Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Retracted Article: Cefotaxime incorporated bimetallic silver-selenium nanoparticles: promising antimicrobial synergism, antibiofilm activity, and bacterial membrane leakage reaction mechanism†
Abdelrahman A. Elakraaab,
Salem S. Salem *a,
Gharieb S. El-Sayyad
*a,
Gharieb S. El-Sayyad *cd and
Mohamed S. Attiaa
*cd and
Mohamed S. Attiaa
aBotany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo 11884, Egypt. E-mail: salemsalahsalem@azhar.edu.eg
bChemical Industries Department, Industrial Control Authority, Cairo, Egypt
cDepartment of Microbiology and Immunology, Faculty of Pharmacy, Galala University, New Galala City, Suez, Egypt. E-mail: Gharieb.Elsayyad@gu.edu.eg; Gharieb.S.Elsayyad@eaea.org.eg
dDrug Microbiology Lab., Drug Radiation Research Department, National Center for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority (EAEA), Cairo, Egypt
First published on 20th September 2022
Abstract
In this research, we reported for the first time the simple incorporation of antibiotic cefotaxime (CFM) with the synthesized Ag NPs, Se NPs, and bimetallic Ag–Se NPs by gamma rays, as a promising cost-effective, and eco-friendly method. The synthesized nanocomposites were characterized by UV-Vis. spectroscopy, XRD, EDX, HR-TEM, SEM/mapping, and EDX studies. The antimicrobial synergistic potential was investigated after CFM drug incorporation. Antibiofilm activity, growth curve assay, and effect of UV illumination were examined against some pathogenic microbes. The antibacterial reaction mechanism was evaluated by protein leakage assay and SEM imaging. HRTEM imaging confirmed the spherical shape and an average diameter of 10.95, 20.54, and 12.69 nm for Ag NPs, Se NPs, and Ag–Se NPs, respectively. Ag NPs-CFM, Se NPs-CFM, and Ag–Se NPs-CFM possessed antimicrobial activity against Staphylococcus aureus (40, 42, and 43 mm ZOI, respectively), Escherichia coli (33, 35, and 34 mm ZOI, respectively) and Candida albicans (25, 22, and 23 mm ZOI, respectively). CFM-incorporated Ag–Se NPs were able to inhibit biofilm formation of S. aureus (96.09%), E. coli (98.32%), and C. albicans (95.93%). Based on the promising results, the synthesized nanocomposites showed superior antimicrobial potential at low concentrations and continued-phase durability; they may find use in pharmaceutical, and biomedical applications.
Introduction
The nanotechnology revolution has resulted in incredible advancements in the production of smart nano-materials for application in medicine, particularly therapeutic drugs.1–4 Bimetallic NPs are made up of two metal nanoparticles that have different structures.5 Bimetallic nanoparticles (NPs) feature a unique mixing pattern and geometrical structure that improves their usefulness.6,7 Certain chemical transformations are possible using as catalysts that are not possible with monometallic NPs.8 This is due to the fact that bimetallic NPs have a specific combination of two metals, each of which serves a specific potential function in the overall reaction process.9 Bimetallic NPs have received much attention because of their distinctive chemical, physical and physiological features, resulting from the synergistic impacts of incorporating various metallic components together.10 Among several bimetallic NPs, silver-gold nanoparticles (Ag–Au NPs) alloys have been widely investigated due to their structure-dependent tunable Plasmonic characteristics and promising applications in sensor operations and catalysis applications.11 For the synthesis of Ag–Au NPs, many preparation routes are available including the reduction of HAuCl4 and AgNO3 precursors at the same time in the presence of gamma-rays and Gum Arabic as an extremely-powerful stabilizing agent.The preparation and characterization of many bimetallic NPs have been widely investigated over the past decades, not only due to their novel features, but also due to their multiple uses in optoelectronics, biosensors, bio-imaging, catalysis, antimicrobial and different biomedical applications.12
Bimetallic NPs have piqued researchers' attention in pathogen-fighting applications due to their unique characteristics, which are based on novel physicochemical qualities that play a role in their numerous uses.13 Previous studies have reported that NPs can be used as an alternative to antibiotics in the treatment of pathogenic microorganisms, whether fungal or bacterial.14–17 Silver (Ag) NPs showed remarkable activity against the pathogens of Urinary Tract Infection (UTI) represented by E. coli, S. aureus, and Pseudomonas aeruginosa.18 The size and geometry of Ag NPs determine their interactions with microbes in most cases.19,20 In a recent study, selenium (Se) NPs were shown to be effective against pathogenic bacteria in general, as well as UTI pathogens.14 The discovery of new efficient and strong antimicrobial medicines is urgently needed due to the significant tolerance that urethral bacteria have evolved to antibiotics.
UTI is one of the most serious and prevalent forms of infection in humans, with around 150 million individuals afflicted each year all over the world.21 Individuals afflicted with this UTIs are treated at a cost of around $6 billion, which is a global health and economic burden due to the spread of infectious epidemics.22 Through hospitals and the community, it is easy to get UTI from infected people and it is diagnosed through the appearance of pus cells in large quantities that are produced as a result of the presence of microbial pathogens (Fungi and bacteria) capable of invading the tissues in the urinary tract cells,23 Gram-negative E. coli is the most common cause of UTI.24 There are also important pathogens that cause UTI, which are Enterococcus faecalis, S. aureus, P. aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, and Candida sp.25 The microbial pathogens of UTI have become more resistant to many antibiotics due to the wrong use in treatment, as well as the development and virulence of the pathogen cells.26 Furthermore, pathogenic bacterial cells have the ability to generate biofilms that protect them from therapeutic medications.27 Therefore, it has become necessary for those interested in scientific research and scientists to devise modern strategies capable of eliminating the pathogens of UTI. Cefotaxime (CFM) is one antibiotic of oxyimino-cephalosporins. In light of this, they were the “most significant antibacterial” for human medicine. As a member of its third generation, it belongs to the beta-lactam family of cephalosporins. It is more effective than first- and second-generation cephalosporins in destroying both gram-negative and gram-positive bacteria and has broad-spectrum action.28 Although a number of antibiotics are generally available for the treatment of both gram-negative and gram-positive bacteria, their widespread usage has resulted in the development of resistance in both pathogens; drug resistance represents a serious concern in public health globally.29
For instance, it has been claimed that bacteria have developed a unique version of the β-lactamase enzyme, which confers antibiotic resistance with a β-lactam ring, such CFM. Two crucial methods for combating bacterial resistance include altering the -lactam constituent of antibiotics and producing novel antibiotics with greater potency. However, these methods take a lot of time, and they generally are not profitable.30 As a feasible alternative to the aforementioned issues brought up by bacterial resistance, nano-strategies for battling drug–resistant microorganisms have evolved.1,31
In this study, we reported for the first time a simple incorporation of CFM antibiotic with the synthesized Ag NPs, Se NPs, and bimetallic Ag–Se NPs by gamma rays which used as a promising cost-effective and eco-friendly method. Optimization was implemented to obtain clear and superior results about purity, morphology, size, stability, and crystallinity of the synthesized NPs. The incorporation with CFM was performed to test the synergistic effect against selected pathogenic microbes at low concentrations to avoid toxicity. Additionally, examination of the antibiofilm capability of the prepared NPs against various pathogenic bacteria and yeasts were studied. The scientific soundness of this research concerns the promising antimicrobial activity at low concentrations against pathogenic microbes resistant to CFM which advanced their property for the possible application after synergistic incorporation in long-run purposes.
Materials and methods
Chemicals and reagents
Analytical grade chemicals such as Na2SeO3 (Sigma Aldrich, UK), and Ag NO3 (Sigma Aldrich, UK), were utilized for NPs synthesis. On the other hand, media used in microbiological tests were purchased from Oxoid, UK.Gamma radiation
Gamma irradiation as an eco-friendly method32–34 were carried out in the NCRRT, Cairo, Egypt. The employed radiation source was 60Co-gamma chamber 4000 A-India, and the samples were gamma-irradiated in a solution form; after dissolving the starting precursors at a radiation time was determined to be as 1.236 kGy hour−1 (dose rate).Synthesis of Ag NPs, Se NPs and bimetallic Ag–Se NPs
Ag NPs, Se NPs and bimetallic Ag–Se NPs were synthesized in the presence of gamma-rays (as a direct reducing agent). Gamma-rays provoked reduction of metal ions due to the liberated reducing electrons, (e−aq), in aqueous solutions which can be considered as direct reduction.For the synthesis of Ag NPs, Se NPs, and bimetallic Ag–Se NPs, several solution samples of (1.0 mM) Ag NO3 were prepared; similarly, for Se NPs synthesis, several solution samples of (1.0 mM) Na2SeO3 were prepared. While, for the bimetallic Ag–Se NPs synthesis, several solution samples of (1.0 mM) Ag NO3 and (1.0 mM) Na2SeO3, were mixed together. Before gamm-irradiation, all samples were checked for their pH and adjusted to be neutral (pH = 7). Then, solutions were gamma-irradiated with different doses such as 0, 1.0, 3.0, 5.0, 10.0, 15.0, 20.0, and 25.0 kGy.
The aim was to specify the most effective dose, and this is carried out by recording the optical density (O.D.) of the formed NPs at a specific and fixed wavelengths (406 nm for Ag NPs, 518 nm for Se NPs, and 420 nm for bimetallic Ag–Se NPs) using UV-Vis. spectrum measurements. For the antibiotic (CFM) incorporation, the prepared solutions were mixed with CFM powder. The conditions had adjusted to obtain the best antibiotic incorporation like incubation temperature 30 °C, reaction time 24 h under agitation at 150 rpm in shaking incubator.
Characterization of the synthesized NPs
The absorbance and the optical characteristics of the synthesized Ag NPs, Se NPs, and bimetallic Ag–Se NPs were investigated via UV-Vis. spectrophotometer (JASCO V-560) at specific wavelengths. A sample without any metal ions was included for auto-zero purposes. All the sample were firstly-screening for the optical properties and to determine the fixed wavelengths that conducted for absorbance determination.Dynamic light scattering (DLS-PSS-NICOMP 380-ZLS particles sized system St. Barbara, California, USA) measurements were conducted to determine the average size distribution of the synthesized NPs. 200 μL of NPs samples carried to disposable cuvette. Following equilibration to a temperature of 25.0 ± 2 °C for 2.0 min, five measures were implemented.
