Open Access Article
Open Access ArticleDiamond-rich crystalline nanosheets seeded with a Langmuir monolayer of arachidic acid on water
Toshihiko Tanaka†
 *abcd,
Yasuhiro F. Miura†*b,
Tetsuya Aoyamacd,
Kazunori Miyamoto
*abcd,
Yasuhiro F. Miura†*b,
Tetsuya Aoyamacd,
Kazunori Miyamoto e,
Yoshiya Akagib,
Masanobu Uchiyamace and
Eiji Osawaf
e,
Yoshiya Akagib,
Masanobu Uchiyamace and
Eiji Osawaf
aChemistry and Biochemistry, Fukushima College, National Institute of Technology, 30 Azanagao, Tairakamiarakawa, Iwaki 970-8034, Fukushima, Japan. E-mail: 3116566701@jcom.home.ne.jp
bHamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu 431-3192, Shizuoka, Japan
cElements Chemistry Laboratory, RIKEN Cluster for Pioneering Research (CPR), 2-1 Hirosawa, Wako 351-0198, Saitama, Japan
dUltrahigh Precision Optics Technology Team, RIKEN Center for Advanced Photonics (RAP), 2-1 Hirosawa, Wako 351-0198, Saitama, Japan
eGraduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku 113-0033, Tokyo, Japan
fNano Carbon Research Institute Ltd, Asama Research Extension Centre, Shinshu University, 3-15-1 Tokita, Ueda 386-8567, Nagano, Japan
First published on 20th September 2022
Abstract
The enigmatic self-assembling ability of nanodiamond (ND) particles has been discovered herein. Diamond-rich crystalline nanosheets with thickness of approximately ∼25 nm were grown from a Langmuir monolayer of arachidic acid (AA) at the interface between air and a dilute aqueous ND solution. Their fine rectangular shapes with uniform uniaxial birefringence indicate appreciable crystallinity, thus supporting that they are hydrated colloidal crystals of homogeneous ND particles.
Introduction
Nanodiamond (ND) particles have attracted growing attention due to their promising potential applications,1–4 and their self-assembly5–10 from aqueous colloidal solutions is enigmatic because of the anisotropic precipitates produced. One of the authors (E. O.) reported the preparation of ND whiskers5 through the slow drying of his commercialized solution, NanoAmando®, and Huang et al.6 prepared fine microfibers from solutions of acid-treated ND particles. Petit et al. also reported an interesting cubic aggregate (1.7 × 1.7 mm; 0.8 mm thick) prepared from a colloidal solution of plasma-hydrogenated ND with HCl.8 Why are these materials so anisotropic? Why are the aggregates rectangular?We presume that the precipitates consist of similar ND particles in terms of both size and shape and that the particles can form a periodic crystal-like structure. If the particles differed in size and shape, the precipitates would not form, regardless of whether they were n-diamonds7 or doped diamonds.8 The concept of homogeneous ND particles was first predicted from DFT calculations11 and we call them elementary diamond nanoparticles (EDIANs). The precipitates also appear to consist of similar EDIANs, however so far it has not been possible to isolate them and to determine their detailed structure experimentally.
