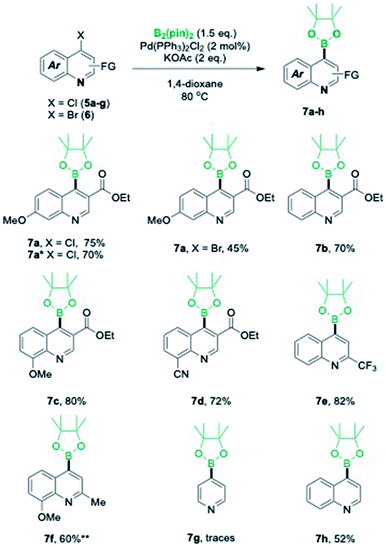
With the standardized reaction conditions in hand, we synthesized the required intermediate 7a in good yield for the synthesis of BT173-C (Scheme 2). A gram scale synthesis of 7a was also performed under the developed conditions for the broader utility of this valuable synthetic intermediate. To our delight, there was not much loss in the yields and the product was isolated in 70% yield. During the course of reaction, chloroquinolines were found better substrates under the developed reaction conditions for the C-4 borylation. To further check the effect of substituent and to expand the substrate scope, C-4 borylated-quinolines 7b–7d were also synthesized. To our delight, the reaction conditions were found efficient for the substrates with electron neutral 7b, electron donating 7c, and electron withdrawing 7d groups in good yields (Scheme 2). To check the effect of ester group at C-3 position, we next tested the outcome of this reaction with 4-chloro-2-(trifluoromethyl)quinoline 5e as a substrate. It was noteworthy that 5e was also converted efficiently into 7e in 82% yield. The efficacy of this catalytic system was further intended to explore with the electron-donating group on the pyridine ring instead of phenyl ring. To achieve this transformation 4-chloro-8-methoxy-2-methylquinoline 5f was chosen as substrate. Our initial efforts with the current catalytic system did not work well and desired product 7f was obtained in low yields (15%). However, addition of 2 mol% XPhos was found beneficial and product 7f was obtained in 60% yield. 4-Chloropyridine did not work well under both the conditions and gave desired 7g only in traces however 4-chloroquinoline 5h was successfully converted into desired product 4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)quinoline 7h in moderate yield (52%) using the developed catalytic system (Scheme 2). Further development of this process with differently substituted functional groups is currently under investigation.
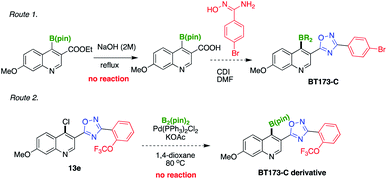
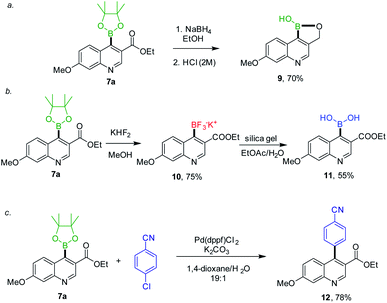
As anticipated, 7a was a potential intermediate for the synthesis of BT173-C, we first attempted to convert compound 7a into the corresponding acid which could be subsequently converted into oxadiazoles (Scheme 3, route 1) but we could not achieve hydrolysis of ester into acid.13 Subsequently, we planned to first synthesize oxadiazoles with chloro-group installed at C-4 position of quinoline and then converting these types of compounds into BT173-C derivatives (Scheme 3, route 2). To check our hypothesis compound 5-(4-chloro-7-methoxyquinolin-3-yl)-3-(2-(trifluoromethoxy)phenyl)-1,2,4-oxadiazole 13e was synthesized (ESI, Scheme 3†). However our preliminary efforts to transform 13e into BT173-C via our developed protocol did not give desired product (Scheme 3). Currently, we are investigating suitable conditions to synthesize our target compound BT173-C. Having a valuable synthon 7a in hand, we decided to explore the synthetic utility of 7a in various synthetic transformations. First, 7a was subjected to reduction using NaBH4 in ethanol followed by neutralization with 2 M HCl to convert it into corresponding alcohol. Interestingly, this reaction yielded in situ formation of 7-methoxy-[1,2]oxaborolo[4,3-c]quinolin-1(3H)-ol 9 in a tandem manner (Scheme 4a). Oxaboroles have shown broad range of biological activities previously.7,14 Compound 7a was also anticipated as a suitable intermediate to develop the series of other boron-based biologically important scaffolds. Similar to oxaboroles, aryltrifluoroborate salts, and arylboronic acids are valuable from synthetic as well as from medicinal chemistry view point.15
Therefore, we next intended the synthesis of potassium (3-(ethoxycarbonyl)-7-methoxyquinolin-4-yl)trifluoroborate 10 and (3-(ethoxycarbonyl)-7-methoxyquinolin-4-yl)boronic acid 11 starting from 7a. The synthesis of compound 10 was achieved by the treatment of compound 7a with KHF2 in MeOH in good yield (75%). Compound 10 was then converted into compound 11 in 55% yield with silica gel in ethyl acetate/water (Scheme 4b). Besides this, boron pinacol esters were also found suitable intermediate for the Suzuki reaction to construct biaryls via in situ generation from aryl halides16a or separately.16b This transformation has added benefits for exploring possibility of generating a library of C-4 functionalized quinolines. Therefore, we opted for intermediate 7a for the Suzuki reaction (Scheme 4c). In our initial efforts, the reaction proceeded with 5 mol% of Pd(dppf)Cl2 and K2CO3 in 1,4-dioxane/H2O (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to provide desired product ethyl 4-(4-cyanophenyl)-7-methoxyquinoline-3-carboxylate 12 in 78% yield. The further development of this transformation is under progress.