In addition, high-resolution transmission electron microscope (HR-TEM, JEM2100, Jeol, Japan) was used to investigate the shape, appearance and the average particle size of the prepared NPs. Drop coating NPs samples produced HRTEM examinations onto carbon-coated TEM grids after drying by incubation at 37.0 ± 2 °C in an incubator.
While, X-ray diffraction analysis (XRD-6000, Shimadzu Scientific Instruments, Japan) was used to determine the crystalline nature and the crystal size of the synthesized NPs. It was adjusted with the XRD-6000 lists, including outstanding austenite quantitation, crystallinity estimation, stress examination, and crystallite size/lattice strain matters. The investigation of extended X-ray diffraction models was employed Cu-Kα target, and nickel filter. Working by a Cu anode at 50.0 mA and 40.0 kV in the state of 2θ value inside 20° and 100° with a flow of 2°/min.; the intensity of the diffracted X-rays estimated as a function of the diffracted angle 2θ.
Finally, SEM analysis (SEM, ZEISS, EVO-MA10, Germany) was used to investigate surface morphology, boundary size and the distribution of the synthesized NPs around CFM. To study the elemental composition, purity, and the distribution of elements founded in the synthesized NPs, EDX, BRUKER, Nano GmbH, D-12489, 410 M, Germany was conducted.
Antimicrobial activity (zone of inhibition (ZOI) assay)
Antimicrobial activity of the synthesized Ag NPs, Se NPs, and Ag Se nanocomposite (without and with cefotaxime (CFM) incorporation) was carried out using the agar well diffusion method towards six isolated microorganisms, including Gram-negative bacteria (E. coli, P. aeruginosa, and K. pneumoniae), Gram-positive bacteria (S. aureus, and Enterococcus sp.), and unicellular fungi (C. albicans).These microbial strains were selected based on their resistance to the antibiotic cefotaxime (CFM), as shown in Table S1.† The tested bacteria were inoculated on nutrient agar for one day at 37 °C, while fungal strains were inoculated on malt extract agar (MEA) plates then incubated for 3–5 days at 28 ± 2 °C; and then kept at 4 °C for further use.35
Additionally, the minimum inhibitory concentration (MIC) of the synthesized Ag NPs, Se NPs, and bimetallic Ag–Se NPs before and after incorporation with CFM was determined according to the microdilution method in agar diffusion. Different concentrations for each compound (10.0 to 0.625 μg mL−1) were performed to determine MIC. The results are statistically examined by using ONE WAY ANOVA, Duncan's multiple series, and the least significant difference (LSD) that are determined by specific software (SPSS version 15).
Antibiofilm potential
The antibiofilm potential of the synthesized Ag–Se NPs (without and with CFM incorporation; 10 μg mL−1) was assessed after conducting the assay of the test tube and tested against some selected pathogenic microbes (tested in ZOI assay), and the results were compared with the control non-treated samples, and finally the semi-qualitative assay regarding the microbial biofilm hindrance was conducted according to the method described by Christensen et al.36It must be noted that, as in ZOI assay, the inocula of the tested bacteria and unicellular fungi must be fixed according to 0.5 McFarland and adjusted at 2 × 108 CFU mL−1, and then incubated overnight at 37 °C before the antibiofilm assay. Firstly, the antibiofilm test stated after mixing about 0.5 mL of the liquid nutrient broth with the fixed microbes in the designed test tubes, and incubated overnight at 37 °C. Secondly, after incubation, all the treated and non-treated tubes were discarded, and all the investigated tubes were cleaned with phosphate buffer saline (PBS; pH 7.0), and finally, washed several times with deionized water.37
After that, the adhered microbial cells in the investigated tubes must be fixed with 3.5% sodium acetate (5 mL) for about 15 minutes and finally cleaned several times with deionized water. After that, the cleaned tubes with the fixed microbial biofilm must be stained with 0.15% crystal violet (CV; 5 mL) for about 15 minutes to estimate the semi-qualitative antibiofilm activity of the synthesized samples.38
Finally, to determine the semi-quantitative antibiofilm potential of the synthesized samples, the CV stained microbial cells were dissolved by the action of ethanol solution (5 mL), and the O.D. of the dissolver CV was tested and counted after applying the UV-Vis. spectrophotometry method at a fixed wavelength (570 nm),38 and the microbial biofilm inhibition % was estimated according to the subsequent eqn (1):37
| Biofilm inhibition % = [(O.D.Control sample–O.D.treated sample)/O.D.Control sample] × 100 | (1) |
Growth curve assay
The method of Huang et al.,39 was conducted to estimate the effect of the synthesized Ag–Se NPs (without and with CFM incorporation) on S. aureus kinetic growth curve. Before testing, the inoculum of the tested bacteria was adjusted by standardized 0.5 McFarland in 2 × 108 CFU mL−1, after that, the synthesized Ag–Se NPs (without and with CFM incorporation) mixed in the tested tube-containing the investigated bacteria. In the test protocol, the O.D. at fixed wavelength (600 nm) was measured after 2 hours for about 24 hours, and the final spectrum and relationship was conducted between the average of duplicate assignments and a time (hours) to get the standard growth curve,40 and assess the impact of the synthesized NPs samples on the kinetic growth of the tested S. aureus.Effect of UV-irradiation on the antimicrobial activity
The adjusted bacterial cells; according to standard 0.5 McFarland (2 × 108 CFU mL−1), were mixed with the synthesized bimetallic Ag–Se NPs (without and with CFM incorporation). Following the mixing, all tubes were UV-irradiated, and the antimicrobial potency of the UV-irradiated samples were tested against the most sensitive S. aureus through the method of the optical density,41 and compared with the control non-UV irradiated samples.The investigated tubes were classified into two conditions, tubes including non-UV irradiated the synthesized bimetallic Ag–Se NPs (without and with CFM incorporation), and tubes with the synthesized bimetallic Ag–Se NPs (without and with CFM incorporation) and UV-irradiated. They were exposures at various times (0, 15, 30, 45, 60, and 75 minutes). It must be noted that the treated samples were noted to form turbidity and were determined at fixed wavelength at 600 nm. The UV-lamp source was fixed on the tested samples at 37 °C (7.0 mW cm−2 disturbance). The method conducted Abd Elkodous et al.,42 was used to determine the inhibition % after using the eqn (1).
Protein leakage assay
To determine the estimated bacterial reaction mechanism of the synthesized samples, the assay of protein leakage was assessed. The bacterial inocula were fixed by standard 0.5 McFarland (2 × 108 CFU mL−1), and mixed with different concentrations of the synthesized bimetallic Ag–Se NPs (without and with CFM incorporation).The synthesized bimetallic Ag–Se NPs (without and with CFM incorporation)-free broth mixed with culture were used as the negative control. After incubation at 37 °C for about 5 hours, all the tested samples were separated by centrifugation operated for 20 minutes at 5000 rpm.43 After centrifugation, the separated supernatant (100 μL) for the examined samples were mixed with the solution of Bradford reagent (1 mL), and O.D. at fixed wavelength (595 nm) was measured in the dark after incubation for 10 minutes at 37 °C.43
SEM reaction mechanism
To prepare the bacterial samples for the SEM imaging, the cells of S. aureus must be cleaned several times with PBS, and subsequently fixed with the solution of glutaraldehyde (3.5%). The fixed S. aureus cells were again washed several times with PBS and finally mixed with the solution of ethanol for 30 minutes at room temperature and then air-dried. The fixed bacterial cells (S. aureus) were placed on the aluminum stump to begin the SEM imaging.44 The surface morphology and any changes in the treated and untreated bacterial cells were investigated with the SEM imaging.Results and discussions
Effect of gamma ray's dosage on Ag NPs, Se NPs and Ag–Se NPs synthesis and the optimal dose
Fig. 1 shows the gamma-ray screening by UV-Vis. spectrophotometry for the synthesized NPs. From Fig. 1(a), we can found that 15 kGy is the most effective dose for Ag NPs synthesis with the highest O.D. = 3.25 (diluted 2 times) at 406 nm. Similarly, Fig. 1(b) shows that 10 kGy is the best conducted dose for Se NPs synthesis with O.D. = 1.65 (diluted 2 times) at 518 nm. While, for the synthesis of bimetallic Ag–Se NPs, Fig. 1(c) elucidates that 15.0 kGy is the most potent dose with high O.D. = 3.65 (diluted 2 times) at 420.0 nm. In this case, the role of gamma-ray as a magic tool is noted for the production of uniform NPs with high relative yield and without the need of high temperature and any other reducing chemical agent.45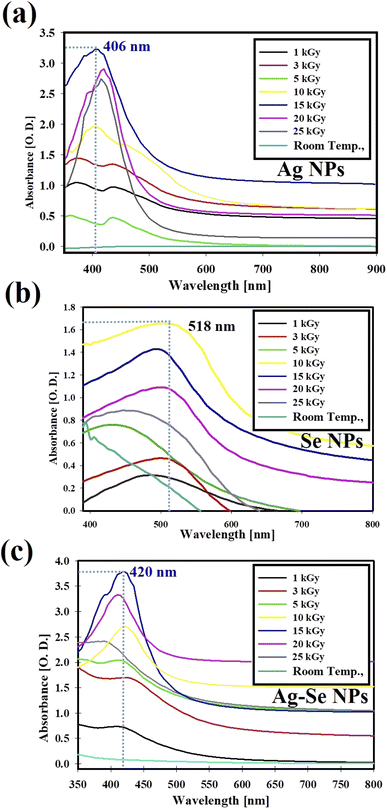 | ||
| Fig. 1 Gamma-rays screening by UV-Vis. spectrophotometry for the synthesis of Ag NPs (a), Se NPs (b), and bimetallic Ag–Se NPs (c). | ||
Fig. 1, explained that the maximum gamma-ray dose used for Ag NPs, and bimetallic Ag–Se NPs synthesis was 15 kGy and 10 kGy in the case of Se NPs. After increasing the dose more than 15 kGy (in the case of Ag NPs, and bimetallic Ag–Se NPs) and more than 10 kGy (in the case of Se NPs), the relative yield of the synthesized NPs had been decreased.