Here we demonstrate the preparation of rectangular crystalline nanosheets (∼25 nm thick) of ND from a monolayer of arachidic acid (AA: C19H39COOH) at the interface between air and a dilute ND solution. Such crystalline ND nanosheets have not come to our knowledge and their anisotropy suggests that they consist of EDIANs containing ordered water molecules. Alternating layers of ND and polyelectrolyte were reported, being neither rectangular nor crystalline.9
Experimental
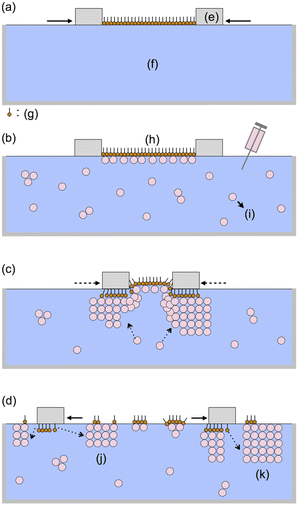
The general procedure for nanosheet formation was as follows. AA (>99%) was purchased from Fluka AG (St. Gallen, Switzerland) and was used as received without further purification. Ultrapure water (18 MΩ cm) was obtained using a filter system (Direct-Q UV 3 Remote, Merck Millipore) to remove ions, organic matter, and small particles, and was used for all experiments and all cleaning steps. Chloroform (spectrophotometric grade) was purchased from Dojin Chemicals.First, we prepared a Langmuir monolayer of AA on the water surface with a commercially available Langmuir–Blodgett trough (model 622, NIMA Instruments Ltd; area: 68.4 × 20 cm2) at 25 °C by moving together two polytetrafluoroethylene bars (21.2 × 1.6 × 0.6 cm3) as shown in Fig. 1a. The surface pressure was measured using a Wilhelmy-type balance. AA was spread on the water from a 10−3 M chloroform solution. After 5 min of air-drying, floating AA molecules were compressed to a surface pressure P of 35 mN m−1 at a rate of 0.73 cm2 s−1. The area per AA molecule SA was 0.23 nm2 immediately after compression, which is close to the reported value.13 Then, a dilute aqueous solution of ND (0.25 wt%, NanoAmando® Nanocarbon Research Institute, Inc.; diameter: 3.6 nm at 2.5 wt% by dynamic light scattering; ζ-potential: 61 mV) was injected into the subphase (1500 mL water), further diluting the ND to 0.005 wt% (Fig. 1b).
After the injection of ND (Fig. 1c), the SA value decreased remarkably while keeping the P value at 35 mN m−1 using the computer-controlled barriers; the compressed area SA decreased by 46% for 0.5 h. The computer-controlled surface pressure was then decreased to 10 mN m−1, and the further decrease in SA was modest: 10% in 16.7 h. Up to this point, no sheets were observed at any position in the trough. However, immediately after pulling the bars apart, we observed innumerable ultrathin rectangular nanosheets (Fig. 1d).
For microscopic measurements including polarized microsopy, atomic force microscopy (AFM) and microscopic Raman spectra, the nanosheets were transferred on glass or silicon substrates as follows. Prior to the formation of the nanosheets at the air/water interface, the substrates were immersed in the subphase and placed on the bottom of the trough. After the growth, the floating nanosheets were transferred on the substrates located at the bottom of the trough by natural lowering of the air/water interface, which takes a few days through air-dry. The sheets were so fragile that we avoided mechanical shocks in the slow transfer process.
Samples for Raman spectra were deposited on silicon substrates and the spectra were recorded with a Raman spectrometer (NRS-4500, JASCO) equipped with a microscope. The exciation beam (16.1 mW) focused on a spot of nearly 2 μm on each sample. The AA monolayer with ND (Fig. 1b) was also transferred onto a hexamethyldisilazane-modified silicon substrate utilizing horizontal lifting method (Langmuir–Schaefer method).14 For reference spectra, an air-dried drop of the ND aquaous colloidal solution (2.5 wt%) or that of AA the chloroform solution (10−3 M) was prepared on the substrates.