1) to provide desired product ethyl 4-(4-cyanophenyl)-7-methoxyquinoline-3-carboxylate 12 in 78% yield. The further development of this transformation is under progress.
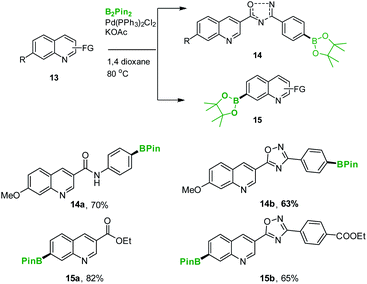
After exploring the synthesis and utility of 7a, we again turned our focus on the synthesis of BT173-A and BT173-B. The developed C-4 borylation of quinolones was found suitable for the borylation of differently functionalized complex heteroarenes 13a–d (ESI, Scheme 2†). Using our newly established borylation-methodology, the synthesis of target compounds 14a–b and 15a–b was achieved efficiently and in good yields (Scheme 5). The reaction well-tolerated numerous medicinally important functional groups e.g. amide 14a, oxadiazole 14b and 15b, ester 7a–d and 15a and nitrile 7d (Schemes 2 and 5). These results clearly demonstrate the suitability of the current borylation protocol for the synthesis of various borylated heteroarenes even at gram scales making them viable synthons for synthetic as well as medicinal applications. This protocol also leads to the synthesis of target compounds BT173-A and BT173-B (14b and 15b) and other related boron based HIPK2 inhibitors 16, 17 and 18.
For the synthesis of 16 and 17, 14b was first converted into corresponding trifluoroborate salt 16 using KHF2 in 80% yield and 16 was subsequently converted into boronic acid 17 in 60% yield (Scheme 6). Our efforts to hydrolyse 15b into carboxylic acids in the presence of 2 M NaOH gave compound 18 in 50% yield (Scheme 6).
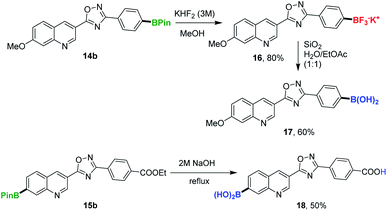 | ||
| Scheme 6 Synthetic utility of 14b and 15a in other biologically interesting boron containing scaffolds. | ||
In conclusion, we report here the design and synthesis of novel first-in-class boron-based scaffolds as a potential HIPK2 inhibitors and developed a new methodology to introduce boron atom at C-4 position of substituted quinolines. Borylation at C-4 position of hindered quinolines took place under relatively simple manner with B2(pin)2. The protocol was found suitable for gram scale synthesis borylated quinolines as well. Additionally, the developed molecules were efficiently converted to other biologically as well as synthetically relevant scaffolds such as oxaborol, trifluoroborate salts and boronic acids. The C-4 borylated quinolines was successfully used for Suzuki–Miyaura coupling reaction. Therefore, this strategy also opens up new avenues for the functionalization of quinoline as well as for future drug discovery. The biological studies of these pharmacophores are also being investigated in our laboratory currently.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
BD acknowledges NIH for support R01AI132614-01A1, R21 AA027374-01, 1R01NS109423-01A1. BD conceive the idea, design the molecules, synthesized molecules, write the paper, edit the paper, did data analysis, and coordinated with all authors. PY synthesized compounds, analysed compounds, write and edited the paper, SD, synthesized, analysed the compounds, edit the paper, JH read and edit the paper. BD and JH initially published BT173.Notes and references
- D. Sombroek and T. Hofmann, Cell Death Differ., 2009, 16, 187–194 CrossRef CAS PubMed.
- Y. Aikawa, L. A. Nguyen, K. Isono, N. Takakura, Y. Tagata, M. L. Schmitz, H. Koseki and I. Kitabayashi, EMBO J., 2006, 25, 3955–3965 CrossRef CAS PubMed.