The excessive radiation is not beneficial for NPs synthesis because the excess free radicals and solvated electrons (produced from water radiolysis) may change the pH of the solutions, attack the formed NPs (opposite charges), interact with the formed NPs, and subsequent the aggregated NPs were formed which decrease their intensity in the UV-Vis. spectrum.46
Some literature reviews,47,48 explains that metal-based NPs were easily for the production, and exhibiting antimicrobial activity, and potentially applicable in various areas including food protection against some invading pathogenic microbes, additionally, they were utilized in tissue engineering.
Proposed synthetic reaction mechanism
Kinetics studies revealed that metal ions reduction to NPs, in aqueous media, always starts with gamma irradiation onset. In the present work, the reduction was most effective at 15.0, and 10 kGy, implying that gamma radiation has a vital role in Ag NPs, Se NPs, and Ag–Se NPs' synthesis as noted in Table 1.38| Reaction inputs | Condition | Products | Eqn |
|---|---|---|---|
| H2O | Radiolysis (γ-ray) | e−aq, OH˙, H˙, H2,and H2O2 | (2) |
| AgNO3 + H2O | Hydrolysis | Ag+ + NO3− | (3) |
| Na2SeO3 + H2O | SeO3−2 + 2Na+ | (4) | |
| Ag+ + e−aq | Reduction | Ag NPs | (5) |
| SeO3−2 + e−aq | Se NPs + 3O2 | (6) | |
| SeO3−2 +3 Ag+ | Complexation | Ag–Se NPs + 3O2 | (7) |
Assortment of species was formed in water upon exposure to gamma radiation; e−aq, OH˙, H˙, H2O2, and H2, eqn (2). The advantage of gamma irradiation for metallic NPs synthesis was the production of highly reducing electrons (e−aq), that perform their function without producing any useless byproducts.49 Production of different NPs started with dissolving Ag NO3 and Na2SeO3 giving the hydrated cations (Ag+ and Na+) and anions (SeO3−2 and NO3−), eqn (3) and (4) in Table 1.50
Afterward, there was a probability that both Ag+ and SeO3−2 are plainly reduced by e−aq forming non-capped Ag NPs and Se NPs which are likely to dissolve, i.e. they are not stable, eqn (5) and (6).51 In parallel, the probable reaction of Ag+ with SeO3−2 formed Ag–Se NPs complex, eqn (7) in Table 1.52
Electron excitation in the conduction zone outside Ag–Se NPs causes what is known as Surface Plasmon Resonance (SPR).53 The unique oscillation characteristics are dependent on the shape and size of the particles. It is worth mentioning that, upon excitation of inorganic NPs by a light source, fused combined flow is created by light electromagnetism across the free electrons specifically, conduction line-located electrons of Ag+ and/or Se+ ions.54
The overall reaction showed the role of electrons in reducing Ag+ and SeO3−2 ions for the growth of Ag–Se NPs. It should be noted that, by increasing the dose of gamma rays up to 15.0 kGy, the average particle size and particle size distribution of the prepared Ag NPs, Se NPs, and Ag–Se NPs was increased,46 and was attributed to the agglomeration and precipitation of the synthesized Ag NPs, Se NPs, and Ag–Se NPs under the effect of excess electrons and free radicals, formed during water radiolysis by gamma rays through the reaction as presented in eqn (2).55
Characterization of the synthesized NPs
By making a comparison in the literature regarding the average particle size and shape, it was found that the synthesized Ag, Se, and Ag–Se bimetallic NPs (in this study) were small in size and a major shape is spherical. Castro-Longoria et al.,56 synthesized silver, gold, and silver-gold bimetallic NPs by green method utilized the extract of a filamentous fungus, and the shape of NPs was found to be mainly spherical with an average diameter of 11.0 nm for silver and 32.0 nm for gold, when the fungus was exposed to the aqueous solutions of 10−3 M of AgNO3 and HAuCl4, respectively.
The produced shapes may be varied in that study,56 since the shape of extracted NPs was nearly spherical or ellipsoidal in all cases, although other morphology may be observed due to the synthetic process from extract so the anisotropic shape had been noted. In our study, a fixed shape is displayed due to a single reducing agent (gamma-rays) were applied. Finally, our results were related to the recently-published articles.57–60
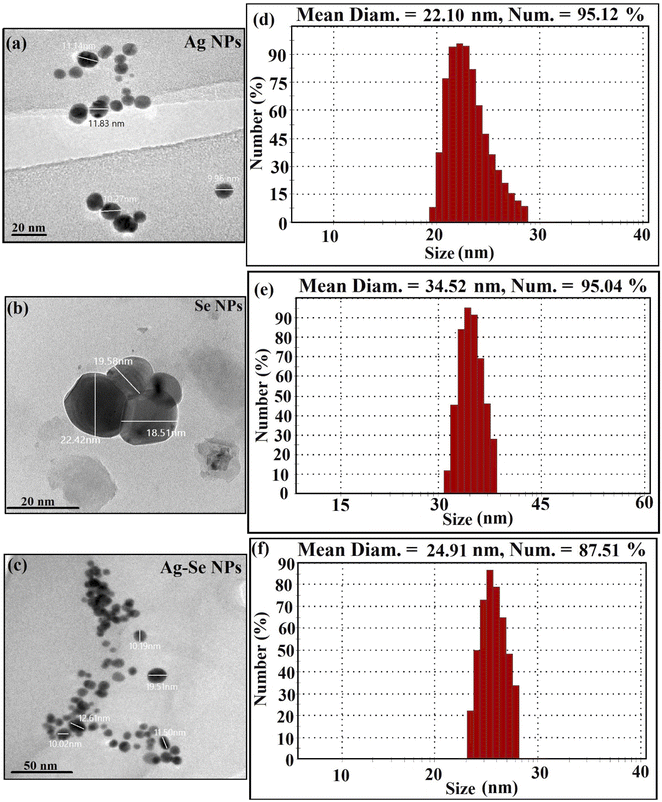
DLS analysis was conducted to evaluate particle size distribution and to calculate the average particle size that was found as 22.10 nm for Ag NPs synthesized by gamma-rays as displayed in Fig. 2(d). Also, it was found to be 34.52 nm for Se NPs (Fig. 2(e)) and 24.91 nm for bimetallic Ag–Se NPs (Fig. 2(f)).
Commonly, DLS size measurements become higher in values than HRTEM measurements, as DLS analysis is measured the hydrodynamic radius of NPs bounded by water molecules (solvent), resulting in larger particle sizes of the capped NPs, while HRTEM analysis is calculated the actual particle size of the material without the solvent layers.61
The present DLS data referred that all the synthesized NPs were distributed well, and the number % is more than 75% which suggested a uniform distribution of the same size according to each sample. For example, Ag NPs was distributed in 22.10 nm by 95.12%, Se NPs in 34.52 by 95.04%, and bimetallic Ag–Se NPs in 24.91 nm by 87.51%. The scientific soundness of DLS results in that the synthesized NPs were highly distributed in the narrow and the same size which in turn effectively-enhanced their properties and biomedical applications.62
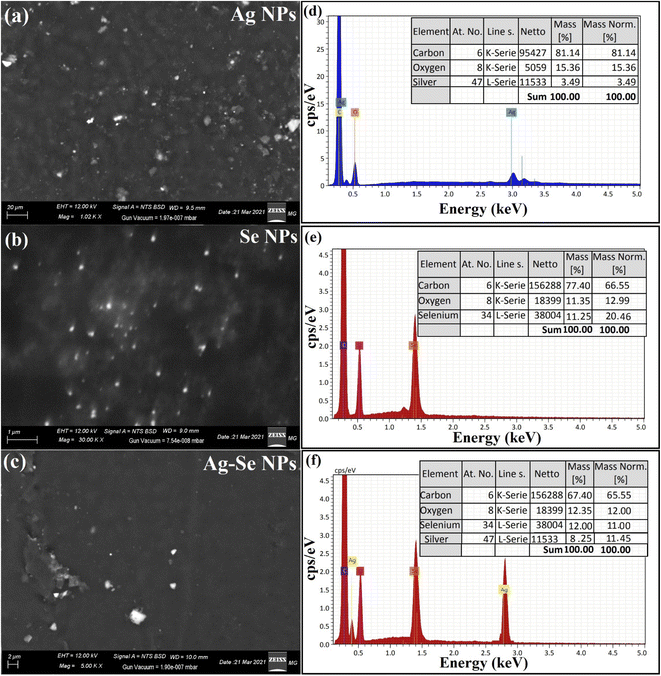
EDX study was used to establish the basic structure of the synthesized Ag NPs, Se NPs, and bimetallic Ag–Se NPs and the purity, as described in Fig. 3. Ag NPs exhibited the main absorption peaks of silver elements at 2.95 keV. The lack of other elemental peaks and a massive quantity of Ag in the spectra validate the Ag element purity, while O and C peaks were for the imaging holder as exhibited in Fig. 3(d).
Se NPs exhibited specific absorption peaks of selenium element at 1.40 keV. The lack of other elemental peaks and a massive quantity of selenium in the spectra validate the selenium element purity while O and C peaks were for the imaging holder as exhibited in Fig. 3(e), finally, bimetallic Ag–Se NPs exhibited specific absorption peaks of selenium, and silver element at 1.40, and 2.89 keV, respectively.
By making a comparison in the literature regarding the morphological shape and elemental analysis, it was founded that the synthesized Ag NPs, Se NPs, and bimetallic Ag–Se NPs (in this study) were uniformly distributed with narrow size and the same spherical shape.