Results and discussion
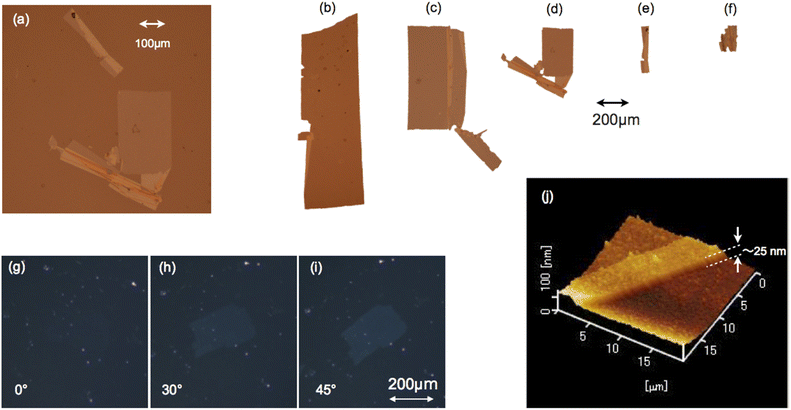
Most of the nanosheets were rectangular and their long sides typically ranged from approximately 0.2 to 10 mm as shown by micrographs of typical deposited sheets in Fig. 2a–f. Their shapes were clear in reflective images, and we found many sheets of various sizes. They were frequently folded or torn by unavoidable water flows while a part of the nanosheets was in contact with the substrate during deposition, creating defects such as voids and folds (Fig. 2a–f). These results indicate that the nanosheets had fine rectangular shapes on the water. Their typical thickness was 25 nm as observed by AFM (NanoNavi IIs/NanoCute, Seiko Instruments Inc.) in dynamic force mode (Fig. 2j).Each nanosheet was uniformly birefringent, indicating crystallinity with uniaxial optical anisotropy. Under crossed-Nicols of a polarizing microscope (Eclipse E600 POL, Nikon), a nanosheet was darkest with the long side parallel to one of the two polarization directions and became brightest at when the long side was rotated laterally 45° with respect to these two directions (Fig. 2g–i). Because of the small thickness of the nanosheets, the brightness was too low to show clear contrast in printed materials; nevertheless, the contrast was clearly seen through careful observations.
ND particles are highly hygroscopic5 and the nanosheets on the water contain significant amount of water at the air–water interface. Hence, the EDIANs should organize into a kind of hydrated colloidal crystal there.
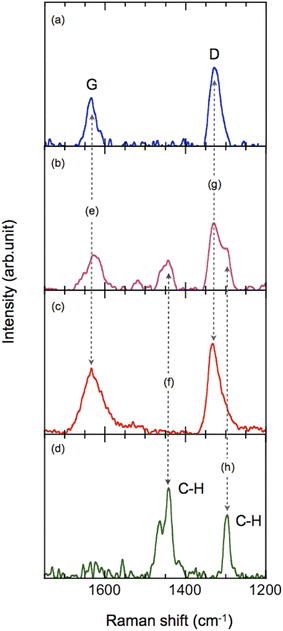
Raman spectra showed that, after injection, the ND particles adsorbed onto the AA monolayer. The deposited monolayer (Fig. 3b) showed the D and G bands15–21 of ND (Fig. 3e and g) together with the CH stretching mode of AA (Fig. 3f and h).
The rectangular nanosheets consist of ND particles. The D and G bands of ND were observed whereas the CH stretching mode of AA was not observed (Fig. 3a). The thickness of 25 nm indicates that the nanosheets contain at least 4–7 layers of EDIANs. Presumably the crystals also contain tiny amounts of AA molecules because they formed from ND adsorbed on the AA monolayer, but we consider that most parts of the nanosheets were not covered with AA as illustrated in Fig. 1k. Hence, they are diamond-rich nanosheets.
The Raman spectra of ND (Fig. 3a–c) were successfully obtained using the fluorescence subtraction method with a laser (532 nm) as the excitation source. The ND particles having a diameter of less than 4 nm possibly contributed this success. The fluorescence should decrease with decreasing ND particle size because of the significant structural deformation recently confirmed by high-resolution transmission electron microscopy (TEM)22 and should affect nitrogen-vacancy centers.23–27
Most of the nanosheets were found near the PTFE bars and thus crystallization of ND took place during or after the decrease in SA upon contact with the PTFE bars, thus yielding the nanosheets along the soaked fringe of the bars (Fig. 1d). Without AA molecules, a dilute ND solution (0.005 wt%) never generated any sheets at the air–water interface. Hence, the nanosheets are seeded by the AA monolayer. Polyanionic nanosheets of [(Ca2Nb3O10)−]n (CNO) can be obtained from dilute colloidal suspensions of CNO in a similar process involving crystallization of CNO after adsorption to a [+N(CH3)2(C18H37)2] (DOA) monolayer.28 There should be Coulombic attractive forces in both of these cases: between CNO anions and DOA cations and between AA− (C19H39COO−) and positively charged ND particles, as indicated by the positive ζ-potential of NanoAmand®. Most of the AA molecules should be dissociated in the subphase (pH ≈ 6).