- G. D'Orazi, B. Cecchinelli, T. Bruno, I. Manni, Y. Higashimoto, S. i. Saito, M. Gostissa, S. Coen, A. Marchetti and G. Del Sal, Nat. Cell Biol., 2002, 4, 11–19 CrossRef PubMed.
- G. Cozza, S. Zanin, R. Determann, M. Ruzzene, C. Kunick and L. A. Pinna, PLoS One, 2014, 9, e89176 CrossRef PubMed.
- (a) Y. Fan, N. Wang, P. Chuang and J. C. He, Kidney Int. Suppl., 2014, 4, 97–101 CrossRef CAS PubMed; (b) J. T. Metz, E. F. Johnson, N. B. Soni, P. J. Merta, L. Kifle and P. J. Hajduk, Nat. Chem. Biol., 2011, 7, 200–202 CrossRef CAS PubMed.
- R. Liu, B. Das, W. Xiao, Z. Li, H. Li, K. Lee and J. C. He, J. Am. Soc. Nephrol., 2017, 28, 2133–2143 CrossRef CAS PubMed.
- (a) B. C. Das, M. A. Shareef, S. Das, N. K. Nandwana, Y. Das, M. Saito and L. M. Weiss, Bioorg. Med. Chem., 2022, 116748 CrossRef CAS PubMed; (b) B. C. Das, N. K. Nandwana, S. Das, V. Nandwana, M. A. Shareef, Y. Das, M. Saito, L. M. Weiss, F. Almaguel and N. S. Hosmane, Molecules, 2022, 27, 2615 CrossRef CAS PubMed.
- (a) B. C. Das, X. Zhao, X.-Y. Tang and F. Yang, Bioorg. Med. Chem. Lett., 2011, 21, 5638–5641 CrossRef CAS PubMed; (b) M. A. B. Siddik, B. C. Das, L. Weiss, N. V. Dhurandhar and V. Hegde, Adipocyte, 2019, 8, 240–253 CrossRef CAS PubMed.
- (a) M. H. Tse, R.-L. Zhong and F. Y. Kwong, ACS Catal., 2022, 12, 3507–3515 CrossRef CAS; (b) Y. Zhang, J. Gao, W. Li, H. Lee, B. Z. Lu and C. H. Senanayake, J. Org. Chem., 2011, 76, 6394–6400 CrossRef CAS PubMed; (c) P. Lassalas, C. Berini, J.-B. E. Rouchet, J. Hédouin, F. Marsais, C. Schneider, C. Baudequin and C. Hoarau, Org. Biomol. Chem., 2018, 16, 526–530 RSC.
- (a) K. L. Billingsley, T. E. Barder and S. L. Buchwald, Angew. Chem., Int. Ed., 2007, 46, 5359–5363 CrossRef CAS PubMed; (b) C. Valente, S. Calimsiz, K. H. Hoi, D. Mallik, M. Sayah and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 3314–3332 CrossRef CAS PubMed.
- (a) J. Anguiano, T. P. Garner, M. Mahalingam, B. C. Das, E. Gavathiotis and A. M. Cuervo, Nat. Chem. Biol., 2013, 9, 374–382 CrossRef CAS PubMed; (b) B. C. Das, N. K. Nandwana, D. P. Ojha, S. Das and T. Evans, Tetrahedron Lett., 2022, 153657 CrossRef CAS PubMed.
- L. Kuehn, M. Huang, U. Radius and T. B. Marder, Org. Biomol. Chem., 2019, 17, 6601–6606 RSC.
- B. C. Das, X.-Y. Tang, P. Rogler and T. Evans, Tetrahedron Lett., 2012, 53, 3947–3950 CrossRef CAS PubMed.
- X. Hui, S. J. Baker, R. C. Wester, S. Barbadillo, A. K. Cashmore, V. Sanders, K. M. Hold, T. Akama, Y. K. Zhang and J. J. Plattner, J. Pharm. Sci., 2007, 96, 2622–2631 CrossRef CAS PubMed.
- D. M. Volochnyuk, A. O. Gorlova and O. O. Grygorenko, Chem.–Eur. J., 2021, 27, 15277–15326 CrossRef CAS PubMed.
- (a) W. Tang, S. Keshipeddy, Y. Zhang, X. Wei, J. Savoie, N. D. Patel, N. K. Yee and C. H. Senanayake, Org. Lett., 2011, 13, 1366–1369 CrossRef CAS PubMed; (b) E. G. Occhiato, F. Lo Galbo and A. Guarna, J. Org. Chem., 2005, 70, 7324–7330 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2ra05063c |
| This journal is © The Royal Society of Chemistry 2022 |