Muhammad Mohsin et al.64 synthesized bimetallic silver and gold NPs by citrate reduction method at different temperatures and pH. The obtained morphological shape and boundary size indicated that they have possessed a size ranging from 50 to 65 nm and appear as spherical particles, so the temperature and pH plays a vital part in the production process. The elemental structure of Ag–Au bimetallic NPs was examined by EDX and compared with the recent publication,64 which proves that bimetallic NPs were formed of Ag and Au. The relative elemental percentage for Au was 55.98% and Ag was 44.02% at 25 °C, which was changed to Au (69.51%) and Ag (30.49%) at 100 °C, proving the efficient reduction at elevated temperatures. Finally, our results were related to the recently-published articles.65–68
Crystal structure and phase of the prepared NPs were analyzed using XRD analysis.69 XRD pattern of the synthesized NPs is presented in Fig. 5. It is clearly shown in the pattern that there are no characteristic peaks for the starting precursors (silver nitrate or sodium selenite). XRD results confirmed the formation of nano-complex (Ag–Se NPs). Fig. 5 presents XRD diffraction peaks of Ag NPs including peaks at 2θ = 38.49°, 44.09°, 64.90°, and 78.31° which matched with a standard card JCPDS-ICDD card 04-0783, and corresponding to (111), (200), (220), and (311) Bragg's reflections.70 Also, Fig. 5 shows XRD diffraction peaks of Se NPs, and displayed the diffraction characteristics regarding 2θ at 27.88°, 33.00°, 46.09°, 57.33°, 67.12°, 75.12°, and 84.84°, which represented the Bragg's reflections at (100), (101), (111), (201), (210), (113), and (301), respectively. All the peaks were similar to the Joint Committee on Powder Diffraction Standards (JCPDS) of Se NPs with a standard card JCPDS File no 06-0362.71
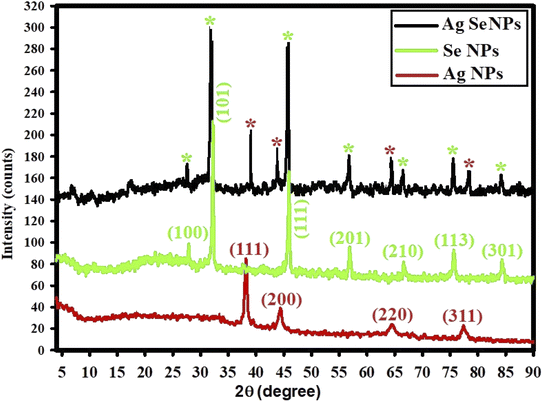 | ||
| Fig. 5 XED spectra for the synthesized Ag NPs, Se NPs, and bimetallic Ag–Se NPs; red stars for detected Ag NPs peaks, and green stars for detected Se NPs peaks. | ||
XRD data for the prepared bimetallic Ag–Se NPs show the diffraction characteristics at 2θ such as those recorded for both Ag NPs and Se NPs where red stars for detected peaks in Ag NPs, and green stars for detected peaks in Se NPs. This implies that the generated Ag–Se NPs were crystalline. It should be noted that a small 2θ shifting was detected which can be due to the development of bimetallic Ag–Se NPs.69
The absence of peaks at 2θ = 31.30°, 32.62°, and 33.68° indicated that the synthesized Ag NPs, Se NPs, and Ag–Se NPs were pure and free of AgO NPs and SeO NPs.72 The XRD results indicate that the produced NPs were highly-crystalline for better biomedical application.73
Ag NPs, Se NPs and bimetallic Ag–Se NPs stability
The stability of the synthesized Ag NPs, Se NPs and bimetallic Ag–Se NPs were tested after seven months, which stored in light place by conducting UV-Vis. spectrophotometry analysis. Fig. S1(a)† shows the stability of the synthesized Ag NPs. After conducting UV-Vis. optical identification, the results indicated that the stored Ag NPs have high-stability which indicated by the small change in their O.D. from 3.23 to 2.65 (diluted 2 times) after seven months, and the absorption peak was slightly-changed from 406 nm to 413 nm.Additionally, the stability of the prepared Se NPs is presented in Fig. S1(b)† which confirmed good-stability and the O.D. was slightly changed from 1.65 to 1.53 (diluted 2 times) after seven months, and the absorption peak was slightly-changed from 518 nm to 538 nm.
Also, Fig. S1(c)† shows the stability of the synthesized Ag–Se NPs and the results revealed that the stored Ag–Se NPs have unique-stability by O.D. slightly changed from 3.79 to 3.50 (diluted 2 times) after seven months, while the absorption peak was slightly-changed from 420 nm to 437 nm, these stabilities should be attributed to the intermolecular hydrogen bonding which connected the produced NPs with CFM.74,75 The slightly-decrease in O.D. was due to the slightly-aggregation of the synthesized NPs and therefore the size was slightly increased as proved by the change in wavelengths.
The scientific soundness of this results concerns the highest stability of the produced NPs for a long time due to the hydrogen bonding between CFM and the synthesized NPs which advanced their property for the possible application in long-run purposes such as in cosmetics, inhibiting the pathogenic microbes, and pharmaceutical fields.76–79
Antimicrobial activity
Agar well diffusion technique is used to investigate the antimicrobial activity of Ag NPs, Se NPs, bimetallic Ag–Se NPs, and after incorporation with antibiotic CFM as Ag NPs-CFM, Se NPs-CFM, and Ag–Se NPs-CFM against isolated clinical pathogenic microorganisms.The findings of the in vitro inhibition zones test show that Ag NPs-CFM, Se NPs-CFM, and Ag–Se NPs-CFM have antibacterial activity against S. aureus (40, 42 and 43 mm, respectively), E. coli (33, 35 and 34 mm, respectively) and P. aeruginosa (25, 30 and 28 mm, respectively) as are shown in Fig. 6. On the other hand, Ag NPs, Se NPs, and Ag–Se NPs exhibited less activity against S. aureus (12, 19, and 17 mm, respectively), E. coli (21, 22, and 20 mm, respectively), and P. aeruginosa (18, 9 and 20 mm, respectively) in Fig. 6 which explains the synergistic effect as reported in an earlier study.80
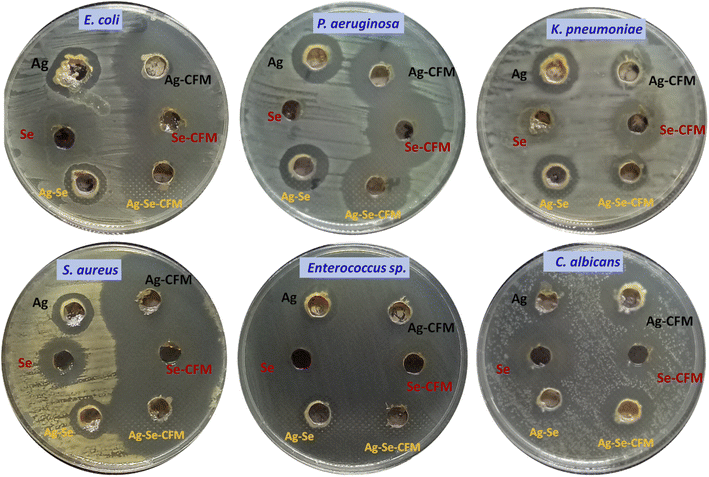 | ||
| Fig. 6 Antimicrobial activity of the synthesized Ag NPs, Se NPs, and bimetallic Ag–Se NPs before and after incorporation with CFM against some pathogenic microbes. | ||
While the nanostructures are efficient against both K. pneumoniae and Enterococcus sp. bacteria, the process of integration between the nanostructures and the antibiotic CFM is successful. It is obvious from this that the antibacterial activity of Ag NPs-CFM, Se NPs-CFM, and Ag–Se NPs-CFM enhanced after antibiotics were added to them, compared to their counterparts before antibiotics were added (Ag NPs, Se NPs, and Ag–Se NPs).
Furthermore, after loading CFM with Ag NPs, Se NPs, and Ag–Se NPs, the maximum antimicrobial activity is reported, and it is important to note that Ag NPs-CFM, Se NPs-CFM, and Ag–Se NPs-CFM nanostructure are more active against S. aureus (Gram-positive) bacteria than E. coli (Gram-negative) bacteria.
Gram-negative bacteria's cell walls are mostly made up of thin layers of peptidoglycan, lipopolysaccharide, and lipid. Gram-positive bacteria, on the other hand, have highly thick peptidoglycan structures in their cell walls. While Ag NPs, Se NPs, Ag–Se NPs, Ag NPs-CFM, Se NPs-CFM, and Ag–Se NPs-CFM (smart antifungal agent) nanostructures were shown to be active against C. albicans (single-celled fungus), producing 17, 25, 15, 25, 22, and 23 mm, respectively (Fig. 6). Finally, Fig. 7 explains the antimicrobial presentation regarding the smarting inhibitory effects of the synthesized nanostructure before and after incorporation with CFM.
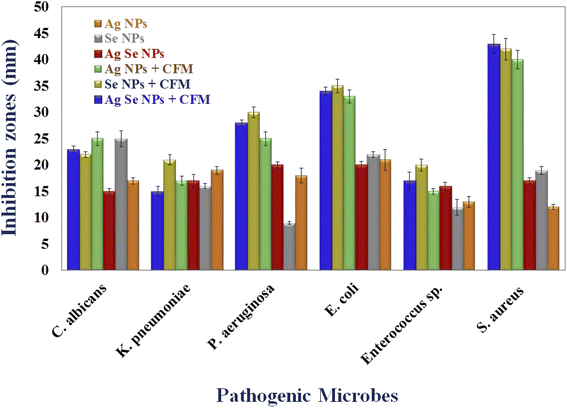 | ||
| Fig. 7 Antimicrobial activity of the synthesized Ag NPs, Se NPs, and bimetallic Ag–Se NPs before and after incorporation with CFM against some pathogenic microbes. | ||
According to our published article;80 we confirm that the interaction between CFM and the formed NPs is by the formation of intermolecular hydrogen bonding as indicated in the published articles,74,75 so, the chemical mechanism for loading CFM to the synthesized NPs and chemical reactions on the surface of NPs by the intermolecular hydrogen bonding.
Across the literature point of view, Halawani et al.,81 conducted the biogenic CFM-conjugated Ag NPs, and studding their effects as antibacterial and anticancer agents. The result indicating encourage enhancement in the antibacterial potential after CFM conjugation against multidrug-resistant bacteria.