The details of this self-organized structure remain an enigmatic open question. In excellent TEM images,22 ND particles are usually so different that they cannot form crystals. Why are they crystallized?
We do not presume that the ND particles in the nanosheets are as different as those in the images. It should be noted that homogeneous EDIANs are likely to be purified through crystallization. If NanoAmando® contains the EDIANs appreciably, they may be crystallized. Another consideration must be the possible deformation due to the electron beam. Although the use of a low voltage (80 keV) with aberration correction successfully minimized the deformation,22 we can not exclude its possibility entirely.
After hydrated colloidal crystals are deposited on solid substrates, their crystalline order is expected to be decreased significantlly by drying. After losing water molecules, the hydrated colloidal crystals must be broken. Small grains can be seen on their surface in Fig. 2j and therefore were formed by aggregation during drying. Perhaps the crystals do not lose all their water molecules and keep some of them depending on ambient humidity and/or temperature. Heat treatment will also remove water molecules from the nanosheets and the focused laser light may cause such heating in the Raman measurements. It should be noted therefore that we measured the nanosheets after such drying.
The potentially organized structure of the ND nanosheets and their small thickness also suggest various applications, particularly on surfaces, as lubricants,29–31 catalysts32–36 chemical vapor deposition seeds,37–39 and drug delivery films.12,40–43 In these applications, does the organized structure have any effect? That will be fascinating.
Conclusions
Rectangular crystalline nanosheets (∼25 nm thick) of ND can be prepared from an AA monolayer on a water subphase containing ND particles in the following two steps: (1) adsorb ND particles onto the AA monolayer and (2) crystallize the ND particles by compressing the AA monolayer horizontally. The nanosheets are hydrated colloidal crystals of ND particles.We believe that the nanosheets are the hydrated colloidal crystals of homogeneous EDIANs. Otherwise, there is not a plausible explanation for their rectangular shapes, ultra thin thickness, and uniform birefringence. To determine their crystal structure, further analysis under wet conditions is expected and we are now proceeding with in situ X-ray diffraction measurements44 of the ND nanosheets on water. The hydrated colloidal crystals on water may keep their water molecules. However, the crystals move easily and are hardly fixed for the measurements. Although a long time will be required to complete the measurements, it could be the rediscovery of ND as a novel chemical species.
Author contributions
Toshihiko Tanaka: writing-original draft, methodology, nanosheet formation, conceptualization and supervision; Yasuhiro F. Miura: methodology, nanosheet formation, conceptualization and supervision; Tetsuyta Aoyama: microscopic analysis; Kazunori Miyamoto: Raman Analysis; Masanobu Uchiyama: Raman analysis; Yoshia Akagi: nanosheet formation; and Eiji Osawa: ND solutions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to acknowledge the following five students of Fukushima College for their excellent contribution on the engineering for Langmuir films: Mr Masamichi Hoshi, Mr Hyuga Kaneko, Mr Takumi Sato, Mr Nagito Haga, and Mr Daiki Ayai. This work was partly supported by JSPS KAKENHI grant number JP21K04813.References
- E. Osawa, et al., Nanodiamonds: Applications in Biology and Nanoscale Medicine, ed. D. Ho, Springer Science+Business Media, Inc., Norwell, MA, 2010 Search PubMed.
- A. Vul', et al., Detonation Nanodiamonds: Science and Applications, ed. Alexander Vul' and Olga Shenderova, CRC Press, FL, 2013 Search PubMed.
- A. S. Barnard, et al., Nanodiamond, RSC Nanoscience & Technology Book No. 31, ed. Oliver A Williams, The Royal Society of Chemistry, London, 2014 Search PubMed.