The MIC results of Ag NPs, Se NPs, Ag–Se NPs, Ag NPs-CFM, Se NPs-CFM, and Ag–Se NPs-CFM against all the tested microbes are in the range from 5 μg mL−1 to 0.625 μg mL−1 and started with concentration 10 μg mL−1 as are presented in Table 2.
| Strains Tested samples | S. aureus | Enterococcus sp | E. coli | P. aeruginosa | K. pneumoniae | C. albicans |
|---|---|---|---|---|---|---|
| Ag NPs | 2.5 μg mL−1 | 2.5 μg mL−1 | 2.5 μg mL−1 | 2.5 μg mL−1 | 2.5 μg mL−1 | 2.5 μg mL−1 |
| Se NPs | 2.5 μg mL−1 | 2.5 μg mL−1 | 2.5 μg mL−1 | 5.0 μg mL−1 | 2.5 μg mL−1 | 2.5 μg mL−1 |
| Ag–Se NPs | 2.5 μg mL−1 | 2.5 μg mL−1 | 2.5 μg mL−1 | 2.5 μg mL−1 | 2.5 μg mL−1 | 2.5 μg mL−1 |
| Ag NPs + CFM | 0.625 μg mL−1 | 2.5 μg mL−1 | 1.25 μg mL−1 | 1.25 μg mL−1 | 2.5 μg mL−1 | 1.25 μg mL−1 |
| Se NPs + CFM | 0.625 μg mL−1 | 2.5 μg mL−1 | 1.25 μg mL−1 | 1.25 μg mL−1 | 2.5 μg mL−1 | 1.25 μg mL−1 |
| Ag–Se NPs + CFM | 0.625 μg mL−1 | 2.5 μg mL−1 | 1.25 μg mL−1 | 1.25 μg mL−1 | 2.5 μg mL−1 | 1.25 μg mL−1 |
Antibiofilm potential
Some specific pathogenic microorganisms are identified for the potential of biofilm creation as exopolysaccharide molecules.37 A tube method,82 was designed for the antibiofilm assessment of the synthesized CFM-incorporated Ag–Se NPs, and Ag–Se NPs.A negative result regarding biofilm repression of the tested S. aureus represented as air-liquid interface distinct whitish-yellow matt, and attached to the walls of designed tubes which staining with blue color after the treatment with CV. This noted negative results is exhibited in the absence of the synthesized CFM-incorporated Ag–Se NPs, and Ag–Se NPs which serve as control. In difference, a positive result regarding the inhibition impact of the synthesized samples represented a faint blue color or the absence of any CV color. Additionally, bacterial ring development was limited, and the antibiofilm results of the synthesized CFM-incorporated Ag–Se NPs more than the activity of Ag–Se NPs.
Semi-quantitative antibiofilm inhibition % is conducted by a method of UV-Vis. spectroscopy at fixed wavelength (570.0 nm). Detailed method is started after dissolving the stained microbial matt with ethanol and the O.D. is assessed to calculate the inhibition % by eqn (1). The inhibition % is calculated and represented in Table 3 for the synthesized CFM-incorporated Ag–Se NPs, and Ag–Se NPs. The most elevated inhibition of CFM-incorporated Ag–Se NPs (10 μg mL−1) percentage was inhibited for S. aureus is 96.09%, for E. coli is 98.32%, and for C. albicans is 95.93% as described in Table 3 and Fig. 8.
| Test organism | O.D. of crystal violet stain at 570.0 nm | Inhibition % | |||
|---|---|---|---|---|---|
| Control | Treated with Ag–Se NPs | Treated with CFM-incorporated Ag–Se NPs | Ag–Se NPs | CFM-incorporated Ag–Se NPs | |
| a Values are means ± SD (n = 3). Data within the groups are analyzed using one-way analysis of variance (ANOVA) followed by a, b, c, d, e, f Duncan's multiple range test (DMRT). | |||||
| Escherichia coli | 0.912b ± 0.0080 | 0.125cd ± 0.0021 | 0.049 cd ± 0.0021 | 86.29 | 94.62 |
| Pseudomonas aeruginosa | 0.941a ± 0.0062 | 0.222ab ± 0.0047 | 0.109b ± 0.0047 | 76.40 | 88.41 |
| Klebsiella pneumoniae | 0.780c ± 0.0070 | 0.127d ± 0.0053 | 0.059c ± 0.0053 | 83.71 | 92.43 |
| Staphylococcus aureus | 0.715de ± 0.0025 | 0.058f ± 0.0062 | 0.012f ± 0.0062 | 91.88 | 98.32 |
| Enterococcus sp. | 0.656f ± 0.0046 | 0.109e ± 0.0036 | 0.041e ± 0.0036 | 83.38 | 93.75 |
| Candida albicans | 0.777d ± 0.0046 | 0.200b ± 0.0036 | 0.187a ± 0.0036 | 74.93 | 75.93 |
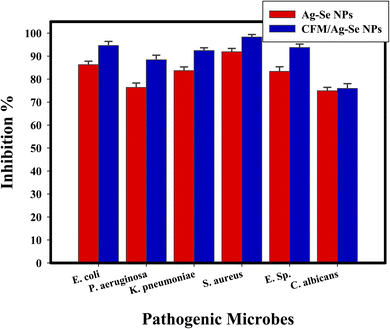 | ||
| Fig. 8 Antibiofilm efficiency CFM-incorporated Ag–Se NPs, and Ag–Se NPs against different pathogenic microbes as inhibition %. | ||
The synthesized CFM-incorporated Ag–Se NPs inhibits biofilm structure at its irreversible adhesion phase.82 However, the automatic action of the synthesized CFM-incorporated Ag–Se NPs upon biofilm structure has yet to be approved. The understanding of the inhibitory % might be determined by numerous factors, such as antimicrobial activity, physicochemical property, penetration abilities, and other chemical outcomes about the charge connection, CFM incorporation, and CFM synergistic effect.83 It was noticed that the synthesized CFM-incorporated Ag–Se NPs inhibited microbial growth and biofilm production. After controlling the exopolysaccharide secretion, the inhibition of the biofilm creation may be positively occurred.37,84,85
Fig. 8 presents a review diagram concerning the antibiofilm activity of CFM-incorporated Ag–Se NPs, and Ag–Se NPs (as inhibition %) toward some pathogenic microbes.
It must be noted that, in our recently published paper,46 Gum Arabic polymer is used only as a stabilizing agent, and in the present paper, the cefotaxime (CFM) drug is not used as a stabilizing agent but used for incorporation with the synthesized bimetallic NPs (nano-drug) to determine the synergistic effect and to assess the effect of NPs incorporation on the antimicrobial effect of CFM against the tested microbes. Additionally, we are selected CFM after making an antibiotic sensitivity test (ESI Table 1†), and we noted that CFM has not any antimicrobial activity, but after the incorporation with the synthesized NPs we noted the synergistic effect, and the tested microbes become more sensitive.
Growth curve assay (kinetic study)
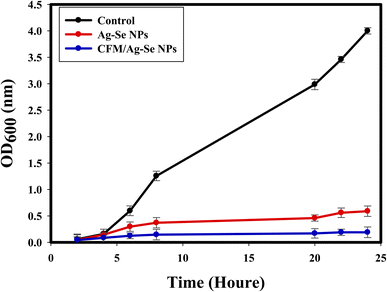
The kinetic study regarding the effect of the synthesized CFM-incorporated Ag–Se NPs, and Ag–Se NPs on the growth and multiplication of the tested S. aureus is exhibited in Fig. 9. The growth kinetic of the non-treated control S. aureus occurred normally, and measured O.D. value at specific wavelength (600 nm) reached 4.00 nm. While, Ag–Se NPs produced a noted differences with the control sample, and the O.D. was measured to be 0.589 nm. Positively, after CFM-incorporated Ag–Se NPs addition, a notable influence on the kinetic growth curve was detected, and the measured O.D. was noted at 0.190 nm, indicating the promising inhibition effect on S. aureus growth kinetic. CFM-incorporated Ag–Se NPs show additionally repressing potential more than Ag–Se NPs, and the superior effect was due to the catalytic potential of the antibacterial loaded CFM.86On the surface of nanocomposites, the generation of ROS may occur as a lethal factor against bacterial cells.87,88 CFM-incorporated Ag–Se NPs is specific for creating ROS, causing protein oxidation, bacterial DNA damage, and lipid peroxidation that can destroy the tested microbes. Additionally, the strong attraction between the synthesized nanocomposite and S. aureus membrane is due to the negative charge of the cells, the release of positive ions like (Ag+, and Se+), from the surface of the synthesized CFM-incorporated Ag–Se NPs, and the silver ions released having a positive charge and increasing the lethal connection. Another fit reactivity was duo to the nano-size, stability, purity, surface charge, and chemical configuration of the synthesized CFM-incorporated Ag–Se NPs, which could elevate the possibility to connect with more pathogenic bacteria.
In the related paper, Xu et al.,89 reported that after nanocomposite treatment and UV-exposure for about 80 minutes, the membrane of E. coli was malformed, and the death of bacteria occurred. Another report,90 stated that, novel synthesized nanocomposites demonstrated strong antibacterial behaviors against the tested E. coli and S. aureus.
Effect of UV on the antimicrobial potential of the synthesized nanocomposite
Fig. 10 indicates that the antimicrobial potential of the synthesized CFM-incorporated Ag–Se NPs, and Ag–Se NPs strongly improved with the increasing UV exposure period, and conformed to the deactivation of the S. aureus cells following UV illumination. The favorable impacts on the extension and development of S. aureus were reported with the exhibit time. The UV assay results confirmed that, the growth of bacterial cells positively influenced following the UV illumination of the synthesized CFM-incorporated Ag–Se NPs corresponded with the non-treated control, and the synthesized Ag–Se NPs.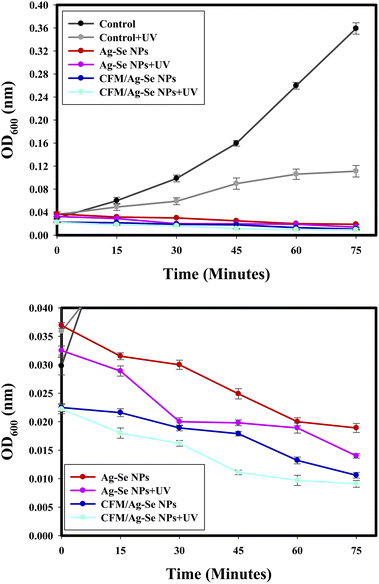 | ||
| Fig. 10 (a) The UV effect on the antibacterial activity of Ag–Se NPs, and CFM-incorporated Ag–Se NPs against S. aureus, and (b) Zooming area. | ||
UV investigation results indicated that the growth of bacteria is influenced and decreases after the UV illumination of the synthesized nanocomposites. UV radiation raises the possibility for CFM-incorporated Ag–Se NPs photo-activation more than Ag–Se NPs. CFM-incorporated Ag–Se NPs is a perfect biocidal agent after the excitation of UV irradiation.
Generally, most of the synthesized nanocomposites contributed to the formation of ROS following the acceptance of photons from the UV illumination process.91 The created ROS (as hydrogen peroxide) after the UV excitation may be the promising factor for the bacterial biocidal process after the interaction with the bacterial membrane. Finally, the infusion of the created ROS within the bacterial cell caused the formation of stable hydroxyl free radical, and the death of the bacterial cell was noted.87,88
Determination of protein leakage from bacterial cell membranes
In the protein leakage assay, the bacterial cell-free supernatant has conducted for the determination of the quantity of bacterial proteins released from the bacterial cells. Fig. 11, illustrated that the quantity of bacterial protein released from the cell is directly symmetrical to the increased concentrations of the synthesized Ag–Se NPs, and CFM-incorporated Ag–Se NPs and was found to be 256.25 μg mL−1, and 298.65 μg mL−1, respectively at the concentration of 1.0 mg mL−1. The present results presented the positive effect of the synthesized CFM-incorporated Ag–Se NPs, and confirmed the catalytic nature as antibacterial agent. CFM-incorporated Ag–Se NPs performs a noted spots around the tested bacterial cells and around S. aureus membrane, and the present facts confirmed the bleeding out of protein molecules from the membrane of S. aureus and approved their present in the bacterial cytoplasm.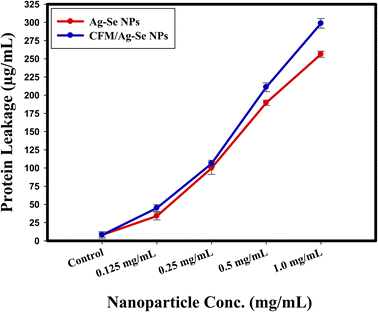 | ||
| Fig. 11 The effect of Ag–Se, and CFM-incorporated Ag–Se nanocomposites on the protein leakage from S. aureus cell membranes. | ||
The presented results indicated that the synthesized CFM-incorporated Ag–Se NPs improved the bacterial membrane leakage and changed the permeability of the bacterial cell membrane more than Ag–Se NPs. Additionally, the bacterial death was due to the interference with the plasma membrane through the protein leakage after the treatment with the synthesized CFM-incorporated Ag–Se NPs, which destroy the line defense of the bacterial cell.
From the literature point of view, recent articles like92 and,93 represent the exact inhibition results following the usage of novel nanocomposites and define the mode of action according to the concentration-dependence which entirely effected the bacterial protein leakage and subsequently destroy the plasma membrane of some selected pathogenic bacteria. A positive report conducted by Paul, et al.,94 verified that the exact determination of the protein leakage must be performed as a step for the reaction mechanism determination, and confirmed to be a critical method.
SEM reaction mechanism
The method of SEM imaging is considered the confirmatory test for the bacterial reaction mechanism determination, as stated in Fig. 12. The SEM imaging of the control S. aureus demonstrated the normal shape of the tested bacteria and normal number and conformation with the normal surface morphology as displayed in Fig. 12(a).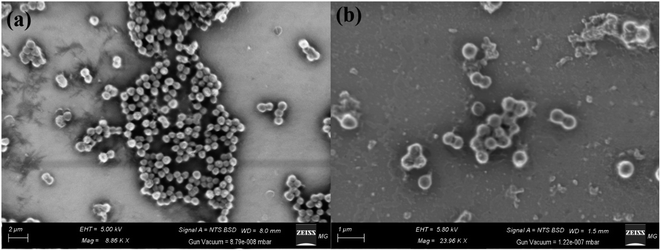 | ||
| Fig. 12 Reaction mechanism determination of CFM-incorporated Ag–Se NPs using SEM analysis where (a) control untreated S. aureus, and (b) Treated S. aureus. | ||
Positively, by the treatment of the synthesized CFM-incorporated Ag–Se NPs, abnormalities were noted as the irregular shape of the S. aureus cells as noted in Fig. 12(b). There are noted malformations as the semi-lysis of the external surface and deformations of the S. aureus cells. Additionally, the synthesized CFM-incorporated Ag–Se NPs achieved the semi-complete lysis of the cells, and a noted decrease in the total viable count. The treated cells in Fig. 12(b) confirms the formation of pits and spots along with the treated bacterial cell, which approved by the assay of protein leakage. Finally, we noted a layers of the synthesized CFM-incorporated Ag–Se NPs across the treated cells which may be because of the charge attractions between positively charged Ag+ founded in the synthesized CFM-incorporated Ag–Se NPs, and negatively charged bacterial cells.
From the literature point of view, the reaction mechanism in Fig. 13, and the inhibition potential of the synthesized CFM-incorporated Ag–Se NPs may be due to the creation of stable ROS from the reactive Ag NPs and Se NPs on the CFM-incorporated Ag–Se NPs surface.
The synthesized CFM-incorporated Ag–Se NPs may attach to the bacterial cells through the charge attraction which in turn altering the bacterial membrane and changing the microbial permeability due to the membrane break down. The response of bacterial cells seems in production of genes making an oxidative stress due to the positive action of the created ROS from the surface of the synthesized CFM-incorporated Ag–Se NPs.95
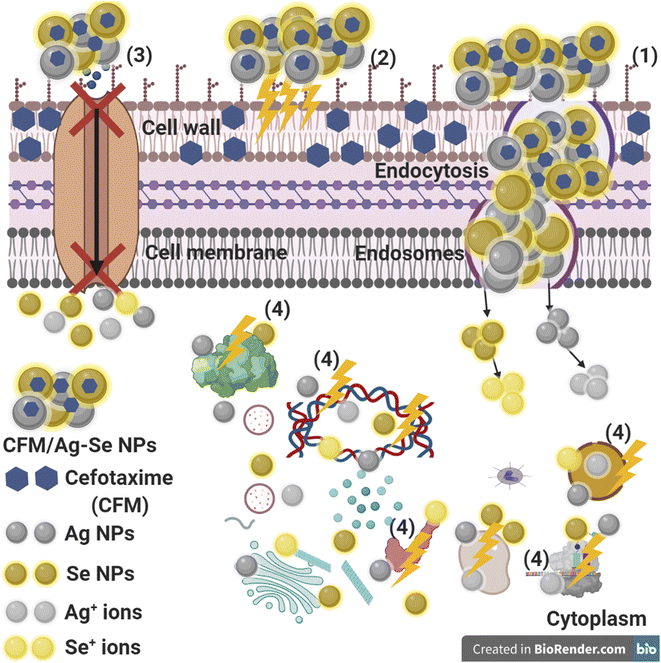
We recognize that the synthesized CFM-incorporated Ag–Se NPs start the action after the adhesion to the microbial surface by the charge attraction (Fig. 13), allowing the leakage of the bacterial membrane, which results in the formation of holes and cavities, and finally stops the ions transport from and inside the bacterial cells (Fig. 13).96 In response to the Ag NPs and Se NPs, ROS is created and penetrated inside the treated bacterial cell, with the response of harming the main micro organelles (like DNA, and plasmid). Finally, genotoxicity and cellular toxicity occurs due to the harmful effect and stress of the produced ROS.96
It is recommended that metal NPs could change the microbial morphology and their biofilm formation, reduce the microbial membrane permeability and provide the residence of oxidative stress genes concerning their responses because of the H2O2 generation.95 We understand that CFM-incorporated Ag–Se NPs begin their performance by wrapping and adhesion at the exterior surface of the microbial cell, producing membrane destruction and changing transport potential.96 Then, the distribution of metal NPs (Ag, and Se) inside the microbial cell divides all intracellular constructions like plasmid, DNA, and other essential organelle. Lastly in at acidic medium, the ionic species were created (Ag+, and Se+ ions), which making cellular toxicity, and genotoxicity due to the interaction among the negatively charged vital organs.96 Recently, Terzioglu et al.,97 explains that Ag NPs were shown to be more effective than Ag+ ions in E. coli, by interacting with the inner and outer membranes and releasing Ag+ ions locally upon Ag NP dissolution.
Conclusion
For the first time, this study is proposed an alternative eco-friendly method for the production of Ag NPs, Se NPs, and bimetallic Ag–Se NPs after the display to gamma rays and their incorporation with CFM drug. Complete characterization is performed to analyze the shape, crystallinity, distribution, and size of the synthesized NPs, which are shown to be spherical with an average size of 10.95, 20.54, and 12.69 nm for Ag NPs, Se NPs, and Ag–Se NPs, respectively. A suggested reaction mechanism describing the potential and constant reduction of ions due to the reduction by 15.0 kGy gamma rays (for Ag NPs, and Ag–Se NPs) and 10 kGy (for Se NPs) is introduced. Antimicrobial synergism potential is studied as ZOI assay and MIC toward some pathogenic microbes. CFM-incorporated Ag–Se NPs were able to inhibit the biofilm formation for S. aureus (96.09%), E. coli (98.32%), and C. albicans (95.93%). The growth rate of S. aureus in the control sample happened quickly, with the most potent optical density at λ = 600 nm (OD600) value having arrived at about 4.00 nm. After the addition of Ag–Se NPs, changes had been detected and OD600 was calculated to be 0.589 nm. In indifference, the OD600 value of CFM-incorporated Ag–Se NPs was the lowest (0.190 nm), showing the repression impact on the growth of S. aureus, and the effect of CFM addition. CFM-incorporated Ag–Se NPs are an excellent disinfectant once it had excited by UV light. In water and air, Ag NPs, and Se NPs receive photons, stopping in the construction of active hydroxyl (.OH) and new ROS (O2−, and H2O2) in the presence of O2 and H2O. It is observed that the quantity of cellular protein discharged from S. aureus is directly proportional to the concentration of Ag–Se NPs, and CFM-incorporated Ag–Se NPs and was found to be 256.25 μg mL−1, and 298.65 μg mL−1, respectively after the treatment with 1 mg mL−1 which proves the antibacterial characteristics of the Ag–Se NPs, and CFM-incorporated Ag–Se NPs, and explains the creation of holes in the cell membrane of S. aureus producing in the oozing out of the proteins from the S. aureus cytoplasm. The prepared nanocomposites can be promising agents for many fields in pharmaceutical and medical areas, especially antimicrobial agents against pathogenic microbe isolates from UTIs. The scientific soundness of this research concerns the promising antimicrobial activity at low concentrations against pathogenic microbes resistant to CFM which advanced their property for the possible application after synergistic incorporation for long-run purposes.Author's contributions
AAE suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. SSS supervision, suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. GSE supervision, suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing. MSA supervision, suggested the research topic, investigated the article, planned the research methodology, wrote the original draft, and participated in data representation and article revising and editing.Conflicts of interest
The authors declare that they have no conflict of interest.References
- P. V. Baptista, M. P. McCusker, A. Carvalho, D. A. Ferreira, N. M. Mohan, M. Martins and A. R. Fernandes, Front. Microbiol., 2018, 9, 1441 CrossRef PubMed.