- C. E. Nebel, et al., Nanodiamonds: Advanced Material Analysis, Properties and Applications, ed. Jean-Charles Arnault, Elsevier, 2017 Search PubMed.
- E. Osawa, Diamond Relat. Mater., 2007, 16, 2018 CrossRef CAS.
- H. Huang, L. Dai, D. H. Wang, L.-S. Tanc and E. Osawa, J. Mater. Chem., 2008, 18, 1347 RSC.
- M. L. Terranova, D. Manno, M. Rossi, A. Serra, E. Filippo, S. Orlanducci and E. Tamburri, Cryst. Growth Des., 2009, 9, 1245–1249 CrossRef CAS.
- T. Petit, H. A. Girard, A. Trouvé, I. Batonneau-Gener, P. Bergonzoa and J.-C. Arnault, Nanoscale, 2013, 5, 8958 RSC.
- T. Yoshikawa, V. Zuerbig, F. Gao, R. Hoffmann, C. E Nebel, O. Ambacher and V. Lebedev, Langmuir, 2015, 31, 5319 CrossRef CAS PubMed.
- S. S. Batsanov, D. L. Guriev, S. M. Gavrilkin, K. A. Hamilton, K. Lindsey, B. G. Mendis, H. J. Riggs and A. S. Batsanov, Mater. Chem. Phys., 2016, 173, 325 CrossRef CAS.
- A. S. Barnard, J. Mater. Chem., 2008, 18, 4038 RSC.
- H. Huang, E. Pierstorff, Eiji Osawa and D. Ho, ACS Nano, 2008, 2, 203 CrossRef CAS.
- M. A. Valdes-Covarrubias, R. D. Cadena-Nara, E. Vásquez-Martínez, D. Valdez-Péres and J. Ruiz-García, J. Phys.: Condens. Matter, 2004, 16, S2097 CrossRef CAS.
- A. Ulman, An Introduction to Ultrathin Organic Films: from Langmuir-Blodgett to Self-Assembly, Academic Press, San Diego, CA, 1991, p. 127 Search PubMed.
- A. C. Ferrari and J. Robertson, Philos. Trans. R. Soc. London,Ser. A, 2004, 362, 2477 CrossRef CAS PubMed.
- B. V. Spitsyn, L. Davidson, M. N. Gradoboev, T. B. Galushko, N. V. Serebryakov, T. A. Karpukhina, I. I. Kulakovac and N. N. Melnik, Diamond Relat. Mater., 2006, 15, 296 CrossRef CAS.
- V. Mochalin, S. Osswald and Y. Gogotsi, Chem. Mater., 2009, 21, 273 CrossRef CAS.
- S. Osswald, V. N. Mochalin, M. Havel, G. Yushin and Y. Gogotsi, Phy. Rev. B, 2009, 80, 075419 CrossRef.
- V. I. Korepanov, H. Witek, H. Okajima, E. Osawa and H. Hamaguchi, J.Chem.Phys., 2014, 140, 041107 CrossRef PubMed.
- M. Popov, V. Churkin, A. Kirichenko, V. Denisov, D. Ovsyannikov, B. Kulnitskiy, I. Perezhogin, V. Aksenenkov and V. Blank, Nanoscale Res. Lett., 2017, 12:561, 1 Search PubMed.
- M. Mermoux, L.-Y. Chang, H. A. Girardd and J.-C. Arnault, Diamond Relat. Mater., 2018, 87, 248 CrossRef CAS.
- S. L. Y. Chang, C. Dwyer, E. Osawa and A. S. Barnard, Nanoscale Horiz., 2018, 3, 213 RSC.
- A. Gruber, A. Drabenstedt, C. Tietz, L. Fleury, J. Wrachtrup and C. von Borczyskowski, Science, 1997, 276, 2012 CrossRef CAS.
- M. W. Doherty, N. B. Manson, P. Delaney, F. Jelezko, J. Wrachtrupe and L. C. L. Hollenberg, Phys. Rep., 2013, 528, 1 CrossRef CAS.