- A. M. H. Al-Rajhi, S. S. Salem, A. A. Alharbi and T. M. Abdelghany, Arabian J. Chem., 2022, 15, 103927 CrossRef CAS.
- S. S. Salem, M. S. E. M. Badawy, A. A. Al-Askar, A. A. Arishi, F. M. Elkady and A. H. Hashem, Life, 2022, 12, 893 CrossRef CAS PubMed.
- A. H. Hashem, T. A. Selim, M. H. Alruhaili, S. Selim, D. H. M. Alkhalifah, S. K. Al Jaouni and S. S. Salem, J. Funct. Biomater., 2022, 13, 112 CrossRef CAS PubMed.
- A. Zaleska-Medynska, M. Marchelek, M. Diak and E. Grabowska, Adv. Colloid Interface Sci., 2016, 229, 80–107 CrossRef CAS PubMed.
- T. Mazhar, V. Shrivastava and R. S. Tomar, J. Pharm. Sci. Res., 2017, 9, 102 CAS.
- S. S. Salem and A. Fouda, Biol. Trace Elem. Res., 2021, 199, 344–370 CrossRef CAS PubMed.
- M. Prakash, H. P. Kavitha, S. Abinaya, J. P. Vennila and D. Lohita, Sustainable Chem. Pharm., 2022, 25, 100547 CrossRef CAS.
- G. Sharma, A. Kumar, S. Sharma, M. Naushad, R. P. Dwivedi, Z. A. ALOthman and G. T. Mola, J. King Saud Univ., Sci., 2019, 31, 257–269 CrossRef.
- S. Godipurge, S. Yallappa, N. J. Biradar, J. Biradar, B. Dhananjaya, G. Hegde, K. Jagadish and G. Hegde, Enzyme Microb. Technol., 2016, 95, 174–184 CrossRef CAS PubMed.
- R. Liu, J. Guo, G. Ma, P. Jiang, D. Zhang, D. Li, L. Chen, Y. Guo and G. Ge, ACS Appl. Mater. Interfaces, 2016, 8, 16833–16844 CrossRef CAS PubMed.
- G. Maduraiveeran, M. Sasidharan and W. Jin, Prog. Mater. Sci., 2019, 100574 CrossRef CAS.
- C. M. Crisan, T. Mocan, M. Manolea, L. I. Lasca, F.-A. Tăbăran and L. Mocan, Appl. Sci., 2021, 11, 1120 CrossRef CAS.
- G. S. El-Sayyad, H. S. El-Bastawisy, M. Gobara and A. I. El-Batal, Biol. Trace Elem. Res., 2020, 195, 323–342 CrossRef CAS PubMed.
- S. S. Salem, O. M. Ali, A. M. Reyad, K. A. Abd-Elsalam and A. H. Hashem, J. Fungi, 2022, 8, 126 CrossRef CAS PubMed.
- S. S. Salem, Appl. Biochem. Biotechnol., 2022, 194, 1898–1910 CrossRef CAS PubMed.
- A. H. Hashem and S. S. Salem, Biotechnol. J., 2022, 17, 2100432 CrossRef CAS PubMed.
- H. B. H. Rahuman, R. Dhandapani, V. Palanivel, S. Thangavelu, R. Paramasivam and S. Muthupandian, PLoS One, 2021, 16, e0256748 CrossRef CAS PubMed.
- S. S. Salem, E. F. El-Belely, G. Niedbała, M. M. Alnoman, S. E.-D. Hassan, A. M. Eid, T. I. Shaheen, A. Elkelish and A. Fouda, Nanomaterials, 2020, 10, 2082 CrossRef CAS.
- S. S. Salem, BioNanoScience, 2022 DOI:10.1007/s12668-022-01026-5.
- T. Jarzembowski, A. Daca and M. A. Dębska-Ślizień, Urinary Tract Infection: The Result of the Strength of the Pathogen, or the Weakness of the Host, BoD–Books on Demand, 2018 Search PubMed.
- S. V. Sánchez, N. Navarro, J. Catalán-Figueroa and J. O. Morales, Front. Cell. Infect. Microbiol., 2021, 11, 656496 CrossRef PubMed.
- M. Rawal, A. Singh and M. M. Amiji, Pharm. Res., 2019, 36, 1–20 CrossRef CAS PubMed.
- Y. Hiyama, T. Sato, S. Takahashi, S. Yamamoto, Y. Fukushima, C. Nakajima, Y. Suzuki, S.-i. Yokota and N. Masumori, J. Infect. Chemother., 2020, 26, 1272–1277 CrossRef CAS PubMed.
- A. L. Flores-Mireles, J. N. Walker, M. Caparon and S. J. Hultgren, Nat. Rev. Microbiol., 2015, 13, 269–284 CrossRef CAS PubMed.
- M. M. Al-Ansari, P. Dhasarathan, A. Ranjitsingh and L. A. Al-Humaid, J. King Saud Univ., Sci., 2020, 32, 3145–3152 CrossRef.
- R. Thombre, K. Jangid, R. Shukla and N. K. Dutta, Front. Microbiol., 2019, 2173 CrossRef.
- M. F. Al-Hakkani, Sustainable Chem. Eng., 2020, 33–42 CrossRef.
- A. K. Jindal, K. Pandya and I. D. Khan, Med J. Armed Forces India, 2015, 71, 178–181 CrossRef CAS PubMed.
- T. Al Hagbani, S. M. D. Rizvi, T. Hussain, K. Mehmood, Z. Rafi, A. Moin, A. S. Abu Lila, F. Alshammari, E.-S. Khafagy, M. Rahamathulla and M. H. Abdallah, Polymers, 2022, 14, 771 CrossRef CAS.
- I. E. Mba and E. I. Nweze, World J. Appl. Microbiol. Biotechnol., 2021, 37, 1–30 CrossRef.
- S. Sivaselvam, R. Selvakumar, C. Viswanathan and N. Ponpandian, Chemosphere, 2021, 275, 130061 CrossRef CAS PubMed.
- A. I. El-Batal, H. G. Nada, R. R. El-Behery, M. Gobara and G. S. El-Sayyad, RSC Adv., 2020, 10, 9274–9289 RSC.
- R. M. Fathy and A. Y. Mahfouz, J. Nanostruct. Chem., 2021, 11, 301–321 CrossRef CAS.
- A. H. Hashem, A. M. A. Khalil, A. M. Reyad and S. S. Salem, Biol. Trace Elem. Res., 2021, 1–11 Search PubMed.
- G. D. Christensen, W. A. Simpson, A. L. Bisno and E. H. Beachey, Infect. Immun., 1982, 37, 318–326 CrossRef CAS PubMed.
- M. A. Ansari, H. M. Khan, A. A. Khan, S. S. Cameotra and R. Pal, Appl. Nanosci., 2014, 4, 859–868 CrossRef CAS.
- A. I. El-Batal, G. S. El-Sayyad, N. E. Al-Hazmi and M. Gobara, J. Cluster Sci., 2019, 30, 947–964 CrossRef CAS.
- W. Huang, J.-Q. Wang, H.-Y. Song, Q. Zhang and G.-F. Liu, Asian Pac. J. Trop. Med., 2017, 10, 663–669 CrossRef CAS PubMed.
- M. Bekhit, S. H. El-Sabbagh, R. M. Mohamed, G. S. El-Sayyad and R. Sokary, J. Inorg. Organomet. Polym. Mater., 2021, 1–15 Search PubMed.
- M. Abd Elkodous, A. M. El-Khawaga, M. I. A. Abdel Maksoud, G. S. El-Sayyad, N. Alias, H. Abdelsalam, M. A. Ibrahim, M. A. Elsayed, G. Kawamura, Z. Lockman, W. K. Tan and A. Matsuda, Nanoscale, 2022, 14, 8306–8317 RSC.
- M. Abd Elkodous, G. S. El-Sayyad, S. M. Youssry, H. G. Nada, M. Gobara, M. A. Elsayed, A. M. El-Khawaga, G. Kawamura, W. K. Tan and A. I. El-Batal, Sci. Rep., 2020, 10, 1–22 CrossRef.
- H. Agarwal, A. Nakara, S. Menon and V. Shanmugam, J. Drug Delivery Sci. Technol., 2019, 53, 101212 CrossRef CAS.
- M. Relucenti, G. Familiari, O. Donfrancesco, M. Taurino, X. Li, R. Chen, M. Artini, R. Papa and L. Selan, Biology, 2021, 10, 51 CrossRef CAS PubMed.
- A. I. El-Batal, F. M. Mosallam, M. Ghorab, A. Hanora, M. Gobara, A. Baraka, M. A. Elsayed, K. Pal, R. M. Fathy and M. Abd Elkodous, Int. J. Biol. Macromol., 2020, 156, 1584–1599 CrossRef CAS PubMed.
- A. I. El-Batal, M. Abd Elkodous, G. S. El-Sayyad, N. E. Al-Hazmi, M. Gobara and A. Baraka, Int. J. Biol. Macromol., 2020, 165, 169–186 CrossRef CAS PubMed.
- D. Chudobová, K. Cihalova, P. Kopel, B. Ruttkay-Nedecky, M. Vaculovicova, R. Krozek and V. Adam, Kvasny Prum., 2015, 61, 51–56 CrossRef.
- X. Wang, M. Ramalingam, G. Chen, P. Ma and F.-Z. Cui, Biomimetics: Advancing Nanobiomaterials and Tissue Engineering, John Wiley & Sons, 2013 Search PubMed.
- A. I. El-Batal, G. S. El-Sayyad, A. El-Ghamry, K. M. Agaypi, M. A. Elsayed and M. Gobara, J. Photochem. Photobiol., B, 2017, 173, 120–139 CrossRef CAS.
- O. Borokhov and D. Schbert, ACS Symp. Ser., 2007, 967, 412–435 CrossRef CAS.
- H. Kolya, S. Pal, A. Pandey and T. Tripathy, Eur. Polym. J., 2015, 66, 139–148 CrossRef CAS.
- W. Yuzheng, X. Xiangxin and Y. He, Chin. J. Chem. Eng., 2014, 22, 474–479 CrossRef.
- A. I. El-Batal, G. S. El-Sayyad, A. El-Ghamery and M. Gobara, J. Cluster Sci., 2017, 28, 1083–1112 CrossRef CAS.
- H. Barabadi, S. Honary, P. Ebrahimi, A. Alizadeh, F. Naghibi and M. Saravanan, Inorg. Nano-Met. Chem., 2019, 49, 33–43 CrossRef CAS.
- A. I. El-Batal, G. S. El-Sayyad, N. E. Al-Hazmi and M. Gobara, J. Cluster Sci., 2019, 30, 947–964 CrossRef CAS.
- E. Castro-Longoria, A. R. Vilchis-Nestor and M. Avalos-Borja, Colloids Surf., B, 2011, 83, 42–48 CrossRef CAS PubMed.
- S. W. Han, Y. Kim and K. Kim, J. Colloid Interface Sci., 1998, 208, 272–278 CrossRef CAS.
- K. Govindaraju, S. K. Basha, V. G. Kumar and G. Singaravelu, J. Mater. Sci., 2008, 43, 5115–5122 CrossRef CAS.
- P. R. Garcia, O. Prymak, V. Grasmik, K. Pappert, W. Wlysses, L. Otubo, M. Epple and C. L. Oliveira, Nanoscale Adv., 2020, 2, 225–238 RSC.
- X. Zou, E. Ying and S. Dong, J. Colloid Interface Sci., 2007, 306, 307–315 CrossRef CAS.
- A. Baraka, S. Dickson, M. Gobara, G. S. El-Sayyad, M. Zorainy, M. I. Awaad, H. Hatem, M. M. Kotb and A. Tawfic, Chem. Pap., 2017, 71, 2271–2281 CrossRef CAS.
- A. S. Awed, G. S. El-Sayyad, A. El-ghandour, M. F. O. Hameed, M. I. A. Abdel Maksoud, A. I. El-Batal and S. S. A. Obayya, J. Cluster Sci., 2021, 32, 1–16 CrossRef CAS.
- A. Ashour, A. I. El-Batal, M. A. Maksoud, G. S. El-Sayyad, S. Labib, E. Abdeltwab and M. El-Okr, Particuology, 2018, 40, 141–151 CrossRef CAS.
- M. Mohsin, M. Jawad, M. A. Yameen, A. Waseem, S. H. Shah and A. J. Shaikh, PLASMONICS, 2020, 15, 1599–1612 CrossRef CAS.
- K. Gopinath, S. Kumaraguru, K. Bhakyaraj, S. Mohan, K. S. Venkatesh, M. Esakkirajan, P. Kaleeswarran, N. S. Alharbi, S. Kadaikunnan and M. Govindarajan, Microb. Pathog., 2016, 101, 1–11 CrossRef CAS PubMed.
- K. Loza, M. Heggen and M. Epple, Adv. Funct. Mater., 2020, 30, 1909260 CrossRef CAS.
- R. Borah and S. W. Verbruggen, J. Phys. Chem. C, 2020, 124(22), 12081–12094 CrossRef CAS.
- M. Clément, I. Abdellah, C. Martini, F. Fossard, D. Dragoe, H. Remita, V. Huc and I. Lampre, Nanoscale Adv., 2020, 2, 2768–2773 RSC.
- M. A. Maksoud, G. S. El-Sayyad, A. Ashour, A. I. El-Batal, M. S. Abd-Elmonem, H. A. Hendawy, E. Abdel-Khalek, S. Labib, E. Abdeltwab and M. El-Okr, Mater. Sci. Eng. C, 2018, 92, 644–656 CrossRef PubMed.
- K. Jyoti, M. Baunthiyal and A. Singh, J. Radiat. Res. Appl. Sci., 2016, 9, 217–227 CrossRef CAS.
- K. Bai, B. Hong, J. He, Z. Hong and R. Tan, Int. J. Nanomed., 2017, 12, 4527 CrossRef CAS.
- A. Gannoruwa, B. Ariyasinghe and J. Bandara, Catal. Sci. Technol., 2016, 6, 479–487 RSC.
- S. Poyraz, I. Cerkez, T. S. Huang, Z. Liu, L. Kang, J. Luo and X. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 20025–20034 CrossRef CAS.
- J. Russell, Clay Miner., 1979, 14, 109–114 CrossRef CAS.
- S. M. Montemayor, L. García-Cerda and J. Torres-Lubián, Mater. Lett., 2005, 59, 1056–1060 CrossRef CAS.
- K. M. Khazaei, S. Jafari, M. Ghorbani and A. H. Kakhki, Carbohydr. Polym., 2014, 105, 57–62 CrossRef PubMed.
- T. Moschakis, B. S. Murray and C. G. Biliaderis, Food Hydrocolloids, 2010, 24, 8–17 CrossRef CAS.
- R. Sukhotu, S. Guo, J. Xing, Q. Hu, R. Wang, X. Shi, K. Nishinari, Y. Fang and S. Guo, LWT--Food Sci. Technol., 2016, 68, 432–438 CrossRef CAS.
- A. I. El-Batal, N. M. Balabel, M. S. Attia and G. S. El-Sayyad, J. Cluster Sci., 2020, 31, 1021–1040 CrossRef CAS.
- A. I. El-Batal, N. E. Al-Hazmi, A. A. Farrag, M. A. Elsayed, A. M. El-Khawaga, G. S. El-Sayyad and A. A. Elshamy, Microb. Pathog., 2022, 164, 105440 CrossRef CAS PubMed.
- E. M. Halawani, A. M. Hassan and S. M. F. Gad El-Rab, Int. J. Nanomed., 2020, 15, 1889–1901 CrossRef CAS.
- C. Ashajyothi, K. H. Harish, N. Dubey and R. K. Chandrakanth, J. Nanostruct. Chem., 2016, 6, 329–341 CrossRef CAS.
- H.-J. Park, H. Y. Kim, S. Cha, C. H. Ahn, J. Roh, S. Park, S. Kim, K. Choi, J. Yi and Y. Kim, Chemosphere, 2013, 92, 524–528 CrossRef CAS PubMed.
- A. A. Mohamed, M. Abu-Elghait, N. E. Ahmed and S. S. Salem, Biol. Trace Elem. Res., 2021, 199, 2788–2799 CrossRef CAS PubMed.
- M. Abu-Elghait, M. Hasanin, A. H. Hashem and S. S. Salem, Int. J. Biol. Macromol., 2021, 175, 294–303 CrossRef CAS PubMed.
- Y. Zhang, T. P. Shareena Dasari, H. Deng and H. Yu, J. Environ. Sci. Health, Part C, 2015, 33, 286–327 CrossRef CAS PubMed.
- R. M. Fathy and A. Y. Mahfouz, J. Nanostruct. Chem., 2021, 11, 301–321 CrossRef CAS.
- A. Joe, S.-H. Park, D.-J. Kim, Y.-J. Lee, K.-H. Jhee, Y. Sohn and E.-S. Jang, J. Solid State Chem., 2018, 267, 124–133 CrossRef CAS.
- Y. Xu, Q. Liu, M. Xie, S. Huang, M. He, L. Huang, H. Xu and H. Li, J. Colloid Interface Sci., 2018, 528, 70–81 CrossRef CAS PubMed.
- I. Matai, A. Sachdev, P. Dubey, S. U. Kumar, B. Bhushan and P. Gopinath, Colloids Surf., B, 2014, 115, 359–367 CrossRef CAS PubMed.
- L. F. Gaunt, C. B. Beggs and G. E. Georghiou, IEEE Trans. Plasma Sci., 2006, 34, 1257–1269 CAS.
- S. Rajesh, V. Dharanishanthi and A. V. Kanna, J. Exp. Nanosci., 2015, 10, 1143–1152 CrossRef CAS.
- Z. Azam, A. Ayaz, M. Younas, Z. Qureshi, B. Arshad, W. Zaman, F. Ullah, M. Q. Nasar, S. Bahadur and M. M. Irfan, Microb. Pathog., 2020, 144, 104188 CrossRef CAS PubMed.
- D. Paul, S. Maiti, D. P. Sethi and S. Neogi, Adv. Powder Technol., 2021, 32, 131–143 CrossRef CAS.
- M. Abd Elkodous, G. S. El-Sayyad, I. Y. Abdelrahman, H. S. El-Bastawisy, A. E. Mohamed, F. M. Mosallam, H. A. Nasser, M. Gobara, A. Baraka, M. A. Elsayed and A. I. El-Batal, Colloids Surf., B, 2019, 180, 411–428 CrossRef CAS PubMed.
- M. A. Maksoud, G. S. El-Sayyad, A. M. El-Khawaga, M. Abd Elkodous, A. Abokhadra, M. A. Elsayed, M. Gobara, L. Soliman, H. El-Bahnasawy and A. Ashour, J. Hazard. Mater., 2020, 399, 123000 CrossRef PubMed.
- E. Terzioğlu, M. Arslan, B. G. Balaban and Z. P. Çakar, World J. Appl. Microbiol. Biotechnol., 2022, 38, 158 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: [DETAILS]. See https://doi.org/10.1039/d2ra04717a |
| This journal is © The Royal Society of Chemistry 2022 |