- S.-J. Yu, M.-W. Kang, H.-C. Chang, K.-M. Chen and Y.-C. Yu, J. Am. Chem. Soc., 2005, 127, 17604 CrossRef CAS PubMed.
- R. Igarashi, Y. Yoshinari, H. Yokota, T. Sugi, F. Sugihara, K. Ikeda, H. Sumiya, S. Tsuji, I. Mori, H. Tochio, Y. Harada and M. Shirakawa, Nano Lett., 2012, 12, 5726 CrossRef CAS PubMed.
- S. Sotoma, D. Terada, T. F. Segawa, R. Igarashi, Y. Harada and M. Shirakawa, Sci. Rep., 2018, 8, 5463 CrossRef PubMed.
- K. Ikegami, H. Tetsuka, Y. Hoshi, T. Ebina and H. Takashima, Langmuir, 2010, 26, 2514 CrossRef CAS PubMed.
- J.-Y. Lee and D.-S. Lim, Surf. Coat. Technol., 2004, 188–189, 534 CrossRef CAS.
- A. V. Sumant, D. S. Grierson, J. E. Gerbi 2, J. A. Carlisle, O. Auciello and R. W. Carpick, Phys.Rev. B, 2007, 76, 235429 CrossRef.
- O. Elomaa, T. J. Hakala, V. Myllymäki, J. Oksanen, H. Ronkainen, V. K. Singh and J. Koskinen, Diamond Relat. Mater., 2013, 34, 89 CrossRef CAS.
- W. W. Zheng, Y. H. Hsieh, Y. C. Chiu, S. J. Cai, C. L. Cheng and C. Chen, J.Mat.Chem., 2009, 19, 8432 RSC.
- J. Zhang, D. S. Su, R. Blume, R. Schlog, R. Wang, X. Yang and A. Gajovic, Angew. Chem., Int. Ed., 2010, 49, 8640 CrossRef CAS PubMed.
- Y. Liu, S. Chen, X. Quan, H. Yu, H. Zhao, Y. Zhang and G. Chen, J. Phys. Chem. C, 2013, 117, 14992 CrossRef CAS.
- A. Ahmed, S. Mandal, L. Gines, O. A. Williams and C.-L. Cheng, Carbon, 2016, 110, 438 CrossRef CAS.
- Y. Ding, X. Huang, X. Yi, Y. Qiao, X. Sun, A. Zheng and D. S. Su, Angew. Chem., Int. Ed., 2018, 57, 13800 CrossRef CAS PubMed.
- O. A. Williams, O. Douhéret, M. Daenen, K. Haenen, E. Osawa and M. Takahashi, Chem. Phys. Lett., 2007, 445, 255 CrossRef CAS.
- N. Yang, H. Uetsuka, E. Osawa and C. E. Nebel, Nano Lett., 2008, 8, 3572 CrossRef CAS PubMed.
- E. L. H. Thomas, S. Mandal, A. Ahmed, J. E. Macdonald, T. G. Dane, J. Rawle, C.-L. Cheng and O. A. Williams, ACS Omega, 2017, 2, 6715 CrossRef CAS PubMed.
- H. Huang, E. Pierstorff, E. Osawa and D. Ho, Nano Lett., 2007, 7, 3305 CrossRef CAS PubMed.
- R. Lam, M. Chen, E. Pierstorff, H. Huang, E. Osawa and D. Ho, ACS Nano, 2008, 2, 2095 CrossRef CAS.
- H. Huang, M. Chen, P. Bruno, R. Lam, E. Robinson, D. Gruen and D. Ho, J. Phys. Chem. B, 2009, 113, 2967 CrossRef PubMed.
- X. Li, J. Shao, Y. Qin, C. Shao, T. Zheng and L. Ye, J. Mater. Chem., 2011, 21, 7966 RSC.
- K. Kjaer, J. Als-Nielsen, C. A. Helm, P. Tippman-Krayer and H. Möhwald, J. Phys. Chem., 1989, 93, 3200 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |